Abstract
Background
Glycine is a major inhibitory neurotransmitter in the spinal cord, the concentration of which is regulated by two types of glycine transporters (GlyTs): GlyT1 and GlyT2. We hypothesized that the inhibition of GlyTs could ameliorate bladder overactivity and/or pain sensation in the lower urinary tract.
Objective
Investigate the effects of GlyT inhibitors on bladder overactivity and pain behavior in rats.
Design, setting, and participants
Cystometry was performed under urethane anesthesia in cyclophosphamide (CYP)–treated rats. In behavioral studies using conscious rats, nociceptive responses were induced by intravesical administration of resiniferatoxin (3 µM). Selective GlyT1 or GlyT2 inhibitors were administered intrathecally to evaluate their effects.
Measurements
Cystometric parameters, nociceptive behaviors (licking and freezing), and messenger RNA (mRNA) levels of GlyTs and glycine receptor (GlyR) subunits in the dorsal spinal cord (L6–S1) were measured.
Results and limitations
During cystometry in CYP-treated rats, significant increases in intercontraction interval and micturition pressure threshold were elicited by ALX-1393, a selective GlyT2 inhibitor, but not by sarcosine, a GlyT1 inhibitor. These effects were completely reversed by strychnine, a GlyR antagonist. ALX-1393 also significantly suppressed nociceptive behaviors in a dose-dependent manner. In sham rats, GlyT2 mRNA was expressed at a much higher level (23-fold) in the dorsal spinal cord than GlyT1 mRNA. In CYP-treated rats, mRNA levels of GlyT2 and the GlyR α1 and β subunits were significantly reduced.
Conclusions
These results indicate that GlyT2 plays a major role in the clearance of extracellular glycine in the spinal cord and that GlyT2 inhibition leads to amelioration of CYP-induced bladder overactivity and pain behavior. GlyT2 may be a novel therapeutic target for the treatment of overactive bladder and/or bladder hypersensitive disorders such as bladder pain syndrome/interstitial cystitis.
Keywords: Glycine, Glycine transporter, Overactive bladder, Rat, Spinal cord, Bladder pain syndrome
1. Introduction
Sensitization of bladder afferent pathways and a subsequent increase in sensory processing in the spinal cord have been proposed as important mechanisms inducing irritative symptoms associated with overactive bladder (OAB) [1,2] and/or pain symptoms in bladder hypersensitive disorders such as bladder pain syndrome (BPS)/interstitial cystitis (IC) [3]. Glycine, a major inhibitory neurotransmitter, is well known to have a role in the control of spinal nociceptive pathways [4] and lower urinary function in both physiologic and pathologic conditions [5–7]. The extracellular concentration of glycine at synapses is regulated by two types of Na+/Cl−-dependent glycine transporters (GlyTs): GlyT1 and GlyT2 [8]. GlyT1 is widely distributed in the central nervous system and predominantly expressed in glial cells nearby both excitatory and inhibitory neurons, while GlyT2 is specifically distributed in the spinal cord, cerebellum, and brainstem and localized in the presynaptic terminals of inhibitory glycinergic neurons [9,10]. Recent studies have suggested the therapeutic potential of GlyT inhibitors for the treatment of schizophrenia [11,12], cognitive disorders [13], and acute and chronic pain [14,15]. However, the effects of GlyT inhibitors on bladder overactivity and pain sensation in the lower urinary tract have not been clarified.
In this study, we examined the effects of selective GlyT inhibitors on bladder overactivity and pain behavior in rats in response to nociceptive stimuli in the bladder. We also examined changes in expression levels of GlyT and glycine receptor (GlyR) subunits in the lumbosacral spinal cord after bladder irritation.
2. Material and methods
2.1. Animals
Adult Sprague Dawley female rats (n = 131) were used (202–268 g). All experiments were conducted in accordance with institutional guidelines and approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
2.2. Cystometry
Seventy-four rats were divided into cyclophosphamide (CYP; 200 mg/kg, intraperitoneally treated) or sham (vehicle-treated) groups. After 48 h, rats were anesthetized with urethane (1.2 g/kg, subcutaneously), and a polyethylene catheter (PE-50; Clay Adams, Parsippany, NJ, USA) was inserted into the bladder through the dome after a laparotomy. Cystometry was performed by continuously infusing saline (0.04 ml/min) into the bladder. After baseline cystometrograms (CMGs) were obtained, drugs were administered intrathecally in a volume of 1 µl followed by 9-µl flush with saline via a polyethylene catheter (PE-10; Clay Adams), which was implanted into the subarachnoid space at the L6–S1 spinal cord level 2 d before cystometry. In the antagonist study, strychnine (a GlyR antagonist) was administered intrathecally following three voiding reflexes after administration of a GlyT2 inhibitor. As the cystometric parameters, intercontraction intervals (ICIs), maximum voiding pressure (MVP), baseline pressure, and pressure threshold (PT; ie, intravesical pressure just prior to the initiation of voiding bladder contraction) were measured and analyzed with Chart5 software (ADInstruments, Milford, MA, USA). Each individual parameter was calculated as the percent change after administration of drugs.
2.3. Quantification of messenger RNA for glycine transporters and glycine receptor subunits
A separate group of 10 rats was divided into CYP-treated or sham group without GlyT treatment (n = 5 each group). Forty-eight hours after administration of CYP or vehicle, the L6–S1 spinal cord and forebrain were harvested under isoflurane anesthesia. One µg of total RNA extracted from the forebrain or the dorsal half of L6–S1 spinal cord was reverse-transcribed into complementary DNA using the ThermoScript RT-PCR System (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s manual. Quantitative polymerase chain reaction (PCR) was performed with an Mx3000P Real-Time PCR System (Stratagene, La Jolla, CA, USA) in a 25-µl volume using SYBR Green PCR Master Mix (QIAGEN, Valencia, CA, USA).
2.4. Histology
The bladders from some sham and CYP-treated rats were removed 48 h after the treatments, then fixed in an ice-cold 4% paraformaldehyde solution containing 0.21% picric acid in 0.1 M phosphate buffer (PB) for 48 h and soaked overnight at 4°C in 0.1 M PB containing increasing concentrations of sucrose (10–30%). The frozen tissues were cut at 10-µm thickness (transverse sections) and stained with hematoxylin and eosin.
2.5. Nociceptive behavioral study
Thirty-nine rats were used for analyses of nociceptive behavior after bladder irritation, as we previously described [16]. Briefly, rats were acclimated in metabolic cages (Nalgene, Rochester, NY, USA) for 3 h. Then, each GlyT inhibitor was administered intrathecally at the level of L6–S1 spinal cord, and after 15 min, animals placed in a Bollman cage were instilled with resiniferatoxin (RTX; 3 µM, 0.3 ml) into the bladder via a temporally inserted urethral catheter (PE-50; Clay Adams) for 1 min. Thereafter, rats were immediately placed in metabolic cages, and both licking and freezing behaviors were scored during 5-s intervals for a period of 15 min in the metabolic cages.
