Abstract
Background
Neurons containing proopiomelanocortin (POMC) derived peptides, known to control stress axis, metabolic and immune functions, have a lower function in patients with a family history of alcoholism, raising the possibility that alcohol effects on the POMC system may transmit through generations. Here we describe epigenetic modifications of Pomc gene that transmit through generation via male germline and may be critically involved in alcoholism-inherited diseases.
Methods
Whether an epigenetic mechanism is involved in causing a Pomc expression deficit in fetal alcohol exposed rats is studied by determining Pomc gene methylation, expression and functional abnormalities and their normalization following suppression of DNA methylation or histone acetylation. Additionally, transgenerational studies were conducted to evaluate the germline-transmitted effect of alcohol.
Results
Fetal alcohol exposed male and female rat offspring showed a significant deficit in POMC neuronal functions. Associated with this was an increased methylation status of several CpG dinucleotides in the proximal part of the Pomc promoter region and altered level of histone modifying proteins and DNA methyltransferases levels in POMC neurons. Suppression of histone deacetylation and DNA methylation normalized Pomc expression and functional abnormalities. Fetal alcohol-induced Pomc gene methylation, expression and functional defects persisted in the F2 and F3 male but not in female germline. Additionally, the hypermethylated Pomc gene was detected in sperms of fetal alcohol exposed F1 offspring that was transmitted through F3 generation via male germline.
Conclusions
Trangenerational epigenetic studies should spur new insight into the biological mechanisms that influence the sex-dependent difference in genetic risk of alcoholism-inherited diseases.
Keywords: Transgenerational epigenetic, fetal alcohol, male germline, DNA methylation, stress hormone, proopiomelanocortin
INTRODUCTION
The gene encoding POMC produces two different classes of peptides, melanocortin and β-endorphin (BEP), which have diverse functions including the regulation of energy homeostasis, the stress response, immune system functions and the brain reward system (1–5). Association analysis connects POMC system abnormalities with metabolic diseases, increased alcohol preference and cancer in human patients (6–10). One of the environmental factors that produce abnormalities to the POMC system is alcohol drinking by parents. Children who are exposed to alcohol during fetal life often show behavioral and physiological changes such as depression, anxiety, hyperactivity and an inability to deal with stressful situations (11–13), as well as increased neonatal infection and childhood leukemia (14, 15). Rodents exposed to alcohol during fetal life have shown to express a lower level of POMC as well as pathologies including stress hyperresponsiveness, obesity, diabetes, and immune problems (4, 16–18). Paternal alcohol consumption has been shown to affect POMC neuronal functions in the offspring (19, 20). Furthermore, patients with a family history of alcoholism often show an increased risk for POMC system-related abnormalities (21). Epigenetic changes are now being considered as potential mechanisms of the long-term effects of many toxicants when individuals are exposed to them during development (22). Hence, by using the animal model of fetal alcohol exposure we determined whether alcohol exposures during the developmental period incite epigenetic marks leading to permanent alteration of Pomc gene expression in the hypothalamus. Furthermore, we tested whether fetal alcohol-induced epigenetic marks on the Pomc gene propagate through generations.
Methods and Materials
Animals and treatments
Sprague-Dawley rats obtained from Charles River (Wilmington, MA) were bred and individually housed with 12-h light/12-h dark cycles at a constant temperature (22°C) throughout the study. These rats were bred in our animal facility. On gestational day (GD) 7–21, pregnant rats were fed rat chow ad libitum (AD), a liquid diet containing ethanol (BioServe Inc., Frenchtown, NJ) (AF), or pair-fed an isocaloric liquid control diet (with the ethanol calories replaced by maltose-dextrin) (PF). AF, PF and AD male and female rat offspring of F1, F2 and F3 generations were used in this study. Transgenerational studies were conducted by breeding FAE rats with control animals of the opposite gender to produce two germlines, male germline by breeding male fetal alcohol rats and their male offspring with normal females (AFM), and female germline by breeding female fetal alcohol rats and their offspring with normal males (AFF). F1~F3 generations rats at 60 and 80 days after birth were used in this study.
Some of the AF, PF and AD F1 offspring were injected subcutaneously with trichostatin (TSA; 2 mg/kg; Sigma), 5-azadeoxycytidine (AZA; 5 mg/kg; Sigma) or vehicle (control) on day 1, 3 and 5 after birth. We administered TSA and AZA during the neonatal period rather than the prenatal period because long-term suppression of DNA methylation during pregnancy could potentially be harmful to the maintenance of pregnancy and fetal growth (23). Rats were sacrificed between 60 and 80 days after birth. Brain tissues of these rats were used for the collection of arcuate nucleus (ARC) or paraventricular nucleus (PVN) areas (24). Peripheral blood mononuclear cells (PBMC) were isolated using Histopaque from trunk blood samples of some of these rats. In some animals, sperm samples were collected from caudal epididymis as previously described (25). Stress responses were evaluated by measuring plasma levels of stress hormones following intraperitoneal injection of 100 µg/kg lipopolysaccharide (LPS; Sigma) or saline at various time period. We used LPS model to determine changes in the POMC neuronal function in fetal alcohol exposed rats because this model allow us to detect the changes in the BEP-regulated hypothalamic-pituitary adrenal (HPA) axis function. In this model, LPS simultaneously activates both corticotrophin-releasing hormone (CRH) and BEP secretion from the hypothalamus (4). However because BEP inhibits CRH release, the amount of CRH release from the hypothalamus and thereby ACTH release from the pituitary and corticosterone release from the adrenal gland following LPS challenge increase when BEP peptide levels in the hypothalamus is lower as in fetal alcohol exposed animals (16). Further support to this concept comes from the data showing that transplantation of BEP-producing neurons in the paraventricular nucleus (PVN) of the hypothalamus normalizes ACTH and corticosterone response to the LPS challenge in fetal alcohol-exposed rats (4). In addition, recent evidence suggests that alcohol drinking increases blood level of LPS and LPS binding proteins (26, 27). Animal surgery and care were performed in accordance with institutional guidelines and complied with the National Institutes of Health policy.
Detailed methods for animal breeding, alcohol feeding, methylation assays, RNA quantitation analysis, immunocytochemical detection of histone and DNA methyltransferese proteins in BEP and CRH cells, hormone assays for BEP, ACTH and corticosterone are described in Methods in the Supplement.
Statistics
Data are presented as mean ± s.e.m. The number of animal was indicated within bracket under or above each histogram. Statistical tests employed and the level of significance are given in Tables S2–9 (see Supplement).
