Abstract
Purpose
It has been well established in mammals that circadian behavior as well as the molecular clockwork can be synchronized to the light-dark (LD) cycle via the suprachiasmatic nucleus of the hypothalamus (SCN). In addition to light, it has been demonstrated that non-photic time cues, such as restricting the time of food availability, can alter circadian behavior and clock gene expression in selected peripheral tissues such as liver. Studies have also suggested that scheduled physical activity (exercise) can alter circadian rhythms in behavior and clock gene expression, however currently the effects of exercise alone are largely unknown and have not been explored in skeletal muscle.
Methods
Period2∷Luciferase (Per2∷Luc) mice were maintained under 12 hours of light followed by 12 hours of darkness (12L:12D) then exposed to 2 hours of voluntary or involuntary exercise during the light phase for 4 weeks. Control mice were left in home cages or moved to the exercise environment (sham). A second group of mice had restricted access to food (4 hours per day for 2 weeks) in order to compare the effects of two non-photic cues on PER2∷LUC bioluminescence. Skeletal muscles, lung and SCN tissue explants were cultured for 5-6 days to study molecular rhythms.
Results
In the exercised mice, the phase of peak PER2∷LUC bioluminescence was shifted in the skeletal muscle and lung explants but not the SCN suggesting a specific synchronizing effect of exercise on the molecular clockwork in peripheral tissues.
Conclusions
These data provide evidence that the molecular circadian clock in peripheral tissues can respond to the time of exercise suggesting that physical activity contributes important timing information for synchronization of circadian clocks throughout the body.
Keywords: PERIOD2∷LUCIFERASE, CIRCADIAN, NON-PHOTIC, TIME CUE
Introduction
It has been well documented that light-dark (LD) cycles can synchronize the expression of circadian clock genes in the suprachiasmatic nucleus of the hypothalamus (SCN) through direct innervation from the retina via the retinohypothalmic tract (15, 16). Rodents in which the SCN is ablated lose the ability to synchronize to the light-dark (LD) cycle and rhythms in locomotor behavior and physiology, such as plasma glucagon levels are lost (5, 26). These daily endogenous oscillations are thought to be driven by molecular clockwork within the organism (25). The circadian clock in mammals consists of several core components that work together through transcriptional and translational feedback loops and form the molecular basis for circadian rhythms in physiology (25). The core components of the molecular clockwork consist of positive regulators of transcription, Bmal1 and Clock and negative components Cry1/2 and Per1/2 whose protein products associate with the BMAL1:CLOCK hetereodimer in the nucleus and repress the activity of the transcription factor complex (4, 28). These circadian clock genes are expressed within most mammalian cells, are cell autonomous and continue to oscillate outside of the organism (36). Like any other timepiece, the molecular clockwork can be “set” to a specific time through external manipulation e.g. environmental time cues.
The most well characterized time cue or Zeitgeber is light. Through manipulation of the LD cycle, molecular and behavioral rhythms can be set experimentally or naturally through seasonal rotation around the sun (25). In addition to light, restricting a rodent’s food availability to a particular time of day has been shown to alter locomotor behavior, and shift gene expression of molecular clockwork components in a tissue specific manner (10, 14). Restricted feeding (RF) alters circadian rhythms in both SCN-intact and SCN-ablated mammals suggesting this non-photic time cue acts independently of the SCN (9). The synchronizing effects of food create robust locomotor responses in the hours preceding food presentation (31). This heightened activity is termed food anticipatory activity (FAA) and is characterized as an intense increase in locomotor behavior approximately 2-4 hours before the food is presented (10). RF also affects the expression pattern of molecular clockwork components (8). The effect of RF on circadian clocks appears to be tissue specific, e.g. the molecular clockwork in the liver exhibits altered gene expression in response to RF while there is no change in the SCN (10, 31). The ability of restricting feeding to alter animal behavior, molecular and physiological rhythms has lead researchers to conclude that there may exist a network of pacemakers with sensitivity to different environmental stimuli (30).
Scheduled exercise on a treadmill or running wheel has been shown to shift behavioral rhythms in mammals kept under constant darkness as well as entrain the free running rhythm (endogenous rhythm) of an animal placed in constant darkness (11, 21). Studies have also demonstrated that scheduled exercise in a novel running wheel can affect how quickly an animal entrains or synchronizes to a new LD cycle. In 2008, Yamanaka et al. demonstrated that scheduled bouts of wheel running could “accelerate re-entrainment” of locomotor activity and molecular rhythms in peripheral tissues to 8-hour shifts in the LD cycle. The LD cycle was phase advanced by 8-hours (one time) and a novel wheel was given at the beginning of the shift. After 4 days in the new lighting schedule, the mice with a running wheel shifted more quickly than the controls. Using Per1-luciferase mice, Yamanaka et al. measured bioluminescence from tissues cultured from the exercised and control mice. Skeletal muscle and lung explants from exercised mice had significantly phase-shifted Per1-luciferase bioluminescence rhythms, which is a measure of circadian clock function. This suggests that the molecular clockwork in the skeletal muscle and lung may be sensitive to scheduled exercise as a time cue (33).