2.6. Drugs
Sarcosine was purchased from Tocris (Ellisville, MO, USA). ALX-1393, cyclophosphamide monohydrate, strychnine hydrochloride, and RTX were purchased from Sigma-Aldrich (St Louis, MO, USA). The doses of sarcosine, ALX-1393, and strychnine were determined based on the results of previous somatic pain studies in rats [15,17].
2.7. Statistical analysis
All data are represented as the mean plus or minus standard error of the mean. Statistical significance was evaluated using the unpaired student t test or one-way analysis of variance (ANOVA) followed by Bonferroni multiple comparison test. P values <0.05 were considered significant.
3. Results
3.1. Effects of glycine transporter inhibitors (intrathecal) on cystometrogram parameters in sham and cyclophosphamide-treated rats
CYP-treated rats showed a significant decrease in ICI and significant increases in MVP, baseline pressure, and PT compared to sham rats (Table 1). They showed histologic changes and inflammatory signs such as inflammatory cell infiltration in the suburothelial layer and mucosal edema (Fig. 1).
Table 1.
Baseline cystometric parameters in rats with or without treatment of cyclophosphamide
| ICI, s | MVP, cm H2O | BP, cm H2O | PT, cm H2O | |
|---|---|---|---|---|
| Sham (n = 27) | 847 ± 52.2 | 32.6 ± 1.23 | 5.10 ± 0.333 | 9.29 ± 0.433 |
| CYP-treated (n = 22) | 353 ± 29.2** | 38.9 ± 2.67* | 10.3 ± 1.05** | 15.9 ± 1.61** |
ICI = intercontraction interval; MVP = maximum voiding pressure; BP = baseline pressure; PT = pressure threshold; CYP = cyclophosphamide.
p < 0.05.
p < 0.01 (unpaired student t test) compared to the sham group.
Fig. 1.
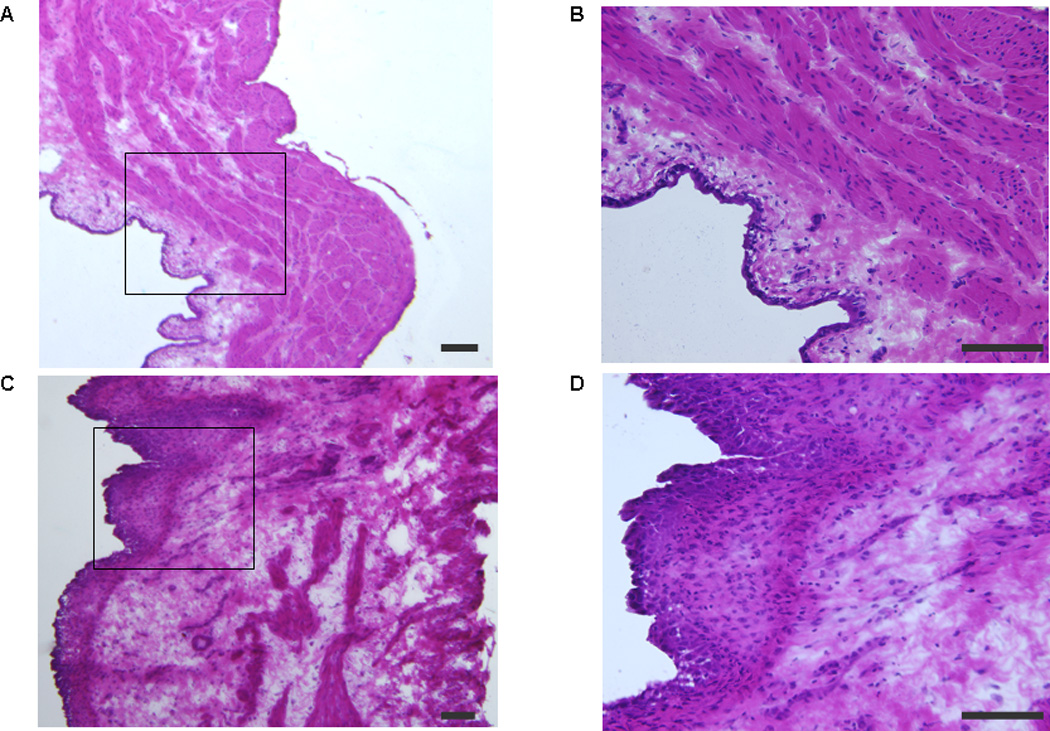
Photomicrographs of hematoxylin and eosin staining of the bladder wall in sham (saline) and cyclophosphamide (CYP)–treated rats: (a) sham rat bladder (×40); (b) sham rat bladder (×200); (c) CYP-treated rat bladder (×40); (d) CYP-treated rat bladder (×200). Figure 1b and 1d show the areas indicated by rectangles in Figure 1a and 1c, respectively, with higher magnification. Inflammatory cell infiltration in the suburothelial layer and tissue edema shown by an increase in the mucosal layer width are observed in the CYP-treated group (c and d). Scale bars: 100 µm.
Sarcosine (250 µg, intrathecal), a selective GlyT1 inhibitor, did not elicit significant changes in any CMG parameter in either sham or CYP-treated rats, although there was a tendency to increase ICI in CYP rats (p = 0.0922;Fig. 2a, 3a, and 4a–4h). In contrast, ALX-1393 (10 µg, intrathecal), a selective GlyT2 inhibitor, evoked a significant increase in PT by 36.5% and an insignificant increase in ICI in sham rats without changing MVP or baseline pressure (Fig. 2b and 4a–4d). In CYP-treated rats, ALX-1393 at a low dose (3 µg) clearly increased both ICI by 43.9% (p < 0.05) and PT by 19.1% (p < 0.01; Fig. 3b, 4e, and 4f). Moreover, ALX-1393 at a higher dose (10 µg) strongly suppressed the micturition reflex, producing a greater increase in PT and overflow incontinence in four of four CYP-treated rats (Fig. 3b). The inhibitory effects of ALX-1393 (3 µg) on ICI and PT in CYP rats were antagonized by strychnine (10 µg, intrathecal), a GlyR antagonist (p < 0.05 and p < 0.01, respectively; Fig. 3c and 5), indicating that ALX-1393-induced inhibition of bladder overactivity in CYP-treated rats is mediated by activation of GlyRs.
Fig. 2.
