Abstract
Background
Twin studies using both clinical and population-based samples suggest that the frequent co-occurrence of attention deficit hyperactivity disorder (ADHD) and reading ability/disability (RD) is largely driven by shared genetic influences. While both disorders are associated with lower IQ, recent twin data suggest that the shared genetic variability between reading difficulties and ADHD inattention symptoms is largely independent from genetic influences contributing to general cognitive ability. The current study aimed to extend the previous findings that were based on rating scale measures in a population sample by examining the generalizability of the findings to a clinical population, and by measuring reading difficulties both with a rating scale and with an objective task. We therefore investigated the familial relationships between ADHD, reading difficulties and IQ in a sample of individuals diagnosed with ADHD combined type, their siblings and control sibling pairs.
Methods
We ran multivariate familial models on data from 1789 individuals at ages 6 to 19. Reading difficulties were measured with both rating scale and an objective task. IQ was obtained using the Wechsler Intelligence Scales (WISC-III / WAIS-III).
Results
Significant phenotypic (0.2–0.4) and familial (0.3–0.5) correlations were observed among ADHD, reading difficulties and IQ. Yet 53% to 72% of the overlapping familial influences between ADHD and reading difficulties were not shared with IQ.
Conclusions
Our finding that familial influences shared with general cognitive ability, though present, do not account for the majority of the overlapping familial influences on ADHD and reading difficulties extends previous findings from a population-based study to a clinically-ascertained sample with combined type ADHD.
Keywords: ADHD, reading difficulties, IQ, familial, sibling-pair, comorbidity
Introduction
Attention deficit-hyperactivity disorder (ADHD) and reading disability (RD) frequently co-occur: 25 to 40% of individuals with one disorder also meet the diagnostic criteria for the other (August & Garfinkel, 1990; Semrud-Clikeman et al., 1992; Willcutt & Pennington, 2000). This is further evident in studies approaching ADHD symptoms (inattentiveness and hyperactivity-impulsivity) and reading ability/disability as continuous traits in population samples (Gilger, Pennington, & DeFries, 1992; Light, Pennington, Gilger, & DeFries, 1995; Martin, Levy, Pieka, & Hay, 2006; Paloyelis, Rijsdijk, Wood, Asherson, & Kuntsi, 2010; Stevenson et al., 2005; Willcutt, Pennington, & DeFries, 2000; Willcutt, Pennington, Olson, & DeFries, 2007). Twin studies on general population samples and samples selected for RD consistently indicate a largely genetic aetiology for the phenotypic association between ADHD symptoms and reading ability/disability (Martin et al., 2006; Paloyelis et al., 2010; Willcutt & Pennington, 2000; Willcutt et al., 2000; Willcutt et al., 2007).
ADHD and RD are associated with IQ scores that are, on average, 7 to 16 points lower than comparison samples (Crosbie & Schachar, 2001; Kuntsi et al., 2004; Mariani & Barkley, 1997; Marzocchi et al., 2008; Rucklidge & Tannock, 2001; Tiffin-Richards, Hasselhorn, Woerner, Rothenberger, & Banaschewski, 2008; Wadsworth, DeFries, Olson, & Willcutt, 2007; Wadsworth, Olson, & Defries, 2000). Correlations between continuous ADHD symptom scores and IQ range from −0.20 to −0.40 (Fergusson, Lynskey, & Horwood, 1993; Goodman, Simonoff, & Stevenson, 1995; Kuntsi et al., 2004; Rapport, Scanlan, & Denney, 1999; Wood, Asherson, van der Meere, & Kuntsi, 2009). Similarly, correlations between reading ability and IQ range from 0.43 to 0.50 (Harlaar, Spinath, Dale, & Plomin, 2005; Haworth et al., 2009) and between reading difficulties and IQ from −0.37 to −0.40 (Cardon, Dialla, Plomin, DeFries, & Fulker, 1990; Paloyelis et al., 2010). Correlations between reading difficulties and ADHD inattention symptoms range from 0.28 to 0.51, and between reading difficulties and hyperactivity-impulsivity symptoms from 0.19 to 0.26 (Martin et al., 2006; Paloyelis et al., 2010; Trzesniewski, Moffitt, Caspi, Taylor, & Maughan, 2006; Willcutt et al., 2007). For both ADHD and RD, twin studies indicate that the association with IQ is largely due to shared genes (Haworth, Meaburn, Harlaar, & Plomin, 2007; Kuntsi et al., 2004; Plomin & Kovas, 2005; Polderman et al., 2006; Wood, Asherson, van der Meere, & Kuntsi, 2010; Wood, Rijsdijk, et al., 2010). These findings raise the question of whether the covariation between ADHD and RD is due to specific factors contributing to these deficits, or to possible “generalist” genes that are involved in both general cognitive processes and reading ability (Haworth et al., 2009). Recent evidence from a population-based twin study suggests that the covariation between ADHD inattention symptoms and reading difficulties is largely independent of the aetiology underlying IQ (Paloyelis et al., 2010). This finding is in line with previous twin analyses, where the genetic relationship between ADHD symptoms and reading difficulties did not change significantly after regressing out IQ (Light et al., 1995; Willcutt et al., 2000).
This study is a novel extension of the previous population-based twin analyses (Paloyelis et al., 2010) to a clinical sample of diagnosed cases selected for combined type ADHD (ADHD-CT) (Wood, Asherson, et al., 2010; Wood, Rijsdijk, et al., 2010), while incorporating both rating scale and objective task measures of reading. Our aim is to investigate the aetiological association between ADHD-CT, reading difficulties and IQ, and specifically the extent to which the familial influences shared between ADHD-CT and reading difficulties are also shared with those on IQ. The focus on familial influences, which refer to the combined effects of genes and shared environment, reflects the sibling design: the sample consists of ADHD-CT sibling pairs (ADHD-CT proband and closest-age sibling) and control sibling pairs. In contrast to the well-known twin method, quantitative genetic model-fitting analyses on sibling-pair samples have remained under-utilised (but see Kuntsi et al., 2010; Wood, Asherson, Rijsdijk, & Kuntsi, 2009; Wood, Rijsdijk, et al., 2010); yet their power and potential lies in the use of clinically diagnosed probands, rarely available in twin populations in adequate numbers, enabling comparisons across clinically-referred and population-based samples.
It is important to complement previous work on an unselected population twin sample with data from a clinical sample selected for ADHD-CT before we can generalise our previous findings to this clinical group. Our previous study used only parent ratings to assess reading difficulties, the present study therefore addressed this possible methodological limitation by taking into account data from both a parent report of reading difficulties and scores on an objective measure of reading ability.
