Abstract
Human immunodeficiency virus (HIV) particles that remain in the blood of patients are frequently ignored as targets for AIDS treatment. We therefore investigated the use of photodynamic therapy (PDT) with hematoporphyrin monomethyl ether (HMME) as a means of inactivating cell-free HIV in vitro. Virus particles including HIV-1IIIB, resistant HIV-1 variants, HIV-1 clinical variants, and HIV-2 variants were incubated with HMME for 40 min, followed by irradiation with a 630-nm semiconductor laser at an energy density of 0.3 J/cm2. The antiviral effects were evaluated by counting syncytium formation or measuring p24 antigen expression levels in supernatants by enzyme-linked immunosorbent assay. The relationships between photoinactivation and HMME concentrations, energy density, power density and antioxidants (NaN3 and d-mannitol) were also assessed using the above methods. All the tested virus particles were completely responsive to HMME-PDT. HMME concentration and energy density were positively correlated with photoinactivation of HIV, while power density was negatively correlated. Both sodium azide and d-mannitol weakened the inhibitory effect of PDT on virus-induced membrane fusion, with d-mannitol having a stronger effect. HMME-PDT can inactivate HIV particles, and may therefore represent a promising treatment for AIDS patients.
Keywords: Photodynamic therapy, Human immunodeficiency virus, Photosensitizer, Energy density, Power density, Antioxidants
Introduction
HIV (human immunodeficiency virus) was identified as the causative agent of AIDS (acquired immunodeficiency syndrome) about 28 years ago [1, 2]. HIV replicates in CD4+ T cells and macrophages, producing infectious particles from multiple intracellular events. Chemicals that act against these intracellular events, especially reverse transcription, integration, and processing, have been successfully generated, and HIV replication and disease progression can now be effectively suppressed by administration of a combination of these chemicals [3, 4]. However, the therapeutic relevance of cell-free HIV particles has been neglected. HIV particles persist in the blood vessels at varying loads from the time of infection until death. Although some of the particles can be eliminated by the patient’s immune system, others remain to infect new host cells and promote disease progression. AIDS progression could thus be effectively suppressed if these cell-free particles could be promptly eradicated as they are produced.
Photodynamic therapy (PDT) is a two-step therapeutic technique in which the topical or systemic delivery of photosensitizing drugs is followed by irradiation with visible light. Activated photosensitizers transfer energy to molecular oxygen, generating reactive oxygen species. The subsequent oxidation of lipids, amino acids and proteins induces cell necrosis and apoptosis [5]. The study of photoinactivation of HIV in blood products was a focus of intense investigation in the 1990s, and PDT was shown to be effective against HIV particles [6, 7], though the side effects, especially the potential teratogenicity of photosensitizers, and technical issues associated with the use of lasers have hampered research in vivo. However, many new photosensitizers with weak side effects are now emerging. Hematoporphyrin monomethyl ether (HMME) is a novel, second-generation porphyrin-related photosensitizer that displays many of the ideal characteristics of effective photoantimicrobials. Experimental studies and clinical trials have shown that it has a simple, monomolecular structure, is selectively taken up by tumor tissues, has a stronger photodynamic effect that yields eight times more singlet oxygens and demonstrates lower toxicity and shorter-term skin photosensitization than hematoporphyrin derivatives [8, 9]. HMME is thus a promising photosensitizer for PDT, but no reports on the antiviral activity of HMME-PDT have yet been published. In this study, we therefore investigated the efficacy of HMME-PDT as a particle inhibitor to inactivate HIV, and analyzed the factors influencing outcome.
Materials and methods
Materials
HMME (12 mg/ml, 10 ml) was purchased from Shanghai Fudan-zhangjiang Bio-Pharmaceutical Company (China). Methylene blue (MB) injection (1 mg/ml) was purchased from the Emergency Pharmacy of Tianjin Medical University General Hospital (China). A semiconductor laser with an output at a wavelength of 630 nm was manufactured by the Laser Medicine Laboratory, Institute of Biomedical Engineering, Chinese Academy of Medical Sciences. NaN3 and d-mannitol were purchased from Sigma. Horseradish peroxidase-labeled goat anti-human IgG was purchased from Sino-America Biotechnology Company (China). The p5F1 monoclonal antibody against HIV-1 p24 was prepared in the laboratory of Professor Zheng [10] (Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, China). Human polyclonal anti-HIV-1 serum was kindly donated by Dr. Hiroo Hoshino (Gunma University School of Medicine, Japan).
