Abstract
Background
The hyperacute rejection mediated by pre-existing antibodies is a major impediment to the success of transplants across allogeneic and xenogeneic barriers. We report a new mouse model that allows us to not only monitor the sensitization of B cells mediating the hyperacute response, but also validate therapeutic strategies for tolerizing them.
Model
The new model system uses 5C.C7,RAG2−/− TCR transgenic T cells and B10.S(9r),CD3ε−/− hosts for adoptive transfer experiments.
Results and Conclusion
In the allogeneic hosts, transgenic T cells expanded briefly before being chronically deleted. Once the deletion was initiated, a second graft of donor cells was used to assess a hyper-acute response. The rapid rejection of the second cohort correlated with the appearance of donor-specific antibodies in the serum. Interestingly, chronically stimulated T cells were relatively resistant to hyperacute rejection suggesting an explanation for the slower rejection kinetics of the first cohort even as the second cohort of identical donor cells was being hyper-acutely rejected. Finally, we could tolerize the potential for a hyperacute response, by pre-treating recipients with a single infusion of naïve donor B cells prior to the first T cell transfer. This treatment not only abrogated the development of a hyperacute response, but also allowed the primary graft to survive in vivo for extended periods of time.
Keywords: Hyperacute rejection, Tolerance, Cellular therapy, B cell tolerance, T cell activation
Introduction
A swift humoral response, usually mediated by pre-existing antibodies or memory B cells specific for transplant antigens, can lead to rapid rejection of allo and xenografts. This response – known as hyperacute rejection (1), was first observed in human renal grafts and extensively studied in more recent models of xenotransplantation (6–12). Clinically, the transplant rapidly (within minutes and often during the surgery) blackens from extensive coagulation and complement mediated tissue destruction. The organ has to be quickly removed to prevent a subsequent systemic inflammatory response syndrome (SIRS)-like sequelae from developing. Hyperacute rejection has been most debilitating to prospects for xenotransplantation (2).
The mechanistic insights gained from early studies have led to the development of several therapeutic strategies to prevent or ameliorate antibody-mediated hyperacute responses. These include treatment with iv-IG, B cell depletion with rituximab (anti-CD20), complement inhibitors such as eculizumab, chemicals which directly target plasma cells (bortezomib), etc. (3–10) However, since most of these approaches broadly target the antibody response in the host, there are considerable side effects. The transplant recipient is severely immuno-compromised and must be supported by antibiotics and other strategies. In the case of xenotransplantation, a more focused effort has been made – by removing potential antibody targets on the xenografts. This includes the generation of genetically modified pigs that lack the alpha-galactoside (a-gal) carbohydrate groups predominantly eliciting the hyperacute response (11), as well as animals expressing negative regulators of human complement cascade activity on their cell surface (12). Unfortunately, these strategies have not been as widely successful as initially hoped. The α-gal knockout grafts for example, continue to elicit accelerated rejection responses involving antibodies targeting alternate antigens on the surface (13). Therefore, it is clear that further efforts are required to develop more effective strategies capable of suppressing antibody-mediated rejection responses, preferably in a donor-specific fashion.
In this study we describe a new mouse model that uses a simple adoptive transfer strategy to sensitize a hyperacute response. Monoclonal helper T cells from the B10.A(H-2a), 5C.C7,RAG2−/− TCR transgenic mouse (5C.C7) were found to be alloreactive against the B10.S(9R) strain of the MHC haplotype H-2t4. Soon after the initial transfer, the host displayed a hyperacute antibody response against subsequent H-2a grafts. Interestingly, chronic antigen stimulation rendered the 5C.C7 T cells somewhat resistant to this rejection. Finally, we were able to validate a form of donor-specific tolerization against the hyperacute response in this model, by adoptively transferring a small number of H-2a B cells to the host, a few days before the first graft of 5C.C7 T cells.
Materials and Methods
Mice
All mice were bred in the NIAID contract barriers at Taconic Farms Inc. (Germantown, NY) and housed at the AAALAC certified SPF facilities maintained by NIAID, NIH. The B10.S(9R)/SgSn.Ai stain was originally obtained from Dr. Jack Stimpfling in 1975. The CD3ε−/− and RAG2−/− backgrounds were crossed onto the strain from the B10.A,CD3ε−/− and B10.A,RAG2−/− lines of mice. The resulting progeny were intercrossed and then selected for homozygosity at the H-2a MHC locus and the respective knockout alleles. The B10.A,5C.C7,RAG2−/− (14) and the B10.A,5C.C7,RAG2−/−,Ly5.1+/+ mice have been previously described (15). All animal protocols were approved by the NIAID/DIR Animal Care and Use Committee.