Method
Sample
ADHD probands and siblings
Participants were recruited from specialist clinics, through five Centres (Amsterdam and Nijmegen in The Netherlands, UK-London, UK-Southampton and Spain) participating in the International Multicentre ADHD Genetics (IMAGE) project (see Chen et al., 2008 for details of ascertainment and diagnostic procedures). All participants were of European Caucasian decent, aged 6 to 19 years. All probands had a clinical diagnosis of DSM-IV ADHD combine type (ADHD-CT) and had one or more full siblings available for ascertainment of clinical information. Siblings within the same age range as the ADHD probands were included in the study and were therefore unselected for ADHD status. Exclusion criteria applying to both probands and siblings included autism, epilepsy, IQ<70, brain disorders and any genetic or medical disorder associated with externalizing behaviour that might mimic ADHD. Of the 1386 ADHD probands and their siblings who participated, 73 were excluded. Of these, 15 were excluded due to IQ<70; 7 had incomplete IQ data; 33 probands did not meet the ADHD DSM-IV criteria and a further 18 probands did not meet the ADHD-CT criteria. The final sample (Table 1) consisted of 1304 individuals, which comprised 615 complete ADHD and sibling pairs and 74 singletons. Singletons are defined as those whose co-siblings had incomplete cognitive or reading data, or were excluded. Singletons were included in our analysis as they provide information on within-subject covariance and therefore increase statistical power. The receiver operating characteristic (ROC) analysis (with 95% sensitivity and specificity) was used to determine the affection status of the siblings of ADHD probands. Those who had a combined parent-rated T-score greater than 137.5 on the Strength and Difficulties Questionnaire (SDQ; Goodman, 1997; Goodman, Meltzer, & Bailey, 1998) and the Conners ADHD/DSM-IV scale (Conners, 2003) were classified as “affected”; those who scored between 118.5 and 137.5 were classified as “subthreshold”; and the remaining who had a score lower than 118.5 were unaffected (Table 1 & 2). Of the 712 individuals with ADHD-CT, there was an overlap of comorbid disorders as follows: 180 had conduct disorder, 441 had oppositional defiant disorder, and 143 had evidence of a mood disorder (excluding possible bipolar disorder), as derived using the Parental Account of Child Symptoms (PACS) parental interview (Taylor et al., 1986; Taylor et al., 1987).
Table 1.
Means (and standard deviations) for gender, age, IQ, and reading difficulties questionnaire (RDQ) in ADHD probands, siblings of ADHD probands and unaffected controls.
| ADHD Probands (n=630) | ADHD siblings | Controls (n=485) | F / χ2 | p | ||||
|---|---|---|---|---|---|---|---|---|
| Affected (n=84) | Subthreshold (n=77) | Unaffected (n=513) | ||||||
| Male % | 88 | 71 | 57 | 47 | ||||
| Age | 11.46 (2.69) | 11.06 (3.10) | 11.53 (2.74) | 11.77 (3.25) | 12.11 (2.76) | 7.09 | 0.01 | |
| IQ | 100.64 (14.74) | 99.78 (14.11) | 98.13 (14.12) | 104.04 (13.86) | 107.13 (12.17) | 29.32 | 0.01 | |
| RDQ | 16.33 (7.55) | 15.53 (8.14) | 14.95 (7.44) | 10.36 (5.99) | 9.86 (5.45) | 142.64 | 0.01 | |
| Centre groups % | a | 53 | 71 | 49 | 48 | 49 | ||
| b | 27 | 21 | 27 | 28 | 51 | |||
| c | 8 | 2 | 18 | 11 | 0 | 163.38 | 0.01 | |
| d | 12 | 6 | 6 | 13 | 0 | |||
a= Netherlands
b= UK- London
c= UK- Southampton
d= Spain
Table 2.
Means and (standard deviation) of gender. age, IQ and TOWRE scores in ADHD probands, siblings of ADHD probands and unaffected controls.
| ADHD Probands (n=221) | ADHD siblings | Controls (n=245) | F / χ2 | p | ||||
|---|---|---|---|---|---|---|---|---|
| Affected (n=20) | Subthreshold (n=35) | Unaffected (n=201) | ||||||
| Male % | 89 | 55 | 60 | 45 | ||||
| Age | 12.19 (2.58) | 10.29 (2.77) | 11.81 (3.24) | 11.89 (2.97) | 12.58 (2.33) | 4.64 | 0.01 | |
| IQ | 99.06 (14.66) | 98.13 (15.66) | 93.75 (14.46) | 101.82 (13.39) | 108.30 (13.78) | 17.44 | 0.01 | |
| TOWRE | 92.46 (16.73) | 92.81 (16.92) | 93.18 (15.94) | 98.94 (14.94) | 100.52 (14.63) | 8.77 | 0.01 | |
| Centre % | a | 0 | 0 | 0 | 0 | 0 | ||
| b | 77 | 90 | 60 | 73 | 100 | |||
| c | 23 | 10 | 40 | 27 | 0 | 85.18 | 0.01 | |
| d | 0 | 0 | 0 | 0 | 0 | |||
a= Netherlands
b=UK-London
c= UK-Southampton
d= Spain
Control sample
The control group was recruited from primary (ages 6–11 years) and secondary (ages 12–19 years) schools in the UK and The Netherlands. The same exclusion criteria were applied as for the clinical sample. Nine controls were excluded for having both parent and teacher subscale T scores on the Conners ADHD/DSM-IV Scale (Conners, 2003) greater than 63, to exclude potential undiagnosed ADHD cases. The final control sample consisted of 485 individuals, which comprised 211 sibling pairs and 63 singletons. Ethical approval was obtained from local ethical review boards and all participants and/or their parents gave written informed consent for participation in the study.
Measures
General cognitive ability
To estimate IQ, we used the vocabulary, similarities, picture completion and block design subtests from the Wechsler Intelligence Scales for Children, Third Edition (WISC-III; Wechsler, 1991) or, for participants older than 16 years, the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III; Wechsler, 1997). These subtests correlate between 0.90 and 0.95 with the full-scale IQ (Groth-Marnat, 1984).
ADHD Diagnosis
All cases were referred from clinics with a diagnosis of ADHD-CT. The PACS interview (Taylor et al., 1986; Taylor et al., 1987) was subsequently conducted with the parents to derive the 18 DSM-IV symptoms for ADHD index cases plus siblings who were thought, on the basis of parents’ descriptions of behaviour or Conners’ scores of 65 or greater, to have ADHD. The PACS interview is a semi-structured and standardised clinical interview used to obtain an objective measure of child behaviour in a range of specified situations, including home and school. Situational pervasiveness was defined as some symptoms occurring within 2 or more different situations from the PACS as well as the presence of 1 or more symptoms scoring 2 or more from the DSM-IV ADHD subscale of the teacher-rated Conners subscale (Conners, Sitarenios, Parker, & Epstein, 1998). Impairment criteria were based on the severity of symptoms identified in the PACS.
