Abstract
BACKGROUND
Screening and early diagnosis tools are lacking for pancreatic adenocarcinoma; most patients are diagnosed with metastatic disease. Autoantibodies to tumor-associated antigens (TAAs) can be present months to years before diagnosis and hold promise as biomarkers for early detection.
METHODS
TAAs to pancreatic cancer autoantibodies CTDSP1, MAPK9, and NR2E3, identified as potentially promising biomarkers in exploratory studies, were evaluated in serum from participants (cases=300, controls=300) in a population-based case-control pancreatic cancer study in the San Francisco Bay Area. Patients were identified through cancer registry rapid case ascertainment, newly diagnosed from 1995-1999 and followed-up through 2008. Autoantibody levels were analyzed as continuous and grouped (quartiles) variables. Multivariable unconditional logistic regression was used to compute odds ratios (OR) as estimates of autoantibody levels associated with disease status. Kaplan-Meier product limit estimates and multivariable Cox proportional hazards regression were used to assess autoantibody levels associated with case survival duration.
RESULTS
Cases had higher levels of CTDSP1 (P=0.004), MAPK9 (P=0.0002), and NR2E3 (P≤0.0001) autoantibodies than controls (4th vs. 1st quartile:CTDSP1 OR=1.7, MAPK9 OR=2.5, NR2E3 OR=4.0). High BMI and tobacco use were associated with levels in controls but were not statistical confounders. High CTDSP1 levels were somewhat associated with better survival (HR=0.77, P=0.07).
CONCLUSIONS
Combined with previous results, our study contributes evidence that cancer-related host immune-response factors may be useful diagnostic screening tools and prognostic indicators for pancreatic cancer. Further studies are needed to critically assess the value of autoantibody panels to TAAs in diagnostic screening, prognosis and immunotherapy of pancreatic and other cancers.
Keywords: Pancreatic cancer, autoantibodies, antigens, biomarkers, case-control
Pancreatic cancer is among the most fatal cancers diagnosed in adult men and women in the U.S. Each year a similar number of newly diagnosed pancreatic cancer cases and deaths occur and the number of new cases continues to increase (1). Recently published data suggest that pancreatic cancer develops slowly over many years (2); however, most patients are diagnosed with late stage disease that is refractory to current therapies. Vague and non-specific symptoms that are consistent with other benign gastrointestinal conditions are likely to contribute to the typically late diagnosis. Increasing age, smoking, diabetes, obesity, heavy alcohol consumption, family history and several rare genetic syndromes are known risk factors for pancreatic cancer but explain little of the disease incidence. Pancreatic cancer, like brain cancers and lymphoma is clearly associated with allergies and allergic conditions (3-6), indicative of an interaction between the immune system and the cancer. Currently used biomarkers, CA19-9 and CEA, have high false-positive rates for pancreatic cancer and while new imaging technologies are helpful they tend to identify more advanced disease. Biomarkers are critically needed to better understand the etiology of this disease, to identify new therapeutic targets and to develop early detection tests to reduce incidence and improve patient prognosis.
Identification of sensitive and specific biomarkers that can be screened using biospecimens obtained through minimally invasive methods (i.e. peripheral blood) has been challenging. Autoantibodies to tumor-associated antigens (TAAs) have been reported for a number of other cancers [ovarian (7-9), breast (10), lung (11), hepatocellular (12), colorectal(13-15)]. Originally explored for development of immunogenic cancer vaccines, autoantibodies to TAAs have more recently been studied for their potential as biomarkers for cancer screening as they may be present in serum months to years before the cancer is symptomatic (16). Specific autoantibodies have been associated with several cancers or with non-cancer conditions whereas others have shown promise as biomarkers for specific types of cancer (17). Further data show that because cancer is a heterogeneous disease, and those with cancer respond to their own tumors in an individual, HLA-restricted fashion, the frequency of specific autoantibodies to TAAs is only about 30% (18).
In addition to their potential role as diagnostic markers, there is some evidence to suggest that autoantibodies to tumor-associated antigens may be useful prognostic or clinical indicators for cancer including ovarian, lung and breast(19-22). While poor clinical response and reduced survival in platinum resistant/refractory ovarian cancer was observed for patients with high serum anti-MUC1 antibody levels(22), separate studies showed improved survival or clinical prognosis with detectable serum autoantibodies to p53 in serous ovarian cancer patients(20), to endostatin in metastatic breast cancer patients(21) and to alpha-2-glycoprotein 1, zinc (AZGP1, a protein overexpressed in smokers) in early stage lung adenocarcinoma patients(19). Further, results also suggest that a specific marker may be useful for diagnosis, prognosis, or both diagnosis and prognosis, emphasizing the importance of separately evaluating serum autoantibodies for use in diagnostic and prognostic biomarker panels.
Given the current evidence, a panel of autoantibodies will be needed to provide the level of sensitivity and specificity necessary for an effective screening tool. Recent intensive screens for autoantibodies to pancreatic cancer have produced several candidates; we selected 3 promising biomarkers [CTDSP1(23), MAPK9 (8), NR2E3 (8)] to explore their association with pancreatic cancer and pancreatic cancer survival in our San Francisco Bay Area population-based epidemiological case-control study.
Materials and Methods
Study Population
Serum from 300 cases and 300 controls in our large population-based case-control pancreatic cancer study (532 cases, 1701 controls) was analyzed for tumor autoantibodies to carboxy-terminal domain, RNA polymerase II, polypeptide A small phosphatase 1, (SCP-1) formally known as CTDSP1, mitogen-activated protein kinase 9 (MAPK9) and nuclear receptor subfamily 2, group E, member 3 (NR2E3) that were selected based on published results suggesting their potential as pancreatic cancer biomarkers (8, 23). The parent study population and design have been published previously (4, 24). Briefly, eligible patients were identified using the Greater Bay Area Cancer Registry rapid case ascertainment, were diagnosed with incident pancreatic adenocarcinoma from 1994-1999, were between 21 and 74 years of age at diagnosis, residents of six San Francisco Bay Area counties, alive at first contact and able to compete and interview in English. Additional out-of –area cases were identified through the University of California. Controls from the same catchment area were identified using random-digit-dial methods and were frequency-matched to cases by age in 5-year groups, sex and county of residence. All participants provided written consent and completed interviewer administered in-person interviews using a structured questionnaire (participation rates 67% cases, 67% controls). Blood specimens were obtained from 309 cases (68% participation) and 964 controls (59% participation) who were eligible for the optional laboratory portion of the study (no portacath in place, Bay Area resident) and who provided separate consent. Patient clinical data were obtained from SEER abstracts and interviews. All cases were followed-up through December 2008 using active and passive methods to ascertain vital status and date of death(25). Median survival for all study patients was 10.1 months (interquartile range, 12.2 months). The study was approved by the University of California Committee on Human Research.
