Abstract
Recent studies have demonstrated that the interaction between the cancer and the stroma, play a key role in the development of pancreatic cancer. The desmoplasia, which consists of fibroblasts, pancreatic stellate cells, lymphatic and vascular endothelial cells, immune cells, pathologic increased nerves, and the extracellular matrix (ECM), creates a complex tumor microenvironment that promotes pancreatic cancer development, invasion, metastasis, and resistance to chemotherapy. Thus, the potential approach for targeting the components of this desmoplastic reaction or the pancreatic tumor microenvironment might represent a novel therapeutic approach to advanced pancreatic carcinoma. Novel therapies that target on the pancreatic tumor microenvironment should become one of the more effective treatments for pancreatic cancer.
Keywords: Pancreas carcinoma, stroma, tumor desmoplasia, mechanism, anticancer treatment, therapeutic targets
INTRODUCTION
Pancreatic cancer is the most lethal human malignancy and has a ratio of death to incidence up to 96% [1]. Pancreatic adenocarcinoma is locally invasive and is surrounded by a dense desmoplastic reaction, which can involve adjacent vital structures. The desmoplasia, which consists of fibroblasts, pancreatic stellate cells, lymphatic and vascular endothelial cells, immune cells, pathologic increased nerves and the ECM, creates a complex tumor microenvironment that promotes pancreatic cancer development, invasion, metastasis and resistance to chemotherapy (Fig. (1)). The molecular mechanisms of the tumor-stroma interaction are very complex. This review focuses on the mechanisms by which the interaction between the cancer cell and the stroma influence pancreatic cancer progression. Studies on the pancreatic tumor microenvironment will bring new concepts that will ultimately contribute to new diagnoses and treatments for this disease.
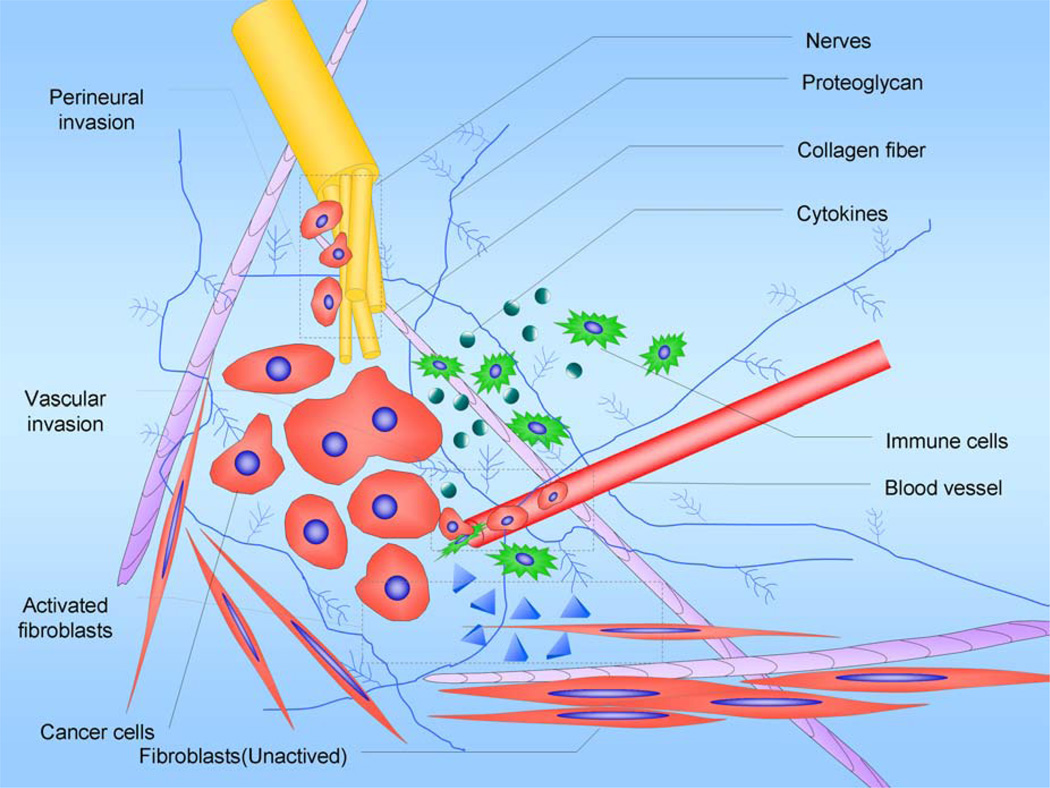
Fig. (1). Desmoplasia.
The desmoplasia consisting of fibroblasts, pancreatic stellate cells, lymphatic and vascular endothelial cells, immune cells, pathologic increased nerves, and ECM creates a complex tumor microenvironment that promotes pancreatic cancer development, invasion, metastasis and resistance to chemotherapy. Targeting the interaction between cancer and stroma in the pancreatic tumor microenvironment may contribute to the design of new diagnosis techniques and treatments for this disease.
PANCREATIC STELLATE CELLS
From a histological point of view, pancreatic cancers may have the most prominent stromal reaction of all of the epithelial tumors; there is a remarkable increase in the connective tissue that infiltrates and envelopes the neoplasm [2]. Studies of human pancreatic cancer have shown that the mesenchymal cells secrete many of cytokines such as insulin-like growth factor-I (IGF-I) and fibroblast growth factor (FGF), which have an impact on disease prognosis (Fig. (2)). Because it is possible to isolate and culture pancreatic stellate cells (PSC) in vitro [3, 4] and these cells are responsible for producing the stromal reaction in pancreatic cancer [5], determining which mechanisms mediate the epithelial-stromal interactions in pancreatic cancer is important.
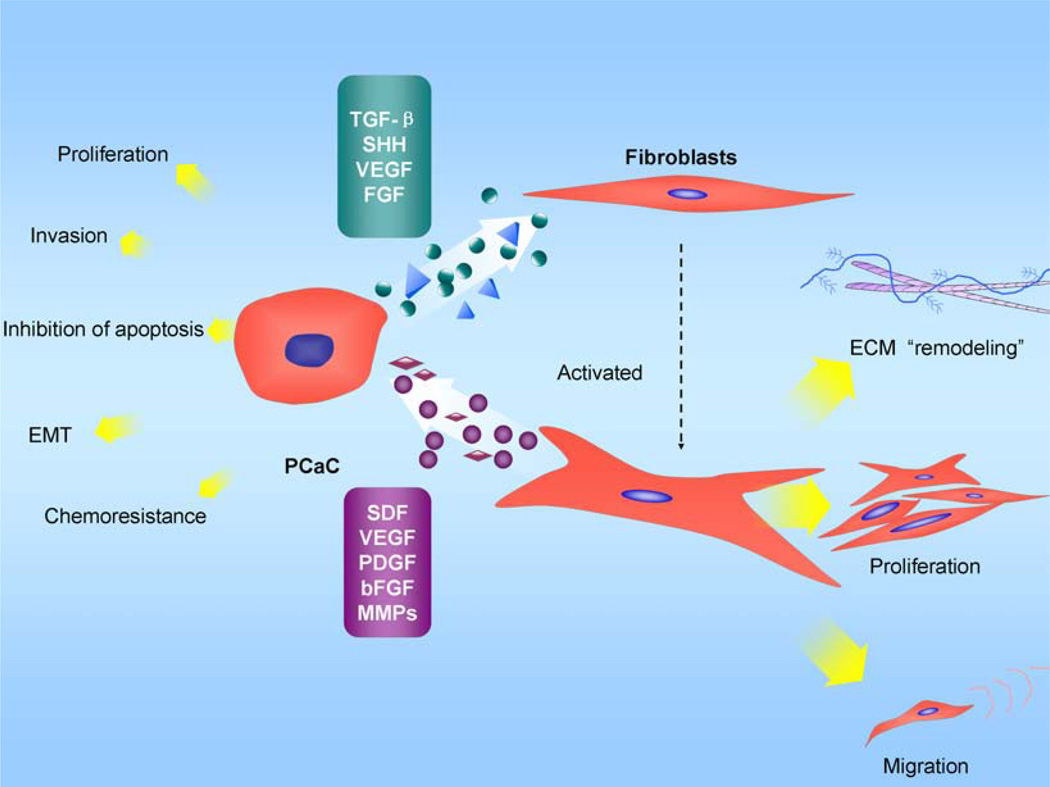
Fig. (2). The interaction between pancreatic cancer cells and PSC.
Selected mediators and their effects on biological behavior changes are depicted. Transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), and VEGF, which are secreted by pancreatic cancer cells, are known to induce PSC activation. Under the influence of these growth factors, PSCs are activated and transformed into a myofibroblast-like phenotype; the PSCs then secret excess amounts of extracellular matrix (ECM) and matrix degrading enzymes to remodel the cancer microenvironment. Simultaneously, the transformation promotes their proliferation and migration capacity. PSCs can also act on pancreatic cancer cells, which can affect their biological behavior. PSCs have a marked influence on promoting cancer cell proliferation through PDGF. Stromal-derived factor-1(SDF-1), EGF, IGF-1, or FGF, when secreted by PSCs, are also likely to display mitogenic effects on cancer cells. EMMPRIN, which is secreted by cancer cells, can increase the MMP-2 secretion by PSCs; MMP-2 has been associated with the invasive phenotype of pancreatic cancer cell lines. Resistance to apoptosis might be related to the increased expression of the anti-apoptotic proteins Bcl-2 and Bcl-xL and of the growth factors IGF-1 and FGF. Tumor-derived pancreatic stellate cells stimulate pancreatic cancer cell invasion likely through the release of thrombospondin-2 and β1 integrins. PDGF receptors of PSCs increase interstitial hypertension and reduce transcapillary transport in tumors and thus influence the transcapillary transport of drugs. SDF-1 can also influence the therapeutic outcome.
In healthy tissue, PSCs are quiescent; in diseased states, under the influence of growth factors, cytokines, and oxidative stress, PSCs are activated and adopt a myofibroblast-like phenotype and then secret excess amounts of extracellular matrix (ECM) and matrix degrading enzymes [3]. Growth factors that are known to induce PSC activation such as transforming growth factor-h1 (TGF-h1), platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) are secreted by pancreatic cancer cells [5, 6]. Additionally, it has been shown that cancer cells can also secrete the ECM metalloproteinase inducer (EMMPRIN) [4]. This secretion leads to increased matrix metalloproteinase 2 secretions by PSCs; matrix metalloproteinase 2 has been associated with the invasive phenotype of pancreatic cancer cell lines [7].
PSCs can also act on pancreatic cancer cells, which affect their biological behavior. How PSCs and the desmoplasia promote the growth of tumor cells in adenocarcinomas is only partially understood [8].
Proliferation and Apoptosis
The growth rate of a tumor that forms when both cancer cells and PSCs are injected subcutaneously into the flanks of nude mice is significantly increased compared to the tumors that form when only the cancer cells are injected [6]. As opposed to the tumors that are initiated by injection of only the cancer cells, the tumors initiated with the co-injection of cancer cells and PSCs have a desmoplasia similar to that observed in human pancreatic adenocarcinoma [6]. The growth advantage of pancreatic cancer cells in the presence of PSCs may be mediated by two mechanisms: increased mitosis and decreased programmed cell death (apoptosis). As observed in the co-injection model [9], PSC secretions displayed a marked influence on the promotion of cancer cell proliferation, and this effect was partially abolished by neutralizing antibodies against the mitogenic factor PDGF. Other factors secreted by PSCs such as stromal-derived factor-1, EGF, IGF-1, or FGF are also likely to exert mitogenic effects on cancer cells, and studies examining the role of these and other factors are currently underway [2].