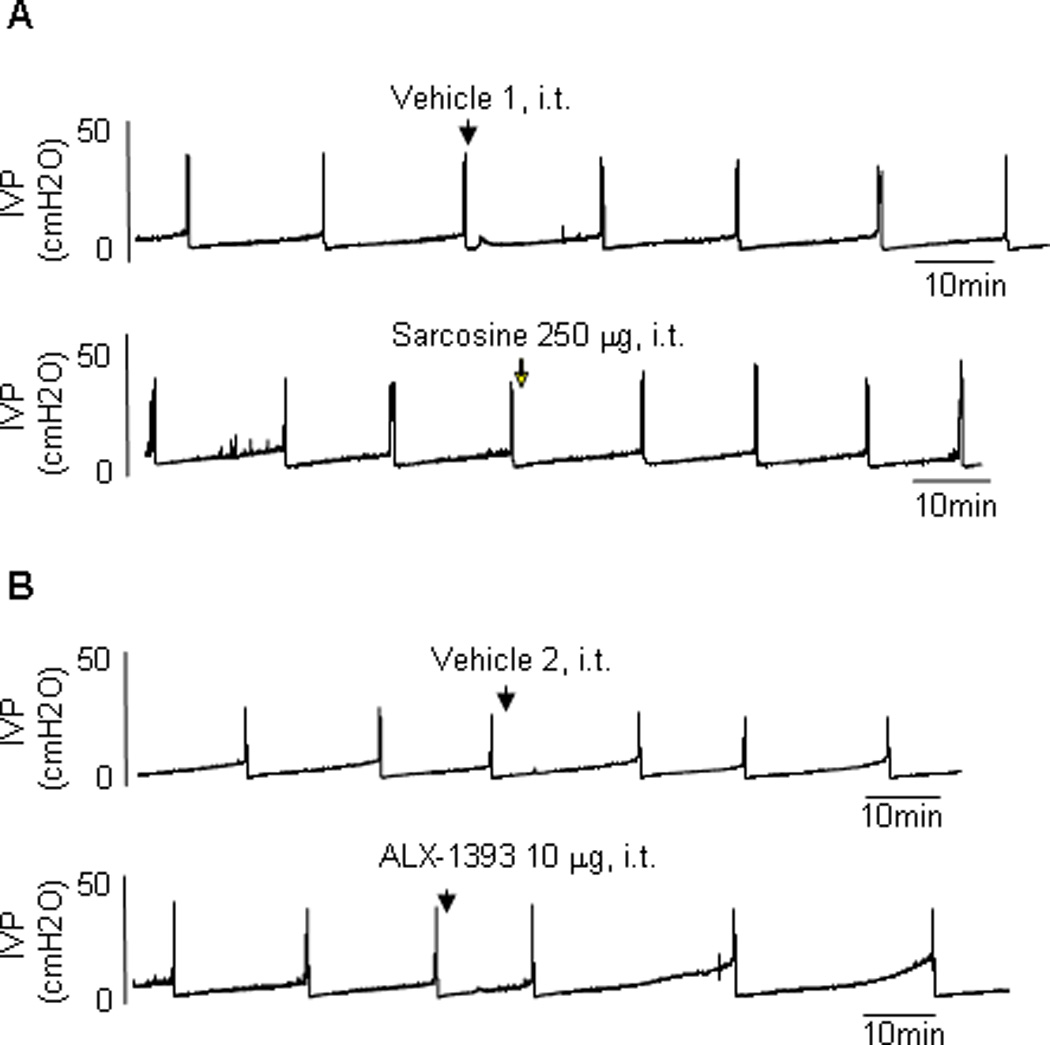
Typical traces showing the effect of intrathecal administration of (a) sarcosine (glycine transporter [GlyT] type 1 inhibitor) and (b) ALX-1393 (GlyT2 inhibitor) on continuous cystometrograms in sham rats. Arrows indicate the timing of drug administration. ALX-1393 showed some inhibitory effects on bladder activity (b), while sarcosine has no apparent effects (a). Vehicle 1: distilled water. Vehicle 2: 100% dimethyl sulfoxide.
IVP = intravesical pressure; i.t. = intrathecal.
Fig. 3.
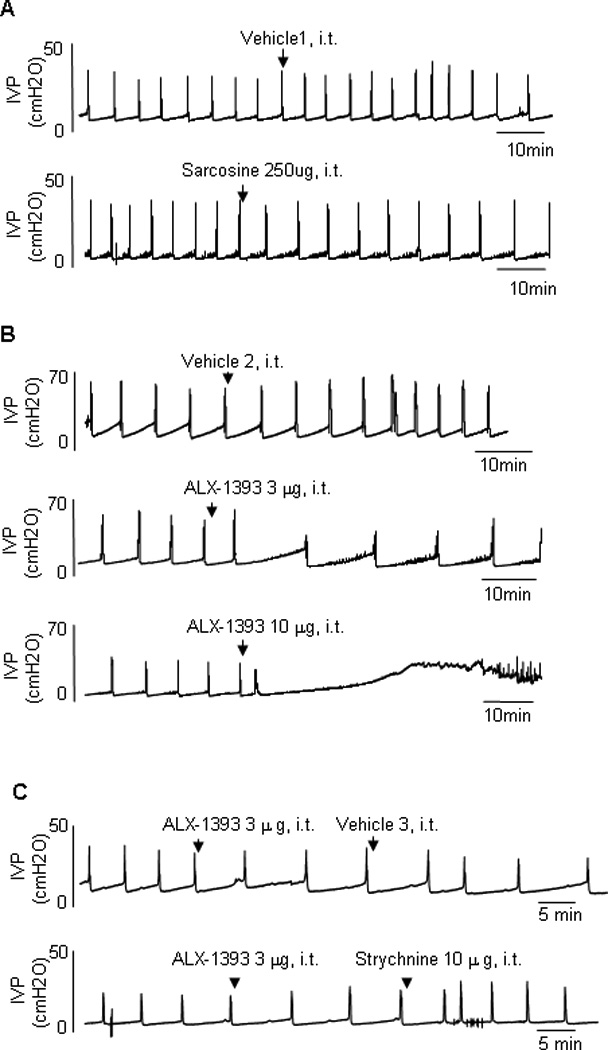
Typical traces showing the effect of intrathecal administration of (a) sarcosine, (b) ALX-1393, and (c) the combined application of ALX-1393 and strychnine, a glycine receptor antagonist, on continuous cystometrograms in cyclophosphamide (CYP)–treated rats. Arrows indicate the timing of drug administration. ALX-1393 showed inhibitory effects on CYP-induced bladder overactivity (b), while sarcosine has no apparent effects (a). Strychnine antagonized the inhibitory effect of ALX-1393 (c). Vehicle 1: distilled water. Vehicle 2: 100% dimethyl sulfoxide. Vehicle 3: saline).
IVP = intravesical pressure; i.t. = intrathecal.