Measurement of Serum Autoantibody Levels
Autoantibody targets were produced as recombinant GST-tagged proteins in cell-free wheat germ extracts (Abnova, Taipei, Taiwan). Proteins were purified on glutathione columns, and GST tags removed by proteolytic digestion and further purified using size exclusion chromatography. Twenty-five μg protein was attached to carboxylated magnetic Luminex microspheres using a labeling kit (Bio-Rad, Hercules, CA). Human serum albumin (Sigma catalog A3782) was used as a control for nonspecific binding (serum “matrix effect”), and Varicella Zoster protein used as a positive control (Fitzgerald, Acton MA; catalog 30R-AV004). Five separate beads were therefore used in multiplex. Incubation and washes were performed as follows: Sera were diluted and incubated in 150 μl assay buffer with 106 labeled beads for 2 hours at room temperature with shaking, followed by three washes in wash buffer (Bio-Plex automated wash station). Secondary biotin-labeled mouse anti-human IgG (BD Biosciences, catalog 555869) diluted 1:500 in 100 μl detection antibody diluent (Bio-Rad) was incubated for 1 hour with shaking, followed by two washes in wash buffer. Assays were built by performing limiting dilutions of test sera in assay buffer (Bio-Rad) to determine the assay titre for each target protein. A 1:50 dilution of sera:sample diluent [PBS+10%FBS+2.5%CBS-K(Millipore)] was determined to be optimal, and all assays were run singly, paired, and together on a test series of sera to determine cross-reactivity. Lack of cross-reactivity interaction was confirmed. The pancreatic cancer sera case-control series were randomized, run in duplicates, and normalized across all plates to median values of total combined autoantibody levels on each plate. Standard reference samples also were run on each plate to confirm assay consistency.
Serum autoantibody levels were examined as the log-transformed values of the ratio of the antibody measure to the bovine serum albumin (BSA) level and dichotomized based on the 75th percentile. A discrete variable representing the total number (0 to 3) of autoantibodies with titer levels in the highest quartile was created to assess the total effect of autoantibodies combined. Variables also were created to explore the specific two-way combinations of ‘high’ levels (per dichotomized variables).
Statistical Analysis
Data were analyzed using SAS 9.2 (SAS Institute, Cary NC). Wilcoxon rank-sum and Kruskal-Wallis tests were used in univariate analyses among controls to assess the association between autoantibody levels and potential confounders including sex, education, obesity, tobacco use, allergies and diabetes. Spearman rank tests were used for pair-wise correlations among autoantibody levels, demographic, clinical and epidemiological factors. Odds ratios (OR) were computed as estimates of the relative risk of pancreatic cancer related to autoantibody levels in age- and sex-adjusted multivariable logistic models. Measures of sensitivity (true positives/ [true positives + false positives]) and specificity (true negatives/ [true negatives + false negatives]), and area under the receiver operator characteristic (ROC) curve (plotted as sensitivity vs. 1- specificity) were computed for each autoantibody. Area under the curve (AUC) for each autoantibody also was tested to determine differences from chance. Kaplan-Meier estimates and log-rank tests were used to evaluate survival probabilities for categories of autoantibody levels and cancer stage, and between autoantibody levels and initial cancer treatment. Hazard ratios (HR) for the association between autoantibody level and patient survival measured in days from diagnosis to death were computed using multivariable Cox proportional hazards models where those alive or lost to follow-up were considered censored. Potential confounding by known clinical prognostic factors including stage at diagnosis and initial treatment type also were evaluated. All models were adjusted for age and sex. All statistical tests were considered statistically significant for a two-sided p< 0.05 and borderline or somewhat statistically significant for 0.05 ≤ p ≤ 0.10.
Results
Demographic and risk factor characteristics of the analyzed population are presented in Table 1. Approximately 46% of cases and of controls were women. Both cases and controls ranged in age from 32 to 85 years at diagnosis/interview with a mean age of 64.5 years (data not shown). Cases were more likely to be smokers and overweight, and somewhat less likely to have had allergies. Physician diagnosed diabetes of ≥5 years duration was similar between cases and controls. Serum autoantibody levels (anti-CTDSP1, anti-MAPK9, and anti-NR2E3 referred to as “CTDSP1,” “MAPK9,” and “NR2E3” hereafter) were higher in cases than controls (CTDSP1 P =0.0085, MAPK9 and NR2E3 P <0.0001, Wilcoxon rank-sum test).
Table 1.
Distribution of covariates in analyzed case-control population and covariate association with serum autoantibody levels in control participants
| Factor | NR2E3 | MAPK9 | CTDSP1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case N=300 | Control N=300 | Case | Control | Case | Control | Case | Control | |||||||
| n (%) | n ( %) | 50% | 75% | 50% | 75% | 50% | 75% | 50% | 75% | 50% | 75% | 50% | 75% | |
| Sex | ||||||||||||||
| Men | 163 (54) | 162 (54) | 0.79 | 0.99 | 0.75 | 0.84 | 0.80 | 0.90 | 0.77 | 0.85 | 1.09 | 1.30 | 1.03 | 1.26 |