Reading difficulties and fluency
Reading Difficulties Questionnaire (RDQ) is a subscale of the Colorado Learning Difficulties Questionnaire (CLDQ; Willcutt et al., 2011). This 6-item parent rating scale is part of an instrument screening for learning disorders. On a scale which ranges from 1 (“Never/ not at all”) to 5 (Always/ a great deal”), parents reported the extent of their child’s difficulties with spelling, learning letter names, sounding words out, and to what extent their child reads slowly, below expectancy level or has required extra help at school. The total score ranges from 5 to 30, with higher scores indicating greater difficulties with reading. This scale has been shown to have excellent internal consistency (mean Cronbach’sα = 0.90) and high inter-rater (r = 0.83) and one-year test-retest reliabilities (r = 0.81; Willcutt et al., 2011). RDQ has shown high correlations with other objective reading and spelling measures (average r = 0.64) but low correlations with measures of other learning difficulties (r = 0.07 to 0.02), which attest to its good criterion and discriminant validity (Willcutt et al., 2011). Moreover, RDQ scores have demonstrated moderate to high heritability (h2= 53 to 83%) and high genetic correlations (0.71 to 0.89) with a composite measure of reading performance (Astrom, DeFries, Pennington, Wadsworth, & Willcutt, 2009; Martin et al., 2006).
Test of Word Reading Efficiency (TOWRE; Torgesen, Wagner, & Rashotte, 1999). This is a standardised measure of fluency and accuracy in word reading skill. It includes two subtests: (a) Sight-word Efficiency (SWE), a measure of accuracy and fluency in reading regular and irregular words, based on the ability to real aloud accurately a graded list of 104 real words in 45 seconds. The total raw score on this subtest ranges from 0 to 104 (b) Phonemic Decoding Efficiency (PDE), a measure of phonological awareness, based on the ability to read aloud accurately a graded list of 63 pronounceable printed non-words. Each child is given 45 seconds to read as many words and non-words as possible. The total raw score of this subtest ranges from 0 to 63. The raw score from each subtest is then standardised based on the age of the participants, and the final score is the sum of the standardised scores from both subtests. A lower overall score indicates greater difficulties with reading. Both subtests have demonstrated excellent test-retest reliability of above 0.90 (Torgesen et al., 1999) and a strong correlation (0.63, p<0.05) with teacher-reported school performance (Trzesniewski et al., 2006). TOWRE composite scores were used in our analyses, obtained by standardising and summing the sub-test scores. TOWRE composite scores were also used in other studies due to the high correlation between the subtests (r = 0.82; Harlaar, Dale, & Plomin, 2007); r = 0. 78 in the present study). The TOWRE was only administered in the UK-London subgroup. The subgroup with both RDQ and TOWRE data was older (p<0.01) than the subgroup without TOWRE data. There were no differences in gender, IQ or RDQ between the two subgroups (further details are available from first author).
Analyses
Multivariate modelling on sibling data
We are interested in the extent to which the traits of ADHD, RDQ and IQ share etiological influences. The power to ascertain this comes from sibling data, because we know the amount of additive genetic (A), shared environmental (C) and child-specific environmental (E) influences shared between members of a sibling pair. Twin modelling is a common application of such quantitative genetic methodology, where comparisons between monozygotic (MZ) and dizygotic (DZ) twin pairs uses known amount of A, C and E sharing between members of twin pairs to decompose the variance in traits into these influences as etiological factors. Familial modelling, using sibling pairs, is an extension of this methodology. As sibling pairs all share 50% of their alleles, unlike MZ vs. DZ pairs that share 100 vs. 50% respectively, we can only combine A and C into familial (F) influences. Thus, under the assumption that siblings reared together share approximately 50% of their alleles, and 100% of their C influences, sibling correlations on a trait allow us to decompose the variance between traits into F and E influences, where E also subsumes possible measurement error. As with DZ twin data, the covariance between members of a sibling pair is considered to arise from A and C influences. Without twin data, it is impossible to know the exact A:C ratio, and A and C are subsumed together in the F parameter. If the covariance between members of a sibling pair is entirely due to A, the sibling covariance (like DZ covariance) will be exactly half the actual F. If, on the other hand, the covariance between members of a sibling pair is entirely due to C, the sibling covariance (like DZ covariance) will be exactly equal to F. Without a comparison group, the exact constituent of F is unknown. Therefore, F could be specified as equal to, or half, the sibling covariance (or somewhere in between). Under the assumption that ADHD is broadly genetic (~80%; Burt, 2009; Faraone & Biederman, 2005), we chose to specify F as half the sibling covariance. This conservative estimate prevents an over estimation of familiality; however, as it is a conservative estimate we here focus on shared F influences, which are not subject to such limitations.
Multivariate familial modelling on sibling data also uses sibling correlations on a trait (e.g. correlations between sibling 1 and sibling 2 for IQ), but also includes information on phenotypic correlations (e.g. correlations between IQ and reading difficulties), and cross-sibling-cross-trait correlations (e.g. correlations between the reading difficulties score of sibling 1 and IQ score for sibling 2). Using the same logic as above, we can decompose the covariance between traits into F and E influences.
The Cholesky (triangular) decomposition describes the extent to which traits share common F influences (Fig. 1). To correct for the selected nature of the sample, the selection variable (ADHD) is included in all models. As this necessitates ordinal data analysis, 95% confidence intervals are not available. However, the significance of parameters in the main model (Fig. 1 & Fig. 2) was tested by dropping each parameter in turn, and comparing the χ2 of the reduced model to that of the full model with a 1-df test of freedom at the p< 0.05 level. A significant result indicates that the dropped parameter is significant with an α level of 0.05 (Wood, Rijsdijk, et al., 2010).
Figure 1.
