Abstract
Aim
To examine the feasibility of using the pOBCol3.6GFPtpz (3.6-GFP) transgenic mice as an in vivo model for studying the biological sequence of events during pulp healing and reparative dentinogenesis.
Methodology
Pulp exposures were created in the first maxillary molar of 12-16 week old 3.6-GFP transgenic mice with CD1 and C57/Bl6 genetic background. Direct pulp capping on exposed teeth were performed using mineral trioxide aggregate (MTA) followed by restoration with a light-cured adhesive system (AS) and composite resin. In control teeth, the AS was placed in direct contact with the pulp. Animals were euthanized at various time points after pulp exposure and capping. The maxillary arch was isolated, fixed and processed for histological and epifluorescence analysis to examine reparative dentinogenesis.
Results
Analysis of teeth immediately after pulp exposure revealed absence of odontoblasts expressing 3.6-GFP at the injury site. Evidence of reparative dentinogenesis was apparent at 4 weeks in 3.6-GFP mice in CD1 background and at 8 weeks in 3.6-GFP mice with C57/Bl6 background. The reparative dentine with both groups contained newly formed atubular-mineralized tissue resembling a dentine bridge and/or osteodentine that was lined by cells expressing 3.6-GFP as well as 3.6-GFP expressing cells embedded within the atubular matrix.
Conclusion
This study was conducted in a few animals and did not allow statistical analysis. The results revealed that the 3.6-GFP transgenic animals provide a unique model for direct analysis of cellular and molecular mechanisms of pulp repair and tertiary dentinogenesis in vivo. The study also shows the effects of the capping material and the genetic background of the mice in the sequence and timing of reparative dentinogenesis.
Keywords: Adhesive system, dentine bridge, green fluorescent protein, mineral trioxide aggregate, odontoblast-like cells, reparative dentinogenesis
Introduction
The dentine-pulp complex has a regenerative potential that leads to the formation of tertiary dentine (Sloan & Smith 2007, Waddington et al. 2009,Simon et al.). Tertiary dentine is divided into reactionary and reparative dentine (Sloan & Smith 2007, Waddington et al. 2009, Simon et al.). Reactionary dentine is secreted by pre-existing odontoblasts that survive the injury/insult. Following more intense injury/insult leading to odontoblast death, stem/progenitor cells are recruited to the injury site and differentiate in odontoblast-like cells that secrete reparative dentine (Sloan & Smith 2007, Waddington et al. 2009, Simon et al.).
Transgenic mouse lines in which Green Fluorescent Protein (GFP) expression is under the control of tissue- and stage-specific regulatory elements of genes involved in dentinogenesis have provided valuable tools for examining the stepwise progression and differentiation of progenitors into odontoblasts (Braut et al. 2003, Mina & Braut 2004, Balic & Mina 2005, Balic et al. 2009, Balic et al. 2010, Balic & Mina 2011). Previous studies using 3.6-GFP transgenic mice in which GFP expression is under control of the rat 3.6-kb collagen type I promoter fragments showed that this transgene is first activated at low levels in cells in early stages of polarization (Balic et al. 2010). The expression of the transgene is significantly upregulated in more advanced stages of odontoblast differentiation. In fully differentiated odontoblasts, the expression of the transgene extends into the odontoblast processes (Mina & Braut 2004).
Additional experiments using 2.3-GFPand DMP-1GFP transgenic mice showed that the 2.3-GFP transgene was activated at a later stage of polarization, just before or at the time of formation of secretory/functional odontoblasts, and DMP1-GFP was activated in secretory/functional odontoblasts producing predentine (Balic & Mina 2005, Balic et al. 2010, Balic & Mina 2011). Together these observations indicated that activation and subsequent expression of these transgenes provide valuable markers for identification of cells at different stage of odontoblast differentiation.
Therefore, in the present study 3.6-GFP transgenic animals were used to gain insight into the sequence of events during reparative dentinogenesis.