| Women | 137 (46) | 138 (46) | 0.84 | 1.10 | 0.76 | 0.86 | 0.81 | 0.93 | 0.74 | 0.85 | 1.06 | 1.28 | 1.01 | 1.18 |
| P-value * | 0.75 | 1.0 | 0.26 | |||||||||||
| Education (years) | ||||||||||||||
| <12 | 37 (12) | 22 (7) | 0.79 | 1.00 | 0.76 | 0.88 | 0.78 | 0.86 | 0.83 | 0.88 | 1.05 | 1.18 | 1.03 | 1.22 |
| 12 | 91 (30) | 65 (22) | 0.80 | 1.09 | 0.75 | 0.85 | 0.81 | 0.93 | 0.74 | 0.82 | 1.03 | 1.26 | 0.94 | 1.16 |
| >12 -<16 | 76 (26) | 82 (27) | 0.77 | 1.04 | 0.76 | 0.86 | 0.77 | 0.88 | 0.75 | 0.88 | 1.06 | 1.24 | 1.02 | 1.20 |
| 16+ | 96 (32) | 131 (44) | 0.86 | 1.10 | 0.75 | 0.84 | 0.82 | 0.91 | 0.76 | 0.84 | 1.15 | 1.43 | 1.07 | 1.28 |
| P-value * | 0.003 | 0.88 | 0.16 | 0.30 | ||||||||||
| Tobacco use | ||||||||||||||
| never | 85 (28) | 110 (37) | 0.78 | 1.13 | 0.75 | 0.83 | 0.78 | 0.92 | 0.74 | 0.82 | 1.04 | 1.19 | 0.97 | 1.16 |
| pipe/cigar | 10 (3) | 10 (3) | 0.85 | 1.00 | 0.77 | 0.80 | 0.78 | 0.88 | 0.79 | 0.87 | 1.22 | 1.38 | 0.99 | 1.16 |
| Former | 135 (45) | 143 (48) | 0.83 | 1.00 | 0.77 | 0.87 | 0.81 | 0.91 | 0.78 | 0.86 | 1.10 | 1.37 | 1.06 | 1.30 |
| current | 69 (23) | 34 (11) | 0.85 | 1.06 | 0.74 | 0.82 | 0.82 | 0.93 | 0.77 | 0.85 | 1.09 | 1.45 | 0.93 | 1.26 |
| P- value * | 0.002 | 0.70 | 0.67 | 0.01 | ||||||||||
| Obese | ||||||||||||||
| BMI<30 | 269 (90) | 273 (92) | 0.82 | 1.05 | 0.75 | 0.84 | 0.81 | 0.92 | 0.76 | 0.85 | 1.08 | 1.31 | 1.01 | 1.21 |
| BMI 30+ | 29 (10) | 25 (8) | 0.87 | 1.12 | 0.80 | 1.20 | 0.79 | 0.93 | 0.80 | 1.00 | 1.06 | 1.20 | 1.15 | 1.47 |
| P- value * | 0.57 | 0.04 | 0.10 | 0.21 | ||||||||||
| Overweight | ||||||||||||||
| BMI<25 | 154 (52) | 177 (59) | 0.82 | 1.02 | 0.75 | 0.84 | 0.81 | 0.91 | 0.74 | 0.84 | 1.07 | 1.30 | 0.98 | 1.18 |
| BMI 25+ | 144 (48) | 121 (41) | 0.83 | 1.10 | 0.77 | 0.88 | 0.80 | 0.92 | 0.78 | 0.87 | 1.09 | 1.29 | 1.08 | 1.27 |
| P- value * | 0.06 | 0.14 | 0.05 | 0.12 | ||||||||||
| Diabetes | ||||||||||||||
| No diabetes | 258 (94) | 271 (92) | 0.82 | 1.06 | 0.75 | 0.85 | 0.81 | 0.92 | 0.75 | 0.85 | 1.07 | 1.26 | 1.02 | 1.21 |
| Diabetes 5+ yrs | 18 (6) | 22 (8) | 0.86 | 1.13 | 0.78 | 0.84 | 0.82 | 0.93 | 0.82 | 0.87 | 1.13 | 1.40 | 1.07 | 1.31 |
| P- value * | 0.65 | 0.22 | 0.78 | 0.29 | ||||||||||
| Allergy history | ||||||||||||||
| No | 168 (56) | 148 (49) | 0.82 | 1.08 | 0.76 | 0.84 | 0.80 | 0.93 | 0.75 | 0.85 | 1.08 | 1.31 | 1.02 | 1.20 |
| Yes | 132 (44) | 152 (51) | 0.83 | 1.05 | 0.75 | 0.86 | 0.81 | 0.90 | 0.77 | 0.85 | 1.09 | 1.27 | 1.03 | 1.26 |
| P- value * | 0.10 | 0.63 | 0.46 | 0.40 | ||||||||||
| Plants/pollen** | 105 (35) | 113 (38) | 0.82 | 1.04 | 0.75 | 0.84 | 0.80 | 0.92 | 0.74 | 0.85 | 1.11 | 1.32 | 1.02 | 1.22 |
| Other allergen** | 25 (8) | 33 (11) | 0.86 | 1.32 | 0.78 | 0.84 | 0.81 | 1.00 | 0.78 | 0.85 | 0.95 | 1.19 | 1.03 | 1.16 |
| P- value * | 0.27 | 0.66 | 0.32 | 0.31 | ||||||||||
| No. of allergies | ||||||||||||||
| 0 | 168 (56) | 148 (49) | 0.83 | 1.05 | 0.76 | 0.86 | 0.81 | 0.90 | 0.77 | 0.85 | 1.09 | 1.27 | 1.03 | 1.26 |
| 1 | 67 (23) | 66 (22) | 0.84 | 1.27 | 0.75 | 0.84 | 0.80 | 1.00 | 0.74 | 0.85 | 1.08 | 1.36 | 1.00 | 1.20 |
| 2 | 33 (11) | 40 (14) | 0.75 | 0.95 | 0.76 | 0.83 | 0.78 | 0.90 | 0.74 | 0.85 | 1.09 | 1.21 | 1.08 | 1.21 |
| 3 or more | 30 (10) | 40 (14) | 0.86 | 1.02 | 0.75 | 0.86 | 0.82 | 0.91 | 0.74 | 0.85 | 1.05 | 1.32 | 1.04 | 1.24 |
| P- value * | 0.34 | 0.50 | 0.44 | 0.61 | ||||||||||
H0: disease status is independent of the covariate; In controls only, H0: autoantibody level (grouped by 75th percentile) is independent of covariate
Referent is no allergy history
BMI= Body Mass Index (weight in kg/ height in m2); yrs= years; No.= number
Correlation among autoantibodies was variable (data not shown). CTDSP1 was weakly correlated with NR2E3 and MAPK9 (controls: spearman rho=0.44, 0.46; P <0.0001; cases: spearman rho=0.56, 0.55; P <0.0001, respectively). NR2E3 and MAPK9 were strongly positively correlated (controls: spearman rho=0.80; P <0.0001; cases: spearman rho=0.86; P <0.0001, respectively). There also was evidence among controls that level of NR2E3 was associated with obesity (BMI≥30), MAPK9 with overweight (BMI ≥25) and somewhat with obesity, and CTDSP1 with tobacco use (Table 1).
Blood samples were collected from patients a median of 3.3 months after diagnosis. Information about any treatment prior to blood collection also was collected. Blood was collected post-surgery for 97% of surgical patients (median 3.2 months), and post chemo-therapy for 49% of patients who reported chemotherapy (median 0.72 months). Autoantibody levels were not correlated with duration between treatment and blood draw or with duration between diagnosis and blood draw (Spearman rho statistics ranged from -0.08 to 0.09, all p-values >0.05). Autoantibody levels were not associated with clinical characteristics among cases (Table 2) although when autoantibodies were analyzed as dichotomized variables (75th percentile), borderline statistically significant associations were observed between initial treatment type and NR2E3 (P =0.08) and between vital status and CTDSP1 (P =0.08). Specifically, high NR2E3 levels were observed among a greater proportion of patients who underwent surgical resection relative to other initial therapies and. high CTDSP1 levels were observed in a greater proportion of patients alive at last follow-up (~8%) compared with those who had died (~2%).