Fig. 4.
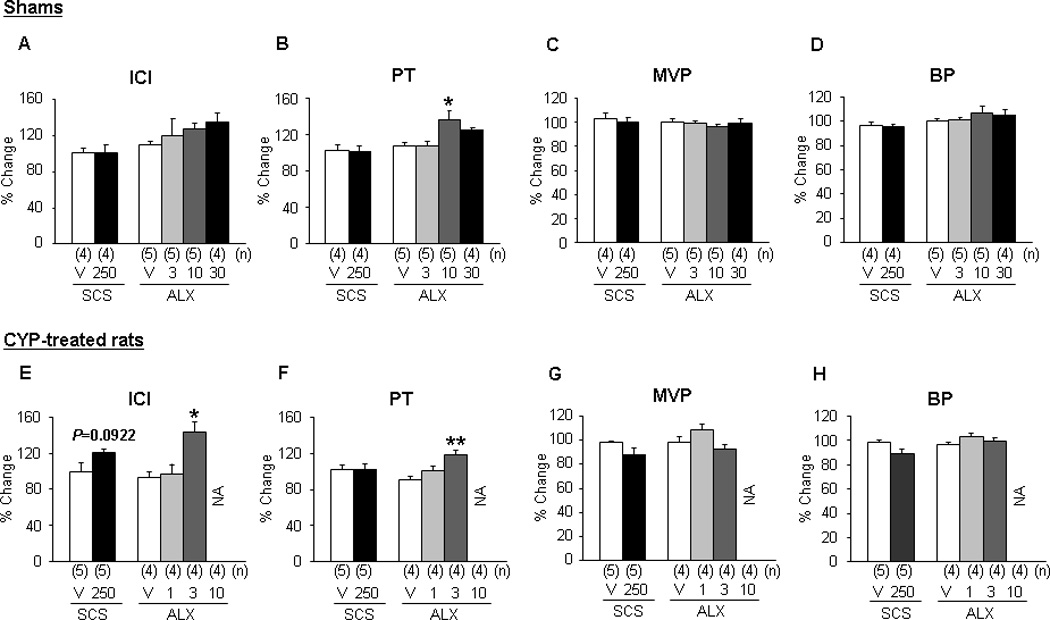
Effects of intrathecal administration of sarcosine and ALX-1393 on cystometric parameters in (a–d) sham and (e–h) cyclophosphamide (CYP)–treated rats. (a) Each bar represents the mean plus or minus standard error of the mean.
ICI = intercontraction interval; PT = pressure threshold; MVP = maximum voiding pressure; BP = baseline pressure; V = vehicle; SCS = sarcosine (in micrograms, intrathecal); ALX = ALX-1393 (in micrograms, intrathecal); CYP = cyclophosphamide; N/A: not analyzed (ie, the analysis of the parameter was impossible because of the strong suppression of the micturition reflex); ANOVA = analysis of variance.
* p < 0.05.
** p < 0.01 (compared to the vehicle group; one-way ANOVA followed by Bonferroni multiple comparison test); the number of animals (n) in each subgroup was shown in parentheses.
Fig. 5.
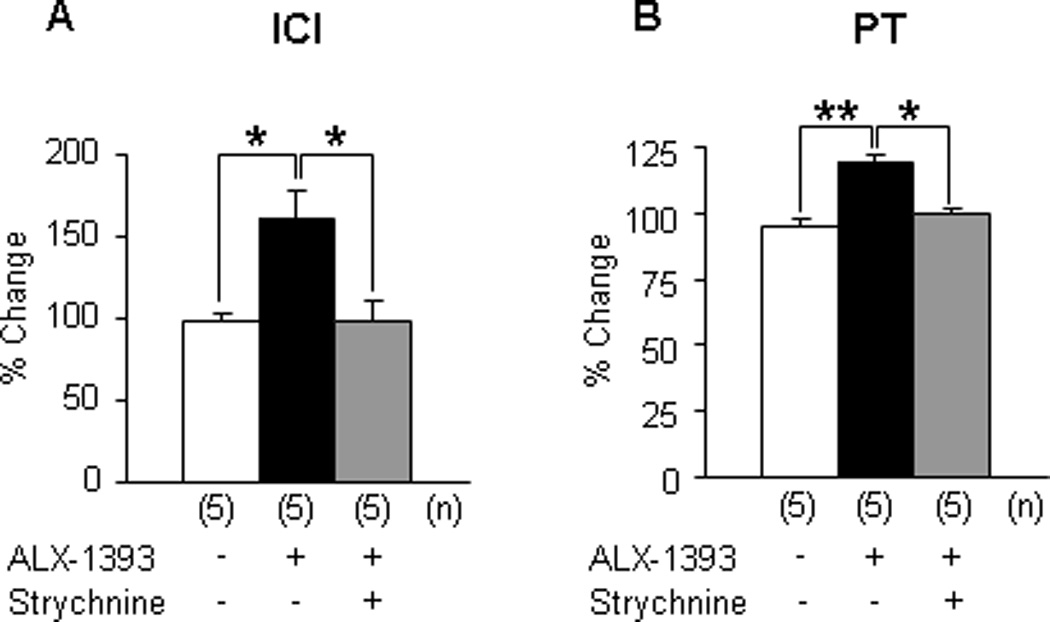
Effects of intrathecal administration of ALX-1393 on (a) intercontraction interval and (b) pressure threshold in the presence or absence of strychnine in cyclophosphamide rats. Each bar represents the mean plus or minus the standard error of the mean.
ICI = intercontraction interval; PT = pressure threshold; - = vehicle (dimethyl sulfoxide or saline); ANOVA = analysis of variance.
* p < 0.05.
** p < 0.01 (compared among three groups; one-way ANOVA followed by Bonferroni multiple comparison test); the number of animals (n) in each subgroup was shown in parentheses.
3.2. Measurement of glycine transporters and glycine receptor subunits in the dorsal spinal cord and forebrain
The GlyT2 messenger RNA (mRNA) level was much higher (23-fold) than that of GlyT1 in the dorsal portion of the L6–S1 spinal cord (p < 0.001) but in the forebrain was much less (13.9-fold) than that of GlyT1 (p < 0.001; data not shown). In CYP-treated rats, the mRNA level of GlyT2 in the dorsal spinal cord (L6–S1) was significantly reduced compared to that in sham rats (p < 0.01; Fig. 6a). However, the mRNA level of GlyT2 was still much higher (24.8-fold) than that of GlyT1 in the dorsal spinal cord of CYP-treated rats (Fig. 6a). Also, significant reductions in mRNA levels of GlyR subunits such as GlyRα1 and GlyRβ were observed in the dorsal spinal cord from CYP-treated rats (p < 0.05) compared to sham rats (Fig. 6b).