Resistance to apoptosis is a common trait of many tumors. It has been shown that PSCs reduce basal level of apoptosis in various pancreatic cancer cell lines in vitro [10, 11]. Importantly, when mice were injected with PSCs and cancer cells, this led to reduced apoptosis in vivo; this result is in contrast to animals that were only injected with cancer cells. The resistance to apoptosis could be related to an increased expression of anti-apoptotic proteins such as Bcl-2 and Bcl-xL. Additionally, growth factors such as IGF-1 and basic fibroblast growth factor (bFGF) may mediate the anti-apoptotic effects of pancreatic cancer cells. PSCs are myofibroblast-like cells that are found in the areas of the pancreas with an exocrine function [8], and abundant producers of ECM components, such as various types of collagen. A conceptually novel study proved the importance of ECM stiffening, which is an abundance of collagen cross-linking, in the process of tumor growth and invasion [12]. During the malignant progression of the oncogene-expressing breast epithelium in transgenic mice, an increase in ECM stiffening and activation of the prototypical PI3 kinase and Akt survival signaling pathway synergestically caused the downstream ligation of integrin by collagen fibers [13].
Invasion and Metastasis
As cancers progress into the malignant state, they acquire the characteristic of invading the surrounding tissue and seeding metastases in the lymphatic system or blood vessels. For invasion to occur, tumor cells need to attain a migratory phenotype, which leads to extensive remodeling of the surrounding ECM. Compared with mice injected with pancreatic cancer cells alone, mice co-injected with human PSCs and cancer cells showed regional and distant metastasis and fibrotic bands (desmoplasia) containing activated PSCs within the tumors [9, 14]. PSCs contribute to the invasive and metastatic process by inducing the epithelial to mesenchymal transition of pancreatic cancer cells [15]. Pancreatic cancer cells co-cultured with PSCs exhibited loose cell contacts and a scattered, fibroblast-like appearance. The expression of E-cadherin, cytokeratin 19, and membrane-associated β-catenin decreased in cancer cells co-cultured with PSCs relative to cells cultured without PSCs whereas vimentin and Snail (Snail-1) expression increased. The migration of pancreatic cancer cells was increased when they were co-cultured with PSCs. The decrease of E-cadherin expression induced by PSCs was not altered by treatment with an anti-TGF-β-neutralizing antibody, which excludes a central role for TGF-β in this process [15]. Moreover, tumor-derived pancreatic stellate cells stimulate pancreatic cancer cell invasion possibly through release of thrombospondin-2 [16]. Additionally, β1 integrins play an essential role in the adhesion and invasion of pancreatic carcinoma cells [11].
Chemotherapy Resistance
The stromal compartment not only provides an abundance of stroma-derived factors that facilitate cancer initiation, growth and progression but also has an effect on the therapeutic outcome and provides ample opportunities for drug targeting. Pancreatic stellate cells could inhibit the effects of chemotherapy and radiation on tumor cells [14]. Indeed, a stroma-derived gene expression pattern can predict the clinical outcome in breast cancer [17]. Further evidence supporting the theory that cancer associated fibroblasts (CAFs) can determine therapeutic outcome in breast cancer patients came from the demonstration that a CAF gene signature is predictive of the response to neoadjuvant chemotherapy [18]. Moreover, preclinical studies suggest that CAFs may also regulate the resistance to antiangiogenic therapy and targeted therapy that uses epidermal growth factor receptor tyrosine kinase inhibitors [19, 20]. In addition to directly modulating the sensitivity of tumor cells to anticancer agents, PDGF receptors of CAFs increase interstitial hypertension and reduce transcapillary transport in tumors to influence the transcapillary transport of drugs [21, 22].
Targeting Therapy
The cell surface serine protease fibroblast activation protein (FAP) is selectively expressed on tumor-associated fibroblasts and pericytes in epithelial tumors; a study showed that genetic deletion and pharmacologic inhibition of FAP inhibited tumor growth in mouse models of epithelial-derived solid tumors. The results indicated that FAP depletion inhibits the tumor cell proliferation indirectly, increases the accumulation of collagen, decreases the myofibroblast content, and decreases the blood vessel density in tumors [23]. The inhibition of the stromal PDGF receptors reduced the proliferation and angiogenesis in cervical lesions by suppressing the expression of the angiogenic factor FGF-2 and the epithelial cell growth factor FGF-7, which are secreted by CAFs. These effects were recapitulated using neutralizing antibodies against the PDGF receptors. When treated with a ligand trap for the FGFs, the angiogenic phenotype was impaired, and this effect was similar to treatment with imatinib [24]. A study showed that the ligand-dependent activation of the hedgehog (Hh) pathway in the stromal microenvironment is a paracrine requirement for Hh signaling in cancer. Specific inhibition of Hh signaling using small molecule inhibitors, a neutralizing anti-Hh antibody or genetic deletion of smoothened (Smo) in the mouse stroma leads to growth inhibition in xenograft tumor models [25]. Moreover, in a mouse model of pancreatic cancer, a co-administration of gemcitabine and IPI-926, a drug that depletes tumor-associated stromal tissue via inhibition of Hedgehog signaling pathway, transiently increase the intratumoral vascular density and intratumoral concentration of gemcitabine and thus lead to a transient stabilization of the disease [26].
VASCULAR MICROENVIRONMENT
The tumor microenvironment is extremely complex and depends on the interaction between the tumor cells and responding host cells. Angiogenesis, new blood vessel growth from the preexisting vasculature, is a preeminent feature of the successful growth of all solid tumors. As one of the hallmarks of cancer of the exocrine pancreas, angiogenesis is an essential event that is involved in pancreatic cancer progression and metastasis.
The proliferative index of tumors decreases as the distance from the nearest capillary blood vessel increases, and the rapid exponential growth of tumors is dependent on the vascularization of the tumor mass. Without angiogenesis, pancreatic tumors are limited in size by the distance that oxygen can diffuse, namely, 1–2 mm. Hypoxia results when the rate of new blood vessel growth is exceeded by the growth of the tumor. Hypoxia in pancreatic cancer results in changes at a transcriptional level, which alter cellular metabolism and stimulates angiogenesis [27].
Angiogenesis is not necessarily linked to invasive pancreatic cancer, but it is an early event in pancreatic cancer genesis; the process of angiogenesis consists of multiple, sequential, and interdependent steps with the involvement of myriad positive and negative regulators of angiogenesis. The survival of pancreatic cancers and their metastases are dependent on the balance of endogenous angiogenic and anti-angiogenic factors such that the outcome favors increased angiogenesis.
Multiple molecules such as VEGF, angiopoietins, FGF, PDGF, and TGF-β regulate angiogenesis. Angiogenesis plays an important role in the growth, progression and metastasis of a tumor. Inhibiting the angiogenic process or targeting existing tumor vessels can be used to treat tumors either as an alternative to or in parallel with conventional chemotherapy. Many anti-angiogenic factors are under investigation, and some are already being used in clinical practice with varying results.
Vascular Endothelial Growth Factor (VEGF)
The VEGF family of growth factors, including the VEGF-A, VEGF-B, VEGF-C, VEGF-D, and VEGF-E factors as well as the placenta growth factor (PIGF), play a critical role in the process of tumor angiogenesis.
VEGF factors regulate endothelial cell proliferation, migration, and vascular permeability by binding to their receptor tyrosine kinases such as VEGFR1, R2, and R3 [28]. However, accumulating evidence shows that VEGFR2 is the crucial and main receptor mediating the angiogenic and vascular permeability activity whereas VEGFR3 is mainly involved in the lymph angiogenic activity [29]. In angiogenesis during pancreatic cancer, VEGFR2 activation leads to the activation of diverse intracellular signaling in endothelial cells and regulates multiple critical steps by phosphorylating different downstream substrates, which results in pancreatic cancer metabolism, growth, proliferation, and survival [30].
The principle form of VEGF is the homodimeric glycoprotein VEGF-A. VEGF-A consists of five major isoforms, all of which act as anti-apoptotic agents, possess vasodilatory abilities, and promote endothelial cell migration and proliferation via binding with to their tyrosine kinase receptors, VEGFR-1 (flt-1) and VEGFR-2. The biological effect of VEGF-A is exerted through its interaction with the cell surface receptors that include VEGFR-1 and VEGFR-2, which are selectively located on vascular endothelium and are upregulated during angiogenesis. The VEGF-A–VEGFR-2 interaction also plays a critical role in pancreatic cancer angiogenesis through the coordinate signaling of endothelial cell proliferation, migration and the recruitment of endothelial cell progenitor cells.
A number of studies [31] have shown that the increased expression of VEGF, a potent mitogen for endothelial cells at the primary site, is correlated with a poor prognosis for pancreatic cancer. Conversely, VEGFR1 contains a classical tyrosine kinase domain; however, the primary function of VEGFR1 may be as a negative regulator in vascular development [32].
Recently, an anti-VEGF antibody (bevacizumab), when used in combination with chemotherapy, was shown to significantly improve the survival and response rates in patients with metastatic colorectal cancer; this finding validates the importance of VEGF pathway inhibitors as a new treatment in cancer therapy [33].
Fibroblast Growth Factor (FGFs)
Fibroblast growth factors (FGFs) comprise a family of 22 members that play important roles during embryogenesis and adulthood, and FGFs regulate many cellular behaviors including proliferation, migration, survival, and differentiation.
The FGF family includes factors such as FGF-1, FGF-2, FGF-5, FGF-7, and FGF receptors are regulated in pancreatic cancer tissue samples and cell lines [34]. These findings suggest that FGF-dependent downstream biologic events are likely to play an important role in the pathobiology of pancreatic cancer.
FGFs stimulate endothelial cell proliferation and migration and the production of collagenase and plasminogen activator. FGFs induce development of blood vessels in vivo in the chick chorioallantoic membrane and cornea, thus supporting their role in angiogenesis.
FGFs are mitogenic, promote angiogenesis and chemotaxis, and participate in the regulation of cellular differentiation and tissue repair. Acidic and basic fibroblast growth factors (aFGF or FGF1 and bFGF or FGF2, respectively) are described as inducers of angiogenesis [35].
PATHOLOGICAL ALTERATIVE NERVES
Perineural invasion (PNI) is the process of the cancer cell invasion of nerves and is a special metastatic route in pancreatic cancer. Pancreatic cancer is characterized by a high frequency of PNI. It is estimated that more than 90% of patients have intra-pancreatic nerves that have been infiltrated by tumor cells, and 69% of these infiltrations involve the extra-pancreatic nerve terminations. Previous articles have reported that 100% of pancreatic tumors would reveal PNI if enough sections were evaluated [36]. PNI is a common but not specific feature of pancreatic carcinoma. Tumor cells in the peripheral space grow in a continuous fashion and may be responsible for some cases of lymphatic spread [37,38].