In the current study, we wanted to test if scheduled exercise alone, independent of changing the LD cycle, could phase shift locomotor and molecular rhythms to an earlier time of day. Period2∷Luciferase (Per2∷Luc) mice (36) housed in a 12L:12D light schedule were exposed to scheduled bouts of either voluntary or involuntary exercise for 2 hours per day between ZT 4-6 for four weeks. We selected this zeitgeber time (ZT 4-6 hours after lights-on) based on previous work with rodents where novel wheel running produced the most significant phase shifts in locomotor activity during the middle of the day (lights-on) (24). In a second experiment we subjected mice to 4 hours of restricted feeding (RF) for 2 weeks. The consequences of RF on behavioral and molecular rhythms have been previously demonstrated (10, 31); therefore we decided to compare the effects of exercise with another well-established non-photic time cue. The primary finding in this study was that there was a significant shift (phase advance) in clock gene expression (PER2∷LUC bioluminescence) in three different skeletal muscles and the lung from exercised mice while the PER2∷LUC rhythm in the SCN remained un-shifted. This study demonstrates that scheduled exercise can alter the molecular clockwork in peripheral tissues of an SCN-intact animal and provides more direct evidence that exercise can act as a non-photic time cue.
Materials and Methods
Animals
23 male and 10 female Per2∷Luc mice on C57BL/6 background (36) approximately six months of age were housed individually in plastic cages measuring 12 × 6 × 5 inches without running wheels. All mice were housed in a light-controlled box with constant air exchange and ad libitum access to food and water. The mice had no prior exposure to running wheels or a treadmill and were housed under the same lighting schedule with 12L:12D (12 hours of light:12 hours of dark, lights on at 6am, Eastern Standard Time). At the conclusion of the exercise study, mice were anesthetized with isoflurane followed by decapitation starting 8 hours before lights-off (ZT 4-6).
Activity Monitoring
Voluntary locomotor activity in the cage was continuously monitored throughout each experiment using passive infrared motion detectors (Aurora by Ademco®, Syosset, NY) and ClockLab analysis software. Activity was recorded in one-minute bins.
Experimental Procedure
All mice had 2 weeks of acclimation to the 12L:12D cycle before 4 weeks of exercise sessions began. ZT 12 signifies the time of lights-off and the onset of locomotor activity for a nocturnal animal. From ZT 4-6 mice were removed from the home cages and placed individually in new cages containing voluntary running wheels n=10, involuntary treadmill running n=5, or exposed to a locked wheel (Sham W, n=6) or non-moving treadmill (Sham T, n=4). Control mice n=8 remained in the cage. Mice on the treadmill were run for two hours; with one ten minute break after the first hour, at a speed of 16m/min 0% grade for a total of approximately 1.76 kilometers (Exer 3/6 Treadmill, Columbus Instruments, Columbus, OH). Treadmill speed was chosen based on previous work (21) with the goal trying to match running intensities with that reported for C57BL6/J mice in voluntary wheels. Others have demonstrated a 60% of VO2 max for mice running at a similar rate (18). Wheel run mice had free access to a running wheel for two hours and averaged approximately 1.06 ± 0.17 kilometers/exercise session, which was similar to the intensity and distance for the treadmill mice and to previously reported values for this strain of mice (13, 19).
For the restricted feeding experiment 10 Per2∷Luc mice (6 male and 4 female) approximately six months of age were housed individually with the same lighting schedule as described above. The mice were split into two groups with either free access to food (FF), n=5 or restricted feeding (RF), n=5. Mice were housed in a 12L:12D cycle with ad libitum access to food and water for 2 weeks. On the first day of restricted feeding, food was removed from the RF group at ZT 8 (or 4 hours before lights off). On the next day food was restricted to four hours between ZT 4-8. It is important to note that this design is to restrict the time of feeding but not to calorically restrict the mice. RF mice consumed approximately 3.4 ± 0.2g of food/day, and previous studies report that in daily food intake for C57BL/6 mice is ~4g of chow (3). At the conclusion of the study, body mass was not different from controls, FF 28.7 ± 2.3g vs. RF 26.3 ± 1.2g.
