Abstract
Objectives:
To evaluate disparities in stroke risk factors and outcome among the Native Hawaiians and other Pacific Islanders (NHPI) in Hawaii who are hospitalized with intracerebral hemorrhage (ICH).
Methods:
We performed a retrospective study on consecutive patients hospitalized for acute ICH at a single tertiary center on Oahu between 2004 and 2010. Clinical data were obtained from the Get With the Guidelines–Stroke database. Multivariable logistic regression was used to assess the predictors for young ICH (age <45).
Results:
A total of 562 patients hospitalized for acute ICH (Asian 63%, NHPI 18%, white 16%, other 3%) were studied. The NHPI were younger (mean ages, NHPI 55 ± 16 vs white 66 ± 16 years, p < 0.0001), and had higher prevalence of diabetes (NHPI 35% vs white 20%, p = 0.01) and history of hypertension (NHPI 77% vs white 64%, p = 0.04) compared to white patients. Independent predictors for young ICH were NHPI race (odds ratio [OR] 3.55; 95% confidence interval [CI] 1.33–9.45), being transferred from another hospital (OR 2.03; 95% CI 1.05–3.93), hypertension (OR 0.49; 95% CI 0.27–0.91), previous stroke or TIA (OR 0.21; 95% CI 0.05–0.91), and dyslipidemia (OR 0.15; 95% CI 0.05–0.50).
Conclusions:
NHPI with ICH are younger and have higher burden of risk factors compared to white patients. Further studies controlling for socioeconomic modifiers are needed to determine factors contributing to the younger age at presentation in this racial group.
Intracerebral hemorrhage (ICH) accounts for 10%–15% of the approximately 700,000 annual strokes in the Unites States1 and results in disproportionately high morbidity and mortality.2 In Hawaii, the age-adjusted incidence of ICH is estimated to be 0.6/1,000 person-years3 in a population that consists of 25% white subjects, 39% Asians, and 10% Native Hawaiians and other Pacific Islanders (NHPI) compared to 0.3/1,000 person-years in a population in Northern Manhattan that consists of 23% white subjects, 12% black subjects, and 65% Hispanic subjects.4 Recent evidence suggests that the burden of ICH is not borne equally by all, with racial minority groups reported to have a higher incidence and poorer outcomes than white subjects.5–7 Although NHPI have been reported to have higher prevalence of major cardiovascular risk factors8–13 and die at a younger age from various cardiovascular disease–related complications compared to other major racial–ethnic groups,14,15 the specific disease burden related to ICH in this population has not been studied. We hypothesized that NHPI with ICH are younger and have higher burden of risk factors compared to non-Hispanic white subjects.
METHODS
We conducted a retrospective observational study using our institution's Get With the Guidelines–Stroke (GWTG-Stroke) registry, a national quality improvement initiative and stroke registry used by many participating hospitals nationwide.16 Since its inception in 2004, GWTG-Stroke registry has been used in our institution to measure and monitor the quality of hospital-based stroke care delivery.
Patients.
The Queen's Medical Center (QMC) is a 505-bed medical center located on Oahu, the largest hospital in Hawaii and the tertiary referral center for the Pacific Basin (Hawaii, American Samoa, the Commonwealth of the Northern Mariana Islands, Micronesia, and the US territories of Guam). QMC has the only Joint Commission–certified Primary Stroke Center and the only Neuroscience Intensive Care Unit in Hawaii. Since ICH is a condition that is preferably treated in the Neuroscience Intensive Care Unit,17 QMC is the primary referral center for acute management of ICH patients from other major islands.
All patients hospitalized at QMC with a diagnosis of spontaneous ICH between January 1, 2004, and August 31, 2010, were identified using the QMC GWTG-Stroke database. The patients with ICH were identified and registered in the database at the time of admission by imaging and admission diagnosis. A trained nurse reviewer (S.M.A.) at QMC identified consecutive patients admitted to QMC with a principal clinical diagnosis of ischemic stroke, hemorrhagic stroke, or TIA and categorized as such. Case ascertainment of admissions for ICH was conducted by prospective clinical identification and retrospective identification with International Classification of Diseases (9th revision) discharge codes (431 for ICH), followed by chart review of an electronic medical record to confirm case eligibility, or a combination of both approaches. Each subject's medical record was reviewed and recorded into an electronic database using standard criteria and definitions. Patients with recurrent ICH who were previously admitted to QMC with ICH were excluded. We were unable to identify and exclude those who may have had ICH in the past and were hospitalized at another hospital. Patients with ICH related to trauma, ruptured cerebral aneurysm, or ischemic stroke with hemorrhagic conversion were also excluded.
