Abstract
Background
Although endoplasmic reticulum (ER) stress has been implicated in the pathophysiology of organ ischemia and reperfusion injury (IRI), the underlying mechanisms have yet to be fully elucidated. In particular, as tissue pro-inflammatory immune response is the key mediator of local IRI, how ER stress impacts liver immune cell activation cascade remains to be determined.
Methods
In vitro, ER stress in macrophages and hepatocytes were induced by pharmacological agents. Macrophage TLR4 and hepatocyte TNF-α responses were studied. In vivo, the induction of ER stress by IR and the impact of ER stress amelioration by a small molecule chaperon 4-phenyl butyric acid (PBA) on liver immune response were studied in a murine partial liver warm ischemia model.
Results
ER stressed macrophages generated a significantly enhanced pro-inflammatory immune response against TLR4 stimulation; while ER stressed hepatocyte became more susceptible to TNF-α induced cell death. IR resulted in upregulations of sXBP-1 and ATF6 levels in affected livers. Mice pre-treated with PBA were protected from liver IRI, in parallel with diminished local pro-inflammatory gene induction program.
Conclusions
Our study documents a potential immune regulatory role of ER stress in the mechanism of liver IRI, and provides a rationale for targeting stress response as a new therapeutic means to ameliorate tissue inflammation in organ transplant recipients.
Keywords: ER stress, Liver ischemia, TLR4, Inflammation
Introduction
Endoplasmic reticulum (ER), the primary cellular organelle of protein synthesis, post-translational modification/folding and traffic, is highly sensitive to stress stimulations that perturb cellular energy levels, redox state and Ca2+ concentration. Stressed ER is characterized by reduced capacity to handle newly synthesized proteins, leading to the accumulation and aggregation of unfolded proteins in ER lumen. This triggers an adaptive intracellular “unfolded protein response” (UPR) (1). In mammals, ER stress responses are triggered by activation of three distinct signaling pathways mediated by PKR-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1) and activating transcription factor 6 (ATF6), respectively, with resultant global inhibition of protein synthesis, but increased transcription of genes for chaperones and degradation machinery of misfolded proteins in ER (2). Although resolution of ER stress by UPR protects cells from apoptosis (3), a sustained ER stress and prolonged UPR may trigger cell death and inflammatory response (4-6).
Ischemia/reperfusion injury (IRI) is a key contributing factor in liver dysfunction and failure after hepatic trauma, resection and liver transplantation (7-10). The IR insult disturbs the homeostasis of liver cells, of both parenchymal and non-parenchymal types, and creates intracellular stresses. In addition to the oxidative stress in mitochondria, ATP/nutrient deprivation may also trigger ER stress, leading to liver parenchymal cell death, and immune responses in NPCs. As ER stress is associated with metabolic disorders, such as obesity and diabetes (type II), it becomes a common pre-existing condition in many liver disease patients. Thus, understanding how ER stress response regulates IRI is an integral part of our mechanistic study of the disease, and will provide us rationale to expand our therapeutic window beyond IR itself, i.e., to tackle with pre-existing pro-pathological conditions in patients.
The role of ER stress in the pathogenesis of tissue IRI has been studied in brain, heart, and liver settings (11-17). However, the mechanistic focus has been limited mostly to cell apoptosis. For instance, Bax inhibitor-1 deficient (KO) mice exhibited enhanced ER stress response against IR and developed more severe hepatocellular injury by sensitizing hepatocytes to stress-induced apoptosis (11). In addition, small chemical chaperon sodium 4-phenylbutyrate acid (PBA) has been shown to protect brain, spine and liver against IRI by inhibiting ER stress-mediated apoptosis (13, 14, 17, 18). Although the ultimate result of IRI is the death of liver parenchymal cells, the full development of injury is critically dependent on liver inflammatory immune response. TLR4 activation by cell death-associated endogenous ligands has been documented recently as a key event triggering liver IR immune response (19-23). Thus, the question of whether and how ER stress interplays with TLR4 response and regulates liver immune cascade against IR is of high interest to further our understanding of the liver IRI pathology.
In this study, we determined how ER stress regulated TLR4-mediated immune responses both in vivo and in vitro, by utilizing chemical ER stress inducers in macrophages/hepatocytes, and small molecule chaperon, PBA, in mice. Our results show that ER stress significantly enhanced pro-inflammatory immune activation by TLR4 and promoted cell death in synergy with TNF-α. Moreover, the amelioration of ER stress was sufficient to disrupt liver pro-inflammatory immune response against IR and to reduce the hepatocellular damage.
