Abstract
INTRODUCTION
Repolarization alternans (Re-ALT), a beat-to-beat alternation in action potential repolarization, promotes dispersion of repolarization, wavebreaks and reentry. Recently, Re-ALT has been shown to play an important role in the transition from rapid pacing to atrial fibrillation (AF) in humans. The detailed kinetics of atrial Re-ALT, however, has not been reported so far. We developed a chronic free-behaving ovine pacing model to study the kinetics of atrial Re-ALT as a function of pacing rate.
METHODS
Thirteen sheep were chronically implanted with two pacemakers for the recording of broadband right atrial unipolar electrograms and delivery of rapid pacing protocols. Beat-to-beat differences in atrial T-wave apex amplitude as a measure of Re-ALT and activation time were analyzed at incremental pacing rates until the effective refractory period (ERP) defined as stable 2:1 capture.
RESULTS
Atrial Re-ALT appeared intermittently but without periodicity, and increased in amplitude as a function of pacing rate until ERP. Intermittent 2:1 atrial capture was observed at pacing cycle lengths 40ms above ERP, and increased in duration as a function of pacing rate. Episodes of rapid pacing-induced AF were rare, and were preceded by Re-ALT or complex oscillations of atrial repolarization, but without intermittent capture.
CONCLUSION
We show in vivo that atrial Re-ALT developed and increased in magnitude with rate until stable 2:1 capture. In rare instances where capture failure did not occur, Re-ALT and complex oscillations of repolarization surged and preceded AF initiation.
Keywords: reentry, atrial fibrillation, excitability, repolarization alternans
Introduction
Atrial Fibrillation (AF) initiates when triggers such as pulmonary vein (PV) tachycardias interact with substrates1, 2. The exact nature of the electrophysiological substrates favoring transition from PV tachycardia to persistent AF remains unclear. Repolarization alternans (Re-ALT), a beat-to-beat alternation in action potential duration (APD) or amplitude, has been identified as a mechanism by which dispersion of repolarization is promoted, leading to wavebreaks and reentry3–6, and has been implicated in transitions to AF from pacing7–9, following atrial flutter5 or myocardial infarction7. Recently, Re-ALT and complex APD oscillations have been shown to play an important role in the transition from rapid pacing to AF10. Atrial Re-ALT required progressively faster rates for patients with persistent AF, patients with paroxysmal AF, and controls. However, the detailed kinetics of atrial Re-ALT has not been reported so far. We developed a chronic free-behaving ovine pacing model to study in vivo the kinetics of atrial Re-ALT as a function of pacing rate.
Methods
ANIMAL EXPERIMENTAL SETUP AND DATA ACQUISITION
Two pacemakers, each with a single lead screwed into the right atrium (RA), were implanted in 13 male sheep (74±18kg) under general anesthesia (2mg/kg i.v. propofol, 1–5% isoflurane)11 : a Vitatron™ T70 model was used for the recording of atrial unipolar electrogram (EGM) because of its unique broadband signal characteristics (sampling frequency 800Hz, 0.4Hz high pass filter), and a Vitatron™ Prevent AF pacemaker to deliver customized electrophysiological pacing protocols because of its reprogramming capacity. Atrial EGM and subcutaneous ECG were recorded with a wireless Holter device (TMSi™, B_Holter). At many pacing cycle lengths (PCLs), 2:1 AV conduction prevented the reliable measurement of Re-ALT as the far-field ventricular depolarization impinged on the preceding atrial repolarization. In order to prevent 2:1 AV ratios or other far-field ventricular interferences, the AV junction was ablated and a right ventricular lead was implanted to maintain AV synchrony in a subset of sheep (n=4, 63±6kg)11. Recordings were acquired at least one month following device and leads implantation after light sedation (Xylazine 0.2mg/kg). Experiments were carried out in accordance with the European convention for the protection of vertebrate animals used for scientific purposes.
PACING PROTOCOLS
Atrial Re-ALT thresholds, kinetics of Re-ALT amplitude and activation time were determined as a function of PCL. The pacing protocols consisted of atrial pacing for 400 beats starting at PCL 400ms with 10ms decrement until stable 2:1 capture (i.e., approximating the effective refractory period, ERP). The amplitude of stimulation (2.3±0.6V, 0.5ms) was at least twice diastolic threshold and remained constant over the pacing protocols.
