Abstract
Pancreatic cancer is one of the most fatal human malignancies. Though a relatively rare malignancy, it remains one of the deadliest tumors, with an extremely high mortality rate. The prognosis of patients with pancreatic cancer remains poor; only patients with small tumors and complete resection have a chance of a complete cure. Pancreatic cancer responds poorly to conventional therapies, including chemotherapy and irradiation. Tumor-specific targeted therapy is a relatively recent addition to the arsenal of anti-cancer therapies. It is important to find novel targets to distinguish tumor cells from their normal counterparts in therapeutic approaches. In the past few decades, studies have revealed the molecular mechanisms of pancreatic tumorigenesis, growth, invasion and metastasis. The proteins that participate in the pathophysiological processes of pancreatic cancer might be potential targets for therapy. This review describes the main players in perineural invasion, hypoxia and desmoplasia and the molecular mechanisms of these pathophysiological processes.
Keywords: Pancreatic cancer, perineural invasion, hypoxia, desmoplasia, therapeutic targets
INTRODUCTION
Pancreatic cancer is one of the major causes for cancer mortality worldwide and is now the fifth most frequent cause of cancer death after lung, prostate, breast, and colorectal cancer [1–2]. Though a relatively rare malignancy, it remains one of the deadliest tumors, with an extremely high mortality rate approaching 100% [3]. The prognosis of patients with pancreatic cancer is not optimistic. Around 75% of patients die within 1 year of diagnosis, whereas about 5% or less will survive for 5 years [4]. Pancreatic cancer survival has not improved substantially over the past 25 years [2]. Most cases are advanced at the time of diagnosis. Only about 10% to 20% of patients are considered eligible for resection. Usually by the time symptoms are present, there are no curative options because of the invasion and metastasis of tumors [4].
One of the main causes of local recurrence of the tumor is perineural invasion (PNI), which is frequently found in pancreatic cancer (incidence as high as 90%–100%) [3,4]. As a common but nonspecific feature of pancreatic cancer, PNI is an important prognostic factor for pancreatic cancer. Further, PNI increases as the cancer becomes more undifferentiated (i.e., MIA PaCa-2 cells) [5].
During growth and development of pancreatic cancer, there are regions of low oxygen tension (hypoxia) because of an imbalance in oxygen supply and demand. However, cancer cells undergo genetic and adaptive changes that allow them to survive and even proliferate in these hypoxic conditions [6]. In human cancer cells, both intratumor hypoxia and genetic alterations affecting signal transduction pathways lead to an increased hypoxia induced factor 1 (HIF-1) activity, which in turn promotes angiogenesis, metabolic adaptation, and other critical aspects of tumor progression [7].
A hallmark in pancreatic ductal adenocarcinoma is the presence of ‘desmoplasia,’ which is defined as proliferation of fibrotic tissue with an altered extracellular matrix (ECM) conducive to tumor growth and metastasis [8]. The desmoplasia, which consists of fibroblasts, pancreatic stellate cells, lymphatic and vascular endothelial cells, immune cells, pathologic increased nerves and ECM, creates a complex tumor microenvironment that promotes pancreatic cancer development, invasion, metastasis and resistance to chemotherapy.
Here we review experimental studies on PNI, hypoxia and desmoplasia in pancreatic cancer. Based on these studies, several key factors are focused on as potential therapeutic and diagnostic targets.
PERINEURAL INVASION
Perineural invasion, a special metastatic route in pancreatic cancer, is the process of cancer cell invasion of nerves. PNI is a distinct pathologic entity that can be observed in the absence of lymphatic or vascular invasion. Pancreatic cancer is characterized by extremely high frequency of PNI, which is an early event. Our previous study reported an 86.9% (53/61) PNI rate in pancreatic cancer patients [5]. Pour et al. have reported that 100% of pancreatic tumors would reveal PNI if enough sections are evaluated [9]. Recent studies have clearly demonstrated that PNI is an independent prognostic factor in tumors arising from the prostate, biliary tract, skin and pancreas. In pancreatic tumors, nerve plexus invasion is regarded as one of the most important prognostic factors [10].
Neurotropism to intra-pancreatic nerves and continuous extension of tumor cells into the extra-pancreatic nerve plexus are hallmarks of ductal adenocarcinoma of the pancreas and a major cause of recurrence after curative resection [9]. In addition to clinical recurrence, neural infiltration by cancer cells and ultimate nerve damage may in part explain the severe pain experienced by advanced patients, as in pancreatic cancer [11].
The Mechanism of Perineural Invasion
The mechanism of PNI in pancreatic cancer is not clear. There are two prominent theories: one is “path of low resistance” and the other is reciprocal signaling interactions. Because of the anatomical proximity between the pancreatic and celiac artery neural plexus, the human pancreas have plenty of neural tissues, including ganglia, being innervated by the autonomic nervous system, through plexi from the celiac and superior mesenteric artery ganglia [9]. Perineurium damage has been identified at the end of the nerve, at the site where the nerve is invaded by the blood vessels around nerves, and at the site invaded by reticular fibers [12–13]. Many previous studies presumed that the tumor cells grow along the “path of low resistance”, and the path serves as a route for their distant migration [14].
Another possible explanation of PNI in pancreatic cancer is reciprocal signaling interactions, because advanced pancreatic cancer with PNI expressed many types of neuroendocrine markers including S-100, synaprophysin, substance-P, enkephalin, and neural cell adhesion molecules (NCAM). More recently, studies have demonstrated that PNI may involve reciprocal signaling interactions between tumor cells and nerves. These invading tumor cells may have acquired the ability to respond to pro-invasive signals within the peripheral nerve milieu [14]. Promising contributors of these neuro–cancer interactions and supporting factors in the pathogenesis of neural invasion in pancreatic cancer seem to be represented by various neurotrophic factors. The detection of increased neurotrophic factors like nerve growth factor (NGF), Glial cell line-derived neurotrophic factor (GDNF), and brain-derived neurotrophic factor (BDNF) in intra-pancreatic nerves and tumor cells, and of their receptors on tumor cells led to increased attention to these molecules in recent years [15–17]. Ceyhan et al. use dorsal root ganglia (DRG) cells and tumor cells to imitate the extrinsic and intrinsic innervations of the pancreas, delivering a much more realistic model for studying the intrapancreatic innervation [18–19]. These findings indicated that nerves provide a suitable environment for tumor growth and the interaction has a very positive effect on the growth of both nerves and tumors.
Therapeutic Targets to PNI in Pancreatic Cancer
Kayahara et al. found that tumor cells grew mainly in a continuous fashion along the branches of nerves by examining histopathologically a huge amount of consecutive sections (almost 5000) of tumor specimens [20]. Tumor cells directly destruct the perineurium to invade into the perineural cavities via the perforating vessels of the perineurium. They also noted that tumor cells spread in the perineural space in a continuous fashion, branch where the nerve itself branches and tends to directly invade the adjacent lymph nodes. Thus, PNI can be regarded as an important route to disseminate to distant organs.
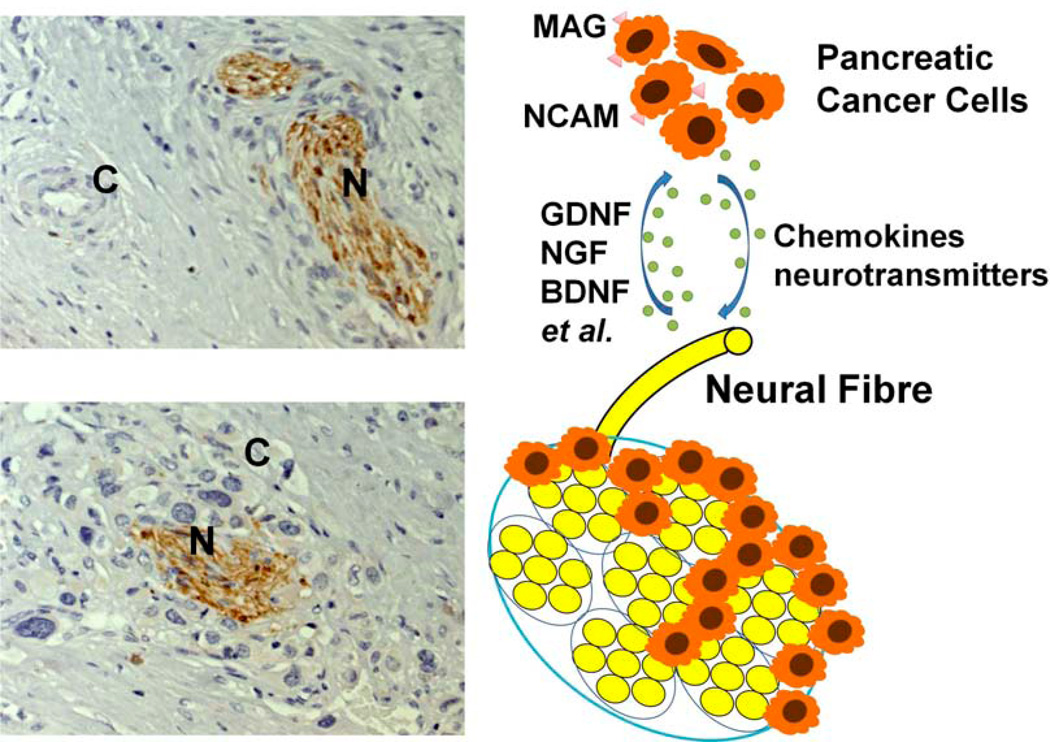
Some neurotrophic factors secreted by nerves enhanced the cancer–nerve interaction, providing biological and physical parameters that would explain their frequent and intimate relationship [17, 21]. Several neurotrophins, including NGF, BDNF, and neurotrophin -3(NT-3) have been implicated in promoting tumor cell invasion and may be key mediators in the pathogenesis of PNI [22–24]. Researchers have begun searching for viable therapeutic targets among these neurotrophins and their receptors (Fig. (1)) [23, 25–27].