Baseline characteristics.
Patient demographics, whether the patient was transferred from another hospital, medical history including history of diabetes mellitus, hypertension, atrial fibrillation/atrial flutter, congestive heart failure, prior stroke or TIA, coronary artery disease (CAD), or prior myocardial infarction (MI), peripheral vascular disease, smoking, dyslipidemia, and prosthetic valve replacement were obtained through the GWTG-Stroke database. The race and ethnicity information were collected by the administrative personnel during the registration process or by the nurses during the intake process. Race was initially categorized as NHPI, Asian, white, black, American Indian/Alaska native, or “other” race. Because of the low number of black and American Indian/Alaska native patients in Hawaii, these racial groups were combined with the “other” group in the analysis. Since mixed racial background is relatively common in Hawaii, race was defined as the racial/cultural background that the patient most closely associated with and was based on patient self-identification or family's identification if the patient was incapacitated. The patients were further dichotomized to young ICH (age <45) and older ICH (age ≥45) groups, a generally accepted age dichotomization that has been used to define ischemic strokes in young adults.18 Additional data on the use of antihypertensive medications, body mass index (BMI), total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides were also collected if they were available. The patients were considered to have hypertension if there was a known history of hypertension prior to admission (self-report, family's report of hypertension, or confirmed history of hypertension in the existing medical record). The patients were considered to have untreated hypertension if they were not taking any antihypertensive medications prior to admission despite the known history of hypertension. The patients were considered to have dyslipidemia if there was a known history of dyslipidemia prior to admission (self-report, family's report, confirmed history of dyslipidemia in the existing medical record, or current use of lipid-lowering agent).
All initial head CT scans were retrospectively reviewed by one of the study investigators (K.N.) using a standardized protocol, blinded to race, ethnicity, and clinical data. Hematoma volume was measured using the previously described ABC/2 method.19 Presence of intraventricular hemorrhage (IVH) was recorded, and ICH location was coded as lobar, basal ganglia, thalamus, brainstem, cerebellum, or primary IVH.
Outcome measures.
Hospital length of stay (LOS), in-hospital mortality, discharge ambulatory status, and discharge destination were the selected clinical outcomes.
Standard protocol approvals, registrations, and patient consents.
We received approval from the QMC Research and Institutional Review Committee to conduct this retrospective review of the prospectively collected QMC GWTG-Stroke database, supplemented by additional clinical data that were obtained from a chart review. Waiver of consent was obtained to conduct this study.
Statistical analysis.
Data were analyzed using commercially available statistical software (SPSS 19.0, Chicago, IL). Patient characteristics were summarized using descriptive statistics appropriate to variable type. The NHPI and Asian racial groups were compared to white subjects (reference group) using χ2 test or Fisher exact test for categorical data and 2-tailed t test for normally distributed, continuous variables. Multivariable regression analyses using a logistic regression model with a forward stepwise procedure, with p < 0.1 for addition of variables, were performed to identify factors predictive of young ICH. Odds ratio (OR) and 95% confidence interval (CI) were calculated from the β coefficients and their standard errors. Data are presented as means ± SD, and levels of p < 0.05 were considered statistically significant.
RESULTS
Between January 2004 and August 2010, 573 consecutive patients with a possible diagnosis of ICH were identified. After case confirmation with retrospective CT analyses, the following patients were excluded: 3 patients with traumatic ICH, 3 patients with ICH related to subarachnoid hemorrhage from ruptured cerebral aneurysm, 2 patients with ischemic stroke with hemorrhagic transformation, 2 patients with recurrent ICH at QMC, and 1 patient without radiographic evidence of ICH. In the final analyses 562 consecutive patients admitted to QMC with a diagnosis of acute nontraumatic, nonaneurysmal, spontaneous ICH were included (Asian 63%, NHPI 18%, white 16%, other 3%). Demographics, cardiovascular risk factors, hospital LOS, and in-hospital mortality data for the NHPI, Asians, and white subjects are summarized in table 1. Univariate analyses showed that NHPI were younger (p < 0.0001) and had higher prevalence of diabetes and hypertension (p < 0.05), higher hospital LOS (p = 0.01), and lower in-hospital mortality (p = 0.03) compared to white subjects. Head CT data were available in 556 (99%) ICH patients. The hematoma size, location, and prevalence of secondary IVH were not different across racial groups.
Table 1.