Kayahara et al. used histopathology to investigate many consecutive sections (almost 5000) of tumor specimens; the study revealed that tumor cells grew mainly in a continuous fashion along the branches of the nerves [38]. Continuity was found between cancer cells within the perineural space and the cancer cells inside some lymph nodes. This finding suggests that neural invasion might also be a pathway that could eventually lead to lymphatic spread. Thus, PNI can be viewed as an important pathway to metastasis. In pancreatic tumors, nerve plexus invasion is regarded as one of the most important prognostic factors for pancreatic cancer [39].
The mechanism of PNI in pancreatic cancer is not clear. It can be partly explained by the anatomical proximity of the pancreatic and celiac artery neural plexus. The human pancreas has plenty of neural tissue including ganglia; the pancreas is innervated by the autonomic nervous system through the plexi from the celiac and superior mesenteric artery ganglia [40]. The perineurium is considered to be deficient at three sites: near the nerve ending, at the site invaded by the blood vessels present in nerves, and at the site invaded by reticular fibers [39, 41]. PNI has long been presumed to simply be the growth of the tumor cells along a “path of low resistance”. The predominant theory behind the pathogenesis of PNI has been that the tumor cells spreading along neural sheaths are privileged to a low-resistance plane, which serves as a route for their migration. Once the tumor cells get into the nerve sheath, they may be in a privileged growth environment that facilitates metastasis, but the multiple layers of collagen and basement membrane that compose the nerve sheath make access to this path anything but low-resistance. The reason that some carcinomas exhibit a predilection for PNI and that others do not remains unknown [42].
Another possible explanation of PNI in pancreatic cancer is neurotropism. Advanced pancreatic cancer with PNI expresses many types of neuroendocrine markers including S-100, synaptophysin, substance-P, enkephalin, and neural cell adhesion molecules (NCAM) [43]. Recently, studies have demonstrated that PNI may involve reciprocal signaling interactions between the tumor cells and the nerves, and these invading tumor cells may have acquired the ability to respond to proinvasive signals within the peripheral nerve milieu [42]. In an in vitro model of PNI, directional outgrowth of mouse dorsal root ganglia (DRG) was seen growing toward prostate tumor cells and migrated along the neurites thus establishing PNI [44]. Similarly, in pancreatic cancer, tumor cells experienced early morphologic changes at the migration front, and neural cells that elongated neurites targeting tumor cells eventually leading to malignant cells around the neuritis [45, 46]. These findings reveal a mutual tropism and paracrine interaction between neurons and cancer cells. Nerves provide a prosperous environment for tumor growth, and the interaction provides a positive influence on the growth of both the nerves and the tumor.
Some neurotrophic factors secreted by the nerves enhanced the cancer–nerve interaction, which provides the biological and physical parameters that would explain their frequent and intimate relationship [47, 48]. Several neurotrophins including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) have been implicated in promoting tumor cell invasion and may be key mediators in the pathogenesis of PNI [49–51] (Fig. (3)).
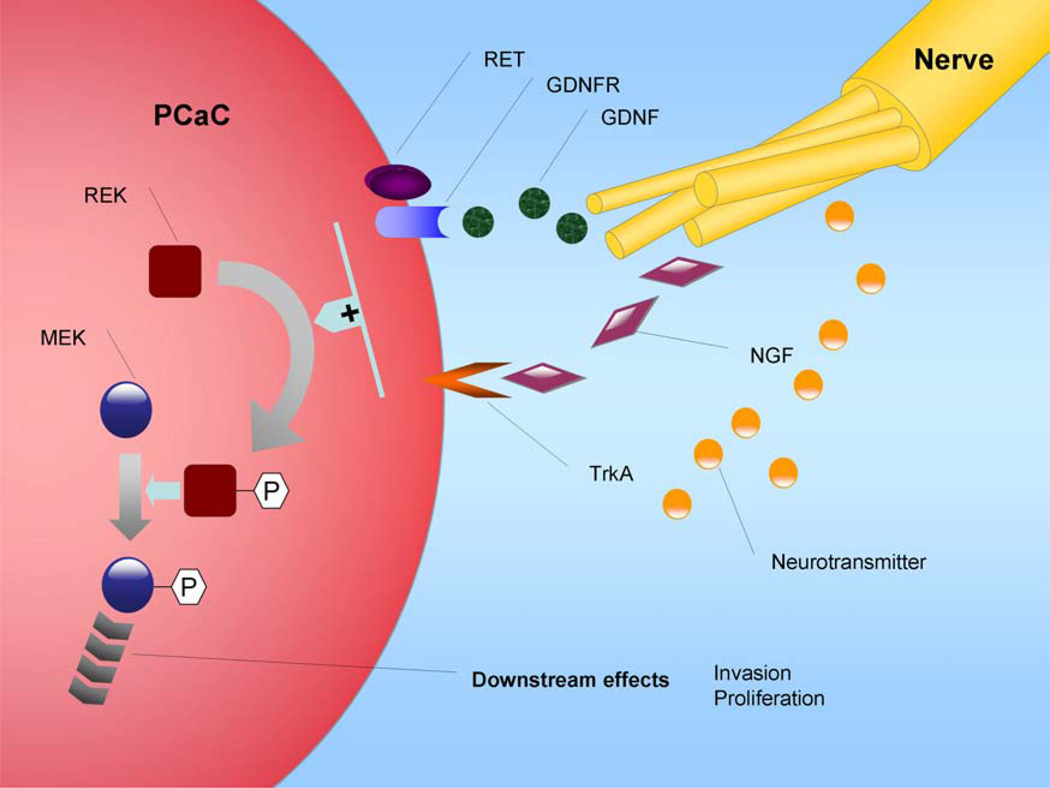
Fig. (3). Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves via NGF, GDNF, and neurotransmitter.
Peripheral nerves in a tumor’s microenvironment may influence the biological behavior of cancer cells, which can contribute to tumor progression. Paracrine regulation of the pancreatic cancer invasion by nerves may have important implications for potential therapy directed against nerve invasion by cancer.
Neurotrophin
The neurotrophin (NT) growth factor family, which includes NGF, BDNF, NT-3, NT-4/5, and their cognate receptors (Trks A, Trks B, Trks C, and the low-affinity NGF receptor, p75NGFR), has been implicated in the paracrine growth regulation of a number of neuronal and non-neuronal tumor types. NTs have also been shown to increase tumor invasiveness, enhance clonal growth, and cause changes in the cell morphology in some kinds of non-neuronal cancer types including melanoma, prostatic, and pancreatic carcinoma [50–52]. NGF that is released from the neural tissue may stimulate epithelial cancer cell growth and may mediate nerve invasion through its interaction with TrkA, an NGF-specific receptor; binding of NGF to TrkA leads to the activation of the p44/42 MAPK signaling pathway and the upregulation of MMP-2, a proinvasive mediator. NGF and its receptor TrkA are overexpressed in pancreas cancer cell lines and the perineurium of peripheral nerves [53].
Glial Cell-Derived Neurotrophic Factor
In pancreatic cancer, members of the glial cell-derived neurotrophic factor (GDNF) family promote both pancreatic cancer invasion of peripheral nerves and the growth and survival of cancer cells [54–56]. In a recent study, it was reported that GDNF is over-expressed in specimens of human neural plexi, and multiple pancreatic cancer cell lines express the RET protein tyrosine kinase receptor for GDNF [56]. GDNF-secreting glioma cells have been reported to increase the migration of pancreatic cancer cells in a dose-dependent fashion, suggesting both a chemotactic effect and a chemokinetic effect of GDNF on tumor cells [56]. Furthermore, GDNF can increase the expression and activity of MMP-9 [57].
Neural Cell Adhesion Molecules
Neural cell adhesion molecules (NCAMs), homophilic adhesion molecules expressed on the nerve cells, have been examined in 15 pancreatic cancer resection specimens, including in neural invasive lesions [58]. NCAMs play critical navigation and docking roles by binding to the target cells during the growth and development of the nervous system. NCAM is highly expressed in the peripheral nerve tissue. Evidence suggests that PNI is correlated with NCAM expression, indicating that NCAM molecules on the surface of cancer cells might induce the cancer cells to migrate and adhere to nerve cells after the tumors breach their capsules [59]. Recent evidence demonstrates that the activation of the proto-oncogene K-Ras in pancreatic cancer cells could induce the upregulation of polysialic acid neural cell adhesion molecule (PSA-NCAM) on tumor cell surfaces. PSA-NCAM could bind to N-cadherin and block N-cadherin mediated cell adhesion, which could increase the ability of the pancreatic cancer cell to migrate and could facilitate tumor cell metastasis to nerve tissue [60].
G-CSF and GM-CSF
Recently, high levels of the hematopoietic colony stimulating factors G-CSF and GM-CSF have been found in pancreatic biopsy specimens, and the expression of their receptors (G-CSFR and GM-CSFRa) on pancreatic nerve cells indicates that these cytokines may play a role in tumor–nerve interactions and, importantly, in tumor-induced pain [61]. The presence of hematopoietic factors in the tumor microenvironment is a very common finding, and hematopoietic factors are known to act on both myeloid and tumor cells, which stimulates the proliferation of the cells [62].
Myelin-Associated Glycoprotein
Myelin-associated glycoprotein (MAG) is a membrane-bound protein expressed by myelinating Schwann cells in the periaxonal membrane. On Schwann cells, MAG binds both to gangliosides on the axons and to the mucin, MUC1, expressed by pancreatic tumor cells [63]. Laminin-5 released from the cancer cells has been reported to correlate with PNI in head and neck squamous carcinomas, which supports the hypothesis that the deposition of basement membrane components may be required in the process of nerve invasion [64].
Chemokines
A recent study indicated that tumor cells from human pancreatic cancers strongly upregulate the chemokine receptor CX3CR1, which is not expressed in the normal pancreatic epithelium, and that the CX3CL1/CX3CR1 axis mediates PNI in pancreatic cancer [65]. CX3CR1 exclusively binds the transmembrane chemokine CX3CL1 (also known as fractalkine or neurotactin) expressed by neurons, nerve fibers and activated endothelial cells [66–69]. CX3CR1+ pancreatic ductal adenocarcinoma (PDAC) tumor cells migrate in response to the ligand CX3CL1 and specifically adhere to neural cells. To determine the relationship between the high CX3CR1 expression by tumor cells and neural tropism, a systematic evaluation of perineural invasion was performed. High expression of the receptor was significantly associated with pathologically detected prominent PNI. The biological significance of PNI and the expression of CX3CR1 in pancreatic cancer were investigated in the clinical outcome of the patients. Higher CX3CR1 expression and perineural invasion were strongly associated with local and earlier tumor recurrence. Thus, CX3CR1 expression by cancer cells is an independent factor predicting local tumor recurrence in resected pancreatic carcinoma patients [65].
ECM
One of the most important features of PDAC is the development of the desmoplastic reaction around tumor cells; this phenomenon is mainly due to excess ECM production, which is mainly produced by the activated pancreatic stellate cells [70–72]. The ECM, which is composed of collagens, noncollagenous glycoproteins, glycosaminoglycans, proteoglycans, proteinases, growth factors, and matricellular proteins, is an important component of the tumor microenvironment. Koninger et al. has described that malignant cells can alter the composition of the ECM and transform their microenvironment in a tumor-favorable way. Although some studies focus on the tumor's impact on its microenvironment [73], much research focuses on the reverse effect: the ability of the surrounding microenvironment to influence the tumor. The ECM is one of the main constituents of the tumor microenvironment; however, it has been thought that the dense ECM around pancreatic cancer cells may represent a host barrier against malignant invasion [74]. Accumulating evidence shows that the ECM is an important component that regulates the biological behaviors of pancreatic cancer such as tumor growth, differentiation, survival and motility [75, 76] (Fig. (4)).