Results
ER stress increases TLR4-induced pro-inflammatory gene expression in macrophages
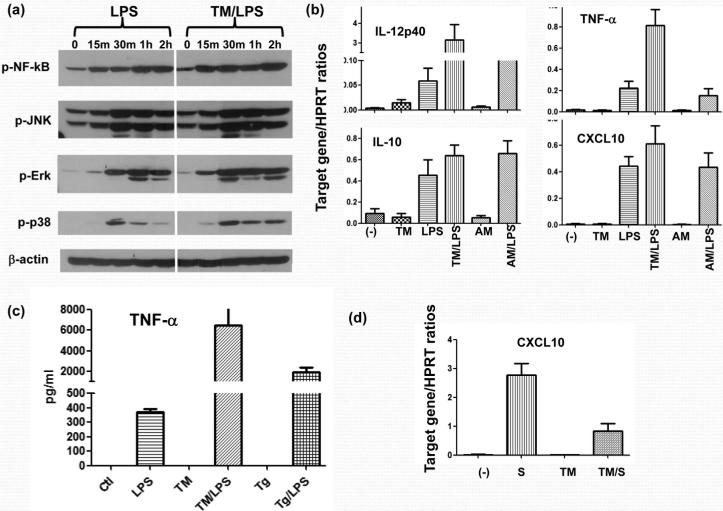
To determine how ER stress modulates macrophage TLR4 response, RAW267.4 cells were first incubated with Tunicamycin (Tm), followed by LPS stimulation. Western blot analysis showed that Tm-treated macrophages had enhanced responses to LPS stimulation, evidenced by significantly higher levels of phosphorylated NF-κB, JNK, Erk and p38, as compared with controls (Fig. 1a). These ER-stressed macrophages selectively upregulated their pro-inflammatory gene induction program in response to LPS. Indeed, while IL-12p40 and TNF-α gene expression levels were consistently higher in the Tm/LPS group than in the LPS group, IL-10 levels, although showed a trend of slight increases, were not statistically significantly different between the two groups (p>0.05) Fig. 1b). We observed similarly altered gene expression pattern in primary bone-marrow derived macrophages (BMM) and with different chemical ER stress inducers, such as thapsigargin (Tg), (data not shown). Cytokine productions in ER stressed macrophages were confirmed by measuring TNF-α levels in BMM culture supernatants. ELISA data (Fig.1c) clearly showed that both ER stress agents (Tm and Tg) enhanced macrophage TNF-α production in response to LPS. These agents by themselves did not significantly affect signaling molecules or gene expression profiles. The ER stress enhancement of macrophage pro-inflammatory response was cell-type specific, as CXCL10 induction in Tg-conditioned hepatocytes in response to inflammatory stimuli (LPS-stimulated macrophage culture supernatants), was largely suppressed (Fig.1d). Thus, ER stress exerts a cell-type specific pro-inflammatory function in macrophages and synergizes with TLR4 activation.
Figure 1.
ER stress enhances TLR4-induced macrophage pro-inflammatory immune response. RAW cells were first incubated with Tunicamycin (TM) or Antimycin (AM), followed by LPS stimulation, as described in Material and Methods. Cells were harvested after 15 min, 30 min, 1 h or 2 h after LPS stimulation, and cell lysates were analyzed for NF-kB, JNK, Erk and p38 phosphorylation, as well as β-actin protein levels by Western blots (a). In a separate experiment, RAW cells were harvested after 4 h-long LPS stimulation and total RNA was isolated and subjected to qRT-PCR analysis (b). Bone-marrow-derived macrophages were stimulated first with ER stress agent Tm or Tg, followed by 24h LPS stimulation. Culture supernatants were harvested and TNF-a levels were measured by ELISA (c). Mouse hepatoma cell line Hepa-1, was stimulated with either control (-) or LPS-stimulated (S) RAW cell culture supernatant w/ or w/o pre-incubation with Thapsigargin (Tg). Cells were harvested after 4 h of stimulation and total RNA was isolated and subjected to qRT-PCR analysis (d). Representative results of 3 separate experiments.
