Abstract
Major depressive disorder (MDD) has been linked to changes in function and activity of the hippocampus, one of the central limbic regions involved in regulation of emotions and mood. The exact cellular and molecular mechanisms underlying hippocampal plasticity in response to stress are yet to be fully characterized. In this study, we examined the genetic profile of micro-dissected subfields of post-mortem hippocampus from subjects diagnosed with MDD and comparison subjects matched for sex, race and age. Gene expression profiles of the dentate gyrus and CA1 were assessed by 48K human HEEBO whole genome microarrays and a subgroup of identified genes was confirmed by real-time polymerase chain reaction (qPCR). Pathway analysis revealed altered expression of several gene families, including cytoskeletal proteins involved in rearrangement of neuronal processes. Based on this and evidence of hippocampal neuronal atrophy in MDD, we focused on the expression of cytoskeletal, synaptic and glutamate receptor genes. Our findings demonstrate significant dysregulation of synaptic function/structure related genes SNAP25, DLG2 (SAP93), and MAP1A, and 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)propanoic acid receptor subunit genes GLUR1 and GLUR3. Several of these human target genes were similarly dysregulated in a rat model of chronic unpredictable stress and the effects reversed by antidepressant treatment. Together, these studies provide new evidence that disruption of synaptic and glutamatergic signalling pathways contribute to the pathophysiology underlying MDD and provide interesting targets for novel therapeutic interventions.
Keywords: AMPA, depression, hippocampus, post-mortem, stress
Introduction
With lifetime prevalence of >16 % (Kessler et al. 2003) and high economic burden (Greenberg et al. 2003; Simon, 2003), major depressive disorder (MDD) is one of the most prominent psychiatric disorders. Clinically, MDD is a heterogeneous mental illness with a broad range of symptoms likely due to a combination of environmental and genetic factors that result in altered gene expression, dysregulated neuronal function and morphological changes in limbic brain regions. Current antidepressant treatments targeting serotonin (5-HT) and norepinephrine brain systems are effective in only a subset of MDD patients (50–70%) and require long-term administration to achieve therapeutic response (Fava & Davidson, 1996; Little, 2009). To date, limited understanding of MDD pathophysiology at the cellular and molecular level has been a major setback in development of more effective therapeutic agents.
The hippocampus has been one of the main limbic structures implicated in the aetiology of MDD. Brain imaging and post-mortem studies provide evidence of changes in cellular architecture and/or morphology within this brain region, including reduction in hippocampal volume in MDD patients (Neumeister et al. 2005; Sheline et al. 1996, 2003), atrophy of hippocampal pyramidal neurons and decreased neuropil (Stockmeier et al. 2004). Preclinical studies further show that exposure to either acute or chronic stressor decreases adult neurogenesis in the subgranular zone of dentate gyrus (DG) and causes atrophy of dendritic structures of pyramidal neurons (Magarinos et al. 1999; Schmidt & Duman, 2007; Watanabe et al. 1992).
Recent studies have largely focused on identifying mechanisms and cellular targets that may elucidate the aetiology of MDD and provide novel substrates for development of more effective and faster-acting therapeutic agents. In the present study, we used a microarray approach to carry out gene profiling of two hippocampal subfields, the DG granule and CA1 pyramidal cell layers, of subjects diagnosed with MDD. Pathway analysis of the resulting gene profiles revealed altered expression of several gene families, including cytoskeletal proteins that could be involved in structural alterations of neuronal processes. Based on this and previous preclinical and clinical studies demonstrating atrophy of neuronal processes, spines and synapses in response to stress and depression (Banasr & Duman, 2007; Duman, 2009), we focused on the expression of genes involved in synaptic function, including pre- and post-synaptic proteins and glutamate receptor subtypes. In addition, we have examined 5-HT receptor subtypes that have been implicated in the depression and treatment response.
Materials and method
Human subjects and tissue preparation
As previously described (Duric et al. 2010), brain tissues from 21 subjects diagnosed with MDD (Supplementary Table S1) and 18 age-matched psychiatrically healthy control subjects (Supplementary Table S2) were collected at autopsy at the Cuyahoga County Coroner’s Office (USA). An ethical protocol, used in this study, was approved by the Institutional Review Board of the University Hospitals of Cleveland, while informed written consent was acquired from the legal next of kin of all subjects. The coroner’s office examined blood and urine samples from all subjects for psychotropic medications and substances of abuse. Overall, no evidence of a neurological disorder was found in depressed subjects, except for one subject who suffered a stroke and two others who were co-morbid for Parkinson’s disease. Informant-based psychiatric assessments were retrospectively conducted for all control and depressed subjects (Supplementary Tables S1 and S2) by trained interviewers. The Schedule for Affective Disorders and Schizophrenia : lifetime version (SADS-L) (Stockmeier et al. 1998) was administered to knowledgeable next of kin of 19 of the depressed subjects, while the Structured Clinical Interview for DSM-IV Psychiatric Disorders (SCID) was administered to next of kin of the two remaining depressed subjects (First et al. 1996). Responses for 19 depressed subjects evaluated with the SADS-L were also recorded in the SCID. Information from the interview and medical records were subsequently used to assess Axis I psychopathology and reach consensus diagnosis.
According to DSM-IV (APA, 1994), all depressed subjects met diagnostic criteria for MDD. The MDD cohort consisted of eight female and 13 male subjects and all except one of them met criteria for a major depressive episode within the last 2 wk of life. The coroner’s office ruled that the cause of death of 15 of the 21 subjects with MDD was suicide. Analysis of post-mortem blood detected the presence of sertraline in one depressed subject, while nine others had a prescription for an antidepressant drug filled during the last month of life (Supplementary Table S2). The control group, closely matched with the depressed group, consisted of seven female and 11 male healthy subjects who never met criteria for an Axis I disorder at any time in their lives. Gene expression analysis was conducted on samples of DG and CA1 from 15 pairs of MDD and control subjects who were matched for age (DG : ±5.1 yr, CA1: ±4.9 yr), gender, tissue pH and post-mortem interval (PMI ; average values for tissue pH and PMI were not statistically different between the two cohorts) (Table 1). Depending on the availability of tissue samples, there was an overlap in the sample pairs used for each brain region.
Table 1.
Case demographics of matched control and depressed subjects
| Group | Region | Age (yr) | Gender | PMI (h) | pH | Cause of death (suicide/other) |
Medicationsa |
|---|---|---|---|---|---|---|---|
| Control | DG | 61.1 | 9 M/6 F | 20.5 | 6.61 | 0/15 | n.d. |
| CA1 | 58.5 | 9 M/6 F | 21.8 | 6.56 | 0/15 | n.d. | |
| MDD | DG | 61.3 | 10 M/5 F | 18.2 | 6.56 | 10/5 | 10 |
| CA1 | 59.2 | 9 M/6 F | 19.7 | 6.54 | 10/5 | 10 |
PMI, Post-mortem interval ; DG, dentate gyrus; M, male; F, female; n.d., no psychotropic medication detected ; MDD, major depressive disorder.
Psychotrophic prescriptions within last month (see Supplementary Tables 1 and 2 for more detail).
During autopsy, the hippocampal formation was dissected from the right temporal lobe, quick-frozen in isopentane cooled by solid CO2 and stored at —80 °C. Frozen blocks of tissue from age-matched pairs of control and subjects with depression were first coded and then sectioned on a cryostat at 60 μm in thickness. Micro-punches from the granule cell layer of the DG and the CA1 pyramidal cell layer were collected from between six and 27 sections, depending on the profile size of the hippocampus (an average of 15 punches, 2 mm in diameter, weighing a total of ~30 mg per region per subject).
