Abstract
Background
Perhaps the most difficult thing to ascertain concerning the behavior of another animal is its motivation. The motivation underlying the preference of Drosophila melanogaster for ethanol-rich food has long been ascribed to its value as a food. A recently introduced idea is that, as in humans, the pharmacological effects of ethanol also motivate the fly to choose ethanol-rich food over non-alcoholic food.
Methods
Flies are given a choice between pipets that contain liquid food and liquid food supplemented with ethanol. In some experiments, carbohydrates are added to the non-ethanol-containing food to balance the calories for ethanol.
Results
We confirm that Drosophila melanogaster indeed prefer food that is supplemented with ethanol. However, if the alternative food choice is isocaloric, Drosophila melanogaster usually do not show any preference for a 10% ethanol solution. Even after ethanol preference has been established, it can be completely reversed if the alternative food is calorically supplemented. This occurs even when the carbohydrate solution used to balance calories is not gustatorily attractive. Furthermore, if the alternative food contains more calories than the ethanol food, the flies will prefer the non-ethanol food. We go on to show that during the preference assay that ethanol in the fly does not exceed 4 mM, which in mammals is a non-intoxicating dose.
Conclusions
We conclude that preference for ethanol in this assay arises not from the pharmacological effects of ethanol but rather because of its nutritive value.
Keywords: preference, Drosophila, calorie, nutrition
Introduction
Alcoholism is a devastating disease that has both large financial and social costs. The manifestation of this disease is complex and both genetic and environmental factors are believed to play important roles (Mayfield et al., 2008; van der Zwaluw and Engels, 2009). The fruit fly, Drosophila melanogaster, has been a useful model system for identifying genes associated with rudimentary facets of alcohol-associated behavior (Atkinson, 2009). As in mammals, low doses of ethanol cause an excitatory effect while higher doses cause sedation in flies (Moore et al., 1998). Additionally, flies are capable of acquiring both rapid and chronic tolerance to the drug (Berger et al., 2004). A variety of Drosophila genes, most of which are conserved in mammals, have been shown to be involved in both locomotor sensitivity and tolerance to ethanol vapor (Moore et al., 1998; Bainton et al., 2000; Scholz et al., 2000; Rothenfluh et al., 2006; Cowmeadow et al., 2005; Dzitoyeva et al., 2003; Corl et al., 2009).
Over 50 years of research documents the ecological relationship between D. melanogaster and ethanol. In natural habitats, these flies feed on rotting fruits that can contain over 5% ethanol. They have also been found to breed inside wineries, where the concentration of ethanol is even higher (McKenzie and McKechnie, 1979; Gibson et al., 1981). One would expect that an animal whose food regularly undergoes ethanol fermentation to be attracted to the odor of ethanol because it is the odor of its food. Furthermore, if the food were to regularly contain potentially debilitating amounts of ethanol, then one would expect the flies to be resistant to the pharmacological effects of ethanol (either via innate resistance or rapid-inducible resistance). Perhaps the most tantalizing support for these suppositions are the comparative studies of the distribution of D. melanogaster and D. simulans in wineries. These species are thought to have diverged from a common ancestor only 3.4 million years ago and in the laboratory can productively interbreed (Capy and Gibert, 2004). Both species are found in the grounds and superstructures of wineries, with D. simulans out-competing D. melanogaster in the non-alcohol-rich regions. However, D. melanogaster occupies all of the high alcohol content niches, such as the wine cellars, to the exclusion of its sister species. In all developmental stages, D. melanogaster proves to be more resistant to ethanol toxicity than D. simulans. Additionally, D. melanogaster females lay eggs in ethanol-containing mash where larvae thrive. It seems that once fermentation begins, the high alcohol content niche belongs to D. melanogaster, both as a food source and a nursery. These differences in distribution are believed to be a product of the capacity to catabolize and tolerate ethanol (McKenzie and Parsons, 1972; McKenzie and Parsons, 1974; McKenzie and McKechnie, 1979).
D. melanogaster shows an obvious preference for ethanol-containing food over non-ethanol-containing food. Many investigators have concluded that D. melanogaster are attracted to ethanol as a food source. D. melanogaster larvae will spend more time on the ethanol-containing half of an agar plate compared to the half without ethanol (Parsons, 1980; Depiereux et al., 1985; Gelfand and McDonald, 1980) and adult flies will extend their proboscis more on the ethanol-containing half of a food plate compared to the half without ethanol (Cadieu et al., 1999). D. melanogaster are capable of breaking down ethanol and using its calories. D. melanogaster that consume only ethanol as a calorie source live significantly longer than flies that receive no calories (Libion-Mannaert, 1976; Pecsenye et al., 1994).
Recently, the role of ethanol in the life of D. melanogaster has been revisited in the context of alcoholism. The question addressed by Devineni and Heberlein (2009) was whether flies show an interest in ethanol because of its neuropharmacological effects and whether these effects could precipitate increased consumption. These experiments used the CAFE (CApillary FEeder) assay in which the flies choose between pipets that contain food alone and pipets that contain food supplemented with ethanol (Ja et al., 2007). Devineni and Heberlein (2009) showed that flies prefer ethanol, and that preference is maintained when the caloric ratio between the ethanol-containing and non-ethanol-containing solutions is varied. However, in these experiments, the food containing ethanol had at least twice the calories as the control food. This leaves open the possibility that the flies can detect this caloric difference and prefer the ethanol-containing food for the calories that it provides. Recently it has been shown that, independent of taste, flies sense and value food for its caloric content (Dus et al., 2011). Furthermore, high calorie tasteless food can be successfully used as an unconditional stimulus in learning assays (Burke and Waddell, 2011; Fujita and Tanimura, 2011). Therefore, when evaluating preference one needs to account for the caloric content of the solution.
Here we further investigate whether flies prefer food containing ethanol because it is more caloric or because of its pharmacological effects. Distinguishing between the nutritive and pharmacological drive to consume ethanol is a maddeningly difficult question to answer because it requires determining the motivation of a fly. Indeed, this issue has not been completely resolved in rodents where the pharmacological effects of ethanol have been studied much longer (Forsander, 1998). In this paper, we show that when we use a variety of carbohydrates to balance the calories between the ethanol and non-ethanol-containing pipets that the preference for ethanol is eliminated. This occurs even if the carbohydrates used to balance calories are not gustatorily attractive to the fly. We conclude that the preference of D. melanogaster for ethanol is primarily due to its nutritive value rather than its pharmacological effects.