Fig. (1). The interaction between neural cells and cancer cells in the generation of perineural invasion (PNI) in pancreatic cancer.
Several neurotrophic factors (e.g., NGF, GDNF, BDNF), chemokines (e.g., CSF, CX3CL1), neurotransmitters (e.g., substance P) and cell-surface molecules (e.g., NCAM, MAG) are involved in neuro-cancer interactions. Growth factors secreted by cancer cells attract neural fibers to grow towards and into the cancer. Simultaneously, molecular factors derived from neural tissues induce the proliferation and direct the migration of cancer cells. (C, cancer cells; N, neural fiber)
NGF
NGF and its cognate receptors have been implicated in the paracrine growth regulation of a number of neuronal and non-neuronal tumor types. J. Miknyoczki et al. have shown that NGF could enhance clonal growth, increase tumor invasiveness, and cause changes in cell morphology in some kinds of non-neuronal cell types including melanoma, prostatic and pancreatic carcinoma [22–23, 28]. Li et al. used histopathology to investigate many consecutive sections of tumor specimens; the study revealed that nerves damage and regeneration occur simultaneously in the tumor microenvironment of pancreatic cancer patients with hyperglycemia; the simultaneous occurrence may aggravate the process of perineural invasion. The abnormal expression of NGF and p75 may also be involved in this process and subsequently leads to a lower rate of curative surgery [29]. NGF and its receptor trkA ars overexpressed in pancreatic cancer cell lines and the perineurium of peripheral nerves. Binding of NGF to trkA leads to activation of the p44/42 MAPK signaling pathway, promotes cancer cell growth, increases the invasiveness and metastasis and eventually mediates nerve invasion [30].
GDNF
In the recent studies, researchers found that nerve invasion was dependent on GDNF secretion and mitogen-activated protein kinase activity. GDNF coreceptors RET (rearranged during transfection) and GDNF family receptor alpha 1 (GFRα-1) were highly expressed in human pancreatic carcinomas by the same population of cells [15, 31–33]. GDNF-secreting glioma cells could increase matrix metalloproteinase-9 (MMP-9) expression and activity, promote the migration of pancreatic cancer cells in a dosedependent manner, suggesting both a chemotactic effect and a chemokinetic effect of GDNF on tumor cells [32, 34].
BDNF
Brain-derived neurotrophic factor may be overexpressed by tumor cells to promote neurite growth, and BDNF stimulates tumor cell invasion at low-to-moderate concentrations. BDNF is overexpressed in pancreatic cancer and adenoid cystic carcinoma, but the expression does not correlate with the presence of PNI, suggesting that the BDNF-expressing phenotype may appear before nerves [21, 35–36].
NCAM
neural cell adhesion molecules (NCAM) and homophilic adhesion molecules, expressed on the nerve cells, as a factor of neurotropism, were examined in 15 pancreatic cancer resection specimens, especially in neural invasive lesions [37]. A histopathologic study was performed on 24 patients with bile duct carcinoma who underwent resections. NCAM expression was shown to be positive in 16 (76%) out of 21 cases in whom perineural invasion was observed. A significant positive correlation was found between the expression of NCAM and perineural invasion in bile duct cancer. These results highlight the important role of NCAM in the development of perineural invasion in bile duct cancer. NCAM play critical navigation and docking roles by binding to target cells during the growth and development of the nervous system. NCAM are highly expressed in peripheral nerve tissue. Some reported that PNI is correlated with NCAM expression, indicating that NCAM molecules on the surface of cancer cells might induce them to migrate and adhere to nerve cells after the tumors breach their capsules [38]. Recent evidence demonstrated that activation of the proto-oncogene K-Ras in pancreatic cancer cells could induce the up-regulation of polysialic acid neural cell adhesion molecule (PSA-NCAM) on tumor cell surfaces. PSA-NCAM could bind to N-cadherin, blocking N-cadherin mediated cell adhesion, increasing pancreatic cancer cell migration ability and facilitating tumor cell metastasis to nerve tissue [39]. NCAM modulate neurite outgrowth and matrix adhesion of beta-cells from pancreatic tumors by assembling a fibroblast-growth-factor receptor-4 (FGFR-4) signaling complex, stimulate beta1-integrin-mediated cell-matrix adhesion and promote the dissemination of metastatic tumor cells [40].
MAG
Myelin-associated glycoprotein (MAG) is a membrane-bound protein expressed by myelinating Schwann cells in the periaxonal membrane. MAG on Schwann cells binds to gangliosides on axons and also the mucin MUC1 expressed by pancreatic tumor cells. The results of Tumor cell–Schwann cell adhesion assay and immunohistochemical analysis showed that the adhesive interactions between MUC1 and MAG are of biological significance in pancreatic cancer perineural invasion [41]. Some researchers found that laminin-5 released from the cancer cells has a close relation with PNI in head and neck squamous carcinoma, supporting the hypothesis that the deposition of basement membrane components may be required in the process of nerve invasion [42].
Chemokines
Marchesi et al. found that tumor cells from human pancreatic cancer strongly up-regulate the chemokine receptor CX3CR1, not expressed in the normal pancreatic epithelium, which is involved in the migration of cancer cells to the distant organs and nerves, thus highlighting the role of CX3CL1/CX3CR1 axis in metastasis [43]. CX3CR1 exclusively binds the transmembrane chemokine CX3CL1 expressed by neurons, nerve fibers and activated endothelial cells [44–47]. CX3CR1+ PDAC tumor cells migrating in response to the ligand CX3CL1, specifically adhere to neural cells. Higher CX3CR1 presence and perineural invasion were strongly associated with local and earlier tumor recurrence. Studies of the role of fractalkine and CX3CR1 in human malignancies provide the rationale to evaluate them as potential targets for therapeutics aimed at reducing tumor-associated pain and tumor progression [43, 48].
γ-synuclein
Recent study indicated that-synuclein is aberrantly expressed by tumor cells. The overexpression is the key biological marker of increased malignant potential and is closely involved in perineural invasion and liver/lymph node metastasis in pancreatic cancer. In surgically resected cases, γ-synuclein is a significant prognostic factor. γ-synuclein may serve as a novel molecular target of early diagnosis as well as antimetastatic therapy [49].
HYPOXIA
Solid tumors often contain heterogeneous hypoxic areas. In addition to diffusion-limited chronic hypoxia in cells that are 100–150 mm away from blood vessels, some tumor cells may also experience perfusion-limited intermittent hypoxia because of intermittent blood supply caused by abnormal tumor vasculature. Interaction among cancer, stellate and inflammatory cells leads to a critically hypoxic microenvironment in pancreatic tumor tissues because of the inhibition of angiogenesis and aberrant extracellular matrix deposition around the periacinar capillary network [50].
HIF-1 is the Key Factor in Tumor Hypoxia Response
HIF-1 is a heterodimeric transcription factor that consists of HIF-1α and β subunits. Under normoxia conditions, the newly produced HIF-1α subunit is quickly hydroxylated by proline hydroxylase (PHD) in a reaction requiring oxygen, ubiquitinated by the von Hippel-Lindau (VHL) protein and then rapidly degraded in the proteosome. However, under conditions of hypoxia in many cancers, oxygen-stimulated PHD is inactivated, and HIF-1α thus cannot be degraded. HIF-1α accumulates in the nucleus, leading to the upregulation of many hypoxia-response proteins, including glycolytic enzymes, angiogenic factors and proteins involved in cell viability, such as glucose transporter 1 (GLUT-1) and vascular endothelial growth factor (VEGF) [51–52].