Material and methods
Transgenic mice
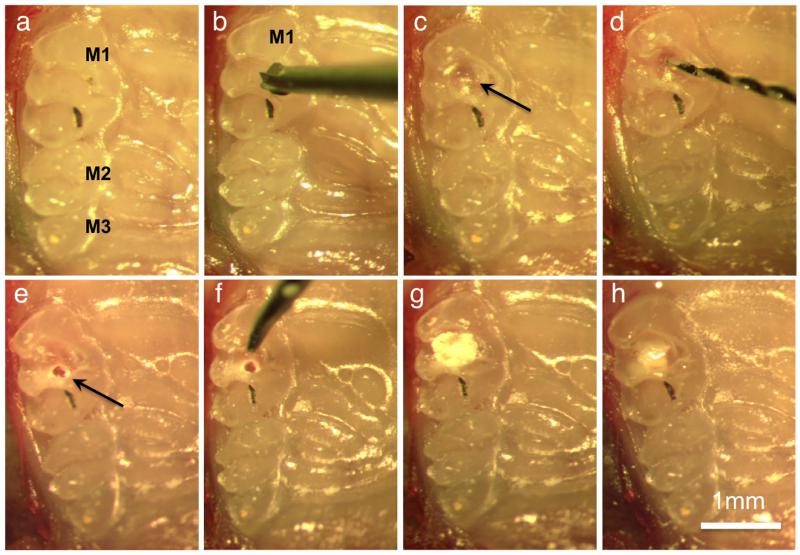
All animal procedures were performed in accordance with Animal Care Committee (ACC) guidelines from University of Connecticut Health Center (UCHC). To stimulate the reparative dentinogenesis, dental pulp exposure was performed in the maxillary first right molar of thirty 3.6-GFP mice (12 to 16 weeks old) with CD1 (outbred) genetic background and C57/Bl6 (inbred) background as described by Simon et al. (2008), with some modification. C57/Bl6 is the most widely used inbred strain used for genetic studies because of the isogenicity (having the same genotype) within a strain. Mice were anaesthetized and a cavity was prepared with a carbide burr (diameter 0.40 mm) on the occlusal surface of the molar (Class I cavity), in the mesial half of the crown, centered on the labial-palatal aspect of the tooth until the pulp was visible through the transparency of the dentine floor of the cavity. A pulp exposure was subsequently created mechanically using an endodontic hand file with 0.15 mm diameter tip with a 2% taper, this approach controled the pulp exposure size to approximately 150 μm (size of the file tip) (Fig. 1). Exposed pulps were capped using mineral trioxide aggregate (MTA Angelus®; Angelus S/A, Paraná, Brazil) mixed with sterile water following the manufacturer’s recommendations. Mineral trioxide aggregate was placed in contact with the pulp using a probe tip, and condensed gently with a sterile paper point (size 35) (Dentsply Maillefer, Ballaigues, Switzerland). Subsequently, the cavity was sealed with light-cured composite resin (Z100® 3M, São Paulo, Brazil), associated with a two-step self-etching adhesive system (AS) (Clearfill® SE Bond, Kuraray, Okayama, Japan) (Fig. 1). As a control group pulp exposures were directly capped with AS overlaid with light-cured composite resin without any MTA or without capping material analyzed immediately after exposure.
Figure 1.
Procedure of pulp exposure, capping, and restoration. (a) Occlusal view of the first (M1), second (M2) and third (M3) molars. In all images the palatal side of the arch is on the right. (b) Position of the carbide bur on the centre of the first molar (M1). (c) Small cavity (arrow) on centre of the occlusal surface. (d) Endodontic hand file used to mechanically expose the pulp. (e) Pulp exposure (arrow). (f) Probe used to apply the MTA. (g) Pulp capping with MTA. (h) Cavity restoration with resin composite.
Tissue isolation and analysis
To analyze the sequence of reparative dentinogenesis animals were euthanized after pulp exposure at different periods of time by intracardiac perfusion with 10% buffered formalin as described before (Palermo et al. 2005). The number of mice analyzed in each time point for each genetic background is shown in Table 1. After perfusion, maxillary arches were isolated, cleaned from soft tissue, trimmed, and fixed in 10% formalin solution for additional 24 hours. Samples were decalcified for 7 days in 15% EDTA, 0.5% of formalin (pH 7.5) at 4°C and then embedded in paraffin following the standard protocols. Serial cross sections of 7μm were placed onto ProbeOn Plus slides (Fisher Scientific, Pittsburgh, PA, USA) deparaffinized with xylene, rehydrated and processed for epifluorescence analysis. To visualize GFP signal deparaffinized sections were mounted with glycerol/PBS (50%: 50%). The fluorescence signal in these sections was examined in at least 20 sections through the region of injury and repair from each animal using an Axio Observer.Z1 for epifluorescence microscope (Carl Zeiss, Thornwood, NY, USA). Sections were examined in GFPtpz filter to allow the detection of 3.6-GFP signal and in GFPmcherry filter to avoid the detection of any GFP auto fluorescence. The images were overlaid to eliminate tissue auto fluorescence using user-defined computation program AxioVision Rel 4.7 software (Carl Zeiss, Thornwood, NY,USA). After epifluorescence analysis the same or adjacent sections were washed in PBS, processed for hematoxylin and eosin (H&E) staining using standard protocols and analyzed by light microscopy.
Table 1.