Materials and Methods
Flies and Media
Flies for all experiments, unless otherwise stated, were female 1–3 day old flies of the Canton-S strain. For the M100 switch assay, we additionally used 1–3 day old male flies of the w;Berlin strain which were kindly provided to us by Ulrike Heberlein. At this age, all experiments can be concluded while the flies are still young adults. Flies were raised on standard cornmeal/molasses/agar medium that had no detectable ethanol.
Preference Assay
We used an assay adapted from Devineni and Heberlein (2009). Groups of eight flies were placed into standard plastic fly vials (Genesee Scientific, San Diego, CA) in which holes were made to allow air to circulate. Filter paper was placed around the vial to prevent visual stimuli for the flies. One-third of a cellulose acetate fly vial plug (Flug, Genesee Scientific, San Diego, CA) was placed at the bottom of the vial to minimize the collection of moisture. Four holes were made in another one-third of a Flug which was placed at the top of the vial. 200 uL yellow pipet tips were cut to the appropriate length to hold a capillary tube and placed in each of the holes of the top Flug. Four uL of food was filled by capillary action into 1–5 uL capillary tubes (Catalog number 21-180-11, Drummond Scientific Company, Broomall, PA) and placed into the yellow tips so that the flies have access to them. Unless otherwise stated, two pipets (facing opposite of each other) contain ethanol-rich food and two contain food without ethanol. All vials were placed in a 5 gallon aquarium, which contained wet sponges on the bottom and wet filter paper on the sides to increase humidity. The top of the aquarium was sealed with plastic wrap and the aquarium was kept in a 12:12 light:dark cycle. Pipets were scored and replaced daily for five days. Unless otherwise stated, sixteen vials of 8 flies were used for each experiment.
Liquid Food Information and Caloric Determination
The control food solution contained 5% sucrose (Certified ACS Crystalline, Fisher Scientific, Fair Lawn, NJ) and 5% yeast extract (Bacto™, Becton, Dickinson and Company, Sparks, MD). In preference assays, half the pipets were supplemented with ethanol (Fisher Scientific, Fair Lawn, NJ). In calorie balancing experiments, we supplemented the non-ethanol-containing food with sucrose, glucose (Certified ACS, Fisher Scientific, Fair Lawn, NJ), mannose (Acros Organics, New Jersey), ribose (Acros Organics, New Jersey), or maltodextrin. We calculated caloric content using the FAO (Food and Agricultural Organization of the United Nations) values of 4 kcal/g for disaccharides (sucrose), 3.75 kcal/g for monosaccharides (glucose, mannose, ribose), and 7 kcal/g for ethanol (FAO, 1998). The caloric content for maltodextrin M100 and maltodextrin M180 is assumed to be 3.76 kcal/g based on information from the manufacturer (Grain Processing Corporation, Muscatine, IA). The caloric content for the yeast extract is estimated to be 1.58 kcal/g (Ja et al., 2007; Devineni and Heberlein, 2009).
Proboscis Extension Response
We used a method adapted from Shiraiwa and Carlson (2007). Flies were starved with only access to a water-soaked Kimwipe for 24–48 hours. Flies are then individually immobilized by placing them into 200 uL yellow pipet tips in which the end was cut so that just the head of the fly protrudes. Wicks made from thin strips of Kimwipes are dipped into the food and applied to the labellum of the fly. To minimize sensitization from the sweet sugars, we only tested two carbohydrates per day plus a positive (4% sucrose) and negative control (water) at the beginning and end of the experiment. For each food source, the labellum was touched five times in one minute intervals, and the number of responses noted. We waited five minutes when switching to a new food source. Any fly that responded less than three times to the initial 4% sucrose or more than two times to the initial water was discarded.
Gas Chromatography
Single flies were placed in the 10% ethanol preference assay. On the third day, we observed the vials. Immediately after a fly was observed to drink from the ethanol-containing pipet, we immediately transferred it into a vial with 7 uL of toluene. The fly was then crushed with a 200 uL pipet tip. Three microliters of the extract was manually injected into an SRI-310C Gas Chromatograph (SRI Instruments, Torrance, CA). The temperature protocol is: 50° for 1 minute, ramp for 10 minutes to 150°, and hold for 10 minutes. An ethanol peak is observed at approximately 2.2 minutes and toluene at approximately 10 minutes. All data was analyzed using PeakSimple software (SRI Instruments, Torrance, CA). The area of the ethanol peak was determined using the integration tool with a threshold area size of 100. Determination of the ethanol content of the fly was determined by comparison to a known standard curve of ethanol. Water content of the female was ascertained by taking the difference in weight immediately after being removed from the CAFE assay and after desiccation at 65°C for 24 hours and was 0.86 uL per fly. This value is close to that reported by (Cowmeadow et al., 2005; Folk and Bradley, 2004; Telonis-Scott and Hoffmann, 2003)
Statistical Analysis
All statistical analysis was performed using Prism 5 (GraphPad Software, Inc.). To determine statistical significance in the preference assays, a Student’s t test was used to compare cumulative consumption from days 2 to 5 from the pipets with ethanol to the pipets without ethanol. To determine significance in the proboscis extension response assay, a one-way ANOVA was performed with a Dunnett’s post-hoc test to compare each sample to the appropriate control.
Results
D. melanogaster prefer food that contains ethanol when the non-ethanol choice contains fewer calories
We used the CAFE assay to verify that flies prefer food that contains ethanol (Ja et al., 2007; Devineni and Heberlein, 2009). Eight flies were placed in vials with access to four capillary pipets. Two pipets were loaded with control food (5% sucrose and 5% yeast extract). Two other pipets contained the control food supplemented with ethanol. The volume of liquid consumed from each pipet was scored daily for five days and each day the pipet was replaced with a new pipet with fresh food. For each day, a preference index was determined by the following formula: (Amount of ethanol food consumed - Amount of control food consumed)/Total food consumed. In this scale, flies showing no preference would receive a preference index of 0, whereas flies that only consumed ethanol food would receive preference index of 1 and flies that consumed only the non-ethanol food would receive a preference index of −1.