RESULTS
Effects of fetal alcohol exposure on Pomc gene expression and stress axis function in offspring
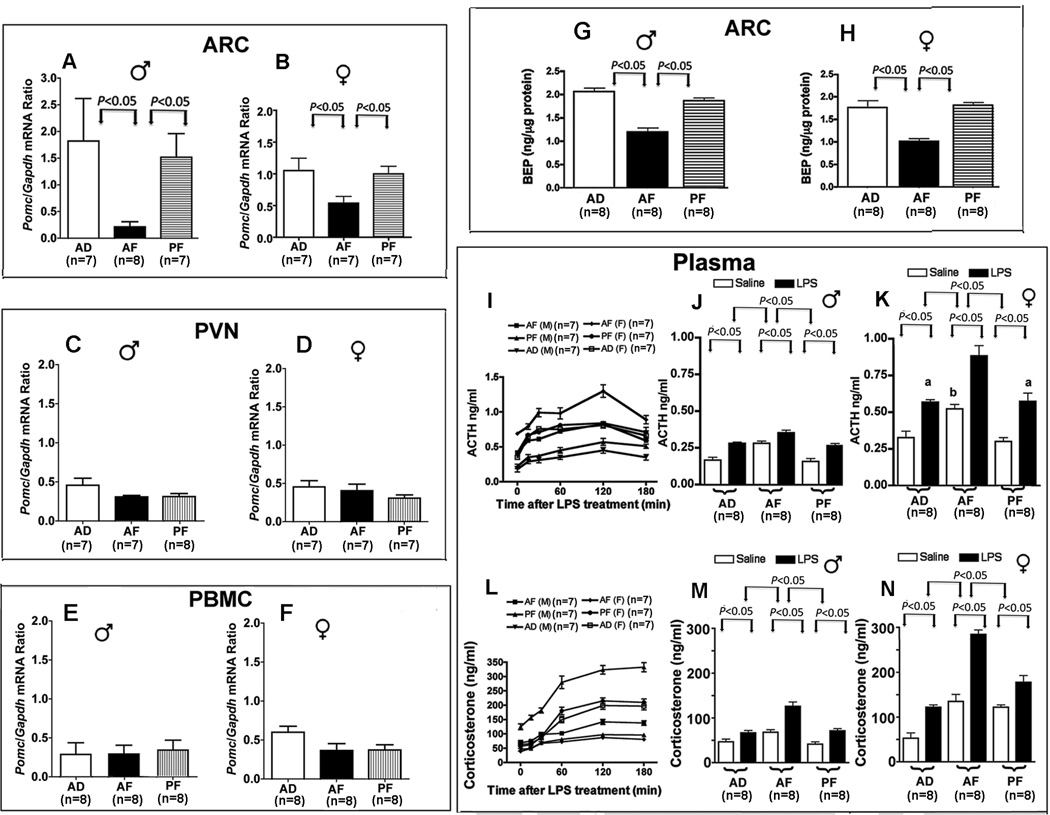
We used a well established liquid diet model of alcohol feeding in pregnant rats, which produces offspring with endophenotypes similar to those found in human fetal alcohol spectrum disorders (e.g., anxiety behaviors, stress hyper-responsiveness, metabolic diseases; 4, 16–18). Alcohol feeding or pair feeding did not affect body growth of dams or their litter size (Figure S1B, C in the Supplement) nor did these procedures lead to having uncompromised body growth of AF and PF offspring (Fig. S1D, E in the Supplement). Determination of Pomc mRNA levels expressed by the ratio of Gapdh (Fig. 1A, B), β-actin (Pomc:Actin mRNA Ratio; n=7–8; AD, 1.44 ± 0.23; AF, 0.08 ± 0.04; PF, 1.07 ± 0.11; P<0.01) and 18S (Pomc:18S mRNA Ratio; n=7–8; AD, 0.91± 0.16; AF, 0.06 ± 0.04; PF, 0.67 ± 0.04; P<0.01) as housekeeping genes in the ARC (where majority of POMC cells are located) revealed that they were similar in AD and PF male and female rats during adulthood, suggesting a minimum impact of the liquid diet feeding paradigm on Pomc gene expression. However, Pomc mRNA levels in the ARC were lower in AF compared to AD and PF rats, without any sex difference. The inhibitory effect of fetal alcohol exposure on Pomc gene expression is targeted to the ARC, since it was not observed in the Pomc gene of low-expressing PVN tissues (Fig. 1C, D) or in the PBMC (Fig. 1E, F). The effect of fetal alcohol on Pomc gene expression in the pituitary and ARC were not compared, since the expression of hypothalamic but not the pituitary Pomc gene is regulated by several enhancer elements located approximately 10 to 12 kb upstream of its transcriptional units (28). Additionally, hypothalamic POMC by producing BEP inhibits pituitary POMC production via suppressing CRH secretion (4).
Figure 1. Consequences of fetal exposure to alcohol on proopiomelanocortin (POMC neuronal function in adult male (♂) and female (♀) rat offspring of pregnant rats fed alcohol (AF), pair-fed isocaloric diet (PF), or ad libitum-fed rat chow (AD).
Changes in the level of Pomc mRNA in the arcuate nucleus (ARC; A, B), paraventricular nucleus (PVN; C, D) and peripheral blood mononuclear cells (PBMC; E, F), the levels of β-endorphin (BEP; G, H) in the ARC of male and female fetal alcohol exposed rat as compared to control rat offspring. Also shown are the effects of fetal alcohol exposure on the time-dependent response of plasma ACTH (I) and corticosterone (L) levels in plasma following treatment with lipopolysaccharide (LPS; 100 µg/kg body weight; sc) of males and females; the basal and LPS-stimulated ACTH levels in plasma at 2 hours in males (J) and females (K); the basal and LPS-stimulated corticosterone levels in plasma at 2 hours in males (M) and females (N). Data presented are mean ± s.e.m. The number of animals was indicated within brackets under each histogram. Data presented in panels A-H were analyzed using one-way ANOVA followed by the Student Newmann-Keuls posthoc test (see also Table S2 in the Supplement). Data presented in panels I and L were analyzed with the two-way analysis of variance, which identified interaction between feeding and time (P<0.001). Bonferroni posthoc test showed that both mean ACTH levels and corticosterone levels at 120 min in AF rats were higher than the rest of the groups (P<0.05). Significance of differences between other groups were shown by a bar above histograms.
Determination of the level of BEP in the ARC verified the inhibitory action of fetal alcohol on production of this peptide in both male and female rats (Fig. 1G, H). Since a reduced BEP level in the ARC is known to result in an increase in responsiveness of the stress axis to LPS (3, 4, 16), we also determined the changes in corticosterone and ACTH responses to LPS in fetal alcohol exposed rats. As expected, both basal and LPS-stimulated ACTH levels (Fig. 1I–K) and corticosterone levels (Fig. 1L–N) were higher in AF rats than in control-fed rats. These data suggest that fetal alcohol-exposure programs the hypothalamus, such that the adult expression of the Pomc gene is reduced resulting in stress axis hyperresponsiveness.
Effects of fetal alcohol on the methylation status of the CpG island of Pomc gene
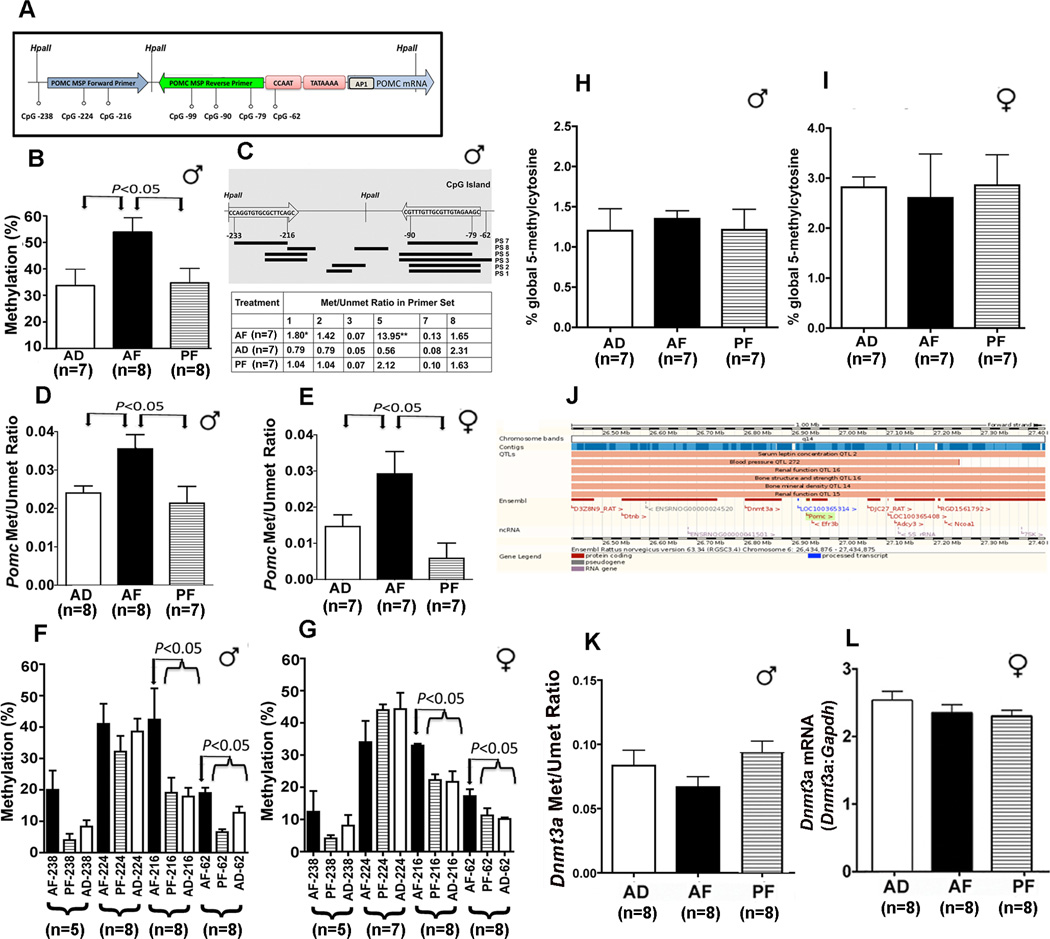
Global or site-specific methylation of CpG sites near and within regulatory regions of genes is often associated with transcriptional inactivity and gene suppression (29). The rat Pomc gene contains a 5' CpG island that surrounds the Pomc transcription start site. Methylation of the CpG island in the human Pomc promoter was shown to be responsible for gene silencing in non-expressing tissues (30). In order to look into epigenetic changes in the Pomc gene, initial digestion with the methylation-sensitive enzyme HpaII and subsequently by real-time PCR with primers flanking recognition sites were performed to determine if there were any change in DNA methylation in the proximal part of the Pomc gene. The rat Pomc proximal promoter region contains three HpaII sites upstream of the transcriptional start site in the positions −107, −152, and −238 (Fig. 2A). The percentage of methylation assessed by the ability of HpaII to digest these sites was significantly higher in the AF group compared to control groups (Fig. 2B). In order