Number of animals analyzed at each time point with different treatments
| Time of analysis after pulp exposure |
3.6-GFP transgenic mice with CD1 background |
3.6-GFP transgenic mice with C57/Bl6 background |
||
|---|---|---|---|---|
| Immediately (no capping) |
2 | 2 | ||
| MTA | AS | MTA | AS | |
|
|
|
|||
| 1 week | 2 | 2 | 2 | 2 |
| 4 weeks | 4 | 2 | 4 | 2 |
| 8 weeks | - | - | 4 | 2 |
Results
Analysis of reparative dentinogenesis immediately and 7 days after pulp exposure
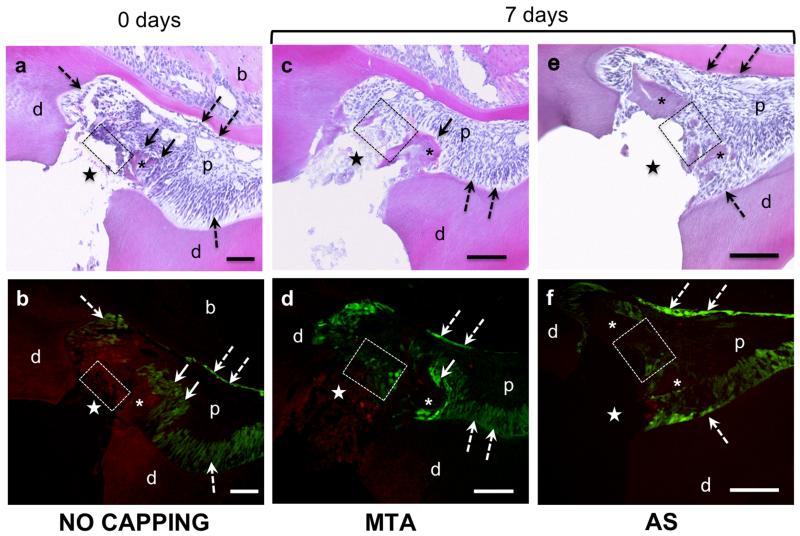
Histological and epifluorescence analyses of teeth immediately after pulp exposure showed absence of dentine and odontoblasts expressing 3.6-GFP transgene at the pulp exposure site in transgenic animals with both CD1 (Fig. 2a, b) and C57/Bl6 background (data not shown) confirming the destruction of the odontoblast at this location. Analysis of pulps capped with MTA or AS, 7 days after pulp exposure showed no evidence of reparative dentinogenesis in transgenic animals with both CD1 (Fig. 2c-f) and C57/Bl6 background (data not shown). However, in transgenic animals with CD1 background (but not with C57/Bl6) the exposure site underneath the MTA and AS contained cells expressing high and low levels of 3.6-GFP (Fig. 2d, f).
Figure 2.
Histological sections stained with H & E (a, c, e) and epifluorescence analyses of adjacent sections (b, d, f) of teeth from 3.6-GFP transgenic mice with CD1 background immediately after pulp exposure (a and b) and 7 days after exposure (c-f). (a and b) show the absence of odontoblasts (indicated by dashed rectangle) and dentine at the exposure site (indicated by stars). Dentine chips in the exposure site are marked by an asterix. Note the expression of 3.6-GFP in the odontoblasts layer around pulp-dentine complex (dashed arrows) and around a dentine chip (indicated by full arrows). Also note the absence of cells expressing 3.6-GFP underneath the exposure site (indicated by dashed rectangle). (c and d) represent images from a tooth capped with MTA. (e and f) represent images from a tooth capped with AS. Note the expression of 3.6-GFP in cells in close contact with the capping materials in d and f (marked by dashed boxes). Also note that the number of cells expressing 3.6-GFP in the tooth capped with MTA (dashed box in d) is higher than in the tooth capped with AS (dashed box in f). Dashed arrows mark expression of 3.6-GFP in the odontoblast layer around pulp-dentine. Full arrows mark expression of 3.6-GFP in odontoblast around dentine chips. Abbreviations: d=dentine; p=pulp; (*)= dentine chips; (dashed arrow)= original odontoblasts; star= exposure site. Scale bar= 100μm.
Despite the lack of statistical analysis the observations in transgenic mice with CD1 background showed that the number of 3.6-GFP expressing cells in teeth capped with, AS (Fig. 2f) were lower than in teeth capped with MTA (Fig. 2d). Small pieces of dentine were frequently observed in the dental pulp (Fig. 2a-f). These chips consisted of tubular dentine matrix surrounded by numerous odontoblasts expressing high levels of 3.6-GFP transgene (Fig. 2a-f).