Lymphocyte isolation and adoptive transfer
T cells were isolated from the lymph nodes of 5C.C7 mice and single cell suspensions prepared in PBS supplemented with 5% FCS and antibiotics. Typically these preparations were 93–95% CD4+ and were not further purified. B cells were purified from the spleens of B10.A, CD3ε−/− mice using the MACS B cell isolation kit (Miltenyii Biotec, Germany) as per the manufacturers recommendations and using an AutoMACS separator (Miltneyii Biotec). In some experiments, the enriched cells (already >98% purity) were further purified by FACS sorting for CD19+ and negative for CD11c, CD11b, DX5, NK1.1 and GR-1. All antibodies were purchased from BD Biosciences (CA). Isolated cells were re-suspended in PBS and 1–5 million injected intravenously by the suborbital route. The expansion of T cells in vivo was monitored by recovering the lymph nodes and spleen from transfer recipients, preparing single cell suspensions, counting viable cells and staining for the TCR (Vβ3), CD4 and Ly5.1 among the 7AAD− cells. Data were collected on either a BD FACS Calibur or a BD FACS Sort upgraded by Cytek Inc, and analyzed with CellQuest or FlowJo software.
Results
1. 5C.C7 TCR transgenic T cells are alloreactive against B10.S(9R) splenocytes
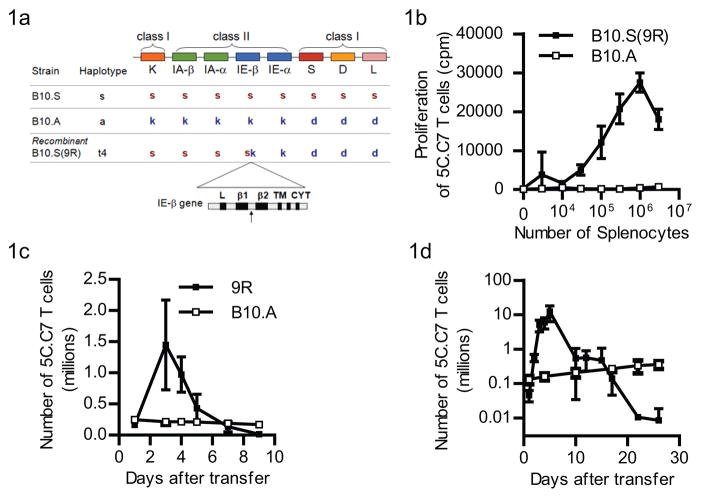
The B10.S(9R) strain was originally obtained as a result of a cross between the B10.S (H-2s) and B10.A (H-2a) strains of mice. A recombination in the H-2 locus resulted in a novel MHC Class II molecule with a crossover within the IEβ gene, after the β1 exon (16). The resulting H-2 locus (Figure 1a) consists of the 5′ elements from the H-2s chromosome and the 3′ elements from the H-2a chromosome and was named H-2t4. Since this allele had been reported to allogeneically stimulate certain PCC-specific T cell clones, we asked if T cells from the MCC(88-103)-specific TCR-5C.C7 transgenic mouse (on a RAG2-deficient background), were also alloreactive against H-2t4. 5C.C7 T cells showed a clear proliferative response, in vitro, to as few as 10,000 B10.S(9R), CD3ε−/− splenocytes (solid squares - Figure 1b). In contrast, they showed no significant proliferative response to the syngeneic B10.A splenocytes (open squares –Figure 1b).
Figure 1. B10.S(9R) presents an allo-antigen to 5C.C7 T cells.
(a) The mouse MHC locus (not to scale) showing the location of the Class I genes relative to the IE-β locus. The B10.S contains a full complement of ‘s’ alleles of each gene, while the B10.A is a combination of ‘k’ and ‘d’ alleles. The recombination hotspot in the IE-β gene between the β1 and β2 exons where the B10.A and B10.S chromosomes crossover in the B10.S(9R) recombinant, is shown by the black arrow below the enlarged schematic of the IE-b gene. As a result, one of the MHC class I molecules (H-2K) is different between the B10.A and B10.S(9R) strains.
(b) Proliferation of 5C.C7 T cells in response to varying numbers of B10.S(9R) (filled squares) or B10.A (open squares) splenocytes (each data point is average of triplicates).
(c) The proliferation of Ly5.1 marked 5C.C7 T cells in an Ly5.2,B10.S(9R) host (filled squares) or Ly5.2,B10.A host (open squares) was assayed by enumerating Ly5.1+,CD4+ T cells in the spleen and lymph nodes of either host, various days after the adoptive transfer of 1 million 5C.C7 cells. Data from three experiments for the B10.S(9R) (n=4) and two experiments with the B10.A recipients (n=3). In independent experiments 5C.C7 T cells routinely do not proliferate in a B10.A host for up to 45 days (data not shown)
(d) Similar experiments as in (c) were performed with B10.S(9R),CD3ε−/−(filled squares) or B10.A, CD3ε−/− (open squares) hosts. The data are averaged from two experiments for the B10.S(9R), CD3ε−/− group (total n=6, per time point) and B10.A, CD3ε−/− recipients (n=4).