SIGNAL ANALYSIS
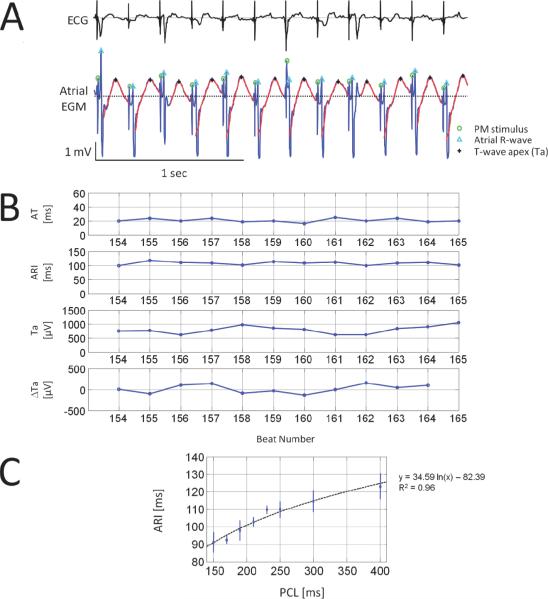
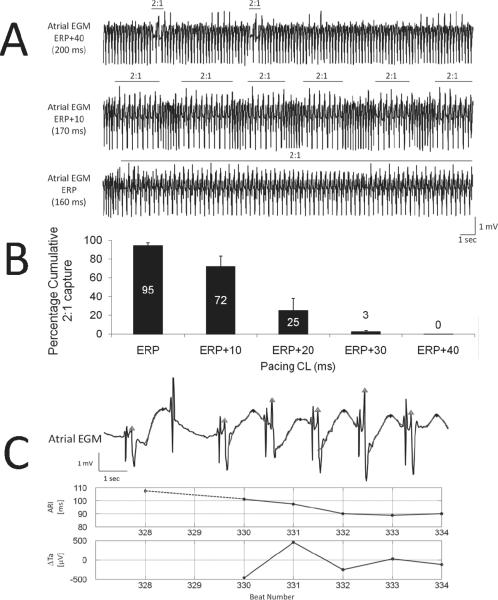
ECG baseline wandering was suppressed with a Butterworth high-pass filter with a 1.5Hz cut-off frequency. A wavelet denoising method was applied on both EGM and ECG signals and ventricular activity was cancelled in sheep with AV block to improve Re-ALT quantification. Pacemaker stimuli, atrial depolarization and repolarization waves, and T-wave apex (Ta) were then identified on the EGM (Figure 1, panel A). The filtering, ventricular cancellation, identification and Ta error in time and amplitude are detailed in the Supplemental material. Re-ALT sequences were determined from time series of beat-to-beat differences of atrial Ta, similarly to the method developed by Swerdlow et al. for unipolar EGMs provided by ICD12. Re-ALT amplitude, when present, was also measured and averaged for the last 9 beats preceding episodes of rapid pacing-induced AF. Because complex oscillations of atrial repolarization have also been reported preceding arrhythmia onset10, we measured the mean beat-to-beat difference in Ta for the last 9 beats before induced AF; oscillations of atrial Ta were considered complex when beat-to-beat Ta varied in a non alternating pattern by >50μV, which has been measured as the background level of Ta variations in our model (see Figure 2C). Activation time (AT, panel B of Figure 1) of unipolar EGM was defined as the time interval between the pacemaker stimulus and the maximum of the atrial depolarization wave (Ra), and alternans of AT (AT-ALT) as beat-to-beat variation in AT >1.25ms (i.e., time interval between two samples). Diastolic intervals (DIs) were measured as the time interval elapsed from the preceding Ta to the following Ra. Activation recovery intervals (ARIs) were measured from Ra to the following Ta because at fast pacing rates the end of the atrial repolarization wave could not be reliably determined as it was impinged by the following depolarization. Although ARIs are moderately correlated with local APD or refractory periods13–15, they are able to track adequately APD changes15. To support the relationship of ARIs to APDs in our model, the rate dependence of ARI was evaluated in a subset of 8 sheep. Panel C of Figure 1 shows that ARI decreased significantly (p<0.05) as a function of PCL from 123±7ms at 400ms PCL to 91±16ms at 150ms PCL. Panel B of Figure 1 also illustrates representative examples of time series of AT, ARI, Ta amplitude and its beat-to-beat differences (ΔTa) at PCL 210ms from the EGM shown in panel A. Although subtle variations in ΔTa were observed, they did not fulfill criteria for Re-ALT (see Statistical Analysis).
Figure 1. Ovine atrial unipolar EGM and time series.
A representative example of subcutaneous bipolar ECG (top) and corresponding unipolar electrograms (EGM, bottom) at a pacing cycle length (PCL) of 210ms is shown in panel A. Green circles denote pacemaker (PM) stimuli, cyan triangles the first atrial depolarization wave (R-wave) and black stars atrial T-wave apexes (Ta). Repolarization waves are highlighted by red bold curves. Panel B shows from top to bottom time series of AT, activation recovery interval (ARI), Ta and beat-to-beat difference in Ta (ΔTa) from the EGM shown above. Panel C illustrates the non linear (i.e. logarithmic fit) relationship between ARI (mean±SD) and PCL determined in a subset of 8 sheep.
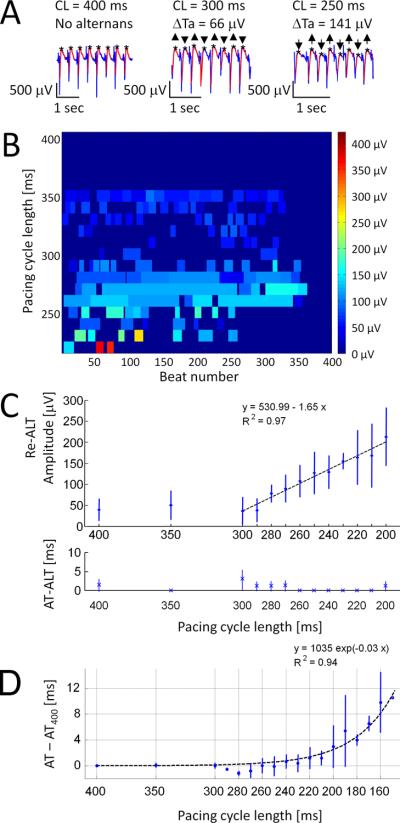
Figure 2. Rate dependence of Re-ALT.
Panel A shows examples of atrial EGM at decreasing PCLs. Panel B displays Re-ALT magnitude over a 400-beat pacing protocol. Significant Re-ALT sequences are displayed as rectangles of variable duration (number of beats, x-axis) and amplitude (0 to 400μV, colorscale) at decreasing PCLs (400 to 220ms, y-axis). Panel C reports (n=4 sheep with AV block) mean±SD of Re-ALT amplitude (top) and activation time alternans (AT-ALT, bottom), and panel D mean±SD of AT prolongation (defined as the difference from baseline value (AT-AT400ms)) as a function of PCL.
STATISTICAL ANALYSIS
Statistical tests were performed using Matlab version 7.9 (Mathworks Inc., Natick MA). Time series of Ta were used to determine Re-ALT sequences, which were considered significant when the two following conditions were fulfilled: 1) Ta was alternating for ≥5 consecutive beats; 2) even and odd Ta distributions were statistically different based on a Student t-test (p<0.01, two sided). The same criteria were applied to AT alternans (AT-ALT), which was considered significant if >1.25ms. Multiple one-way ANOVA was used to test differences in ARI, Re-ALT amplitude, AT and AT-ALT as a function of PCL. Fisher's g-statistic was used to assess whether Re-ALT sequences appeared in a periodic manner16. Fisher's exact test was used to analyze the 2×2 contingency table. A p<0.05 was considered significant.