Explant Cultures
Explants were taken from the Per2∷Luc reporter mice on the day after the final exercise session or following the final day of restricted feeding and analyzed for 6 days in order to measure circadian characteristics of the bioluminescence rhythms. Tissue culture dissection media and culturing media were used as reported previously (34-36). Briefly, the brain was removed and placed in chilled Hanks balanced salt solution supplemented with 25 units/mL penicillin, 25ug/mL streptomycin (Invitrogen), 10mM HEPES (Sigma), and 4mM NaHCO3 (Fisher Scientific). The brain was sectioned using a vibrating microtome (Vibratome series 1000 EM Corp, Chestnut Hill, MA). The SCN was dissected and placed on a Millicell insert (Millipore) in a 35mm tissue culture dish (Sigma) containing 1 mL of DMEM without phenol red (Invitrogen) supplemented with 4mM NaHCO3, 10mM HEPES, 25 units/mL penicillin, 25ug/mL streptomycin, 3.5g d-glucose (Sigma), 2%B27 (Gibco), and 0.1mM D-luciferin firefly, potassium salt (Biosynth). Peripheral tissues included lung, and three different skeletal muscles; flexor digitorum brevis (FDB), extensor digitorum longus (EDL), and soleus. These tissues were dissected from the mouse, the lung was hand sliced in dissection media and the muscles were removed from tendon to tendon and cultured in the same culture media containing 5% FBS (Invitrogen). Tissue bioluminescence was measured using a LumiCycle (Actimetrics, Wilmette, IL) housed in a light-tight, water-jacketed incubator at 36.5° C. LumiCycle software was used to collect raw bioluminescence data in 1.2 minute bins every 10 minutes that was stored on an attached computer. Similar to what others have published (36), the raw data were smoothed by 0.5 hour adjacent averaging using LumiCycle analysis software. Baseline-subtracted data were then used to calculate the phase of the PER2∷LUC bioluminescence rhythms of the cultured tissues, using ClockLab (Actimetrics, Wilmette, IL). The phase was measured as the time of the first peak of the PER2∷LUC bioluminescence rhythm after 24 hours in culture.
Histological Sections
Following 6 days in culture, soleus muscles were removed from the LumiCyle and placed in a vial with 4% paraformaldehyde and stored at 4°C for 24 hours, then placed in 70% ethanol until embedded in paraffin. Control soleus muscles were prepared immediately following euthanasia. Five micrometer cross-sections were taken starting at the tendon. Sections were stained with hemotoxylin and eaosin (H&E) (Sigma). Images of the muscle cross sections were taken with a Nikon DS-Ri1 using a Nikon Eclipse (E600) microscope at 20X.
Animal Care and Use
All procedures, which complied with the guidelines of the American Association for Accreditation of Animal Care (AAALAC), were approved by the University of Kentucky Institutional Animal Care and Use Committee. All procedures adhere to the American College of Sports Medicine standards for animal care.
Statistics
To analyze the locomotor behavior for all experiments, ANOVA was used with post hoc Dunnett’s. Note: 33 mice began the exercise study but only 30 were used for locomotor behavioral analysis due to technical problems with the motion detectors. The phase of peak PER2∷LUC bioluminescence between exercise groups within each tissue were compared using ANOVA with post hoc Tukey. The phase of peak PER2∷LUC bioluminescence was compared using Student’s t-test.
Results
Restricted feeding alters the pattern of locomotor activity over the day without decreasing overall cage activity
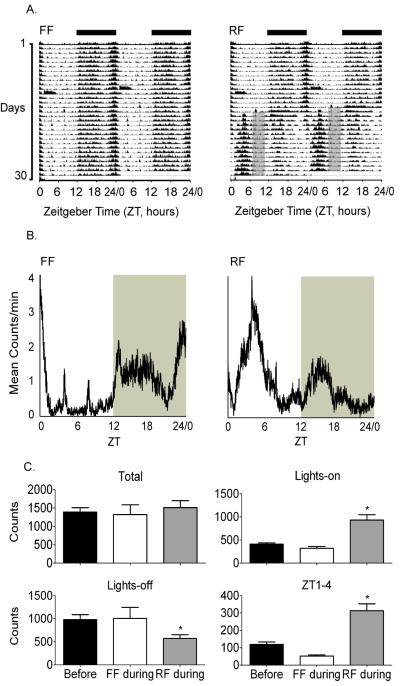
Total cage activity was not different between FF or RF mice during restricted feeding. During lights-on, activity was increased in the RF group and during lights-off locomotor cage activity was decreased. The double-plotted actograms in Figure 1A represent total locomotor cage activity over the day for the entire experiment. Days are listed on the y-axis and time on the x-axis. Zeitgeber time refers to the time of day relative to the LD cycle with ZT 12 indicating the time of lights-off. Figure 1B contains cage activity profiles averaged from the last two weeks of the experiment. In the FF group, activity was relatively low during lights-on and higher during lights-off. In contrast, the RF mice had significantly increased cage activity during lights-on especially during the 2-3 hours before food was presented. This anticipatory activity occurred in the hours immediately before food was presented, ZT 1-4, Figure 1C. Locomotor cage activity, before the start of restricted feeding, was averaged for both groups and is presented in Figure 1C, black bars.