Cells and viruses
The laboratory-derived viruses HIV-1IIIB, HIV-2CBL-20, HIV-2ROD, HIV-1IIIB A17, protease-inhibitor resistant HIV-1 variants (HIV-1 L10R/M46I/L63P/V82T/I84V; HIV-1 protease gene mutants RF/V82F/184V) and enfuvirtide-resistant HIV-1 variants (pNL4-3 gp41 (36G) N42S; pNL4-3 gp41 (36G) V38A, N42T) were obtained from the NIH AIDS Research and Reference Reagent Program and the MRC AIDS Reagent Project. Clinically isolated HIV-1KM018 was obtained from a naive HIV-1-infected individual from Yunnan Province, China, as previously described [11]. All the virus variants were cultured in Professor Zheng’s laboratory. Cell lines (C8166, MT-4, H9/HIV-1IIIB) were maintained in RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum (Gibco). The cells used in all experiments were in log-phase growth. Peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated by Ficoll-Hypaque centrifugation, and incubated in complete medium containing 5 μg/ml phytohemagglutinin (Sigma) for 72 h prior to use in antiviral assays. The 50% tissue culture infectious dose (TCID50) of HIV strains in C8166 cells was determined and calculated using the method of Reed and Muench [12]. Virus stocks were stored in aliquots at −70°C. The titer of the virus stock was 3.4 × 106 TCID50 per ml.
PDT
HMME was used as the photosensitizer in the experiments, with MB as a positive control. MB is an established biological stain and effective photosensitizer with limited in vivo use because of its potential mutagenic effects. A 630-nm wavelength semiconductor laser was developed in our laboratory. The core diameter of the output optical fiber was 400 μm and the numerical aperture was 0.22. The PDT protocol was as follows. Virus suspensions were incubated with HMME or MB at concentrations in the range 0–100 μg/ml for a specific time, followed by irradiation using the semiconductor laser. The power density and irradiation duration were set at different values in different experiments. After PDT, viruses were incubated with host cells and photoinactivation was assessed by detecting the infection rate.
Incubation periods
A total of 50 μl HIV-1IIIB stock solution was mixed with 50 μl HMME (10 μg/ml) and incubated in 5% CO2 at 37°C for various times (0–80 min). The mixture was then irradiated with 20 mW/cm2 for 1 min, diluted 100 times and mixed with 104 C8166 (multiplicity of infection, MOI, 0.3) and incubated for up to 3 days in a CO2-enriched atmosphere in an incubator at 37°C. The number of syncytia was counted and the percentage deactivation was calculated relative to the control group.
Photoinactivation
The HIV-1IIIB, HIV-2 strains (HIV-2CRL20 and HIV-2ROD), resistant HIV-1 strains (HIV-1IIIBA17, HIV-1 L10R/M46I/L63P/V82T/I84V, HIV-1 protease gene mutants RF/V82F/184V, pNL4-3 gp41 (36G) N42S, pNL4-3 gp41 (36G) V38A, N42T) and HIV-1 clinical strains (HIV-1KM018) were subjected to PDT as described above. After PDT, HIV-1IIIB, HIV-2 strains and resistant HIV-1 strains were used to infect C8166 cells (105/ml, MOI 0.3) for 3 days. The number of syncytia was counted for HIV-2 strains, and the level of p24 antigen in the culture supernatant was tested by enzyme-linked immunosorbent assay (ELISA), as described by Zheng et al. [10] for HIV-1-resistant strains. Briefly, Triton X-100-treated cell-free culture supernatant was added to 96-well microtiter plates coated with monoclonal antibody p5F1. Plates were then incubated with diluted human polyclonal anti-HIV-1 serum, followed by incubation with horseradish peroxidase-labeled goat anti-human IgG, and o-phenylenediamine substrate was added. The optical density of each well was read using a Bio-Tek ELx 800 ELISA reader at 490/630 nm. The percentage inhibition of p24 antigen expression was calculated for each well and a dose-response curve was constructed.