The poor perfusion frequently found in tumors causes intratumoral hypoxia and therefore stabilizes HIF-1α. On the other hand, cancer cells may accumulate metabolites that directly inhibit PHD and thereby stabilize HIF-1α independent of hypoxia [51].
In both normal development and tumor growth, hypoxia results in the development of additional vasculature through the increased vascular growth factors, notably VEGF-A [53]. VEGF-A was first identified as a vascular permeability factor (VPF) before it was rediscovered as a mitogenic chemo-attractant and fenestrating factor for vascular endothelial cells. Microvascular development is determined by the interplay between tissue cells and microvascular endothelial cells. Vascular endothelial cells migrate to the source of VEGF-A, proliferate and form blood vessels in response to VEGF-A (Fig. (2)) [54]. Because of the importance of HIF-1α in cancer, targeting HIF-1α could become a novel approach in cancer therapy.
Fig. (2). HIF-1α is the key factor in hypoxia response.
Hypoxic conditions can lead to several cellular and molecular changes, many of which are transduced through the basic helix-loop-helix transcription factor HIF-1α. Under normal oxygen conditions, the HIF-1α protein is rapidly ubiquitinated and degraded. Under hypoxic conditions, the protein is stabilized, heterodimerizes with ARNT, and translocates to the nucleus where it activates transcription from a number of hypoxia-responsive genes.
Cancer Therapy Targeting HIF
Oxygen availability affects tumor therapy efficacy not only by the direct effects of severe hypoxia (anoxia), but also by genetic components involving altered HIF-1 and HIF-1 target gene expression in malignant cells. HIF-1α-deficient cells are more susceptible to chemotherapeutic agents and radiotherapy [55].
Tirapazamine
Tirapazamine (TPZ) is a newer class of cytotoxic drugs with selective toxicity towards hypoxic mammalian cells. It represents a class of hypoxia-selective cytotoxins and is currently in phase II and III clinical trials for the treatment of head and neck cancers, cervical cancer and lung cancer [56]. TPZ also functions as a hypoxia-activated topoisomerase IIa poison [57].
PX-478
PX-478 (S-2-amino-3-[4V-N,N,-bis(2-chloroethyl) amino]-phenyl propionic acid N-oxide dihydrochloride) is an experimental small-molecule agent that suppresses constitutive and hypoxia-induced HIF-1α in cancer cells at multiple levels [58]. In xenograft mice with Panc-1 pancreatic cancer cells, treatment of mice with 125 mm3 tumors with PX-478 at a dose of 100 mg/kg/day i.p. for 5 days caused a 40% tumor regression and a tumor growth delay of 35 days. Treatment of mice with established 288 mm3 MIA PaCa-2 cancer xenografts with PX-478 on the same schedule resulted in a 20% tumor regression and a tumor growth delay of 6 days, whereas mice with initial 100 mm3 BxPC-3 cell xenografts displayed 20% tumor regression and 30 days tumor growth delay. The antitumor response to PX-478 positively correlated with tumor HIF-1α levels. An important feature of PX-478 is that it appears to cause a greater response in larger tumors with increased HIF-1α staining than in smaller tumors. Tumor xenografts showing high levels of HIF-1α staining showed marked tumor regression and growth delay caused by treatment with PX-478. The inhibition of tumor growth appears to correlate with inhibition of glucose metabolism rather than inhibition of angiogenesis because PX-478 gave a relatively small change in tumor VEGF and a marked and prolonged decrease in tumor GLUT-1 [59]. In orthotopic human non-small cell lung cancer cell PC14-PE6 mouse models, treatment with 20 mg/kg PX-478 daily oral treatment significantly reduced the median primary lung tumor volume and mediastinal metastasis and prolonged survival. In small cell lung cancer models, PX-478 was even more effective [60]. In addition to producing cytotoxicity, PX-478 enhanced the radiosensitivity of PC3 and DU 145 prostate carcinoma cells in vitro [61]. PX-478 also causes in vivo radiosensitization to prevent postradiation HIF-1 signaling, and treatment also abrogates downstream stromal adaptation through blockade of HIF-1-dependent reconstitution of tumor stromal function in C6 glioma and HN5 head and neck squamous carcinoma cells [62]. No experimental research in orthotopic mouse models of pancreatic cancer has been reported.
Fusion protein
Kizaka-Kondoh et al. developed and validated a fusion protein called TAT-PTD-ODD-procaspase-3 (TOP3) to specifically eradicate HIF-1–active hypoxic cells. TOP3 consists of three domains: 1) a protein transduction domain (TAT-PTD) for efficient delivery into cells, 2) an ODD domain containing a VHL-mediated protein destruction motif from the human HIF-1α protein that confers hypoxia-dependent stabilization to the fusion protein for oxygen-dependent regulation, and 3) a functional domain (procaspase-3, an inactive proenzyme form of human caspase-3) for tumor cell killing via activation by preexisting apoptotic signals in hypoxic cells [63–64]. The biologically active fusion protein was specifically stabilized in solid tumors but was barely detected in the normal tissue. It did not cause any obvious side effects in tumor-bearing mice, suggesting specific stabilization and activation of the fusion protein in the hypoxic tumor cells [65].
Recently, another HIF-1–targeting fusion protein, POP33, was reported, which has a novel protein transduction domain PTD3 that mediates significantly higher protein transduction activity than TAT-PTD [63]. In an orthotopic SUIT-2/HRE-Luc cell pancreatic cancer mouse model, POP33 suppressed peritoneal dissemination and improved survival of tumor-bearing mice as well as selectively impaired HIF-1–active/hypoxic cells in the xenografts.
Endothelial cells at the sites of angiogenesis express high levels of VEGF receptors and therefore may be particularly sensitive to VEGF-mediated drug delivery [66]. A SLT-VEGF fusion protein composed of VEGF121 and the catalytically active A subunit of Shiga-like toxin 1 (SLT-1) produced by Escherichia coli O157:H7 [67–68] retained functional activities of both SLT and VEGF121 moieties. The SLT-VEGF fusion protein is internalized into cells through VEGFR-2 mediated endocytosis, and its cytotoxicity correlates with cellular VEGFR-2 expression. It selectively inhibits growth of endothelial cells but does not influence proliferation of pancreatic cancer cells. The SLT-VEGF fusion protein reduced tumor growth, metastasis and microvascular density and increased 14-week survival in an orthotopic pancreatic cancer model in nude mice.
DESMOPLASIA
Pancreatic adenocarcinoma has an extraordinary fibrotic component, the extent of which is often greater than the epithelial component [69–73]. Several excellent reviews have reported the molecular and cellular regulation of pancreatic fibrosis [74–75].
Role of Pancreatic Stellate Cells in Desmoplasia
In the desmoplasia progress of pancreatic cancer, the pancreatic stellate cells (PSCs) play an important role. Activated PSCs, which express alpha-smooth muscle actin (α-SMA) and colocalize with mRNA encoding procollagen αI, participate in the desmoplasia of human pancreatic cancers [69] and contribute to most of the extracellular matrix (ECM) proteins that constitute the desmoplasia [69, 73, 76–80]. PSCs are quiescent in healthy conditions. In diseased states, under the influence of growth factors, cytokines or oxidant stress, PSCs begin to morph into myofibroblast-like phenotypes that secrete significant levels of ECM as well as matrix-degrading enzymes [81].
The pivotal regulation that orchestrates the persistent activation of PSCs in vivo is similar to the regulation of the activation of primary PSCs in culture. Studies on human and rodent primary PSCs in culture have found a large number of growth factors, cytokines, hormones, intracellular signaling molecules, and transcription factors to be mediators of PSC activation. Potential activators of PSCs in vivo include growth factors (platelet derived growth factor (PDGF) and Transforming growth factor beta 1 (TGF-β1), paracrine factors, such as cytokines (interleukin-1 (IL-1), IL-6, IL-8, and tumor necrosis factor alpha (TNF-α), angiotensin II, and reactive oxygen species, which are released by damaged neighboring cells, and leukocytes, which are recruited in response to pancreatic injury [82–88].