Microarray analysis
Microarray analysis of human post-mortem samples was conducted as previously described in our laboratory (Duric et al. 2010). Total RNA was extracted with RNAqueous kit (Ambion, USA) from two microdissected hippocampal subfields, the DG granule cell layer (n = 15) and CA1 (n = 15) pyramidal cell layer, and cleaned up with RNeasy MinElute kit (Qiagen, USA). The integrity of extracted RNA was similar for the depressed and control samples [RNA integrity (RIN) values for both DG and CA1 samples were not statistically different between the two cohorts (DG: control RIN average=6.3, MDD RIN average=6.1; CA1: control RIN average=5.5, MDD RIN average=5.4); Supplementary Table S3]. Extracted RNA from matched pairs of depressed and control human samples were reverse-transcribed into cDNA, indirectly labelled with highly sensitive fluorescent dendrimers (Genisphere, USA) and subsequently hybridized to human whole genome MI ready microarrays (Microarray Inc., USA) containing oligonucleotide (70mer) probes from the Human Exonic Evidence Based Oligonucleotide set. As previously described in our laboratory (Newton et al. 2003), after an overnight hybridization process, array chips were subjected to a series of stringent washes to reduce non-specific probe binding, followed by post-staining with fluorescent Cy3 and Cy5 dendrimers. Following dendrimer hybridization, microarrays were subjected to another set of stringent washes and scanned using a GenePix scanner (Axon Instruments, USA).
GenePix Pro 6.0 (Axon Instruments) software was used for initial two-channel analysis of scanned microarray images. The individual GenePix format files were converted to ASCII format using the read. GenePix function from the R/Bioconductor library marray and the red and green channels and associated metadata were written to an ASCII text format file after dropping probe sets lacking gene symbols. The resulting data were then subjected to linear-log followed by lowess normalization using the R/Bioconductor library maanova. In addition, photo-multiplier tube (PMT) values that were <0.2 or missing were imputed using the impute.knn function from the R/Bioconductor library impute as long as no more than 10% of values were missing or out of bounds. Data were than analysed using mixed-effects linear models using the R/Bioconductor library maanova. The array effect was fit as a random effect and case vs. control status (affected) was fit as a fixed effect. A series of models were also fit that included potential confounding variables such as antidepressant usage, neurological disease (Parkinson’s or stroke), pH, PMI, mode of death (suicide vs. not suicide), age and sex. In the case of pH, PMI and age, computational difficulties with model fitting arose likely due to multi-collinearity and these values were therefore dichotomized using a median-split approach before analysis. Covariates were included in models one at a time because insufficient degrees of freedom were available to include all of the covariates simultaneously. The resulting p values corresponding to the F statistics (Cui et al. 2005) were adjusted for multiple comparisons by false-discovery rate correction method (Storey & Tibshirani, 2003). To provide additional assessment of the effects of mode of death (suicide vs. non-suicide), we also calculated model-based expression estimates using the R/Bioconductor library maanova. A variable ’affected suicide’ was created with the values A0S0 A1S1 and A1S0 for controls, depressed suicide and depressed non-suicide subjects, respectively, and a linear model with array as a random effect and affected suicide as a fixed effect was fit using the fitmaanova function. The model-based estimate (BLUE/BLUP) for affected suicide as well as the error variance was extracted from the object created by the function. Furthermore, to generate multi-dimensional scaling plots, data for the genes of interest (see Supplementary Tables S4 and S5) were used to generate distances after removing the array effect. These values were calculated from the maanova model object as the sum of the mean log2-transformed intensity for each case, the estimate for the affected term, the estimate for the gene effect, and the error term. These values were then converted to distances based on the metric distance (x, y)=1–r(x, y), where r(x, y) is the Pearson’s correlation between the data vector for case x and case y. The values were then subjected to two-dimensional, non-metric, multidimensional scaling using the isoMDS function from the MASS library in R. The fitted points were plotted with the sample RIN values superimposed using the calibrate library in R (see Supplementary Figs 1S and 2S).
Quantitative real-time polymerase chain reaction (qPCR) in post-mortem samples
qPCR was used to validate microarray findings on a subset of samples from the same cohort (see Supplementary Table S6 for a list of control and MDD samples used). Total RNA (500 ng) extracted from human DG and CA1 tissues was reverse-transcribed into cDNA using random hexamer primer mix and SuperScriptIII qRT-PCR kit (Invitrogen, USA). Hot-start SYBR Green PCR kit (Qiagen) was used in 16 μl reactions containing 1 μl cDNA, and 1 μl 5 pmol/μl primer mixture. qPCR was performed with ABI 7900 instrument (Applied Biosystems, USA), followed by melt-curve analysis to further verify specificity and well-to-well consistency of specific product generation. Fold changes in gene expression (MDD vs. controls) were calculated using ΔΔCt (where Ct is cycle number at threshold) analytical method that includes normalization against housekeeping genes GAPDH (encoding glyceraldehyde 3-phosphate dehydrogenase), HMBS (encoding hydroxymethylbilane synthase) and/or TUBB (encoding β-tubulin). Forward and reverse primers (Supplementary Table S7) for human housekeeping genes and genes of interest were designed using Primer3 v. 0.4.0 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi; Whitehead Institute for Biomedical Research, USA) based on coding DNA sequences acquired from GenBank [National Center for Biotechnology Information (NCBI)]. Primer specificity was verified using nucleotide blast software (BLAST Interface, NCBI).
In situ hybridization analysis
Expression of target genes was evaluated using specific antisense cRNA probes and in situ hybridization protocol previously described in our laboratory (Newton et al. 2002). Briefly, probes were generated by an in vitro transcription reaction using PCR product-derived template and MAXIscript kit T7 polymerase (Ambion, USA). Forward and reverse primers used for generation of probe templates are shown in Supplementary Table S8; specificity of the PCR product for each gene of interest was additionally verified by sequencing. Probes were first radio-labelled with [35S]rCTP, purified through a NucAway spin column (Ambion) and hybridized [hybridization buffer: 50 % (v/v) formamide, 3×SSC, 50 mM NaPO4,10 mM dithiothreitol, 1×Denhart’s, 0.25 g/l tRNA, 10% dextran SO4] to post-fixed (4% paraformaldehyde) fresh frozen coronal rat brain sections (16 μm) mounted on Probe-On Plus slides (Fisher Scientific Co., USA). Following overnight hybridization at 55 °C, slides were first washed in several SSC solutions with increasing stringency and then rinsed in dH2O and 100% ethanol, air dried for several hours and exposed to Kodak MR autoradiographic film. Corresponding mRNA expression images were captured using a computer-controlled digital camera (Cohu, USA) and imported into Image J (Scion Corp., USA) for densitometric analysis. Raw densitometry data were converted to nCi 14C/g tissue according to manufacturer’s calibration scale (range 40–1070 nCi/g), which are linearly related to the tissue levels of the specific mRNA.
Animal housing and chronic unpredictable stress (CUS)
Adult male Sprague–Dawley rats (Charles River, USA) were housed in groups of 2–4 per cage under a 12-h light/dark cycle (lights on 07:00 hours) at constant temperature (25 °C) and humidity with ad libitum access to food and water. Animals were allowed at least 1 wk habituation to the housing conditions before exposure to any treatments or experiments. All animals were age and weight matched (rats 250–300 g) at the beginning of the stress paradigm. All rat treatments, housing and maintenance procedures were in accordance with NIH laboratory care standards and approved by the Yale University Care and Use of Laboratory Animals guidelines.
CUS is a rodent model of depression consisting of long-term, daily exposures of animals to a series of mild and unpredictable stressors designed to prevent habituation (Willner, 2005). As previously described (Banasr & Duman, 2008; Banasr et al. 2007) in our laboratory, CUS rats were subjected to the sequence of 12 different stressors (two per day for 35 consecutive days) including cold stress, cold swim stress, cage rotation, isolation, lights on/off, isolation, food and water deprivation, odour exposure, stroboscope, wet bedding, crowding and cage tilt. A subset of CUS animals was also administered fluoxetine [Flx; 5 mg/ kg, i.p.; Eli Lilly, USA] daily for 21 d with continued CUS (Banasr et al. 2007). The effects of stress and anti-depressant treatment on helplessness and hedonic state were assessed by active avoidance test (day 28) and sucrose preference test (day 35), respectively (Duric et al. 2010). Rats were additionally tested for locomotor activity on days 15, 28 and 35.
Statistical analysis
qPCR-derived gene expression experiments were analysed using paired Student’s t test for two-group comparisons. Two-way analysis of variance (ANOVA) with Fisher’s PLSD post-hoc comparison tests was used in rat experiments with four treatment groups. The differentially expressed genes in the DG and CA1 were submitted to data mining using MetaCore software version 6.7 build 28822 (GeneGo; Thomson Reuters Business, USA). Using lists of significantly up and down-regulated genes (fold change >1.5 and <0.7, p<0.05), MetaCore allowed for classification according to canonical pathways and signalling networks containing one or more regulated genes. Over-represented pathways were ranked according to the p values that were calculated based on hypergeometric distribution.