to characterize the extent of cytosine methylation of other CpG dinucleotides in the Pomc proximal promoter, we designed six sets of primers specific for either the methylated or the unmethylated state of various CpG-rich sites adjacent to the Pomc gene transcription start site. Using these primers and the SYBR Green methylation specific real-time PCR (MSP), several locations on the Pomc promoter region were identified where the cytosine methylation in AF animals was significantly higher than in control groups (Fig. 2C). In particular, in the more proximal part of the promoter spanning the region −81 to −154, as detected by the use of primer 5, the ratio of methylated to unmethylated DNA was markedly higher in AF rats in comparison to control groups. TaqMan MSP assay with the probes derived from the sequence in the region −81 to −154 confirmed significant differences in cytosine methylation between AF and control-fed animals in both male and female sexes (Fig. 2D, E). Pyrosequencing techniques were also employed to further verify Pomc gene methylation (Fig. 2F, G). The methylation status of four CpG dinucleotides (−238, −224, −216 and −62) in the proximal promoter of the Pomc gene was determined by the pyrosequencing technique (the rest of the CpG's in the area could not be resolved). The positions −238, −224 and −216 are covered by our forward MSP primer and position −62 is immediately downstream of the reverse MSP primer (Table S1 in the Supplement). Pyrosequencing data confirmed the MSP values showing increased methylation of the CpG dinucleotides in AF animals in comparison to control-fed groups. Both MSP and pyrosequencing showed an increase in the DNA methylation of the proximal part of the rat Pomc promoter in AF animals in comparison to control-fed rats in both sexes. In order to determine whether Pomc DNA hypermethylation reflects the overall global DNA methylation status, we compared the % of global 5-methylcytosine levels in the ARC of AF, PF and AD male and female rats. 5-methycytosine levels were comparable between treatment groups (Fig. 2H, I), suggesting that the hypermethylation effect of fetal alcohol on Pomc DNA is not universal but rather gene specific. This view is further supported by the observation that fetal alcohol exposure failed to alter methylation of Dnmt3a gene (Fig. 2K), which is located close to Pomc gene (Fig. 2J) and whose mRNA expression is unresponsive to fetal alcohol treatment (Fig. 2L). Additionally, we determined the ratio of methylated to unmethylated Pomc gene in the PVN tissue where the expression of Pomc gene is low (Fig. 1C,D). We found no significant differences between the ratio of methylated to unmethylated Pomc gene in the PVN of AF, PF and AD rats (Pomc Met/Umet Ratio; n=4; AD, 0.092 ± 0.013; AF, 0.091 ± 0.009; PF, 0.08 ± 0.005; Kruskal-Wallis ANOVA, P=0.79). Overall, these data indicate that fetal alcohol alters methylation of Pomc DNA in adulthood, even though the exposure to ethanol ended at the prenatal period.
Figure 2. Fetal alcohol- Pomc gene methylation in the arcuate of the hypothalamus of F1 adult rat offspring.
Showing the different CpG sites and HpaII sites on the rat Pomc proximal promoter and the area covered by the primers used in the MSP assay used in this study (A). The percentage of methylation as assessed by HpaII digestion followed by real-time PCR with the primers chosen from the regions flanking the restriction sites of the Pomc gene in DNA prepared from the ARC of alcohol-fed (AF), pair-fed (PF) and ad lib-fed (AD) male rats (B). Changes in methylation state of different CpG sites in the Pomc promoter as assessed by bisulfite conversion followed by real-time PCR using primer sets spanning different CpG sites of the Pomc promoter (top; C) and determining the methylation-to-unmethylation ratio presented in the table (bottom; C). Methylation-to-unmethylation ratio of the CpG pairs in the −81 to −154 region of the Pomc promoter as measured using TaqMan methylation-specific real-time PCR in the ARC of males (♂) and female (♀) AF, PF and AD rats (D, E). DNA methylation status of four CpG dinucleotides from proximal POMC promoter determined by pyrosequencing analysis of the ARC of AF, PF and AD male (F) and female (G) rats. After bisulfite conversion, the DNA was subjected to pyrosequencing in regions encompassing four CpG dinucleotides. The numeration starts from the POMC gene transcription start site. 5-methylcytosine levels in the DNA of ARC of AD, AF and PF male and female rats were measured using MethylFlash Methylated Quantification kit (Epigentek, NY) and shown in panels (H and I). Showing the location of the Rattus norvegicus Dnmt3a gene relative to Pomc gene as illustrated in Ensembl Genome Browser (J). Showing the effects of fetal alcohol exposure on Dnmt3a gene methylation (K) and Dnmt3a mRNA (L) in the ARC of the hypothalamus in rats prenatally exposed to alcohol (AF) or control diets (PF and AD). Data presented are mean ± s.e.m. The number of animals was indicated within brackets under each histogram. Data shown in panels were analyzed using one-way ANOVA followed by Student-Newman-Keuls posthoc test, except those of Pomc methylation and unmethylated ratio, which was analyzed using Kruskal-Wallis ANOVA followed by Dunn's posttest (see Table S3 in the Supplement). The significance of difference between groups was identified by a bar above the histograms.