To maintain the activated phenotype, activated PSCs produce autocrine factors, such as PDGF, TGF-β1, cytokines (e,g. IL-1, IL-6, and TRAIL), and proinflammatory molecules (e.g. cyclooxygenase 2 (COX-2) [85, 87, 89–90]. Moreover, activin-A, a member of the soluble factors of the TGF-β family, also performs its functions in an autocrine manner, increasing collagen secretion and upregulating TGF-β1 expression and secretion [91]. Also, rat PSCs in primary culture express endothelin-1, which in turn can stimulate their migration and contraction [92]. Ample experimental evidence demonstrates that PDGF acts as a major modulator, inducing the proliferation of PSCs and contributing to the migration capacity of PSCs, and TGF-β1 and angiotensin II induce PSCs to express α-SMA and ECM proteins to transform into an activated phenotype. Those three are considered modulators of the persistently activated and profibrotic phenotype of PSCs (Fig. (3)) [74–75, 82, 86–91, 93–94]. Moreover, several inflammatory factors released during pancreatitis have the potential to activate PSCs. Recently, IL-13 has been shown to promote the proliferation of rat pancreatic stellate cells through the suppression of the NF-γB/TGF-β1 pathway. Alcohol metabolites and oxidative stress have also been considered to have the potential to activate PSCs. Ethanol can be metabolized in pancreatic acinar cells, leading to toxic metabolites and oxidative stress that can cause pancreatic damage [95]. In vitro cultured rat PSCs display ethanol-induced alcohol dehydrogenase activity, implying that PSCs may also participate in metabolizing ethanol [95–96]. Ethanol and its metabolite acetaldehyde not only promote the activation of rat PSCs but also cause lipid peroxidation in these cells [95]. Moreover, the antioxidant vitamin E can prevent ethanoland acetaldehyde-induced activation of PSCs, thereby indicating that oxidative stress regulates PSC activation [96]. Carbon monoxide releasing molecule-2 inhibits PSC proliferation by activating p38 MAPK/HO-1 signaling [97]. Conditioned medium from hypoxia-treated PSCs induced migration of PSCs, which could be inhibited by an antibody against VEGF but not by an antibody against hepatocyte growth factor. PSCs also express several angiogenesis-regulating molecules, including VEGF receptors, angiopoietin-1, and Tie-2, and hypoxia induced type I collagen expression in PSCs [98]. Fibrinogen induced the expression of IL-6, IL-8, monocyte chemoattractant protein-1, VEGF, angiopoietin-1 and type I collagen, but not proliferation or intercellular adhesion molecule-1. Fibrinogen increased α-smooth muscle actin expression and induced the activation of NF-κB, Akt and three classes of MAPK (ERK, c-Jun N-terminal kinase and p38 MAPK). IL-6 and IL-8 production induced by fibrinogen was inhibited by antibodies against αvβ3 and α5β1 integrins, indicating that these integrins served as counter receptors for fibrinogen in PSCs. In addition, fibrinogen-induced production of these cytokines was eliminated by an inhibitor of NF-κB and partially suppressed by inhibitors of ERK and p38 MAPK [99]. Multiple studies have demonstrated that major signaling pathways are involved in the regulation of PSC function [88, 92, 100–103]. MAPKs are pivotal activating signal mediators initiated by growth factors, angiotensin II, and ethanol [91, 103]. Other signaling pathways mediating PSC activation include PI3K, RHO kinase, the activator protein-1, and the NF-κB, JAK/STAT, and TGF-β/SMAD–related pathways [92, 101, 104]. Additionally, PPARγ ligands have been implicated in the downregulation of PSC activation [100].
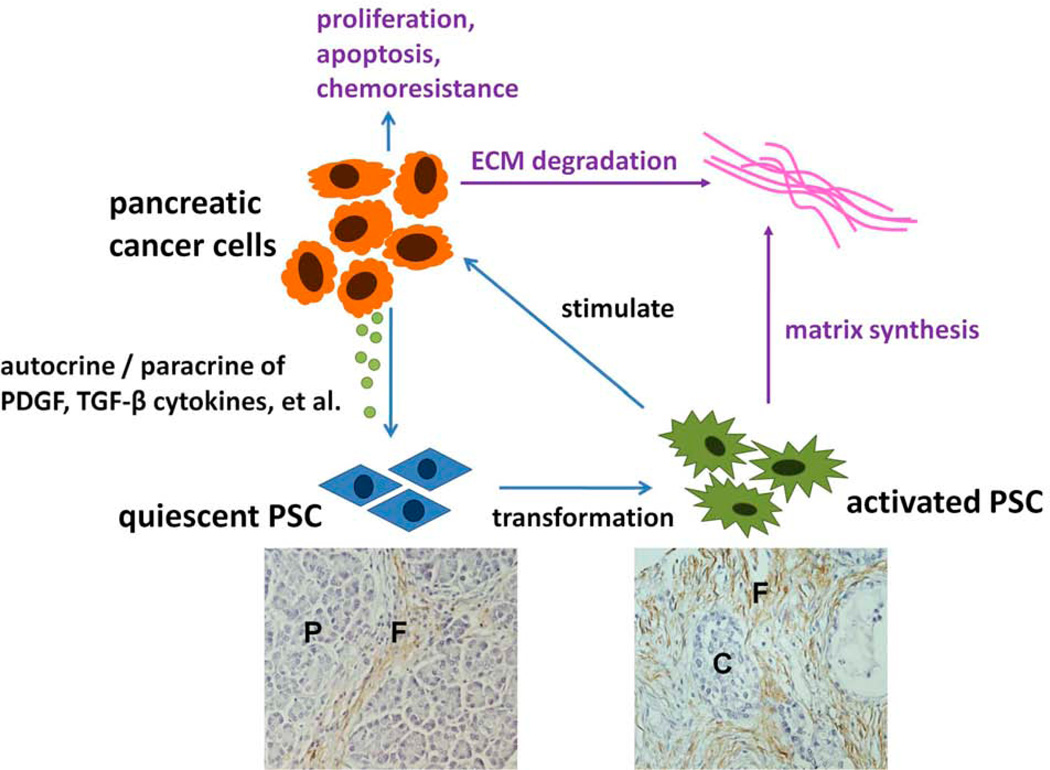
Fig. (3). Interaction of PSCs with pancreatic cancer cells.
Cancer cells accelerate transformation of quiescent PSCs to an activated phenotype in a paracrine manner. Activated PSCs are attracted by cancers and stimulated to proliferate and produce ECM and growth factors. Further, PSCs stimulate the proliferation, chemoresistance, invasion, and motility of pancreatic cancers. (P, pancreatic acinar; F, stroma fibrosis; C, cancer cells)
Therapeutic Potential of Desmoplasia
In theory, treatments for pancreatic cancer by targeting PSCs should focus on the key mechanisms involved in their activation and proliferation. Blocking the receptors for PDGF, TGF-β, and angiotensin II, as well as blocking the intracellular signaling pathways downstream of these receptors, seems to provide effective therapeutic benefits [93, 102, 105–106]. In vivo experiments have shown the important role of the angiotensin II system in the development of pancreatic fibrosis [106]. Also, several studies in vitro using PSCs showed pivotal roles for MAPK pathways in the activation and/or proliferation process: in particular, ERK1/2, p38 kinase, and JNK [101, 103–104, 107–109], PKC and PI3K, PPARγ [110], carbon monoxide releasing molecule-2 [97]; and ethanol metabolism to acetaldehyde [75]. In the aforementioned studies, inhibition of most of these pathways attenuates the activation and proliferation of PSCs, but activation of PPARγ and carbon monoxide releasing molecule-2 is likely to block the PSC activation [97, 104, 110].
Studies have also shown that activated rat PSCs express COX-2 when stimulated by TGF-β1 and other cytokines [90] or conditioned medium from human pancreatic tumor cells [111]. Pharmacological blockade of COX-2 and inhibition of the TGF-β1 signaling pathway reduced the expression of COX-2, α-SMA, and collagen I, indicating that COX-2 might be a relevant therapeutic target for pancreatic cancer. Furthermore, inducing PSC transdifferentiation from an activated to a quiescent state and increasing PSC apoptosis are also attractive approaches. For example, administration of vitamin A transformed culture-activated rat PSCs in a quiescent state [112]. As for the approach of targeting fibrosis in pancreatic tumor models, antibodies to CTGF, which activates PSCs through their α5β1 integrin receptor [89], decreased tumor growth and metastasis in a xenograft mouse model [113–114]. In addition, efforts aimed at blocking TGF-β signaling pathways [111, 115] have proven effective in reducing experimental pancreatic fibrosis in rodents.