Results
Decreased expression of synapse-related genes in the hippocampus of MDD subjects
Pathway analysis identified apoptosis and cell survival signalling, immune response signalling, cell adhesion and cytoskeletal rearrangement as some of the most dysregulated functional pathways in both DG and CA1 of depressed subjects. Neuronal survival, hippocampal neurogenesis and immune response have previously been linked with stress and depression (Banasr & Duman, 2007; Koo & Duman, 2009; Pittenger & Duman, 2008). Genes involved in cytoskeletal rearrangement have also been implicated in remodelling of neuronal processes and synapses, including pre- and post-synaptic cytoskeletal and signalling proteins, as well as glutamate receptor subtypes. Based on strong preclinical and clinical evidence for atrophy and loss of hippocampal neurons, we decided to focus on this category of genes. Alterations of 5-HT receptors have also been demonstrated in depression and stress-related illnesses prompting our interest in this class of receptors (Graeff et al. 1996; Hsiung et al. 2003; Stockmeier et al. 1998; Vaidya et al. 1999).
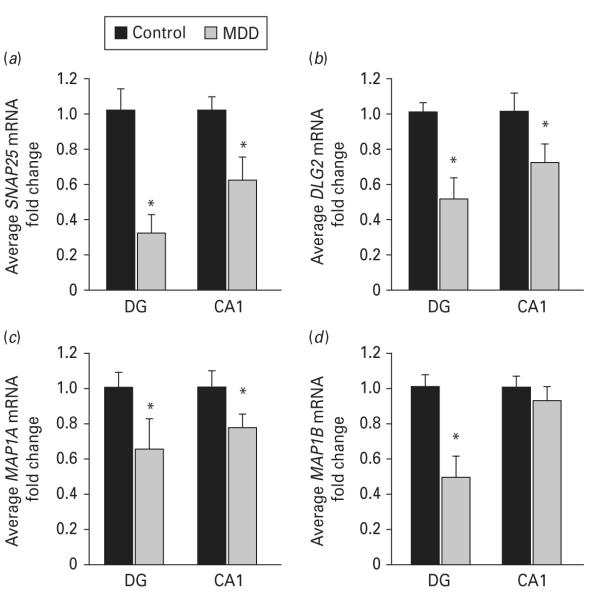
Expression levels of several presynaptic genes were decreased in the DG and CA1 of depressed subjects (Table 2), including synapsin 3 (encoded by SYN3) and synaptosomal-associated protein 25 kDa (encoded by SNAP25). A greater number of post-synaptic genes were also significantly down-regulated in the DG and CA1 of MDD subjects, including synapse-associated proteins (SAP) 93 and 102 (encoded by DLG2 and 3, respectively) and microtubule-associated proteins 1a, 1b, 2 and τ (encoded by MAP1A, MAP1B, MAP2 and MAPT, respectively ; Table 2). These microarray findings were confirmed by qPCR, demonstrating decreased levels of MAP1B (0.50-fold decrease, p=0.04) in the DG and decreased SNAP25 (DG: 0.32-fold decrease, p=0.03; CA1: 0.62-fold decrease, p=0.03), DLG2 (SAP93; DG: 0.52-fold decrease, p=0.05; CA1 : 0.72-fold decrease, p=0.03) and MAP1A (DG: 0.64-fold decrease, p=0.05; CA1: 0.78-fold decrease, p=0.05) in both hippocampal subfields of brains from subjects with MDD (Fig. 1a–d).
Table 2.
Results of microarray experiments ; dysregulated synaptic genes in the hippocampal sub-regions of subjects with major depressive disorder
| DG |
CA1 |
|||||
|---|---|---|---|---|---|---|
| Gene name | Symbol | RefSeq ID | Fold change | p value | Fold change | p value |
| Synaptic function | ||||||
| Pre-synaptic | ||||||
| Synapsin 1 | SYN1 | NM_006950.2 | 0.97 | 0.936 | 0.86 | 0.628 |
| Synapsin 3 | SYN3 | NM_133632.1 | 0.93 | 0.673 | 0.57 | 0.0005* |
| Synaptosomal-associated protein, 25 kDa |
SNAP25 | NM_130811.1 | 0.40 | 0.036* | 0.39 | 0.004* |
| Post-synaptic | ||||||
| Synapse-associated protein 97 | DLG10/SAP97 | NM_004087.2 | 0.86 | 0.225 | 0.84 | 0.248 |
| Synapse-associated protein 93 | DLG2/SAP93 | NM_001364 | 0.41 | 0.007* | 0.50 | 0.008* |
| Synapse-associated protein 102 | DLG3/SAP102 | NM_020730.1 | 0.81 | 0.014* | 0.81 | 0.003* |
| Synapse-associated protein 95 | DLG4/PSD95 | NM_001365.1 | 1.05 | 0.773 | 0.86 | 0.570 |
| Microtubule-associated protein 1a | MAP1a | NM_002373.4 | 0.75 | 0.127 | 0.53 | 0.025* |
| Microtubule-associated protein 1b | MAP1b | NM_005909.2 | 0.68 | 0.130 | 0.56 | 0.004* |
| Microtubule-associated protein 2 | MAP2 | NM_002374.2 | 0.83 | 0.003* | 0.80 | 0.011* |
| Microtubule-associated protein τ | MAPT | NM_016841.1 | 0.29 | 0.0002* | 0.61 | 0.005* |
DG, Dentate gyrus.
Indicates statistically significant change in fold change where p<0.05.
Fig. 1.
Expression levels of synapse-related genes are altered in major depressive disorder (MDD). Microarray findings were validated by real-time polymerase chain reaction on subset of samples from the same cohort. mRNA levels of selected genes involved in synaptic functioning (a) SNAP25, (b) DLG2 (SAP93), (c) MAP1A, and (d) MAP1B) are shown. Data are expressed as mean fold change ±S.E.M.(n=6) ; * p≤0.05 compared to the healthy controls (paired Student’s t test). DG, dentate gyrus.
Altered expression of glutamate and serotonin-related genes in the hippocampus of MDD subjects
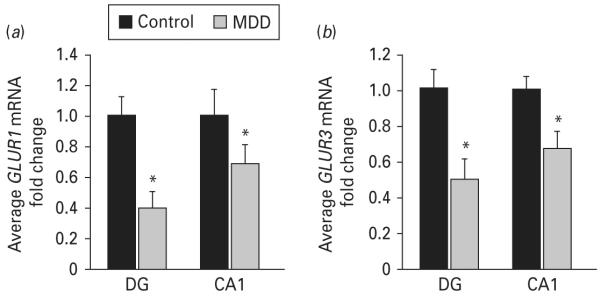
Expression levels of genes encoding subunits of two main ionotropic transmembrane glutamate receptors, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-d-aspartic acid (NMDA), are shown in Table 3. Several AMPA receptor subunits (encoded by GRIA family) are significantly dysregulated in both the DG and CA1 subfields of subjects with MDD. Levels of glutamate receptors genes GLUR1 and GLUR3 (encoded by GRIA1 and GRIA3, respectively) were significantly down-regulated in both DG and CA1 of depressed subjects, while GLUR4 (encoded by GRIA4) was decreased only in the DG (Table 3). Secondary validation of microarray results using qPCR confirmed that GLUR1 and GLUR3 mRNA levels are significantly reduced in the DG (0.4-fold decrease, p=0.04 ; 0.50-fold decrease, p=0.01, respectively) and CA1 (0.69-fold decrease, p=0.05 ; 0.67-fold decrease, p=0.03, respectively) of subjects with MDD (Fig. 2a, b).
Table 3.