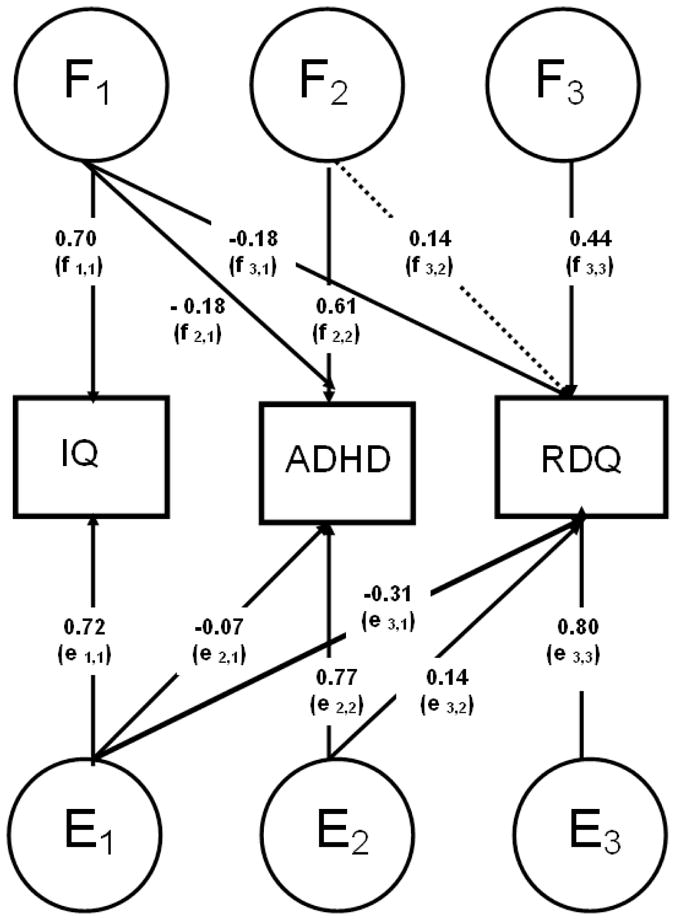
Parameters F1–F3 and parameters E1–E3 are estimates from Cholesky models estimating the familial and child-specific environmental factors across IQ, ADHD and Reading Difficulties Questionnaire (RDQ), respectively. Significant paths (p<0.05) are indicated as solid lines and non-significant paths (p≥0.05) are indicated as dotted lines.
Note: Parameters F1–F3 represent familial influences on and between the traits. f1,1 represents the sum of the familial influences underlying IQ. f2,1 represents the familial influences from IQ that are also shared with ADHD, while parameter f2,2 represents those familial influences that underlie ADHD, and are not shared with IQ. The sum of familial influences underlying ADHD is therefore the sum of f1,1 (those shared with IQ) and f2,2 (those not shared with IQ). The same inferences apply to RDQ, such that f3,1 represents familial influences underlying RDQ that are shared with ADHD (and to some extent ADHD), f3,2 represents familial influences shared between ADHD and RDQ only, and f3,3 represents familial influences specific to RDQ only. The sum of these three paths represents the sum of all familial influences underlying RDQ. The same inferences can be made for the E parameters, which represent child-specific environmental influences (and subsume any measurement error).
Figure 2.
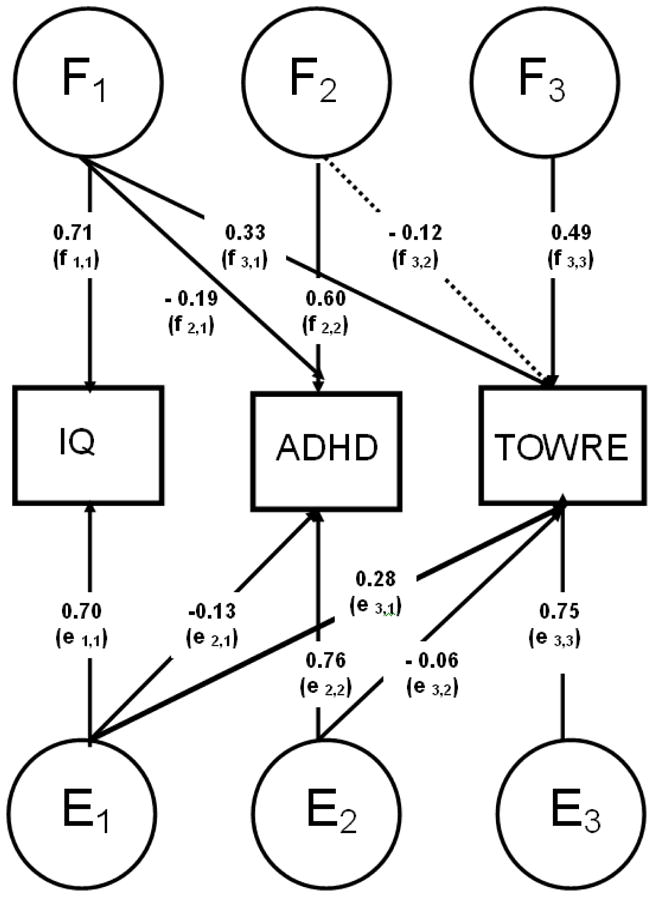
Parameters F1–F3 and parameters E1–E3 are estimates from Cholesky models estimating the familial and child-specific environmental factors across IQ, ADHD and TOWRE, respectively. Significant paths (p<0.05) are indicated as solid lines and non-significant paths (p≥0.05) are indicated as dotted lines.
Familial structural equation models (SEM)
The SEM program Mx (Neale, Boker, Xie, & Maes, 2006) was used to conduct the genetic analyses and to estimate phenotypic correlations. To account for the selected nature of the sample, the selection variable (ADHD status) was included in all models with its prevalence and familiality parameters fixed (Rijsdijk et al., 2005). The Mx program cannot include both ordinal and continuous data in the same analysis, and, as the selection variable is ordinal, the age- and sex-regressed residual scores of the cognitive variables were ordinalised into five equal size categories. Regression analyses were done in STATA. The cluster command was used to cluster by family, to account for the non-independence of the sibling sample. Ordinal data analysis assumes the combination of ordered categories to reflect measurements of an underlying multivariate normal distribution of traits, with one or more thresholds per liability distribution to distinguish between the ordered categories (Rijsdijk et al., 2005). The threshold for ADHD status was fixed to a z-value of 1.64 to give a population prevalence of 5%, and its parameters fixed to expected population estimates, with the familiality of ADHD fixed to 80% based on a sibling correlation of 0.40 (see Rijsdijk et al., 2005 for further explanation and validation of this approach).
Phenotypic correlation
Sibling correlations were estimated from a phenotypic correlation model, specified in a Gaussian decomposition to give maximum likelihood phenotypic correlations between the measures and to allow for additional constraints. The first imposed constraint is fixing sibling correlation for ADHD status to 0.40 to correct for ascertainment bias, by means of a method developed and validated in an earlier stimulation study (Rijsdijk et al., 2005). Additional constraints reflect the assumptions of the familial model: that phenotypic correlation across traits is the same across individuals and that cross-trait cross-sibling correlations are independent of sibling order.
Results
To account for group differences in IQ and reading performance across age, gender and centre group (Tables 1 & 2), IQ and reading data were regressed for these variables. Centre group differed significantly in age and IQ (p<0.01) but not in gender or reading performances. The residual scores obtained from the regression were then used to derive the phenotypic, familial and child-specific environmental correlations, and the familial parameter estimates (Table 3). The correlation between RDQ and TOWRE reading measures was r = − 0.54 (p<0.01).