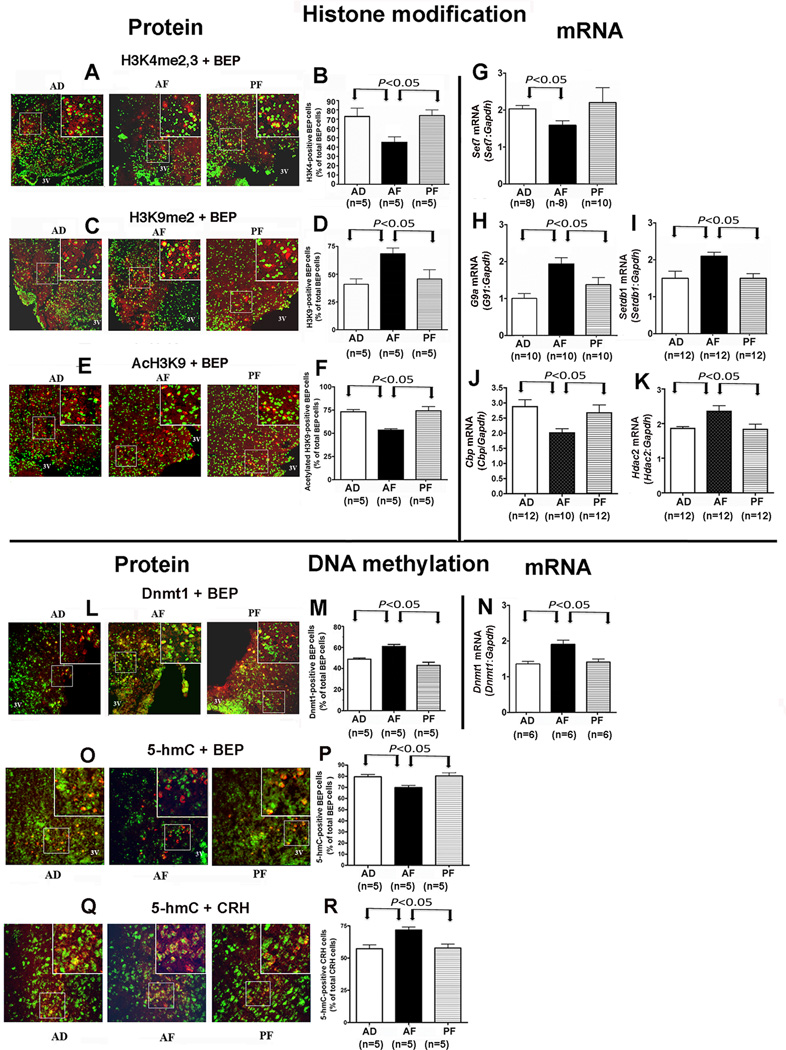
Effects of fetal alcohol on the level of enzymes involved in histone modifications and DNA methylation in POMC cells in the hypothalamus
Increased gene methylation can be caused by altered chromatin remodeling, which is known to be accomplished through posttranslational modifications of the amino termini of histone tails at specific residues, and/or by the addition of methyl groups by DNA methyltransferases (Dnmts) to the DNA occurring mostly at CpG sites (29). Whether fetal alcohol exposure caused long-lasting histone marks in POMC-neurons was evaluated by immunohistochemical localization of H3K9me2, H3K4me2,3, and AcH3K9 in BEP-positive cells in the ARC of male offspring. Preliminary experiment did not identify any sex differences in the fetal alcohol effect on these protein levels and therefore only male rats were used in this study. A reduced number of H3K4me2,3-positive BEP cells (Fig. 3A, B), an increased number of H3K9me2-positive BEP cells (Fig. 3C, D) and a reduced number of AcH3K9-positive BEP cells (Fig. 3E, F) were observed in AF group as compared to two control groups. In agreement with these protein data, a decreased mRNA levels of Set7/9 that mono-methylates H3k4an increased in mRNA levels of the histone methyl transferase G9awhich dimethylates H3k9me2and of Setdb1, which mono-, di-, and trimethylates H3K9 (31) were observed in the ARC of the hypothalamus of AF rats (Fig. 3G–I). The reduced acH3K9 protein data also positively correlated with the mRNA levels of CREB-binding protein (Cbp; Fig. 3J), which has significant histone acetyltransferase activity to increase acH3K9 (32), and negatively correlated with histone deacetylase 2 (HDAC2; Fig. 3K), which is widely expressed in the brain and has significant deacetylation activity (33). Increased H3K9 methylation level is known to repress while increased H3K4 methylation level is known to activate gene expression (34). Additionally, alcohol has been shown to increase HDAC2 levels while reducing Cbp levels to suppress acH3K9 and gene expression in the brain (35). Hence, fetal alcohol induced changes in the POMC gene might involve increased H3K9 methylation/deacetylation.
Figure 3. Fetal alcohol-induced alteration in the levels of histone and DNA methyltransferases in POMC neurons in the arcuate area of the hypothalamus.
Showing the changes in the number of BEP (a protein product of POMC) cell-positive to di and trimethylated histone H3 at lysine 4 (H3K4me2,3; A, B), dimethylated histone H3 at lysine 9 (H3K9me2; C, D), acetylated H3 at lysine 9 (AcH3K9; E, F), DNA methyltransferase 1 (Dnmt1; L, M), 5-hydroxymethylcytosine (5–hmC; O,P), corticotropin releasing hormone (CRH) cell positive 5-hmC (Q, R), and mRNA levels of Set7 (G), G9a (H), Setdb1 (I), CREB binding protein (Cbp; J), histone deacetylase 2 (Hdac2; K) and Dnmt1 (N) in the hypothalamus of male adult offspring of pregnant rats ad lib-fed (AD), alcohol-fed (AF) or pair-fed isocaloric diet (PF). Representative photographs show the double-labeled cells, indicated by arrows, in each treatment group, and histograms show the mean ± SEM values of the percentage of BEP cells that were double-labeled or the gene to Gapdh mRNA ratios. Data were analyzed using one-way ANOVA followed by the Student-Newman-Keuls posthoc test (Table S4 in the Supplement). The number of animals was indicated within brackets under each histogram. The significance of difference between groups was identified by a bar above the histograms.
It has been proposed that Dnmts could only access the DNA that is wrapped around nucleosomal histones with H3K9 methylation signal (36). Hence, we tested whether significant changes in one of the Dnmts level has also occurred in POMC cells of fetal alcohol exposed animals. Dnmts (Dnmt1, Dnmt3a and Dnmt3b) are known to catalyze the transfer of a methyl group to DNA. Since Dnmt3a mRNA level did not change in fetal alcohol exposed animals (Fig. 3L) and Dnmt3b immunoreactivity levels were very low in BEP cells, we measured only the changes in Dnmt1 levels in BEP cells. The data revealed a stimulatory effect of fetal alcohol exposure on the level of Dnmt1 in BEP cells (Fig.3L, M). Dnmt1 mRNA levels in the ARC of AF rats were also higher than those in PF and AD rats (Fig.3N). These results identify a possible involvement of Dnmt1 in fetal alcohol induced changes in Pomc gene methylation and expression.
The methylation of C5 of cytosine 5-methylcytosine (5–mC) by Dnmts is the most stable heritable chromatin modification, which is conducive for inhibition of transcription (37). It is suggested that the oxidation of 5-mC to 5-hydroxymethylcytosine (5–hmC) by hydroxylases could modulate the binding of proteins or transcription factors to the chromatin and hence alter transcriptional outcome. 5-hmC also facilitates demethylation and promotes gene transcription (38). Thus, the modulation of the balance between methylation and hydroxymethylation by external factors could alter gene expression. Hence, we determined the protein levels of 5-hmC in β-endorphin-producing neurons of the ARC of AF, PF and AF rats. We found that fetal alcohol exposure decreased significantly the protein level of 5-hmC in BEP-producing POMC neurons (Fig. 3O, P). In order to determine that the changes in DNA methylation modifier mark specific to ARC, we measured 5-hmC levels, as representative marker of methylation modifier, in a CRH-producing cell population whose functions are known to be increased in fetal alcohol-exposed rats (4, 16). We found that 5-hmC levels in CRH neurons were elevated in AF rats as compared to AD and PF rats (Fig. 3Q, R). Thus, the opposing data of 5-hmC in BEP and CRH cells could indicate that fetal alcohol-induced Pomc gene hypermethylation by Dnmts in the ARC area is not global, but rather is gene and cell specific.