Table.
Re-ALT and AT prolongation before capture failure - Prevalence of the four patterns illustrated in Figure 4.
| Presence of Re-ALT | Absence of Re-ALT | ||
|---|---|---|---|
| Stable AT | 40% (B1) | 11% (B4) | 51% |
| Increased AT | 33% (B2) | 16% (B3) | 49% |
|
| |||
| 73% | 37% | ||
AT, activation time; Re-ALT, repolarization alternans
Results
KINETICS OF ATRIAL REPOLARIZATION ALTERNANS
Atrial Re-ALT was detected in all sheep (with and without AV block), and its amplitude increased as a function of pacing rate. Panel A of Figure 2 illustrates representative examples of atrial unipolar EGMs at decreasing PCLs. Smoothed repolarization waves are shown in red and Ta by black stars. Re-ALT was absent at 400ms PCL, but appeared and increased in magnitude at shorter PCLs. The decrease of PCL to 300ms and 250ms was associated with progressive increase in Re-ALT amplitude from a mean peak-to-peak difference (ΔTa) of 66μV at 300ms to 141μV at 250ms PCL. Panel B shows a color-map of amplitude and beat locations (x-axis, from 1 to 400 beats) of Re-ALT for an entire stimulation protocol (y-axis, range 400 to 220ms PCL) in an AV block sheep with ventricular pacing at 40 bpm (1500ms) to dissociate far-field ventricular activity from atrial EGMs. Subtle Re-ALT was observed at long PCLs (400 to 300ms, dark blue). For PCLs ≤290ms, a progressive increase in duration and amplitude (light blue) of Re-ALT was noticed. Importantly, Re-ALT amplitude was much higher (red) at short PCL (220ms) before the first instance of 2:1 capture (dark blue following the red marks at PCL 220ms). Panels C and D show summary data of the kinetics (mean±SD) of Re-ALT, AT-ALT and AT prolongation at decremental PCLs (x-axis) in sheep with AV block (n=4) during right ventricular pacing at 40 bpm. Notably, Re-ALT amplitude increased linearly (R2=0.971, p<0.001) from PCL 290ms and became significant from background level (40±25μV at PCL400ms) at PCLs ≤230ms (p<0.05), which was defined as the atrial Re-ALT threshold. We further evaluated the presence and amplitude of Re-ALT induced by realistic noise on simulated EGMs. Noise-induced Re-ALT (5.2±0.5μV) was present but appeared much smaller than the amplitude of experimentally measured Re-ALT (i.e., 155±19.5μV at PCL 230ms) as detailed in the Supplementary material. Panel D of Figure 2 shows the kinetics of AT minus its mean value measured at PCL 400ms (AT400) as function of PCL. AT remained stable until PCL 210ms, increased exponentially, and became significantly different from baseline value at PCLs ≤190ms (p<0.05). Then, the potential contribution of alternans of AT to Re-ALT was evaluated. Panel C shows that AT-ALT was absent over the entire pacing protocol. In summary, both the occurrence and amplitude of atrial Re-ALT, and the kinetics of AT were rate-dependent, but alternans of AT was not observed.
ATRIAL RE-ALT WAS INTERMITTENT DESPITE CONTINUED PACING
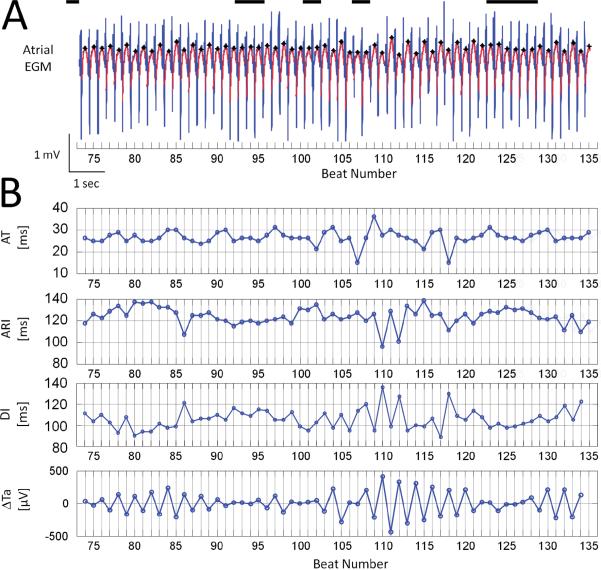
The color-map (panel B) of Figure 2 shows that once atrial Re-ALT had developed, it was not steady during the time course of the pacing protocol. Atrial Re-ALT amplitude fluctuated over time but without periodic pattern (Fisher's g-statistic, p=ns). Panel A of Figure 3 shows a representative example of atrial unipolar EGM taken between beats 74 and 135 of a 400-beat protocol at PCL 230ms. Ta are emphasized by black stars. Sequences without significant Re-ALT are highlighted by black lines of variable duration at the top of the recording. Re-ALT appeared intermittently and in a non-periodic pattern with rises and declines of magnitude separated by periods of no Re-ALT of variable duration as well. Panel B of Figure 3 shows the corresponding time series of AT, ARI, DI and Re-ALT (ΔTa). Notably, depolarization wave amplitudes in panel A and AT in panel B did not show any beat-to-beat alternation, suggesting that the mechanisms underlying atrial Re-ALT at intermediate PCLs primarily involve repolarization rather than depolarization. In contrast, ΔTa clearly exhibited periods of Re-ALT of variable magnitude and duration. At the maximum amplitude of ΔTa alternans (beat number 103 to 119 and 131 to 135), ARI and DI also showed significant beat-to-beat alternation, but of shorter length compared to ΔTa. ARIs alternating at maximal ΔTa alternans showed a concordant relationship with DIs: short ARIs (e.g. beats 110 and 112) were preceded by short DIs (e.g., beats 109 and 111), and long ARIs (e.g., beats 111 and 113) by long DIs (e.g., beats 110 and 112). The dependency of APD (and its surrogate: ARI) on the preceding DI4, 6, 10, 17, 18, termed the ARI-DI restitution, has been thought to be mechanistically involved in Re-ALT when the slope of the relationship is >119. Figure 3B shows that the ARI-DI restitution does not drive ΔTa alternans as ARIs and DIs alternated less frequently than ΔTa did. To further examine whether the ARI-DI restitution may play a limited role in driving ARI alternans at maximal ΔTa alternans, an ARI-DI restitution limited to beats 103–119 showing both ARI and DI alternans was drawn (data not shown). Although ARIs decreased as function of DIs, the maximum restitution slope determined from a linear fit10, 18 for the shortest 40ms DI segment (range: 89 to 111ms) was <1 (0.6). Altogether, these observations suggest that atrial Re-ALT is not driven by the ARI-DI restitution.