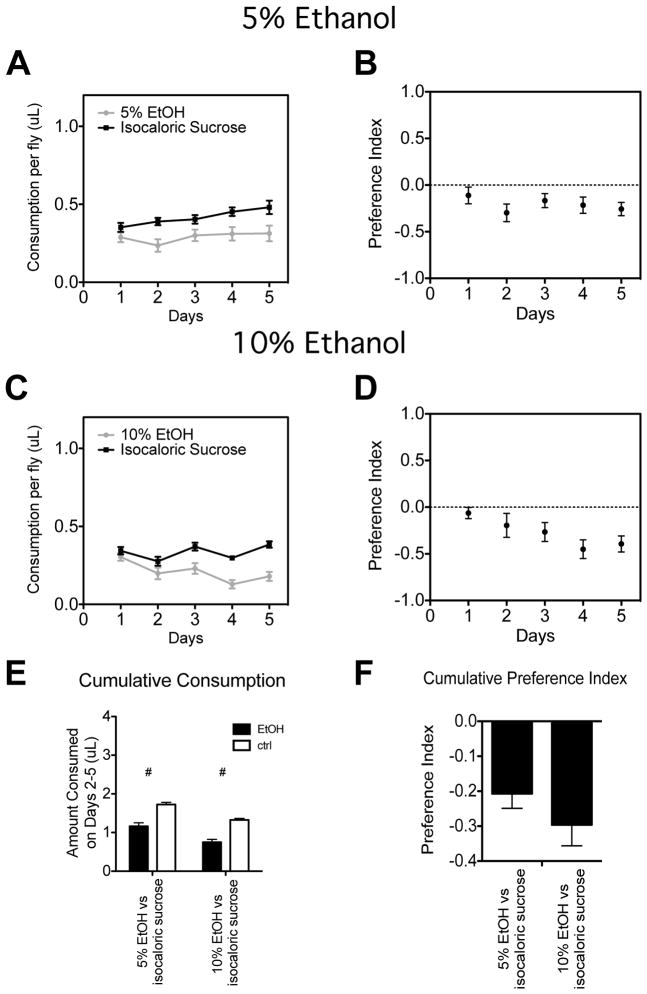
As previously demonstrated, flies prefer food that contains a high concentration of ethanol (Figure 1 and (Devineni and Heberlein, 2009)). Flies show no preference for food supplemented with 1% ethanol (Figure 1A, 1B). However, flies show a strong preference for food supplemented with 5%, 10%, or 15% ethanol when compared to ethanol-free food (Figure 1C–1J). As reported by Devineni and Heberlein (2009), the ethanol preference in flies is highly variable during the first day of the assay. Therefore, we calculate the total amount of the ethanol food consumed over the final four days and compared it to the amount of control food consumed. Only at 1% ethanol was the difference not statistically significant (Figure 1I). Preference indices for each condition are shown in Figure 1J.
Figure 1. Drosophila show preference for liquid food supplemented with ethanol.
Food consumption plots are shown for five-day preference assays in which flies had a choice between liquid food (5% sucrose, 5% yeast) and liquid food to which we added either (A) 1% ethanol, (C) 5% ethanol, (E) 10% ethanol, or (G) 15% ethanol. Panels B, D, F, and H are preference index plots for the data presented in panels A, C, E and G, respectively. Panel I shows the cumulative consumption for both the ethanol-containing and ethanol-free food (control) for each concentration of ethanol tested during days 2 to 5. Panel J shows the cumulative preference indices calculated from the cumulative data. Significance was determined using Student’s t test (*= p<0.05). n=16 vials for all experiments. Total calories consumed and total volume of food consumed for these experiments are shown in Supplemental Figures S1A and S1D.
Preference for ethanol is eliminated when the control food is balanced for calories
Ethanol is rich in calories, and can be used as a food by D. melanogaster (Libion-Mannaert, 1976; Pecsenye et al., 1994). Indeed, ethanol is estimated to contain 7 kcal/g, compared to 4 kcal/g for sucrose and 1.58 kcal/g for yeast extract (Ja et al., 2007; Devineni and Heberlein, 2009). In our 5%, 10%, and 15% ethanol assays, the ethanol pipet contained 1.99, 2.98, and 3.97 times the number of calories than the control pipet, respectively. This led us to hypothesize that the flies were choosing the ethanol-containing food because it is richer in calories. To test this hypothesis, we added sufficient carbohydrate to the control pipet to equal the calories provided by ethanol in the ethanol-containing pipet. We first used sucrose to balance calories. In a balanced assay with 5% ethanol, flies not only failed to show a preference for ethanol, but instead showed a preference for sucrose (Figure 2A, 2B). This was also the outcome when ethanol was raised to 10% and the amount of sucrose added to the control pipet matched the calories provided by 10% ethanol (Figure 2C, 2D). Cumulative plots from days 2–5 show a significant preference for the balanced sucrose solution (Figure 2E, 2F). These data indicate that the presence of an isocaloric sucrose mix is sufficient to eliminate a preference for ethanol.
Figure 2. Balancing calories with sucrose eliminates preference for ethanol.
Food consumption plots are shown for five-day preference assays in which flies had a choice between two pipets that contained liquid food (5% sucrose, 5% yeast) and ethanol and two pipets that contained liquid food supplemented with sufficient sucrose to counter-balance the calories contributed by ethanol in the other pipets. In panel A, 5% ethanol was tested while in panel C, 10% ethanol was tested. Panels B and D are preference index plots for the data in A and C, respectively. Panel E shows the cumulative consumption from days 2–5 for both the ethanol and non-ethanol-containing food. Panel F shows the preference index calculated from the cumulative data. Significance was determined using Student’s t test (*= p<0.05 for preference for ethanol, #=p<.05 for preference for control food). n=16 vials for all experiments. Total calories consumed and total volume of food consumed for these experiments are shown in Supplemental Figures S1B and S1E.