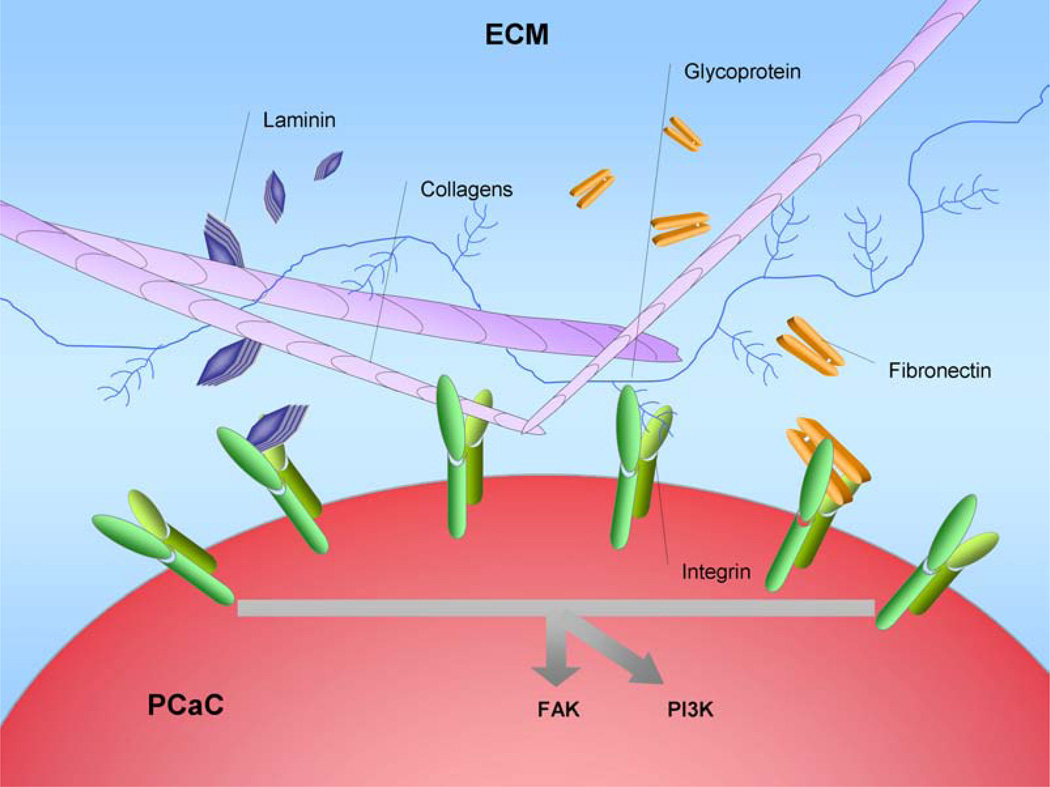
Fig. (4). Excess extracellular matrix (ECM) production in pancreatic tumor microenvironment.
From a histologic standpoint, the prominent stromal reaction in the pancreatic tumor local microenvironment is one of the most remarkable characteristics of pancreatic cancers, and the stroma is thought to be an active participant in the process of pancreatic cancer initiation, progression, metastasis and chemoresistance. Increased deposition of the ECM, which is mainly composed of collagens, glycoproteins, proteoglycans, proteinases, and growth factors, is the culprit of this desmoplastic reaction. Most members of the ECM, including collagens, fibronectins, and laminins, can bind to integrins, a large family of type I transmembrane heterodimeric receptors that play key roles in downstream cell processes. When several members of the ECM bind to integrins, they then cause the activation of multiple downstream signaling cascades, which include the Src–FAK, Ras–MEK–MAPK, and PI3K signaling pathways that control biological and cellular functions such as cell adhesion, migration, proliferation, cell differentiation, and apoptosis. A more complete understanding of the interactions between the altered ECM surrounding tumor cells and pancreatic ductal adenocarcinoma will help to elucidate the mechanisms of tumor biological behaviors and to develop new targets for pancreatic cancer prevention.
Collagens
Collagens are the main ingredients of the ECM. Abnormal expression of type I collagen, the predominant component of the desmoplastic reaction on pancreatic cancer cells, facilitates the malignant phenotype of pancreatic cancer cells and promotes gemcitabine resistance [77, 78]. The activation of α2β1 integrin, which is mediated by type I collagen, can promote pancreatic cancer cell proliferation and migration and contributes to the generation of the malignant phenotype [79]. Work done by Yasushi S and his colleagues revealed that type I collagen can activate the c-Jun-NH-2–terminal-kinase-1 and upregulate N-cadherin expression to promote pancreatic cancer metastasis [80]. Studies also show that type I collagen can modulate the expression of Snail and E-cadherin; both of these proteins are important transcription factors of the epithelial-mesenchymal transition (EMT) process, which results in the increased invasion of pancreatic cancer cells [81,82]. Type IV collagen may be a useful biomarker to evaluate pancreatic cancer prognosis and to predict the effect of pancreatic cancer treatments [83].
Glycoproteins
Pancreatic cancer cells with high levels of laminin γ2 expression that are associated with long nerve invasion reveal an important role for laminin γ2 in nerve invasion[84]. Laminin-1 adheres to α6β1 integrin, and it can induce CXCR4 and IL-8 expression, which may play a mechanistic role in pancreatic cancer metastasis [85, 86].
Proteoglycans
Proteoglycans, which are abundantly present in normal and neoplastic tissues, can modulate paracrine growth factor signaling events both directly and indirectly [87]. Versican, a member of the proteoglycan family, can regulate the cell adhesion, survival, proliferation, and migration of cancer cells in pancreatic carcinoma [88, 89].
Proteinases
Before the invasion and distant metastasis can occur, cancer cells must degrade the surrounding basement membrane; this process largely relies on the role of proteinases, especially the matrix metalloproteinases (MMPs). MMPs are zinc-dependent proteinases that are frequently expressed in cancer; MMPs play important roles in tumor growth and invasion, and they are positively correlated with a poorer prognosis and shorter patient survival time in pancreatic ductal adenocarcinoma.
Some data suggest that the activation of MMP-2 and MMP-9 plays an key role in cancer invasion and metastasis by digesting the ECM and potentially aiding in the development of the desmoplastic reaction in pancreatic cancer [90–93]. RNA interference targeting MMP-2 may be an effective therapeutic strategy for cancer [94]. MMP-7 is a member of the MMP family, and overexpression of MMP-7 may be involved in oncogenesis and degradation of extracellular matrix and is thought to promote the subsequent invasion of pancreatic cancer cells [95, 96]. MMP-21, which may be upregulated by EGF, is a marker of differentiation, and MMP-26 expression is associated with metastases in pancreatic cancer [97]. Johnson et al. demonstrated the role of human kallikrein 7 (hK7), a serine protease with aberrant expression in pancreatic cancer, in cell invasion by showing hK7’s ability to cleave E-cadherin [98].
Matricellular Proteins
Thrombospondin-1 (TSP-1) and TGF-β1 can upregulate the urokinase plasminogen activator (uPA) and its receptor (uPAR) and promote pancreatic tumor cell invasion [99]. In a study by Tobita et al., TSP-1 was found to play an important role in cancer cell growth and metastasis, and its immunoreactivity was found to act as a good prognostic predictor of human pancreatic cancer [100]. Thrombospondin-2 (TSP-2), a secreted extracellular matrix glycoprotein released by tumor-derived pancreatic stellate cells, stimulated the spread of pancreatic cancer [101]. Increased levels of MMP-2 found in TSP-2-null fibroblasts revealed a different role for TSP-2 in cancer; this conflicting result may be due to the different sources of cancer cells [102].
Tenascin-C (TNC), synthesized by pancreatic stellate cells, is upregulated in pancreatic cancer and potentially promotes pancreatic cancer progression [103, 104]. It has also been reported that TNC expression correlates with cell differentiation [105]. Fibronectin and laminin can stimulate reactive oxygen species (ROS) production and thus increase pancreatic cancer cell survival [106]. Recent data by Wu have demonstrated that laminin-induced FAK phosphorylation led to the increased chemoresistance to gemcitabine in pancreatic cancer cell lines [107]. The overexpression of fibrinogen (FBG), a central protein in the hemostasis pathway, induced cytokine and collagen production, which contributed to the desmoplastic reaction in pancreatic cancer [108–110].
Clearly, the articles mentioned above do not cover all the details of the ECM; these papers studied the extensively researched components of the ECM that are altered in pancreatic carcinoma microenvironments and produce various effects on tumor biological behaviors during cancer development. Hence, the ECM components may be effective targets for pancreatic cancer therapeutic strategies.
INFLAMMATORY CELLS
The relationship between inflammatory cells and tumors has been highlighted in recent years. Recent data have shown that inflammation is a critical constituent of the tumor microenvironment and that it plays an important role in carcinogenesis, tumor proliferation, angiogenesis, metastasis and resistance to chemotherapy [111, 112]. Evidence suggests that pancreatic inflammation may be involved in the progression of pancreatic malignancy [113, 114]. Inflammation is mainly mediated by cytokines, most of which are produced by the cancer cells and inflammatory cells. Here, we will review the cytokines involved in pancreatic cancer that may play key roles in the progression of the tumor.
Tumor Necrosis Factor (TNF)
Tumor necrosis factor, a cytokine involved in systemic inflammation, possesses a wide range of proinflammatory actions. Although its anticancer and cancer-promoting effects have be studied in the past [115], it is still used as anticancer agent. Here, we discuss its effects on pancreatic cancer. Human pancreatic tumor cells are highly resistant to TNF; however, the delivery of TNF to HER-2/neu expressing pancreatic tumor cells may be an effective therapy for pancreatic cancer especially when utilized in combination with 5-fluorouracil (5-FU) [116]. Tumor necrosis factor-alpha (TNF-α) is the most frequently mentioned member of TNF family; its anti-tumor activity is undermined by the activation of I-kappa-B-alpha kinase (IKK), which in turn activates nuclear factor-κB (NF-κB) to help cancer cells survive; a specific inhibitor or small interfering RNA against IKK improves the anticancer efficacy of TNF-α [117].
Another report showed that TNF-α induces claudin-1 expression and is thus responsible for the proliferation of pancreatic cancer cells [118]. The combination of human TNF-alpha and gemcitabine may be a potentially useful therapeutic approach for the improved treatment of pancreatic cancer [119]. Preoperative chemoradiation using TNFerade, a replication deficient adenovirus vector carrying the human TNF-α gene, may be a promising therapeutic strategy for advanced pancreatic cancer surgical resection [120].
Interleukins
The interleukins (ILs) are a class of cytokines that act to stimulate, regulate, or modulate lymphocytes such as T cells. Recent investigations have demonstrated that ILs, which can be produced by inflammatory cells, can also modulate the behavior of cancer cells. Although some interleukins, such as IL2, exert antitumor effects [121, 122], most members of this family have tumor-promoting effects; here, we review the complex roles of this family.