Analysis of reparative dentinogenesis 4 and 8 weeks after pulp exposure
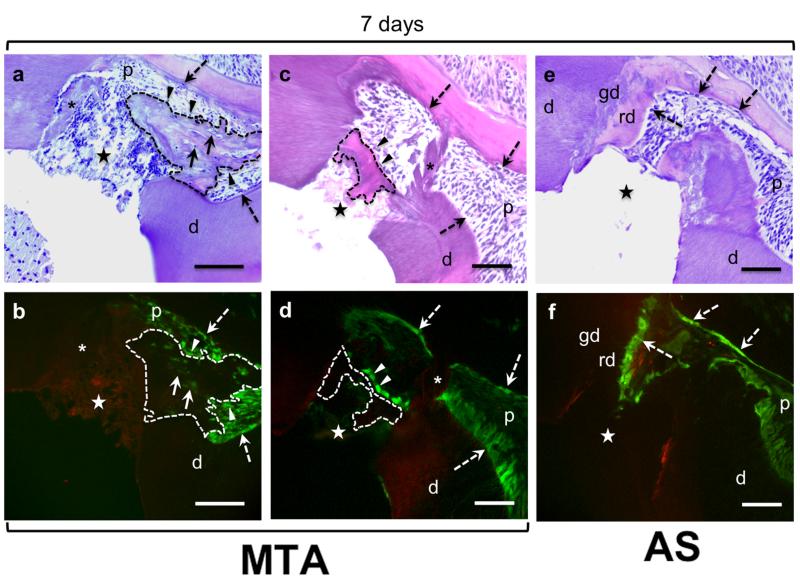
Histological analysis 4 weeks after injury revealed a clear evidence of reparative dentinogenesis in pulps capped with MTA in transgenic animals with CD1 (but not with C57/Bl6 background). In these animals two patterns of reparative dentinogenesis were observed. One was the formation of an extensive atubular matrix in close contact with MTA, resembling an osteodentine, extending to the pulp (Fig. 3a). This matrix was lined with cells expressing 3.6-GFP and contained 3.6-GFP expressing cells embedded within the matrix (Fig. 3b). The second pattern of reparative dentinogenesis was the formation of a well-defined dentine bridge that appears to seal the exposure site (Fig. 3c). This dentine bridge contained atubular matrix lined with cells expressing high levels of 3.6-GFP but did not contain a significant number of 3.6-GFP expressing cells embedded within the matrix (Fig. 3d). After 4 weeks, dentine chips, although still present in the pulp were devoid of cells expressing 3.6-GFP transgene (Fig. 3c, d). In teeth capped with AS with CD1 background, no evidence of reparative dentinogenesis (dentine bridge or osteodentine) was evident after 4 weeks (Fig. 3e). However, in these animals there was a thickened layer of dentine indicating extensive reactionary dentinogenesis over a new globular calcified dentine (Fig. 3e, f). This new reactionary dentine was lined with odontoblasts expressing high levels of 3.6-GFP transgene (Fig. 3f).
Figure 3.
Histological sections stained with H & E (a, c, e) and epifluorescence analyses of adjacent sections (b, d, f) of teeth from 3.6-GFP transgenic mice with CD1 background 4 weeks after pulp exposure. (a - d) are representative images of sections from teeth capped with MTA. (e and f) are representative images of adjacent section from a tooth capped with AS. (a and b) show the formation of newly synthesized matrix (outlined with dashed lines) that extends into the pulp from exposure site. This matrix is atubular and contains 3.6-GFP expressing cells (indicated by arrowheads) and 3.6-GFP expressing cells embedded in the matrix (indicated by full arrows) resembling osteodentine. (c and d) show the formation of a well-defined dentine bridge (outlined with dashed lines). Note the 3.6-GFP expressing cells (indicated by arrowheads) lining this bridge. Note the presence of dentine chips (indicated by asterix) in images (a-d), dentine chips at this time point are devoid of 3.6-GFP expressing odontoblast. (e and f) show the thickened layer of reactionary dentine (rd) over a globular calcified dentine (gd) at the borders of the pulp close to the exposure site. Note the high expression of 3.6-GFP in original odontoblasts underneath the reactionary dentine around the pulp (indicated by dashed arrows). Abbreviations: d=dentine; gd=globular calcified dentine, p=pulp; rd=reactionay dentine; (*)= dentine chips; (dashed arrow)= original odontoblasts; star= exposure site. Scale bar= 100μm.