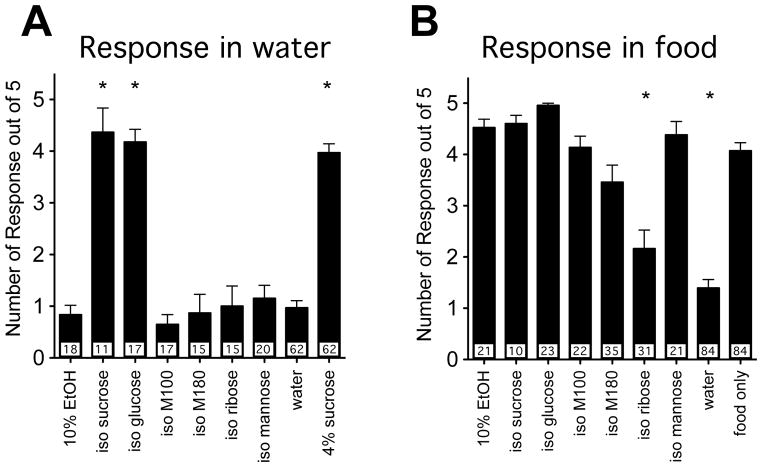
The fact that the flies no longer preferred ethanol in the balanced sucrose assay could be due to an overwhelmingly strong attraction for the taste of sucrose. To find a calorie source that is less gustatorily attractive, we tested a palette of calorie sources for gustatory attractiveness using the Proboscis Extension Response (PER) assay. In this assay, a solution is applied to the proboscis of the fly, and if it is gustatorily attractive, the fly will extend its proboscis. The PER assay has been used by many labs to determine the attractiveness of many carbohydrates and other substances (Tompkins et al., 1979; Dahanukar et al., 2007; Gordesky-Gold et al., 2008). The carbohydrates were tested to identify those that were more palatable than ethanol, less palatable than ethanol, or equally palatable to ethanol. All of the carbohydrates were tested at a concentration calculated to have the same caloric content as 10% ethanol. We tested sucrose, glucose, mannose, ribose, maltodextrin M100, and maltodextrin M180. Mannose and ribose have been previously shown not to activate the sugar receptor of the fly (Dahanukar et al., 2007). When tested in the PER assay, only sucrose and glucose showed a response higher than the water control (Figure 3A). These results indicate that the maltodextrin M100, maltodextrin M180, ribose, and mannose are not gustatorily attractive to the fly.
Figure 3. Proboscis extension response to different calorie sources.
A) Proboscis extension response to the substances in water. Flies were given 5 trials with each carbohydrate solution and the number of proboscis extensions counted. Shown are the mean number of extensions to each carbohydrate solution. Water was used as a negative control and 4% sucrose as a positive control. Significance was determined using a One-way ANOVA with a Dunnett’s correction to compare to the water control (*=p<.05). B) Proboscis extension response to the same carbohydrate series dissolved in liquid food (5% sucrose, 5% yeast). Water was used as a negative control and liquid food as a positive control. Significance was determined using a One-way ANOVA with a Dunnett’s correction to compare to the liquid food control (*=p<0.05). The number of flies tested for each solution is indicated by the number in the bottom of each bar.
To determine whether any of these carbohydrates were gustatorily aversive to the fly, we asked whether the addition of the other carbohydrates to the standard food solution (5% yeast extract, 5% sucrose) would reduce the number of responses in the PER assay. All of the carbohydrates were tested at a concentration calculated to have the same caloric content as 10% ethanol. When these carbohydrates are added to the food solution of 5% sucrose and 5% yeast, only ribose supplementation reduced the attractiveness of the standard food solution indicating that only ribose is aversive (Figure 3B).
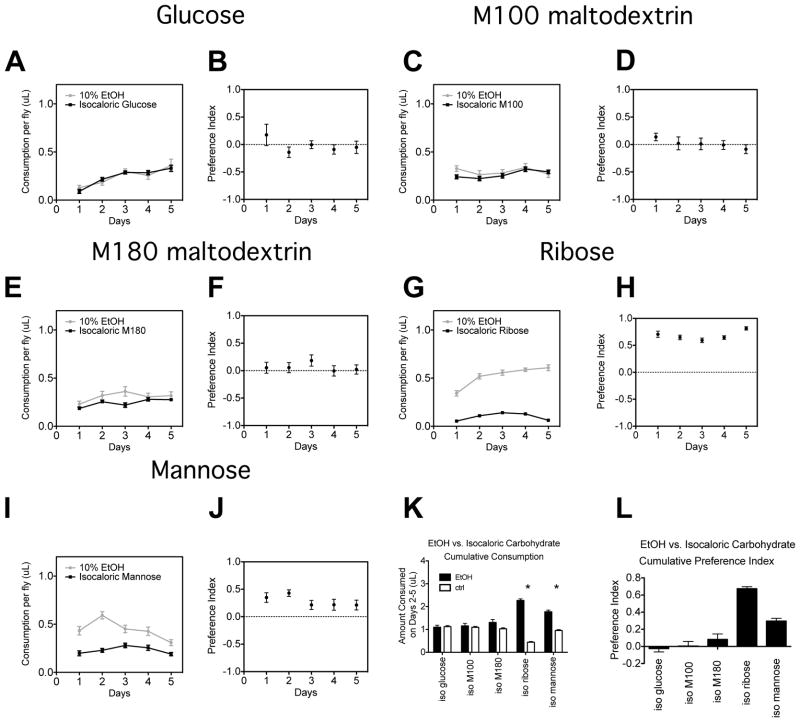
The six carbohydrates characterized in the PER assay were used in the CAFE assay to supplement the control pipet to a level calculated to counterbalance the calories provided by the ethanol in the ethanol-supplemented pipet (Figure 4). When calorie balancing was performed with glucose, ethanol preference was eliminated. Furthermore, despite not being gustatorily attractive, ethanol preference was also eliminated when the control solution was balanced with either maltodextrin M100 or maltodextrin M180. However, preference for ethanol persisted when calorie balancing was performed with ribose. Ribose was shown to be strongly aversive in the PER assay and therefore, in this instance the fly may choose the isocaloric ethanol solution merely as a way to avoid ribose. These data support a model in which the fly chooses ethanol-containing food only if another isocaloric (and palatable) calorie source is not available. However, we did encounter one carbohydrate that belied this model. When mannose was used to balance the calories in the ethanol pipet, we observed a preference for ethanol despite the fact that the PER assay shows that the taste of mannose is neither gustatorily aversive nor attractive. It is possible that the rate of mannose catabolism is sufficiently slow that the caloric content cannot be registered in time to influence the pipet choice. Nevertheless, the model in which ethanol is sought for its caloric content should not be discounted since four out of five of the non-aversive carbohydrates that were used to balance the calories in the ethanol pipet produced results that are consistent with this long-standing ecologically-based model.