Fig. 6.
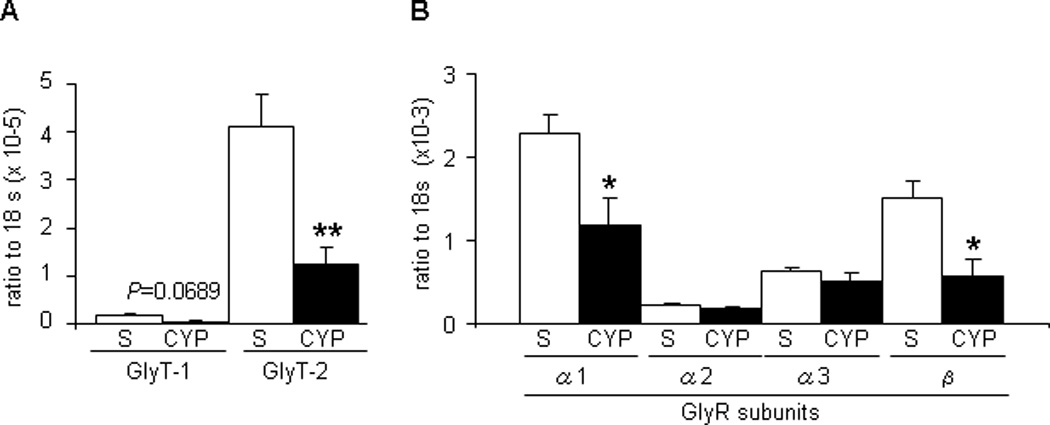
Changes in messenger RNA levels of (a) glycine transporters and (b) glycine receptor subunits in the dorsal L6–S1 spinal cord at the after cyclophosphamide treatment. Each bar represents the mean plus or minus the standard error of the mean from five different rats.
S = sarcosine; CYP = cyclophosphamide; GlyT = glycine transporter; GlyR = glycine receptor; PCR = polymerase chain reaction; NCBI = National Center for Biotechnology Information.
* p < 0.05.
** p < 0.01 (unpaired student t test); the real-time PCR was run by 40 cycles (denaturation at 95°C for 15 s; primer annealing at 55°C for 60 s; elongation at 72°C for 30 s); relative expression data were quantified using the 2-ΔΔCT method, where CT is the cycle threshold. All target messenger RNA (mRNA) expression levels were normalized to that of the constitutive 18S ribosomal RNA. Primer sequences used for real-time PCR were as described in Table 2. All primers for PCR reaction were designed based on the NCBI database sequence of rat reference mRNA and checked for specificity with BLAST software from the NCBI Web site. PCR products were also validated by size determination after separation on 2% agarose gel.
3.3. Effects of glycine transporter inhibitors (intrathecal) on nociceptive behaviors in rats
Sarcosine (250 µg, intrathecal) did not significantly change either licking or freezing behavior induced by intravesical application of RTX (Fig. 7c and 7d). In contrast, ALX-1393 significantly decreased both licking (at 0–5 min) and freezing behaviors (at 10–15 min) by RTX in a dose-dependent (10–30 µg, intrathecal) manner (Fig. 8c and 8d).
Fig. 7.
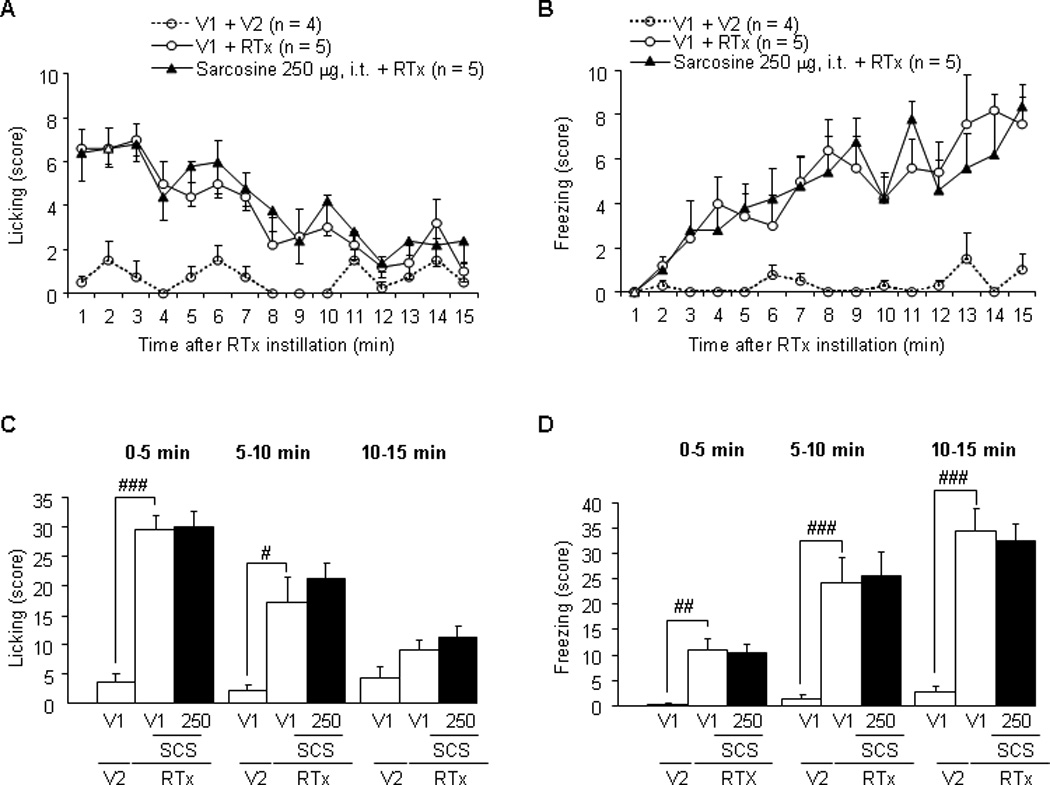
Effects of intrathecal administration of sarcosine on licking and freezing nociceptive behaviors in rats treated with intravesical resiniferatoxin (RTX; 3 µM). (a, b) The number of licking or freezing behaviors counted every minute. Intravesical application of RTX, which stimulates TRPV1 receptor-expressing C-fiber afferent nerves, increased the licking behavior, with a peak in the early phase (0–5 min) as described previously [14]. (c, d) The total number of licking or freezing behaviors for each 5-min period, showing that no significant effect of intrathecal administration of glycine transporter type 1 inhibitor was observed.
V1 = vehicle for sarcosine; V2 = vehicle 10% ethanol, 10% tween 80, and 80% saline for RTX; RTX = resiniferatoxin (3 µM, intravesically); i.t. = intrathecal; SCS = sarcosine (250 µg, intrathecal).