Characteristics of ICH patients at The Queen's Medical Center: 2004–2010a
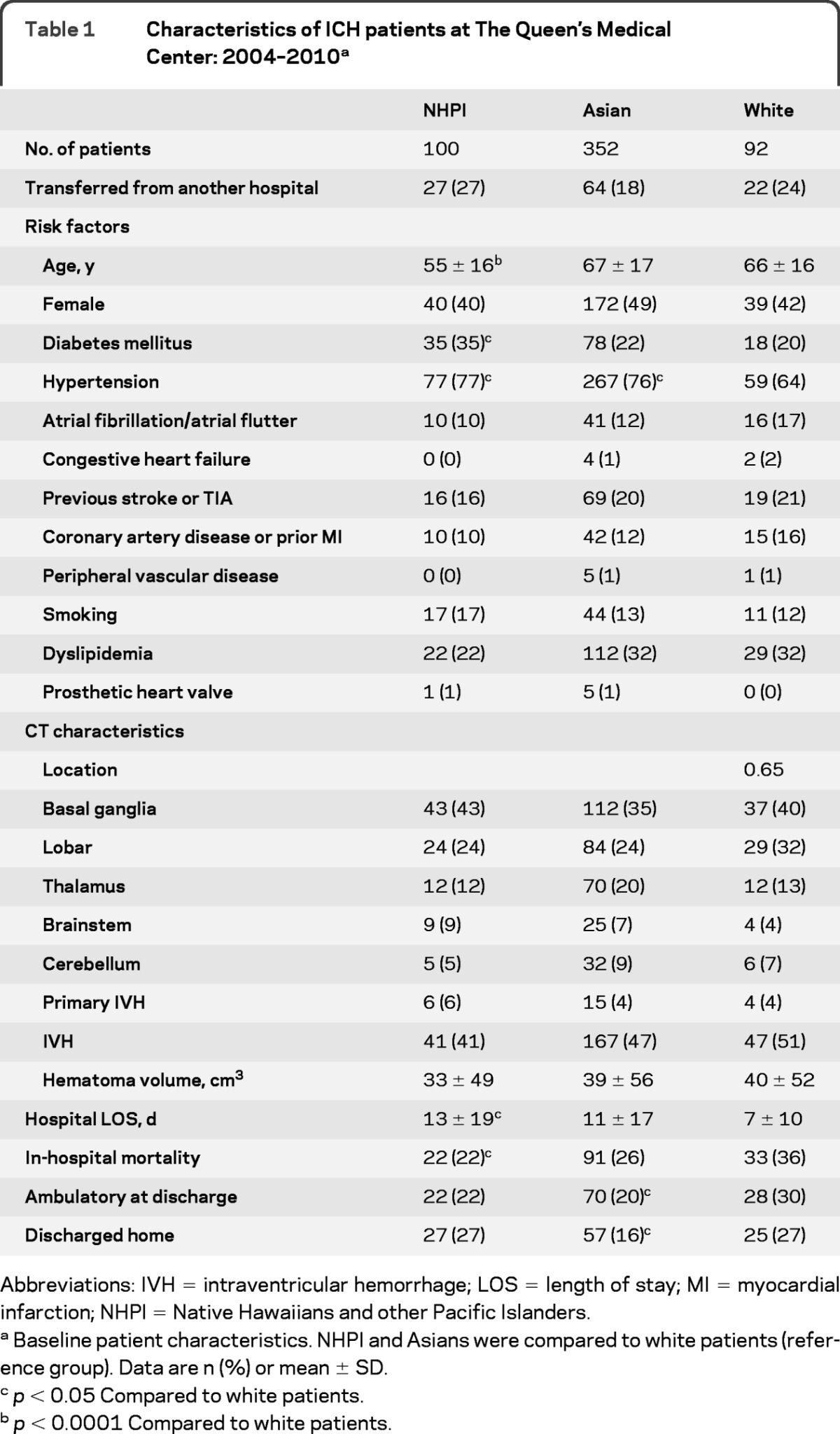
Abbreviations: IVH = intraventricular hemorrhage; LOS = length of stay; MI = myocardial infarction; NHPI = Native Hawaiians and other Pacific Islanders.
Baseline patient characteristics. NHPI and Asians were compared to white patients (reference group). Data are n (%) or mean ± SD.
p < 0.05 Compared to white patients.
p < 0.0001 Compared to white patients.