Figure 4. Preference for ethanol food can be eliminated if the other solution has equal caloric content.
Consumption plots are shown for five-day preference assays in which flies had a choice between two pipets that contained liquid food (5% sucrose, 5% yeast) and ethanol and two pipets that contained liquid food supplemented with an isocaloric amount of (A) glucose, (C) M100 maltodextrin, (E) M180 maltodextrin, (G) ribose, or (I) mannose. Panels B, D, F, H, and J are preference index plots for the data presented in panels A, C, E, G, and I, respectively. Panel K shows the cumulative consumption for both the ethanol-containing and ethanol-free food (control) for each concentration of ethanol tested during days 2 to 5. Panel L shows the cumulative preference indices calculated from the cumulative data. Significance was determined using Student’s t test (*= p<0.05). n=16 vials for all experiments. Total calories consumed and total volume of food consumed for these experiments are shown in Supplemental Figures S1C and S1F.
The peak ethanol concentration in the unbalanced ethanol CAFE assay does not exceed 4mM
Previously, Devineni and Heberlein (2009) found that flies with the option of drinking 15% ethanol food had about 1 mM more ethanol in them than control flies that had no access to ethanol. However, the authors point out that the sample consisted of multiple flies, that although collected at a single moment in time, was likely to be a mixture of flies that had recently consumed ethanol and flies that had consumed ethanol a long time ago and may have already metabolized it. To determine the maximum possible ethanol concentration that accumulates when flies are showing strong ethanol preference, we determined the amount of ethanol inside single flies immediately after ethanol consumption in an unbalanced CAFE assay. One set of pipets contained standard food and one set of pipets contained standard food supplemented with 10% ethanol. All samples were collected on the third day of the assay at which point the flies were showing strong ethanol preference (Figure 1). We assayed single flies immediately after they drank from the ethanol pipet. The ethanol concentration maxima is likely to occur immediately after the sip is taken because the inter-sip interval is very long (~120 minutes, data not shown and also as reported in Ja et al., 2007 and Devineni and Heberlein, 2009) and that the rate of ethanol catabolism is very large (Middleton and Kacser, 1983; Moore et al., 1998; Cowmeadow et al., 2005). Thus, there is little opportunity for ethanol to accumulate between sips.
The ethanol content of each fly was measured by gas chromatography (Figure 5A). Water content of the flies was determined as described (Cowmeadow et al., 2005). The minimum concentration of ethanol that can be detected by this method is 0.5 mM. Flies drinking non-ethanol food contained no detectable ethanol above background (<0.5 mM, n=4). The highest observed concentration of ethanol in a fly immediately after drinking was 3.08 mM. Figure 5B shows the distribution of ethanol content.
Figure 5. Quantification of internal ethanol concentration.
A) Chromatograph tracings at the elution time of ethanol from a standard curve of ethanol dilutions and representative extracts from flies. The standard curve consists of two-fold ethanol dilutions into toluene. The labels on the x-axis pertain to the molarity of an amount equivalent to the water weight of one fly. Individual flies were prepared by crushing them in 7 uL of toluene immediately after they were observed to drink from a pipet. Tracings from a representative fly that only got control food (ctrl fly) and two representative flies that were in a 10% ethanol assay (EtOH fly) are shown. B) A histogram showing the ethanol content observed in flies that only received control food (ctrl) and flies that were immediately assayed after drinking from an ethanol pipet.
Flies prefer the non-ethanol-containing food if it contains more calories
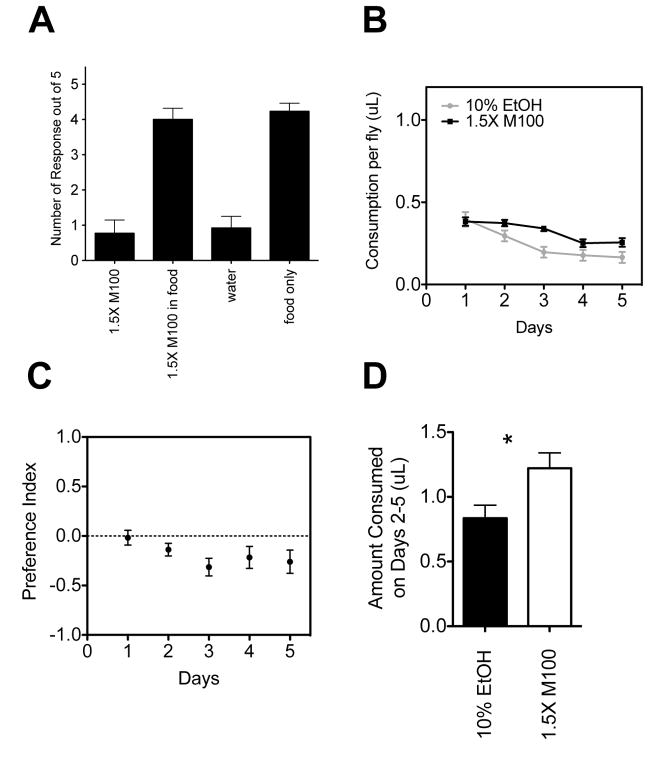
We have shown that in an unbalanced assay in which flies have a choice between food supplemented with ethanol and unsupplemented food that flies prefer the ethanol food. We have shown that this preference can be eliminated if we calorically balance the control pipet by adding non-sweet M100 maltodextrin. Given the hypothesis that flies are choosing the pipets based on calories, one would expect that the flies would prefer the non-ethanol-containing pipet if it contained more calories than the ethanol pipet. To test this hypothesis, we tested preference between a pipet supplemented with 10% EtOH and a pipet supplemented with enough M100 to provide 1.5-fold more calories than the ethanol. Like the isocaloric M100, this concentration of maltodextrin was neither gustatorily attractive nor aversive in the PER assay (Figure 6A). When tested in the CAFE assay, flies showed a significant preference for the M100 solution (Figure 6B–D).
Figure 6. Drosophila prefer the non-ethanol solution if it contains more calories.