The in vitro alloreactivity we discovered allowed us to consider the B10.S(9R) mouse as an in vivo model for studying GVH responses using the 5C.C7 T cells. Adoptively transferred 5C.C7 (Ly5.1+) T cells expanded rapidly in B10.S(9R) (Ly5.2+) mice for up to 3 days after transfer (Figure 1c – filled squares) but not in a B10.A host, which does not express any stimulatory antigen for the 5C.C7 TCR (Figure 1c – open squares). Subsequently the number of T cells dropped precipitously. Such a pattern is similar to the behavior of 5C.C7 T cells in hosts that express their cognate antigen – PCC(15). However, we have previously reported that if such PCC transgenic hosts were devoid of endogenous T cells, the deletional phase could be largely eliminated. In order to examine that in this model, B10.S(9R),CD3ε−/− mice were generated wherein endogenous T cell development is abrogated. Although, adoptive transfer of 5C.C7 T cells into these mice resulted in a more robust T cell expansion (Figure 1d – filled squares) than observed in the intact B10.S(9R) host, the recovery of T cells still declined after the fifth day and was below detection beyond 30–35 days. As previously reported, 5C.C7 T cells in syngeneic B10.A,CD3ε−/− hosts persisted, with a characteristic “homeostatic” expansion (Figure 1d, open squares).
2. Deletion of 5C.C7 T cells is accompanied by the development of an H-2a specific hyperacute response
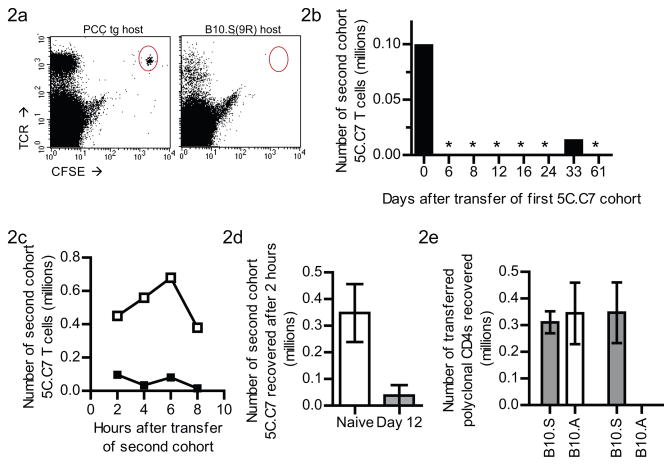
The deletion of the alloreactive 5C.C7 T cell population in the B10.S(9R),CD3ε−/− host could be due to T cell autonomous changes over the course of their response or due to changes in the allogeneic environment, induced by the T cell response. We attempted to distinguish the two, by transferring a fresh cohort of CFSE-labeled naïve 5C.C7 T cells into T cell-experienced B10.S(9R),CD3ε−/− recipients (that had begun to delete an initial cohort of 5C.C7 T cells administered 14 days previously). Surprisingly, even one day after the second transfer we could not recover the fresh cohort of 5C.C7 T cells from the T cell experienced B10.S(9R),CD3ε−/− mice (Figure 2a –right panel). A similar transfer to a PCC transgenic host (Figure 2a – left panel) resulted in successful engraftment. This rapid deletion of a second cohort was evident as early as six days after sensitization by an initial transfer of 5C.C7 T cells into a B10.S(9R),CD3ε−/− mouse (day 6 – Figure 2b) and persisted as long as 61 days afterwards. The transferred T cells do reach the lymphoid organs of T cell experienced B10.S(9R),CD3ε−/− mice since a small number could be seen 2 hours after transfer (Figure 2c); but this number further reduces over the next 6 hours (solid squares, Figure 2c). Therefore, the rejection process is quite acute, starting as early as 2 hours after grafting (Figure 2d).
Figure 2. A second graft of 5C.C7 T cells is hyper-acutely rejected in B10.S(9R),CD3ε−/− hosts that received an earlier transfer of alloreactive T cells.
(a) FACS profiles, one day after adoptively transferring a new cohort of CFSE-labeled naïve 5C.C7 T cells into B10.A, PCC-transgenic, CD3ε−/−(left) or B10.S(9R), CD3ε−/− mice (right), both of which had received an earlier infusion of 5C.C7 T cells 14 days before. One of 3 similar plots are shown.
(b) Similar experiments as in (a) were performed with B10.S(9R),CD3ε−/− mice that had received the primary infusion of 5C.C7 cells at various days (Y-axis) before the second transfer. Number of the second transfer cohort recovered one day later are shown. (* = below the limit of detection, n=1 per time point).
(c) Kinetics of the rejection of a new cohort of 5C.C7 T cells in a B10.S(9R),CD3ε−/− host with a previous infusion of 5C.C7 T cells (filled squares) compared to a naïve B10.S(9R),CD3ε−/− host (open squares).