# p < 0.05.
## p < 0.01 (unpaired student t test).
Fig. 8.
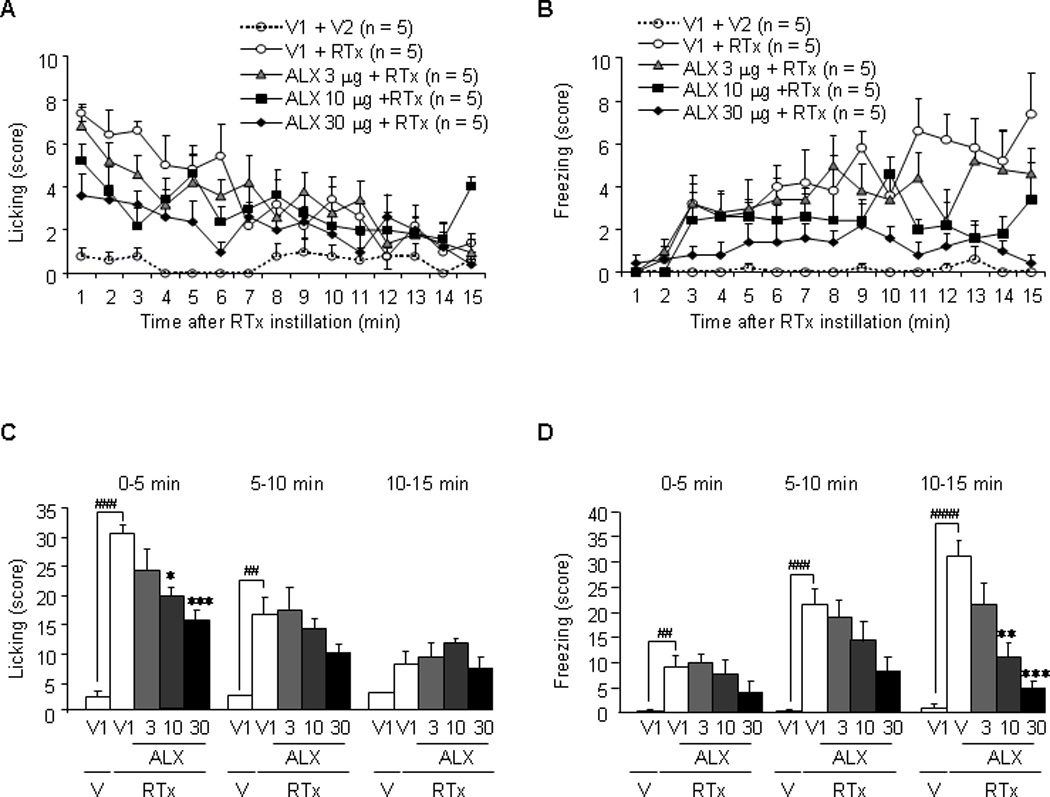
Effects of intrathecal administration of ALX-1393 on licking and freezing nociceptive behaviors in rats treated with intravesical resiniferatoxin (RTX; 3 µM). (a, b) The number of licking or freezing behaviors counted every minute. Intravesical application of RTX, which stimulates TRPV1 receptor-expressing C-fiber afferent nerves, increased the licking behavior, with a peak in the early phase (0–5 min) as described previously [14]. (c, d) The total number of licking or freezing behaviors for each 5-min period. The glycine transporter type 2 inhibitor significantly suppressed both nociceptive behaviors in a dose-dependent manner. Each point or the bar represents the mean plus or minus the standard error of the mean from five rats.
V1 = vehicle for ALX-1393; V2 = vehicle for RTX; RTX = resiniferatoxin (3 µM, intravesically); ALX = ALX-1393 (in µg, intrathecal); V = vehicle.
# p < 0.05.
## p < 0.01 (unpaired student t test).
* p < 0.05.
** p < 0.01 (compared to the vehicle group).
4. Discussion
In the present study, we demonstrated the following new findings: (1) intrathecal administration of an inhibitor of GlyT2 but not GlyT1 suppressed reflex micturition in both normal and CYP-irritated bladders with greater effects in CYP-treated rats; (2) mRNA levels of GlyT2 and GlyR subunits (α1 and β) were significantly decreased in the dorsal portion of lumbosacral spinal cord in CYP-treated rats; and (3) an inhibitor of GlyT2 but not GlyT1 suppressed the nociceptive behaviors induced by intravesical administration of RTX. Previous studies have indicated that exogenous application of glycine suppresses isovolumetric bladder contractions in rats [18]. The present study further demonstrated that the GlyT2 inhibitor, which increases extracellular glycine levels in the synaptic cleft in the spinal cord [19], exerts inhibitory effects on bladder overactivity and pain responses induced by nociceptive stimuli in the bladder.
During cystometry in rats with CYP-induced bladder overactivity, the GlyT2 inhibitor clearly induced increases in ICI and PT, which were completely reversed by strychnine, the GlyR antagonist. Because intrathecal administration of ALX-1393 at the level of L6–S1 spinal cord increased PT and ICI without suppressing MVP, the mechanism of action of GlyT2 inhibitor is assumed to be mainly through the enhancement of glycinergic inhibition of the afferent limb rather than the efferent limb of the micturition reflex pathway in the lumbosacral spinal cord. In contrast, the GlyT1 inhibitor did not produce any significant changes in CMG parameters in either sham or CYP-treated rats. It is postulated that GlyT1 is expressed on glial cells adjacent to glutamatergic excitatory neurons expressing N-methyl-D-aspartate (NMDA) receptors, where glycine acts as a coagonist and possibly participates in excitatory neurotransmission [20,21]. Therefore, the inhibitory effects through postsynaptic GlyRs could be masked by the counteracting excitatory mechanisms through NMDA receptor activation. In contrast, GlyT2 is considered to be primarily involved in the clearance of the extracellular glycine at the inhibitory presynaptic terminals of glycinergic neurons [22,23]. A previous study with an in vivo microdialysis technique also demonstrated that the perfusion of a selective GlyT2 inhibitor increased the extracellular glycine in the lumbar spinal cord, while a selective GlyT1 inhibitor increased the extracellular glycine accompanied by a progressive increase in citrulline, which is used as an index of NMDA receptor activity [19]. Moreover, we demonstrated in this study that mRNA levels of GlyT2 were much higher than that of GlyT1 in the L6–S1 spinal cord, while GlyT1 mRNA is highly expressed in the brain in both sham and CYP-treated rats. Taken together, a high expression level of GlyT2 in the spinal cord and association of GlyT2 with the glycinergic inhibitory pathways could account for the superiority of GlyT2 over GlyT1 inhibitors for suppression of bladder overactivity and pain responses in in vivo studies.