There were 123 patients (22%) who were transferred from other hospitals. Among these patients (Asian 52%, NHPI 22%, white 18%, other 8%), the racial distribution was different compared to those who were directly admitted (Asian 65%, NHPI 17%, white 16%, other 2%, p = 0.001). Furthermore, these patients were younger (mean age: 58 ± 18 vs 66 ± 17, p < 0.0001), had higher prevalence of CHF (4% vs 0.2%, p = 0.0003) and IVH (60% vs 44%, p = 0.003), and lower prevalence of prior stroke or TIA (10% vs 22%, p = 0.003) and lower mortality (20% vs 29%, p = 0.049) compared to those who were directly admitted. The distribution of hematoma location was different between the 2 groups (transferred patients: basal ganglia 33%, lobar 29%, thalamus 16%, brainstem 3%, cerebellum 12%, primary IVH 8%; vs directly admitted patients: basal ganglia 39%, lobar 24%, thalamus 18%, brainstem 8%, cerebellum 7%, primary IVH 4%, p = 0.014). There was no difference in the prevalence of other risk factors, hematoma size, or other discharge outcomes between the 2 groups.
Univariate analyses showed that the young ICH group (age <45 years) had different racial distribution (p = 0.001), higher prevalence of patient being transferred from another hospital (p < 0.0001), higher hospital LOS (p < 0.05), and lower prevalence of hypertension (p < 0.0001), atrial fibrillation/atrial flutter (p = 0.001), previous stroke or TIA (p = 0.001), CAD or prior MI (p = 0.004), and dyslipidemia (p < 0.0001) compared to the older ICH group (table 2). In multivariable analyses, independent predictors for young ICH were NHPI race (OR 3.55; 95% CI 1.33–9.45; p = 0.01) compared to white subjects, being transferred from other hospital (OR 2.03; 95% CI 1.05–3.93, p = 0.04), hypertension (OR 0.49; 95% CI 0.27–0.91; p = 0.02), previous stroke or TIA (OR 0.21; 95% CI 0.05–0.91; p = 0.04), and dyslipidemia (OR 0.15; 95% CI 0.05–0.50; p = 0.002).
Table 2.
Characteristics of young ICH patientsa
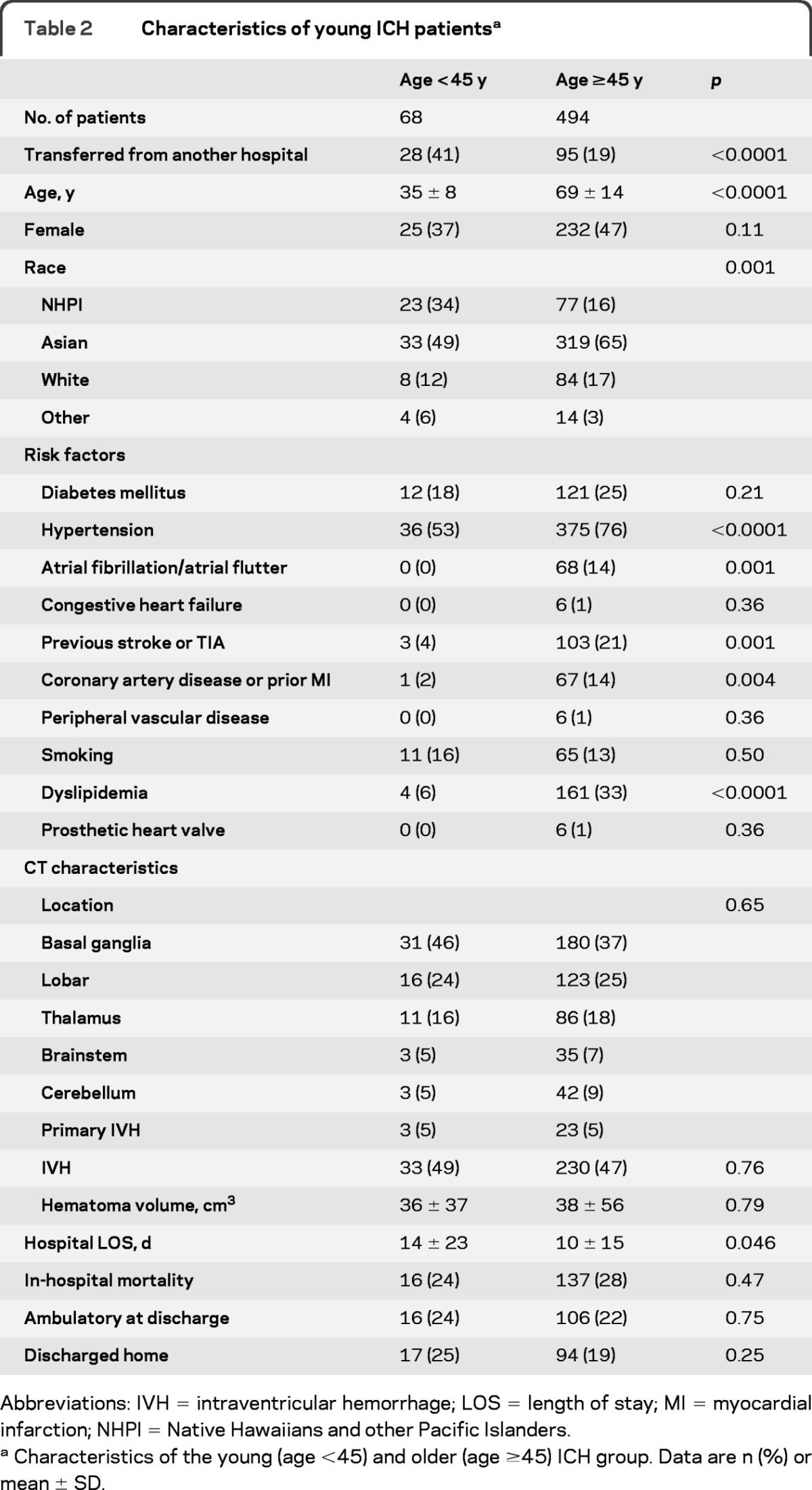
Abbreviations: IVH = intraventricular hemorrhage; LOS = length of stay; MI = myocardial infarction; NHPI = Native Hawaiians and other Pacific Islanders.