The expression of EMMPRIN (extracellular matrix metalloproteinase inducer) by cancer cells plays a very important role in the interaction of stellate cells with tumor cells, the shedding of the extracellular part of EMMPRIN by MMPs in cancer cells, and the induction of MMPs in stellate cells by soluble EMMPRIN [116]. EMMPRIN serum levels were remarkably increased in pancreatic cancer patients [117]. Thus, EMMPRIN may be a therapeutic target. Due to the important role of PSC pathobiology in pancreatic cancer, increasing efforts should be mad to addressing PSC functions in this disease and the development of therapeutic strategies targeting PSCs.
CONCLUDING REMARKS
In the present work, we shows the value of viable therapeutic targets among these molecular factors, which also participate in the pathophysiological processes of perineural invasion, hypoxia and desmoplasia. Various approaches have already been developed to modulate the activities of these mediators, and develop new contexts for them in pancreatic cancer may open up new opportunities for innovative therapeutic strategies.
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation (Grant serial No.30900705), 13115 Major Project (2010ZDKG-49), Scientific Grant of Xi'an City (2009 No.SF09027), and ND EPSCoR funds and Pilot Project Grant (EW) from the Centers of Biomedical Research Excellence (COBRE) grant NIH P20 RR020151 from the National Center for Research Resources (NCRR). NCRR is a component of the National Institutes of Health (NIH). The contents of this report are solely the responsibility of the authors and do not necessarily reflect the official views of the NIH or NCRR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LIST OF ABBREVIATION
- HIF-1
Hypoxia induced factor 1
- PNI
Perineural invasion
- NCAM
Neural cell adhesion molecules
- DRG
Dorsal root ganglia
- NGF
Nerve growth factor
- GDNF
Glial cell line-derived neurotrophic factor
- BDNF
Brain-derived neurotrophic factor
- NT
Neurotrophin
- MMP
Matrix metalloproteinases
- PHD
Proline hydroxylase
- VHL
Von Hippel-Lindau
- GLUT
Glucose transporter
- VEGF
Vascular endothelial growth factor
- PSCs
Pancreatic stellate cells
- ECM
Extracellular matrix
- PDGF
Platelet derived growth factor
- TGF-β1
Transforming growth factor beta 1
- IL
Interleukin
- COX-2
Cyclooxygenase 2
- MAPK
Mitogen-activated protein kinase
- HO-1
Heme oxygenase 1
- NF-κB
Nuclear factor kappa B
- ERK
Extracellular signal-regulated kinase
REFERENCES
- 1.Shaib YH, Davila JA, El-Serag HB. The epidemiology of pancreatic cancer in the United States: changes below the surface. Aliment Pharmacol Ther. 2006;24:87–94. doi: 10.1111/j.1365-2036.2006.02961.x. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:197–209. doi: 10.1016/j.bpg.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Cartwright T, Richards DA, Boehm KA. Cancer of the pancreas: are we making progress? A review of studies in the US Oncology Research Network. Cancer Control. 2008;15:308–313. doi: 10.1177/107327480801500405. [DOI] [PubMed] [Google Scholar]
- 5.Li JH, Ma QY, Liu H, et al. Relationship between Neural Alteration and Perineural Invasion in Pancreatic Cancer Patients with Hyperglycemia. Plos One. 2011;6(2):e17385. doi: 10.1371/journal.pone.0017385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang M, Ma Q, Hu H, et al. Stem cell factor/c-kit signaling enhances invasion of pancreatic cancer cells via HIF-1alpha under normoxic condition. Cancer Lett. 2011;303:108–117. doi: 10.1016/j.canlet.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Hu HT, Ma QY, Zhang D, et al. HIF-1 alpha links beta-adrenoceptor agonists and pancreatic cancer cells under normoxic condition. Acta Pharmacol Sin. 2010;31:102–110. doi: 10.1038/aps.2009.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6:1186–1197. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 9.Pour PM, Bell RH, Batra SK. Neural invasion in the staging of pancreatic cancer. Pancreas. 2003;26:322–325. doi: 10.1097/00006676-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Kayahara M, Nagakawa T, Konishi I, Ueno K, Ohta T, Miyazaki I. Clinicopathological study of pancreatic carcinoma with particular reference to the invasion of the extrapancreatic neural plexus. Int J Pancreatol. 1991;10:105–111. doi: 10.1007/BF02924113. [DOI] [PubMed] [Google Scholar]
- 11.Ceyhan GO, Michalski CW, Demir IE, Muller MW, Friess H. Pancreatic pain. Best Pract Res Clin Gastroenterol. 2008;22:31–44. doi: 10.1016/j.bpg.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Liu B, Lu KY. Neural invasion in pancreatic carcinoma. Hepatobiliary Pancreat Dis Int. 2002;1:469–476. [PubMed] [Google Scholar]
- 13.Nagakawa T, Kayahara M, Ueno K, Ohta T, Konishi I, Miyazaki I. Clinicopathological study on neural invasion to the extrapancreatic nerve plexus in pancreatic cancer. Hepatogastroenterology. 1992;39:51–55. [PubMed] [Google Scholar]
- 14.Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379–3391. doi: 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- 15.Ceyhan GO, Giese NA, Erkan M, et al. The neurotrophic factor artemin promotes pancreatic cancer invasion. Annals of Surgery. 2006;244:274–281. doi: 10.1097/01.sla.0000217642.68697.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Z, Friess H, diMola FF, et al. Nerve growth factor expression correlates with perineural invasion and pain in human pancreatic cancer. J Clin Oncol. 1999;17:2419–2428. doi: 10.1200/JCO.1999.17.8.2419. [DOI] [PubMed] [Google Scholar]
- 17.Zhu ZW, Friess H, Wang L, et al. Nerve growth factor exerts differential effects on the growth of human pancreatic cancer cells. Clinical Cancer Research. 2001;7:105–112. [PubMed] [Google Scholar]
- 18.Dai H, Li R, Wheeler T, et al. Enhanced survival in perineural invasion of pancreatic cancer: an in vitro approach. Hum Pathol. 2007;38:299–307. doi: 10.1016/j.humpath.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Ceyhan GO, Demir IE, Altintas B, et al. Neural invasion in pancreatic cancer: a mutual tropism between neurons and cancer cells. Biochemical and Biophysical Research Communications. 2008;374:442–447. doi: 10.1016/j.bbrc.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 20.Kayahara M, Nakagawara H, Kitagawa H, Ohta T. The nature of neural invasion by pancreatic cancer. Pancreas. 2007;35:218–223. doi: 10.1097/mpa.0b013e3180619677. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Z, Kleeff J, Kayed H, et al. Nerve growth factor and enhancement of proliferation, invasion, and tumorigenicity of pancreatic cancer cells. Molecular Carcinogenesis. 2002;35:138–147. doi: 10.1002/mc.10083. [DOI] [PubMed] [Google Scholar]
- 22.Djakiew D, Pflug BR, Delsite R, et al. Chemotaxis and chemokinesis of human prostate tumor cell lines in response to human prostate stromal cell secretory proteins containing a nerve growth factor-like protein. Cancer Res. 1993;53:1416–1420. [PubMed] [Google Scholar]
- 23.Miknyoczki SJ, Lang D, Huang L, Klein-Szanto AJ, Dionne CA, Ruggeri BA. Neurotrophins and Trk receptors in human pancreatic ductal adenocarcinoma: expression patterns and effects on in vitro invasive behavior. Int J Cancer. 1999;81:417–427. doi: 10.1002/(sici)1097-0215(19990505)81:3<417::aid-ijc16>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 24.Geldof AA, De Kleijn MA, Rao BR, Newling DW. Nerve growth factor stimulates in vitro invasive capacity of DU145 human prostatic cancer cells. J Cancer Res Clin Oncol. 1997;123:107–112. doi: 10.1007/BF01269888. [DOI] [PubMed] [Google Scholar]