Figure 3. Re-ALT is intermittent but not periodic.
Panel A shows an EGM with intermittent Re-ALT at PCL 230ms. Ta are marked by black stars and non alternating periods by black lines. Panel B depicts the corresponding time series of AT, ARI, diastolic intervals (DI) and beat-to-beat differences in Ta (ΔTa).
INTERMITTENT ATRIAL CAPTURE
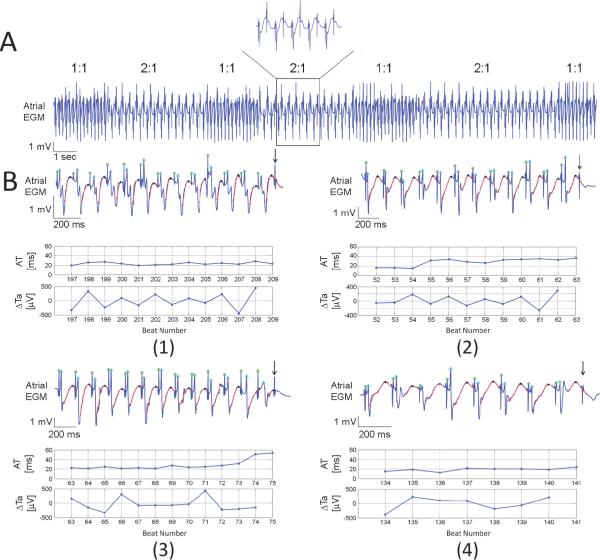
The pacing protocol was pursued until stable 2:1 atrial capture which occurred at mean PCL 128±19ms (i.e., steady state ERP). Interestingly, we observed periods of intermittent 2:1 atrial capture intermingling with 1:1 capture as shown in Figure 4. The mean PCL at which the first periods of intermittent 2:1 atrial capture appeared was 154±24ms. Panel A of Figure 4 illustrates an example of intermittent 1:1 and 2:1 atrial capture. No clear periodicity was observed as periods of 2:1 capture varied in duration. Panel B shows representative examples of atrial unipolar EGMs and their corresponding AT and ΔTa time series prior to 2:1 atrial capture. Four different patterns of AT and Re-ALT dynamics were observed among a total of 186 transitions from 1:1 to 2:1 capture. Atrial EGMs display 1:1 atrial capture until the first non-captured beat (arrow). Panel B1 shows Re-ALT with a progressive increase in ΔTa magnitude (maximum ΔTa 450μV) and no AT change preceding 2:1 capture. Panel B2 shows the second pattern characterized by a similar increase in Re-ALT (maximum ΔTa 280μV) but with a gradual increase in AT (from 26 to 36ms). Panel B3 shows the third pattern characterized by the lack of any significant Re-ALT. AT, however, markedly increased four beats prior to capture failure (from 28 to 54ms). Panel B4 shows the last pattern characterized by the absence of any Re-ALT and AT changes preceding 2:1 capture. The table displays the prevalence of each of the four patterns of AT and ΔTa changes among the 186 episodes. 40% of the episodes were of the type as shown in panel B1 and 33% in B2. Importantly, Re-ALT was observed in 73% and AT prolongation in 49% of episodes. Only 11% of the overall episodes exhibited neither changes in AT nor Re-ALT (panel B4). Using Fisher's exact test, no significant difference in Re-ALT prevalence (p=0.14) was observed between sequences with and without AT prolongation, supporting the lack of relationship between Re-ALT occurrence and AT prolongation before capture failure. In the 49% of episodes showing AT prolongation before capture failure, the mean number of beats over which AT prolonged was 4.9±2.5 (95% CI, 2–8).
Figure 4. Intermittency of atrial capture.
An illustrative unipolar EGM with intermittent 1:1 and 2:1 atrial capture of variable duration at PCL 160ms is shown in panel A. Panel B illustrates the four different patterns of EGM and corresponding AT, ARI and ΔTa time series until the first beat of 2:1 capture (arrow).
Also, the faster the pacing rate, the longer the periods of intermittent 2:1 capture until steady state ERP. Panel A of Figure 5 shows (top tracing) the first episodes of 2:1 capture at PCL 200ms. The middle tracing shows the same sheep at a shorter PCL (170ms), with more frequent and longer periods of 2:1 capture. The bottom tracing shows stable 2:1 atrial capture at steady state ERP (PCL 160ms). For all pacing protocols between the first beats of capture failure and steady state ERP, durations of 2:1 capture sequences were totaled, divided by the duration of the pacing protocol and expressed as the percentage of cumulative 2:1 atrial capture. Panel B of Figure 5 shows summary data based on 35 recordings in 7 sheep starting at PCL that was 40ms above steady state ERP and decremented by steps of 10ms. Importantly, the cumulative percentage of intermittent atrial capture increased as the PCL shortened. At ERP, cumulative 2:1 capture was 95% as 1:1 capture of a few beats' duration was observed at the initiation of the pacing protocol. In a subset of 4 sheep (20 recordings) whose ARIs were reliable enough, the last beat of 2:1 capture and the first beat of 1:1 capture were compared (panel C of Figure 5). AT remained similar (from 34±14 to 31±12ms, p=NS) but ARI decreased significantly (from 97±18 to 89±19ms, p<0.05) at resumption of 1:1 capture, which is consistent with a decrease in APD promoting the recovery of 1:1 capture. Also, note the transient Re-ALT at resumption of 1:1 capture.