Characteristics of the young (age <45) and older (age ≥45) ICH group. Data are n (%) or mean ± SD.
Further comparative analyses of cholesterol and BMI were performed in a subset of patients with available data. Cholesterol data were available in 238 (42%) patients. NHPI had lower HDL level than white subjects (p < 0.05) but similar LDL, total cholesterol, and triglyceride levels (table 3). The BMI data were available in 376 (67%) patients. The NHPI group had higher BMI compared to white subjects (p < 0.0001; table 3), suggesting higher prevalence of obesity.
Table 3.
Comparison of cholesterol and BMI between different racial groupsa
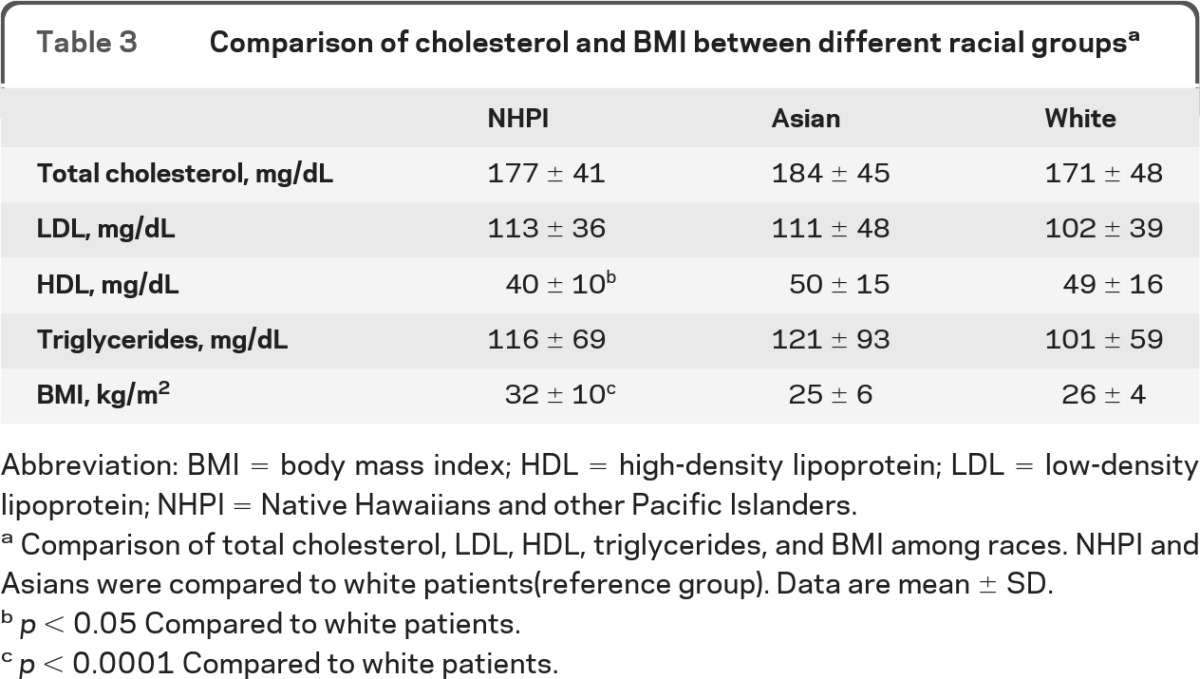
Abbreviation: BMI = body mass index; HDL = high-density lipoprotein; LDL = low-density lipoprotein; NHPI = Native Hawaiians and other Pacific Islanders.