- 25.Adriaenssens E, Vanhecke E, Saule P, et al. Nerve growth factor is a potential therapeutic target in breast cancer. Cancer Res. 2008;68:346–351. doi: 10.1158/0008-5472.CAN-07-1183. [DOI] [PubMed] [Google Scholar]
- 26.Desmet CJ, Peeper DS. The neurotrophic receptor TrkB: a drug target in anti-cancer therapy? Cell Mol Life Sci. 2006;63:755–759. doi: 10.1007/s00018-005-5490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papatsoris AG, Liolitsa D, Deliveliotis C. Manipulation of the nerve growth factor network in prostate cancer. Expert Opin Investig Drugs. 2007;16:303–309. doi: 10.1517/13543784.16.3.303. [DOI] [PubMed] [Google Scholar]
- 28.Marchetti D, McQuillan DJ, Spohn WC, Carson DD, Nicolson GL. Neurotrophin stimulation of human melanoma cell invasion: selected enhancement of heparanase activity and heparanase degradation of specific heparan sulfate subpopulations. Cancer Res. 1996;56:2856–2863. [PubMed] [Google Scholar]
- 29.Li J, Ma Q, Liu H, et al. Relationship between neural alteration and perineural invasion in pancreatic cancer patients with hyperglycemia. PLoS One. 2011;6:e17385. doi: 10.1371/journal.pone.0017385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okada Y, Eibl G, Guha S, Duffy JP, Reber HA, Hines OJ. Nerve growth factor stimulates MMP-2 expression and activity and increases invasion by human pancreatic cancer cells. Clin Exp Metastasis. 2004;21:285–292. doi: 10.1023/b:clin.0000046131.24625.54. [DOI] [PubMed] [Google Scholar]
- 31.Ayala GE, Dai H, Tahir SA, et al. Stromal antiapoptotic paracrine loop in perineural invasion of prostatic carcinoma. Cancer Research. 2006;66:5159–5164. doi: 10.1158/0008-5472.CAN-05-1847. [DOI] [PubMed] [Google Scholar]
- 32.Okada Y, Takeyama H, Sato M, et al. Experimental implication of celiac ganglionotropic invasion of pancreatic-cancer cells bearing c-ret proto-oncogene with reference to glial-cell-line-derived neurotrophic factor (GDNF) Int J Cancer. 1999;81:67–73. doi: 10.1002/(sici)1097-0215(19990331)81:1<67::aid-ijc13>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 33.Gil Z, Cavel O, Kelly K, et al. Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves. J Natl Cancer Inst. 2010;102:107–118. doi: 10.1093/jnci/djp456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okada Y, Eibl G, Duffy JP, Reber HA, Hines OJ. Glial cell-derived neurotrophic factor upregulates the expression and activation of matrix metalloproteinase-9 in human pancreatic cancer. Surgery. 2003;134:293–299. doi: 10.1067/msy.2003.239. [DOI] [PubMed] [Google Scholar]
- 35.Ketterer K, Rao S, Friess H, Weiss J, Buchler MW, Korc M. Reverse transcription-PCR analysis of laser-captured cells points to potential paracrine and autocrine actions of neurotrophins in pancreatic cancer. Clin Cancer Res. 2003;9:5127–5136. [PubMed] [Google Scholar]
- 36.Kowalski PJ, Paulino AF. Perineural invasion in adenoid cystic carcinoma: Its causation/promotion by brain-derived neurotrophic factor. Hum Pathol. 2002;33:933–936. doi: 10.1053/hupa.2002.128249. [DOI] [PubMed] [Google Scholar]
- 37.Kameda K, Shimada H, Ishikawa T, et al. Expression of highly polysialylated neural cell adhesion molecule in pancreatic cancer neural invasive lesion. Cancer Lett. 1999;137:201–207. doi: 10.1016/s0304-3835(98)00359-0. [DOI] [PubMed] [Google Scholar]
- 38.Seki H, Tanaka J, Sato Y, Kato Y, Umezawa A, Koyama K. Neural cell adhesion molecule (NCAM) and perineural invasion in bile duct cancer. J Surg Oncol. 1993;53:78–83. doi: 10.1002/jso.2930530205. [DOI] [PubMed] [Google Scholar]
- 39.Schreiber SC, Giehl K, Kastilan C, et al. Polysialylated NCAM represses E-cadherin-mediated cell-cell adhesion in pancreatic tumor cells. Gastroenterology. 2008;134:1555–1566. doi: 10.1053/j.gastro.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 40.Cavallaro U, Niedermeyer J, Fuxa M, Christofori G. N-CAM modulates tumour-cell adhesion to matrix by inducing FGF-receptor signalling. Nat Cell Biol. 2001;3:650–657. doi: 10.1038/35083041. [DOI] [PubMed] [Google Scholar]
- 41.Swanson BJ, McDermott KM, Singh PK, Eggers JP, Crocker PR, Hollingsworth MA. MUC1 is a counter-receptor for myelin-associated glycoprotein (Siglec-4a) and their interaction contributes to adhesion in pancreatic cancer perineural invasion. Cancer Research. 2007;67:10222–10229. doi: 10.1158/0008-5472.CAN-06-2483. [DOI] [PubMed] [Google Scholar]
- 42.Anderson TD, Feldman M, Weber RS, Ziober AF, Ziober BL. Tumor deposition of laminin-5 and the relationship with perineural invasion. Laryngoscope. 2001;111:2140–2143. doi: 10.1097/00005537-200112000-00011. [DOI] [PubMed] [Google Scholar]
- 43.Marchesi F, Piemonti L, Fedele G, et al. The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Cancer Res. 2008;68:9060–9069. doi: 10.1158/0008-5472.CAN-08-1810. [DOI] [PubMed] [Google Scholar]
- 44.Cardona AE, Pioro EP, Sasse ME, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 45.Bazan JF, Bacon KB, Hardiman G, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 46.Pan Y, Lloyd C, Zhou H, et al. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387:611–617. doi: 10.1038/42491. [DOI] [PubMed] [Google Scholar]
- 47.Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci. 2004;20:1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- 48.Marchesi F, Locatelli M, Solinas G, Erreni M, Allavena P, Mantovani A. Role of CX3CR1/CX3CL1 axis in primary and secondary involvement of the nervous system by cancer. J Neuroimmunol. 2010;224:39–44. doi: 10.1016/j.jneuroim.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 49.Hibi T, Mori T, Fukuma M, et al. Synuclein-gamma Is Closely Involved in Perineural Invasion and Distant Metastasis in Mouse Models and Is a Novel Prognostic Factor in Pancreatic Cancer. Clinical Cancer Research. 2009;15:2864–2871. doi: 10.1158/1078-0432.CCR-08-2946. [DOI] [PubMed] [Google Scholar]
- 50.Li DD, Guo JF, Huang JJ, et al. Rhabdastrellic Acid-A Induced Autophagy-Associated Cell Death through Blocking Akt Pathway in Human Cancer Cells. Plos One. 2010;5 doi: 10.1371/journal.pone.0012176. -. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang F, Li SS, Segersvard R, et al. Hypoxia inducible factor-1 mediates effects of insulin on pancreatic cancer cells and disturbs host energy homeostasis. Am J Pathol. 2007;170:469–477. doi: 10.2353/ajpath.2007.060489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Couvelard A, O'Toole D, Turley H, et al. Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumours: negative correlation of microvascular density and VEGF expression with tumour progression. Br J Cancer. 2005;92:94–101. doi: 10.1038/sj.bjc.6602245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li XQ, Zhang LJ, Meshinchi S, et al. Islet microvasculature in islet hyperplasia and failure in a model of type 2 diabetes. Diabetes. 2006;55:2965–2973. doi: 10.2337/db06-0733. [DOI] [PubMed] [Google Scholar]
- 54.Konstantinova I, Lammert E. Microvascular development: learning from pancreatic islets. Bioessays. 2004;26:1069–1075. doi: 10.1002/bies.20105. [DOI] [PubMed] [Google Scholar]
- 55.Unruh A, Ressel A, Mohamed HG, et al. The hypoxia-inducible factor-1 alpha is a negative factor for tumor therapy. Oncogene. 2003;22:3213–3220. doi: 10.1038/sj.onc.1206385. [DOI] [PubMed] [Google Scholar]