Results of microarray experiments ; dysregulated glutamatergic and serotonergic genes in the hippocampal sub-regions of subjects with major depressive disorder
| DG |
CA1 |
|||||
|---|---|---|---|---|---|---|
| Gene name | Symbol | RefSeq ID | Fold change | p value | Fold change | p value |
| Glutamate signalling | ||||||
| AMPA receptor 1 (GLUR1) | GRIA1 | NM_000827.3 | 0.26 | 0.0005* | 0.44 | 0.001* |
| AMPA receptor 2 (GLUR2) | GRIA2 | NM_000826 | 1.20 | 0.098 | 0.95 | 0.819 |
| AMPA receptor 3 (GLUR3) | GRIA3 | NM_181894.1 | 0.13 | 0.0003* | 0.09 | 1×10−5* |
| AMPA receptor 4 (GLUR4) | GRIA4 | NM_000829.1 | 0.82 | 0.007* | 0.99 | 0.932 |
| NMDA receptor 1 (NR1) | GRIN 1 | NM_000832.4 | 1.30 | 0.102 | 1.10 | 0.730 |
| NMDA receptor 2a (NR2A) | GRIN 2a | NM_000833.2 | 0.82 | 0.280 | 0.94 | 0.607 |
| NMDA receptor 2b (NR2B) | GRIN 2b | NM_000834.3 | 0.95 | 0.531 | 1.12 | 0.101 |
| NMDA receptor 2c (NR2C) | GRIN 2c | NM_000835.3 | 0.94 | 0.776 | 1.01 | 0.973 |
| NMDA receptor 2d (NR2D) | GRIN 2d | NM_000836.1 | 1.09 | 0.721 | 0.97 | 0.915 |
| Serotonin signalling | ||||||
| 5-HT receptor 1a | HTR1A | NM_000524.2 | 0.82 | 0.002* | 0.95 | 0.509 |
| 5-HT receptor 1b | HTR1B | NM_000863.1 | 0.91 | 0.024* | 1.04 | 0.740 |
| 5-HT receptor 2a | HTR2A | NM_000621.2 | 0.92 | 0.201 | 0.82 | 0.156 |
| 5-HT receptor 2b | HTR2B | NM_000867.2 | 0.90 | 0.345 | 0.87 | 0.058 |
| 5-HT receptor 2c | HTR2C | NM_000868.1 | 1.78 | 0.021* | 1.06 | 0.638 |
| NM_213621.1 | ||||||
| 5-HT receptor 3a | HTR3A | NM_130770.2 | 1.20 | 0.250 | 1.21 | 0.015* |
| 5-HT receptor 3c | HTR3C | NM_199453.1 | 1.13 | 0.325 | 1.32 | 0.076 |
| NM_000871.1 | ||||||
| 5-HT receptor 4 | HTR4 | NM_000872.3 | 0.60 | 0.0003* | 0.67 | 0.002* |
| 5-HT receptor 6 | HTR6 | NM_000524.2 | 1.25 | 0.047* | 1.04 | 0.783 |
| 5-HT receptor 7 | HTR7 | NM_000863.1 | 0.61 | 0.0001* | 0.68 | 0.0003* |
DG, Dentate gyrus ; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid ; NMDA, N-methyl-d-aspartic acid.
Indicates statistically significant change in fold change where p<0.05.
Fig. 2.
Expression levels of glutamate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors are altered in major depressive disorder (MDD). Microarray findings were validated by real-time polymerase chain reaction on a subset of samples from the same cohort. mRNA levels of (a) GLUR1 and (b) GLUR3 are shown. Data are expressed as mean fold change±S.E.M. (n=6) ; * p≤0.05 compared to the healthy controls (paired Student’s t test). DG, dentate gyrus.
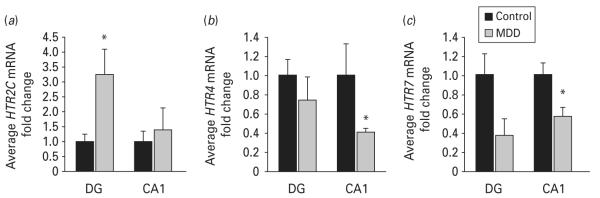
The results of our microarray study also indicate that several 5-HT receptor (HTR) genes are significantly dysregulated in the hippocampus of subjects with MDD (Table 3). HTR2C was robustly over-expressed in the DG, but was not altered in CA1. In contrast, HTR4 and HTR7 were strongly down-regulated in both the DG and CA1 in tissues from depressed subjects. Moreover, qPCR results confirmed that HTR2C levels were up-regulated by more than three-fold (3.25-fold increase, p=0.03) in the DG of depressed subjects (Fig. 3a). Decreases in HTR4 and HTR7 mRNA levels were confirmed by qPCR in the CA1 (0.41-fold decrease, p=0.02 and 0.56-fold decrease, p=0.05, respectively), but not in the DG (Fig. 3b, c).
Fig. 3.
Expression levels of serotonin receptors are altered in major depressive disorder (MDD). Microarray findings were validated by real-time polymerase chain reaction on a subset of samples from the same cohort. mRNA levels of (a) HTR2C, (b) HTR4 and (c) HTR7 are shown. Data are expressed as mean fold change ±S.E.M.(n=5–6) ; * p≤0.05 compared to the healthy controls (paired Student’s t test). DG, dentate gyrus.
Assessment of potential confounds in the microarray analysis
To determine the role of potential confounds in the microarray analysis, we fit a series of linear models to the data that included a number of relevant covariates. Because of limitations in the available degrees of freedom, each covariate was assessed per model. These analyses are described in detail in the Method section and the results are shown in Supplementary Tables S4 (DG) and S5 (CA1). The bottom row of Supplementary Tables S4 and S5 indicates the number of probes that were significant (Q value <0.05) on the entire array. There were only minor effects of age, sex, PMI or tissue pH. Co-morbid neurological disease and the status of antidepressants therapy at the time of death had moderate effects, the latter suggesting that some gene expression patterns are responsive to treatment. In the DG, out of 14 genes of interest that were significantly dysregulated, six genes lost significance after controlling for antidepressant usage [DLG3 (SAP102), GRIA4, HTR1B, HTR2C, HTR6 and SNAP25.2] and one after controlling for central nervous system disease (SNAP25.2). The effects of antidepressant usage on expression of the 5-HT receptors in MDD further support involvement of serotonergic pathways in depression pathophysiology and treatment (Jones & Blackburn, 2002; Lopez et al. 1997). In the CA1, out of 13 dysregulated genes of interest, five were affected by antidepressant treatment [DLG2 (SAP93), HTR3A, MAP1A.2, MAP1B.3 and SNAP25.2] and none by comorbid neurological disease. The most prominent adjustment was for mode of death, which had the largest impact on the results in both DG and CA1. To allow for interpretation of the effects of mode of death, we present model-based expression estimates for each gene for the control (A0S0), depressed non-suicidal (A1S0) and depressed suicidal (A1S1) subjects. For the DG samples, DLG2 (SAP93), DLG3 (SAP102), GRIA4, HTR2C, HTR6, MAP2.5 and SNAP25.2 lost significance after controlling for mode of death. For the CA1 samples, DLG3 (SAP102), HTR3A, HTR4.4, MAP1A.2, MAP2.5, MAPT and SNAP25.2 lost significance after controlling for mode of death. It is important to note that, because most of the depressed subjects died by suicide, suicide and depression were highly collinear in our analyses (r~0.7 for DG and CA1) and power for these analyses was likely reduced as a consequence.
In addition, we assessed the role of variation in RNA integrity (RIN values ; see Method for details). Plots of multi-dimensional scaling analysis from the DG and CA1 samples on the basis of the genes in Supplementary Tables S4 and S5, respectively, showed strong discrimination between the control and depressed samples (see Supplementary Figures S1 and S2). RIN values were plotted adjacent to each coordinate. These plots show that RIN values do not cluster with the samples, suggesting that the results were not biased by variation in RIN values.