ER stress promotes TNF-α-induced hepatocyte death
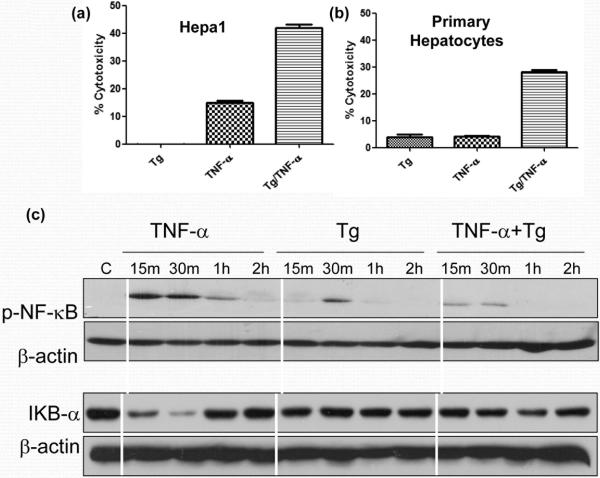
The major outcome of IR-mediated liver immune activation is the hepatocyte death via TNF-α pathway. As both pro- and anti-apoptotic signaling pathways are triggered simultaneously, TNF-α does not kill cells by itself, unless the anti-apoptotic pathway is blocked or additional cytotoxic mechanisms are operational. To test the role of ER stress in TNF-α-induced hepatocyte death, we treated hepa-1 cells with Tg, prior to the addition of TNF-α, and measured the cell death by LDH assay (culture supernatants). As shown in Fig. 2a, Tg by itself did not exert any cytotoxicity in hepa-1 cells. As expected, the hepatocyte death after addition of TNF-α alone was relatively low (15%). However, the induction of ER stress after adjunctive treatment with Tg, significantly increased TNF-α cytotoxicity in hepa-1 cells (41%). This was confirmed in primary murine hepatocytes that Tg significantly increased hepatocyte death in response to TNF-α (Fig.2b). To address putative mechanism of ER stress-enhanced TNF-α cytotoxicity, we analyzed the activation of NF-κB, the essential player in the anti-apoptotic signaling pathway. Western blot data shows Tg reduced TNF-α-induced NF-κB (p65) phosphorylation, as well as Iκb degradation (Fig. 2c). Thus, ER stress may increase the hepatocyte death by suppressing NF-κB activation.
Figure 2.
ER stress increases hepatocyte sensitivity to TNF-α induced cell death. Hepa-1 (a) or primary hepatocytes from B6 mice (b) cells were pre-incubated with vehicle control or Tg, followed by TNF-α stimulation. Cell death was measured at 24 or 48 h by LDH assay. In a separate experiment, Tg-treated hepa-1 cells were harvested after 15 min, 30 min, 1 h and 2 h of TNF-α stimulation. Cell lysates were subjected to Western blot analysis of p-NF-κB, IκB and β-actin (c). Representative results of 3 separate experiments.
PBA ameliorates IR-induced hepatocellular damage and liver immune response
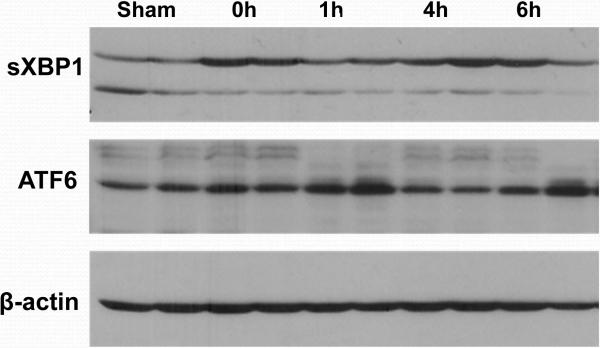
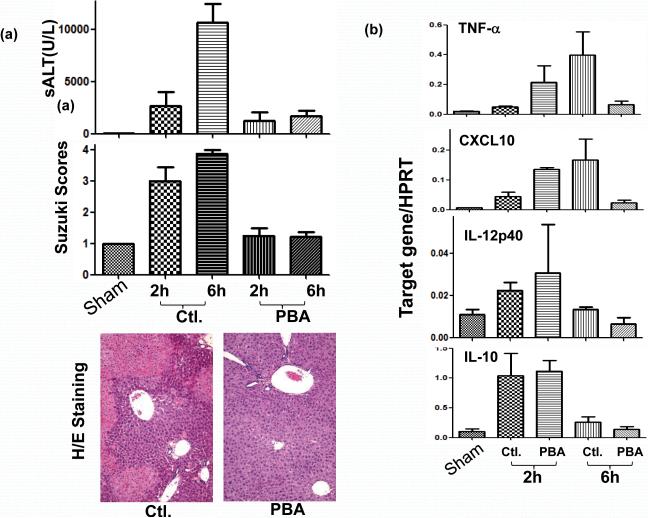
We first determined whether liver IR activated ER stress response. Liver tissue samples were harvested serially post-reperfusion following 90 min. of warm ischemia and subjected to Western blot analysis of UPR signature markers. Indeed, spliced xbp1 and cleaved ATF6, but not ATF4, were readily detected in livers and their levels increased rapidly after reperfusion (Fig. 3; xbp1 at 0 h and ATF6 at 1 h). To analyze how ER stress amelioration impacted the development of IRI, we administered a small molecule chaperon, phenylbutyrate acid (PBA), which has been shown to reduce ER stress in vivo in various models (13, 14, 17), prior to the onset of liver ischemia. As compared with vehicle controls, PBA-treated mice had markedly reduced sALT levels at 6 h of reperfusion (p<0.001, n=8/group), and better preserved liver histological structures, evidenced by lower Suzuki's scores of the liver IR-damage at both 2 h and 6 (p≤0.04 and 0.001 respectively, n=3-4/group) (Fig.4a). We then analyzed liver inflammatory gene induction pattern to determine whether targeting ER stress did affect local immune response against IR. Indeed, PBA treatment decreased TNF-α and CXCL10 gene expression levels selectively at 6 h, but not at 2 h, post reperfusion (Fig. 4b). Interestingly, PBA did not affect the induction of IL-12p40 or IL-10 at either time points. Thus, targeting ER stress disrupted the sustainment of liver pro-inflammatory immune response against IR, although it did not prevent the early immune activation.