Table 2.
Clinical characteristics of pancreatic cancer patients (N=300) associated with serum autoantibody level evaluated using 75th percentile cutpoint based on the population distribution
| 75th percentile cutpoint | |||
|---|---|---|---|
| Clinical Factor | NR2E3 <Q4/Q4 | MAPK9 <Q4/Q4 | CTDSP1 <Q4/Q4 |
| Tumor Stage | |||
| Local | 21 / 12 | 22 / 11 | 22 / 11 |
| Regional | 87 / 52 | 95 / 44 | 103 / 36 |
| Distant | 66 / 32 | 68 / 30 | 73 / 25 |
| Unstaged | 20 / 6 | 22 / 4 | 18 / 8 |
| Chi-square P-value | 0.53 | 0.39 | 0.79 |
| Initial Treatment | |||
| None/unknown/palliative | 72 / 36 | 77 / 31 | 79 / 29 |
| Chemo/radiation | 67 / 25 | 70 / 22 | 68 / 24 |
| Surgery | 55 / 41 | 60 / 36 | 69 / 27 |
| Chi-square p-value | 0.08 | 0.12 | 0.95 |
| Vital Status* | |||
| alive | 5 / 6 | 6 / 5 | 5 / 6 |
| dead | 189 / 96 | 201 / 84 | 211 / 74 |
| Fisher's exact P-value | 0.20 | 0.32 | 0.08 |
Followed-up through December 2008
Case-control Comparisons
Relatively consistent positive associations were observed between higher autoantibody levels and pancreatic cancer (Table 3). The odds of pancreatic cancer increased with increased autoantibody level assessed on a continuous scale (CTDSP1, P =0.004; MAPK9, P =0.0002; NR2E3, P <0.0001) and by population quartiles (CTDSP1, P for trend=0.04; MAPK9, P for trend=0.0002; NR2E3, P for trend<0.0001). Further, compared with those in the lowest quartile, participants with the highest CTDSP1, MAPK9 or NR2E3 levels had nearly 2 to 4-fold increased odds of pancreatic cancer (Table 3). When additional analyses were conducted to compare those in the highest quartile with all others, risk of pancreatic cancer was increased for those with the highest MAPK9 and NR2E3 levels (all P -values ≤0.0009). Tobacco use, overweight and obesity were neither confounders nor effect modifiers of the associations.
Table 3.
Association between pancreatic cancer and level of serum autoantibody, odds ratios (OR) and 95% confidence intervals (CI) from age and sex-adjusted logistic models
| Cases (N=300) | Controls (N=300) | OR | 95% CI | P-value | |
|---|---|---|---|---|---|
| CTDSP1 | |||||
| Q1 | 65 | 89 | 1.0 | ||
| Q2 | 77 | 70 | 1.5 | (0.96-2.4) | |
| Q3 | 77 | 75 | 1.4 | (0.90-2.2) | |
| Q4 | 81 | 66 | 1.7 | (1.1 -2.6) | |
| P trend | 0.04 | ||||
| MAPK9 | |||||
| Q1 | 61 | 91 | 1.0 | ||
| Q2 | 69 | 77 | 1.3 | (0.84-2.1) | |
| Q3 | 80 | 77 | 1.5 | (0.98-2.5) | |
| Q4 | 90 | 55 | 2.4 | (1.5 -3.9) | |
| P trend | 0.0002 | ||||
| NR2E3 | |||||
| Q1 | 50 | 89 | 1.0 | ||
| Q2 | 76 | 85 | 1.6 | (1.0 -2.5) | |
| Q3 | 71 | 79 | 1.6 | (1.0 -2.6) | |
| Q4 | 103 | 47 | 4.0 | (2.4 -6.5) | |
| P trend | <0.0001 | ||||
| CTDSP1 | |||||
| <Q4 | 219 | 234 | 1.0 | ||
| Q4 | 81 | 66 | 1.3 | (0.90-1.9) | |
| 0.15 | |||||
| MAPK9 | |||||
| <Q4 | 210 | 245 | 1.0 | ||
| Q4 | 90 | 55 | 1.9 | (1.3 -2.8) | |
| 0.0009 | |||||
| NR2E3 | |||||
| <Q4 | 197 | 253 | 1.0 | ||
| Q4 | 103 | 47 | 2.9 | (1.9 -4.3) | |
| <0.0001 | |||||
| No. of AAs with levels in Q4 | |||||
| 0 (none) | 158 | 198 | 1.0 | (referent) | |
| 1 of 3 | 55 | 55 | 1.2 | (0.81-1.9) | |
| 2 of 3 | 42 | 28 | 1.9 | (1.1 - 3.2) | |
| 3 of 3 | 45 | 19 | 3.0 | (1.7 -5.4) | |
| P trend | 1.4 | (1.2 -1.7) | <0.0001 | ||
* Autoantibody serum level fit as a continuous variable in age and sex-adjusted logistic model
Q4 = 4th quartile, AAs= autoantibodies
Sensitivity and AUC values were in the poor range (AUC: NR2E3=0.62, MAPK9=0.59, CTDPS1=0.56, data not shown). However, all AUC values were different from chance (AUC for chance=0.50, all P ≤0.01). Also, the AUC for NR2E3 differed from MAPK9 (P =0.04) and CTDSP1 (P =0.01) whereas AUC for MAPK9 and CTDSP1 did not differ (P =0.19).
Survival Analysis
The survival duration for the 300 patients (median= 10.5 months) included in these analyses did not differ from the total case population (10.1 months, log-rank p=0.36) or from that of the eligible patients who did not provide a blood sample (median 9.7 months, log-rank p=0.07) . Further, for the 300 patients included in these analyses, survival was longest for those who had had surgical resection (palliative or no treatment, 8.6 months; chemotherapy and/or radiation, 9.8 months; surgical resection, 17.7 months). Among the 16 long-term survivors (>=5 years) included in these analyses, median survival was 111.2 months (IQR, 42.1 months).
Patients with the highest autoantibody levels consistently had longer median survival with borderline statistically significant associations observed for CTDSP1 and NR2E3 levels (log-rank P=0.05, P=0.07; Table 4). Analyses of autoantibody combinations showed that cases with more than one autoantibody at a high level had longer survival (log rank: high MAPK9 and high NR2E3, P =0.05; high CTDSP1 and high NR2E3, P =0.04; high CTDSP1 and high MAPK9, P =0.03; all three high P =0.02, data not shown). Correlation between autoantibody levels and survival was poor (all Spearman rho <0.09, all p-values >0.15, data not shown).
Table 4.