A) Proboscis extension response data is shown for M100 maltodextrin at a concentration sufficient to provide 1.5 times the amount of calories of 10% EtOH (1.5X M100). This concentration was tested both in water and in food and compared to the appropriate control. The response to the 1.5X M100 solution was not statistically different from the controls (Student’s t test). n=13 flies. B) Food consumption plots are shown for five-day preference assays in which flies had a choice between two pipets that contained liquid food supplemented with 10% ethanol and two pipets that contained liquid food supplemented with 1.5X M100. C) Preference indices based on the data from Panel B. D) Cumulative consumption from days 2–5 for both the ethanol and non-ethanol-containing food. Significance was determined using Student’s t test (p<0.05). Total calories consumed and total volume of food consumed for these experiments are shown in Supplemental Figures S1C and S1F.
Switching to a calorically-balanced alternative after preference has developed eliminates ethanol preference
Flies prefer ethanol in the calorically-unbalanced but not in the calorically-balanced assay. One reason that the flies do not show ethanol preference in the calorically-balanced assay might be that they do not consume sufficient ethanol to experience the pharmacological or intoxicating effects due to the presence of a gustatorily attractive and high calorie ethanol-free pipet. Indeed, Shtonda and Avery (Shtonda and Avery, 2006) have shown that C. elegans are not interested in other food if presented with a high quality food. To address this issue, we performed assays which began in the unbalanced state but were switched to a balanced state once clear preference had been established (3 days). For the first three days, the flies had the standard choice between food supplemented with 10% EtOH and non-ethanol containing food. Then, for the fourth and fifth days, the non-ethanol containing food was supplemented with M100 maltodextrin, chosen because of its lack of gustatory attractiveness, at a level sufficient to balance the calories from ethanol. Flies demonstrated preference for ethanol on days 2 and 3, but immediately following the switch to a balanced assay, preference was eliminated (Figure 7A–D). The switching assay provides the clearest evidence that ethanol preference was in response to the caloric content of ethanol and was not in response to the pharmacological effect of ethanol. Devineni and Heberlein (2009), who reached the opposite conclusion, did so using an calorically-unbalanced CAFE assay, male flies, and a different strain of Drosophila (w;Berlin strain). To test if w;Berlin males show a fundamentally different response to ethanol, we repeated the switch assay with male flies of the w;Berlin strain and found that they respond in the same way as the Canton-S strain (Figure 7E–H). These results indicate that even when given sufficient time to develop preference for ethanol, flies do not prefer ethanol if presented with an isocaloric alternative.
Figure 7. Switching to a balanced assay after preference has been established eliminates the preference for ethanol.
A and E) Food consumption plots for five-day preference assays in which flies had a choice between pipets containing liquid food (ctrl) or liquid food supplemented with 10% ethanol (EtOH). On days 1–3, the control food was 5% sucrose, 5% yeast. On days 4 and 5, the control food is supplemented with enough M100 maltodextrin to balance the calories contributed by ethanol in the 10% EtOH pipets. B and F) Preference index plots for the data presented in panels A and E respectively. C and G) Cumulative consumption of control or ethanol-containing food before and after the flies were switched to a balanced assay. For days 2–3, the calories in the control pipet did not counterbalance the calories from ethanol in the EtOH pipet (Days 2–3 unbalanced). During days 4–5 (Days 4–5 balanced), the calories from ethanol in the EtOH pipet was counterbalanced in the control pipet with M100 maltodextrin. Significance was determined using Student’s t test (*= p<0.05 for preference for ethanol food, #=p<0.05 for preference for control food). D and H) Preference indices calculated from the cumulative data in C and G respectively. Significance was determined using Student’s t test (*= p<0.05). Panels A–D use females from the Canton-S strain. Panels E–H use males from the w;Berlin strain. n=16 vials for all experiments. Total calories consumed and total volume of food consumed for these experiments are shown in Supplemental Figures S2A and S2B.
Discussion
In this paper, we show that preference for ethanol in D. melanogaster is not due to the pharmacological effects of ethanol. While flies show preference for ethanol over control food that contains fewer calories, this preference is lost once the control food is adjusted to be isocaloric with the ethanol food. This holds true even for food supplemented with maltodextrin M100 and maltodextrin M180, which are not gustatorily attractive to the fly. Flies will even shift their preference toward the maltodextrin M100 solution if it contains more calories. In this study, we use values for calories based on FAO (1998) estimates. It is possible that the number of calories available to Drosophila from its foodstuffs are different from the FAO estimates. An accurate measurement of effective number of calories provided by each food ingredient would be invaluable for studies of this kind. Because we could not be certain of the actual number of calories available to the fly, we balanced calories using a variety of carbohydrates. Four of the five non-repulsive carbohydrates that were tested abolished ethanol preference, which is consistent with the idea that the flies would reject ethanol as long as the calorically balanced alternative was not gustatorily repugnant. This is consistent with the interpretation that it is calories and not pharmacological effects that are motivating flies to drink ethanol.
One might argue that in the balanced assay flies do not prefer ethanol as in the unbalanced assay because the presence of a calorically-balanced alternative means that they never drink sufficient quantities of ethanol to induce pharmacological preference. A related counter argument is that flies can develop pharmacological preference to ethanol but not if an alternative is simultaneously presented. However, both these caveats were discredited by our food switching experiment in which we allowed ethanol preference to develop under non-calorically balanced conditions and then switched to a calorically balanced alternative food. Once offered another choice with equal caloric value flies immediately lost their preference for ethanol and showed strong preference for the non-alcoholic food. If flies prefer ethanol for pharmacological reasons, then one would assume that they would continue to prefer it even after presented with a calorie-balanced alternative.
The role of calories in ethanol intake in rodents has been a contentious issue (Forsander, 1998; Gill et al., 1996). Early work showed that rats will eat considerably less food when ethanol is present and will drink more ethanol on a calorically restricted diet, suggesting that the calories provided by ethanol are not without consequence (Richter, 1926; Westerfieid and Lawrow, 1953). Furthermore, it was more recently demonstrated that blocking the leptin pathway, which is involved in feeding and appetite, reduces ethanol consumption in mice (Forsander, 1998; Blednov et al., 2004). For rodents, it is probably reasonable to discount the possibility that this animal seeks ethanol for its caloric value because this would be such an unnatural situation. Rodents do not consume ethanol in the wild. However, wild Drosophila melanogaster do consume ethanol. The evolutionary history of D. melanogaster supports the interpretation that this species uses ethanol as a food source. In the wild, ethanol is a common component of high quality D. melanogaster food and high value egg-laying sites. While ethanol-rich foods are avoided by competitor Drosophila species–probably because ethanol reduces the fecundity of these competitors–D. melanogaster is better able to metabolically handle high concentrations of ethanol than its close relatives (McKenzie and Parsons, 1972). This makes the ethanol-rich niche a protected area for D. melanogaster feeding and reproduction. Further support for a non-pharmacological role for ethanol in Drosophila natural history is the recent demonstration by Ogueta et al., (2010) of a linkage between ethanol preference in an olfactory assay and ethanol metabolism in adult flies.