Figure 3.
Liver IR triggers ER stress response. Ischemic liver lobes were harvested at different time points of reperfusion (after 90 min of warm ischemia), as described in Material and Methods. Tissue lysates were subjected to Western blot analysis for XBP1, ATF6, ATF4, and β-actin. Representative results of 2 separate experiments.
Figure 4.
PBA protects livers from IRI by depressing local pro-inflammatory immune response. PBA was administered 12 h prior to the ischemia insult (10 mg/kg, i.p). Liver injury and immune response were evaluated at 2 h and 6 h of reperfusion by (a) measuring sALT levels, Suzuki's scores of liver tissue damage and liver histology (H&E staining); n=8/group. (b) qRT-PCR of liver inflammatory gene expression programs (IL-12p40, IL-10, TNF-α, CXCL10); n=3/group.
Discussion
Although ER stress has been implicated in multiple organ IRI models, its pathogenic role has been mostly limited to regulation of the cell death (11-15, 17, 24, 25). Roles of ER stress in chronic-type tissue inflammation have been documented recently, however, the underlying mechanisms remain to be fully elucidated (4, 6). Our present study presents evidence that ER stress contributes to liver pro-inflammatory response against IR in both the activation stage in macrophages and the effector stage in hepatocytes, in an acute tissue inflammation/injury model. Indeed, ER-stressed macrophages were hyper-responsive to TLR4 activation in vitro, in both intracellular signaling pathways and pro-inflammatory gene transcription patterns. In parallel, ER-stressed hepatocytes became more sensitive to TNF-α induced cell death. Liver IR disturbs cellular metabolism, leading to ATP/nutrient deprivation and redox alterations. ER stress, as the direct consequence of these metabolic disorders in liver cells, can, therefore, function very early pathologically on the development of IRI. Indeed, our Western blot data showed that sXBP1 and ATF6 were upregulated in the ischemic liver lobes as early as at 0 h, i.e., at the very beginning of reperfusion phase when local immune response had yet to be fully activated. Consistent with published data in liver IRI models (17, 25), in vivo pretreatment with small molecule chaperons effectively ameliorated liver injury and reduced tissue inflammation. Interestingly, the liver immune response against IR in the treated animals was not prevented, but rather terminated prematurely, evidenced by a robust pro-inflammatory gene induction at 2 h but diminished at 6 h of reperfusion. This result is consistent with a finding by Martinon et al that the ER stress signaling molecule xbp1 is required for the optimal TLR response that its absence will lead to reductions, but not complete inhibition, of pro-inflammatory gene inductions (26). Hence, although ER stress may not be essential in triggering the acute immune response against IR per se, it contributes to its maintenance, by either direct regulation of macrophage activation or indirectly via cell death-associated pro-inflammatory events. Although our in vivo experiments could not differentiate between these two mechanisms, we provide compelling evidence that IR-triggered ER stress is relevant in liver immune response against IR.