IL-1α plays an important role in metastasis through the constitutive activation of NF-κB. Thus, blocking IL-1α is a potential novel therapeutic strategy for treating pancreatic adenocarcinoma [123, 124]. Mononuclear cell-derived IL-1β stimulates the expression of cyclooxygenase-2, which contributes to chemoresistance in pancreatic cancer cells [125, 126]. IL-4 may enhance the growth of several pancreatic cancer cell lines in a dose-dependent manner, and this effect can be inhibited by neutralizing IL-4 antibodies [127]. The use of the IL-4-Pseudomonas exotoxin targeting IL-4 receptors is an effective therapy for pancreatic cancer [128]. IL-8, which was originally discovered as a chemotactic factor for leukocytes, plays an important role in tumor angiogenesis and contributes to the aggressive biology of human pancreatic cancer through its autocrine growth effects [129–133]. Targeting IL-8 activity in combination with chemotherapeutics may be an effective pancreatic cancer therapy [134, 135]. IL-13, an autocrine growth factor expressed in human pancreatic cancer, may cause immunosuppression in the host, and its expression correlates with lymph node metastases. Both neutralizing IL-13 antibodies and IL-13-Pseudomonas exotoxin can be useful agents for PDA therapy [136–139]. It has been shown that 5-fluorouracil can induce tumor cells to secrete biologically active IL-18, which has anti-tumor effects [140]. IL-32, a recently described proinflammatory cytokine that is upregulated in pancreatic cancer cells, may play an important role in pancreatic cancer growth. The knockdown of IL-32 led to the decrease of the antiapoptotic proteins Bcl-2, Bcl-xL, and Mcl-1 [141].
There are many other interleukins whose roles in CP remains unknown; more research is needed to elucidate the mechanisms of interleukins during the development and progression of pancreatic cancer.
Other Chemokines
Chemokines are a group of cytokines produced and released by a wide variety of cell types; they play a crucial role in acute and chronic inflammation. The chemokine family is divided into four groups: CXC, CC, C, and CX3C molecules. Their receptors are named CXCR, CCR, XCR, CX3CR, respectively [142, 143]. Recent discoveries have also highlighted the pivotal role of chemokines in the growth and progression of different cancers [144–147], including pancreatic cancer. Definitive roles of the chemokines in the pathogenesis of pancreatic cancer will provide a firm foundation for future therapies of pancreatic cancer.
CXCL12, the ligand for CXCR4, can induce the proliferation of pancreatic cancer cells; this proliferation is mediated by both AKT and ERK signaling [148]. CXCL17 and intercellular adhesion molecule 2 (ICAM2) are involved in immune surveillance by inducing infiltration and accumulation in the tumor epithelial layer by immature myeloid dendritic cells during pancreatic cancer development [149].
A study conducted by Matsuo Y and his colleagues demonstrates that the CXC/CXCR2 axis promotes pancreatic cancer tumor-associated angiogenesis both in vitro and in vivo and that this effect can be inhibited by targeted treatment against CXCR2 [150, 151]. CXCL8 and CXCL12, produced by tumor cells and fibroblast cells, respectively, can synergistically induce angiogenesis in vitro in pancreatic cancer [152].
Stromal cell-derived factor-1, a secretory product of pancreatic stellate cells, has been suggested to cause the invasion of human pancreatic cancer cells by activating the SDF-1/CXCR4 axis [153–155]. Therapeutic strategies targeting the SDF-1/CXCR4 or SDF-1/CXCR7 pathways may represent a potential therapeutic intervention for PDA [156]. Zerumbone, a component of subtropical ginger that can inhibit CXCR4 expression, can inhibit the CXCL12-induced invasion of pancreatic tumor cells [157]. CXCL14, a member of the CXC chemokine family, is upregulated in pancreatic cancer tissues and is associated with increased invasiveness of pancreatic cancer cells [158]. CXCL16 and its receptor CXCR6 are both upregulated in pancreatic ductal adenocarcinoma (PDAC); CXCL16 and CXCR6 have a major influence on the invasiveness of PDAC cells [159].
A previous study has demonstrated that the CXCL12–CXCR4 signaling pathway contributes to drug resistance in pancreatic cancer cells; thus, the CXCL12–CXCR4 axis represents a novel therapeutic target for pancreatic cancer therapy either in monotherapy or in combination with other cytotoxic drugs [160]. Intratumoral injection of the CCL2 chemokine (C-C motif) promotes the infiltration of immune cells in cancers and may become a tool for immunotherapy in pancreatic cancer [161]. Another study has shown that CCL21, which also belongs to C-C motif chemokines, has a similar function as CCL2 [162].
Although it is now becoming clear that various cytokines are indispensable participants in the neoplastic process and that they foster the proliferation, survival and migration of cancer cells, the detailed mechanisms remain to be elucidated; the search for drugs that act on these targets is still a challenge for cancer prevention.
CONCLUSION
In conclusion, the dense desmoplastic reaction is an important character of pancreatic adenocarcinomas. The role of the stroma tissue in the initiation, maintenance and aggravation of pancreatic cancer suggests that cancer-stroma interaction may be a promising target for new therapeutic approaches for the treatment of pancreatic carcinoma.
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation (Grant serial No.81172360 and No.30900705), 13115 Major Project (2010ZDKG-49), Scientific Grant of Xi'an City (2009 No.SF09027), and Pilot Project Grant from NIH P20 RR020151.
ABBREVIATIONS
- PSC
Pancreatic stellate cells
- IGF-I
Insulin-like growth factor-I
- FGF
Fibroblast growth factor
- TGF-h1
Transforming growth factor-h1
- PDGF
Platelet-derived growth factor
- VEGF
Vascular endothelial growth factor
- EMMPRIN
ECM metalloproteinase inducer
- bFGF
Basic fibroblast growth factor
- CAFs
Cancer associated fibroblasts
- FAP
Fibroblast activation protein
- Hh
Hedgehog
- Smo
Smoothened
- VEGF
Vascular endothelial growth factor
- PIGF
Placenta growth factor
- PNI
Perineural invasion
- NCAM
Neural cell adhesion molecules
- DRG
Dorsal root ganglia
- NGF
Nerve growth factor
- BDNF
Brain-derived neurotrophic factor
- NT
Neurotrophin
- GDNF
Glial cell-derived neurotrophic factor
- PSA
Polysialic acid
- MAG
Myelin-associated glycoprotein
- ECM
Eextracellular matrix
- EMT
Epithelial-mesenchymal transition
- MMPs
Matrix metalloproteinases
- hK7
Human kallikrein 7
- TSP
Thrombospondin
- uPA
Urokinase plasminogen activator
- TNC
Tenascin-C
- ROS
reactive oxygen species
- FBG
fibrinogen
- TNF
Tumor necrosis factor
- 5-FU
5-fluorouracil
- TNF-α
Tumor necrosis factor-alpha
- NF-κB
Nuclear factor-κB
- IKK
I-kappa-B-alpha kinase
- ILs
Interleukins
- ICAM2
Intercellular adhesion molecule 2
- PDAC
Pancreatic ductal adenocarcinoma
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Vonlaufen A, Phillips PA, Xu Z, et al. Pancreatic stellate cells and pancreatic cancer cells: an unholy alliance. Cancer Res. 2008;68:7707–7710. doi: 10.1158/0008-5472.CAN-08-1132. [DOI] [PubMed] [Google Scholar]
- 3.Apte MV, Wilson JS. Stellate cell activation in alcoholic pancreatitis. Pancreas. 2003;27:316–320. doi: 10.1097/00006676-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Bachem MG, Zhou S, Buck K, Schneiderhan W, Siech M. Pancreatic stellate cells--role in pancreas cancer. Langenbecks Arch Surg. 2008;393:891–900. doi: 10.1007/s00423-008-0279-5. [DOI] [PubMed] [Google Scholar]
- 5.Apte MV, Park S, Phillips PA, et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179–187. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Bachem MG, Schunemann M, Ramadani M, et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907–921. doi: 10.1053/j.gastro.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 7.Ellenrieder V, Alber B, Lacher U, et al. Role of MT-MMPs and MMP-2 in pancreatic cancer progression. Int J Cancer. 2000;85:14–20. doi: 10.1002/(sici)1097-0215(20000101)85:1<14::aid-ijc3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 8.Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117:50–59. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vonlaufen A, Joshi S, Qu C, et al. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res. 2008;68:2085–2093. doi: 10.1158/0008-5472.CAN-07-2477. [DOI] [PubMed] [Google Scholar]
- 10.Logsdon CD, Simeone DM, Binkley C, et al. Molecular profiling of pancreatic adenocarcinoma and chronic pancreatitis identifies multiple genes differentially regulated in pancreatic cancer. Cancer Res. 2003;63:2649–2657. [PubMed] [Google Scholar]
- 11.Arao S, Masumoto A, Otsuki M. Beta1 integrins play an essential role in adhesion and invasion of pancreatic carcinoma cells. Pancreas. 2000;20:129–137. doi: 10.1097/00006676-200003000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316:1324–1331. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 14.Hwang RF, Moore T, Arumugam T, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kikuta K, Masamune A, Watanabe T, et al. Pancreatic stellate cells promote epithelial-mesenchymal transition in pancreatic cancer cells. Biochem Biophys Res Commun. 2010;403:380–384. doi: 10.1016/j.bbrc.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 16.Farrow B, Berger DH, Rowley D. Tumor-derived pancreatic stellate cells promote pancreatic cancer cell invasion through release of thrombospondin-2. J Surg Res. 2009;156:155–160. doi: 10.1016/j.jss.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 17.Finak G, Bertos N, Pepin F, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- 18.Farmer P, Bonnefoi H, Anderle P, et al. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat Med. 2009;15:68–74. doi: 10.1038/nm.1908. [DOI] [PubMed] [Google Scholar]
- 19.Crawford Y, Kasman I, Yu L, et al. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15:21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Li Q, Yamada T, et al. Crosstalk to stromal fibroblasts induces resistance of lung cancer to epidermal growth factor receptor tyrosine kinase inhibitors. Clin Cancer Res. 2009;15:6630–6638. doi: 10.1158/1078-0432.CCR-09-1001. [DOI] [PubMed] [Google Scholar]