Figure 1. Restricted feeding alters the pattern of locomotor activity over the day without decreasing overall activity.
(A) Double-plotted actograms of locomotor activity counts measured in the home cage averaged from free feeding (FF) or restricted feeding (RF), n=5 mice per group, dark bars indicate lights-off and gray shaded areas indicate time of restricted feeding in RF mice. (B) Activity profiles calculated during the two weeks of restricted feeding, averaged from n=5 per group. Gray shaded area indicates lights-off. (C) Mean activity counts over the entire day (Total), during lights-on, lights off, and anticipatory activity from ZT 1-4. Before refers to the averaged locomotor cage activity from all groups during time points indicated on each graph. * indicates significant from before (*p<0.05, mean ± SEM, ANOVA, post hoc Dunnett’s).
Restricted feeding phase shifts PER2∷LUC bioluminescence in peripheral tissues
Figure 2 includes bioluminescence data from the Per2∷Luc mice exposed to restricted feeding. Figure 2A is a graph of raw bioluminescence data from two representative soleus muscle explants. The explants remained rhythmic for several days in culture. Figure 2B is a phase plot of quantified bioluminescence data from lung and soleus cultures. In the RF group PER2∷LUC bioluminescence rhythms from both tissues displayed a phase-shift to an earlier time of day suggesting that the molecular clockwork in these tissues had been reset to a different time of day following RF. The phase shift was greater in the lung, ~13hours (anti-phase to FF) than in the soleus, ~3hours. Figure 2C provides histological data from soleus muscles fixed and stained with H&E either immediately following dissection (Control) or following 5 days in culture (LumiCycle). The fibers within the soleus muscles remain structurally intact following the static culture with no indication of necrosis, suggesting that the integrity and organization of the muscle is maintained. This morphological data are consistent with the bioluminescent results and provide confirmation that the muscle explants are transcriptionally and translationally active during the 5 days in culture.
Figure 2. Restricted feeding phase shifts PER2∷LUC bioluminescence in peripheral tissues.
(A) Representative raw bioluminescence data from FF in black or RF in gray. (B) Phase plot of cultured explants (mean ± SEM), black squares indicate FF, open squares RF. Mean phase data from Per2∷Luc explants was calculated using the time of peak bioluminescence following 24 hours in culture. In the RF group PER2∷LUC bioluminescence demonstrated a significant phase shift in both the lung and soleus explants (*p<0.05, mean ± SEM, Student’s t-test). (C) Representative sections from control soleus (fixed immediately) and one soleus that had been in the LumiCyle for 5 days, stained with H&E, imaged at 20X.
Locomotor cage activity is decreased during the dark phase in wheel, treadmill, and sham treadmill mice
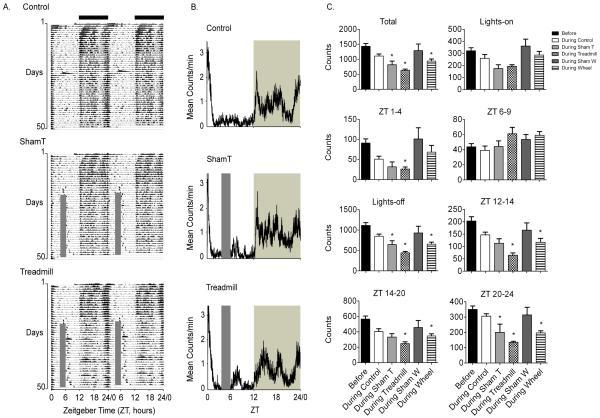
Figure 3A shows locomotor cage activity (double-plotted actograms) for control, sham treadmill and treadmill mice for the entire study, dark horizontal bars indicate lights-off and gray vertical bars (ZT 4-6) indicate exercise time. Control mice were primarily active in the dark, ZT 12-24/0, throughout the experiment. Treadmill and wheel mice (wheel data not shown) displayed locomotor cage activity that was similar to controls for the first 2 weeks (before exercise) in LD, but began to change after the exercise regimen started. Sham wheel mice exposed to wheels during ZT 4-6 displayed home cage locomotor activity that was similar to the 2 weeks before exercise began (not shown) while sham treadmill (ShamT) mice placed on a non-moving treadmill exhibited diminished home cage locomotor activity during the weeks of exercise compared to the weeks before exercise began. Figure 3B shows mean activity profiles for the same three groups in 3A calculated by averaging the home cage locomotor activity during the last 2 weeks of exercise (gray vertical bars indicate exercise time and shaded area denotes time of lights-off). Both 3A and 3B demonstrate that the mice continued to display a short burst of locomotor activity at the dark to light transition around ZT24/0. This pattern is maintained in the exercised and sham animals (as well as control) and is common for C57BL/6 mice (4). Before ZT 4-6 (exercise) values were averaged from all groups during the 2 weeks prior to start of the exercise paradigm, Figure 3C black bars. During the weeks of the exercise treatment, there was no significant increase in locomotor cage activity during lights-on and no anticipatory activity between ZT 1-4. Total locomotor cage activity (22 hours in the home cage) decreased in wheel (~40%) and treadmill (~60%) groups across the lights-off period. The decrease in activity in the sham treadmill (~40%) group was primarily due to a decrease in activity between ZT 20-24 at the end of the lights-off period (Figure 3C).