Survival distributions associated with autoantibody levels stratified by stage and initial treatment. Log-rank test comparison of survival distributions by grouped level (4th quartile [Q4]) and, hazard ratios (HR) and 95% confidence intervals (CI) from age- and sex-adjusted Cox-proportional hazards models
| Clinical Factor | Autoantibody | <Q4 N Cases (days) | Q4 N Cases (days) | p-value* | HR | 95% CI | HR | 95%CI** |
|---|---|---|---|---|---|---|---|---|
| N (med. survival) | N (med. survival) | |||||||
| CTDSP1 | 216 (313) | 80 (345) | 0.05 | 0.77 | 0.59-1.01 | 0.85 | 0.60-1.2 | |
| MAPK9 | 207 (305) | 89 (388) | 0.16 | 0.90 | 0.69-1.2 | 0.84 | 0.46-1.5 | |
| NR2E3 | 194 (301) | 102 (387.5) | 0.07 | 0.86 | 0.66-1.1 | 0.91 | 0.66-1.3 | |
| Stage | ||||||||
| CTDSP1 | ||||||||
| local | 22 (420.5) | 11 (533) | 0.28 | 0.76 | 0.31-1.9 | 0.95 | 0.23– 3.9 | |
| regional | 103 (391) | 36 (417) | 0.23 | 0.72 | 0.52-1.1 | 0.88 | 0.54– 1.4 | |
| distant | 18 (297.5) | 8 (334.5) | 0.28 | 0.81 | 0.51-1.3 | 0.82 | 0.44– 1.5 | |
| unknown | 73 (221) | 25 (314) | 0.89 | 1.3 | 0.50-3.1 | 1.7 | 0.48– 6.1 | |
| MAPK9 | ||||||||
| local | 22 (343.5) | 11 (515) | 0.27 | 0.81 | 0.33-2.0 | 0.57 | 0.07–4.9 | |
| regional | 95 (343) | 44 (430) | 0.42 | 0.87 | 0.60-1.3 | 0.72 | 0.31–1.7 | |
| distant | 68 (330.5) | 30 (225.5) | 0.54 | 2.4 | 0.70-8.0 | 1.4 | 0.44–5.1 | |
| unknown | 22 (224.5) | 4 (238) | 0.35 | 0.98 | 0.63-1.5 | 11 | 1.06- 113 | |
| NR2E3 | ||||||||
| local | 21 (388) | 12 (538.5) | 0.10 | 0.65 | 0.25-1.7 | 0.77 | 0.23–2.6 | |
| regional | 87 (336) | 52 (443.5) | 0.35 | 0.85 | 0.60-1.2 | 0.85 | 0.55–1.3 | |
| distant | 6 (370.5) | 32 (242) | 0.80 | 1.2 | 0.74-1.8 | 1.5 | 0.77–8.2 | |
| unknown | 20 (226.5) | 6 (256.5) | 0.95 | 1.1 | 0.38-3.5 | 2.4 | 0.73–8.2 | |
| Initial Treatment | ||||||||
| CTDSP1 | ||||||||
| None/palliative | 79 (255) | 29 (272) | 0.96 | 1.1 | 0.68–1.6 | 1.1 | 0.58–2.1 | |
| Chemo/radiation | 68 (291) | 24 (350.5) | 0.04 | 0.64 | 0.39–1.06 | 0.75 | 0.40–1.4 | |
| Surgical resection | 69 (515) | 27 (562) | 0.11 | 0.63 | 0.38–1.04 | 0.70 | 0.39–1.3 | |
| MAPK9 | ||||||||
| None/palliative | 77 (262) | 31 (255) | 0.85 | 1.05 | 0.69–1.6 | 1.8 | 0.65–4.7 | |
| Chemo/radiation | 70 (282) | 22 (361) | 0.21 | 0.84 | 0.50–1.4 | 0.76 | 0.25–2.3 | |
| Surgical resection | 60 (549) | 36 (538.5) | 0.78 | 0.95 | 0.60–1.5 | 0.64 | 0.21–1.9 | |
| NR2E3 | ||||||||
| None/palliative | 72 (256.5) | 36 (285) | 0.45 | 0.86 | 0.57 – 1.3 | 1.2 | 0.73 – 1.9 | |
| Chemo/radiation | 67 (282) | 25 (339) | 0.37 | 0.95 | 0.57 – 1.6 | 0.83 | 0.45 – 1.5 | |
| Surgical resection | 55 (589) | 41 (515) | 0.61 | 0.91 | 0.58 – 1.4 | 0.83 | 0.46 – 1.5 |
Log-rank test
For serum autoantibody level as a continuous variable
Adjustment for known prognostic factors, stage at diagnosis or initial treatment, had little effect on the magnitude and direction of HRs although most p-values were increased (Table 4). In stratified analyses, the greatest survival differences by autoantibody level were observed in patients who typically have better prognosis, those with localized disease and those who had surgical resection (Figure 1), although results were not statistically significant. Formal tests of statistical interaction between these prognostic factors and autoantibody levels were largely null. A suggestive association between MAPK9 level and survival by stage (P-interaction=0.08) was likely driven by patients with unstaged disease whose risk of dying increased nearly 11-fold for each unit increase in MAPK9 level. Further analyses of autoantibodies mutually adjusted for in Cox models that included age, sex and stage, showed the best fitting model per AIC and -2 log likelihood statistics, was that for CTDSP1 alone. No other associations were observed between survival and autoantibody levels on a discrete or continuous scale.
Figure 1.
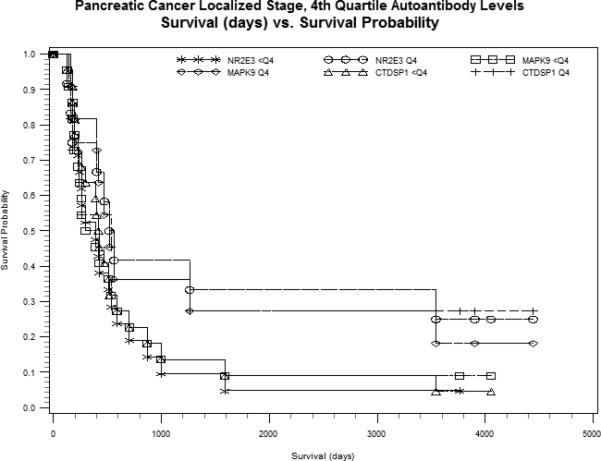
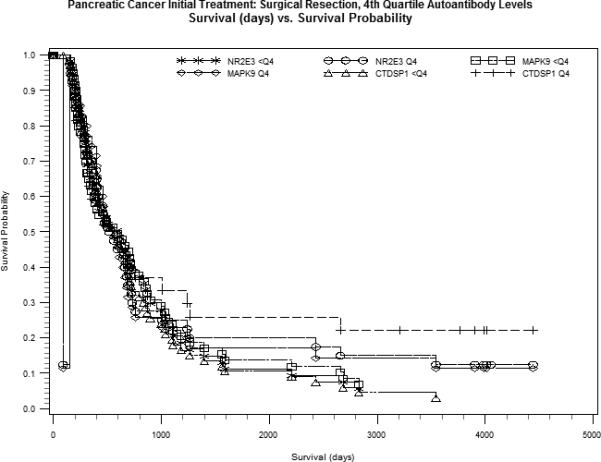
Kaplan-Meier estimates for serum autoantibody levels <4th quartile (<Q4) versus 4th quartile (Q4) for CTDSP1, MAPK9 and NR2E3, plotted as survival probability (y-axis) versus survival in days (x-axis). A. Grouped serum autoantibody levels within localized disease. B. Grouped serum autoantibody levels among surgical resection patients.