(d) Similar experiments as in (c) with n=3 mice for B10.S(9R),CD3ε−/− hosts that had a previous 5C.C7 transfer (Gray bars) or not (white bars)
(e) CFSE-labeled polyclonal CD4+ T cells from B10.S (Gray bars) or B10.A (white bars) mice were transferred to naïve (left two bars) or T cell pre-infused (right bar and below detection(*)) B10.S(9R),CD3ε−/− mice. Recovery of the transferred T cells from lymph nodes and spleens of the recipients, one day after transfer is shown (n=3 per bar).
Polyclonal T cells from B10.A mice (white bars in Figure 2e), but not the ones from B10.S(9R) mice (Gray bars in Figure 2e) were also rejected in Day12-B10.S(9R),CD3ε−/− mice (right two bars in Figure 2e), but not in naive B10.S(9R),CD3ε−/− (left two bars in Figure 2e). Thus, the rejection process was neither TCR transgene specific nor required antigen-specific interactions mediated through the 5C.C7 TCR on the second graft of T cells. In fact, it also applied to B10.A B cells and CD8+ T cells as well (data not shown), suggesting that it is a rejection of H-2a tissue in general.
3. Antibodies mediate hyperacute rejection of secondary grafts
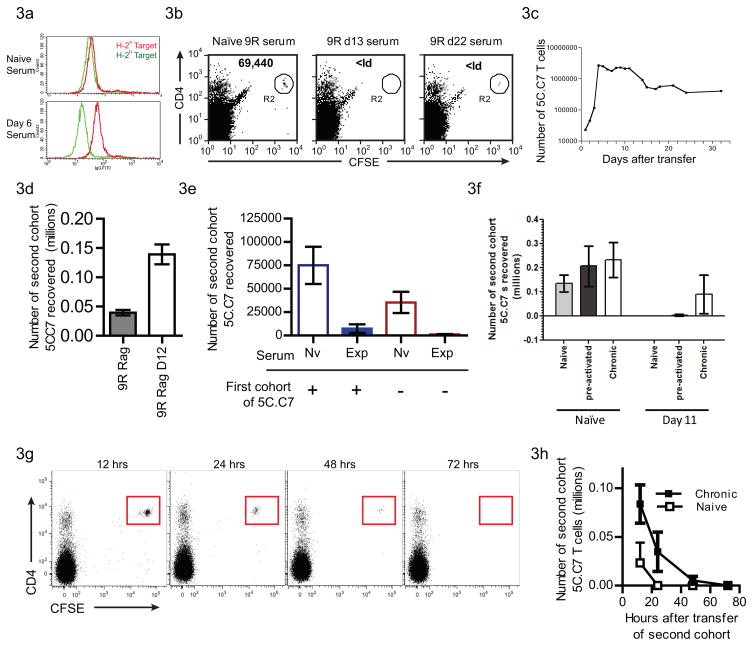
We examined the production of antibodies against the H-2a-derived T cells, using serum from B10.S(9R),CD3ε−/− recipients of 5C.C7 T cells (Figure 3). Serum from B10.S(9R),CD3ε−/− mice that received 5C.C7 T cells 6 days before (bottom panel–Figure 3a) clearly stained H-2a T cells (Red lines) and did not label the H-2b CD4+ T cells (Green lines), while sera from naïve B10.S(9R),CD3ε−/− mice (top panel – Figure 4a) stained neither. We also tested the ability of these antibodies to target cytotoxicity to H-2a T cells by incubating naïve 5C.C7 T cells with serum from 5C.C7-experienced mice, at 4°C, prior to transfer into a naïve B10.S(9R),CD3ε−/− host (Fig 3b). Incubation with serum from B10.S(9R),CD3ε−/− mice (13 or 22 days after first T cell transfer) resulted in deletion confirming the ability of H-2a-specific antisera produced by these hosts to acutely reject H-2a cells in vivo.
Figure 3. The hyperacute response is mediated by the rapid generation of allo-specific antibodies.
(a) Serum from naïve B10.S(9R),CD3ε−/− mice (top panel) or B10.S(9R),CD3ε−/− mice that received 5C.C7 T cells 6 days before (bottom panel) was incubated with H-2a cells (B10.A,5C.C7 - Red line) or H-2b cells (from C57/B10(H-2b),Marilyn TCR transgenic mice - Green line) and then stained with FITC-conjugated anti-mouse IgG. Similar experiments used day 12 or day 18 serum for n=8.
(b) Serum from B10.S(9R),CD3ε−/− mice (as labeled above the dot plots) was incubated with CFSE-labeled, 5C.C7 T cells before transfer into naïve B10.S(9R), CD3ε−/− mice. A day after transfer the spleens and lymph nodes of recipients were stained for CD4 and TCR. FACS plots are gated on live cells based on FSC/SSC and the numbers in the inset indicate the calculated recovery of 5C.C7 T cells.
(c) Expansion of 5C.C7 T cells in a B10.S(9R),RAG2−/− host, enumerating the TCR-Vβ3+CD4+ cells recovered from lymph nodes and spleen of transfer recipients. Data are shown from a single experiment with one recipient assayed per time point. An additional experiment yielded a similar profile
(d) Rejection of a second cohort of 5C.C7 in a B10.S(9R), RAG2−/− host was assayed a day after adoptive transfer of CFSE-labeled T cells into naïve (gray bar, n=5) or 12 day 5C.C7 experienced (open bar, n=5) recipients.