Comparison of total cholesterol, LDL, HDL, triglycerides, and BMI among races. NHPI and Asians were compared to white patients(reference group). Data are mean ± SD.
p < 0.05 Compared to white patients.
p < 0.0001 Compared to white patients.
Since hypertension is one of the major causes of ICH, we also analyzed the ICH patients with a pre-existing diagnosis of hypertension (NHPI = 77, Asian = 267, white = 59) and assessed the proportion of those with untreated hypertension prior to admission. The results demonstrate that NHPI had higher prevalence of untreated hypertension compared to Asians (NHPI 45% vs Asians 31%, p = 0.02), and a trend toward higher prevalence of untreated hypertension compared to white subjects (NHPI 45% vs white subjects 31%, p = 0.11), suggesting a possible racial disparity in primary prevention.
DISCUSSION
Our results based on a single-center study demonstrate that NHPI admitted with spontaneous ICH are significantly younger, by more than 10 years, and have higher prevalence of diabetes and hypertension compared to white patients. In multivariable analyses adjusting for ICH risk factors, NHPI race was an independent predictor of young ICH. The age disparity seen in our study is similar to the age disparities reported in other ICH observational studies comparing non-Hispanic white subjects to Maoris from New Zealand,20 Mexican Americans,21 and black patients22,23 with ICH, and supports the idea that minority racial groups have a significantly younger age at presentation with ICH. Since NHPI have been historically grouped with Asians into a single racial category in many studies, prior ICH studies may have masked substantial differences in disease characteristics and outcomes among the NHPI group. Indeed, to our knowledge, this is the first study to characterize the clinical characteristics of NHPI who were hospitalized with acute ICH.
Although we acknowledge the limitation of a single-center study and its lack of generalizability, we believe this is an important first glance at the possible racial disparities in stroke risk factors seen in the state of Hawaii. Interestingly, NHPI had longer hospital LOS compared to white patients even though they shared similar ICH size and location, which suggests that the disease severity was likely similar between the 2 groups. We speculate that the insurance status, the disposition environment, and the geographical location of patients' homes may have played a major role in this observation.
Our study also demonstrates that NHPI with ICH are more likely to have characteristics of the metabolic syndrome (diabetes, obesity, and lower HDL) compared to white subjects, which is consistent with the prior studies that showed higher prevalence of cardiometabolic risk factors among the community-dwelling NHPI compared to other racial-ethnic groups.8–13 However, it is uncertain how these cardiometabolic health disparities impact the incidence and long-term outcome of ICH among the NHPI population. Although low levels of LDL cholesterol have been linked with increased risk of death after ICH,24 no studies have investigated the effect of HDL cholesterol on ICH. Furthermore, the proportion of hypertensive ICH patients who were untreated for hypertension prior to admission was significantly higher among NHPI compared to Asians even though there was no difference in the prevalence of reported hypertension. This finding suggests a possible disparity in community-based primary prevention between these 2 races. Further study addressing the socioeconomic status, access to health care, insurance status, and cultural beliefs toward health care is needed to determine the important factors driving this disparity.
This study has several limitations. First, the data on sympathomimetic drug abuse (i.e., methamphetamine and cocaine) were not available and were not accounted for in the analyses. Since sympathomimetic drug abuse is considered one of the major etiologies of spontaneous ICH in the young, and NHPI have a higher prevalence of methamphetamine abuse compared to other major racial-ethnic groups in Hawaii,25–27 it is unclear whether younger age at ICH observed in NHPI is related to high prevalence of methamphetamine abuse. Second, Asian race was not further specified (i.e., Japanese, Filipinos, Chinese, Korean), and thus it is unclear if similar age disparities exist between NHPI and each of the specific Asian races. Third, our prediction model for young ICH, based on a stepwise logistic regression, is limited due to the few subjects with young ICH. Fourth, due to the lack of long-term outcome data in our study, we were unable to assess any potential disparities that may exist in long-term outcome among the NHPI population as previously shown in black stroke patients in the United States,28,29 and the Pacific people in New Zealand.30 Fifth, we do not have complete data on anticoagulation use and were not able to incorporate it in the final model, which could have affected our results. However, we have attempted to adjust for this to the best of our ability by incorporating the pertinent risk factors in the model such as atrial fibrillation/atrial flutter, prosthetic heart valve, peripheral vascular disease, and history of prior stroke that are often associated with anticoagulation use. Finally, since this is a single-center observational study, our results may not be generalizable to other populations since there may have been a referral bias at our institution; for example, because our institution is a tertiary referral center, there may have been a referral bias toward more severe ICH patients as ICH patients with small hematomas and minor neurologic symptoms may not have been transferred to our facility. Also, it is possible that some of the older ICH patients with preexisting do-not-resuscitate orders or those with terminal illness may not have transferred to our facility, creating a possible selection bias toward younger ICH patients. However, only 22% of our patients were transferred from another hospital, and the rest of the patients were brought directly to our Emergency Department from the scene; thus, we believe that our study population is reasonably representative of the Honolulu County. We have adjusted for this referral bias factor in our final multivariable model.