Figure 3. Alterations in locomotor cage activity following exercise in mice.
(A) Double-plotted actograms of locomotor behavior throughout the experiment and (B) averaged activity profiles (calculated from the last two weeks) from control (n=3), sham treadmill (n=4) and treadmill (n=5) mice. (C) Mean activity counts throughout the day in control, sham treadmill (ShamT), treadmill, sham wheel (ShamW) and wheel groups. Before (black bars) is the locomotor cage activity in the two weeks prior to exercise averaged from all groups during time points indicated on each graph. During control, during ShamT, during treadmill, during ShamW, and during wheel were calculated from the final two weeks of exercise, * indicates significant from before (*p<0.05, mean ± SEM, ANOVA, post hoc Dunnett’s). Dark bars (A) and shaded area (B) represent time of lights-off, gray bars (A and B) indicate when animals were exposed to exercise.
Scheduled voluntary or involuntary exercise shifts the circadian clock in skeletal muscles and the lung
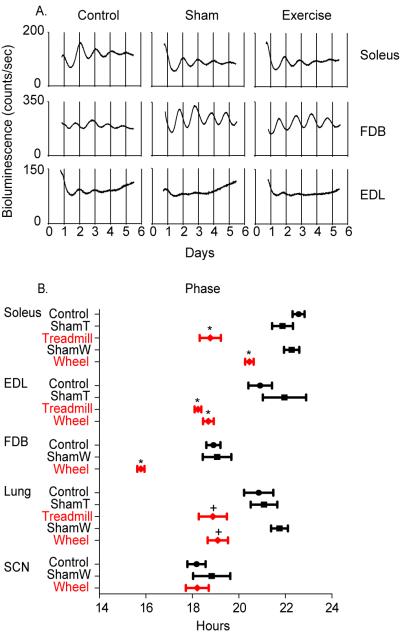
Raw bioluminescence data from three different skeletal muscles demonstrated rhythmic expression of PER2∷LUC for 5 days in static culture, Figure 4A. For soleus and extensor digitorum longus (EDL) representative images are from control, sham treadmill (Sham) and treadmill (Exercise). For the flexor digitorum brevis (FDB) representative images are from control, sham wheel (Sham) and wheel (Exercise) groups. The phase of the PER2∷LUC bioluminescence rhythm was significantly shifted in all three different skeletal muscles explanted from mice exposed to voluntary or involuntary exercise but not controls or shams, Figure 4B. The phase of the PER2∷LUC bioluminescence rhythm in the soleus muscle (postural muscle) was advanced by approximately 3 hours in the treadmill and 2 hours in the wheel running groups. In the extensor digitorum longus (EDL) and flexor digitorum brevis (FDB) muscles (recruited intermittently, used to extend/flex the toes) there were approximate 3 hours and 2 hours (3 hours in FDB, wheel only) advances in the treadmill and wheel running groups, respectively. In the lung the phase of PER2∷LUC bioluminescence was advanced by approximately 2 hours in both exercise conditions but was only statistically significant when compared with sham wheel. The phase of PER2∷LUC bioluminescence in the SCN remained unchanged with exercise.
Figure 4. Scheduled voluntary or involuntary exercise shifts PER2∷LUC bioluminescence in several skeletal muscles and lung.
(A) Representative graphs of raw bioluminescence data (counts/sec) from Per2∷Luc tissue cultured explants over 5-6 days. For soleus and EDL representative raw data from control, ShamT, and treadmill groups. For FDB representative raw data from control, ShamW, and wheel. (B) The phase of PER2∷LUC bioluminescence was calculated using the time of peak bioluminescence following 24 hours in culture. Phase plot of cultured explants (mean ± SEM), black circles indicate control, black squares for sham groups, and red diamonds indicate both treadmill and wheel. In all three skeletal muscles from exercised mice the phase of PER2∷LUC bioluminescence was shifted to an earlier time (advanced) compared to control and in the lung compared to ShamW. There was no significant shift in the SCN. (*p<0.05, mean ± SEM, ANOVA within each tissue, post hoc Tukey, +p<0.05 compared with ShamW).