HIV-1KM018 strain was added to PBMCs (20% of total, MOI 0.04). After incubation for 2 h, the remaining PBMCs (80% of total) were added and the cultures were incubated in RPMI-1640 with interleukin-2 (50 U/ml) at 37°C for 7 days, with fresh medium supplied every 3 days. The level of p24 antigen in the culture supernatant was tested by ELISA as described above.
Factors affecting photoinactivation
Energy density, power density, photosensitizer, and oxygen can potentially influence the outcome of PDT. We determined the effects of these factors on the inactivation of HIV-1IIIB by PDT by varying their values. Concentrations of HMME were set at fivefold dilution in the range 0–100 μg/ml. In the experiments for energy density, the power density was set at 20 mW/cm2, and the irradiation time was varied from 15 s to 4 min. For power density, the energy density was fixed at 1.2 J/cm2, and the power density was set at 5, 20 or 80 mW/cm2. After PDT, all treated viruses were used to infect MT-4 cells, and the protection rates of infected MT-4 cells were used as a measure of the inhibitory effects on the virus.
In the case of oxygen, one of two antioxidants, sodium azide (10 μM) or d-mannitol (40 μM), or both, was added to the reaction medium throughout the whole procedure. We performed two different antioxidant experiments, one using cell-free HIV-1 particles and the other using cell-associated HIV-1 in the process of virus–cell membrane fusion. The protocol for the latter experiment was as follows. Photosensitizer was added to HIV-1IIIB virus stocks at a 1:1 ratio and incubated at 37°C for 40 min. The mixture was then diluted 100-fold and added to 104 C8166 cells (MOI 0.3) and incubated for 1 h at 4°C to allow virus binding. The virus–cell mixture was washed three times with phosphate-buffered saline to remove extra photosensitizer and free virus. The virus–cell mixture was resuspended in RPMI-1640 and seeded in 96-well microtiter plates. After incubation for different times at 37°C, the cells were irradiated at an energy density of 0.3 J/cm2. p24 antigen expression in the culture supernatant was measured after 3 days, as described previously. The percentage inactivation of virus was calculated relative to the control group.
Statistical analysis
Data were processed using the SPSS software package (version 13.0). Data are shown as means ± SD or SE, unless otherwise stated, and p values of <0.05 were considered significant. Experiments were performed at least three times and data from representative experiments are presented.
Results
Optimal incubation time for photosensitizers
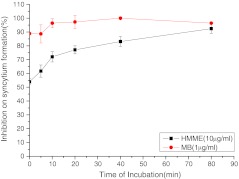
We determined the efficacy of photoinactivation with photosensitizers for different incubation times and determined the optimal incubation duration to be used in subsequent experiments. The results are shown in Fig. 1. The change in the curve from a sharp to a gentle gradient occurred at 40 min for HMME and 10 min for MB. We therefore selected 40 min as the optimal incubation period in subsequent experiments.
Fig. 1.
Duration of incubation with photosensitizers for HIV-1IIIB. The efficacy of PDT was determined by counting syncytium formation. The concentration of photosensitizer was set at 10 μg/ml. Error bars represent the standard deviation
Virus inactivation
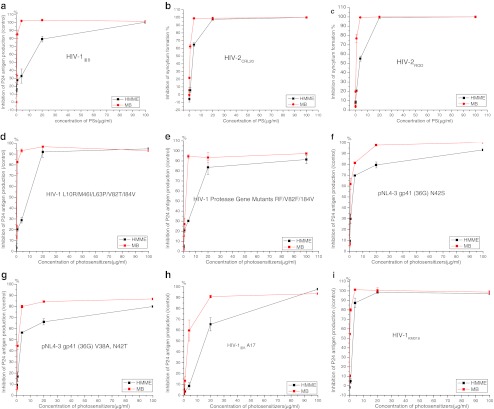
To determine if PDT had broad-spectrum activity against HIV, we evaluated the responses of HIV-1, HIV-2, resistant HIV, and HIV clinical strains to PDT. The inactivating effects of PDT were assessed in terms of the inhibition of syncytium formation or p24 antigen production after infection, compared to nontreated virus. As shown in Fig. 2, all the tested variants were significantly deactivated by PDT treatment. When the dose of HMME was increased to 20 μg/ml or that of MB to 5 μg/ml, the response curves for almost all variants changed from a sharp to a gentle gradient. When the photosensitizer concentrations were increased to 100 μg/ml, the virus inactivation rates were almost 100%.