Figure 5. Duration of 2:1 atrial capture is rate-dependent.
Panel A displays representative examples of unipolar EGM at decreasing PCLs. Rare 2:1 atrial captures of short duration are seen at PCL 40ms above effective refractory period (ERP, top). Middle tracing illustrates the increase in 2:1 capture duration 10ms above ERP. Bottom tracing shows 1:1 atrial capture of short duration followed by stable 2:1 capture. Panel B shows mean±SD of cumulative percentage of 2:1 atrial capture as a function of PCL in a subset of 7 sheep. In panel C are shown EGM (top), ARI (middle) and Re-ALT (ΔTa, bottom) time series at resumption of 1:1 capture. ARI decreased before, and Re-ALT emerged at resumption of 1:1 capture.
ATRIAL FIBRILLATION EPISODES
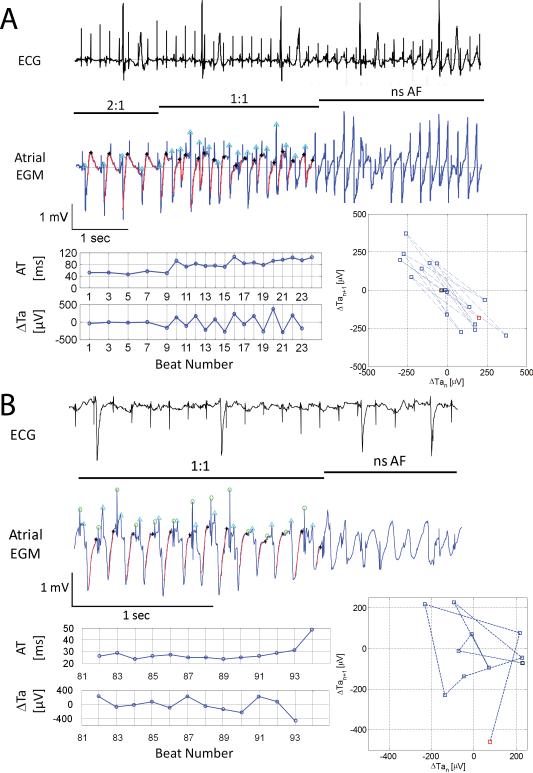
Forty-four protocols totaling 758 bursts of 400 beats were performed starting at PCL 400ms until steady state ERP, of which 12 (1.6%) triggered 20 episodes of nonsustained AF (nsAF) at mean PCL 150±36ms. Seventeen episodes occurred during 1:1 capture, and 3 following the transition from 2:1 to 1:1 capture. Eighty percent (16/20) of the AF episodes were preceded by Re-ALT (mean magnitude of the 8 last ΔTa: 366±285μV) and 15% (3/20) by complex Ta oscillations (mean magnitude of the 8 last ΔTa: 180±5μV). One episode was triggered by a single S1 at resumption of 1:1 capture at PCL 120ms. Prolongation of AT was observed in 55% (11/20), absent in 40% (8/20) and not analyzable in one episode (5%). Note that the amplitude of Re-ALT (366±285μV) preceding AF was well above the maximal value (210±70μV) measured at PCL 200ms (shown in Figure 2C). Panel A of Figure 6 shows an illustrative example where Re-ALT (ΔTa) emerged and AT prolonged at resumption of 1:1 capture, leading directly to AF. Note the similar dynamics of Re-ALT and AT prolongation with episodes of 2:1 capture (see Figure 4). Panel B of Figure 6 shows an example of complex Ta oscillations preceding the initiation of AF by rapid pacing. To further illustrate the observed atrial repolarization dynamics preceding nsAF, Poincaré plots of the beat-to-beat Ta difference (ΔTan+1) as a function of its preceding value (ΔTan) were built. ΔTa alternans (Figure 6A) tended to aggregate at two distinct locations on either side of the “zero” alternans line, while complex ΔTa oscillations spanned the graph (Figure 6B) before nsAF. In summary, both alternans and complex oscillations of atrial repolarization were commonly observed before rapid pacing-induced AF.
Figure 6. Rapid pacing-induced AF.
From top to bottom are shown ECG, atrial EGM, corresponding AT and ΔTa time series and Poincaré plots of ΔTa during rapid pacing. Panel A shows an episode of non sustained AF (nsAF) at resumption of 1:1 capture at PCL 180ms. Note the gradual increase in atrial Re-ALT (ΔTa) and AT prolongation, but the lack of capture failure before nsAF. Panel B shows an example of pacing-induced nsAF at PCL 130ms preceded by complex Ta fluctuations. Note the large beat-to-beat difference in Ta (range ~200μV) and AT prolongation without capture failure. The Poincaré plots show that ΔTa alternans (upper graph) tends to aggregate at two distinct locations on either side of the “zero” alternans line, while complex ΔTa oscillations span the graph (lower plot) before nsAF.
Discussion
This study reports the detailed kinetics of atrial Re-ALT during rapid atrial pacing. We show in vivo that Re-ALT developed and increased in magnitude with rate until stable 2:1 capture. In rare instances where 1:1 capture was maintained during rapid pacing, nsAF surged. Our work also shows the feasibility of recording atrial Re-ALT using available pacemaker technology to provide a surrogate of APD alternans.