Discussion
In the present study, scheduled exercise during lights-on, produced significant phase shifts in PER2∷LUC bioluminescence rhythms in skeletal muscles and the lung. These data indicate that the circadian clock in skeletal muscle and lung tissues, are sensitive to scheduled bouts of exercise while in a normal lighting environment. In addition we found that 4 weeks of low intensity endurance exercise is sufficient to significantly shift the clock. Similar to what has been published previously (8, 10, 31), restricted access to food for 4 hours/day for two weeks produced a significant phase shift in PER2∷LUC bioluminescence rhythms in skeletal muscle and lung explants. Interestingly the magnitude of the phase shift (~2-3hrs) in the skeletal muscle (soleus) was comparable in both the RF and exercise studies. However in the lung the phase advance was much larger with RF (~13hrs) than scheduled exercise (~2hrs). This demonstrates that 2 weeks of RF and 4 weeks of exercise have a similar effect on the clock in skeletal muscle, but RF has a greater effect on the lung. Together these data support the concept that the endogenous rhythms of circadian clocks in peripheral tissues can be altered by non-photic environmental stimuli such as RF and exercise, however the pathways may be different.
The RF mice demonstrated anticipatory activity in the hours preceding the feeding time cue unlike what we saw in the mice given the scheduled exercise time cue. The mechanisms behind anticipatory activity are not well understood, but food anticipatory activity (FAA) has been observed in rodents with SCN lesions or lacking core clock genes suggesting that FAA is not dependent on the canonical clock or SCN driven pathways (8, 32). Some evidence suggests that FAA is the result of RF altering circulating metabolic and hormonal factors (e.g. glucagon, insulin, corticosterone and melatonin) thereby setting peripheral oscillators that then feedback to the brain (12). Scheduled exercise did not produce anticipatory activity demonstrating that non-photic environmental cues alter locomotor activity differentially and may be driven by separate pathways. Both RF and scheduled exercise are able to “set” behavioral rhythms when rodents are housed in the absence of light, but the results of this study indicate that only RF can phase shift locomotor activity in the presence of a normal LD cycle (8, 11).
Although exercise did not induce anticipatory activity, locomotor behavior in the wheel running group, the treadmill group and, interestingly, the sham treadmill group was reduced throughout the dark-phase. Although exercise did not induce anticipatory activity, we did see a reduction in cage activity behavior in the wheel running group, the treadmill group the sham treadmill group but not the sham wheel group throughout the dark-phase. The observation that the sham treadmill group also exhibited a lower cage activity indicates that this effect is not solely due to exercise. For this study, the mice in the wheel running cages were moved to a separate light box similar to the one containing the home cages. In contrast the treadmill exercise and sham treadmill mice were moved outside the box but in the same room in the animal facility for that 2-hour period during the light phase. The reason why sham treadmill mice were less active in the home cage while the sham wheel mice (moved to a new cage with a locked wheel) showed no change in locomotor activity levels is not clear. One explanation could be the increased stress of moving the mice out of the light box into the room with the treadmill. One study demonstrated that mice exposed to social stress had decreased activity around the home cage (7). We cannot determine if the sham treadmill mice were more stressed however, the fact that the wheel mice but not sham wheel mice had less locomotor activity during the dark argues that this lowered behavior is not as simple as one factor. It is also important to point out that the phase of the molecular circadian clock was not altered in the peripheral tissues of the sham treadmill mice so whatever factor(s) influenced locomotor cage activity did not contributing to the non-photic cue with exercise.
It has been well demonstrated that altering lighting conditions and restricting an animal’s feeding to a specific time of day, can shift locomotor behavior and molecular clockwork gene expression as well as clock controlled genes with tissue specificity (27, 37). The consequences of scheduled exercise on the circadian transcriptome in skeletal muscle are unknown at this time, however it has been demonstrated that several genes involved in metabolism are expressed in a circadian manner (22, 23). In skeletal muscle, genes involved in fatty acid metabolism such as PGC-1β, UCP3, Dbt, Dgat2, and Acat2 have been shown to oscillate in a circadian manner (period ~24hrs) and are important for metabolic functions such as fatty acid oxidation (17, 20), fatty/cholesterol synthesis (1, 6). Because daily activities for most species occur with some circadian rhythmicity (active phase-inactive phase every 24hrs) and skeletal muscle is a highly metabolic tissue, it is reasonable that genes involved in energy storage/utilization would also be under circadian control in order to anticipate the daily demands on the system. The current study has demonstrated that low intensity scheduled exercise can shift the circadian molecular clock in skeletal muscle, and we suggest that this could lead to altered metabolic processes, in the presence of an LD cycle. With increasing associations between clock disruption and metabolic disease, scheduled exercise may be useful as a lifestyle treatment to synchronize rhythms in humans (2, 29).