Genes regulated by chronic stress in a rat model of depression
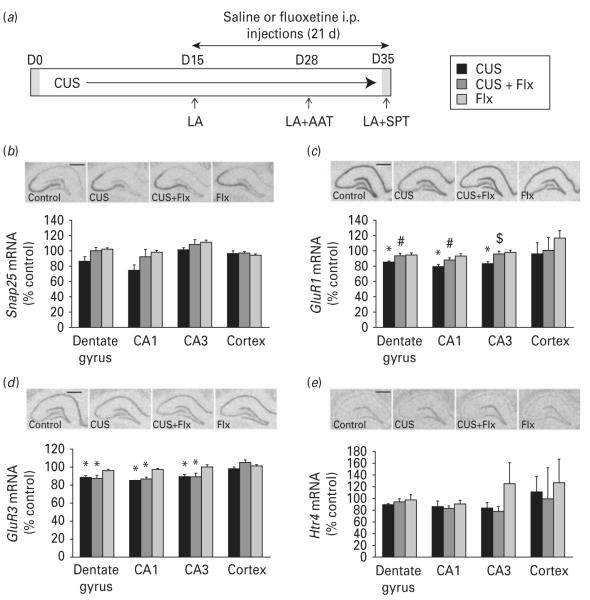
To further investigate the regulation of the genes identified in the MDD microarray study, we conducted expression studies in rats exposed to CUS (Fig. 4a). CUS is considered one of the more valid rodent models of depression. CUS exposure results in anhedonia and helplessness, core symptoms of MDD that are reversed by chronic, but not acute or short-term anti-depressant treatment (Banasr et al. 2007, 2010; Murua et al. 1991; Willner, 2005). In addition, CUS causes neuronal atrophy and decreased neurogenesis in the hippocampus. We previously showed that exposing rats to 35 d of CUS decreases sucrose preference and increases escape failures in an active avoidance test (reflecting anhedonia and helplessness, respectively), depressive-like behaviours that were reversed by long-term Flx treatment (5 mg/kg for 21 d ; Duric et al. 2010). Gene expression levels, measured by in situ hybridization analysis, are shown for Snap25, GluR1, GluR3 and Htr4 (Fig. 4). Previous studies in rodents have also shown that exposure to CUS produces alterations in levels of various neuro-transmitters and their receptors, in particular ones involved in serotonergic- and glutamatergic-related pathways (Farley et al. 2010; Li et al. 2011; Ossowska et al. 2001).
Fig. 4.
Effects of chronic unpredictable stress (CUS) and antidepressant treatment on expression of selected human target genes in the rat hippocampus. (a) Rats were exposed to CUS or control housing conditions ; saline (vehicle) or fluoxetine (Flx) were administered for the last 21 d of stress. CUS had no effect on the locomotor activity (LA), but evoked increased helpless behaviour in active avoidance test (AAT) and reduced sucrose intake in sucrose preference test (SPT) ; both of these behavioural effects of CUS were reversed by chronic Flx treatment (data not shown). Representative autoradiographs and quantitative analysis of (b) Snap25, (c) GluR1, (d) GluR3 and (e) Htr4 mRNA levels by in situ hybridization on coronal sections of dorsal rat hippocampus (scale bar, 1.0 mm). Results are expressed as mean±s.e.m. percent change over non-stressed control group (n=4or 5) ; * p<0.05 compared to the non-stressed control group, # p<0.05 compared to CUS group, $ p<0.07 compared to CUS group (two-way analysis of variance and Fisher’s PLSD post-hoc analysis).
ANOVA analysis revealed that CUS exposure resulted in a trend for decreased levels of Snap25 in the rat hippocampus (CA1 26% and DG 14 % decrease relative to control ; F3,16,16=2.31, p=0.115 ; Fig. 4b). CUS exposure caused significant decreases in levels of AMPA receptor subunit GluR1 mRNA in the DG (15%, F1,16,=6.59, p=0.021), CA1 (20%, F1,16=21.64, p=0.0003) and CA3 (17%, F1,16=4.87, p=0.042) (Fig. 4c). Two-way ANOVA analysis reveals a significant CUS×Flx interaction in the DG and CA1 (F1,16=5.99, p=0.026; F1,16=7.47, p=0.015, respectively) and a trend in CA3 (F1,16=3.90, p=0.066). Flx treatment, which blocked CUS-induced anhedonia and helpless behaviours (not shown), resulted in~10 % up-regulation of GluR1 mRNA levels in both DG and CA1 when compared to CUS alone (p=0.044 and p=0.044, respectively). Similarly, CUS exposure also caused significant down-regulation of GluR3 levels in DG (12 %, F1,16=16.18, p=0.001), CA1 (15%, F1,16=54.28, p<0.0001) and CA3 (11 %, F1,16=12.26, p=0.003) (Fig. 4d). However, there was no CUS ×Flx interaction as antidepressant treatment did not reverse CUS-induced decreases in GluR3 mRNA levels.
Neither exposures to CUS (35 d) nor administration of Flx had a significant effect on expression levels of Htr2c, Htr4 and Htr7 genes in different hippocampal subfields (data for Htr4 shown in Fig. 4e). Similar results for expression of these 5-HT receptors were also observed using qPCR analysis of homogenized whole rat hippocampus tissues, indicating that chronic stress does not produce the same effect on regulation of 5-HT receptor expression in animal models as observed in human subjects with MDD.
Expression levels of all genes addressed in this experiment were not altered in the cortex of rats exposed to either CUS or Flx, demonstrating that observed changes in the hippocampus are not a global effect.
Discussion
Exposure to stress can result in considerable structural and molecular alterations in neuronal populations within the limbic system. The hippocampus may be especially vulnerable to stress, evident from reports of neuronal atrophy and decreased neurogenesis (Duman, 2002; Schmidt & Duman, 2007). Post-mortem studies of the hippocampus of subjects diagnosed with mood disorders show changes in neuronal synaptic density and levels of neurotrophic factors, as well as signalling activity of several neurotransmitters, especially glutamate, monoamines and γ-aminobutyric acid (Knable et al. 2004). However, the exact inter- and intra-cellular events, including complex molecular interactions, involved in the pathogenesis of MDD are still being investigated.
Pre- and post-synaptic vesicle and cytoskeletal proteins, as well as glutamate receptors are critical mediators of synaptic plasticity, learning and memory (Bear & Malenka, 1994; Malenka, 2003). Previous reports have shown impairments in hippocampal neuroplasticity, including synaptic abnormalities, in both patients with mood disorders and animal stress models (Manji et al. 2003). Expression levels of GAP-43 and synapsin, known markers of synaptic plasticity, were reported to be decreased in post-mortem brains of subjects with bipolar disorder (Benowitz & Perrone-Bizzozero, 1991; Vawter et al. 2002). Studies in rodents have also reported that CUS decreases levels of synaptic proteins, including synapsin I and PSD95 (Li et al. 2011).
Here, our microarray results demonstrate down-regulation of several pre- and post-synaptic genes in MDD subjects. At the pre-synaptic level, notably, synapsin 3 and SNAP25, both of which are essential for vesicular release of neurotransmitters, including glutamate and 5-HT, are significantly decreased in MDD subjects. SNAP25 encodes a protein that is a key component of the soluble N-ethylmaleimide-sensitive factor attachment receptor complex that controls fusion of the synaptic vesicle and membrane during the process of exocytosis (Wang et al. 2006). We also found Snap25 to be down-regulated in the DG and CA1 of rats exposed to CUS, while Flx treatment protected against these stress-induced changes. These findings support previous reports that chronic antidepressant treatments modulate expression of synaptic vesicle proteins that may lead to altered pre-synaptic release of neurotransmitters involved in therapeutic action of these drugs (Rapp et al. 2004; Yamada et al. 2002).
Significant decreases in expression of genes encoding microtubule-associated proteins, especially MAP1A, MAP1B, MAP2 and MAPT, were also observed in both the DG and CA1 of subjects with MDD. MAPs are a family of cytoskeletal proteins that regulate tubulin stability and play important roles in the maintenance of neuronal morphology, neurite out-growth and stabilization of synapses (Tucker, 1990). MAP1A, in particular, was shown to interact with disrupted-in-schizophrenia 1 and epidermal growth factor receptor, which are involved in formation and maturation of synapses as well as neurite outgrowth (Kim et al. 2009; Lajoie-Mazenc et al. 2008; Morris et al. 2003). Thus, decreased levels of MAPs, observed in both hippocampal subfields of human subjects with MDD, may also have profound effects on the overall stability of synaptic connections and neuronal function.