ER stress activates an evolutionally conserved transcriptional program, UPR, by three distinct signaling pathways, mediated by IRE1, ATF6 and PERK (2). In our model, spliced XBP1 and cleaved ATF6 proteins were readily detectable in IR-livers and their levels increased early after reperfusion (Fig.3). Although the expression of ER signature molecules in liver IRI has been reported, their kinetic profiles have not been characterized. Our finding of a rather transient induction of sXBP1 and cATF6 in livers right after the ischemia insult is somewhat unexpected. However, considering an atypical (transient) nature of ER stress in IR cascade and impacts of other IR-associated events, it is not totally surprising. The concurrent tissue inflammation during IR may alter UPR. Indeed, TLR4 activation may suppress ATF4-CHOP pathway, and ATF4 translation in particular can be halted by a TRIF-mediated mechanism (27).
The interaction between ER stress and tissue inflammation occurs at multiple levels. We focused on the activation and effector stages, occurring in different liver cells during the course of IR. In macrophages, UPR signaling pathways may directly activate inflammatory signaling molecules. Phosphorylated IRE1 (kinase) is able to bind TRAF2, leading to IKK activation and subsequent IkB degradation. Together with the PERK-mediated translational decrease, IkB level becomes insufficient to suppress NF-kB activation (5). The IRE1-TRAF2 complex is also able to activate JNK via ASK1 (28). In our study, ER-stressed macrophages responded to TLR4 stimulation, with enhanced NF-kB and MAP kinase phosphorylation. It has been shown that XBP1 is directly involved in the regulation of TLR-induced cytokine production (26). In particular, it is required for the optimal induction of a subset of inflammatory genes by Toll ligands, e.g., IL-6. Our results extend this finding, as LPS-induced IL-12p40 and TNF-α expression in vitro significantly increased in ER stressed macrophages, whereas IL-10 and CXCL10 genes were only marginally affected. In vivo, we found that chaperon-assisted reduction of ER stress terminated liver IR pro-inflammatory response and ameliorated hepatocellular injury. We further showed that ER stress regulation of the inflammatory response was cell-type specific, as pre-existing ER stress suppressed hepatocyte CXCL10 expression. Additionally, stressed hepatocytes became more sensitive to TNF-α induced cell death. Both of these phenomena could be explained by the fact that inflammation-induced NF-κB activation in hepatocytes was inhibited by ER stress. Other than cell types, the nature of stimulus may also be a critical determinant of distinctive impact of ER stress on cell immune responses. In liver IR, ER stress may enhance TLR4 induced NF-kB activation in macrophages, leading to increased inflammatory gene expression program. However, it may also suppress TNF-α-induced NF-κB activation in hepatocytes, leading to the augmented cell death. Distinctive effects of ER stress on inflammatory response have been reported. In the very same macrophage cell line as ours, thapsigargin (Tg) was shown to increase log-fold LPS-mediated IFN-β induction (29, 30); while UPR triggered by a sublethal dose of cytotoxin Subtilase inhibited LPS-induced MCP-1 and TNF-α (31). In renal mesangial cells, pre-existing ER stress blunted cellular response to TNF-α (32). Thus, mechanism details by which ER stress regulates inflammation in the context of different cells and stimulations, remain to be delineated.
ER stress and inflammation are two-way interactions. As previously shown by Martinon et al (26), TLR activation triggers a partial UPR response that activates XBP1 but suppresses ATF4 pathway. Thus, ER stress in liver IR could be induced initially by ischemia, tissue inflammation would also alter/regulate ER stress. Our in vivo liver inflammatory gene induction data by IR being unaffected early in PBA-treated mice indicates that ER stress may not be involved in triggering, but sustaining, the liver immune activation cascade. As ER stress regulates the hepatocyte death response to inflammation, which in turn releases endogenous TLR ligands driving sterile liver IR inflammation forward, one may envision at least two mechanisms by which ER stress may promote liver inflammation. Thus, UPR may directly enhance TLR4 activation in macrophages via XBP1, or alternatively, ER stress-enhanced hepatocyte death may indirectly augment macrophage activation by release of endogenous inflammatory stimuli, such as HMGB1. Although our study reveals the potential impact of ER stress on liver IR immune response, a causal link between these two events and the exact mechanism remain to be elucidated. As PBA is not a specific ER targeting agent (33), the question of whether our in vivo findings of its effect on tissue inflammation/injury is ER stress dependent needs to be further elaborated. Our in vitro experiment using PBA in macrophage cultures exclude its direct immunosuppressive function (data not shown).