(e) Sera (100 μl) from naïve B10.S(9R),CD3ε−/− mice (open bars) or B10.S(9R),CD3ε−/− mice that received 5C.C7 T cells (shaded bars) were injected into B10.S(9R),RAG2−/− mice that received a primary infusion of 5C.C7 T cells (left 2 bars) or were naive (right 2 bars). After a second injection of serum from the same donors, a CFSE-labeled second cohort of T cells was injected and their recovery calculated on the next day.
(f) Naïve (light grey bars), in vitro pre-activated (dark grey bars) or chronically stimulated in vivo (open bars) 5C.C7 T cells were labeled with CFSE and grafted into B10.S(9R),CD3ε−/− mice that were either naïve or received a primary 5C.C7 T cell cohort 11 days before. Recovery of the second cohort a day later is plotted from n=2 (naïve) and n=4 (pre-activated and chronic) transfer recipients.
(g) CFSE labelled Chronically stimulated 5C.C7 T cells were transferred to 12 day T cell experienced B10.S(9R),CD3ε−/− mice and their rejection monitored over time
(h) Quantitation of data from (g) comparing the rejection of naive (open squares) or chronically stimulated (closed squares) T cells. n=3 or 4 per time point
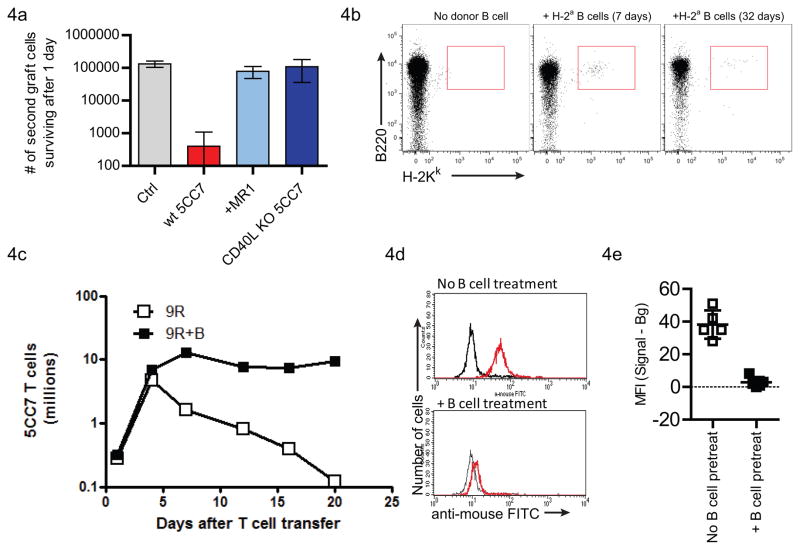
Figure 4. Hyperacute responses can be prevented by CD40L blockade or donor B cell pretreatment.
(a) Tolerization of the primary T cell response by blocking CD40L. CD40L engagement during the interaction of primary grafted 5C.C7 T cells and anti-H2a B cells was abrogated by blocking antibody (MR1) treatment (Bar #3, n=3) or by using CD40L-deficient 5C.C7 T cells as the primary graft (Bar #4, n=3). 7 days after transfer, a new graft of 5C.C7 was transferred to naïve B10.S(9R),CD3ε−/− mice (Bar #1, control), mice that had received wt 5C.C7 T cells (Bar #2) or to either treatment group. The recovery of the transferred T cells a day later is enumerated.
(b) Prolonged microchimerism of donor B cells in B10.S(9R),CD3ε−/− mice
(c) Tolerization of the hyperacute response by cellular therapy. A single infusion of 5 million purified B cells from B10.A,CD3ε−/− mice were used to treat B10.S(9R),CD3ε−/− mice 5 days before grafting 5C.C7 T cells. T cells transferred to B cell-treated mice (closed squares) did not show a significant deletional component, compared to untreated mice (open squares). n=2 mice per time point.
(d) Serum from naïve (black lines) or 5C.C7 grafted (red lines) mice were used to stain H-2a (5C.C7) T cells. The B cell treatment (bottom panel) abolishes the development of anti-H-2a antibodies.
(e) Quantitation of staining intensities from (d).
In order to further validate the role of the B cells, we generated a RAG2-deficient B10.S(9R) strain lacking B and T cells. As in the B10.S(9R),CD3ε−/− hosts, the 5C.C7 T cells expanded quickly in the B10.S(9R).Rag2−/− hosts, peaking by day 4 (Figure 3c). Although the T cell numbers did subsequently undergo a significant decline (80% loss between days 4 to 15), complete deletion of T cells did not occur. In fact, T cells could be recovered from this host even two months after transfer. The B10.S(9R),Rag2−/− hosts also did not develop a hyperacute response. Interestingly, we recovered a greater number of the newly transferred T cell cohort from the 12 day T-experienced B10.S(9R),Rag2−/− recipients, relative to naïve B10.S(9R),Rag2−/−, after the second transfer (Figure 3d).