Effects of DNA methyltransferase and histone deacetylase inhibitors on fetal alcohol-induced changes in Pomc gene expression and stress axis function
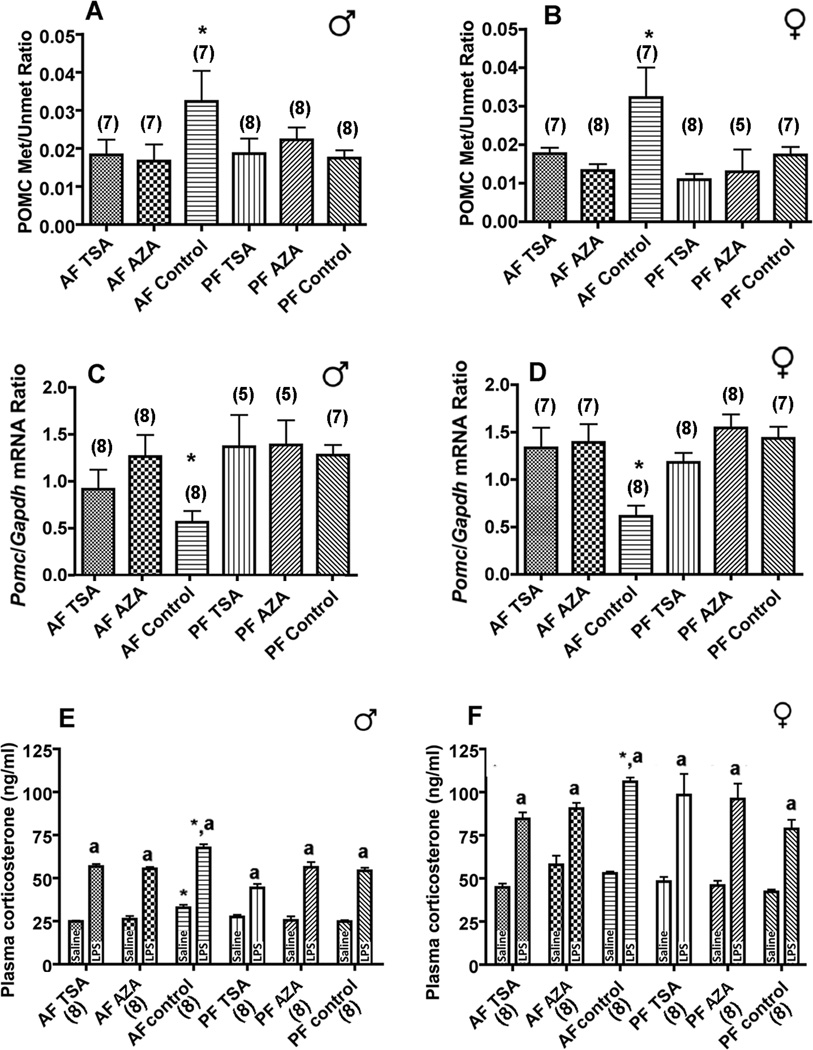
Since fetal alcohol exposure caused significant changes in levels of histone modifiers and Dnmt1, we tested whether transcriptional repression can be relieved to normalize POMC gene expression and neurotransmission by the Dnmt blocker AZA or the histone deacetylase inhibitor TSA (39, 40). We found that treatment with TSA or AZA significantly reduced the fetal alcohol-induced Pomc gene hypermethylation and normalized Pomc mRNA levels in male and female rat offspring (Fig. 4A–D). We also found that both AZA and TSA reduced the high corticosterone-response to LPS in these rats (Fig. 4E, F). These data suggest that DNA hypermethylation in the proximal Pomc promoter, possibly caused by a significant alteration in histone modifications and DNA methylation, results in fetal alcohol-induced reduction of Pomc gene expression and functions. However, the functional role of this promoter domain in methylated and unmethylated form need to be determined in order to establish this view.
Figure 4. Suppression of histone and DNA methylation prevents fetal alcohol effects on POMC neuronal function.
Effects of DNA methyltransferase blocker 5-azadeoxycytidine (AZA) and the histone deacetylase inhibitor trichostatin (TSA) on Pomc gene methylation (male, A; female, B) and Pomc mRNA expression in ARC tissues (male, C; female, D) and on corticosterone levels in plasma (male, E; female, F). Data presented are mean ± s.e.m. The number of animals was indicated within brackets under each histogram. Data shown in panels A and B were analyzed using using Kruskal-Wallis ANOVA followed by Dunn's posttest, panels C and D were analyzed using one-way ANOVA followed by Student-Newman-Keuls posthoc test, panels E and F were analyzed using two-way ANOVA followed by Bonferroni posthock test (see Table S5 in the Supplement). The significance of difference between groups was identified by a bar above histograms.
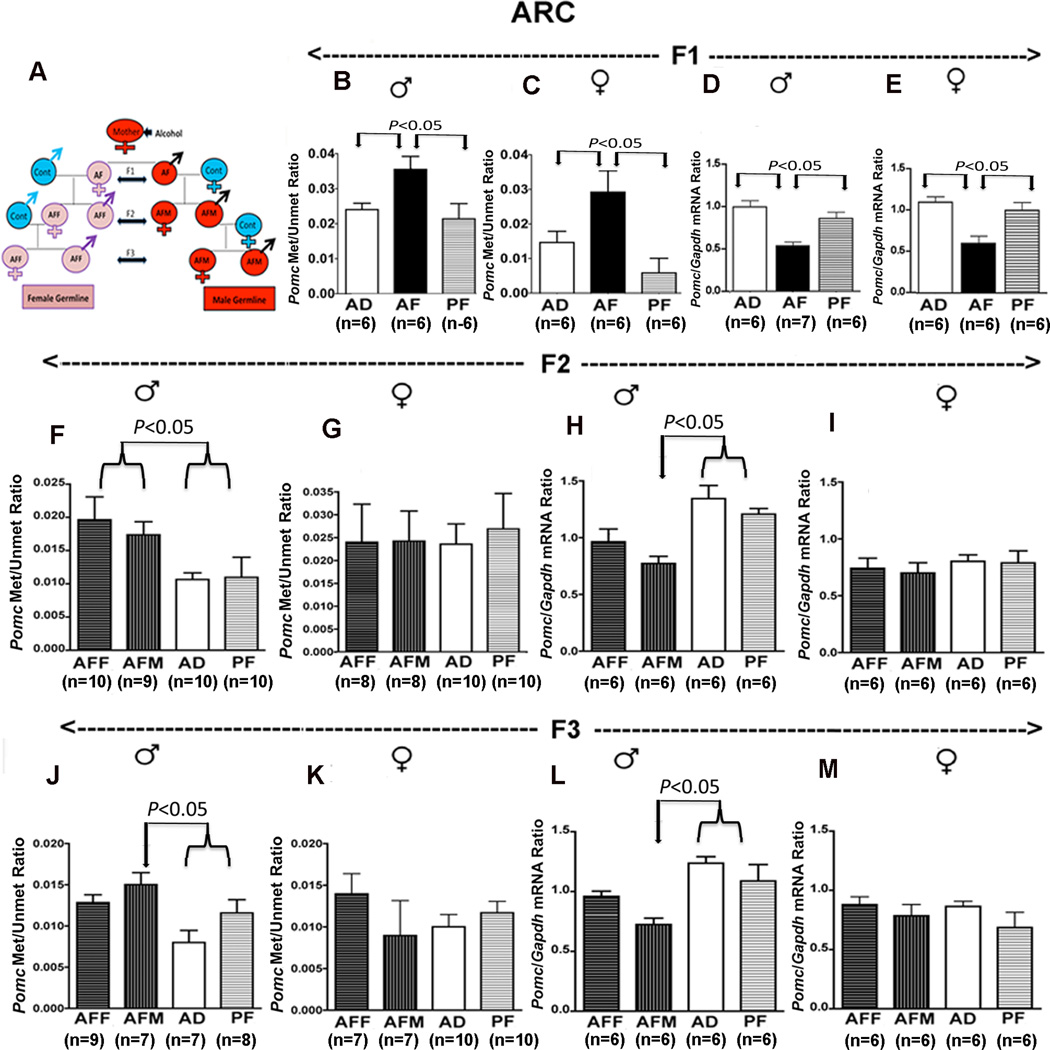
Fetal alcohol induced transgenerational changes in POMC neuronal function
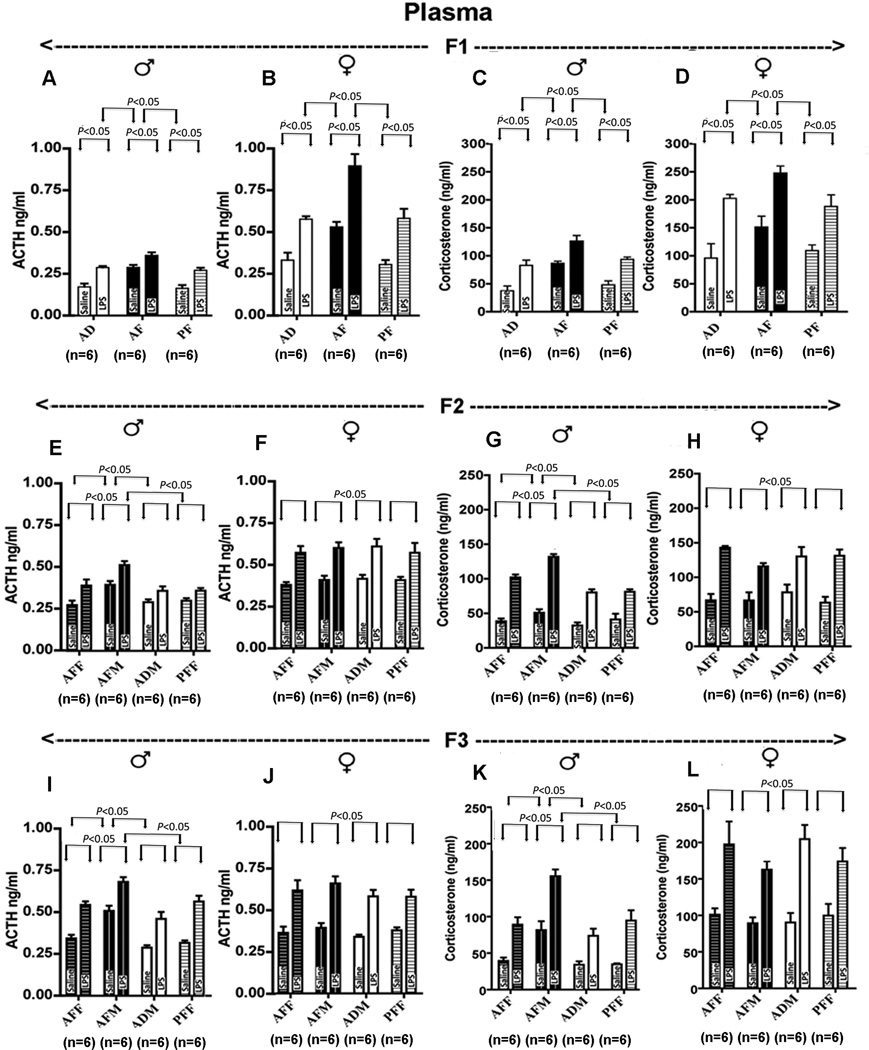
Whether the fetal alcohol effect is transmitted transgenerationally was tested by studying POMC neuronal function in the F1-F3 male (AFM) and female germlines (AFF). We produced two different germlines—a male germline by breeding male fetal alcohol exposed rats and their male offspring with normal females, and a female germline by breeding female fetal alcohol exposed rats and their female offspring with normal males (Fig. 5A). The DNA methylation status of the proximal part of the Pomc promoter in the AF animals in comparison to the control group was increased, and the Pomc gene expression level was decreased in both the male and female F1 progeny (Fig. 5B–E). The F2 and F3 male progeny of male germline demonstrated significant increase in DNA methylation levels with the concomitant reduction in Pomc gene expression levels (Fig. 5F–M). Only the male offspring of F2 generation from female germline showed a moderate increase in Pomc methylation but not Pomc gene expression. There were no differences in the degree of DNA methylation or Pomc gene expression among the groups in female F2 and F3 rats irrespective of their germline differences. Because the offspring (F1) and its germ cells (F2) were exposed to alcohol during development via the F0 female, the persistence of alcohol effects in the F3 male but not in the F3 female suggest transgenerational transmission of fetal alcohol effects on Pomc gene methylation and expression via the male germline. To evaluate whether the transgenerational