In summary, our study documents an immune regulatory function of ER stress in the mechanism of liver IRI. Our findings provide a rationale for targeting stress response as a novel anti-inflammatory therapeutic option in liver transplant recipients.
Materials and Methods
Mice
Male wide-type (WT; C57BL/6) mice (8-12 weeks old) were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were housed in the UCLA animal facility under specific pathogen-free conditions, and received human care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institute of Health (NIH publication 86-23 revised 1985).
Model of warm liver IRI
A model of partial liver warm IRI was used, as described (34). In brief, an atraumatic clip was used to interrupt the arterial and portal venous blood supply to the cephalad lobes of the liver for 90 min. Mice were sacrificed after various times of reperfusion. Liver tissue and serum samples were collected for future analysis.
Serum alanine aminotransferase (sALT) levels were measured by IDEXX Laboratories. Part of liver specimens were fixed in 10% buffered formalin and embedded in paraffin. Liver sections (4 μm) were stained with hematoxylin and eosin, and then analyzed blindly. Sham WT controls underwent the same procedure, but without vascular occlusion.
To reduce ER stress in vivo, mice were treated with a single dose of PBA (100μg/g, Sigma, St. Luis, MO) dissolved in PBS, i.p.12 h prior to the onset of liver ischemia or LPS (1μg/mouse) injection.
Cell cultures
Mouse macrophage RAW264.7 (from ATCC, Manassas, VA) cells were maintained in DMEM medium supplemented with 10% heat inactivated fetal bovine serum (FBS). LPS (10ng/ml, Invivogen, San Diego, CA) was used to activate cells. Pre-incubation of cells with Tunicamycin (Tm; 10μg/ml, 6 h) or Thapsigargin (Tg; 1μM/ml, 1 h) or Antimycin A (Am; 10μg/ml, 1 h) (all from Sigma, St. Luis, MO) was used to induce ER or oxidative stress.
Murine bone marrow-derived macrophages (BMM) were differentiated from bone marrow from 6-10-week old C57B/6 mice, as described (26) by culturing in 1×DMEM, 10% fetal bovine serum, 1% penicillin/streptomycin, and 20% L929 conditioned medium for 6 days. The cell purity was assayed to be 94-99% CD11b+.
Mouse Hepa 1 cells were plated in 96 or 48-well plate at appropriate concentration. After o/n culture, recombinant mouse TNF-α (20ng/ml, R&D System, Minneapolis, MN) was added into the culture wells w/ or w/o Thapsigargin (1μM/ml) pre-incubation (1 h). Cell death was evaluated at 24 h by LDH assay (Biochain Institute, Hayward, CA) of culture supernatants, according to the manufacturer instruction.
Quantitative RT-PCR
Total RNA was reverse-transcribed into cDNA using SuperScriptTM III First-Strand Synthesis System (Invitrogen, Carlsbad, CA). Quantitative-PCR was performed using the DNA Engine with Chromo 4 Detector (MJ Research, Waltham, MA). In a final reaction volume of 25 μl, with the following components: 1xSuperMix (Platinum SYBR Green qPCR Kit, Invitrogen, Carlsbad, CA), cDNA and 0.5 μM of each primer. Amplification conditions were: 50°C (2 min), 95°C (5 min) followed by 50 cycles of 95°C (15 s), 60°C (30 s). Primers used to amplify a specific mouse gene fragments were described previously.