In the hippocampus and most of the brain, endogenous AMPA receptors are mainly expressed as GluR1/GluR2 and GluR2/GluR3 heteromers (Wenthold et al. 1996). Under pathological conditions elevated glutamate may result in impairments of synaptic plasticity and even excitotoxicity. Post-mortem MDD studies report increased glutamate levels and dysregulation of genes involved in glutamate signalling (Bernard et al. 2010; Hashimoto et al. 2007). In the current study, we found that the expression of AMPA receptors, specifically GLUR1 and GLUR3 subunits, are decreased in both the DG and CA1 of MDD subjects. Previous reports showed decreased GLUR1 expression in the striatum of individuals with bipolar disorder (BPD) and decreased GLUR3 levels in the dorsolateral prefrontal cortex of individuals with MDD (Beneyto & Meador-Woodruff, 2006; Meador-Woodruff et al. 2001). We further show that GluR1 and GluR3 are similarly down-regulated in the DG, CA1 and CA3 of rats exposed to CUS. Chronic administration of Flx protected against stress-evoked decreases of GluR1, but had no effect on GluR3, suggesting that chemical antidepressants, such as 5-HT reuptake inhibitors (SSRIs), may regulate only specific subtypes of AMPA receptors. These results are consistent with preclinical studies showing that chronic antidepressant treatment increases GluR1 expression or function in the rat hippocampus (Naylor et al. 1996; Svenningsson et al. 2002; Wong et al. 1993). Targeted deletion of GluR1 decreases LTP and impairs hippocampus-mediated learning, as the GluR1 sub-unit plays a key role in AMPA receptor trafficking and synaptic delivery (Bruneau & Akaaboune, 2006; Sheng & Lee, 2001). Decreased levels of GLUR1 in the hippocampal subfields of subjects with MDD and rats exposed to CUS could thereby lead to abnormalities in overall glutamatergic signalling and impairment of hippocampal function.
Generally, we did not observe dysregulation of genes encoding NMDA receptor subunits in either the DG or CA1 of MDD subjects. Previous studies have reported a reduction in NMDA receptor binding and expression of NMDA receptor 1 (NR1) and NR2A transcripts or protein levels in the hippocampus, prefrontal cortex or superior temporal cortex of subjects with MDD and BPD (Feyissa et al. 2009; Law & Deakin, 2001; McCullumsmith et al. 2007; Nudmamud-Thanoi & Reynolds, 2004). However, another study reported no changes in NR1 protein levels in the hippocampus of depressed patients (Thompson et al. 2003). Differences between subfields examined in the current study vs. whole hippocampus, as well as differences in MDD cohorts and methodology, could contribute to discrepancies between these reports.
The potential role of 5-HT receptors is supported by the monoamine theory of MDD, most notably the therapeutic effectiveness of SSRIs (Coppen & Doogan, 1988; Millan, 2006). Furthermore, compounds that either activate or inhibit the function of specific 5-HT receptor subtypes are reported to evoke an anti-depressant response in preclinical stress models (Middlemiss et al. 2002). Previous studies in post-mortem brain tissues of MDD subjects report abnormalities in binding and expression levels of 5-HT receptor subtypes in multiple brain regions (Lopez-Figueroa et al. 2004; Mintun et al. 2004; Pandey et al. 2002; Stockmeier, 2003). Here, we report altered expression of genes encoding 5-HT2C, 5-HT4 and 5-HT7 receptors in the hippocampus of depressed subjects. In particular, we show >2-fold increase in HTR2C gene expression in the DG. 5-HT2C receptors have been implicated in the aetiology of depression and anxiety and current available antidepressants, such as nefazodone or mirtazapine, act as direct 5-HT2C antagonists (Millan, 2005). However, regulation of 5-HT2C function in mood disorders is complex and still poorly understood, evident from reports that both 5-HT2C agonist and antagonists can produce antidepressant activity (Heisler et al. 2007; Millan, 2005; Rosenzweig-Lipson et al. 2007a, b). Previous studies have reported altered expression of 5-HT4 receptor binding in MDD (Rosel et al. 2004) and recent reports indicate that 5-HT4 receptor-selective agonists have rapid onset anti-depressant efficacy (Lucas et al. 2007). Decreased HTR7 expression in MDD is consistent with the idea that reduction of Gs-coupled receptors, which stimulate production of neurotrophic factors (e.g. brain-derived neurotrophic factor and vascular endothelial growth factor) could contribute to the pathophysiology of MDD (Duman et al. 1997, 2004; Errico et al. 2001).
In conclusion, the results provide novel evidence of robust dysregulation of genes involved in glutamate-mediated neuronal and synaptic plasticity in hippocampal subfields from MDD subjects. Further characterization of the molecular and cellular mechanisms underlying these changes and the functional consequences may lead to a better understanding of the pathophysiology underlying depression and provide potential targets for novel therapeutic interventions for the treatment of this disorder.
Supplementary Material
Acknowledgements
This work is supported by USPHS grants MH45481 (R.S.D.), 2 P01 MH25642 (R.S.D.), the Connecticut Mental Health Center (R.S.D.), MH67996 (C.A.S.) and P20 RR17701 (C.A.S.). We acknowledge the invaluable contributions made by the families consenting to donate brain tissue and be interviewed. We also thank the Cuyahoga County Coroner and staff, Cleveland, Ohio, for their willing assistance. The contributions of Drs Herbert Y. Meltzer and Bryan Roth and Ginny Dilley, Lisa Konick, Nicole Herbst, Timothy M. DeJong and Gouri Mahajan, in the psychiatric assessment and human tissue dissection and preparation are gratefully noted. We also thank Rosemarie Terwilliger and H. J. Kang for technical assistance with gene expression analysis.
Footnotes
Statement of Interest None.
Accession codes Microarray data have been deposited in Gene Expression Omnibus with accession code GSE24095.
Note Supplementary material accompanies this paper on the Journal’s website (http://journals.cambridge.org/pnp).
References
- 1.APA . Diagnostic and Statistical Manual of Mental Health Disorders. 4th edn American Psychiatric Association; Washington DC: 1994. [Google Scholar]
- 2.Banasr M, Chowdhury GM, Terwilliger R, Newton SS, et al. Glial pathology in an animal model of depression : reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Molecular Psychiatry. 2010;15:501–511. doi: 10.1038/mp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banasr M, Duman RS. Regulation of neurogenesis and gliogenesis by stress and antidepressant treatment. CNS and Neurological Disorders Drug Targets. 2007;6:311–320. doi: 10.2174/187152707783220929. [DOI] [PubMed] [Google Scholar]
- 4.Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biological Psychiatry. 2008;64:863–870. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banasr M, Valentine GW, Li XY, Gourley SL, et al. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biological Psychiatry. 2007;62:496–504. doi: 10.1016/j.biopsych.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Bear MF, Malenka RC. Synaptic plasticity : LTP and LTD. Current Opinion in Neurobiology. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 7.Beneyto M, Meador-Woodruff JH. Lamina-specific abnormalities of AMPA receptor trafficking and signaling molecule transcripts in the prefrontal cortex in schizophrenia. Synapse. 2006;60:585–598. doi: 10.1002/syn.20329. [DOI] [PubMed] [Google Scholar]
- 8.Benowitz LI, Perrone-Bizzozero NI. The relationship of GAP-43 to the development and plasticity of synaptic connections. Annals of the New York Academy of Sciences. 1991;627:58–74. doi: 10.1111/j.1749-6632.1991.tb25914.x. [DOI] [PubMed] [Google Scholar]
- 9.Bernard R, Kerman IA, Thompson RC, Jones EG, et al. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Molecular Psychiatry. 2010;16:634–646. doi: 10.1038/mp.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruneau EG, Akaaboune M. Running to stand still : ionotropic receptor dynamics at central and peripheral synapses. Molecular Neurobiology. 2006;34:137–151. doi: 10.1385/MN:34:2:137. [DOI] [PubMed] [Google Scholar]