effect of fetal alcohol exposure applies to some endophenotypes of the Pomc defect, we determined stress hormone response to LPS in F1–F3 rat germlines. It was observed that fetal alcohol exposure increased the basal and LPS-stimulated ACTH and corticosterone levels in both male and female offspring of F1 progeny (Fig. 6A–D), but were observed only in males of the male germline in F2 and F3 progeny (Fig. 6E–L). These results suggest that fetal alcohol's effects on Pomc gene hypermethylation and stress axes abnormalities persist throughout adulthood and perpetuate into subsequent generations through the male germline.
Figure 5. Transgenerational changes in hypothalamic Pomc gene methylation, expression and functions after alcohol feeding in pregnant female rats (F0).
A schematic diagram to indicate how F1, F2 and F3 male germline (AFM) and female germline (AFF) fetal alcohol-exposed offspring were generated (A). Methylation-to-unmethylation ratio of the CpG pairs in the −81 to −154 region of the Pomc promoter in ARC tissues of F1 (B, C), F2 (F, G) and F3 (J, K) male and female rat offspring of male and female germlines. Pomc mRNA levels in the ARC tissues of F1 (D, E), F2 (H, I) and F3 (L, M) male and female rat offspring from different germlines. Data presented are mean ± s.e.m. The number of animals was indicated within brackets under each histogram. Pomc methylation and unmethylation ratio data were analyzed using using Kruskal-Wallis ANOVA followed by Dunn's posttest. Pomc mRNA levels data were analyzed using a one-way ANOVA followed by the Student-Newman-Keuls posthoc test (see Table S6 in the Supplement). The significance of difference between groups was identified by a bar above histograms.
Figure 6. Transgenerational changes in plasma ACTH and corticosterone responses to the lipopolysaccharide challenge after alcohol feeding in pregnant female rats (F0).
Basal (saline-treated) and LPS-stimulated ACTH levels in plasma of F1 (A, B), F2 (E, F) and F3 (I, J) male and female rat offspring of different germlines. Basal and LPS-stimulated corticosterone levels in plasma of F1 (C, D), F2 (G, H) and F3 (K, L) male and female rat offspring of different germlines. Data presented are mean ± s.e.m. The number of animals was indicated within brackets under each histogram. Data were analyzed using using two-way ANOVA followed by the Bonferroni posthoc test (see also Table S7 in the Supplement). The significance of difference between groups was identified by a bar above the histograms.
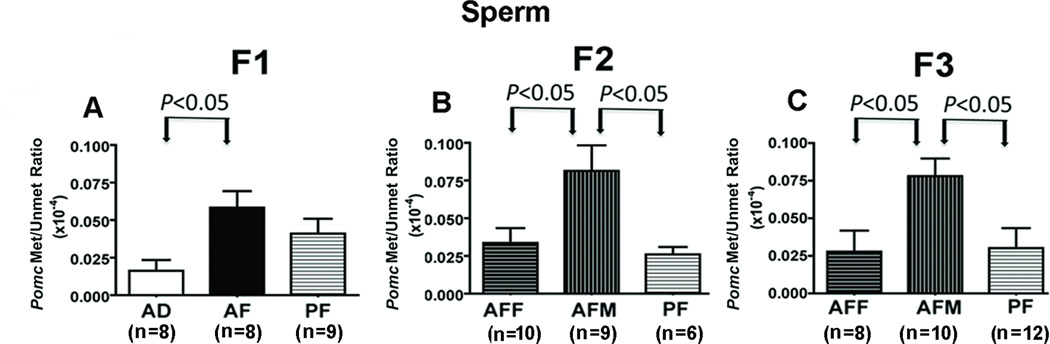
In order to further verify the male germline transmission of fetal alcohol's effects on Pomc, we measured the DNA methylation status of the Pomc promoter in sperm of F1–F3 male rats derived from the male (AFM) or female germline (AFF) and compared their levels with those of F1–F3 pair-fed male rats. The level of methylated and unmethylated Pomc DNA in sperm was detectable in the MSP assay and indicated a higher amount of Pomc gene methylation in AF rats than in control-fed rats (Fig. 7). Additionally, a comparison between male rats of various germlines revealed that the fetal alcohol effect on Pomc methylation in sperm persisted in the F1 through F3 generations of male rats derived from the male germline.
Figure 7. Fetal alcohol effect on Pomc gene in sperm transmitted through generations in the male germline but not in female germline.
Methylation-to-unmethylation ratio of the CpG pairs in the −81 to −154 region of the POMC promoter in sperm of F1 (A), F2 (B) and F3 (C) rat offspring of male (AFM) and female germlines (AFF). Data were analyzed using one-way ANOVA followed by Student-Newman-Keuls posthoc test. *, P < 0.05, compared with AD and PF (see also Table S7 in the Supplement).