A substantial amount of work has been done in the field of chronobiology to understand the relationship between time cues and central and peripheral circadian clocks within the organism (12, 25). The exercised mice in this study were exposed to two zeitgebers, a constant LD cycle and scheduled exercise. We have demonstrated that the molecular clocks in peripheral tissues in mice with an intact central clock (SCN) were shifted with exposure to voluntary or involuntary exercise for 2 hours per day for 4 weeks. These data provide further evidence that exercise alone has effects on the molecular clock and strengthens the hypothesis that exercise has the ability to influence the network of oscillators that exist within the mammalian system.
Acknowledgments
Support for this study was provided by the NIH, grant numbers AR055246 and ES018636 to KAE and T32 fellowship, grant number HL086341-02 to GW. Special thanks to Cynthia Long and Mellani Lefta for assistance with histological sections. There are no disclosures. The results from this study do not constitute endorsement by the American College of Sports Medicine.
The NIH provided support for this study.
Footnotes
Disclosures: None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson RA, Joyce C, Davis M, Reagan JW, Clark M, Shelness GS, Rudel LL. Identification of a form of acyl-CoA:cholesterol acyltransferase specific to liver and intestine in nonhuman primates. The Journal of biological chemistry. 1998;273(41):26747–54. doi: 10.1074/jbc.273.41.26747. [DOI] [PubMed] [Google Scholar]
- 2.Antunes LC, Levandovski R, Dantas G, Caumo W, Hidalgo MP. Obesity and shift work: chronobiological aspects. Nutrition research reviews. 2010;23(1):155–68. doi: 10.1017/S0954422410000016. [DOI] [PubMed] [Google Scholar]
- 3.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behavior genetics. 2002;32(6):435–43. doi: 10.1023/a:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103(7):1009–17. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cailotto C, La Fleur SE, Van Heijningen C, Wortel J, Kalsbeek A, Feenstra M, Pevet P, Buijs RM. The suprachiasmatic nucleus controls the daily variation of plasma glucose via the autonomic output to the liver: are the clock genes involved? Eur J Neurosci. 2005;22(10):2531–40. doi: 10.1111/j.1460-9568.2005.04439.x. [DOI] [PubMed] [Google Scholar]
- 6.Chuang DT, Hu CC, Ku LS, Niu WL, Myers DE, Cox RP. Catalytic and structural properties of the dihydrolipoyl transacylase component of bovine branched-chain alpha-keto acid dehydrogenase. The Journal of biological chemistry. 1984;259(14):9277–84. [PubMed] [Google Scholar]
- 7.Dalm S, de Visser L, Spruijt BM, Oitzl MS. Repeated rat exposure inhibits the circadian activity patterns of C57BL/6J mice in the home cage. Behav Brain Res. 2009;196(1):84–92. doi: 10.1016/j.bbr.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 8.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14(23):2950–61. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson AJ, Stephan FK. Feeding-entrained circadian rhythms in hypophysectomized rats with suprachiasmatic nucleus lesions. Am J Physiol. 1999;277(5 Pt 2):R1376–84. doi: 10.1152/ajpregu.1999.277.5.R1376. [DOI] [PubMed] [Google Scholar]
- 10.Davidson AJ, Stokkan KA, Yamazaki S, Menaker M. Food-anticipatory activity and liver per1-luc activity in diabetic transgenic rats. Physiol Behav. 2002;76(1):21–6. doi: 10.1016/s0031-9384(02)00680-7. [DOI] [PubMed] [Google Scholar]
- 11.Edgar DM, Dement WC. Regularly scheduled voluntary exercise synchronizes the mouse circadian clock. Am J Physiol. 1991;261(4 Pt 2):R928–33. doi: 10.1152/ajpregu.1991.261.4.R928. [DOI] [PubMed] [Google Scholar]
- 12.Escobar C, Cailotto C, Angeles-Castellanos M, Delgado RS, Buijs RM. Peripheral oscillators: the driving force for food-anticipatory activity. Eur J Neurosci. 2009;30(9):1665–75. doi: 10.1111/j.1460-9568.2009.06972.x. [DOI] [PubMed] [Google Scholar]
- 13.Esser KA, Harpole CE, Prins GS, Diamond AM. Physical activity reduces prostate carcinogenesis in a transgenic model. The Prostate. 2009;69(13):1372–7. doi: 10.1002/pros.20987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feillet CA, Mendoza J, Pevet P, Challet E. Restricted feeding restores rhythmicity in the pineal gland of arrhythmic suprachiasmatic-lesioned rats. Eur J Neurosci. 2008;28(12):2451–8. doi: 10.1111/j.1460-9568.2008.06538.x. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Hernandez F, Aguilar-Roblero R, Drucker-Colin R. Transplantation of the fetal occipital cortex to the third ventricle of SCN-lesioned rats induces a diurnal rhythm in drinking behavior. Brain Res. 1987;418(1):193–7. doi: 10.1016/0006-8993(87)90980-2. [DOI] [PubMed] [Google Scholar]