- 11.Coppen AJ, Doogan DP. Serotonin and its place in the pathogenesis of depression. Journal of Clinical Psychiatry. 1988;49(Suppl.):4–11. [PubMed] [Google Scholar]
- 12.Cui X, Hwang JT, Qiu J, Blades NJ, et al. Improved statistical tests for differential gene expression by shrinking variance components estimates. Biostatistics. 2005;6:59–75. doi: 10.1093/biostatistics/kxh018. [DOI] [PubMed] [Google Scholar]
- 13.Duman RS. Structural alterations in depression: cellular mechanisms underlying pathology and treatment of mood disorders. CNS Spectrums. 2002;7:140–142. 144–147. doi: 10.1017/s1092852900017454. [DOI] [PubMed] [Google Scholar]
- 14.Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Medicine. 2004;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- 15.Duman RS. Neuronal damage and protection in the pathophysiology and treatment of psychiatric illness : stress and depression. Dialogues in Clinical Neuroscience. 2009;11:239–255. doi: 10.31887/DCNS.2009.11.3/rsduman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Archives of General Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 17.Duric V, Banasr M, Licznerski P, Schmidt HD, et al. A negative regulator of MAP kinase causes depressive behavior. Nature Medicine. 2010;16:1328–1332. doi: 10.1038/nm.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Errico M, Crozier RA, Plummer MR, Cowen DS. 5-HT(7) receptors activate the mitogen activated protein kinase extracellular signal related kinase in cultured rat hippocampal neurons. Neuroscience. 2001;102:361–367. doi: 10.1016/s0306-4522(00)00460-7. [DOI] [PubMed] [Google Scholar]
- 19.Farley S, Apazoglou K, Witkin JM, Giros B, et al. Antidepressant-like effects of an AMPA receptor potentiator under a chronic mild stress paradigm. International Journal of Neuropsychopharmacology. 2010;13:1207–1218. doi: 10.1017/S1461145709991076. [DOI] [PubMed] [Google Scholar]
- 20.Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatric Clinics of North America. 1996;19:179–200. doi: 10.1016/s0193-953x(05)70283-5. [DOI] [PubMed] [Google Scholar]
- 21.Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Progress in Neuro-psychopharmacology and Biological Psychiatry. 2009;33:70–75. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.First MB, Donovan S, Frances A. Nosology of chronic mood disorders. Psychiatric Clinics of North America. 1996;19:29–39. doi: 10.1016/s0193-953x(05)70271-9. [DOI] [PubMed] [Google Scholar]
- 23.Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacology, Biochemistry, and Behavior. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, et al. The economic burden of depression in the United States : how did it change between 1990 and 2000? Journal of Clinical Psychiatry. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biological Psychiatry. 2007;62:1310–1316. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Heisler LK, Zhou L, Bajwa P, Hsu J, et al. Serotonin 5-HT(2C) receptors regulate anxiety-like behavior. Genes, Brain, and Behavior. 2007;6:491–496. doi: 10.1111/j.1601-183X.2007.00316.x. [DOI] [PubMed] [Google Scholar]
- 27.Hsiung SC, Adlersberg M, Arango V, Mann JJ, et al. Attenuated 5-HT1A receptor signaling in brains of suicide victims : involvement of adenylyl cyclase, phosphati-dylinositol 3-kinase, Akt and mitogen-activated protein kinase. Journal of Neurochemistry. 2003;87:182–194. doi: 10.1046/j.1471-4159.2003.01987.x. [DOI] [PubMed] [Google Scholar]
- 28.Jones BJ, Blackburn TP. The medical benefit of 5-HT research. Pharmacology, Biochemistry, and Behavior. 2002;71:555–568. doi: 10.1016/s0091-3057(01)00745-6. [DOI] [PubMed] [Google Scholar]
- 29.Kessler RC, Berglund P, Demler O, Jin R, et al. The epidemiology of major depressive disorder : results from the National Comorbidity Survey Replication (NCS-R) Journal of the American Medical Association. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 30.Kim JY, Duan X, Liu CY, Jang MH, et al. DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron. 2009;63:761–773. doi: 10.1016/j.neuron.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knable MB, Barci BM, Webster MJ, Meador-Woodruff J, et al. Molecular abnormalities of the hippocampus in severe psychiatric illness : postmortem findings from the Stanley Neuropathology Consortium. Molecular Psychiatry. 2004;9:609–620. doi: 10.1038/sj.mp.4001471. [DOI] [PubMed] [Google Scholar]
- 32.Koo JW, Duman RS. Evidence for IL-1 receptor blockade as a therapeutic strategy for the treatment of depression. Current Opinion in Investigational Drugs. 2009;10:664–671. [PMC free article] [PubMed] [Google Scholar]
- 33.Lajoie-Mazenc I, Tovar D, Penary M, Lortal B, et al. MAP1A light chain-2 interacts with GTP-RhoB to control epidermal growth factor (EGF)-dependent EGF receptor signaling. Journal of Biological Chemistry. 2008;283:4155–4164. doi: 10.1074/jbc.M709639200. [DOI] [PubMed] [Google Scholar]
- 34.Law AJ, Deakin JF. Asymmetrical reductions of hippocampal NMDAR1 glutamate receptor mRNA in the psychoses. Neuroreport. 2001;12:2971–2974. doi: 10.1097/00001756-200109170-00043. [DOI] [PubMed] [Google Scholar]
- 35.Li N, Liu RJ, Dwyer JM, Banasr M, et al. Glutamate N-methyl-d-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biological Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Little A. Treatment-resistant depression. American Family Physician. 2009;80:167–172. [PubMed] [Google Scholar]
- 37.Lopez JF, Vazquez DM, Chalmers DT, Watson SJ. Regulation of 5-HT receptors and the hypothalamic-pituitary-adrenal axis. Implications for the neurobiology of suicide. Annals of the New York Academy of Sciences. 1997;836:106–134. doi: 10.1111/j.1749-6632.1997.tb52357.x. [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Figueroa AL, Norton CS, Lopez-Figueroa MO, Armellini-Dodel D, et al. Serotonin 5-HT1A, 5-HT1B, and 5-HT2A receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biological Psychiatry. 2004;55:225–233. doi: 10.1016/j.biopsych.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 39.Lucas G, Rymar VV, Du J, Mnie-Filali O, et al. Serotonin(4) (5-HT(4)) receptor agonists are putative antidepressants with a rapid onset of action. Neuron. 2007;55:712–725. doi: 10.1016/j.neuron.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 40.McCullumsmith RE, Kristiansen LV, Beneyto M, Scarr E, et al. Decreased NR1, NR2A, and SAP102 transcript expression in the hippocampus in bipolar disorder. Brain Research. 2007;1127:108–118. doi: 10.1016/j.brainres.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Magarinos AM, Deslandes A, McEwen BS. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. European Journal of Pharmacology. 1999;371:113–122. doi: 10.1016/s0014-2999(99)00163-6. [DOI] [PubMed] [Google Scholar]
- 42.Malenka RC. Synaptic plasticity and AMPA receptor trafficking. Annals of the New York Academy of Sciences. 2003;1003:1–11. doi: 10.1196/annals.1300.001. [DOI] [PubMed] [Google Scholar]
- 43.Manji HK, Quiroz JA, Sporn J, Payne JL, et al. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biological Psychiatry. 2003;53:707–742. doi: 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 44.Meador-Woodruff JH, Hogg AJ, II, Smith RE. Striatal ionotropic glutamate receptor expression in schizophrenia, bipolar disorder, and major depressive disorder. Brain Research Bulletin. 2001;55:631–640. doi: 10.1016/s0361-9230(01)00523-8. [DOI] [PubMed] [Google Scholar]
- 45.Middlemiss DN, Price GW, Watson JM. Serotonergic targets in depression. Current Opinion in Pharmacology. 2002;2:18–22. doi: 10.1016/s1471-4892(01)00116-3. [DOI] [PubMed] [Google Scholar]
- 46.Millan MJ. Serotonin 5-HT2C receptors as a target for the treatment of depressive and anxious states : focus on novel therapeutic strategies. Therapie. 2005;60:441–460. doi: 10.2515/therapie:2005065. [DOI] [PubMed] [Google Scholar]