Western blots
Protein was extracted from cultured cells or liver tissue with ice cold lysis buffer (1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 10% glycerol, 137mM sodium chloride, 20mM Tris, pH 7.4). Proteins (20 μg) were subjected to 12% SDS-PAGE electrophoresis and transferred to PVDF nitrocellulose membrane. Antibodies against phosphorylated NF-kB, extracellular signal-regulated kinase (ERK), Jun N-terminal kinase (JNK), and p38 mitogen-activated protein (MAP) kinase proteins, total Ik-B, βactin (Cell Signaling Technology, Santa Cruz, CA), XBP-1 (Abcam, Cambridge, MA), and ATF6 (Imgenex, San Diego, CA) were used for Western blot analysis. Membranes were probed with primary antibody (1:500-1000) in 10 ml blocking buffer overnight at 4°C. After washing, membranes were further probed with appropriate HRP-conjugated secondary antibody (1:2000) in 10 ml of blocking buffer for 1 h at room temperature. SuperSignal® West Pico Chemiluminescent Substrates (Thermo Fisher Scientific, Rockford, IL) were used for chemo-luminescence development.
Statistical analysis
Results are shown as mean±SD. Statistical analyses were performed using unpaired Student's t test with p<0.05 (two tailed) considered as significant.
Acknowledgements
This work was supported NIH Grants RO1 DK083408 (YZ), DK062357 (JWKW), and The Dumont Research Foundation
Abbreviations
- ATF6
Activating transcription factor 6
- ER
endoplasmic reticulum
- IRI
Ischemia reperfusion injury
- KO
Knock-out
- LDH
Lactate dehydrogenase
- LPS
Lipopolysaccharide
- NPC
Non-parenchymal cell
- PBA
phenyl butyric acid
- sXBP-1
Spliced X-box binding protein 1
- TLR4
Toll-like-receptor 4
- TNF-α
Tumor necrosis factor alpha
- UPR
Unfolded protein response
Footnotes
JL, FR, and LB designed experiments and performed in vitro assays; QC, XS, and FG performed in vivo experiments; RWB and JWK revised manuscript; YZ designed, supervise the study and wrote manuscript.
All authors declare no conflict of interest.
Current addresses for JL: Department of General Surgery, Beijing Friendship Hospital, Beijing, China 100050; FR and LB: Institute of Liver Diseases, Beijing You'an Hospital, Beijing, China 100069; QC, XS, FG, RWB, JWK and YZ: 77-120 CHS, 10833 Le Conte Ave, Los Angeles, CA 90095.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332(6163):462. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 2.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 3.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7(12):1013. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 4.Kaser A, Blumberg RS. Endoplasmic reticulum stress and intestinal inflammation. Mucosal Immunology. 2010;3(1):11. doi: 10.1038/mi.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinon F, Glimcher LH. Regulation of innate immunity by signaling pathways emerging from the endoplasmic reticulum. Curr Opin Immunol. 2010 doi: 10.1016/j.coi.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Todd DJ, Lee AH, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol. 2008;8(9):663. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- 7.Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury--a fresh look. Exp Mol Pathol. 2003;74(2):86. doi: 10.1016/s0014-4800(03)00008-x. [DOI] [PubMed] [Google Scholar]
- 8.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284(1):G15. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 9.Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32(2):169. doi: 10.1053/jhep.2000.9323. [DOI] [PubMed] [Google Scholar]
- 10.Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001;181(2):160. doi: 10.1016/s0002-9610(00)00573-0. [DOI] [PubMed] [Google Scholar]
- 11.Bailly-Maitre B, Fondevila C, Kaldas F, et al. Cytoprotective gene bi-1 is required for intrinsic protection from endoplasmic reticulum stress and ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2006;103(8):2809. doi: 10.1073/pnas.0506854103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martindale JJ, Fernandez R, Thuerauf D, et al. Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ Res. 2006;98(9):1186. doi: 10.1161/01.RES.0000220643.65941.8d. [DOI] [PubMed] [Google Scholar]
- 13.Mizukami T, Orihashi K, Herlambang B, et al. Sodium 4-phenylbutyrate protects against spinal cord ischemia by inhibition of endoplasmic reticulum stress. Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2010;52(6):1580. doi: 10.1016/j.jvs.2010.06.172. [DOI] [PubMed] [Google Scholar]
- 14.Qi X, Hosoi T, Okuma Y, Kaneko M, Nomura Y. Sodium 4-phenylbutyrate protects against cerebral ischemic injury. Molecular Pharmacology. 2004;66(4):899. doi: 10.1124/mol.104.001339. [DOI] [PubMed] [Google Scholar]