DISCUSSION
We showed here that fetal alcohol exposure induces long-lasting hypermethylation of the Pomc gene. This conclusion is based on the findings that the percentage of the cytosine methylation of CpG-rich sites adjacent to the Pomc gene transcription start site was higher in alcohol-fed animals than in controls. Methylation of the proximal region of the promoter often correlates closely with a negative effect on gene expression (41). Moreover, this site is located in close proximity to the CCAAT box required for the constitutive transcriptional activation. Methylation at a CpG site just upstream of the CCAAT box can effectively interfere with binding of the transcription factors to the CCAAT sequence (42). Additionally, the pyrosequencing analysis confirmed higher methylation in this region. Both MSP and pyrosequencing show an increase in the DNA methylation of the proximal part of the rat Pomc promoter in alcohol-fed animals in comparison to controls. Additionally, the area covered by the forward MSP primer (−224 to −202) contains an 11 bp region (GTGCTAACGCC), which is highly conserved in all vertebrates and is necessary for high levels of Pomc expression in target tissues (43). Deletions or mutations in this region cause a significant decrease in Pomc gene expression in mice. Hence, increased DNA methylation in this area in alcohol-fed animals could impair the function of this region resulting in lower levels of Pomc gene expression. However, conclusive proof of this view will require further study in determining the functional role of this promoter domain in methylated and unmethylated form.
Our investigation on the histone modification and DNA methylation identified involvement of these epigenetic machineries in fetal alcohol-induced Pomc gene hypermethylation. We found fetal alcohol exposure increases H3K9 me2 level while it decreases the H3K4me2,3 level and acetylation of H3K9 in POMC cells, suggesting a significant aberrant post-translational modification of histones. We also found increased levels of Dnmt1 in POMC cells of fetal alcohol exposed animals, suggesting that the machinery that increases DNA methylation is enhanced in these cells. These data as well as the data showing that TSA or AZA reversed the suppressive effects of fetal alcohol on Pomc gene hypermethylation and gene expression suggest the possibility that fetal alcohol exposure alters histone postranslational modifications and increases DNA methylation machinery to cause Pomc gene suppression. However, the proof of this concept should require the demonstration of an altered Pomc gene transcription following promoter methylation in a functional assay system. Despite this limitation, our study identified altered Pomc gene methylation and expression in association of stress hyperresponse in fetal alcohol exposed animals.
We also provide the first evidence of perpetuation of the fetal alcohol-induced changes in Pomc DNA methylation across generations in two tissues, hypothalamus and sperm, through the male germline. Additionally, we provide evidence for transgenerational effect of fetal alcohol exposure on Pomc gene expression suppression, and one of the endophenotypes of the Pomc defect involving the hyper-stress-response of F1-F3 rat male germlines. Reason for this male-specific transgenerational transfer of the epigenetic modification of Pomc gene is currently unknown and thus a focus of ongoing studies. However, we showed in this study that fetal alcohol-induced hypermethylation of Pomc gene in sperm transferred accross three generations. Recently it has been shown that the non-paring part of the Y-chromosome influences the expression of BEP in the hypothalamus (44). Additionally, the transcription of Mc2Ra POMC receptor, is dependent on the Sry gene (45). Furthermore, paternally induced transgenerational environmental reprogramming of metabolic gene expression has recently been demonstrated (46). Also, transgeneration male germline imprinting of an endocrine disruptor during fetal life has been documented (47). Thus, our data highlight the novel possibility that epigenetic changes contribute to the effects of fetal alcohol exposure on Pomc gene expression as well as the perpetuation of abnormal POMC neuronal functions through subsequent generations via the male. Whether or not this male-specific transgenerational transfer of the epigenetic modification of Pomc gene is mediated via the Y chromosome or the mechanism involved in this process are currently unknown and thus a focus of ongoing studies. Nevertheless, data highlight the possibility that epigenetic changes contribute to fetal alcohol effects on Pomc as well as the perpetuation of stress axis dysfunction through generations. The novel transgenerational epigenetic effect of alcohol that we demonstrated in this study should be considered as an important covariant with genetic factors in the determination of the longitudinal effects of fetal alcohol exposure on physiological processes and possibly behaviors.
Supplementary Material
ACKNOWLEDGMENTS
The pyrosequencing study was conducted under the guidance of Dr. Lourdes Serrano. Supported by NIH R21 grant (AA16695) and R37 award (AA08757).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: All authors reported no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neuroscience. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 2.Mountjoy KG. Functions for iomelanocortin-derived peptides in obesity and diabetes. Biochem J. 2010;428:305–324. doi: 10.1042/BJ20091957. [DOI] [PubMed] [Google Scholar]
- 3.Smart JL, Tolle V, Otero-Corchon V, Low MJ. Central dysregulation of the hypothalamic-pituitary-adrenal axis in neuron-specific pro-opiomelanocortin-deficient mice. Endocrinology. 2007;148:647–659. doi: 10.1210/en.2006-0990. [DOI] [PubMed] [Google Scholar]
- 4.Boyadjieva NI, Ortigüela M, Arjona A, Cheng X, Sarkar DK. Beta-endorphin neuronal cell transplant reduces corticotropin releasing hormone hyperresponse to lipopolysaccharide and eliminates natural killer cell functional deficiencies in fetal alcohol exposed rats. Alcohol Clin Exp Res. 2009;33:931–937. doi: 10.1111/j.1530-0277.2009.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luger TA, Scholzen TE, Brzoska T, Böhm M. New insights into the functions of alpha-MSH and related peptides in the immune system. Ann N Y Acad Sci. 2003;994:133–140. doi: 10.1111/j.1749-6632.2003.tb03172.x. [DOI] [PubMed] [Google Scholar]
- 6.Xuei X, Flury-Wetherill L, Bierut L, Dick D, Nurnberger J, Jr, Foroud T, Edenberg HJ. The opioid system in alcohol and drug dependence: family-based association study. Am J Med Genet B Neuropsychiatr Genet. 2007;144:877–884. doi: 10.1002/ajmg.b.30531. [DOI] [PubMed] [Google Scholar]
- 7.Lissoni P, Barni S, Paolorossi F, Crispino S, Rovelli F, Ferri L, et al. Evidence for altered opioid activity in patients with cancer. Br J Cancer. 1987;56:834–837. doi: 10.1038/bjc.1987.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutton BS, Langefeld CD, Williams AH, Norris JM, Saad MF, Haffner SM, et al. Association of pro-opiomelanocortin gene polymorphisms with obesity in the IRAS family study. Obes Res. 2005;13:1491–1498. doi: 10.1038/oby.2005.180. [DOI] [PubMed] [Google Scholar]
- 9.Baker M, Gaukrodger N, Mayosi BM, Imrie H, Farrall M, Watkins H, et al. Association between common polymorphisms of the proopiomelanocortin gene and body fat distribution: a family study. Diabetes. 2005;54:2492–2496. doi: 10.2337/diabetes.54.8.2492. [DOI] [PubMed] [Google Scholar]
- 10.Rizzato C, Scherer D, Rudnai P, Gurzau E, Koppova K, Hemminki K, et al. POMC and TP53 genetic variability and risk of basal cell carcinoma of skin: Interaction between host and genetic factors. J Dermatol Sci. 2011;63:47–54. doi: 10.1016/j.jdermsci.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Schneider ML, Moore CF, Kraemer GW, Roberts AD, DeJesus OT. The impact of prenatal stress, fetal alcohol exposure, or both on development: perspectives from a primate model. Psychoneuroendocrinology. 2002;27:285–298. doi: 10.1016/s0306-4530(01)00050-6. [DOI] [PubMed] [Google Scholar]