- 47.Millan MJ. Multi-target strategies for the improved treatment of depressive states : conceptual foundations and neuronal substrates, drug discovery and therapeutic application. Pharmacology and Therapeutics. 2006;110:135–370. doi: 10.1016/j.pharmthera.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Mintun MA, Sheline YI, Moerlein SM, Vlassenko AG, et al. Decreased hippocampal 5-HT2A receptor binding in major depressive disorder : in vivo measurement with [18F]altanserin positron emission tomography. Biological Psychiatry. 2004;55:217–224. doi: 10.1016/j.biopsych.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 49.Morris JA, Kandpal G, Ma L, Austin CP. DISC1 (Disrupted-In-Schizophrenia 1) is a centrosome-associated protein that interacts with MAP1A, MIPT3, ATF4/5 and NUDEL : regulation and loss of interaction with mutation. Human Molecular Genetics. 2003;12:1591–1608. doi: 10.1093/hmg/ddg162. [DOI] [PubMed] [Google Scholar]
- 50.Murua VS, Gomez RA, Andrea ME, Molina VA. Shuttle-box deficits induced by chronic variable stress : reversal by imipramine administration. Pharmacology, Biochemistry, and Behavior. 1991;38:125–130. doi: 10.1016/0091-3057(91)90599-w. [DOI] [PubMed] [Google Scholar]
- 51.Naylor P, Stewart CA, Wright SR, Pearson RC, et al. Repeated ECS induces GluR1 mRNA but not NMDAR1AG mRNA in the rat hippocampus. Brain Research. 1996;35:349–353. doi: 10.1016/0169-328x(95)00264-s. [DOI] [PubMed] [Google Scholar]
- 52.Neumeister A, Wood S, Bonne O, Nugent AC, et al. Reduced hippocampal volume in unmedicated, remitted patients with major depression vs. control subjects. Biological Psychiatry. 2005;57:935–937. doi: 10.1016/j.biopsych.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 53.Newton SS, Collier EF, Hunsberger J, Adams D, et al. Gene profile of electroconvulsive seizures : induction of neurotrophic and angiogenic factors. Journal of Neuroscience. 2003;23:10841–10851. doi: 10.1523/JNEUROSCI.23-34-10841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Newton SS, Dow A, Terwilliger R, Duman R. A simplified method for combined immunohistochemistry and in-situ hybridization in fresh-frozen, cryocut mouse brain sections. Brain Research Protocols. 2002;9:214–219. doi: 10.1016/s1385-299x(02)00148-4. [DOI] [PubMed] [Google Scholar]
- 55.Nudmamud-Thanoi S, Reynolds GP. The NR1 subunit of the glutamate/NMDA receptor in the superior temporal cortex in schizophrenia and affective disorders. Neuroscience Letters. 2004;372:173–177. doi: 10.1016/j.neulet.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 56.Ossowska G, Nowa G, Kata R, Klenk-Majewska B, et al. Brain monoamine receptors in a chronic unpredictable stress model in rats. Journal of Neural Transmission. 2001;108:311–319. doi: 10.1007/s007020170077. [DOI] [PubMed] [Google Scholar]
- 57.Pandey GN, Dwivedi Y, Rizavi HS, Ren X, et al. Higher expression of serotonin 5-HT(2A) receptors in the postmortem brains of teenage suicide victims. American Journal of Psychiatry. 2002;159:419–429. doi: 10.1176/appi.ajp.159.3.419. [DOI] [PubMed] [Google Scholar]
- 58.Pittenger C, Duman RS. Stress, depression, and neuroplasticity : a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 59.Rapp S, Baader M, Hu M, Jennen-Steinmetz C, et al. Differential regulation of synaptic vesicle proteins by antidepressant drugs. Pharmacogenomics Journal. 2004;4:110–113. doi: 10.1038/sj.tpj.6500229. [DOI] [PubMed] [Google Scholar]
- 60.Rosel P, Arranz B, Urretavizcaya M, Oros M, et al. Altered 5-HT2A and 5-HT4 postsynaptic receptors and their intracellular signalling systems IP3 and cAMP in brains from depressed violent suicide victims. Neuropsychobiology. 2004;49:189–195. doi: 10.1159/000077365. [DOI] [PubMed] [Google Scholar]
- 61.Rosenzweig-Lipson S, Dunlop J, Marquis KL. 5-HT2C receptor agonists as an innovative approach for psychiatric disorders. Drug News and Perspectives. 2007a;20:565–571. doi: 10.1358/dnp.2007.20.9.1162244. [DOI] [PubMed] [Google Scholar]
- 62.Rosenzweig-Lipson S, Sabb A, Stack G, Mitchell P, et al. Antidepressant-like effects of the novel, selective, 5-HT2C receptor agonist WAY-163909 in rodents. Psychopharmacology. 2007b;192:159–170. doi: 10.1007/s00213-007-0710-6. [DOI] [PubMed] [Google Scholar]
- 63.Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behavioural Pharmacology. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 64.Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. American Journal of Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 65.Sheline YI, Wang PW, Gado MH, Csernansky JG, et al. Hippocampal atrophy in recurrent major depression. Proceedings of the National Academy of Sciences USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sheng M, Lee SH. AMPA receptor trafficking and the control of synaptic transmission. Cell. 2001;105:825–828. doi: 10.1016/s0092-8674(01)00406-8. [DOI] [PubMed] [Google Scholar]
- 67.Simon GE. Social and economic burden of mood disorders. Biological Psychiatry. 2003;54:208–215. doi: 10.1016/s0006-3223(03)00420-7. [DOI] [PubMed] [Google Scholar]
- 68.Stockmeier CA. Involvement of serotonin in depression : evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. Journal of Psychiatric Research. 2003;37:357–373. doi: 10.1016/s0022-3956(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 69.Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, et al. Cellular changes in the postmortem hippocampus in major depression. Biological Psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, et al. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. Journal of Neuroscience. 1998;18:7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proceedings of the National Academy of Sciences USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Svenningsson P, Tzavara ET, Witkin JM, Fienberg AA, et al. Involvement of striatal and extrastriatal DARPP-32 in biochemical and behavioral effects of fluoxetine (Prozac) Proceedings of the National Academy of Sciences USA. 2002;99:3182–3187. doi: 10.1073/pnas.052712799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thompson PM, Egbufoama S, Vawter MP. SNAP-25 reduction in the hippocampus of patients with schizophrenia. Progress in Neuro-psychopharmacology and Biological Psychiatry. 2003;27:411–417. doi: 10.1016/S0278-5846(03)00027-7. [DOI] [PubMed] [Google Scholar]
- 74.Tucker RP. The roles of microtubule-associated proteins in brain morphogenesis : a review. Brain Research Review. 1990;15:101–120. doi: 10.1016/0165-0173(90)90013-e. [DOI] [PubMed] [Google Scholar]
- 75.Vaidya VA, Terwilliger RM, Duman RS. Role of 5-HT2A receptors in the stress-induced down-regulation of brain-derived neurotrophic factor expression in rat hippocampus. Neuroscience Letters. 1999;262:1–4. doi: 10.1016/s0304-3940(99)00006-3. [DOI] [PubMed] [Google Scholar]
- 76.Vawter MP, Thatcher L, Usen N, Hyde TM, et al. Reduction of synapsin in the hippocampus of patients with bipolar disorder and schizophrenia. Molecular Psychiatry. 2002;7:571–578. doi: 10.1038/sj.mp.4001158. [DOI] [PubMed] [Google Scholar]
- 77.Wang M, Yang Y, Dong Z, Cao J, et al. NR2B-containing N-methyl-d-aspartate subtype glutamate receptors regulate the acute stress effect on hippocampal long-term potentiation/long-term depression in vivo. Neuroreport. 2006;17:1343–1346. doi: 10.1097/01.wnr.0000227994.07799.6c. [DOI] [PubMed] [Google Scholar]
- 78.Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Research. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- 79.Wenthold RJ, Petralia RS, Blahos J, II, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. Journal of Neuroscience. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Willner P. Chronic mild stress (CMS) revisited : consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 81.Wong ML, Smith MA, Licinio J, Doi SQ, et al. Differential effects of kindled and electrically induced seizures on a glutamate receptor (GluR1) gene expression. Epilepsy Research. 1993;14:221–227. doi: 10.1016/0920-1211(93)90046-a. [DOI] [PubMed] [Google Scholar]
- 82.Yamada M, Takahashi K, Tsunoda M, Nishioka G, et al. Differential expression of VAMP2/synaptobrevin2 after antidepressant and electroconvulsive treatment in rat frontal cortex. Pharmacogenomics Journal. 2002;2:377–382. doi: 10.1038/sj.tpj.6500135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.