Our study offers a first look at the possible racial disparities in stroke risk factors among NHPI with ICH, which has not been described previously. Prospective statewide, multicenter studies controlling for socioeconomic modifiers and the impact of substance abuse are needed to determine factors contributing to the younger age at ICH presentation in this unique racial group.
Supplementary Material
ACKNOWLEDGMENT
Lyle Oshita, Tracy Stern, and Tina Robertson were the clinical research coordinators who provided support for this project. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCRR, NIH, or The Queen's Medical Center.
GLOSSARY
- BMI
body mass index
- CAD
coronary artery disease
- CI
confidence interval
- GWTG
Get With the Guidelines
- HDL
high-density lipoprotein
- ICH
intracerebral hemorrhage
- IVH
intraventricular hemorrhage
- LDL
low-density lipoprotein
- LOS
length of stay
- MI
myocardial infarction
- NHPI
Native Hawaiians and other Pacific Islanders
- OR
odds ratio
- QMC
Queen's Medical Center
Footnotes
Editorial, page 623
AUTHOR CONTRIBUTIONS
K.N. participated in the conception and design of the study, the analysis and interpretation of data, and was responsible for drafting and finalizing the manuscript. M.A.K. participated in the analysis and interpretation of data and helped to draft and finalize the manuscript. T.B.S. participated in the analysis and interpretation of data, and helped to draft and finalize the manuscript. S.M.A. participated in the acquisition of data, analysis and interpretation of data, and finalization of the manuscript. C.W.C. participated in the conception and design of the study, study supervision, analysis and interpretation of the data, and helped to draft and finalize the manuscript. All authors read and approved the final manuscript.
DISCLOSURE
K. Nakagawa has received research support from the American Heart Association (11CRP7160019), Hawaii Community Foundation (10ADVC-47086), and Queen Emma Research Fund. M. Koenig has received research support from the Hawaii Community Foundation (11ADVC-49231) and Queen Emma Research Fund. T. Seto has received research support from the NIMHD (5P20MD000173), NCCAM (1R21 AT003725–01A1), NHLBI (1UO1 HL079152–01), NCRR (1U54RR0261 36–01A1), and AHRQ (1R01HS019990–01). S. Asai and C. Chang report no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med 2001; 344: 1450– 1460 [DOI] [PubMed] [Google Scholar]
- 2. Elijovich L, Patel PV, Hemphill JC., 3rd Intracerebral hemorrhage. Semin Neurol 2008; 28: 657– 667 [DOI] [PubMed] [Google Scholar]
- 3. Kagan A, Popper J, Reed DM, MacLean CJ, Grove JS. Trends in stroke incidence and mortality in Hawaiian Japanese men. Stroke 1994; 25: 1170– 1175 [DOI] [PubMed] [Google Scholar]
- 4. Labovitz DL, Halim A, Boden-Albala B, Hauser WA, Sacco RL. The incidence of deep and lobar intracerebral hemorrhage in whites, blacks, and Hispanics. Neurology 2005; 65: 518– 522 [DOI] [PubMed] [Google Scholar]
- 5. Stansbury JP, Jia H, Williams LS, Vogel WB, Duncan PW. Ethnic disparities in stroke: epidemiology, acute care, and postacute outcomes. Stroke 2005; 36: 374– 386 [DOI] [PubMed] [Google Scholar]
- 6. Sacco RL, Boden-Albala B, Gan R, et al. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol 1998; 147: 259– 268 [DOI] [PubMed] [Google Scholar]
- 7. Sacco RL, Boden-Albala B, Abel G, et al. Race-ethnic disparities in the impact of stroke risk factors: the Northern Manhattan Stroke Study. Stroke 2001; 32: 1725– 1731 [DOI] [PubMed] [Google Scholar]
- 8. Erber E, Hopping BN, Grandinetti A, Park SY, Kolonel LN, Maskarinec G. Dietary patterns and risk for diabetes: the multiethnic cohort. Diabetes Care 2010; 33: 532– 538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Curb JD, Aluli NE, Kautz JA, et al. Cardiovascular risk factor levels in ethnic Hawaiians. Am J Public Health 1991; 81: 164– 167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mau MK, Grandinetti A, Arakaki RF, Chang HK, Kinney EK, Curb JD. The insulin resistance syndrome in native Hawaiians: Native Hawaiian Health Research (NHHR) Project. Diabetes Care 1997; 20: 1376– 1380 [DOI] [PubMed] [Google Scholar]