- 12.Kelly Y, Sacker A, Gray R, Kelly J, Wolke D, Quigley MA. Light drinking in pregnancy, a risk for behavioural problems and cognitive deficits at 3 years of age? Int J Epidemiol. 2009;38:129–140. doi: 10.1093/ije/dyn230. [DOI] [PubMed] [Google Scholar]
- 13.Kelley ML, Braitman A, Henson JM, Schroeder V, Ladage J, Gumienny L. Relationships among depressive mood symptoms and parent and peer relations in collegiate children of alcoholics. Am J Orthopsychiatry. 2010;80:204–212. doi: 10.1111/j.1939-0025.2010.01024.x. [DOI] [PubMed] [Google Scholar]
- 14.Gauthier TW, Drews-Botsch C, Falek A, Coles C, Brown LA. Maternal alcohol abuse and neonatal infection. Alcohol Clin Exp Res. 2005;29:1035–1043. doi: 10.1097/01.alc.0000167956.28160.5e. [DOI] [PubMed] [Google Scholar]
- 15.Latino-Martel P, Chan DS, Druesne-Pecollo N, Barrandon E, Hercberg S, Norat T. Maternal alcohol consumption during pregnancy and risk of childhood leukemia: systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19:1238–1260. doi: 10.1158/1055-9965.EPI-09-1110. [DOI] [PubMed] [Google Scholar]
- 16.Sarkar DK, Kuhn P, Marano J, Chen C, Boyadjieva N. Alcohol exposure during the developmental period induces beta-endorphin neuronal death and causes alteration in the opioid control of stress axis function. Endocrinology. 2007;148:2828–2834. doi: 10.1210/en.2006-1606. [DOI] [PubMed] [Google Scholar]
- 17.Hellemans KG, Sliwowska JH, Verma P, Weinberg J. Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev. 2010;34:791–807. doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ting JW, Lautt WW. The effect of acute, chronic, and prenatal ethanol exposure on insulin sensitivity. Pharmacol Ther. 2006;111:346–373. doi: 10.1016/j.pharmthera.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Fortuna JL. Sweet preference, sugar addiction and the familial history of alcohol dependence: shared neural pathways and genes. J Psychoactive Drugs. 2010;42:147–151. doi: 10.1080/02791072.2010.10400687. [DOI] [PubMed] [Google Scholar]
- 20.Zimmermann U, Spring K, Wittchen HU, Himmerich H, Landgraf R, Uhr M, et al. Arginine vasopressin and adrenocorticotropin secretion in response to psychosocial stress is attenuated by ethanol in sons of alcohol-dependent fathers. J Psychiatr Res. 2004;38:385–393. doi: 10.1016/j.jpsychires.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Avila CA, Oncken C, Van Kirk J, Wand G, Kranzler HR. Adrenocorticotropin and cortisol responses to a naloxone challenge and risk of alcoholism. Biol Psychiatry. 2002;51:652–658. doi: 10.1016/s0006-3223(01)01334-8. [DOI] [PubMed] [Google Scholar]
- 22.Bernal AJ, Jirtle RL. Epigenomic disruption: the effects of early developmental exposures. Birth Defects Res A Clin Mol Teratol. 2010;88:938–944. doi: 10.1002/bdra.20685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 24.Chen CP, Kuhn P, Advis JP, Sarkar DK. Prenatal ethanol exposure alters the expression of period genes governing the circadian function of beta-endorphin neurons in the hypothalamus. J Neurochem. 2006;97:1026–1033. doi: 10.1111/j.1471-4159.2006.03839.x. [DOI] [PubMed] [Google Scholar]
- 25.Kashiwazaki N, Seita Y, Takizawa A, Maedomari N, Ito J, Serikawa T. Techniques for in vitro and in vivo fertilization in the rat. Methods Mol Biol. 2010;597:311–322. doi: 10.1007/978-1-60327-389-3_22. [DOI] [PubMed] [Google Scholar]
- 26.Frank J, Witte K, Schrödl W, Schütt C. Chronic alcoholism causes deleterious conditioning of innate immunity. Alcohol Alcohol. 2004;39:386–392. doi: 10.1093/alcalc/agh083. [DOI] [PubMed] [Google Scholar]
- 27.Schäfer C, Parlesak A, Schütt C, Bode JC, Bode C. Concentrations of lipopolysaccharide-binding protein, bactericidal/permeability-increasing protein, soluble CD14 and plasma lipids in relation to endotoxaemia in patients with alcoholic liver disease. Alcohol Alcohol. 2002;37:81–86. doi: 10.1093/alcalc/37.1.81. [DOI] [PubMed] [Google Scholar]
- 28.de Souza FS, Santangelo AM, Bumaschny V, Avale ME, Smart JL, Low MJ, Rubinstein M. Identification of neuronal enhancer of the pro-opiomelanocortin gene by transgenic mouse analysis and phylogenetic footprinting. Mol Cell Biol. 2005;25:3076–3086. doi: 10.1128/MCB.25.8.3076-3086.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacDonald JL, Roskams AJ. Epigenetic regulation of nervous system development by DNA methylation and histone deacetylation. Prog Neurobiol. 2009;88:170–183. doi: 10.1016/j.pneurobio.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Newell-Price J, King P, Clark AJ. The CpG island promoter of the human pro-opiomelanocortin gene is methylated in nonexpressing normal tissue and tumors and represses expression. Mol Endocrinol. 2001;15:338–348. doi: 10.1210/mend.15.2.0599. [DOI] [PubMed] [Google Scholar]
- 31.Izzo A, Schneider R. Chatting histone modifications in mammals. Briefings in Functional Genomics. 2011;9:429–443. doi: 10.1093/bfgp/elq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogryzko VV, Schiltz RI, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 33.Agudelo M, Gandhi N, Saiyed Z, Pichili V, Thangavel S, Khatavkar P, et al. Effects of Alcohol on Histone Deacetylase 2 (HDAC2) and the Neuroprotective Role of Trichostatin A (TSA) Alcohol Clin Exp Res. 2011;35:1550–1556. doi: 10.1111/j.1530-0277.2011.01492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li F, Huarte M, Zaratiegui M, Vaughn M, Shi Y, Martienssen R, et al. Lid2 Is Required for Coordinating H3K4 and H3K9 Methylation of Heterochromatin and Euchromatin. Cell. 2008;135:272–283. doi: 10.1016/j.cell.2008.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci. 2008;28:3729–3737. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaissière T, Sawan C, Herceg Z. Epigenetic interplay between histone modifications and DNA methylation in gene silencing. Mutat Res. 2008;659:40–48. doi: 10.1016/j.mrrev.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Jeltsch A. Beyond Watson and Crick: DNA methylation and molecular enzymology of DNA methyltransferases. ChemBiochem. 2002;3:274–293. doi: 10.1002/1439-7633(20020402)3:4<274::AID-CBIC274>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 38.Tahiliani M, Koh K, Shen Y, Pastor WA, Bandukwala H, Brudno Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ou JN, Torrisani J, Unterberger A, Provençal N, Shikimi K, Karimi M, et al. Histone deacetylase inhibitor Trichostatin A induces global and gene-specific DNA demethylation in human cancer cell lines. Biochem Pharmacol. 2007;73:1297–1307. doi: 10.1016/j.bcp.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 40.Cameron EE, Bachman KE, Myöhänen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–107. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 41.Capel E, Fléjou JF, Hamelin R. Assessment of MLH1 promoter methylation in relation to gene expression requires specific analysis. Oncogene. 2007;26:7596–7600. doi: 10.1038/sj.onc.1210581. [DOI] [PubMed] [Google Scholar]
- 42.Deng G, Chen A, Pong E, Kim YS. Methylation in hMLH1 promoter interferes with its binding to transcription factor CBF and inhibits gene expression. Oncogene. 2001;20:7120–7127. doi: 10.1038/sj.onc.1204891. [DOI] [PubMed] [Google Scholar]
- 43.Bumaschny V, de Souza FS, López Leal RA, Santangelo AM, Baetscher M, Levi DH, et al. Transcriptional regulation of pituitary POMC is conserved at the vertebrate extremes despite great promoter sequence divergence. Mol Endocrinol. 2007;21:2738–2749. doi: 10.1210/me.2006-0557. [DOI] [PubMed] [Google Scholar]
- 44.Botbol M, Roubertoux PL, Carlier M, Trabado S, Brailly-Tabard S, Perez-Diaz F, et al. Modulation of Brain β-Endorphin Concentration by the Specific Part of the Y Chromosome in Mice. PLoS One. 2011;6:e16704. doi: 10.1371/journal.pone.0016704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menke DB, Page DC. Sexually dimorphic gene expression in the developing mouse gonad. Gene Expr Patterns. 2002;2:359–367. doi: 10.1016/s1567-133x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- 46.Carone BR, et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143:1084–1096. doi: 10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.