Discussion
To our knowledge, this study is the first to assess previously identified autoantibodies to TAAs among population-based pancreatic cancer patients and controls. Our results showed that serum levels of autoantibodies to the TAAs, MAPK9, CTDSP1 and NR2E3, were statistically significantly higher among cases than controls. Despite these strong associations, measures of sensitivity, specificity and AUC did not attain levels necessary for consideration of these biomarkers as diagnostic screening tools. We also found that combinations of high serum autoantibody levels were more strongly associated with case status than individual markers, providing support for the idea that multiple biomarkers are needed to best differentiate persons with and without pancreatic cancer. Although clinical prognostic factors were not related to biomarker levels, patterns of association within initial treatment categories and disease stage suggested that patients with high levels may have improved survival, especially high CTDSP1 in patients who had surgical resection and high NR2E3 in patients with localized disease. Given small cell sizes and multiple testing however, further assessment is warranted of these and other autoantibodies as potential markers of survival in larger patient populations.
Several studies have considered the autoantibody repertoire in pancreatic cancer (26-36). We chose three targets of the many candidates identified for the current study as a proof of principle for our method: a multiplex bead-based immunoassay using wheat-germ expressed proteins. MAPK9 and NR2E3 were the top 2 of 15 antigens identified to be most immunogenic in pancreatic cancer cases in a study identifying autoantibodies in an agnostic fashion from 9,000 target baculovirus-expressed antigens (8). The autoantibodies were validated against ELISA in the sera of 60 patients, most with stage IIB (regional) and IV (metastatic) disease at the time of surgery (8). A unique algorithm was used to establish a threshold cutpoint that would increase the test specificity and to compute an intensity score. Because their approach did not include typical estimates of sensitivity, specificity or AUC it is not possible for us to directly compare results across studies or to easily determine whether identified autoantibodies to TAAs would be appropriate for diagnostic screening.
Cancer testis (CT) antigens are limited to cancer and germline cells and therefore are thought to be ideal markers for cancer screening. In the published CT antigen studies (23, 37), CTDSP1 was the only CT antigen highly expressed in pancreatic cancer patients. In one study that used a eukaryotic cDNA expression system in yeast that allowed for detection of antigens that undergo post-translation modifications, an immune response to CTDSP1 was detected in nearly 15% of patients (compared with 27% in the 4th quartile in our population) with all of these patients having tumors with a TNM classification of pT3-4 (23). Furthermore, other clinical characteristics, stage, age and sex, were not associated with CTDSP1(23), findings that are similar to the results from our analyses. In contrast, the authors reported that no seroreactivity was detected in the healthy controls (n=48) or pancreatitis patients (n=18)(23), which contrasts with data from our study where 22% of controls had levels above the 75th percentile cutpoint. Overall, the consistency in results suggests that CTDSP1 may be a good candidate component of a biomarker screening panel for pancreatic cancer.
Autoantibodies to TAAs are thought to be part of cancer immune surveillance and may be a result of immune system function related to several factors including over-expression of a protein, response to a viral protein (i.e. to EBV, HPV, KSHV), to an oncogenic or tumor suppressor protein (i.e. to c-MYC, HER2/Neu, p53), or to post-translational protein modifications.(38) Recent work has focused on their potential as early diagnostic markers of cancer development, as markers of progression/prognosis and for their potential use in identifying targets for therapy and cancer vaccines. Many of the autoantibodies to TAAs have been found to be present in high titers across cancers and for other chronic inflammatory diseases. Results from studies of ovarian (39), breast (10), colon (13), lung (11), hepatocellular (12, 40), meningioma (41), and prostate (42) cancer have shown that good sensitivity and specificity can be attained by using a panel of autoantibody markers for cancer screening. The few studies that assessed autoantibodies and pancreatic cancer explored a number of biomarkers but no study formally evaluated the performance of a panel of select autoantibodies to correctly discriminate patients from controls. As noted in a recent review (43), autoantibody biomarker studies have tended to focus on known oncogenic proteins including p53, c-myc, Her-2/Neu and the CT antigen NY-ESO-1. There has been little study overlap or validation of discovered markers especially those that may be unique to a specific cancer. Further, results are sometimes difficult to evaluate across studies as a variety of methods have been used to establish cutpoints and thresholds, and to analyze data. Finally, recent work has highlighted how a high background rate of humoral autoimmunity can negatively impact the overall sensitivity and specificity of screening tests that might use these biomarkers.(44) The significance of this finding in discovery and development of autoantibodies to TAAs as screening tools is difficult to determine as little is known of their role in tumorigenesis. Research is needed that will increase our understanding of whether autoantibodies promote, suppress, or only herald cancer development.
Many factors determine whether an individual will produce autoantibodies to specific tumor antigens, and whether levels of such autoantibodies have any relationship to the levels of particular antigens. Antibody specificity is governed in part by the interplay between tumor protein expression and an individual's capacity to present particular antigens to the immune system based on the highly polymorphic peptide binding pockets of the major histocompatibility proteins. As a pancreatic tumor grows and evolves, it alters expression of immunogenic antigens that elicit an anti-tumor response, likely altering the autoantibody repertoire over time. Because of these variables, an ideal autoantibody assay would encompass enough antigens to yield consistent and correct classification of individuals with different tumors and HLA genotypes, despite that an individual's autoantibodies may alter over time. Typically, a single serum sample will be available on any given individual, necessitating a robust multipoint assay. While the current study represents an important start, a future study would need to address a much larger antigen repertoire including possibly posttranslational modifications, as well as a robust analytical rubric.