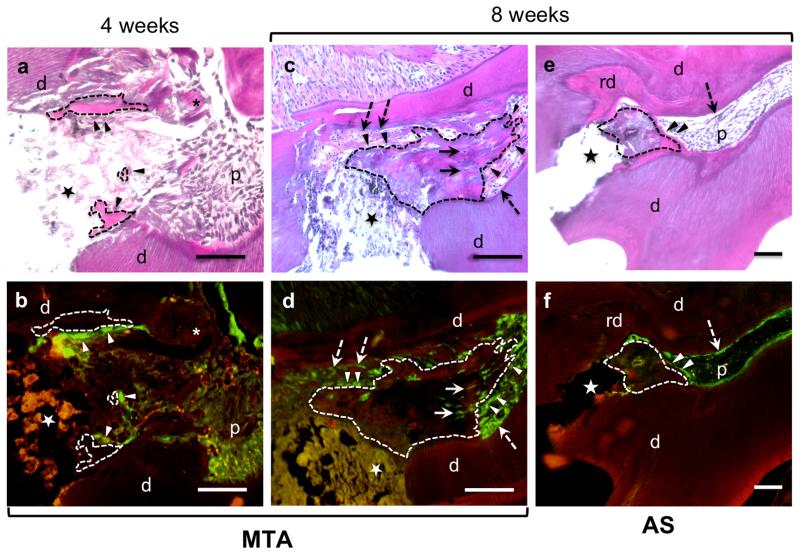
Transgenic animals with C57/Bl6 background capped with MTA 4 weeks after exposure showed no evident osteodentine or dentine bridge. In these animals there were islands of focal dentine matrix lined by a few cells expressing high levels of 3.6-GFP signal (Fig. 4a, b). In transgenic animals with C57/Bl6 background capped with AS, there was no evidence of reparative dentinogenesis or islands of dentine matrix deposition (data not shown).
Figure 4.
Histological sections stained with H&E (a, c, e) and epifluorescence analyses of sections (b, d, f) of teeth from 3.6-GFP transgenic mice with C57/Bl6 background 4 and 8 weeks after pulp exposure. (a and c) are images of a section through a pulp capped with MTA after 4 weeks. Note in (a) the presence of focal islands of matrix (outlined with dashed lines) in close contact with the capping material lined with 3.6-GFP cells (indicated by arrowheads). (c and d) are images from a tooth capped with MTA after 8 weeks. Note the formation of newly synthesized matrix (outlined by dashed lines) that extends into the pulp. This matrix resembling osteodentine is atubular and contains 3.6-GFP expressing cells (indicated by arrowheads) and 3.6-GFP cells embedded in the matrix (indicated by full arrows). (e and f) are images from a tooth capped with AS after 8 weeks. Note in (e) the thickened layer of reactionary dentine (rd) over a calcified dentine at the borders of the pulp close to the exposure site. Note in (f) the high expression of 3.6-GFP in original odontoblasts underneath the reactionary dentine and around the pulp (indicated by dashed arrows). Also note the presence of a small osteodentine (outlined by dashed lines). Abbreviations: d=dentine; p=pulp; rd=reactionary dentine; (dashed arrow)=original odontoblasts; (*)= dentine chips; star= exposure site. Scale bar= 100μm.
Analysis of teeth in transgenic animals with C57/Bl6 background after 8 weeks of pulp exposure capped with MTA revealed the formation of an extensive atubular matrix, resembling osteodentine (Fig. 4c). This matrix also extended from the injury site into the pulp (Fig. 4c), and was lined with cells expressing 3.6-GFP and contained 3.6-GFP expressing cells entrapped within the new matrix (Fig. 4d). In teeth capped with AS, there was evidence of reactionary dentinogenesis characterized by thickened dentine secreted by pre-existing odontoblasts expressing 3.6-GFP (Fig. 4e, f) and reparative dentinogenesis (matrix lined with new secretory cells expressing 3.6-GFP) under the exposure site (Fig. 4e, f). Despite the lack of statistical analysis the extent and frequency of reparative dentinogenesis in teeth treated with AS was lower than in teeth treated with MTA.
Discussion
It has been well documented that after an intense injury that leads to destruction and eliminates the pre-existing odontoblast layer, odontoblast-like cells originating from dental pulp cells and secrete reparative dentine at the injury site (Sloan & Smith 2007, Waddington et al. 2009, Simon et al.). Although the formation of reparative dentinogenesis has been studied, the origin of the cells giving rise to odontoblast-like cells and cellular and molecular events leading to this reparative process are not well understood. Previous in vitro studies have shown that 3.6-GFP animals provide a unique model to examine odontoblast differentiation from a progenitor population (Mina & Braut 2004, Balic et al. 2010). Therefore, in the present study 3.6-GFP transgenic mice was used to gain insight in vivo into the sequence of events during reparative dentinogenesis.
In this study, to stimulate the differentiation of odontoblast-like cells from progenitor or stem cell population, exposure on maxillary first molars of 3.6-GFP transgenic mice was used as described before (Simon et al. 2008) with some modifications. Utilization of this transgenic animals allowed us to gain a better insight into many aspects of this reparative process including destruction of odontoblasts after pulp exposure, presence of dentine chips at the healing pulp, the fate of the pre-existing odontoblasts around these chips, recruitment of progenitors to the injury site and their subsequent differentiation and the formation of different patterns of tertiary dentine.