Finally, we asked whether serum isolated from 5C.C7 experienced B10.S(9R),CD3ε−/− mice can transfer deletion to the RAG2−/− host. B10.S(9R),Rag2−/− mice were treated with T cell experienced B10.S(9R),CD3ε−/− serum for two consecutive days (Figure 3e) before injecting CFSE-labeled naïve 5C.C7 T cells. There was a marked decrease in the number of T cells recovered from the Rag2−/− mice treated with hyper-acute serum compared to those that only received serum from naïve B10.S(9R),CD3ε−/− mice.
The development of a robust B cell-mediated anti H-2a hyperacute response in the B10.S(9R),CD3ε−/− mice can account for the rapid deletion of the 5C.C7 T cells in this model. However, while the secondary cohorts of H-2a tissues are rapidly deleted (within 24 hours), the primary 5C.C7 graft is actually detectable for up to two weeks after, presenting a paradox. This could be due to the primary graft (1) occupying niche(s) not accessible to the toxic antibodies (2) being in an antigen-experienced state; or (3) being under chronic stimulation by the persistent stimulus. In order to distinguish between these possibilities, we adoptively transferred 5C.C7 T cells that were either naïve (Figure 3f – gray bars), activated in vitro to a memory phenotype (Figure 3f – black bars) or chronically stimulated in B10.A,PCC+,CD3ε−/− mice as previously reported (Figure 3f – white bars), into B10.S(9R),CD3ε−/− mice after the development of the hyperacute phase. Interestingly, while both the naïve and antigen-experienced 5C.C7 T cells were rapidly deleted, a significant proportion of the chronically stimulated 5C.C7 T cells survived the onslaught of rejecting antibodies. These cells did indeed succumb to rejection over a 72 hour period (Figure 3g), but this was considerably delayed compared to the rejection of naive T cells (Figure 3h). This suggests that chronic TCR stimulation affords a partial protection to T cells from antibody-mediated hyperacute rejection.
4. Modeling therapeutic strategies to prevent the development of a hyperacute rejection
A transgenic mouse model for the hyperacute response is not only valuable for studying the cellular processes underlying a hyperacute response, but also for developing and validating potential therapeutic strategies for circumventing the process. First, we validated a reputed therapeutic regimen in such transplantation settings – using the blockade of CD40L. Both the blockade of this molecule using antibody treatment (Figure 4a, bar #3) or the abolition of its role in the primary 5C.C7 response by using a CD40L-deficient T cell, resulted in the elimination of the subsequent hyperacute response (Figure 4a).
We then attempted to develop a rational cellular therapy that would eliminate the hyperacute response in this model from first principles. The hyperacute response is likely initiated when 5C.C7 T cells first meet alloantigen presenting B cells, irrespective of the antigen specificity of the B cell receptor. However, if some B cells are also capable of recognizing the H-2a molecule on the 5C.C7 T cell, the combination of Ig signaling and T cell help will rapidly drive an anti-H-2a B cell response. We therefore hypothesized that any strategy that would tolerize the H-2a-specific B cells prior to their encounter with the 5C.C7 T cell would prevent the onset of the hyperacute response. In order to achieve this end, we developed a simple strategy that involved adoptive transfer of purified H-2a B cells (donor haplotype) to the B10.S(9R),CD3ε−/− mice 4–7 days before the infusion of 5C.C7 T cells. These B cells would be expected to migrate to the B cell areas of the recipient mice and allow host B cells to interact with the H-2a antigen. Since these interactions take place in the absence of T cell help, the recipient’s B cell would be expected to be tolerized by the H-2a antigen.
Adoptive transfer of 5 million purified B10.A B cells, four days before the transfer of 5C.C7 T cells demonstrates the viability of this strategy. The transferred B cells engraft and can be detected even 32 days after transfer (Figure 4b). 5C.C7 T cells in the donor B cell-treated mice (filled squares, Figure 4c) expanded similarly to control, untreated mice (open squares, Figure 4c). However after the expansion phase, while the 5C.C7 cells were rapidly deleted in the untreated mice, no appreciable deletion was observed in the B cell-treated animals. This prolonged survival of the alloreactive T cell is similar to previous observations in PCC transgenic CD3ε−/− hosts. Finally, serum samples from B cell-treated mice did not have significant levels of antibodies to H-2a (Figure 4d and 4e) confirming the complete tolerization of the hyper-acute response.
Discussion
The hyperacute rejection response presents a major hurdle to successful transplantation of allo and xeno grafts. We describe a new mouse model system that is highly amenable to the study of a hyperacute response. Interestingly, it also incorporates the phenomenon of adaptation, where the chronically activated T cells are resistant to antibody attack (18). We further demonstrate the utility of this model in formulating new therapeutic strategies for preventing hyperacute rejection in a donor-specific fashion.