In the present study, we demonstrated in CYP-treated rats a significant reduction in the mRNA levels of GlyT2 as well as GlyR α1 and β1 subunits, which are known to compose one of the major GlyR subtypes (α1β) [24,25] in the L6–S1dorsal spinal cord. In contrast, the decreased GlyT2 expression in the dorsal spinal cord of CYP-treated rats might suggest an adaptive mechanism for increasing the extracellular glycine level at inhibitory synapses, which could counteract the enhanced spinal excitatory signaling in the CYP-induced bladder overactivity condition. This study also further demonstrated that additional suppression of GlyT2 by selective GlyT2 inhibitors in the spinal cord is effective in reducing CYP-induced bladder overactivity.
Another notable finding in this study is that the intrathecal application of the GlyT2 inhibitor significantly suppressed nociceptive behaviors elicited by RTX-mediated bladder afferent stimulation. Licking and freezing behaviors in rats induced by intravesical application of RTX are known to be nociceptive responses elicited by activation of urethral afferents in the pudendal nerve and bladder afferents in the pelvic nerve, respectively [16]. In the glycinergic control of pain processing at the level of spinal cord, the following mechanisms have been postulated [14]; (1) spinal glycinergic interneurons can be directly activated by input from mechanosensitive primary afferents, including nociceptive C-fibers; (2) descending antinociceptive mechanisms, including those mediated by noradrenergic and serotonergic axons, can activate local glycinergic interneurons; and (3) glycinergic inputs to the dorsal spinal horn can originate directly from supraspinal regions. Thus, it is likely that the inhibitory effect by the GlyT2 inhibitor on RTX-induced pain behavior such as licking and freezing is the result of an increase in extracellular glycine concentrations at bulbospinal or segmental interneuronal synapses in the dorsal horn of the lumbosacral spinal cord.
In terms of safety issues about GlyT2 inhibitors, previous animal studies indicated that ALX-1393 induced motor dysfunction and respiratory depression at a higher doses (60 or 100 µg, intrathecal) in somatic pain rats models [15,26]. However, in the present study, ALX-1393 significantly ameliorated bladder overactivity and pain behavior at lower doses (3 µg and 10 µg, intrathecal) in CYP- and RTX-treated rats, respectively, than the doses necessary to demonstrate efficacy in rat somatic pain models [15,26]. Therefore, activation of glycinergic inhibitory mechanisms by GlyT2 inhibitors might be more effective in suppressing visceral overactivity and pain in comparison to their effects on the somatic system. In addition, although there are no previous clinical studies using GlyT2 inhibitors, GlyT1 inhibitors have been used for people with schizophrenia with relatively good tolerability [27,28]. Also, this study showed the greater inhibitory effects of GlyT2 inhibitors on bladder activity in CYP-treated rats compared to control rats, possibly because of increased sensitivity of GlyRs in the spinal cord after CYP treatment. Thus, GlyT2 inhibition could be a novel strategy for the treatment of OAB and/or pain symptoms of BPS and IC, although further studies are needed to clarify whether more conventional routes of application, such as oral administration, show similar efficacy with minimal side effects.
5. Conclusions
The findings in this study suggest an important role for spinal glycinergic pathways, which are enhanced by GlyT2 inhibitors, in the suppression of bladder overactivity and pain sensation induced by noxious stimuli in the bladder. Because afferent sensitization and increased signal transduction in the spinal cord have been proposed as important mechanisms inducing irritative symptoms of OAB and/or pain symptoms of BPS and IC, GlyT2 inhibitors might be effective in treating these bladder overactive and hypersensitive disorders.
Take-home message.
Inhibition of glycine transporter type 2 (GlyT2) ameliorated bladder overactivity and pain responses induced by bladder irritation in rats. This result suggests that GlyT2 inhibitors might be useful for the treatment of overactive and hypersensitive bladder conditions, including bladder pain syndrome.
Table 2.
Primers used for real-time reverse transcriptase–polymerase chain reaction
| Name | Forward primer (5′–3′) | Reverse primer (5′–3′) | Size (bp) |
|---|---|---|---|
| GlyT1 | GCTCTGGTCCTTGCTGTTTT | GTCTCTGCTTGGCTTTGTGG | 595 |
| GlyT2 | GATGCTTCTCACGCTTGGAC | CAAACGCCCAGCAGACCTTC | 344 |
| GlyRα1 | GCACCAAGCACTACAACAC | AGGACAGGATGACGATAAGC | 123 |
| GlyRα2 | GAGACAGCAGTGGAACGATTC | TCCGCAGCAACTTGTTATCAG | 167 |
| GlyRα3 | GCCTTCTGATTGTCATTCTGTC | CTCTGCGTGGTCATCGTAAG | 109 |
| GlyRβ | AAGGCACTGGTTACTACAC | CTGAGCACGGAGAAGATG | 181 |
| 18S | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG | 151 |
GlyT = glycine transporter; GlyR = glycine receptor.
Acknowledgments
Funding/Support and role of the sponsor: This study was supported by the Samuel Wilan Research Fund for Interstitial Cystitis and National Institutes of Health grants (DK57267, DK88836 and P01 DK44935).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Satoru Yoshikawa had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Yoshikawa, Yoshimura.
Acquisition of data: Yoshikawa.
Analysis and interpretation of data: Yoshikawa, Yoshimura.
Drafting of the manuscript: Yoshikawa.
Critical revision of the manuscript for important intellectual content: Yoshimura, Oguchi, Funahashi, de Groat.
Statistical analysis: Yoshikawa.
Obtaining funding: Yoshimura.
Administrative, technical, or material support: Oguchi, Funahashi.
Supervision: Yoshimura, de Groat.
Other (specify): None.