- 56.Le QTX, Moon J, Redman M, et al. Phase II Study of Tirapazamine, Cisplatin, and Etoposide and Concurrent Thoracic Radiotherapy for Limited-Stage Small-Cell Lung Cancer: SWOG 0222. J Clin Oncol. 2009;27:3014–3019. doi: 10.1200/JCO.2008.21.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang J, Cao J, Weng QJ, et al. Suppression of Hypoxia-Inducible Factor 1 alpha (HIF-1 alpha) by Tirapazamine Is Dependent on eIF2 alpha Phosphorylation Rather Than the mTORC1/4E-BP1 Pathway. Plos One. 2010;5 doi: 10.1371/journal.pone.0013910. -. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koh MY, Spivak-Kroizman T, Venturini S, et al. Molecular mechanisms for the activity of PX-478, an antitumor inhibitor of the hypoxia-inducible factor-1 alpha. Mol Cancer Ther. 2008;7:90–100. doi: 10.1158/1535-7163.MCT-07-0463. [DOI] [PubMed] [Google Scholar]
- 59.Welsh S, Williams R, Kirkpatrick L, Paine-Murrieta G, Powis G. Antitumor activity and pharmacodynamic properties of PX-478, an inhibitor of hypoxia-inducible factor-1 alpha. Mol Cancer Ther. 2004;3:233–244. [PubMed] [Google Scholar]
- 60.Jacoby JJ, Erez B, Korshunova MV, et al. Treatment with HIF-1 alpha Antagonist PX-478 Inhibits Progression and Spread of Orthotopic Human Small Cell Lung Cancer and Lung Adenocarcinoma in Mice. J Thorac Oncol. 2010;5:940–949. doi: 10.1097/JTO.0b013e3181dc211f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palayoor ST, Mitchell JB, Cerna D, DeGraff W, John-Aryankalayil M, Coleman CN. PX-478, an inhibitor of hypoxia-inducible factor-1 alpha, enhances radiosensitivity of prostate carcinoma cells. Int J Cancer. 2008;123:2430–2437. doi: 10.1002/ijc.23807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwartz DL, Powis G, Thitai-Kumar A, et al. The selective hypoxia inducible factor-1 inhibitor PX-478 provides in vivo radiosensitization through tumor stromal effects. Mol Cancer Ther. 2009;8:947–958. doi: 10.1158/1535-7163.MCT-08-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kizaka-Kondoh S, Itasaka S, Zeng LH, et al. Selective Killing of Hypoxia-Inducible Factor-1-Active Cells Improves Survival in a Mouse Model of Invasive and Metastatic Pancreatic Cancer. Clin Cancer Res. 2009;15:3433–3441. doi: 10.1158/1078-0432.CCR-08-2267. [DOI] [PubMed] [Google Scholar]
- 64.Kizaka-Kondoh S, Inoue M, Harada H, Hiraoka M. Tumor hypoxia: A target for selective cancer therapy. Cancer Sci. 2003;94:1021–1028. doi: 10.1111/j.1349-7006.2003.tb01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harada H, Hiraoka M, Kizaka-Kondoh S. Antitumor effect of TAT-oxygen-dependent degradation-caspase-3 fusion protein specifically stabilized and activated in hypoxic tumor cells. Cancer Res. 2002;62:2013–2018. [PubMed] [Google Scholar]
- 66.Backer MV, Backer JM. Targeting endothelial cells overexpressing VEGFR-2: Selective toxicity of Shiga-like toxin-VEGF fusion proteins. Bioconjugate Chem. 2001;12:1066–1073. doi: 10.1021/bc015534j. [DOI] [PubMed] [Google Scholar]
- 67.Backer MV, Gaynutdinov TI, Patel V, et al. Vascular endothelial growth factor selectively targets boronated dendrimers to tumor vasculature. Mol Cancer Ther. 2005;4:1423–1429. doi: 10.1158/1535-7163.MCT-05-0161. [DOI] [PubMed] [Google Scholar]
- 68.Hotz B, Backer MV, Backer JM, Buhr HJ, Hotz HG. Specific Targeting of Tumor Endothelial Cells by a Shiga-like Toxin-Vascular Endothelial Growth Factor Fusion Protein as a Novel Treatment Strategy for Pancreatic Cancer. Neoplasia. 2010;12:U797–U148. doi: 10.1593/neo.10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Apte MV, Park S, Phillips PA, et al. Desmoplastic reaction in pancreatic cancer - Role of pancreatic stellate cells. Pancreas. 2004;29:179–187. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 70.Logsdon CD, Simeone DM, Binkley C, et al. Molecular profiling of pancreatic adenocarcinoma and chronic pancreatitis identifies multiple genes differentially regulated in pancreatic cancer. Cancer Res. 2003;63:2649–2657. [PubMed] [Google Scholar]
- 71.Schneider G, Siveke JT, Eckel F, Schmid RM. Pancreatic cancer: Basic and clinical aspects. Gastroenterology. 2005;128:1606–1625. doi: 10.1053/j.gastro.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 72.Vonlaufen A, Phillips PA, Xu ZH, et al. Pancreatic stellate cells and pancreatic cancer cells: An unholy alliance. Cancer Res. 2008;68:7707–7710. doi: 10.1158/0008-5472.CAN-08-1132. [DOI] [PubMed] [Google Scholar]
- 73.Yen TNF, Aardal NP, Bronner MP, et al. Myofibroblasts are responsible for the desmoplastic reaction surrounding human pancreatic carcinomas. Surgery. 2002;131:129–134. doi: 10.1067/msy.2002.119192. [DOI] [PubMed] [Google Scholar]
- 74.Menke A, Adler G. TGF beta-induced fibrogenesis of the pancreas. Int J Gastro Cancer. 2002;31:41–46. doi: 10.1385/IJGC:31:1-3:41. [DOI] [PubMed] [Google Scholar]
- 75.Apte MV, Wilson JS. Mechanisms of pancreatic fibrosis. Digest Dis. 2004;22:273–279. doi: 10.1159/000082799. [DOI] [PubMed] [Google Scholar]
- 76.Armstrong T, Packham G, Murphy LB, et al. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10:7427–7437. doi: 10.1158/1078-0432.CCR-03-0825. [DOI] [PubMed] [Google Scholar]
- 77.Binkley CE, Zhang LZ, Greenson JK, et al. The molecular basis of pancreatic fibrosis - Common stromal gene expression in chronic pancreatitis and pancreatic adenocarcinoma. Pancreas. 2004;29:254–263. doi: 10.1097/00006676-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 78.Koninger J, Giese T, di Mola FF, et al. Pancreatic tumor cells influence the composition of the extracellular matrix. Biochem Bioph Res Co. 2004;322:943–949. doi: 10.1016/j.bbrc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 79.Yoshida S, Yokota T, Ujiki M, et al. Pancreatic cancer stimulates pancreatic stellate cell proliferation and TIMP-1 production through the MAP kinase pathway. Biochem Bioph Res Co. 2004;323:1241–1245. doi: 10.1016/j.bbrc.2004.08.229. [DOI] [PubMed] [Google Scholar]
- 80.Bachem MG, Schunemann M, Ramadani M, et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907–921. doi: 10.1053/j.gastro.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 81.Apte MV, Wilson JS. Stellate cell activation in alcoholic pancreatitis. Pancreas. 2003;27:316–320. doi: 10.1097/00006676-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 82.Apte MV, Haber PS, Darby SJ, et al. Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut. 1999;44:534–541. doi: 10.1136/gut.44.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luttenberger T, Schmid-Kotsas A, Menke A, et al. Platelet-derived growth factors stimulate proliferation and extracellular matrix synthesis of pancreatic stellate cells: Implications in pathogenesis of pancreas fibrosis. Lab Invest. 2000;80:47–55. doi: 10.1038/labinvest.3780007. [DOI] [PubMed] [Google Scholar]
- 84.Schneider E, Schmid-Kotsas A, Zhao JS, et al. Identification of mediators stimulating proliferation and matrix synthesis of rat pancreatic stellate cells. Am J Physiol-Cell Ph. 2001;281:C532–C543. doi: 10.1152/ajpcell.2001.281.2.C532. [DOI] [PubMed] [Google Scholar]
- 85.Shek FWT, Benyon RC, Walker FM, et al. Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am J Pathol. 2002;160:1787–1798. doi: 10.1016/s0002-9440(10)61125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mews P, Phillips P, Fahmy R, et al. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut. 2002;50:535–541. doi: 10.1136/gut.50.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Phillips PA, Wu MJ, Kumar RK, et al. Cell migration: a novel aspect of pancreatic stellate cell biology. Gut. 2003;52:677–682. doi: 10.1136/gut.52.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hama K, Ohnishi H, Aoki H, et al. Angiotensin II promotes the proliferation of activated pancreatic stellate cells by Smad7 induction through a protein kinase C pathway. Biochem Bioph Res Co. 2006;340:742–750. doi: 10.1016/j.bbrc.2005.12.069. [DOI] [PubMed] [Google Scholar]