Calories are most likely an important factor in determining food selection by the fly. Indeed, flies have a putative taste-independent metabolic sensor that can be used to assess the caloric content of a food source (Dus et al., 2011). Additionally, flies will learn to be attracted to odors that are associated with tasteless calorie-rich foods (Burke and Waddell, 2011; Fujita and Tanimura, 2011). Our data indicates that flies are able to assess the caloric difference between the ethanol and non-ethanol pipets and prefer the one with a higher caloric value.
Drosophila may prove to be a useful model for alcohol preference in humans. However, the experimental paradigm must induce preference for the pharmacological effects of ethanol and not only for the caloric content of ethanol. Our data do not exclude the possibility that conditions exist that will cause flies to prefer ethanol for its pharmacological effects. The data only indicate that preference does not exist in the CAFE assay under conditions in which the alternative food is calorically balanced. This may be because the ethanol content in the fly is never sufficiently elevated (<4 mM). Devineni and Heberlein (2009) have generated higher ethanol levels in CAFE assays after flies have been starved for 20 hours. In this instance, ethanol preference is still more likely to be calorically based because the flies were extremely hungry and because the alternative food provided far fewer calories (unbalanced CAFE assay). Exposing flies to ethanol vapor or direct injection of ethanol is a standard way of generating high ethanol hemolymph levels (Devineni and Heberlein, 2009; Manev et al., 2003; Moore et al., 1998; Cowmeadow et al., 2005) and thus this approach may help generate pharmacological-based preference that can later be studied in the CAFE assay. In conclusion, our data indicate that D. melanogaster prefer ethanol for its caloric value in the confines of the CAFE assay.
Supplementary Material
Acknowledgments
We would like to thank Benjamin Troutwine, Alfredo Ghezzi, Rudolf Bohm, Roseanna Robles, Brooks Robinson, and Sukant Khurana for critical reading of the manuscript. This work was supported by National Institute of Health Grant R01AA018037 to NSA and T32AA007471 awarded to JBP. The undergraduate contribution was supported by NSF IOS-0641370 to NSA.
References
- Atkinson NS. Tolerance in Drosophila. J Neurogenet. 2009;23:293–302. doi: 10.1080/01677060802572937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton RJ, Tsai LT, Singh CM, Moore MS, Neckameyer WS, Heberlein U. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr Biol. 2000;10:187–194. doi: 10.1016/s0960-9822(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Berger KH, Heberlein U, Moore MS. Rapid and chronic: two distinct forms of ethanol tolerance in Drosophila. Alcohol Clin Exp Res. 2004;28:1469–1480. doi: 10.1097/01.alc.0000141817.15993.98. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Harris RA. Blockade of the leptin-sensitive pathway markedly reduces alcohol consumption in mice. Alcohol Clin Exp Res. 2004;28:1683–1692. doi: 10.1097/01.alc.0000145790.60216.c8. [DOI] [PubMed] [Google Scholar]
- Burke CJ, Waddell S. Remembering nutrient quality of sugar in Drosophila. Curr Biol. 2011;21:746–750. doi: 10.1016/j.cub.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadieu N, Cadieu J, El Ghadraoui L, Grimal A, Lamboeuf Y. Conditioning to ethanol in the fruit fly-a study using an inhibitor of ADH. J Insect Physiol. 1999;45:579–586. doi: 10.1016/s0022-1910(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Capy P, Gibert P. Drosophila melanogaster, Drosophila simulans: so similar yet so different. Genetica. 2004;120:5–16. doi: 10.1023/b:gene.0000017626.41548.97. [DOI] [PubMed] [Google Scholar]
- Corl AB, Berger KH, Ophir-Shohat G, Gesch J, Simms JA, Bartlett SE, Heberlein U. Happyhour, a Ste20 family kinase, implicates EGFR signaling in ethanol-induced behaviors. Cell. 2009;137:949–960. doi: 10.1016/j.cell.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Cowmeadow RB, Krishnan HR, Atkinson NS. The slowpoke gene underlies rapid ethanol tolerance in Drosophila. Alcohol Clin Exp Res. 2005;29:1777–1786. doi: 10.1097/01.alc.0000183232.56788.62. [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Lei YT, Kwon JY, Carlson JR. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56:503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depiereux E, Hougouto N, Lechien J, Libion-Mannaert M, Liétaert MC, Feytmans E, Elens A. Larval behavioral response to environmental ethanol in relation to alcohol dehydrogenase activity level in Drosophila melanogaster. Behav Genet. 1985;15:181–188. doi: 10.1007/BF01065898. [DOI] [PubMed] [Google Scholar]
- Devineni AV, Heberlein U. Preferential ethanol consumption in Drosophila models features of addiction. Curr Biol. 2009;19:2126–2132. doi: 10.1016/j.cub.2009.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dus M, Min S, Keene AC, Lee GY, Suh GS. Taste-independent detection of the caloric content of sugar in Drosophila. Proc Natl Acad Sci U S A. 2011;108:11644–11649. doi: 10.1073/pnas.1017096108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzitoyeva S, Dimitrijevic N, Manev H. gamma-Aminobutyric acid B receptor 1 mediates behavior-impairing actions of alcohol in Drosophila: Adult RNA interference and pharmacological evidence. Proc Natl Acad Sci U S A. 2003;100:5485–5490. doi: 10.1073/pnas.0830111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO. FAO Food and Nutrition Paper. 1998. Carbohydrates in human nutrition. Report of a Joint FAO/WHO Expert Consultation; p. 66. [PubMed] [Google Scholar]