Fig. 2.
a–c Inactivation of HIV-1IIIB, HIV-2, resistant HIV-1 and HIV-1 clinical strains induced by PDT HIV-1IIIB (a), HIV-2CRL20 (b) and HIV-2ROD (c). The efficacy of PDT was expressed as the inhibition rate of syncytium formation. d, e Inactivation of protease inhibitor-resistant HIV-1 variants HIV-1 L10R/M46I/L63P/V82T/I84V (d) and HIV-1 protease gene mutants RF/V82F/184V (e). f, g Efficacy of PDT against the enfuvirtide-resistant HIV-1 variants pNL4-3 gp41 (36G) N42S (f) and pNL4-3 gp41 (36G) V38A, N42T (g). h, i Inactivation of the reverse transcriptase non-nucleoside inhibitor-resistant variant HIV-1IIIB A17 (h) and HIV-1KM018 (i). p24 antigen production was used as a measure of PDT-induced inactivation of HIV-1-resistant and clinical variants
PDT variables
Energy density, power density and photosensitizer concentration are the three basic variables of PDT, and we therefore examined their contributions to the anti-HIV activities of PDT.
Photosensitizer dose
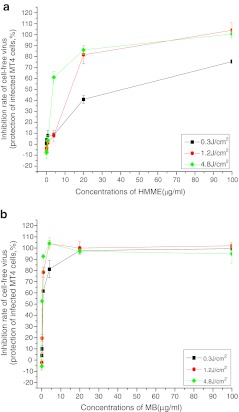
Photoinactivation was represented by the protection of infected MT4 cells (Fig. 3). The survival rate of MT4 cells increased with increasing photosensitizer dose in the range 0–100 μg/ml when the energy density and power density remained the same, suggesting that the efficacy of PDT depended on the concentration of photosensitizer.
Fig. 3.
Anti-HIV activities of PDT at different photosensitizer concentrations and different energy densities. The photosensitizer dose ranged from 0 to 100 μg/ml, and the power density was fixed at 20 mW/cm2 (a HMME, b MB)
Energy density
The efficacy of PDT against HIV was also dependent on energy density, increasing in the order 0.3 J/cm2 < 1.2 J/cm2 < 4.8 J/cm2 at a power density of 20 mW/cm2, as shown in Fig. 3.
Power density
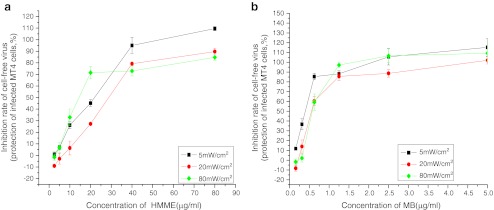
The anti-HIV activity of PDT was also inversely proportional to the power density at 1.2 J/cm2, in the order 5 mW/cm2 > 20 mW/cm2 > 80 mW/cm2 (Fig. 4). However, at HMME doses below 40 μg/ml, the photoinactivation effect was greatest at 80 mW/cm2. The reason for this may be that the energy of light and oxygen is enough for HMME excitation at HMME doses below 40 μg/ml, so the increased virus death at 80 mW/cm2 would be caused by thermal injury from the laser.
Fig. 4.
Anti-HIV activities of PDT at different power densities. The photosensitizer dose ranged from 0 to 100 μg/ml, and the energy density was fixed at 1.2 J/cm2 (a HMME, b MB)
Antioxidants
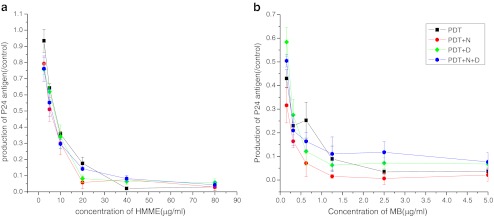
We investigated the abilities of the singlet-oxygen quencher sodium azide and the hydroxyl radical scavenger d-mannitol to protect the virus from PDT-induced inactivation. Virus activity was expressed as p24 antigen production in treated virus as a percentage of that in the control virus. There were no significant differences in p24 antigen production between any of the groups in the cell-free experiments (Fig. 5). This may have been because of a lack of necessary enzymes and substrates in the medium.