- 89.Gao RP, Brigstock DR. Connective tissue growth factor (CCN2) in rat pancreatic stellate cell function: Integrin zeta(5)beta(1) as a novel CCN2 receptor. Gastroenterology. 2005;129:1019–1030. doi: 10.1053/j.gastro.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 90.Aoki H, Ohnishi H, Hama K, et al. Cyclooxygenase-2 is required for activated pancreatic stellate cells to respond to proinflammatory cytokines. Am J Physiol-Cell Ph. 2007;292:C259–C268. doi: 10.1152/ajpcell.00030.2006. [DOI] [PubMed] [Google Scholar]
- 91.Ohnishi N, Miyata T, Ohnishi H, et al. Activin A is an autocrine activator of rat pancreatic stellate cells: potential therapeutic role of follistatin for pancreatic fibrosis. Gut. 2003;52:1487–1493. doi: 10.1136/gut.52.10.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Masamune A, Satoh M, Kikuta K, Suzuki N, Shimosegawa T. Endothelin-1 stimulates contraction and migration of rat pancreatic stellate cells. World J Gastroenterol. 2005;11:6144–6151. doi: 10.3748/wjg.v11.i39.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jaster R, Hilgendorf I, Fitzner B, et al. Regulation of pancreatic stellate cell function in vitro: biological and molecular effects of all-trans retinoic acid. Biochem Pharmacol. 2003;66:633–641. doi: 10.1016/s0006-2952(03)00390-3. [DOI] [PubMed] [Google Scholar]
- 94.Won JH, Zhang Y, Ji BA, Logsdon CD, Yule DI. Phenotypic changes in mouse pancreatic stellate cell Ca2+ signaling events following activation in culture and in a disease model of pancreatitis. Mol Biol Cell. 2011;22:421–436. doi: 10.1091/mbc.e10-10-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Apte MV, Pirola RC, Wilson JS. Battle-scarred pancreas: Role of alcohol and pancreatic stellate cells in pancreatic fibrosis. J Gastroen Hepatol. 2006;21:S97–S101. doi: 10.1111/j.1440-1746.2006.04587.x. [DOI] [PubMed] [Google Scholar]
- 96.Masamune A, Satoh A, Watanabe T, et al. Effects of Ethanol and Its Metabolites on Human Pancreatic Stellate Cells. Digest Dis Sci. 2010;55:204–211. doi: 10.1007/s10620-008-0695-y. [DOI] [PubMed] [Google Scholar]
- 97.Schwer CI, Mutschler M, Stoll P, et al. Carbon monoxide releasing molecule-2 inhibits pancreatic stellate cell proliferation by activating p38 mitogen-activated protein kinase/heme oxygenase-1 signaling. Mol Pharmacol. 2010;77:660–669. doi: 10.1124/mol.109.059519. [DOI] [PubMed] [Google Scholar]
- 98.Masamune A, Kikuta K, Watanabe T, Satoh K, Hirota M, Shimosegawa T. Hypoxia stimulates pancreatic stellate cells to induce fibrosis and angiogenesis in pancreatic cancer. Am J Physiol Gastrointest Liver Physiol. 2008;295:G709–G717. doi: 10.1152/ajpgi.90356.2008. [DOI] [PubMed] [Google Scholar]
- 99.Masamune A, Kikuta K, Watanabe T, et al. Fibrinogen induces cytokine and collagen production in pancreatic stellate cells. Gut. 2009;58:550–559. doi: 10.1136/gut.2008.154401. [DOI] [PubMed] [Google Scholar]
- 100.Haber PS, Keogh GW, Apte MV, et al. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am J Pathol. 1999;155:1087–1095. doi: 10.1016/S0002-9440(10)65211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kuno A, Yamada T, Masuda K, et al. Angiotensin-converting enzyme inhibitor attenuates pancreatic inflammation and fibrosis in male Wistar Bonn/Kobori rats. Gastroenterology. 2003;124:1010–1019. doi: 10.1053/gast.2003.50147. [DOI] [PubMed] [Google Scholar]
- 102.Ohnishi H, Miyata T, Yasuda H, et al. Distinct roles of Smad2-, Smad3-, and ERK-dependent pathways in transforming growth factor-beta1 regulation of pancreatic stellate cellular functions. J Biol Chem. 2004;279:8873–8878. doi: 10.1074/jbc.M309698200. [DOI] [PubMed] [Google Scholar]
- 103.Kikuta K, Masamune A, Satoh M, Suzuki N, Satoh K, Shimosegawa T. Hydrogen peroxide activates activator protein-1 and mitogen-activated protein kinases in pancreatic stellate cells. Mol Cell Biochem. 2006;291:11–20. doi: 10.1007/s11010-006-9189-4. [DOI] [PubMed] [Google Scholar]
- 104.Masamune A, Kikuta K, Suzuki N, Satoh M, Satoh K, Shimosegawa T. A c-Jun NH2-terminal kinase inhibitor SP600125 (anthra[1,9-cd]pyrazole-6 (2H)-one) blocks activation of pancreatic stellate cells. J Pharmacol Exp Ther. 2004;310:520–527. doi: 10.1124/jpet.104.067280. [DOI] [PubMed] [Google Scholar]
- 105.Masamune A, Kikuta K, Satoh M, Suzuki N, Shimosegawa T. Protease-activated receptor-2-mediated proliferation and collagen production of rat pancreatic stellate cells. J Pharmacol Exp Ther. 2005;312:651–658. doi: 10.1124/jpet.104.076232. [DOI] [PubMed] [Google Scholar]
- 106.Yamada T, Kuno A, Ogawa K, et al. Combination therapy with an angiotensin-converting enzyme inhibitor and an angiotensin II receptor blocker synergistically suppresses chronic pancreatitis in rats. J Pharmacol Exp Ther. 2005;313:36–45. doi: 10.1124/jpet.104.077883. [DOI] [PubMed] [Google Scholar]
- 107.Masamune A, Kikuta K, Satoh M, Satoh A, Shimosegawa T. Alcohol activates activator protein-1 and mitogen-activated protein kinases in rat pancreatic stellate cells. J Pharmacol Exp Ther. 2002;302:36–42. doi: 10.1124/jpet.302.1.36. [DOI] [PubMed] [Google Scholar]
- 108.Jaster R, Sparmann G, Emmrich J, Liebe S. Extracellular signal regulated kinases are key mediators of mitogenic signals in rat pancreatic stellate cells. Gut. 2002;51:579–584. doi: 10.1136/gut.51.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McCarroll JA, Phillips PA, Kumar RK, et al. Pancreatic stellate cell migration: role of the phosphatidylinositol 3-kinase(PI3-kinase) pathway. Biochem Pharmacol. 2004;67:1215–1225. doi: 10.1016/j.bcp.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 110.Masamune A, Kikuta K, Satoh M, Sakai Y, Satoh A, Shimosegawa T. Ligands of peroxisome proliferator-activated receptor-gamma block activation of pancreatic stellate cells. J Biol Chem. 2002;277:141–147. doi: 10.1074/jbc.M107582200. [DOI] [PubMed] [Google Scholar]
- 111.Yoshida S, Ujiki M, Ding XZ, et al. Pancreatic stellate cells (PSCs) express cyclooxygenase-2 (COX-2) and pancreatic cancer stimulates COX-2 in PSCs. Mol Cancer. 2005;4:27. doi: 10.1186/1476-4598-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McCarroll JA, Phillips PA, Santucci N, Pirola RC, Wilson JS, Apte MV. Vitamin A inhibits pancreatic stellate cell activation: implications for treatment of pancreatic fibrosis. Gut. 2006;55:79–89. doi: 10.1136/gut.2005.064543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dornhofer N, Spong S, Bennewith K, et al. Connective tissue growth factor-specific monoclonal antibody therapy inhibits pancreatic tumor growth and metastasis. Cancer Res. 2006;66:5816–5827. doi: 10.1158/0008-5472.CAN-06-0081. [DOI] [PubMed] [Google Scholar]
- 114.Aikawa T, Gunn J, Spong SM, Klaus SJ, Korc M. Connective tissue growth factor-specific antibody attenuates tumor growth, metastasis, and angiogenesis in an orthotopic mouse model of pancreatic cancer. Mol Cancer Ther. 2006;5:1108–1116. doi: 10.1158/1535-7163.MCT-05-0516. [DOI] [PubMed] [Google Scholar]
- 115.Yoo BM, Yeo M, Oh TY, et al. Amelioration of pancreatic fibrosis in mice with defective TGF-beta signaling. Pancreas. 2005;30:e71–e79. doi: 10.1097/01.mpa.0000157388.54016.0a. [DOI] [PubMed] [Google Scholar]
- 116.Bachem MG, Zhou S, Buck K, Schneiderhan W, Siech M. Pancreatic stellate cells--role in pancreas cancer. Langenbecks Arch Surg. 2008;393:891–900. doi: 10.1007/s00423-008-0279-5. [DOI] [PubMed] [Google Scholar]
- 117.Zhang W, Erkan M, Abiatari I, et al. Expression of extracellular matrix metalloproteinase inducer (EMMPRIN/CD147) in pancreatic neoplasm and pancreatic stellate cells. Cancer Biol Ther. 2007;6:218–227. doi: 10.4161/cbt.6.2.3623. [DOI] [PubMed] [Google Scholar]