- 11. Henderson SO, Haiman CA, Wilkens LR, Kolonel LN, Wan P, Pike MC. Established risk factors account for most of the racial differences in cardiovascular disease mortality. PLoS One 2007; 2: e377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mau MK, Sinclair K, Saito EP, Baumhofer KN, Kaholokula JK. Cardiometabolic health disparities in native Hawaiians and other Pacific Islanders. Epidemiol Rev 2009; 31: 113– 129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moy KL, Sallis JF, David KJ. Health indicators of Native Hawaiian and Pacific Islanders in the United States. J Community Health 2010; 35: 81– 92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aluli NE, Reyes PW, Tsark J. Cardiovascular disease disparities in native Hawaiians. J Cardiometab Syndr 2007; 2: 250– 253 [DOI] [PubMed] [Google Scholar]
- 15. Balabis J, Kromer Baker K, Tottori C, Salvail F. The Burden of Cardiovascular Disease in Hawaii 2007. Hawaii State Department of Health. 2007 [Google Scholar]
- 16. Schwamm LH, Fonarow GC, Reeves MJ, et al. Get With the Guidelines–Stroke is associated with sustained improvement in care for patients hospitalized with acute stroke or transient ischemic attack. Circulation 2009; 119: 107– 115 [DOI] [PubMed] [Google Scholar]
- 17. Morgenstern LB, Hemphill JC, 3rd, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2010; 41: 2108– 2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bevan H, Sharma K, Bradley W. Stroke in young adults. Stroke 1990; 21: 382– 386 [DOI] [PubMed] [Google Scholar]
- 19. Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke 1996; 27: 1304– 1305 [DOI] [PubMed] [Google Scholar]
- 20. Feigin V, Carter K, Hackett M, et al. Ethnic disparities in incidence of stroke subtypes: Auckland Regional Community Stroke Study, 2002–2003. Lancet Neurol 2006; 5: 130– 139 [DOI] [PubMed] [Google Scholar]
- 21. Zahuranec DB, Brown DL, Lisabeth LD, et al. Differences in intracerebral hemorrhage between Mexican Americans and non-Hispanic whites. Neurology 2006; 66: 30– 34 [DOI] [PubMed] [Google Scholar]
- 22. Kuhlemeier KV, Stiens SA. Racial disparities in severity of cerebrovascular events. Stroke 1994; 25: 2126– 2131 [DOI] [PubMed] [Google Scholar]
- 23. Copenhaver BR, Hsia AW, Merino JG, et al. Racial differences in microbleed prevalence in primary intracerebral hemorrhage. Neurology 2008; 71: 1176– 1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramirez-Moreno JM, Casado-Naranjo I, Portilla JC, et al. Serum cholesterol LDL and 90-day mortality in patients with intracerebral hemorrhage. Stroke 2009; 40: 1917– 1920 [DOI] [PubMed] [Google Scholar]
- 25. Sakai JT, Wang C, Price RK. Substance use and dependence among Native Hawaiians, Other Pacific Islanders, and Asian ethnic groups in the United States: contrasting multiple-race and single-race prevalence rates from a national survey. J Ethn Subst Abuse 2010; 9: 173– 185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Winslow BT, Voorhees KI, Pehl KA. Methamphetamine abuse. Am Fam Physician 2007; 76: 1169– 1174 [PubMed] [Google Scholar]
- 27. Edwards C, Giroux D, Okamoto SK. A review of the literature on Native Hawaiian youth and drug use: implications for research and practice. J Ethn Subst Abuse 2010; 9: 153– 172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Horner RD, Matchar DB, Divine GW, Feussner JR. Racial variations in ischemic stroke-related physical and functional impairments. Stroke 1991; 22: 1497– 1501 [DOI] [PubMed] [Google Scholar]
- 29. Horner RD, Swanson JW, Bosworth HB, Matchar DB. Effects of race and poverty on the process and outcome of inpatient rehabilitation services among stroke patients. Stroke 2003; 34: 1027– 1031 [DOI] [PubMed] [Google Scholar]
- 30. McNaughton H, Feigin V, Kerse N, et al. Ethnicity and functional outcome after stroke. Stroke 2011; 42: 960– 964 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