- 21.Pietras K, Ostman A, Sjoquist M, et al. Inhibition of platelet-derived growth factor receptors reduces interstitial hypertension and increases transcapillary transport in tumors. Cancer Res. 2001;61:2929–2934. [PubMed] [Google Scholar]
- 22.Pietras K, Rubin K, Sjoblom T, et al. Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res. 2002;62:5476–5484. [PubMed] [Google Scholar]
- 23.Santos AM, Jung J, Aziz N, Kissil JL, Pure E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest. 2009;119:3613–3625. doi: 10.1172/JCI38988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pietras K, Pahler J, Bergers G, Hanahan D. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:e19. doi: 10.1371/journal.pmed.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yauch RL, Gould SE, Scales SJ, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 26.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcea G, Doucas H, Steward WP, Dennison AR, Berry DP. Hypoxia and angiogenesis in pancreatic cancer. ANZ J Surg. 2006;76:830–842. doi: 10.1111/j.1445-2197.2006.03872.x. [DOI] [PubMed] [Google Scholar]
- 28.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 29.Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res. 2006;12:5018–5022. doi: 10.1158/1078-0432.CCR-06-1520. [DOI] [PubMed] [Google Scholar]
- 30.He L, Wu Y, Lin L, et al. Hispidulin, a small flavonoid molecule, suppresses the angiogenesis and growth of human pancreatic cancer by targeting vascular endothelial growth factor receptor 2-mediated PI3K/Akt/mTOR signaling pathway. Cancer Sci. 2011;102:219–225. doi: 10.1111/j.1349-7006.2010.01778.x. [DOI] [PubMed] [Google Scholar]
- 31.Seo Y, Baba H, Fukuda T, Takashima M, Sugimachi K. High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer. 2000;88:2239–2245. doi: 10.1002/(sici)1097-0142(20000515)88:10<2239::aid-cncr6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 32.Cao Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci Signal. 2009;2:re1. doi: 10.1126/scisignal.259re1. [DOI] [PubMed] [Google Scholar]
- 33.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 34.Tassi E, Wellstein A. The angiogenic switch molecule, secreted FGF-binding protein, an indicator of early stages of pancreatic and colorectal adenocarcinoma. Semin Oncol. 2006;33:S50–S56. doi: 10.1053/j.seminoncol.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makrilia N, Lappa T, Xyla V, Nikolaidis I, Syrigos K. The role of angiogenesis in solid tumours: an overview. Eur J Intern Med. 2009;20:663–671. doi: 10.1016/j.ejim.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Pour PM, Bell RH, Batra SK. Neural invasion in the staging of pancreatic cancer. Pancreas. 2003;26:322–325. doi: 10.1097/00006676-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Kayahara M, Nakagawara H, Kitagawa H, Ohta T. The nature of neural invasion by pancreatic cancer. Pancreas. 2007;35:218–223. doi: 10.1097/mpa.0b013e3180619677. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Ma Q. Hyperglycemia promotes the perineural invasion in pancreatic cancer. 2008;71:386–389. doi: 10.1016/j.mehy.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Liu B, Lu KY. Neural invasion in pancreatic carcinoma. Hepatobiliary Pancreat Dis Int. 2002;1:469–476. [PubMed] [Google Scholar]
- 40.Pour PM, Bell RH, Batra SK. Neural invasion in the staging of pancreatic cancer. Pancreas. 2003;26:322–325. doi: 10.1097/00006676-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Nagakawa T, Kayahara M, Ueno K, Ohta T, Konishi I, Miyazaki I. Clinicopathological study on neural invasion to the extrapancreatic nerve plexus in pancreatic cancer. Hepatogastroenterology. 1992;39:51–55. [PubMed] [Google Scholar]
- 42.Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379–3391. doi: 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- 43.Kenmotsu M, Gochi A, Ishii H, et al. Relationship between perineural invasion and local recurrence of rectal carcinoma: a preliminary study with immunohistochemical staining with anti-NCAM: preliminary report. Nippon Geka Gakkai Zasshi. 1990;91:1759. [PubMed] [Google Scholar]
- 44.Ayala GEW, T. M. Shine HDS, M. Frolov AC, S. Rowley D. In vitro dorsal root ganglia and human prostate cell line interaction: redefining perineural invasion in prostate cancer. Prostate. 2001;49:213–223. doi: 10.1002/pros.1137. [DOI] [PubMed] [Google Scholar]
- 45.Ceyhan GOD, I. E. Altintas BR, U. Thiel GM, M. W. Giese NAF, H. Schafer KH. Neural invasion in pancreatic cancer: a mutual tropism between neurons and cancer cells. Biochemical and Biophysical Research Communications. 2008;374:442–447. doi: 10.1016/j.bbrc.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 46.Dai H, Li R, Wheeler T, et al. Enhanced survival in perineural invasion of pancreatic cancer: an in vitro approach. Hum Pathol. 2007;38:299–307. doi: 10.1016/j.humpath.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Zhu ZW, Friess H, Wang L, et al. Nerve growth factor exerts differential effects on the growth of human pancreatic cancer cells. Clin Cancer Res. 2001;7:105–112. [PubMed] [Google Scholar]
- 48.Zhu Z, Kleeff J, Kayed H, et al. Nerve growth factor and enhancement of proliferation, invasion, and tumorigenicity of pancreatic cancer cells. Mol Carcinog. 2002;35:138–147. doi: 10.1002/mc.10083. [DOI] [PubMed] [Google Scholar]
- 49.Geldof AA, De Kleijn MA, Rao BR, Newling DW. Nerve growth factor stimulates in vitro invasive capacity of DU145 human prostatic cancer cells. J Cancer Res Clin Oncol. 1997;123:107–112. doi: 10.1007/BF01269888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miknyoczki SJ, Lang D, Huang L, Klein-Szanto AJ, Dionne CA, Ruggeri BA. Neurotrophins and Trk receptors in human pancreatic ductal adenocarcinoma: expression patterns and effects on in vitro invasive behavior. Int J Cancer. 1999;81:417–427. doi: 10.1002/(sici)1097-0215(19990505)81:3<417::aid-ijc16>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 51.Djakiew D, Pflug BR, Delsite R, et al. Chemotaxis and chemokinesis of human prostate tumor cell lines in response to human prostate stromal cell secretory proteins containing a nerve growth factor-like protein. Cancer Res. 1993;53:1416–1420. [PubMed] [Google Scholar]
- 52.Marchetti D, McQuillan DJ, Spohn WC, Carson DD, Nicolson GL. Neurotrophin stimulation of human melanoma cell invasion: selected enhancement of heparanase activity and heparanase degradation of specific heparan sulfate subpopulations. Cancer Res. 1996;56:2856–2863. [PubMed] [Google Scholar]
- 53.Okada Y, Eibl G, Guha S, Duffy JP, Reber HA, Hines OJ. Nerve growth factor stimulates MMP-2 expression and activity and increases invasion by human pancreatic cancer cells. Clin Exp Metastasis. 2004;21:285–292. doi: 10.1023/b:clin.0000046131.24625.54. [DOI] [PubMed] [Google Scholar]
- 54.Ayala GE, Dai H, Tahir SA, et al. Stromal antiapoptotic paracrine loop in perineural invasion of prostatic carcinoma. Cancer Res. 2006;66:5159–5164. doi: 10.1158/0008-5472.CAN-05-1847. [DOI] [PubMed] [Google Scholar]
- 55.Ceyhan GO, Giese NA, Erkan M, et al. The neurotrophic factor artemin promotes pancreatic cancer invasion. Ann Surg. 2006;244:274–281. doi: 10.1097/01.sla.0000217642.68697.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okada Y, Takeyama H, Sato M, et al. Experimental implication of celiac ganglionotropic invasion of pancreatic-cancer cells bearing c-ret proto-oncogene with reference to glial-cell-line-derived neurotrophic factor (GDNF) Int J Cancer. 1999;81:67–73. doi: 10.1002/(sici)1097-0215(19990331)81:1<67::aid-ijc13>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 57.Okada Y, Eibl G, Duffy JP, Reber HA, Hines OJ. Glial cell-derived neurotrophic factor upregulates the expression and activation of matrix metalloproteinase-9 in human pancreatic cancer. Surgery. 2003;134:293–299. doi: 10.1067/msy.2003.239. [DOI] [PubMed] [Google Scholar]
- 58.Kameda K, Shimada H, Ishikawa T, et al. Expression of highly polysialylated neural cell adhesion molecule in pancreatic cancer neural invasive lesion. Cancer Lett. 1999;137:201–207. doi: 10.1016/s0304-3835(98)00359-0. [DOI] [PubMed] [Google Scholar]
- 59.Seki HT, J. Sato YK, Y. Umezawa AKK. Neural cell adhesion molecule (NCAM) and perineural invasion in bile duct cancer. J Surg Oncol. 1993;53:78–83. doi: 10.1002/jso.2930530205. [DOI] [PubMed] [Google Scholar]
- 60.Schreiber SC, Giehl K, Kastilan C, et al. Polysialylated NCAM represses E-cadherin-mediated cell-cell adhesion in pancreatic tumor cells. Gastroenterology. 2008;134:1555–1566. doi: 10.1053/j.gastro.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 61.Schweizerhof M, Stosser S, Kurejova M, et al. Hematopoietic colony-stimulating factors mediate tumor-nerve interactions and bone cancer pain. Nat Med. 2009;15:802–807. doi: 10.1038/nm.1976. [DOI] [PubMed] [Google Scholar]
- 62.Marchesi F, Piemonti L, Mantovani A, Allavena P. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev. 2010;21:77–82. doi: 10.1016/j.cytogfr.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Swanson BJ, McDermott KM, Singh PK, Eggers JP, Crocker PR, Hollingsworth MA. MUC1 is a counter-receptor for myelin-associated glycoprotein (Siglec-4a) and their interaction contributes to adhesion in pancreatic cancer perineural invasion. Cancer Res. 2007;67:10222–10229. doi: 10.1158/0008-5472.CAN-06-2483. [DOI] [PubMed] [Google Scholar]
- 64.Anderson TD, Feldman M, Weber RS, Ziober AF, Ziober BL. Tumor deposition of laminin-5 and the relationship with perineural invasion. Laryngoscope. 2001;111:2140–2143. doi: 10.1097/00005537-200112000-00011. [DOI] [PubMed] [Google Scholar]
- 65.Marchesi F, Piemonti L, Fedele G, et al. The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Cancer Res. 2008;68:9060–9069. doi: 10.1158/0008-5472.CAN-08-1810. [DOI] [PubMed] [Google Scholar]
- 66.Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci. 2004;20:1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- 67.Pan Y, Lloyd C, Zhou H, et al. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387:611–617. doi: 10.1038/42491. [DOI] [PubMed] [Google Scholar]