The 5C.C7 TCR transgenic mouse generates a population of naïve CD4+ T cells specific for a peptide from pigeon cytochrome c (PCC) presented on IEk. These T cells have no known cross-reactivity with self antigens and retain a naïve phenotype in intact B10.A mice. The alloreactivity that we now describe against B10.S(9R) cells adds an important extension to this model system. We have previously reported the behavior of 5C.C7 T cells in an adoptive transfer model where they encounter their cognate antigen (PCC) as a self protein. In this model, PCC is expressed in the hematopoietic system and presented by antigen-presenting cells including B cells. Interestingly, in otherwise T cell-deficient animals (CD3ε−/−), the strong anti-PCC response is followed by prolonged survival of the T cells with no appreciable deletional component. This had led us to propose that “clonal deletion” in peripheral T cells is not determined by cell-intrinsic processes; but rather by regulatory interactions originating from neighboring T cells. A prediction of this hypothesis was that deletion would be either absent or severely blunted in T cell-deficient hosts. The development of the alloreactive model has allowed us to examine this hypothesis in a different context. Surprisingly, the 5C.C7 T cells were deleted (albeit slowly) in this new model. Our analysis of the mechanism involved, however, revealed a B cell response that actively eliminates the antigen-specific T cell. Similar rejection of lymphocytes by CD8+ T cells has been reported before(19,20). Thus, this further validates the idea that non T-cell-autonomous mechanisms dominate in peripheral clonal deletion/elimination. Furthermore, when we are able to tolerize the B cell response against 5C.C7 MHC molecules in the CD3ε−/− host or to eliminate B cells completely in the RAG2−/− host, the responding T cells did persist
The strategy we developed to tolerize the B cell response is a modification of the concept of microchimerism developed by Starzl, Sykes, Sachs and others (21,22). While this has largely been developed using stem cell transplants, our approach makes use of mature B lymphocytes. The strategy has several advantages: (1) The donor B cells are most likely to migrate to the B cell areas of the host – thereby effectively targeting the very B cells that generate a hyperacute response against the donor. (2) Naïve B cells are relatively poor activators of de novo T cell responses and are therefore likely to tolerize rather than prime anti-donor responses (23). (3) From a practical standpoint, B cells can be obtained from donors (even under clinical settings) in relatively large numbers compared to stem cells.
A potential caveat in the application of this strategy more broadly is the presence of alloreactive T cells in the host. In the model we use, the lack of host T cells (CD3ε−/−) eliminates this difficulty. It also allows the H-2a-reactive B cells to be easily tolerized (by engaging antigens on the donor B cells, in the absence of T cell help). In a more complete in vivo milieu, the host T cells would probably have to be suppressed during the initial engraftment of the donor B cells. While several options are available for this (Cyclosporine, Sirolimus, Abatacept etc.) this is still a major limitation of the current study, as presented. Nevertheless a reductionist yet refined TCR transgenic model will facilitate molecular genetic dissection of hyperacute rejection in a very sensitive fashion. However, those observations should be validated in widely available polyclonal models before further application.
Acknowledgments
We thank Chuan Chen and Andrew Medina-Marino for assistance with experiments, the NIAID flow cytometry facility (Carol Henry and Calvin Eigsti) for flow sorting and members of LCMI, NIAID for discussions. This research was supported by the Intramural Research Program of the NIH, NIAID.
Footnotes
Specific Author Contributions: SO, RHS and NJS designed experiments. SO & NJS performed experiments and analyzed the data. NJS wrote the paper.