Table 3.
Maximum-liklihood phenotypic (r), familial (rF) and child-specific environmental (rE) correlations across ADHD, IQ, reading difficulties (RDQ) and TOWRE scores.
| IQ | RDQ | TOWRE | |
|---|---|---|---|
| Phenotypic correlations (r) | |||
| ADHD | −0.17** | 0.25** | −0.22** |
| IQ | 1 | −0.34** | 0.43** |
| Familial correlations (rF) | |||
| ADHD | −0.29** | 0.38** | −0.35* |
| IQ | 1 | −0.36** | 0.54** |
| Child - specific environmental correlations (rE) | |||
| ADHD | −0.09* | 0.19** | −0.13* |
| IQ | 1 | −0.35** | 0.35** |
p<0.001
p< 0.01
Reading difficulties questionnaire
We calculated the sum of F influences underlying the covariance between ADHD and RDQ that are not shared with IQ (path f2,2 × f3,2 in Fig. 1) as a percentage of the total F influences underlying the covariance (i.e. including those shared with IQ; f2,1 × f3,1 + f2,2 × f3,2 ). In total; 72% of the shared F between ADHD and RDQ was not shared with IQ.
We calculated the sum of E influences underlying the covariance between ADHD and RDQ that are not shared with IQ in the same manner. By summing F and E influences we obtain all the etiological influences accounting for the covariance between phenotypes, which leads us to deduce that 78% of the phenotypic covariation between ADHD and reading difficulties was driven by aetiological influences that were not shared with IQ.
A separate analysis was conducted to assess the relationship between ADHD, RDQ and IQ in the London-only subgroup, which provided both RDQ and TOWRE data. In this sub-sample, shared familial influences accounted for 55% of the covariation between ADHD and RDQ in total; 55% of the shared F was not shared with IQ.
TOWRE
The same analyses were used to assess the aetiological overlap between ADHD-CT and TOWRE scores that was independent of aetiology shared with IQ (Fig. 2). We calculated the sum of F influences underlying the covariance between ADHD and TOWRE scores that are not shared with IQ (path f2,2 × f3,2 in Fig. 2) as a percentage of the total F influences underlying the covariance (i.e. including those shared with IQ; f2,1 × f3,1 + f2,2 × f3,2 ). In total; 53% of the shared F between ADHD and TOWRE scores was not shared with IQ.
We calculated the sum of E influences underlying the covariance between ADHD and TOWRE scores that are not shared with IQ in the same manner. By summing F and E influences we obtain all the etiological influences accounting for the covariance between phenotypes, which leads us to deduce that 54% of the phenotypic covariation between ADHD and TOWRE scores was driven by aetiological influences that were not shared with IQ
Discussion
Our findings from both rating scale and objective measures of reading indicate that over half (53 to 72%) of the overlapping familial influences between ADHD and reading difficulties were not shared with IQ. This finding is consistent with recent evidence from a population-based twin study that focused on the association between parent ratings of reading difficulties and continuous ADHD symptom scores (Paloyelis et al., 2010). This was the first study to examine the relationship between ADHD, reading difficulties and IQ in a sample selected for ADHD-CT. The generalisability of the findings from a population sample to a clinical sample with ADHD-CT is consistent with pre-existing evidence suggesting that both ADHD and RD represent the extreme and impairing tail of continuously distributed traits of ADHD symptoms and reading ability scores (Chen et al., 2008; Harlaar et al., 2005; Levy, Hay, McStephen, Wood, & Waldman, 1997; Shaywitz, Escobar, Shaywitz, Fletcher, & Makuch, 1992). Overall, our results suggest that there are both unique processes that contribute to the co-occurrence between ADHD and reading difficulties and common processes that are shared with general cognitive abilities.
Between 48% and 62% of the phenotypic overlap between ADHD and reading difficulties, measured by the RDQ and the TOWRE, respectively, was due to shared familial influences. This is consistent with previous twin studies of both ADHD and RD that showed the comorbidity between these two disorders are in part (50–75%) due to common genetic influences (Gilger et al., 1992; Light et al., 1995; Paloyelis et al., 2010; Stevenson, Pennington, Gilger, DeFries, & Gillis, 1993). Findings from this study supported the common genetic aetiology hypothesis for the co-occurrence between ADHD and reading difficulties. Alternative explanations such as sampling artefacts (Berkson, 1946), assortative mating for ADHD and RD (Faraone et al., 1993), or a causal relation between ADHD and RD (Pennington, Grossier, & Welsh, 1993) would also be consistent with the shared genes account. Although these alternative hypotheses were not tested in the present study due to insufficient power with ordinal data analysis, previous studies in ADHD and RD have shown that the association between ADHD and RD was not due to sampling or measurement artefacts as the findings were replicated in population-based samples using both objective (Willcutt et al., 2000) and subjective (Martin et al., 2006; Paloyelis et al., 2010) measures of reading. Moreover, assortative mating has not been consistently observed (Doyle, Faraone, DuPre & Biederman, 2001) and significant bivariate heritability between ADHD and RD from twin studies provided evidence against the assortative mating hypothesis (Gilger et al., 1992; Light et al., 1995; Paloyelis et al., 2010; Stevenson et al., 1993; Willcutt et al., 2000; Willcutt et al., 2007), given that assortative mating decreases estimates of shared genetic influences (Willcutt et al., 2000).
The phenocopy hypothesis (Pennington et al., 1993) argues that the co-occurrence between ADHD and RD is a result of the primary disorder causing manifestation of deficits associated with the secondary disorder, in the absence of aetiological influences associated with the secondary disorder. This hypothesis was not supported for ADHD and RD by neuropsychological studies, in which individuals with comorbid ADHD and RD exhibited cognitive deficits that are associated with both ADHD and RD (Willcutt et al., 2007). The present study was the first using a selected sample with ADHD to show shared familial association between ADHD and reading difficulties, and further provided evidence against the phenocopy hypothesis. There is growing evidence including the present study, supporting the existence of common sets of genes which explain the comorbidity between ADHD and reading difficulties (Gilger et al., 1992; Light et al., 1995; Paloyelis et al., 2010; Stevenson et al., 1993; Willcutt et al., 2000; Willcutt et al., 2007). This has potential implications for future clinical intervention to identify treatments that target both ADHD symptoms and reading difficulties, although the presence of shared aetiological influences could be explained by pleiotropic effects (the multiple phenotypic effects of genes) impacting on multiple neurobiological processes, which could be targeted independently of each other. Further work is needed to identify the neurobiological processes that mediate these familial effects on ADHD and RD.