Our use of the Greater Bay Area Cancer Registry rapid case-ascertainment helped us to identify cases shortly after diagnosis and diminish selection bias. However, because most patients are diagnosed with metastatic disease, the sickest patients are under-represented in our study population as suggested by the slightly greater median survival duration for case participants compared with registry statistics. Therefore our results may not pertain to very ill pancreatic cancer patients. Furthermore, blood samples were collected after treatment for many of our study patients. We found no evidence that levels of NR2E3, MAPK9 or CTDSP1 were correlated with duration between blood draw and treatment (surgery or chemotherapy) although earlier studies have shown treatment-related decline in antibodies to tumor antigens for some cancers(43, 45, 46). The relevance for the difference in our findings with those of previous studies is unclear but should be considered when interpreting our findings. Careful analysis of treatment effects on autoantibodies to TAAs in future large studies is needed.
Combined with previously published results from autoantibody studies of pancreatic and other cancers, our results contribute additional evidence that factors related to host immune response to cancer hold promise as diagnostic screening tools and prognostic indicators. The search for highly sensitive and specific markers for pancreatic cancer is challenging. Standardized laboratory and statistical methods for autoantibody discovery and evaluation, replication studies to confirm findings, and studies that will elucidate autoantibody function are needed to critically assess the value of panels of autoantibodies to TAAs in diagnostic screening, prognosis and immunotherapy of pancreatic and other cancers.
Acknowledgments
Funding Sources: This work was supported in part by NIH-NCI grants (R01CA1009767, R01CA109767S1, R01CA59706, R03CA108370, R03CA89726, R01CA109745, P01ES018172, R01CA52689, and P50CA097257) and by the Joan Rombauer Pancreatic Cancer Research Fund. Collection of cancer incidence data was supported by the California Department of Public Health as part of the statewide cancer reporting program; the NCI's SEER Program under contract N01-PC-35136 awarded to NCCC; and the CDC's National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute.
Footnotes
P.M. Bracci and J. Wiemels contributed equally to this work.
Financial disclosures: The authors have no potential financial conflicts.
References
- 1.Altekruse S, Kosary C, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2007. National Cancer Institute; Bethesda, MD: 2010. based on November 2009 SEER data submission, posted to the SEER web site 2010.
- 2.Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2011;467:1114–7. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eppel A, Cotterchio M, Gallinger S. Allergies are associated with reduced pancreas cancer risk: A population-based case-control study in Ontario, Canada. Int J Cancer. 2007;121:2241–5. doi: 10.1002/ijc.22884. [DOI] [PubMed] [Google Scholar]
- 4.Holly EA, Eberle CA, Bracci PM. Prior history of allergies and pancreatic cancer in the San Francisco Bay area. Am J Epidemiol. 2003;158:432–41. doi: 10.1093/aje/kwg174. [DOI] [PubMed] [Google Scholar]
- 5.Maisonneuve P, Lowenfels AB, Bueno-de-Mesquita HB, et al. Past medical history and pancreatic cancer risk: Results from a multicenter case-control study. Ann Epidemiol. 2010;20:92–8. doi: 10.1016/j.annepidem.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Olson SH, Orlow I, Simon J, et al. Allergies, variants in IL-4 and IL-4R alpha genes, and risk of pancreatic cancer. Cancer Detect Prev. 2007;31:345–51. doi: 10.1016/j.cdp.2007.10.002. Epub 2007 Nov 26. [DOI] [PubMed] [Google Scholar]
- 7.Wandall HH, Blixt O, Tarp MA, et al. Cancer biomarkers defined by autoantibody signatures to aberrant O-glycopeptide epitopes. Cancer Res. 2010;70:1306–13. doi: 10.1158/0008-5472.CAN-09-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gnjatic S, Ritter E, Buchler MW, et al. Seromic profiling of ovarian and pancreatic cancer. Proc Natl Acad Sci U S A. 2010;107:5088–93. doi: 10.1073/pnas.0914213107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S, Cho H, Nam EJ, et al. Autoantibodies against stress-induced phosphoprotein-1 as a novel biomarker candidate for ovarian cancer. Genes Chromosomes Cancer. 2010;49:585–95. doi: 10.1002/gcc.20769. [DOI] [PubMed] [Google Scholar]
- 10.Piurax E, Piura B. Autoantibodies to tailor-made panels of tumor-associated antigens in breast carcinoma. J Oncol. 2011 doi: 10.1155/2011/982425. Epub 2011 Mar 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyle P, Chapman CJ, Holdenrieder S, et al. Clinical validation of an autoantibody test for lung cancer. Ann Oncol. 2011;22:383–9. doi: 10.1093/annonc/mdq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang JY, Tan EM. Autoantibodies to tumor-associated antigens as diagnostic biomarkers in hepatocellular carcinoma and other solid tumors. Expert Rev Mol Diagn. 2010;10:321–8. doi: 10.1586/erm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedersen JW, Blixt O, Bennett EP, et al. Seromic profiling of colorectal cancer patients with novel glycopeptide microarray. Int J Cancer. 2011;128:1860–71. doi: 10.1002/ijc.25778. [DOI] [PubMed] [Google Scholar]
- 14.Chan CC, Fan CW, Kuo YB, et al. Multiple serological biomarkers for colorectal cancer detection. Int J Cancer. 2010;126:1683–90. doi: 10.1002/ijc.24912. [DOI] [PubMed] [Google Scholar]
- 15.Babel I, Barderas R, Diaz-Uriarte R, et al. Identification of tumor-associated autoantigens for the diagnosis of colorectal cancer in serum using high density protein microarrays. Mol Cell Proteomics. 2009;8:2382–95. doi: 10.1074/mcp.M800596-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson KS, LaBaer J. The sentinel within: exploiting the immune system for cancer biomarkers. J Proteome Res. 2005;4:1123–33. doi: 10.1021/pr0500814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan HT, Low J, Lim SG, et al. Serum autoantibodies as biomarkers for early cancer detection. Febs J. 2009;276:6880–904. doi: 10.1111/j.1742-4658.2009.07396.x. [DOI] [PubMed] [Google Scholar]
- 18.Casiano CA, Mediavilla-Varela M, Tan EM. Tumor-associated antigen arrays for the serological diagnosis of cancer. Mol Cell Proteomics. 2006;5:1745–59. doi: 10.1074/mcp.R600010-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albertus DL, Seder CW, Chen G, et al. AZGP1 autoantibody predicts survival and histone deacetylase inhibitors increase expression in lung adenocarcinoma. J Thorac Oncol. 2008;3:1236–44. doi: 10.1097/JTO.0b013e318189f5ec. [DOI] [PubMed] [Google Scholar]