- Folk DG, Bradley TJ. The evolution of recovery from desiccation stress in laboratory-selected populations of Drosophila melanogaster. J Exp Biol. 2004;207:2671–2678. doi: 10.1242/jeb.01048. [DOI] [PubMed] [Google Scholar]
- Forsander OA. Dietary influences on alcohol intake: a review. J Stud Alcohol. 1998;59:26–31. doi: 10.15288/jsa.1998.59.26. [DOI] [PubMed] [Google Scholar]
- Fujita M, Tanimura T. Drosophila evaluates and learns the nutritional value of sugars. Curr Biol. 2011;21:751–755. doi: 10.1016/j.cub.2011.03.058. [DOI] [PubMed] [Google Scholar]
- Gelfand LJ, McDonald JF. Relationship between ADH activity and behavioral response to environmental alcohol in Drosophila. Behav Genet. 1980;10:237–249. doi: 10.1007/BF01067770. [DOI] [PubMed] [Google Scholar]
- Gibson JB, May YW, Wilks AV. Genetic variation at the alcohol dehydrogenase locus in Drosophila melanogaster in relation to environmental variation: Ethanol levels in breeding sites and allozyme frequencies. Oecologia. 1981;51:191–198. doi: 10.1007/BF00540600. [DOI] [PubMed] [Google Scholar]
- Gill K, Amit Z, Smith BR. Alcohol as a food: a commentary on Richter. Physiol Behav. 1996;60:1485–1490. doi: 10.1016/s0031-9384(96)00309-5. [DOI] [PubMed] [Google Scholar]
- Gordesky-Gold B, Rivers N, Ahmed OM, Breslin PA. Drosophila melanogaster prefers compounds perceived sweet by humans. Chem Senses. 2008;33:301–309. doi: 10.1093/chemse/bjm088. [DOI] [PubMed] [Google Scholar]
- McKenzie JA, McKechnie SW. A comparative study of resource utilization in natural populations of Drosophila melanogaster and D. simulans. Oecologia. 1979;40:299–309. doi: 10.1007/BF00345326. [DOI] [PubMed] [Google Scholar]
- Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci U S A. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libion-Mannaert M, Delcour J, Deltombe-Lietaert MM, Lenelle-Montfort N, Elens A. Ethanol as a “food” for Drosophila melanogaster: influence of the ebony gene. Experientia. 1976;32:22–24. [Google Scholar]
- Manev H, Dimitrijevic N, Dzitoyeva S. Techniques: fruit flies as models for neuropharmacological research. Trends Pharmacol Sci. 2003;24:41–43. doi: 10.1016/s0165-6147(02)00004-4. [DOI] [PubMed] [Google Scholar]
- Mayfield RD, Harris RA, Schuckit MA. Genetic factors influencing alcohol dependence. Br J Pharmacol. 2008;154:275–287. doi: 10.1038/bjp.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie JA, Parsons PA. Alcohol tolerance: an ecological parameter in the relative success of Drosophila melanogaster and Drosophila simulans. Oecologia. 1972;10:373–388. doi: 10.1007/BF00345738. [DOI] [PubMed] [Google Scholar]
- McKenzie JA, Parsons PA. Microdifferentiation in a natural population of Drosophila melanogaster to alcohol in the environment. Genetics. 1974;77:385–394. doi: 10.1093/genetics/77.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton RJ, Kacser H. Enzyme variation, metabolic flux and fitness: alcohol dehydrogenase in Drosophila melanogaster. Genetics. 1983;105:633–650. doi: 10.1093/genetics/105.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MS, DeZazzo J, Luk AY, Tully T, Singh CM, Heberlein U. Ethanol intoxication in Drosophila: Genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell. 1998;93:997–1007. doi: 10.1016/s0092-8674(00)81205-2. [DOI] [PubMed] [Google Scholar]
- Ogueta M, Cibik O, Eltrop R, Schneider A, Scholz H. The influence of Adh function on ethanol preference and tolerance in adult Drosophila melanogaster. Chem Senses. 2010;35:813–822. doi: 10.1093/chemse/bjq084. [DOI] [PubMed] [Google Scholar]
- Parsons PA. Larval responses to environmental ethanol in Drosophila melanogaster: variation within and among populations. Behav Genet. 1980;10:183–190. doi: 10.1007/BF01066268. [DOI] [PubMed] [Google Scholar]
- Pecsenye K, Lefkovitch LP, Giles BE, Saura A. Does Drosophila-melanogaster use ethanol as an energy-source during starvation. Hereditas. 1994;121:225–236. doi: 10.1111/j.1601-5223.1994.t01-1-00225.x. [DOI] [PubMed] [Google Scholar]
- Richter CP. A study of the effect of moderate doses of alcohol on the growth and behavior of the rat. J Exp Zool. 1926;44:397–418. [Google Scholar]
- Rothenfluh A, Threlkeld RJ, Bainton RJ, Tsai LT, Lasek AW, Heberlein U. Distinct behavioral responses to ethanol are regulated by alternate RhoGAP18B isoforms. Cell. 2006;127:199–211. doi: 10.1016/j.cell.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Scholz H, Ramond J, Singh CM, Heberlein U. Functional ethanol tolerance in Drosophila. Neuron. 2000;28:261–271. doi: 10.1016/s0896-6273(00)00101-x. [DOI] [PubMed] [Google Scholar]
- Shiraiwa T, Carlson JR. Proboscis extension response (PER) assay in Drosophila. J Vis Exp. 2007:193. doi: 10.3791/193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtonda BB, Avery L. Dietary choice behavior in Caenorhabditis elegans. J Exp Biol. 2006;209:89–102. doi: 10.1242/jeb.01955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telonis-Scott M, Hoffmann AA. Isolation of a Drosophila melanogaster desiccation resistant mutant. J Insect Physiol. 2003;49:1013–1020. doi: 10.1016/s0022-1910(03)00184-7. [DOI] [PubMed] [Google Scholar]
- Tompkins L, Cardosa MJ, White FV, Sanders TG. Isolation and analysis of chemosensory behavior mutants in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1979;76:884–887. doi: 10.1073/pnas.76.2.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zwaluw CS, Engels RC. Gene-environment interactions and alcohol use and dependence: current status and future challenges. Addiction. 2009;104:907–914. doi: 10.1111/j.1360-0443.2009.02563.x. [DOI] [PubMed] [Google Scholar]
- Westerfieid WW, Lawrow J. The effect of caloric restriction and thiamine deficiency on the voluntary consumption of alcohol by rats. Q J Stud Alcohol. 1953;14:378–384. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.