Fig. 5.
Effects of antioxidants on the ability of PDT to inactivate cell-free virus (a HMME-PDT, b MB-PDT) (+N addition of 10 μM NaN3, +D addition of 40 μM d-mannitol)
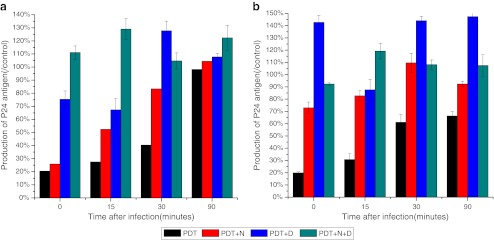
In the cell-associated virus experiments, however, p24 antigen production was lower in the PDT group than in the groups with added sodium azide, d-mannitol, or sodium azide plus d-mannitol, at all time points after adhesion. In the absence of antioxidants, HMME-PDT reduced p24 antigen production in the medium after adhesion to 20.38%, 27.49%, 40.37% and 98.20% of control at 0, 15, 30 and 60 min (p < 0.05), respectively. However, the inhibitory effect of PDT on p24 antigen production was significantly blocked by 10 μM sodium azide, 40 μM d-mannitol, and the combination of the two. d-Mannitol had a greater effect than sodium azide, while the combination of the two had the greatest effect (Fig. 6a). Both antioxidants also weakened the inhibitory effect of MB-PDT on p24 antigen production, with d-mannitol alone having the greatest effect (Fig. 6b).
Fig. 6.
The effects of antioxidants on the ability of PDT to inhibit cell-virus membrane fusion (a HMME-PDT, b MB-PDT) (+N addition of 10 μM NaN3, +D addition of 40 μM d-mannitol)
Discussion
In previous studies we assessed the ability of PDT to inhibit the infection of host cells by HIV, including its effects on viral entry, reverse transcription, integration, duplication, translation, and assembly of the HIV replication cycle, as well as its ability to inactivate cell-free HIV [13]. HMME-PDT inhibited membrane fusion (the process of HIV entry) induced by HIV-1IIIB and deactivated cell-free HIV-1IIIB, suggesting that PDT may represent a promising new treatment for HIV-infected individuals. Cell-free HIV particles represent an important target for PDT, and we therefore investigated the responses of different HIV isoforms, including HIV-1 and HIV-2 variants, resistant HIV-1 variants and HIV-1 clinical variants to HMME-PDT.
Since the initial description of HIV-1 in 1983 [1] and HIV-2 in 1986 [14], these two viruses have been identified as the primary causes of AIDS. HIV-1 is the major cause of AIDS in the world today, but although most anti-AIDS therapies therefore focus on HIV-1 infection, HIV-2 remains a potential problem. The results of the current study showed that not only was PDT with HMME able to inactivate up to 100% of HIV-1IIIB particles, but also up to 100% of HIV-2CRL20 and HIV-2ROD particles, suggesting that it might provide a useful treatment for AIDS patients in most parts of the world. The development of resistant viral strains is one of the main reasons for virological failure of anti-HIV therapy [15]. The current results showed that a series of resistant HIV strains, including those resistant to protease inhibitors (HIV-1 L10R/M46I/L63P/V82T/I84V and HIV-1 protease gene mutants RF/V82F/184V), enfuvirtide (pNL4-3 gp41 (36G) N42S and pNL4-3 gp41 (36G) V38A, N42T) and reverse transcriptase non-nucleoside inhibitors (HIV-1IIIB A17), were all completely responsive to PDT, indicating that this might also represent a useful therapeutic strategy in patients with drug-resistant AIDS. We also determined the effects of PDT on the clinical strain HIV-1KM018 (obtained from a naive HIV-1-infected individual from Yunnan Province, China) and demonstrated complete inactivation at HMME concentrations >20 μg/ml.