- 68.Bazan JF, Bacon KB, Hardiman G, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 69.Cardona AE, Pioro EP, Sasse ME, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 70.Masamune A, Watanabe T, Kikuta K, Shimosegawa T. Roles of pancreatic stellate cells in pancreatic inflammation and fibrosis. Clin Gastroenterol Hepatol. 2009;7:S48–S54. doi: 10.1016/j.cgh.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 71.Masamune A, Shimosegawa T. Signal transduction in pancreatic stellate cells. J Gastroenterol. 2009;44:249–260. doi: 10.1007/s00535-009-0013-2. [DOI] [PubMed] [Google Scholar]
- 72.Pandol S, Edderkaoui M, Gukovsky I, Lugea A, Gukovskaya A. Desmoplasia of pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2009;7:S44–S47. doi: 10.1016/j.cgh.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koninger J, Giese T, di MFF, et al. Pancreatic tumor cells influence the composition of the extracellular matrix. Biochem Biophys Res Commun. 2004;322:943–949. doi: 10.1016/j.bbrc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 74.Erkan M, Michalski CW, Rieder S, et al. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2008;6:1155–1161. doi: 10.1016/j.cgh.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 75.Brakebusch C, Bouvard D, Stanchi F, Sakai T, Fassler R. Integrins in invasive growth. J Clin Invest. 2002;109:999–1006. doi: 10.1172/JCI15468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stupack DG, Cheresh DA. Get a ligand, get a life: integrins, signaling and cell survival. J Cell Sci. 2002;115:3729–3738. doi: 10.1242/jcs.00071. [DOI] [PubMed] [Google Scholar]
- 77.Armstrong T, Packham G, Murphy LB, et al. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10:7427–7437. doi: 10.1158/1078-0432.CCR-03-0825. [DOI] [PubMed] [Google Scholar]
- 78.Dangi-Garimella S, Krantz SB, Barron MR, et al. Three-dimensional collagen I promotes gemcitabine resistance in pancreatic cancer through MT1-MMP-mediated expression of HMGA2. Cancer Res. 2011;71:1019–1028. doi: 10.1158/0008-5472.CAN-10-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grzesiak JJ, Bouvet M. The alpha2beta1 integrin mediates the malignant phenotype on type I collagen in pancreatic cancer cell lines. Br J Cancer. 2006;94:1311–1319. doi: 10.1038/sj.bjc.6603088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shintani Y, Hollingsworth MA, Wheelock MJ, Johnson KR. Collagen I promotes metastasis in pancreatic cancer by activating c-Jun NH(2)-terminal kinase 1 and up-regulating N-cadherin expression. Cancer Res. 2006;66:11745–11753. doi: 10.1158/0008-5472.CAN-06-2322. [DOI] [PubMed] [Google Scholar]
- 81.Imamichi Y, Konig A, Gress T, Menke A. Collagen type I-induced Smad-interacting protein 1 expression downregulates E-cadherin in pancreatic cancer. Oncogene. 2007;26:2381–2385. doi: 10.1038/sj.onc.1210012. [DOI] [PubMed] [Google Scholar]
- 82.Shields MA, Dangi-Garimella S, Krantz SB, Bentrem DJ, Munshi HG. Pancreatic Cancer Cells Respond to Type I Collagen by Inducing Snail Expression to Promote Membrane Type 1 Matrix Metalloproteinase- dependent Collagen Invasion. J Biol Chem. 2011;286:10495–10504. doi: 10.1074/jbc.M110.195628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ohlund D, Lundin C, Ardnor B, Oman M, Naredi P, Sund M. Type IV collagen is a tumour stroma-derived biomarker for pancreas cancer. Br J Cancer. 2009;101:91–97. doi: 10.1038/sj.bjc.6605107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mitsunaga S, Fujii S, Ishii G, et al. Nerve invasion distance is dependent on laminin gamma2 in tumors of pancreatic cancer. Int J Cancer. 2010;127:805–819. doi: 10.1002/ijc.25104. [DOI] [PubMed] [Google Scholar]
- 85.Patrick CW, Jr, Wu X. Integrin-mediated preadipocyte adhesion and migration on laminin-1. Ann Biomed Eng. 2003;31:505–514. doi: 10.1114/1.1566446. [DOI] [PubMed] [Google Scholar]
- 86.Grzesiak JJ, Smith KC, Burton DW, Deftos LJ, Bouvet M. Integrin-mediated laminin-1 adhesion upregulates CXCR4 and IL-8 expression in pancreatic cancer cells. Surgery. 2007;141:804–814. doi: 10.1016/j.surg.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Friedl A. Proteoglycans: master modulators of paracrine fibroblast-carcinoma cell interactions. Semin Cell Dev Biol. 2010;21:66–71. doi: 10.1016/j.semcdb.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- 89.Skandalis SS, Kletsas D, Kyriakopoulou D, Stavropoulos M, Theocharis DA. The greatly increased amounts of accumulated versican and decorin with specific post-translational modifications may be closely associated with the malignant phenotype of pancreatic cancer. Biochim Biophys Acta. 2006;1760:1217–1225. doi: 10.1016/j.bbagen.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 90.Ellenrieder V, Alber B, Lacher U, et al. Role of MT-MMPs and MMP-2 in pancreatic cancer progression. Int J Cancer. 2000;85:14–20. doi: 10.1002/(sici)1097-0215(20000101)85:1<14::aid-ijc3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 91.Yang X, Staren ED, Howard JM, Iwamura T, Bartsch JE, Appert HE. Invasiveness and MMP expression in pancreatic carcinoma. J Surg Res. 2001;98:33–39. doi: 10.1006/jsre.2001.6150. [DOI] [PubMed] [Google Scholar]
- 92.Schneiderhan W, Diaz F, Fundel M, et al. Pancreatic stellate cells are an important source of MMP-2 in human pancreatic cancer and accelerate tumor progression in a murine xenograft model and CAM assay. J Cell Sci. 2007;120:512–519. doi: 10.1242/jcs.03347. [DOI] [PubMed] [Google Scholar]
- 93.Nagai S, Nakamura M, Yanai K, et al. Gli1 contributes to the invasiveness of pancreatic cancer through matrix metalloproteinase-9 activation. Cancer Sci. 2008;99:1377–1384. doi: 10.1111/j.1349-7006.2008.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhi YH, Song MM, Wang PL, Zhang T, Yin ZY. Suppression of matrix metalloproteinase-2 via RNA interference inhibits pancreatic carcinoma cell invasiveness and adhesion. World J Gastroenterol. 2009;15:1072–1078. doi: 10.3748/wjg.15.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li YJ, Wei ZM, Meng YX, Ji XR. Beta-catenin up-regulates the expression of cyclinD1, c-myc and MMP-7 in human pancreatic cancer: relationships with carcinogenesis and metastasis. World J Gastroenterol. 2005;11:2117–2123. doi: 10.3748/wjg.v11.i14.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tan X, Egami H, Abe M, Nozawa F, Hirota M, Ogawa M. Involvement of MMP-7 in invasion of pancreatic cancer cells through activation of the EGFR mediated MEK-ERK signal transduction pathway. J Clin Pathol. 2005;58:1242–1248. doi: 10.1136/jcp.2004.025338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bister V, Skoog T, Virolainen S, Kiviluoto T, Puolakkainen P, Saarialho-Kere U. Increased expression of matrix metalloproteinases-21 and -26 and TIMP-4 in pancreatic adenocarcinoma. Mod Pathol. 2007;20:1128–1140. doi: 10.1038/modpathol.3800956. [DOI] [PubMed] [Google Scholar]
- 98.Johnson SK, Ramani VC, Hennings L, Haun RS. Kallikrein 7 enhances pancreatic cancer cell invasion by shedding E-cadherin. Cancer. 2007;109:1811–1820. doi: 10.1002/cncr.22606. [DOI] [PubMed] [Google Scholar]
- 99.Albo D, Berger DH, Vogel J, Tuszynski GP. Thrombospondin-1 and transforming growth factor beta-1 upregulate plasminogen activator inhibitor type 1 in pancreatic cancer. J Gastrointest Surg. 1999;3:411–417. doi: 10.1016/s1091-255x(99)80058-4. [DOI] [PubMed] [Google Scholar]
- 100.Tobita K, Kijima H, Dowaki S, et al. Thrombospondin-1 expression as a prognostic predictor of pancreatic ductal carcinoma. Int J Oncol. 2002;21:1189–1195. [PubMed] [Google Scholar]
- 101.Farrow B, Berger DH, Rowley D. Tumor-derived pancreatic stellate cells promote pancreatic cancer cell invasion through release of thrombospondin-2. J Surg Res. 2009;156:155–160. doi: 10.1016/j.jss.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 102.Yang Z, Kyriakides TR, Bornstein P. Matricellular proteins as modulators of cell-matrix interactions: adhesive defect in thrombospondin 2-null fibroblasts is a consequence of increased levels of matrix metalloproteinase-2. Mol Biol Cell. 2000;11:3353–3364. doi: 10.1091/mbc.11.10.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Esposito I, Penzel R, Chaib-Harrireche M, et al. Tenascin C and annexin II expression in the process of pancreatic carcinogenesis. J Pathol. 2006;208:673–685. doi: 10.1002/path.1935. [DOI] [PubMed] [Google Scholar]
- 104.Chen J, Chen Z, Chen M, et al. Role of fibrillar Tenascin-C in metastatic pancreatic cancer. Int J Oncol. 2009;34:1029–1036. [PubMed] [Google Scholar]
- 105.Juuti A, Nordling S, Louhimo J, Lundin J, Haglund C. Tenascin C expression is upregulated in pancreatic cancer and correlates with differentiation. J Clin Pathol. 2004;57:1151–1155. doi: 10.1136/jcp.2003.015818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Edderkaoui M, Hong P, Vaquero EC, et al. Extracellular matrix stimulates reactive oxygen species production and increases pancreatic cancer cell survival through 5-lipoxygenase and NADPH oxidase. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1137–G1147. doi: 10.1152/ajpgi.00197.2005. [DOI] [PubMed] [Google Scholar]
- 107.Wu W, Zhiyong L, Xiaohua S, Xinyu R, Kai W, Tonghua L. Intrinsic chemoresistance to gemcitabine is associated with constitutive and laminin-induced phosphorylation of FAK in pancreatic cancer cell lines. Mol Cancer. 2009;8:125. doi: 10.1186/1476-4598-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Simpson-Haidaris PJ, Rybarczyk B. Tumors and fibrinogen. The role of fibrinogen as an extracellular matrix protein. Ann N Y Acad Sci. 2001;936:406–425. [PubMed] [Google Scholar]
- 109.Bloomston M, Zhou JX, Rosemurgy AS, Frankel W, Muro-Cacho CA, Yeatman TJ. Fibrinogen gamma overexpression in pancreatic cancer identified by large-scale proteomic analysis of serum samples. Cancer Res. 2006;66:2592–2599. doi: 10.1158/0008-5472.CAN-05-3659. [DOI] [PubMed] [Google Scholar]
- 110.Masamune A, Kikuta K, Watanabe T, et al. Fibrinogen induces cytokine and collagen production in pancreatic stellate cells. Gut. 2009;58:550–559. doi: 10.1136/gut.2008.154401. [DOI] [PubMed] [Google Scholar]
- 111.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 113.Farrow B, Evers BM. Inflammation and the development of pancreatic cancer. Surg Oncol. 2002;10:153–169. doi: 10.1016/s0960-7404(02)00015-4. [DOI] [PubMed] [Google Scholar]
- 114.Whitcomb DC. Inflammation and Cancer V. Chronic pancreatitis and pancreatic cancer. Am J Physiol Gastrointest Liver Physiol. 2004;287:G315–G319. doi: 10.1152/ajpgi.00115.2004. [DOI] [PubMed] [Google Scholar]