- 20.Anderson KS, Wong J, Vitonis A, et al. p53 autoantibodies as potential detection and prognostic biomarkers in serous ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:859–68. doi: 10.1158/1055-9965.EPI-09-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bachelot T, Ratel D, Menetrier-Caux C, et al. Autoantibodies to endostatin in patients with breast cancer: correlation to endostatin levels and clinical outcome. Br J Cancer. 2006;94:1066–70. doi: 10.1038/sj.bjc.6603037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budiu RA, Mantia-Smaldone G, Elishaev E, et al. Soluble MUC1 and serum MUC1-specific antibodies are potential prognostic biomarkers for platinum-resistant ovarian cancer. Cancer Immunol Immunother. 2011;60:975–84. doi: 10.1007/s00262-011-1010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wadle A, Kubuschok B, Imig J, et al. Serological immune response to cancer testis antigens in patients with pancreatic cancer. Int J Cancer. 2006;119:117–25. doi: 10.1002/ijc.21744. [DOI] [PubMed] [Google Scholar]
- 24.Duell EJ, Holly EA, Bracci PM, et al. A population-based, case-control study of polymorphisms in carcinogen-metabolizing genes, smoking, and pancreatic adenocarcinoma risk. J Natl Cancer Inst. 2002;94:297–306. doi: 10.1093/jnci/94.4.297. [DOI] [PubMed] [Google Scholar]
- 25.Gong Z, Holly EA, Bracci PM. Survival in population-based pancreatic cancer patients: San Francisco Bay area, 1995-1999. Am J Epidemiol. 2011;174:1373–81. doi: 10.1093/aje/kwr267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamanaka Y, Suehiro Y, Fukui M, et al. Circulating anti-MUC1 IgG antibodies as a favorable prognostic factor for pancreatic cancer. Int J Cancer. 2003;103:97–100. doi: 10.1002/ijc.10801. [DOI] [PubMed] [Google Scholar]
- 27.Heller A, Zornig I, Muller T, et al. Immunogenicity of SEREX-identified antigens and disease outcome in pancreatic cancer. Cancer Immunol Immunother. 2010;59:1389–400. doi: 10.1007/s00262-010-0870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong SH, Misek DE, Wang H, et al. An autoantibody-mediated immune response to calreticulin isoforms in pancreatic cancer. Cancer Res. 2004;64:5504–10. doi: 10.1158/0008-5472.CAN-04-0077. [DOI] [PubMed] [Google Scholar]
- 29.Li C, Kim HY, Vuong H, et al. The identification of auto-antibodies in pancreatic cancer patient sera using a naturally fractionated Panc-1 cell line. Cancer Biomark. 2010;7:25–37. doi: 10.3233/CBM-2010-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li C, Simeone DM, Brenner DE, et al. Pancreatic cancer serum detection using a lectin/glyco-antibody array method. J Proteome Res. 2009;8:483–92. doi: 10.1021/pr8007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakatsura T, Senju S, Ito M, et al. Cellular and humoral immune responses to a human pancreatic cancer antigen, coactosin-like protein, originally defined by the SEREX method. Eur J Immunol. 2002;32:826–36. doi: 10.1002/1521-4141(200203)32:3<826::AID-IMMU826>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 32.Nakatsura T, Senju S, Yamada K, et al. Gene cloning of immunogenic antigens overexpressed in pancreatic cancer. Biochem Biophys Res Commun. 2001;281:936–44. doi: 10.1006/bbrc.2001.4377. [DOI] [PubMed] [Google Scholar]
- 33.Okada T, Akada M, Fujita T, et al. A novel cancer testis antigen that is frequently expressed in pancreatic, lung, and endometrial cancers. Clin Cancer Res. 2006;12:191–7. doi: 10.1158/1078-0432.CCR-05-1206. [DOI] [PubMed] [Google Scholar]
- 34.Patwa TH, Wang Y, Simeone DM, et al. Enhanced detection of autoantibodies on protein microarrays using a modified protein digestion technique. J Proteome Res. 2008;7:2553–61. doi: 10.1021/pr800023g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomaino B, Cappello P, Capello M, et al. Autoantibody signature in human ductal pancreatic adenocarcinoma. J Proteome Res. 2007;6:4025–31. doi: 10.1021/pr070281a. [DOI] [PubMed] [Google Scholar]
- 36.Tomaino B, Cappello P, Capello M, et al. Circulating autoantibodies to phosphorylated alpha-enolase are a hallmark of pancreatic cancer. J Proteome Res. 2010;10:105–12. doi: 10.1021/pr100213b. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz-Winnenthal FH, Galindo-Escobedo LV, Rimoldi D, et al. Potential target antigens for immunotherapy in human pancreatic cancer. Cancer Lett. 2007;252:290–8. doi: 10.1016/j.canlet.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Kobold S, Lutkens T, Cao Y, et al. Autoantibodies against tumor-related antigens: incidence and biologic significance. Hum Immunol. 2010;71:643–51. doi: 10.1016/j.humimm.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 39.Hudson ME, Pozdnyakova I, Haines K, et al. Identification of differentially expressed proteins in ovarian cancer using high-density protein microarrays. Proc Natl Acad Sci U S A. 2007;104:17494–9. doi: 10.1073/pnas.0708572104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Han KJ, Pang XW, et al. Large scale identification of human hepatocellular carcinoma-associated antigens by autoantibodies. J Immunol. 2002;169:1102–9. doi: 10.4049/jimmunol.169.2.1102. [DOI] [PubMed] [Google Scholar]
- 41.Ludwig N, Keller A, Heisel S, et al. Novel immunogenic antigens increase classification accuracy in meningioma to 93.84%. Int J Cancer. 2011;128:1493–501. doi: 10.1002/ijc.25467. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Yu J, Sreekumar A, et al. Autoantibody signatures in prostate cancer. N Engl J Med. 2005;353:1224–35. doi: 10.1056/NEJMoa051931. [DOI] [PubMed] [Google Scholar]
- 43.Reuschenbach M, von Knebel Doeberitz M, Wentzensen N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother. 2009;58:1535–44. doi: 10.1007/s00262-009-0733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nolen B, Winans M, Marrangoni A, et al. Aberrant tumor-associated antigen autoantibody profiles in healthy controls detected by multiplex bead-based immunoassay. J Immunol Methods. 2009;344:116–20. doi: 10.1016/j.jim.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angelopoulou K, Diamandis EP, Sutherland DJ, et al. Prevalence of serum antibodies against the p53 tumor suppressor gene protein in various cancers. Int J Cancer. 1994;58:480–7. doi: 10.1002/ijc.2910580404. [DOI] [PubMed] [Google Scholar]
- 46.Korangy F, Ormandy LA, Bleck JS, et al. Spontaneous tumor-specific humoral and cellular immune responses to NY-ESO-1 in hepatocellular carcinoma. Clin Cancer Res. 2004;10:4332–41. doi: 10.1158/1078-0432.CCR-04-0181. [DOI] [PubMed] [Google Scholar]