Financial disclosures: I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
References
- 1.Yoshimura N, Chancellor MB. Current and future pharmacological treatment for overactive bladder. J Urol. 2002;168:1897–1913. doi: 10.1016/S0022-5347(05)64261-9. [DOI] [PubMed] [Google Scholar]
- 2.Birder L, de Groat W, Mills I, Morrison J, Thor K, Drake M. Neural control of the lower urinary tract: peripheral and spinal mechanisms. Neurourol Urodyn. 2010;29:128–139. doi: 10.1002/nau.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshimura N, Birder LA. Interstitial cystitis and related painful bladder syndromes: pathophysiology. In: Pasricha PJ, Willis WD, Gebhart GF, editors. Chronic abdominal and visceral pain: theory and practice. New York, NY: Informa Healthcare USA; 2007. pp. 495–520. [Google Scholar]
- 4.Zeilhofer HU. The glycinergic control of spinal pain processing. Cell Mol Life Sci. 2005;62:2027–2035. doi: 10.1007/s00018-005-5107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyazato M, Sugaya K, Nishijima S, Kadekawa K, Ashimine S, Ogawa Y. Intrathecal or dietary glycine inhibits bladder and urethral activity in rats with spinal cord injury. J Urol. 2005;174:2397–2400. doi: 10.1097/01.ju.0000180415.69705.7a. [DOI] [PubMed] [Google Scholar]
- 6.Miyazato M, Sugaya K, Nishijima S, et al. Changes of bladder activity and glycine levels in the lumbosacral cord after partial bladder outlet obstruction in rats. Int J Urol. 2008;15:843–847. doi: 10.1111/j.1442-2042.2008.02079.x. [DOI] [PubMed] [Google Scholar]
- 7.Nishijima S, Sugaya K, Fukuda T, Miyazato M, Ashimine S, Ogawa Y. Serum amino acids as indicators of cerebrospinal neuronal activity in patients with micturition disorders. Int J Urol. 2006;13:1479–1483. doi: 10.1111/j.1442-2042.2006.01653.x. [DOI] [PubMed] [Google Scholar]
- 8.Eulenburg V, Armsen W, Betz H, Gomeza J. Glycine transporters: essential regulators of neurotransmission. Trends Biochem Sci. 2005;30:325–333. doi: 10.1016/j.tibs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Zafra F, Aragon C, Olivares L, Danbolt NC, Gimenez C, Storm-Mathisen J. Glycine transporters are differentially expressed among CNS cells. J Neurosci. 1995;15:3952–3969. doi: 10.1523/JNEUROSCI.15-05-03952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zafra F, Gomeza J, Olivares L, Aragon C, Gimenez C. Regional distribution and developmental variation of the glycine transporters GLYT1 and GLYT2 in the rat CNS. Eur J Neurosci. 1995;7:1342–1352. doi: 10.1111/j.1460-9568.1995.tb01125.x. [DOI] [PubMed] [Google Scholar]
- 11.Mohler H, Boison D, Singer P, Feldon J, Pauly-Evers M, Yee BK. Glycine transporter 1 as a potential therapeutic target for schizophrenia-related symptoms: evidence from genetically modified mouse models and pharmacological inhibition. Biochem Pharmacol. 2011;81:1065–1077. doi: 10.1016/j.bcp.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Boulay D, Pichat P, Dargazanli G, et al. Characterization of SSR103800, a selective inhibitor of the glycine transporter-1 in models predictive of therapeutic activity in schizophrenia. Pharmacol Biochem Behav. 2008;91:47–58. doi: 10.1016/j.pbb.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Manahan-Vaughan D, Wildforster V, Thomsen C. Rescue of hippocampal LTP and learning deficits in a rat model of psychosis by inhibition of glycine transporter-1 (GlyT1) Eur J Neurosci. 2008;28:1342–1350. doi: 10.1111/j.1460-9568.2008.06433.x. [DOI] [PubMed] [Google Scholar]
- 14.Dohi T, Morita K, Kitayama T, Motoyama N, Morioka N. Glycine transporter inhibitors as a novel drug discovery strategy for neuropathic pain. Pharmacol Ther. 2009;123:54–79. doi: 10.1016/j.pharmthera.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Haranishi Y, Hara K, Terada T, Nakamura S, Sata T. The antinociceptive effect of intrathecal administration of glycine transporter-2 inhibitor ALX1393 in a rat acute pain model. Anesth Analg. 2010;110:615–621. doi: 10.1213/ANE.0b013e3181c7ebbb. [DOI] [PubMed] [Google Scholar]
- 16.Saitoh C, Chancellor MB, de Groat WC, Yoshimura N. Effects of intravesical instillation of resiniferatoxin on bladder function and nociceptive behavior in freely moving, conscious rats. J Urol. 2008;179:359–364. doi: 10.1016/j.juro.2007.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centeno MV, Mutso A, Millecamps M, Apkarian AV. Prefrontal cortex and spinal cord mediated anti-neuropathy and analgesia induced by sarcosine, a glycine-T1 transporter inhibitor. Pain. 2009;145:176–183. doi: 10.1016/j.pain.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyazato M, Sugaya K, Nishijima S, Ashitomi K, Hatano T, Ogawa Y. Inhibitory effect of intrathecal glycine on the micturition reflex in normal and spinal cord injury rats. Exp Neurol. 2003;183:232–240. doi: 10.1016/s0014-4886(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 19.Whitehead KJ, Pearce SM, Walker G, Sundaram H, Hill D, Bowery NG. Positive N-methyl-D-aspartate receptor modulation by selective glycine transporter-1 inhibition in the rat dorsal spinal cord in vivo. Neuroscience. 2004;126:381–390. doi: 10.1016/j.neuroscience.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 21.Nong Y, Huang YQ, Ju W, et al. Glycine binding primes NMDA receptor internalization. Nature. 2003;422:302–307. doi: 10.1038/nature01497. [DOI] [PubMed] [Google Scholar]
- 22.Brasnjo G, Otis TS. Glycine transporters not only take out the garbage, they recycle. Neuron. 2003;40:667–669. doi: 10.1016/s0896-6273(03)00725-6. [DOI] [PubMed] [Google Scholar]
- 23.Jursky F, Nelson N. Localization of glycine neurotransmitter transporter (GLYT2) reveals correlation with the distribution of glycine receptor. J Neurochem. 1995;64:1026–1033. doi: 10.1046/j.1471-4159.1995.64031026.x. [DOI] [PubMed] [Google Scholar]
- 24.Grudzinska J, Schemm R, Haeger S, et al. The beta subunit determines the ligand binding properties of synaptic glycine receptors. Neuron. 2005;45:727–739. doi: 10.1016/j.neuron.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 25.Betz H, Langosch D, Hoch W, et al. Structure and expression of inhibitory glycine receptors. Adv Exp Med Biol. 1991;287:421–429. doi: 10.1007/978-1-4684-5907-4_37. [DOI] [PubMed] [Google Scholar]
- 26.Hermanns H, Muth-Selbach U, Williams R, et al. Differential effects of spinally applied glycine transporter inhibitors on nociception in a rat model of neuropathic pain. Neurosci Lett. 2008;445:214–219. doi: 10.1016/j.neulet.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Tsai GE, Lin PY. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr Pharm Des. 2010;16:522–537. doi: 10.2174/138161210790361452. [DOI] [PubMed] [Google Scholar]
- 28.Lane HY, Lin CH, Huang YJ, Liao CH, Chang YC, Tsai GE. A randomized, double-blind, placebo-controlled comparison study of sarcosine (N-methylglycine) and D-serine add-on treatment for schizophrenia. Int J Neuropsychopharmacol. 2010;13:451–460. doi: 10.1017/S1461145709990939. [DOI] [PubMed] [Google Scholar]