RATE-DEPENDANCE OF ATRIAL REPOLARIZATION ALTERNANS
Atrial Re-ALT has been observed using unipolar recordings20, MAP5, 8, 10, 17, 18 and optical mapping7, 21. The present study is the first to our knowledge to report the detailed kinetics of atrial Re-ALT and its interplay with capture failure based on unipolar EGMs using a method similar to the one developed by Swerdlow et al. in patients with ICD12. Atrial Re-ALT was rarely observed at long PCLs, but progressively increased in duration and linearly in amplitude starting at PCL 280ms. The PCL at which Re-ALT amplitude became significantly different from the background level (230ms) was similar to the value measured in control subjects (i.e. 218±30ms) referred for catheter ablation of supraventricular tachycardia and in cultures of atrial myocyte monolayer (250ms)21, supporting the adequacy of our model to study the kinetics of Re-ALT in vivo10. Importantly, right atrial Re-ALT arose at PCLs where AT was neither prolonged nor alternating, indicating that atrial Re-ALT, as for the ventricles3, 10, 22, primarily involves beat-to-beat alternation in the repolarization time course. This is further supported by Tsai et al. who recently reported Re-ALT in cultures of atrial myocytes during rapid field pacing21. Atrial Re-ALT arose from alternans of intracellular calcium, and was further promoted by atrial stretch-induced defective intracellular calcium re-uptake. Also, no consistent relationship was found between ΔTa alternans and ARI and DI alternans or the kinetics of the ARI-DI restitution. ΔTa alternans was observed at times of no ARI and DI alternans. This is in line with recent clinical10 and experimental studies6, 21 that found no relationship between APD-DI restitution kinetics and alternans, but a role of intracellular calcium cycling alternans in driving repolarization alternans.
INTERMITTENCY OF ATRIAL REPOLARIZATION ALTERNANS
Selvaraj et al. reported in heart failure patients spatially out-of-phase (i.e., discordant) intermittent ventricular Re-ALT using unipolar signals23. Mirinov et al. showed that intermittent Re-ALT is related to unstable nodal lines separating out-of-phase regions spanning the heart surface, which are mechanistically linked to slow APD accommodation following a change in PCL24. Based on atrial unipolar signals, we observed sequences of intermittent Re-ALT appearing in a non-periodic pattern. The rise and decline in Re-ALT amplitude preceding non-alternating periods is suggestive of nodal lines spanning the RA, but cannot be proven as recordings were performed at a single site. Interestingly, atrial ARIs displayed alternation in duration only at the maximum amplitude of Ta alternans. Experimentally, atrial ARIs are able to track APD changes15, hence alternation of ARIs indicates APD alternans. The repolarization wave of bipolar and unipolar signals is the result of repolarization gradient of surrounding excitable tissue13. The peak of the T-wave is timed with the maximal voltage gradient between neighboring APs and the height of the T-wave with the magnitude of that maximal voltage gradient13. Alternation in Ta amplitude without ARI alternans suggests alternation in the steepness of the phase 2 and 3 of neighboring APs but not necessarily of the AP duration. This hypothesis is supported by the recent clinical observation that alternans of phase 2 of MAPs better correlates with T-wave alternans than alternans of the duration of phase 3 of the AP10. However, in general, both phases alternate together (see Figure 1 in25). Alternatively, some inaccuracy in the detection of the timing, but not of the amplitude of Ta, may have postponed the detection of ARI alternans. In summary, ovine right atrial repolarization kinetics share common features with human and rodent ventricles including the rate-dependence and the intermittency of Re-ALT.
POTENTIAL MECHANISMS OF INTERMITTENT ATRIAL CAPTURE
Brooks et al. observed 40 years ago that atrial Re-ALT and intermittent capture occurred at similar rapid pacing rates20. Their observation that an increase in stimulus strength converted 2:1 into 1:1 atrial capture argued for decreased excitability as the underlying mechanism of intermittent capture. More recently, Watanabe et al. showed in canine RA using MAPs that the ERP measured during Re-ALT after a long APD is longer than that after a short APD8. Hence, atrial Re-ALT combined with AT prolongation may have facilitated the first non-captured beat by delaying the ERP to a point where the next stimulus is refractory. This hypothesis is further supported by MAP recordings performed in humans suffering from AF as shown in Figure 1 of the Supplemental material. Panel A shows left atrial (LA) Re-ALT at slow pacing rate, and panels B (LA) and C (right atrial, RA) Re-ALT and capture failure preceded by AT prolongation that progressively postponed the repolarization until the stimulus fell within the ERP. This, in addition to the observation that intermittent capture in the sheep was seen in a range (i.e., 40ms) of PCLs just above ERP, strongly suggests reduced excitability. However, other mechanisms must be involved in the maintenance of intermittent capture. The rate-dependence of the duration of intermittent capture and the decrease in ARI preceding resumption of 1:1 capture are both suggestive of a time-dependent process. Further studies beyond the scope of our research are required to elucidate these mechanisms. Our results may also hint at a potential mechanism by which rapid atrial rhythms may fail to cause AF in certain patients by means of capture failure as recently reported during rapid pacing10.
REPOLARIZATION ALTERNANS AND MECHANISM OF AF
In the seminal work of Haissaguerre et al.1, PV tachycardias triggering AF had a mean CL of 175±30ms that fell within human (<218±30ms)10, ovine (i.e., 230ms to ERP) and atrial myocyte monolayer culture (250ms to ERP)21 PCL range during which atrial Re-ALT occurred. In the present study, during the rare instances where 2:1 capture did not occur with rapid pacing, nsAF episodes were observed and preceded either by overt Re-ALT or by complex Ta oscillations, whereas AT prolonged or remained stable. Interestingly, complex APD oscillations have recently been reported to herald rapid pacing-induced AF in humans as well10. Taken together, these observations suggest that alternans and complex oscillations of atrial repolarization, but not AT prolongation, may be one of the mechanisms facilitating transition from focal tachycardia to AF by promoting dispersion of repolarization and wavebreaks as reported recently in humans with rapid pacing10. Whether digital pacemaker technology with broadband signal characteristics could be used to predict the susceptibility to and imminence of AF in humans and animal models of AF need to be further investigated. Our study may also hint at the limited clinical success of overdrive pacing in suppressing AF burden26 as increasing the pacing rate may promote Re-ALT and dispersion of repolarization. Patients suffering from AF and models of rapid-pacing display multiple atrial alterations including a decrease in ERP27 that may potentially shift Re-ALT to lower rates10 and 2:1 capture to higher rates, both reducing the effectiveness of this potentially protective mechanism.