The observations revealed that the pulp exposure protocol leads to complete destruction of odontoblasts as evident by the absence of cells expressing 3.6-GFP transgene in the pulp tissue under the exposure site immediately after injury. The results revelaed no evidence of dentine bridge formation in pulps capped with MTA or AS in transgenic animals with CD1 and C57/Bl6 backgrounds 7 days after pulp exposure that are consistent with previous results (Simon et al. 2008). Interestingly results demonstrated the 3.6-GFP expressing cells in the pulp tissue underneath the site of injury 7 days after pulp exposure. The appearance of 3.6-GFP transgene in these cells is before the formation of calcified tissue and is related to the activation or up-regulation of 3.6-GFP transgene in cells in close proximity to the injury and/or in cells recruited to the site of injury from a distance. These findings on early appearance of cells committed to dentinogenic lineage at the injury site are in agreement with results reported by others (Harada et al. 2008, Ishikawa et al. 2010) who used rat molars to demonstrate that cells committed to odontoblasts lineage appeared at the injury site 2-5 days after odontoblast death. Based on previous observations (Balic et al. 2010), the results show that these cells at the site of injury expressing 3.6-GFP transgene are in early stages of polarization.
Despite the lack of statistical analysis, the observation revealed that the numbers of 3.6-GFP expressing cells in pulps under MTA were higher than those under AS which is most likely related to the properties of MTA such as its high pH, calcium release, and biocompatibility (Parirokh & Torabinejad 2010). MTA also is known for its good sealing capacity (Barrieshi-Nusair & Hammad 2005) its ability to promote cell proliferation (Paranjpe et al. 2010) and ability to up-regulate the gene expression (Runx2, osteocalcin, alkalin phosphatase and DSPP), and the differentiation of pulp cells into odontoblast-like cells (Paranjpe et al. 2010).
In the present study reparative dentinogenesis and dentine bridges were observed in pulps capped with MTA, after 4 weeks of exposure with transgenic animals with CD1 background and 8 weeks after exposure with C57/Bl6 background. These observations indicated the delayed process of dentine repair and regeneration with C57/Bl6 strains. The differences in the timing of reparative dentinogenesis with the CD1 and with C57/Bl6 backgrounds are consistent with the influences of genetic backgrounds on the expression of various diseases and repair processes (Everett 2011).
Two different patterns of reparative dentinogenesis was observed. One was a bulky, atubular calcified tissue under the capping material that extended into the pulp resulting in the obliteration of the pulp chamber. In this atubular matrix 3.6-GFP expressing cells surrounding the matrix and embedded in the matrix were detetcted. These atubular structures with their cellular contents resemble the osteodentine described in the literature (Sloan & Smith 2007, Waddington et al. 2009) and were similar to the structures reported by others after transplantation of pulp tissue under the kidney capsule (Braut et al. 2003) and after reimplantation of rat molars (Zhao et al. 2007, Ishikawa et al. 2010).
The other pattern of reparative dentinogenesis was a well-defined calcified bridge along the exposure site underneath the MTA. This finding is consistent with previous publications, which reported the secretion of reparative matrix forming a dentine bridge 14 days after pulp exposure and MTA capping in rats molars (Andelin et al. 2003, Kuratate et al. 2008, Simon et al. 2008, Shahravan et al. 2011). The Present study showed that the calcified bridge was lined with 3.6-GFP expressing cells. Previous studies have demonstrated that cells lining the dentine bridge under MTA were immunoreactive for Nestin, osteopontin and for DSP (Andelin et al. 2003, Kuratate et al. 2008).
Previous studies have shown the expression of 3.6-GFP transgene in cells in both dentinogenic and osteogenic lineage, making the distinction between odontoblasts- and osteoblasts-like cells secreting dentine- and bone-like tissues difficult. The distinction between these cell type are important in the light of more recent observations that have provided clear evidence for osteogenic and dentinogenic potential of dental pulp cells (Ogawa et al. 2006, Takamori et al. 2008, Balic et al. 2010).
Transgenic animals with both backgrounds capped with AS had evidence of extensive reactionary dentinogenesis consisting of a thickened dentine over a globular calcified dentine narrowing the exposure site. The extensive amount of reactionary dentine in teeth capped with AS is due to short and long term inflammation in the dental pulp caused by the acid primer (de Souza Costa et al. 2001, Dominguez et al. 2003) that stimulates pre-existing odontoblast to secrete reactionary dentine over the dentine walls.