Reference List
- 1.Williams GM, Hume DM, Hudson RP, Jr, Morris PJ, Kano K, Milgrom F. “Hyperacute” renal-homograft rejection in man. N Engl J Med. 1968;279:611–618. doi: 10.1056/NEJM196809192791201. [DOI] [PubMed] [Google Scholar]
- 2.Platt JL. The immunological barriers to xenotransplantation. Crit Rev Immunol. 1996;16:331–358. [PubMed] [Google Scholar]
- 3.Thomas F, Naff G, Thomas J, Dvorak K. Prevention of hyperacute kidney rejection of decomplementation using purified cobra venom factor. J Surg Res. 1977;22:189–194. doi: 10.1016/0022-4804(77)90133-0. [DOI] [PubMed] [Google Scholar]
- 4.Yoshioka H, McCalmon RT, Jr, Putnam CW, McIntosh RM, Terman DS. Attenuation of hyperacute xenograft rejection in unmodified host by extracorporeal plasma perfusion. Transplantation. 1977;24:78–81. [PubMed] [Google Scholar]
- 5.Terman DS, Garcia-Rinaldi R, McCalmon R, et al. Modification of hyperacute renal xenograft rejection after extracorporeal immunoadsorption of heterospecific antibody. Int J Artif Organs. 1979;2:35–41. [PubMed] [Google Scholar]
- 6.Hammer C, Krebs G, Chaussy C, et al. Mitigation of hyperacute rejection by antilymphocyte globulin (ALG) Transplant Proc. 1979;11:31–35. [PubMed] [Google Scholar]
- 7.Pruitt SK, Baldwin WM, III, Marsh HC, Jr, Lin SS, Yeh CG, Bollinger RR. The effect of soluble complement receptor type 1 on hyperacute xenograft rejection. Transplantation. 1991;52:868–873. doi: 10.1097/00007890-199111000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Gaber LW, Gaber AO, Vera SR, Braxton F, Hathaway D. Successful reversal of hyperacute renal allograft rejection with the anti-CD3 monoclonal OKT3. Transplantation. 1992;54:930–932. doi: 10.1097/00007890-199211000-00032. [DOI] [PubMed] [Google Scholar]
- 9.Dalmasso AP, Platt JL. Prevention of complement-mediated activation of xenogeneic endothelial cells in an in vitro model of xenograft hyperacute rejection by C1 inhibitor. Transplantation. 1993;56:1171–1176. doi: 10.1097/00007890-199311000-00024. [DOI] [PubMed] [Google Scholar]
- 10.Pruitt SK, Baldwin WM, III, Barth RN, Sanfilippo F. The effect of xenoreactive antibody and B cell depletion on hyperacute rejection of guinea pig-to-rat cardiac xenografts. Transplantation. 1993;56:1318–1324. doi: 10.1097/00007890-199312000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Tearle RG, Tange MJ, Zannettino ZL, et al. The alpha-1,3-galactosyltransferase knockout mouse. Implications for xenotransplantation. Transplantation. 1996;61:13–19. doi: 10.1097/00007890-199601150-00004. [DOI] [PubMed] [Google Scholar]
- 12.Bach FH, Robson SC, Winkler H, et al. Barriers to xenotransplantation. Nat Med. 1995;1:869–873. doi: 10.1038/nm0995-869. [DOI] [PubMed] [Google Scholar]
- 13.Kuwaki K. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. 2005. [DOI] [PubMed] [Google Scholar]
- 14.Miller C, Ragheb JA, Schwartz RH. Anergy and cytokine-mediated suppression as distinct superantigen- induced tolerance mechanisms in vivo. J Exp Med. 1999;190:53–64. doi: 10.1084/jem.190.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh NJ, Chen C, Schwartz RH. The impact of T cell intrinsic antigen adaptation on peripheral immune tolerance. PLoS Biol. 2006;4:e340. doi: 10.1371/journal.pbio.0040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padgett KA, Shreffler DC, Saha BK. Molecular mapping of murine I region recombinants. III. Crossing over at two discrete sites within the beta 1-beta 2 intron of the E beta gene. J Immunol. 1991;147:2764–2770. [PubMed] [Google Scholar]
- 17.Tanchot C, Barber DL, Chiodetti L, Schwartz RH. Adaptive tolerance of CD4+ T cells in vivo: multiple thresholds in response to a constant level of antigen presentation. J Immunol. 2001;167:2030–2039. doi: 10.4049/jimmunol.167.4.2030. [DOI] [PubMed] [Google Scholar]
- 18.Platt JL. A perspective on xenograft rejection and accommodation. Immunol Rev. 1994;141:127–149. doi: 10.1111/j.1600-065x.1994.tb00875.x. [DOI] [PubMed] [Google Scholar]
- 19.Xu H, Exner BG, Chilton PM, Schanie C, Ildstad ST. CD45 congenic bone marrow transplantation: evidence for T cell-mediated immunity. Stem Cells. 2004;22:1039–1048. doi: 10.1634/stemcells.22-6-1039. [DOI] [PubMed] [Google Scholar]
- 20.Herndon JM, Stuart PM, Ferguson TA. Peripheral deletion of antigen-specific T cells leads to long-term tolerance mediated by CD8+ cytotoxic cells. J Immunol. 2005;174:4098–4104. doi: 10.4049/jimmunol.174.7.4098. [DOI] [PubMed] [Google Scholar]
- 21.Sharabi Y, Aksentijevich I, Sundt TM, III, Sachs DH, Sykes M. Specific tolerance induction across a xenogeneic barrier: production of mixed rat/mouse lymphohematopoietic chimeras using a nonlethal preparative regimen. J Exp Med. 1990;172:195–202. doi: 10.1084/jem.172.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang YG, deGoma E, Ohdan H, et al. Tolerization of anti-Galalpha1-3Gal natural antibody-forming B cells by induction of mixed chimerism. J Exp Med. 1998;187:1335–1342. doi: 10.1084/jem.187.8.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lassila O, Vainio O, Matzinger P. Can B cells turn on virgin T cells? Nature. 1988;334:253–255. doi: 10.1038/334253a0. [DOI] [PubMed] [Google Scholar]
- 24.Singh NJ, Schwartz RH. The lymphopenic mouse in immunology: from patron to pariah. Immunity. 2006;25:851–855. doi: 10.1016/j.immuni.2006.12.002. [DOI] [PubMed] [Google Scholar]