- 15.Tajiri S, Oyadomari S, Yano S, et al. Ischemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death Differ. 2004;11(4):403. doi: 10.1038/sj.cdd.4401365. [DOI] [PubMed] [Google Scholar]
- 16.Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res. 2006;99(3):275. doi: 10.1161/01.RES.0000233317.70421.03. [DOI] [PubMed] [Google Scholar]
- 17.Vilatoba M, Eckstein C, Bilbao G, et al. Sodium 4-phenylbutyrate protects against liver ischemia reperfusion injury by inhibition of endoplasmic reticulum-stress mediated apoptosis. Surgery. 2005;138(2):342. doi: 10.1016/j.surg.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Ben Mosbah I, Alfany-Fernandez I, Martel C, et al. Endoplasmic reticulum stress inhibition protects steatotic and non-steatotic livers in partial hepatectomy under ischemia-reperfusion. Cell death & disease. 2010;1:e52. doi: 10.1038/cddis.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsung A, Hoffman RA, Izuishi K, et al. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol. 2005;175(11):7661. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 20.Wu HS, Zhang JX, Wang L, Tian Y, Wang H, Rotstein O. Toll-like receptor 4 involvement in hepatic ischemia/reperfusion injury in mice. Hepatobiliary Pancreat Dis Int. 2004;3(2):250. [PubMed] [Google Scholar]
- 21.Tsung A, Sahai R, Tanaka H, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201(7):1135. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhai Y, Qiao B, Shen XD, et al. Evidence for the pivotal role of endogenous toll-like receptor 4 ligands in liver ischemia and reperfusion injury. Transplantation. 2008;85(7):1016. doi: 10.1097/TP.0b013e3181684248. [DOI] [PubMed] [Google Scholar]
- 23.Zhai Y, Shen XD, O'Connell R, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173(12):7115. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Wang JJ, Zhang SX. Preconditioning with endoplasmic reticulum stress mitigates retinal endothelial inflammation via activation of X-box binding protein 1. J Biol Chem. 2010 doi: 10.1074/jbc.M110.199729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ben Mosbah I, Alfany-Fernandez I, Martel C, et al. Endoplasmic reticulum stress inhibition protects steatotic and non-steatotic livers in partial hepatectomy under ischemia-reperfusion. Cell Death Dis. 2010;1:e52. doi: 10.1038/cddis.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11(5):411. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woo CW, Cui D, Arellano J, et al. Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by toll-like receptor signalling. Nat Cell Biol. 2009;11(12):1473. doi: 10.1038/ncb1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urano F, Wang X, Bertolotti A, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287(5453):664. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 29.Zeng L, Liu YP, Sha H, Chen H, Qi L, Smith JA. XBP-1 couples endoplasmic reticulum stress to augmented IFN-beta induction via a cis-acting enhancer in macrophages. J Immunol. 2010;185(4):2324. doi: 10.4049/jimmunol.0903052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith JA, Turner MJ, DeLay ML, Klenk EI, Sowders DP, Colbert RA. Endoplasmic reticulum stress and the unfolded protein response are linked to synergistic IFN-beta induction via X-box binding protein 1. Eur J Immunol. 2008;38(5):1194. doi: 10.1002/eji.200737882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harama D, Koyama K, Mukai M, et al. A subcytotoxic dose of subtilase cytotoxin prevents lipopolysaccharide-induced inflammatory responses, depending on its capacity to induce the unfolded protein response. J Immunol. 2009;183(2):1368. doi: 10.4049/jimmunol.0804066. [DOI] [PubMed] [Google Scholar]
- 32.Hayakawa K, Hiramatsu N, Okamura M, et al. Acquisition of anergy to proinflammatory cytokines in nonimmune cells through endoplasmic reticulum stress response: a mechanism for subsidence of inflammation. J Immunol. 2009;182(2):1182. doi: 10.4049/jimmunol.182.2.1182. [DOI] [PubMed] [Google Scholar]
- 33.Newmark HL, Lupton JR, Young CW. Butyrate as a differentiating agent: pharmacokinetics, analogues and current status. Cancer Lett. 1994;78(1-3):1. doi: 10.1016/0304-3835(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 34.Shen XD, Ke B, Zhai Y, et al. CD154-CD40 T-cell costimulation pathway is required in the mechanism of hepatic ischemia/reperfusion injury, and its blockade facilitates and depends on heme oxygenase-1 mediated cytoprotection. Transplantation. 2002;74(3):315. doi: 10.1097/00007890-200208150-00005. [DOI] [PubMed] [Google Scholar]