The two reading measures we used were highly correlated with one another and yielded similar phenotypic correlations with ADHD. Furthermore, the results obtained with either the RDQ or the TOWRE measures in the London subgroup were comparable, indicating that around 53% to 72% of familial influences shared between ADHD and reading ability/disability were independent of IQ. It should be noted, however, that the RDQ is a general measure of a child’s overall reading difficulties, while the TOWRE measures specific processes in reading such as reading fluency, word recognition and phonemic awareness. Measures that tap specific aspects of the reading process will be required in future research to fully disentangle the aetiological basis for the covariation between ADHD and reading difficulties.
Whereas a sibling design is a powerful tool for studying shared familial effects in samples with clinically-ascertained probands, a limitation is that genetic effects cannot be separated from shared environmental effects. However, previous studies indicate a limited contribution for shared environmental factors in ADHD (Burt, 2009), suggesting that the familial influences that underlie ADHD and reading difficulties in this study reflect mainly genetic effects. Another limitation in the present study is that, due to computational intensity of ordinal data, confidence intervals could not be obtained. However, we did test the significance of each at an alpha level of .05.
Overall, in a sibling-pair sample selected for ADHD combined subtype and controls, a large proportion (53–78%) of the overlapping familial influences on ADHD and reading difficulties are not shared with IQ. The generalisability of the current findings to other populations needs to be examined in future research. Recent studies have explored the relationship between ADHD and reading difficulties beyond a behavioural level, by using cognitive endophenotypes to further understand the genetic aetiology and architecture on a neurocognitive level (McGrath et al., 2010; Willcutt et al., 2010). The results from these multivariate twin studies selected for RD suggest that the comorbidity between ADHD and RD is driven by common genetic influences also shared with slow processing speed. Future studies should replicate these findings in the general population and explore other cognitive endophenotypes associated with ADHD such as reaction time and reaction time variability. Neurocognitive measures that are associated and share common genetic influences with ADHD and RD maybe be useful for a more in depth understanding of the comorbidity between the two disorders on a molecular level.
Key points.
Previous studies have attributed the overlap between reading difficulties and ADHD inattention symptoms to shared genetic causes, with initial evidence from a population twin sample further suggesting that these are largely independent of genetic influences on IQ.
Using a sibling-pair sample selected for ADHD combined type (ADHD-CT) and controls, we obtained further evidence that over half of the overlapping familial influences shared between ADHD and reading difficulties are not shared with IQ.
Overall, the data from both population-based and clinical samples concur on the idea that familial influences shared with general cognitive ability, though present, account for only a small proportion of the overlapping familial and genetic influences on ADHD and reading difficulties.
Acknowledgments
This work was supported in part by National Institutes of Health (NIH) grants R01MH62873 and R01MH081803 to S.V. Faraone and, in London, by a UK Medical Research Council grant G03001896 to J. Kuntsi. We thank all the families who kindly participated in this research. Principal investigators for this study were P. Asherson, J. K. Buitelaar, S.V. Faraone, B. Franke, J. Kuntsi, A. Miranda, F. Mulas, N. Rommelse, E. J. Sonuga-Barke, and J. A. Sergeant.
We thank further team members at data collection sites of Amsterdam, London, Nijmegen, Southampton and Valencia for their important contributions.
Footnotes
The authors have disclosed the following competing or potential conflicts of interest: J.K B. has been in the past 3 years a consultant to / member of Advisory Board of / and/or speaker for Janssen Cilag, Eli Lilly, Bristol-Myer Squibb, Organon/Shering Plough, UCB, Shire, Medice, Servier, Bioprojet, Pfizer, and Servier. J.A.S. is a member of an Advisory Board to Eli Lilly and Shire; has received research funding from Eli Lilly; educational grants from Eli Lilly, Janssen-Cilag and Shire; and a speaker’s fee from Eli Lilly, Janssen-Cilag and Shire. E. S.-B. is a member of an Advisory Board to Shire, Flynn Pharma, UCB Pharma and Astra Zeneca; has received research support from Janssen Cilag, Shire and Qbtech and Flynn Pharma; conference support from Shire; is on speaker board for Shire and UCB Pharma; and has been a consultant for UCB Pharma and Shire. S.V.F. received consulting fees and was on Advisory Boards for Shire Development and received research support from Shire and the National Institutes of Health (NIH) in the past year; in previous years, he received consulting fees or was on Advisory Boards or participated in continuing medical education programs sponsored by: Shire, McNeil, Janssen, Novartis, Pfizer and Eli Lilly; he also receives royalties from a book published by Guilford Press: Straight Talk about Your Child’s Mental Health. P.A. has received funding for educational and research activities from Janssen-Cilag and Shire; and has received consultancy and speaker fees from Janssen-Cilag, Shire, Eli Lilly and Flynn Pharma that have been used for educational and research activities. J.K. has received speaker fees from Eli Lilly that have been used for educational and research activities. The other authors report no such associations and declared that they have no competing or potential conflicts of interest arising from the publication of this work..