The reparative dentinogenesis in teeth capped with AS is in agreement with Akimoto et al. (1998) that reported new dentine bridge formation directly adjacent to the Clearfil system interface. This reparative dentine could be explained by the fact that self-etching AS such Clearfill® SE Bond used in this study contains a phosphoric acid monoester, in the acid primer, with a pH higher than others acid primers, and produces a substantially milder effect on pulp tissue (Akimoto et al. 1998, Koliniotou-Koumpia & Tziafas 2005).
Consistent with results described before (Simon et al. 2008) the results revealed the presence of dentine chips at the periphery of the injury deeply impacted into the pulp as a consequence of the endodontic file penetration. The chips, although present at all time points, were lined with surviving odontoblast expressing 3.6-GFP up to 7 days but not thereafter. Previous results have shown that this dentine debris can stimulate the formation of hard tissue (Jaber et al. 1991). However, a deep impaction of dentine chips can decrease the rate of healing and bridge formation (Dominguez et al. 2003).
Conclusion
The data provided by this study demonstrated the feasibility of using 3.6-GFP transgenic report mice either with CD1 or C57/Bl6 background in combination with the pulp exposure protocol as a powerful in vivo model for analysis of pulp healing process, and biological events involved in reparative and reactionary dentinogenesis. This opens new opportunities for the use of others transgenic animals in which GFP coding sequences is under the control of tissue-specific regulatory elements (i.e., DSPP) and animals with regulatory elements for progenitor or stem cells markers (i.e., α-SMA) for better understanding of various aspects of tertiary dentinogenesis.
Acknowledgements
We would like to thank many individuals including Ms. Barbra Rodgers and all the people that helped with laboratory techniques used in this experiment. This study was supported by grants from NIH (R01-DE016689) to MM and CAPES (3422/09-7) to MF.
References
- Akimoto N, Momoi Y, Kohno A, Suzuki S, Otsuki M, Cox CF. Biocompatibility of Clearfil Liner Bond 2 and Clearfil AP-X system on nonexposed and exposed primate teeth. Quintessence International. 1998;29:177–88. [PubMed] [Google Scholar]
- Andelin WE, Shabahang S, Wright K, Torabinejad M. Identification of hard tissue after experimental pulp capping using dentin sialoprotein (DSP) as a marker. Journal of Endodontics. 2003;29:646–50. doi: 10.1097/00004770-200310000-00008. [DOI] [PubMed] [Google Scholar]
- Balic A, Aguila HL, Mina M. Identification of cells at early and late stages of polarization during odontoblast differentiation using pOBCol3.6GFP and pOBCol2.3GFP transgenic mice. Bone. 2010;47:948–58. doi: 10.1016/j.bone.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balic A, Mina M. Analysis of developmental potentials of dental pulp in vitro using GFP transgenes. Orthodontics and Craniofacial Research. 2005;8:252–8. doi: 10.1111/j.1601-6343.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- Balic A, Mina M. Identification of secretory odontoblasts using DMP1-GFP transgenic mice. Bone. 2011;48:927–37. doi: 10.1016/j.bone.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balic A, Rodgers B, Mina M. Mineralization and expression of Col1a1-3.6GFP transgene in primary dental pulp culture. Cells Tissues Organs. 2009;189:163–8. doi: 10.1159/000154813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrieshi-Nusair KM, Hammad HM. Intracoronal sealing comparison of mineral trioxide aggregate and glass ionomer. Quintessence International. 2005;36:539–45. [PubMed] [Google Scholar]
- Braut A, Kollar EJ, Mina M. Analysis of the odontogenic and osteogenic potentials of dental pulp in vivo using a Col1a1-2.3-GFP transgene. International Journal of Development Biology. 2003;47:281–92. [PubMed] [Google Scholar]
- de Souza Costa CA, Lopes do Nascimento AB, Teixeira HM, Fontana UF. Response of human pulps capped with a self-etching adhesive system. Dental Materials. 2001;17:230–40. doi: 10.1016/s0109-5641(00)00076-2. [DOI] [PubMed] [Google Scholar]
- Dominguez MS, Witherspoon DE, Gutmann JL, Opperman LA. Histological and scanning electron microscopy assessment of various vital pulp-therapy materials. Journal of Endodontics. 2003;29:324–33. doi: 10.1097/00004770-200305000-00003. [DOI] [PubMed] [Google Scholar]