- 16.Johnson RF, Moore RY, Morin LP. Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypothalamic tract. Brain Res. 1988;460(2):297–313. doi: 10.1016/0006-8993(88)90374-5. [DOI] [PubMed] [Google Scholar]
- 17.Kelly DP, Kim JJ, Billadello JJ, Hainline BE, Chu TW, Strauss AW. Nucleotide sequence of medium-chain acyl-CoA dehydrogenase mRNA and its expression in enzyme-deficient human tissue. Proc Natl Acad Sci U S A. 1987;84(12):4068–72. doi: 10.1073/pnas.84.12.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kemi OJ, Loennechen JP, Wisloff U, Ellingsen O. Intensity-controlled treadmill running in mice: cardiac and skeletal muscle hypertrophy. J Appl Physiol. 2002;93(4):1301–9. doi: 10.1152/japplphysiol.00231.2002. [DOI] [PubMed] [Google Scholar]
- 19.Lerman I, Harrison BC, Freeman K, Hewett TE, Allen DL, Robbins J, Leinwand LA. Genetic variability in forced and voluntary endurance exercise performance in seven inbred mouse strains. J Appl Physiol. 2002;92(6):2245–55. doi: 10.1152/japplphysiol.01045.2001. [DOI] [PubMed] [Google Scholar]
- 20.MacLellan JD, Gerrits MF, Gowing A, Smith PJ, Wheeler MB, Harper ME. Physiological increases in uncoupling protein 3 augment fatty acid oxidation and decrease reactive oxygen species production without uncoupling respiration in muscle cells. Diabetes. 2005;54(8):2343–50. doi: 10.2337/diabetes.54.8.2343. [DOI] [PubMed] [Google Scholar]
- 21.Marchant EG, Mistlberger RE. Entrainment and phase shifting of circadian rhythms in mice by forced treadmill running. Physiol Behav. 1996;60(2):657–63. doi: 10.1016/s0031-9384(96)80045-x. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy JJ, Andrews JL, McDearmon EL, Campbell KS, Barber BK, Miller BH, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiological genomics. 2007;31(1):86–95. doi: 10.1152/physiolgenomics.00066.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, Andrews JL, Antoch MP, Walker JR, Esser KA, Hogenesch JB, Takahashi JS. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci U S A. 2007;104(9):3342–7. doi: 10.1073/pnas.0611724104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mrosovsky N. Locomotor activity and non-photic influences on circadian clocks. Biol Rev Camb Philos Soc. 1996;71(3):343–72. doi: 10.1111/j.1469-185x.1996.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 25.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935–41. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 26.Ruiter M, La Fleur SE, van Heijningen C, van der Vliet J, Kalsbeek A, Buijs RM. The daily rhythm in plasma glucagon concentrations in the rat is modulated by the biological clock and by feeding behavior. Diabetes. 2003;52(7):1709–15. doi: 10.2337/diabetes.52.7.1709. [DOI] [PubMed] [Google Scholar]
- 27.Saito H, Terada T, Shimakura J, Katsura T, Inui K. Regulatory mechanism governing the diurnal rhythm of intestinal H+/peptide cotransporter 1 (PEPT1) Am J Physiol Gastrointest Liver Physiol. 2008;295(2):G395–402. doi: 10.1152/ajpgi.90317.2008. [DOI] [PubMed] [Google Scholar]
- 28.Sato TK, Yamada RG, Ukai H, Baggs JE, Miraglia LJ, Kobayashi TJ, Welsh DK, Kay SA, Ueda HR, Hogenesch JB. Feedback repression is required for mammalian circadian clock function. Nature genetics. 2006;38(3):312–9. doi: 10.1038/ng1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106(11):4453–8. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stephan FK. The “other” circadian system: food as a Zeitgeber. J Biol Rhythms. 2002;17(4):284–92. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- 31.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291(5503):490–3. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 32.Storch KF, Weitz CJ. Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. Proc Natl Acad Sci U S A. 2009;106(16):6808–13. doi: 10.1073/pnas.0902063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamanaka Y, Honma S, Honma K. Scheduled exposures to a novel environment with a running-wheel differentially accelerate re-entrainment of mice peripheral clocks to new light-dark cycles. Genes Cells. 2008;13(5):497–507. doi: 10.1111/j.1365-2443.2008.01183.x. [DOI] [PubMed] [Google Scholar]
- 34.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288(5466):682–5. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 35.Yamazaki S, Takahashi JS. Real-time luminescence reporting of circadian gene expression in mammals. Methods Enzymol. 2005;393:288–301. doi: 10.1016/S0076-6879(05)93012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101(15):5339–46. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G, Wu X, Goh BC, Mynatt RL, Gimble JM. Characterization of peripheral circadian clocks in adipose tissues. Diabetes. 2006;55(4):962–70. doi: 10.2337/diabetes.55.04.06.db05-0873. [DOI] [PubMed] [Google Scholar]