STUDY LIMITATIONS
Our study bears limitations that deserve some comments. First, our experimental model of rapid pacing does not provide the typical electroanatomical substrate reported in animal models and humans suffering from AF28. Second, some uncertainties remain as to whether our observations apply to the stimulation site as the recording electrode was remote by 2cm on average, and to the LA as the pacemaker leads were implanted into the RA. Similar APD and Re-ALT dynamics between the RA and LA, however, were recently reported by our group in humans suffering from paroxysmal and persistent AF10. Figure 1 of supplemental material shows in humans RA and LA MAP recordings with atrial Re-ALT and delayed AT preceding capture failure at the pacing site that shared all the characteristics of the transitions to 2:1 capture observed in our ovine model. Third, our model is limited to a single RA site, which prevents us from drawing any conclusion on rapid pacing-induced dispersion of repolarization and discordant (i.e., arrhythmogenic) alternans. Fourth, the analysis of unipolar EGM repolarization presumably provides information about atrial repolarization properties during rapid pacing. It does not provide, however, direct mechanistic insights into cellular and subcellular mechanisms and future studies should analyze the relationship between Ta alternans and local repolarization properties using optical mapping24.
Supplementary Material
Acknowledgments
The authors would like to thank P. van Dam and H. de Bruyn for the development and embedding of the customized pacemaker protocols.
This work was supported by The Swiss National Fund for Scientific Research [grant numbers 325200–112668, 320030–127229] to E. Pruvot, the CardioMet Pôle, Medtronic™ Switzerland and Vitatron™. Dr. Narayan was supported by a grant from the NIH [grant number HL83359].
Etienne Pruvot reports honoraria from Medtronic.
Sanjiv M. Narayan reports Fellow support from Medtronic, Boston Scientific, St. Jude Medical & Biotronik., and honoraria from Medtronic, St. Jude Medical & Biotronik. Dr. Narayan is co-inventor of intellectual property owned by the University of California Regents and Licensed to Topera Inc.
However, Topera does not fund any research.
Martin Fromer reports honoraria from Medtronic & St. Jude Medical.
Drs. Ruchat & Tenkorang report research support in the form of out-dated pacing leads and pacemakers from Medtronic & Vitatron.
Footnotes
Other authors: No disclosures.
References
- [1].Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- [2].Ausma J, Wijffels M, Thone F, Wouters L, Allessie M, Borgers M. Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation. 1997;96:3157–3163. doi: 10.1161/01.cir.96.9.3157. [DOI] [PubMed] [Google Scholar]
- [3].Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS. Mechanism Linking T-Wave Alternans to the Genesis of Cardiac Fibrillation. Circulation. 1999;99:1385–1394. doi: 10.1161/01.cir.99.10.1385. [DOI] [PubMed] [Google Scholar]
- [4].Weiss JN, Karma A, Shiferaw Y, Chen P-S, Garfinkel A, Qu Z. From Pulsus to Pulseless: The Saga of Cardiac Alternans. Circ Res. 2006;98:1244–1253. doi: 10.1161/01.RES.0000224540.97431.f0. [DOI] [PubMed] [Google Scholar]
- [5].Narayan SM, Bode F, L. Karasik P, Franz MR. Alternans of Atrial Action Potentials During Atrial Flutter as a Precursor to Atrial Fibrillation. Circulation. 2002:1968–1973. doi: 10.1161/01.cir.0000037062.35762.b4. [DOI] [PubMed] [Google Scholar]
- [6].Pruvot EJ, Katra RP, Rosenbaum DS, Laurita KR. Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ Res. 2004;94:1083–1090. doi: 10.1161/01.RES.0000125629.72053.95. [DOI] [PubMed] [Google Scholar]
- [7].Miyauchi Y, Zhou S, Okuyama Y, Miyauchi M, Hayashi H, Hamabe A, Fishbein MC, Mandel WJ, Chen LS, Chen PS, Karagueuzian HS. Altered atrial electrical restitution and heterogeneous sympathetic hyperinnervation in hearts with chronic left ventricular myocardial infarction: implications for atrial fibrillation. Circulation. 2003;108:360–366. doi: 10.1161/01.CIR.0000080327.32573.7C. [DOI] [PubMed] [Google Scholar]
- [8].Watanabe I, Masaki R, Nuo M, Oshikawa N, Okubo K, Okumura Y. Effect of 60 Minutes of Rapid Atrial Pacing on Atrial Action Potential Duration in the In-Situ Canine Heart. J Interv Card Electrophysiol. 2003;8:165–171. doi: 10.1023/a:1023909019945. [DOI] [PubMed] [Google Scholar]
- [9].Hiromoto K, Shimizu H, Furukawa Y, Kanemori T, Mine T, Masuyama T, Ohyanagi M. Discordant repolarization alternans-induced atrial fibrillation is suppressed by verapamil. Circ J. 2005;69:1368–1373. doi: 10.1253/circj.69.1368. [DOI] [PubMed] [Google Scholar]
- [10].Narayan SM, Franz MR, Clopton P, Pruvot EJ, Krummen DE. Repolarization alternans reveals vulnerability to human atrial fibrillation. Circulation. 2011;123:2922–2930. doi: 10.1161/CIRCULATIONAHA.110.977827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pruvot E, Jousset F, Ruchat P, Vesin J-M, Prudat Y, Zerm T, Fromer M. Propagation velocity kinetics and repolarization alternans in a free-behaving sheep model of pacing-induced atrial fibrillation. Europace. 2007;9:vi83–88. doi: 10.1093/europace/eum211. [DOI] [PubMed] [Google Scholar]