Overall these studies suggest that HMME-PDT could provide a useful treatment for AIDS patients infected with most kinds of HIV strains. HMME-PDT targets cell-free HIV particles, which could be accessed in clinical practice via extracorporeal circulation.
However, HMME-PDT is a multifactor therapy and its effects can vary depending on the parameters used. Photosensitizer, light, and oxygen form the basic components of PDT. For example, the molecular structure, molecular charge and relative hydrophobicity of the photosensitizer are important determinants of antiviral activity [6]. Positively charged photosensitizers are reportedly cause nucleic acid damage more effectively than their neutral anionic congeners. However, the latter are more sensitive to the membrane, and enveloped viruses thus respond more sensitively to anionic photosensitizers, while non-enveloped viruses are more sensitive to cationic ones. HMME is an anionic photosensitizer and HIV is an enveloped virus. According to the theory above, HMME should thus be more effective against HIV than the cationic MB. Moreover, the potential mutagenic effects of MB restrict its use in clinical practice [16]. Our previous study also confirmed that the cytotoxicity of MB is higher than that of HMME. Both these factors make HMME a more suitable photosensitizer for the treatment of AIDS.
The results of this study confirmed that photosensitizer dose and light are important determinants of the antiviral activity of PDT. The degree of photodamage to HIV strains depended on the concentration of HMME and the energy of the light. Higher concentrations and increased light energy were associated with increased anti-HIV activities. Power density has also been identified as an important modulator of tissue oxygenation and treatment outcome in PDT. Henderson et al. [17] reported that severe oxygen depletion occurs within seconds of illumination at a fluence rate of 75 mW/cm2 in radiation-induced fibrosarcoma tumors photosensitized with AlPcS2, and they found that power density affected the response of tumors to PDT in the Colon 26 tumor model. High power densities reduced or even totally inhibited tumor control, while low power densities promoted tumor control. Our results are in agreement with those of Henderson et al., in that PDT at a low power density (5 mW/cm2) killed more HIV virus particles than at a high density (80 mW/cm2), as long as a high enough concentration of photosensitizer was used. If the concentration of photosensitizer was insufficient, its levels were exhausted before the oxygen was depleted.
The production of reactive oxygen species, specifically singlet oxygen, is another factor determining the outcome of PDT. The photosensitizer in its ground state is excited to its singlet state by a laser emitting at a suitable wavelength. After intersystem crossing to the triplet state, the photosensitizer can react with oxygen via an energy transfer process to generate singlet oxygen (1O2; type II reaction), or can participate in electron transfer processes, leading to radical formation (type I reaction). Both type I and type II reactions can occur during PDT, but type II reactions usually play a major role [18, 19]. Understanding the major reaction type is important for improving the clinical efficacy of PDT. Two antioxidants, the singlet oxygen quencher sodium azide and the hydroxyl radical scavenger d-mannitol, were used to identify the reaction type in the current study, and both type I and type II reactions were found to occur simultaneously during anti-HIV HMME-PDT, with type I reactions playing a major role.
In conclusion, photoinactivation of HIV by HMME was dependent on the photosensitizer used, light and oxygen. Thus the outcome of PDT could be improved by adjusting the parameters used. The results also demonstrated that most kinds of HIV strains are responsive to HMME-PDT, which therefore represents a promising treatment for AIDS patients. Further studies are planned to identify the molecular mechanisms responsible for the inactivation of HIV particles by HMME-PDT, and to investigate the safety and effectiveness of HMME-PDT for treating AIDS using extracorporeal circulation in animal experiments.