References
- Astrom R, DeFries JC, Pennington B, Wadsworth SJ, Willcutt E. Etiology of covariation between a parent-report screening measure and reading performance. Behaviour Genetics. 2009;39(6):634. [Google Scholar]
- August G, Garfinkel B. Comorbidity of ADHD and reading disability among clinic referred children. Journal of Abnormality Child Psychology. 1990;18:29–45. doi: 10.1007/BF00919454. [DOI] [PubMed] [Google Scholar]
- Berkson J. Limitations of the application of fourfold table analysis hospital data. Biometrics Bull. 1946;(2):47–53. [PubMed] [Google Scholar]
- Burt SA. Rethinking environmental contributions to child and adolescent psychopathology: a meta-analysis of shared environmental influences. Psychol Bull. 2009;135(4):608–637. doi: 10.1037/a0015702. [DOI] [PubMed] [Google Scholar]
- Cardon LR, Dialla LF, Plomin R, DeFries JC, Fulker DW. Genetic correlations between reading performance and IQ in the Colorado Adoption Project. Intelligence. 1990;14:245–257. [Google Scholar]
- Chen W, Zhou K, Sham P, Franke B, Kuntsi J, Campbell D, et al. DSM-IV combined type ADHD shows familial association with sibling trait scores: a sampling strategy for QTL linkage. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(8):1450–1460. doi: 10.1002/ajmg.b.30672. [DOI] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN. Revision and restandardization of the Conners Teacher Rating Scale (CTRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26(4):279–291. doi: 10.1023/a:1022606501530. [DOI] [PubMed] [Google Scholar]
- Crosbie J, Schachar R. Deficient inhibition as a marker for familial ADHD. Am J Psychiatry. 2001;158:1884–1890. doi: 10.1176/appi.ajp.158.11.1884. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J. What is the prevalence of adult ADHD? Results of a population screen of 966 adults. J Atten Disord. 2005;9(2):384–391. doi: 10.1177/1087054705281478. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Lehman BK, Keenan K, Norman D, Seidman LJ, et al. Evidence for the independent familial transmission of attention deficit hyperactivity disorder and learning disabilities: results from a family genetic study. Am J Psychiatry. 1993;150(6):891–895. doi: 10.1176/ajp.150.6.891. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Lynskey MT, Horwood LJ. Conduct problems and attention deficit behaviour in middle childhood and cannabis use by age 15. Aust N Z J Psychiatry. 1993;27(4):673–682. doi: 10.3109/00048679309075830. [DOI] [PubMed] [Google Scholar]
- Gilger JW, Pennington BF, DeFries JC. A twin study of the etiology of comorbidity: attention-deficit hyperactivity disorder and dyslexia. J Am Acad Child Adolesc Psychiatry. 1992;31(2):343–348. doi: 10.1097/00004583-199203000-00024. [DOI] [PubMed] [Google Scholar]
- Goodman R. The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry. 1997;38(5):581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Goodman R, Meltzer H, Bailey V. The Strengths and Difficulties Questionnaire: a pilot study on the validity of the self-report version. Eur Child Adolesc Psychiatry. 1998;7(3):125–130. doi: 10.1007/s007870050057. [DOI] [PubMed] [Google Scholar]
- Goodman R, Simonoff E, Stevenson J. The impact of child IQ, parent IQ and sibling IQ on child behavioural deviance scores. J Child Psychol Psychiatry. 1995;36(3):409–425. doi: 10.1111/j.1469-7610.1995.tb01299.x. [DOI] [PubMed] [Google Scholar]
- Groth-Marnat G. Handbook of psychological assessment. 3. NY: Wiley; 1984. [Google Scholar]
- Harlaar N, Dale PS, Plomin R. From learning to read to reading to learn: substantial and stable genetic influence. Child Dev. 2007;78(1):116–131. doi: 10.1111/j.1467-8624.2007.00988.x. [DOI] [PubMed] [Google Scholar]
- Harlaar N, Spinath FM, Dale PS, Plomin R. Genetic influences on early word recognition abilities and disabilities: a study of 7-year-old twins. J Child Psychol Psychiatry. 2005;46(4):373–384. doi: 10.1111/j.1469-7610.2004.00358.x. [DOI] [PubMed] [Google Scholar]
- Haworth CM, Kovas Y, Harlaar N, Hayiou-Thomas ME, Petrill SA, Dale PS, et al. Generalist genes and learning disabilities: a multivariate genetic analysis of low performance in reading, mathematics, language and general cognitive ability in a sample of 8000 12-year-old twins. J Child Psychol Psychiatry. 2009;50(10):1318–1325. doi: 10.1111/j.1469-7610.2009.02114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth CM, Meaburn EL, Harlaar N, Plomin R. Reading and Generalist Genes. Mind Brain Educ. 2007;1(4):173–180. doi: 10.1111/j.1751-228X.2007.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsi J, Eley TC, Taylor A, Hughes C, Asherson P, Caspi A, et al. Co-occurrence of ADHD and low IQ has genetic origins. American Journal of Medical Genetics. 2004;124B(1):41–47. doi: 10.1002/ajmg.b.20076. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Wood AC, Rijsdijk F, Johnson KA, Andreou P, Albrecht B, et al. Separation of cognitive impairments in attention-deficit/hyperactivity disorder into 2 familial factors. Arch Gen Psychiatry. 2010;67(11):1159–1167. doi: 10.1001/archgenpsychiatry.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy F, Hay DA, McStephen M, Wood C, Waldman I. Attention-deficit hyperactivity disorder: a category or a continuum? Genetic analysis of a large-scale twin study. J Am Acad Child Adolesc Psychiatry. 1997;36(6):737–744. doi: 10.1097/00004583-199706000-00009. [DOI] [PubMed] [Google Scholar]
- Light J, Pennington B, Gilger JW, DeFries JC. Reading disability and hyperactivity disorder: evidence for a common genetic eiotiology. Developmental Neuropsychology. 1995;11(3):323–335. [Google Scholar]
- Mariani MA, Barkley RA. Neuropsychological and academic functioning in preschool boys with attention deficit hyperactivity disorder. Dev Neuropsychol. 1997;13:111–129. [Google Scholar]
- Martin NC, Levy F, Pieka J, Hay D. A genetic study of Attention Deficit Hyperactivity Disorder, Conduct Disorder, Oppositional Defiant Disorder and Reading Disability: Aetiological overlaps and implications. International Journal of Disability, Development and Education. 2006;53:21–34. [Google Scholar]
- Marzocchi GM, Oosterlaa J, Zuddas A, Cavolina P, Geurts H, Redigolo D, et al. Contrasting deficits on executive functions between ADHD and reading disabled children. Journal of Child Psychology and Psychiatry. 2008;49(5):543–552. doi: 10.1111/j.1469-7610.2007.01859.x. [DOI] [PubMed] [Google Scholar]
- McGrath LM, Pennington BF, Shanahan MA, Santerre-Lemmon LE, Barnard HD, Willcutt EG, et al. A multiple deficit model of reading disability and attention-deficit/hyperactivity disorder: searching for shared cognitive deficits. J Child Psychol Psychiatry. 2010 doi: 10.1111/j.1469-7610.2010.02346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes H. Statistical Modelling. 7. VCU Box 900126, Richmond, VA 23298: Department of Psychiatry; 2006. [Google Scholar]