- [12].Swerdlow C, Chow T, Das M, Gillis AM, Zhou X, Abeyratne A, Ghanem RN. Intracardiac electrogram T-wave alternans/variability increases before spontaneous ventricular tachyarrhythmias in implantable cardioverter-defibrillator patients: a prospective, multi-center study. Circulation. 2011;123:1052–1060. doi: 10.1161/CIRCULATIONAHA.110.986364. [DOI] [PubMed] [Google Scholar]
- [13].Janse MJ, Sosunov EA, Coronel R, Opthof T, Anyukhovsky EP, de Bakker JMT, Plotnikov AN, Shlapakova IN, Danilo P, Jr., Tijssen JGP, Rosen MR. Repolarization Gradients in the Canine Left Ventricle Before and After Induction of Short-Term Cardiac Memory. Circulation. 2005;112:1711–1718. doi: 10.1161/CIRCULATIONAHA.104.516583. [DOI] [PubMed] [Google Scholar]
- [14].Anyukhovsky EP, Sosunov EA, Feinmark SJ, Rosen MR. Effects of quinidine on repolarization in canine epicardium, midmyocardium, and endocardium: II. In vivo study. Circulation. 1997;96:4019–4026. doi: 10.1161/01.cir.96.11.4019. [DOI] [PubMed] [Google Scholar]
- [15].Vigmond EJ, Tsoi V, Yalin Y, Page P, Vinet A. Estimating Atrial Action Potential Duration from Electrograms. IEEE Trans Biomed Eng. 2009;56:1546–1555. doi: 10.1109/TBME.2009.2014740. [DOI] [PubMed] [Google Scholar]
- [16].Wichert S, Fokianos K, Strimmer K. Identifying periodically expressed transcripts in microarray time series data. Bioinformatics. 2004;20:5–20. doi: 10.1093/bioinformatics/btg364. [DOI] [PubMed] [Google Scholar]
- [17].Kim B-S, Kim Y-H, Hwang G-S, Pak H-N, Lee SC, Shim WJ, Oh DJ, Ro YM. Action potential duration restitution kinetics in human atrial fibrillation. J Am Coll Cardiol. 2002;39:1329–1336. doi: 10.1016/s0735-1097(02)01760-6. [DOI] [PubMed] [Google Scholar]
- [18].Narayan SM, Kazi D, Krummen DE, Rappel W-J. Repolarization and Activation Restitution Near Human Pulmonary Veins and Atrial Fibrillation Initiation: A Mechanism for the Initiation of Atrial Fibrillation by Premature Beats. J Am Coll Cardiol. 2008;52:1222–1230. doi: 10.1016/j.jacc.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Qu Z, Garfinkel A, Chen P-S, Weiss JN. Mechanisms of Discordant Alternans and Induction of Reentry in Simulated Cardiac Tissue. Circulation. 2000;102:1664–1670. doi: 10.1161/01.cir.102.14.1664. [DOI] [PubMed] [Google Scholar]
- [20].Brooks CM, Gilbert JL, Janse MJ. Failure of integrated cardiac action at supernormal heart rates. Proceedings of the Society for Experimental Biology and Medecine. 1964;117:630–634. doi: 10.3181/00379727-117-29655. [DOI] [PubMed] [Google Scholar]
- [21].Tsai CT, Chiang FT, Tseng CD, Yu CC, Wang YC, Lai LP, Hwang JJ, Lin JL. Mechanical stretch of atrial myocyte monolayer decreases sarcoplasmic reticulum calcium adenosine triphosphatase expression and increases susceptibility to repolarization alternans. J Am Coll Cardiol. 2011;58:2106–2115. doi: 10.1016/j.jacc.2011.07.039. [DOI] [PubMed] [Google Scholar]
- [22].Narayan SM, Bayer JD, Lalani G, Trayanova NA. Action Potential Dynamics Explain Arrhythmic Vulnerability in Human Heart Failure: A Clinical and Modeling Study Implicating Abnormal Calcium Handling. J Am Coll Cardiol. 2008;52:1782–1792. doi: 10.1016/j.jacc.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Selvaraj RJ, Picton P, Nanthakumar K, Mak S, Chauhan VS. Endocardial and Epicardial Repolarization Alternans in Human Cardiomyopathy: Evidence for Spatiotemporal Heterogeneity and Correlation With Body Surace T-Wave Alternans. J Am Coll Cardiol. 2007;49:338–347. doi: 10.1016/j.jacc.2006.08.056. [DOI] [PubMed] [Google Scholar]
- [24].Mironov S, Jalife J, Tolkacheva EG. Role of Conduction Velocity Restitution and Short-Term Memory in the Development of Action Potential Duration Alternans in Isolated Rabbit Hearts. Circulation. 2008;118:17–25. doi: 10.1161/CIRCULATIONAHA.107.737254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wan X, Laurita KR, Pruvot EJ, Rosenbaum DS. Molecular correlates of repolarization alternans in cardiac myocytes. J Mol Cell Cardiol. 2005;39:419–428. doi: 10.1016/j.yjmcc.2005.06.004. [DOI] [PubMed] [Google Scholar]
- [26].Camm AJ, Sulke N, Edvardsson N, Ritter P, Albers BA, Ruiter JH, Lewalter T, Capucci PA, Hoffmann E. Conventional and dedicated atrial overdrive pacing for the prevention of paroxysmal atrial fibrillation: the AFTherapy study. Europace. 2007;9:1110–1118. doi: 10.1093/europace/eum253. [DOI] [PubMed] [Google Scholar]
- [27].Anné W, Willems R, Holemans P, Beckers F, Roskams T, Lenaerts I, Ector H, Heidbüchel H. Self-terminating AF depends on electrical remodeling while persistent AF depends on additional structural changes in a rapid atrially paced sheep model. J Mol Cell Cardiol. 2007;43:148–158. doi: 10.1016/j.yjmcc.2007.05.010. [DOI] [PubMed] [Google Scholar]
- [28].Platonov PG, Mitrofanova LB, Orshanskaya V, Ho SY. Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J Am Coll Cardiol. 2011;58:2225–2232. doi: 10.1016/j.jacc.2011.05.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.