Acknowledgments
This work was supported by grants from the Chinese Academy of Medical Sciences (0124, 0217) and the Natural Science Foundation of China (60678047).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vézinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphotropic retrovirus from a patient at risk for AIDS. Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 2.Popovic M, Sarngadharan MG, Read E, Gallo RC. Detection, isolation and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984;224:497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- 3.Ray S, Fatima Z, Saxena A. Drugs for AIDS. Mini Rev Med Chem. 2010;10:147–161. doi: 10.2174/138955710791185145. [DOI] [PubMed] [Google Scholar]
- 4.Delaney M. The development of combination therapies for HIV infection. AIDS Res Hum Retrov. 2010;26:501–509. doi: 10.1089/aid.2010.0064. [DOI] [PubMed] [Google Scholar]
- 5.Calzavara-Pinton PG, Venturini M, Sala R. Photodynamic therapy: update 2006. Part 1: photochemistry and photobiology. J Eur Acad Dermatol Venereol. 2007;21:293–302. doi: 10.1111/j.1468-3083.2006.01902.x. [DOI] [PubMed] [Google Scholar]
- 6.Wainwright M. Photoinactivation of viruses. Photochem Photobiol Sci. 2004;3:406–411. doi: 10.1039/b311903n. [DOI] [PubMed] [Google Scholar]
- 7.Abe H, Yamada-Ohnishi Y, Hirayama J, Owada T, Ikeda H, Ikebuchi K. Elimination of both cell-free and cell-associated HIV infectivity in plasma by a filtration/methylene blue photoinactivation system. Transfusion. 2000;40:1081–1087. doi: 10.1046/j.1537-2995.2000.40091081.x. [DOI] [PubMed] [Google Scholar]
- 8.Ding X, Xu Q, Liu F, Zhou P, Gu Y, Zeng J, An J, Dai W, Li X. Hematoporphyrin monomethyl ether photodynamic damage on HeLa cells by means of reactive oxygen species production and cytosolic free calcium concentration elevation. Cancer Lett. 2004;216:43–54. doi: 10.1016/j.canlet.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Wang JB, Liu LX, Pan SH, Wang CY, Fu QF. Therapeutic effect of photodynamic therapy using hematoporphyrin monomethyl ether (HMME) on human cholangiocarcinoma cell line QBC939. Neoplasma. 2010;57:79–85. doi: 10.4149/neo_2010_01_079. [DOI] [PubMed] [Google Scholar]
- 10.Liu GJ, Wang JP, Xiao JC, Zhao ZW, Zheng YT. Preparation and characterization of three monoclonal antibodies against HIV-1 P24 capsid protein. Cell Mol Immunol. 2007;4:203–208. [PubMed] [Google Scholar]
- 11.Zhang GH, Wang Q, Chen JJ, Zhang XM, Tam SC, Zheng YT. The anti-HIV-1 effect of scutellarin. Biochem Biophys Res Commun. 2005;334:812–816. doi: 10.1016/j.bbrc.2005.06.166. [DOI] [PubMed] [Google Scholar]
- 12.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Epidemiol. 1938;27:493–497. [Google Scholar]
- 13.Yin HJ, Zheng YT, Li YX, Li CY, Zou ZH, Zhao Y. Photochemical inactivation of cell-associated and cell-free human immunodeficiency virus in vitro. Chinese J Lasers. 2009;36:2705–2711. doi: 10.3788/CJL20093610.2705. [DOI] [Google Scholar]
- 14.Clavel F, Guétard D, Brun-Vézinet F, Chamaret S, Rey MA, Santos-Ferreira MO, Laurent AG, Dauguet C, Katlama C, Rouzioux C. Isolation of a new human retrovirus from West African patients with AIDS. Science. 1986;233:343–346. doi: 10.1126/science.2425430. [DOI] [PubMed] [Google Scholar]
- 15.Locarnini S, Bowden S. Drug resistance in antiviral therapy. Clin Liver Dis. 2010;14:439–459. doi: 10.1016/j.cld.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Floyd RA, Jr, Schneider JE, Dittmer DP. Methylene blue photoinactivation of RNA viruses. Antivir Res. 2004;61:141–151. doi: 10.1016/j.antiviral.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Henderson BW, Busch TM, Snyder JW. Fluence rate as a modulator of PDT mechanisms. Lasers Surg Med. 2006;38:489–493. doi: 10.1002/lsm.20327. [DOI] [PubMed] [Google Scholar]
- 18.Harrod-Kim P. Tumor ablation with photodynamic therapy: introduction to mechanism and clinical applications. J Vasc Interv Radiol. 2006;17:1441–1448. doi: 10.1097/01.RVI.0000231977.49263.DE. [DOI] [PubMed] [Google Scholar]
- 19.Zhu TC, Finlay JC. The role of photodynamic therapy (PDT) physics. Med Phys. 2008;35:3127–3136. doi: 10.1118/1.2937440. [DOI] [PMC free article] [PubMed] [Google Scholar]