- Paloyelis Y, Rijsdijk F, Wood AC, Asherson P, Kuntsi J. The Genetic Association Between ADHD Symptoms and Reading Difficulties: The Role of Inattentiveness and IQ. J Abnorm Child Psychol. 2010;38(8):1083–1095. doi: 10.1007/s10802-010-9429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington BF, Grossier D, Welsh MC. Contrasting cognitive deficits in attention deficit hyperactivity disorder versus reading disability. Developmental Psychology. 1993;29:511–523. [Google Scholar]
- Plomin R, Kovas Y. Generalist genes and learning disabilities. Psychol Bull. 2005;131(4):592–617. doi: 10.1037/0033-2909.131.4.592. [DOI] [PubMed] [Google Scholar]
- Polderman TJ, Gosso MF, Posthuma D, Van Beijsterveldt TC, Heutink P, Verhulst FC, et al. A longitudinal twin study on IQ, executive functioning, and attention problems during childhood and early adolescence. Acta Neurol Belg. 2006;106(4):191–207. [PubMed] [Google Scholar]
- Rapport MD, Scanlan SW, Denney CB. Attention-deficit/hyperactivity disorder and scholastic achievement: a model of dual developmental pathways. J Child Psychol Psychiatry. 1999;40(8):1169–1183. [PubMed] [Google Scholar]
- Rijsdijk FV, van Haren NE, Picchioni MM, McDonald C, Toulopoulou T, Hulshoff Pol HE, et al. Brain MRI abnormalities in schizophrenia: same genes or same environment? Psychol Med. 2005;35(10):1399–1409. doi: 10.1017/S0033291705005167. [DOI] [PubMed] [Google Scholar]
- Rucklidge JJ, Tannock R. Psychiatric, psychosocial, and cognitive functioning of female adolescents with ADHD. J Am Acad Child Adolesc Psychiatry. 2001;40:530–540. doi: 10.1097/00004583-200105000-00012. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Biederman J, Sprich-Buckminster S, Lehman BK, Faraone SV, Norman D. Comorbidity between ADDH and learning disability: a review and report in a clinically referred sample. J Am Acad Child Adolesc Psychiatry. 1992;31(3):439–448. doi: 10.1097/00004583-199205000-00009. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Escobar MD, Shaywitz BA, Fletcher JM, Makuch R. Evidence that dyslexia may represent the lower tail of a normal distribution of reading ability. N Engl J Med. 1992;326(3):145–150. doi: 10.1056/NEJM199201163260301. [DOI] [PubMed] [Google Scholar]
- Stevenson J, Langley K, Pay H, Payton A, Worthington J, Ollier W, et al. Attention deficit hyperactivity disorder with reading disabilities: preliminary genetic findings on the involvement of the ADRA2A gene. J Child Psychol Psychiatry. 2005;46(10):1081–1088. doi: 10.1111/j.1469-7610.2005.01533.x. [DOI] [PubMed] [Google Scholar]
- Stevenson J, Pennington BF, Gilger JW, DeFries JC, Gillis JJ. Hyperactivity and spelling disability: testing for shared genetic aetiology. J Child Psychol Psychiatry. 1993;34(7):1137–1152. doi: 10.1111/j.1469-7610.1993.tb01779.x. [DOI] [PubMed] [Google Scholar]
- Taylor E, Everitt B, Thorley G, Schachar R, Rutter M, Wieselberg M. Conduct disorder and hyperactivity: II. A cluster analytic approach to the identification of a behavioural syndrome. Br J Psychiatry. 1986;149:768–777. doi: 10.1192/bjp.149.6.768. [DOI] [PubMed] [Google Scholar]
- Taylor E, Schachar R, Thorley G, Wieselberg HM, Everitt B, Rutter M. Which boys respond to stimulant medication? A controlled trial of methylphenidate in boys with disruptive behaviour. Psychol Med. 1987;17(1):121–143. doi: 10.1017/s0033291700013039. [DOI] [PubMed] [Google Scholar]
- Tiffin-Richards MC, Hasselhorn M, Woerner W, Rothenberger A, Banaschewski T. Phonological short-term memory and central executive processing in attention-deficit/hyperactivity disorder with/without dyslexia--evidence of cognitive overlap. J Neural Transm. 2008;115(2):227–234. doi: 10.1007/s00702-007-0816-3. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. Test of Word Reading Efficiency (TOWRE) Austin, TX: Pro-ed; 1999. [Google Scholar]
- Trzesniewski KH, Moffitt TE, Caspi A, Taylor A, Maughan B. Revisiting the association between reading achievement and antisocial behavior: new evidence of an environmental explanation from a twin study. Child Dev. 2006;77(1):72–88. doi: 10.1111/j.1467-8624.2006.00857.x. [DOI] [PubMed] [Google Scholar]
- Wadsworth SJ, DeFries JC, Olson RK, Willcutt EG. Colorado longitudinal twin study of reading disability. Ann Dyslexia. 2007;57(2):139–160. doi: 10.1007/s11881-007-0009-7. [DOI] [PubMed] [Google Scholar]
- Wadsworth SJ, Olson RK, Defries JC. Differential Genetic Etiology of Reading Difficulties as a Function of IQ: An Update. Behav Genet. 2000 doi: 10.1007/s10519-010-9349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechler Intelligence Scale for Children. 3. London: The Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. London: The Psychological Corporation; 1997. [Google Scholar]
- Willcutt EG, Betjemann RS, McGrath LM, Chhabildas NA, Olson RK, DeFries JC, et al. Etiology and neuropsychology of comorbidity between RD and ADHD: the case for multiple-deficit models. Cortex. 2010;46(10):1345–1361. doi: 10.1016/j.cortex.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Boada R, Riddle M, Chhabildas N, DeFries JC, Pennington B. Colorado Learning Difficulties Questionnaire: Validation of a parent-report screening measure. 2011 doi: 10.1037/a0023290. (Under review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF. Psychiatric comorbidity in children and adolescents with reading disability. J Child Psychol Psychiatry. 2000;41(8):1039–1048. [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, DeFries JC. Twin study of the etiology of comorbidity between reading disability and attention-deficit/hyperactivity disorder. Am J Med Genet. 2000;96(3):293–301. doi: 10.1002/1096-8628(20000612)96:3<293::aid-ajmg12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Olson RK, DeFries JC. Understanding comorbidity: a twin study of reading disability and attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(6):709–714. doi: 10.1002/ajmg.b.30310. [DOI] [PubMed] [Google Scholar]
- Wood AC, Asherson P, Rijsdijk F, Kuntsi J. Is overactivity a core feature in ADHD? Familial and receiver operating characteristic curve analysis of mechanically assessed activity level. J Am Acad Child Adolesc Psychiatry. 2009;48(10):1023–1030. doi: 10.1097/CHI.0b013e3181b54612. [DOI] [PubMed] [Google Scholar]
- Wood AC, Asherson P, van der Meere JJ, Kuntsi J. Separation of genetic influences on attention deficit hyperactivity disorder symptoms and reaction time performance from those on IQ. Psychol Med. 2009:1–11. doi: 10.1017/S003329170999119X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AC, Asherson P, van der Meere JJ, Kuntsi J. Separation of genetic influences on attention deficit hyperactivity disorder symptoms and reaction time performance from those on IQ. Psychol Med. 2010;40(6):1027–1037. doi: 10.1017/S003329170999119X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AC, Rijsdijk F, Johnson KA, Andreou P, Albrecht B, Arias-Vasquez A, et al. The relationship between ADHD and key cognitive phenotypes is not mediated by shared familial effects with IQ. Psychol Med. 2010:1–11. doi: 10.1017/S003329171000108X. [DOI] [PMC free article] [PubMed] [Google Scholar]