- Everett ET. Fluoride’s effects on the formation of teeth and bones, and the influence of genetics. Journal of Dental Research. 2011;90:552–60. doi: 10.1177/0022034510384626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M, Kenmotsu S, Nakasone N, Nakakura-Ohshima K, Ohshima H. Cell dynamics in the pulpal healing process following cavity preparation in rat molars. Histochemistry and Cell Biology. 2008;130:773–83. doi: 10.1007/s00418-008-0438-3. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Ida-Yonemochi H, Suzuki H, et al. Mapping of BrdU label-retaining dental pulp cells in growing teeth and their regenerative capacity after injuries. Histochemistry and Cell Biology. 2010;134:227–41. doi: 10.1007/s00418-010-0727-5. [DOI] [PubMed] [Google Scholar]
- Jaber L, Mascres C, Donohue WB. Electron microscope characteristics of dentin repair after hydroxylapatite direct pulp capping in rats. Journal of Oral Pathology & Medicine. 1991;20:502–8. doi: 10.1111/j.1600-0714.1991.tb00413.x. [DOI] [PubMed] [Google Scholar]
- Koliniotou-Koumpia E, Tziafas D. Pulpal responses following direct pulp capping of healthy dog teeth with dentine adhesive systems. Journal of Dentistry. 2005;33:639–47. doi: 10.1016/j.jdent.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Kuratate M, Yoshiba K, Shigetani Y, Yoshiba N, Ohshima H, Okiji T. Immunohistochemical analysis of nestin, osteopontin, and proliferating cells in the reparative process of exposed dental pulp capped with mineral trioxide aggregate. Journal of Endodontics. 2008;34:970–4. doi: 10.1016/j.joen.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Mina M, Braut A. New insight into progenitor/stem cells in dental pulp using Col1a1-GFP transgenes. Cells Tissues Organs. 2004;176:120–33. doi: 10.1159/000075033. [DOI] [PubMed] [Google Scholar]
- Ogawa R, Saito C, Jung HS, Ohshima H. Capacity of dental pulp differentiation after tooth transplantation. Cell and Tissue Research. 2006;326:715–24. doi: 10.1007/s00441-006-0242-0. [DOI] [PubMed] [Google Scholar]
- Palermo AT, Labarge MA, Doyonnas R, Pomerantz J, Blau HM. Bone marrow contribution to skeletal muscle: a physiological response to stress. Developmental Biology. 2005;279:336–44. doi: 10.1016/j.ydbio.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Paranjpe A, Zhang H, Johnson JD. Effects of mineral trioxide aggregate on human dental pulp cells after pulp-capping procedures. Journal of Endodontics. 2010;36:1042–7. doi: 10.1016/j.joen.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review--Part I: chemical, physical, and antibacterial properties. Journal of Endodontics. 2010;36:16–27. doi: 10.1016/j.joen.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Shahravan A, Jalali SP, Torabi M, Haghdoost AA, Gorjestani H. A histological study of pulp reaction to various water/powder ratios of white mineral trioxide aggregate as pulp-capping material in human teeth: a double-blinded, randomized controlled trial. International Endodontic Journal. 2011;44:1029–33. doi: 10.1111/j.1365-2591.2011.01916.x. [DOI] [PubMed] [Google Scholar]
- Simon S, Cooper P, Smith A, Picard B, Ifi CN, Berdal A. Evaluation of a new laboratory model for pulp healing: preliminary study. International Endodontic Journal. 2008;41:781–90. doi: 10.1111/j.1365-2591.2008.01433.x. [DOI] [PubMed] [Google Scholar]
- Simon SR, Berdal A, Cooper PR, Lumley PJ, Tomson PL, Smith AJ. Dentin-pulp complex regeneration: from lab to clinic. Advances in Dental Research. 2011;23:340–5. doi: 10.1177/0022034511405327. [DOI] [PubMed] [Google Scholar]
- Sloan AJ, Smith AJ. Stem cells and the dental pulp: potential roles in dentine regeneration and repair. Oral Diseases. 2007;13:151–7. doi: 10.1111/j.1601-0825.2006.01346.x. [DOI] [PubMed] [Google Scholar]
- Takamori Y, Suzuki H, Nakakura-Ohshima K, et al. Capacity of dental pulp differentiation in mouse molars as demonstrated by allogenic tooth transplantation. Journal of Histochemistry and Cytochemistry. 2008;56:1075–86. doi: 10.1369/jhc.2008.951558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington RJ, Youde SJ, Lee CP, Sloan AJ. Isolation of distinct progenitor stem cell populations from dental pulp. Cells Tissues Organs. 2009;189:268–74. doi: 10.1159/000151447. [DOI] [PubMed] [Google Scholar]
- Zhao C, Hosoya A, Kurita H, et al. Immunohistochemical study of hard tissue formation in the rat pulp cavity after tooth replantation. Archives of Oral Biology. 2007;52:945–53. doi: 10.1016/j.archoralbio.2007.04.015. [DOI] [PubMed] [Google Scholar]