- 115.Mocellin S, Nitti D. TNF and cancer: the two sides of the coin. Front Biosci. 2008;13:2774–2783. doi: 10.2741/2884. [DOI] [PubMed] [Google Scholar]
- 116.Lyu MA, Kurzrock R, Rosenblum MG. The immunocytokine scFv23/TNF targeting HER-2/neu induces synergistic cytotoxic effects with 5-fluorouracil in TNF-resistant pancreatic cancer cell lines. Biochem Pharmacol. 2008;75:836–846. doi: 10.1016/j.bcp.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 117.Zhang Y, Gavriil M, Lucas J, et al. IkappaBalpha kinase inhibitor IKI-1 conferred tumor necrosis factor alpha sensitivity to pancreatic cancer cells and a xenograft tumor model. Cancer Res. 2008;68:9519–9524. doi: 10.1158/0008-5472.CAN-08-1549. [DOI] [PubMed] [Google Scholar]
- 118.Kondo J, Sato F, Kusumi T, et al. Claudin-1 expression is induced by tumor necrosis factor-alpha in human pancreatic cancer cells. Int J Mol Med. 2008;22:645–649. [PubMed] [Google Scholar]
- 119.Murugesan SR, King CR, Osborn R, et al. Combination of human tumor necrosis factor-alpha (hTNF-alpha) gene delivery with gemcitabine is effective in models of pancreatic cancer. Cancer Gene Ther. 2009;16:841–847. doi: 10.1038/cgt.2009.32. [DOI] [PubMed] [Google Scholar]
- 120.Chadha MK, Litwin A, Levea C, et al. Surgical resection after TNFerade therapy for locally advanced pancreatic cancer. JOP. 2009;10:535–538. [PubMed] [Google Scholar]
- 121.Degrate L, Nobili C, Franciosi C, et al. Interleukin-2 immunotherapy action on innate immunity cells in peripheral blood and tumoral tissue of pancreatic adenocarcinoma patients. Langenbecks Arch Surg. 2009;394:115–121. doi: 10.1007/s00423-008-0393-4. [DOI] [PubMed] [Google Scholar]
- 122.Grande C, Firvida JL, Navas V, Casal J. Interleukin-2 for the treatment of solid tumors other than melanoma and renal cell carcinoma. Anticancer Drugs. 2006;17:1–12. doi: 10.1097/01.cad.0000182748.47353.51. [DOI] [PubMed] [Google Scholar]
- 123.Matsuo Y, Sawai H, Ochi N, et al. Interleukin-1alpha secreted by pancreatic cancer cells promotes angiogenesis and its therapeutic implications. J Surg Res. 2009;153:274–281. doi: 10.1016/j.jss.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 124.Melisi D, Niu J, Chang Z, et al. Secreted interleukin-1alpha induces a metastatic phenotype in pancreatic cancer by sustaining a constitutive activation of nuclear factor-kappaB. Mol Cancer Res. 2009;7:624–633. doi: 10.1158/1541-7786.MCR-08-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Angst E, Reber HA, Hines OJ, Eibl G. Mononuclear cell-derived interleukin-1 beta confers chemoresistance in pancreatic cancer cells by upregulation of cyclooxygenase-2. Surgery. 2008;144:57–65. doi: 10.1016/j.surg.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ramachandran C, Resek AP, Escalon E, Aviram A, Melnick SJ. Potentiation of gemcitabine by Turmeric Force in pancreatic cancer cell lines. Oncol Rep. 2010;23:1529–1535. doi: 10.3892/or_00000792. [DOI] [PubMed] [Google Scholar]
- 127.Prokopchuk O, Liu Y, Henne-Bruns D, Kornmann M. Interleukin-4 enhances proliferation of human pancreatic cancer cells: evidence for autocrine and paracrine actions. Br J Cancer. 2005;92:921–928. doi: 10.1038/sj.bjc.6602416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kawakami K, Kawakami M, Husain SR, Puri RK. Targeting interleukin-4 receptors for effective pancreatic cancer therapy. Cancer Res. 2002;62:3575–3580. [PubMed] [Google Scholar]
- 129.Wigmore SJ, Fearon KC, Sangster K, Maingay JP, Garden OJ, Ross JA. Cytokine regulation of constitutive production of interleukin-8 and -6 by human pancreatic cancer cell lines and serum cytokine concentrations in patients with pancreatic cancer. Int J Oncol. 2002;21:881–886. doi: 10.3892/ijo.21.4.881. [DOI] [PubMed] [Google Scholar]
- 130.Shi Q, Abbruzzese JL, Huang S, Fidler IJ, Xiong Q, Xie K. Constitutive and inducible interleukin 8 expression by hypoxia and acidosis renders human pancreatic cancer cells more tumorigenic and metastatic. Clin Cancer Res. 1999;5:3711–3721. [PubMed] [Google Scholar]
- 131.Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12:375–391. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- 132.Takamori H, Oades ZG, Hoch OC, Burger M, Schraufstatter IU. Autocrine growth effect of IL-8 and GROalpha on a human pancreatic cancer cell line, Capan-1. Pancreas. 2000;21:52–56. doi: 10.1097/00006676-200007000-00051. [DOI] [PubMed] [Google Scholar]
- 133.Li M, Zhang Y, Feurino LW, et al. Interleukin-8 increases vascular endothelial growth factor and neuropilin expression and stimulates ERK activation in human pancreatic cancer. Cancer Sci. 2008;99:733–737. doi: 10.1111/j.1349-7006.2008.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhu YM, Woll PJ. Mitogenic effects of interleukin-8/CXCL8 on cancer cells. Future Oncol. 2005;1:699–704. doi: 10.2217/14796694.1.5.699. [DOI] [PubMed] [Google Scholar]
- 135.Kamohara H, Takahashi M, Ishiko T, Ogawa M, Baba H. Induction of interleukin-8 (CXCL-8) by tumor necrosis factor-alpha and leukemia inhibitory factor in pancreatic carcinoma cells: Impact of CXCL-8 as an autocrine growth factor. Int J Oncol. 2007;31:627–632. [PubMed] [Google Scholar]
- 136.Formentini A, Prokopchuk O, Strater J, et al. Interleukin-13 exerts autocrine growth-promoting effects on human pancreatic cancer, and its expression correlates with a propensity for lymph node metastases. Int J Colorectal Dis. 2009;24:57–67. doi: 10.1007/s00384-008-0550-9. [DOI] [PubMed] [Google Scholar]
- 137.Shimamura T, Fujisawa T, Husain SR, Joshi B, Puri RK. Interleukin 13 mediates signal transduction through interleukin 13 receptor alpha2 in pancreatic ductal adenocarcinoma: role of IL-13 Pseudomonas exotoxin in pancreatic cancer therapy. Clin Cancer Res. 2010;16:577–586. doi: 10.1158/1078-0432.CCR-09-2015. [DOI] [PubMed] [Google Scholar]
- 138.Fujisawa T, Nakashima H, Nakajima A, Joshi BH, Puri RK. Targeting IL-13Ralpha2 in human pancreatic ductal adenocarcinoma with combination therapy of IL-13-PE and gemcitabine. Int J Cancer. 2011;128:1221–1231. doi: 10.1002/ijc.25437. [DOI] [PubMed] [Google Scholar]
- 139.Fujisawa T, Joshi B, Nakajima A, Puri RK. A novel role of interleukin-13 receptor alpha2 in pancreatic cancer invasion and metastasis. Cancer Res. 2009;69:8678–8685. doi: 10.1158/0008-5472.CAN-09-2100. [DOI] [PubMed] [Google Scholar]
- 140.Carbone A, Rodeck U, Mauri FA, et al. Human pancreatic carcinoma cells secrete bioactive interleukin-18 after treatment with 5-fluorouracil: implications for anti-tumor immune response. Cancer Biol Ther. 2005;4:231–241. doi: 10.4161/cbt.4.2.1476. [DOI] [PubMed] [Google Scholar]
- 141.Nishida A, Andoh A, Inatomi O, Fujiyama Y. Interleukin-32 expression in the pancreas. J Biol Chem. 2009;284:17868–17876. doi: 10.1074/jbc.M900368200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pellegrino A, Vacca A, Scavelli C, Dammacco F. Chemokines and tumors. Recenti Prog Med. 2002;93:642–654. [PubMed] [Google Scholar]
- 143.Trakatelli C, Frydas S, Hatzistilianou M, Papadopoulos E, Simeonidou I, Founta A, et al. Chemokines as markers for parasite-induced inflammation and tumors. Int J Biol Markers. 2005;20:197–203. doi: 10.1177/172460080502000401. [DOI] [PubMed] [Google Scholar]
- 144.Yoong KF, Afford SC, Jones R, et al. Expression and function of CXC and CC chemokines in human malignant liver tumors: a role for human monokine induced by gamma-interferon in lymphocyte recruitment to hepatocellular carcinoma. Hepatology. 1999;30:100–111. doi: 10.1002/hep.510300147. [DOI] [PubMed] [Google Scholar]
- 145.Huang F, Geng XP. Chemokines and hepatocellular carcinoma. World J Gastroenterol. 2010;16:1832–1836. doi: 10.3748/wjg.v16.i15.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Barbieri F, Bajetto A, Florio T. Role of chemokine network in the development and progression of ovarian cancer: a potential novel pharmacological target. J Oncol. 2010;2010:426956. doi: 10.1155/2010/426956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Strieter RM, Belperio JA, Burdick MD, Sharma S, Dubinett SM, Keane MP. CXC chemokines: angiogenesis, immunoangiostasis, and metastases in lung cancer. Ann N Y Acad Sci. 2004;1028:351–360. doi: 10.1196/annals.1322.041. [DOI] [PubMed] [Google Scholar]
- 148.Shen X, Artinyan A, Jackson D, Thomas RM, Lowy AM, Kim J. Chemokine receptor CXCR4 enhances proliferation in pancreatic cancer cells through AKT and ERK dependent pathways. Pancreas. 2010;39:81–87. doi: 10.1097/MPA.0b013e3181bb2ab7. [DOI] [PubMed] [Google Scholar]
- 149.Hiraoka N, Yamazaki-Itoh R, Ino Y, et al. CXCL17 and ICAM2 are associated with a potential anti-tumor immune response in early intraepithelial stages of human pancreatic carcinogenesis. Gastroenterology. 2011;140:310–321. doi: 10.1053/j.gastro.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 150.Matsuo Y, Raimondo M, Woodward TA, et al. CXC-chemokine/CXCR2 biological axis promotes angiogenesis in vitro and in vivo in pancreatic cancer. Int J Cancer. 2009;125:1027–1037. doi: 10.1002/ijc.24383. [DOI] [PubMed] [Google Scholar]
- 151.Wente MN, Keane MP, Burdick MD, et al. Blockade of the chemokine receptor CXCR2 inhibits pancreatic cancer cell-induced angiogenesis. Cancer Lett. 2006;241:221–227. doi: 10.1016/j.canlet.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 152.Matsuo Y, Ochi N, Sawai H, et al. CXCL8/IL-8 and CXCL12/SDF-1alpha co-operatively promote invasiveness and angiogenesis in pancreatic cancer. Int J Cancer. 2009;124:853–861. doi: 10.1002/ijc.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gao Z, Wang X, Wu K, Zhao Y, Hu G. Pancreatic stellate cells increase the invasion of human pancreatic cancer cells through the stromal cell-derived factor-1/CXCR4 axis. Pancreatology. 2010;10:186–193. doi: 10.1159/000236012. [DOI] [PubMed] [Google Scholar]
- 154.Wang Z, Ma Q, Liu Q, et al. Blockade of SDF-1/CXCR4 signalling inhibits pancreatic cancer progression in vitro via inactivation of canonical Wnt pathway. Br J Cancer. 2008;99:1695–1703. doi: 10.1038/sj.bjc.6604745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wehler T, Wolfert F, Schimanski CC, et al. Strong expression of chemokine receptor CXCR4 by pancreatic cancer correlates with advanced disease. Oncol Rep. 2006;16:1159–1164. [PubMed] [Google Scholar]
- 156.Liang JJ, Zhu S, Bruggeman R, et al. High levels of expression of human stromal cell-derived factor-1 are associated with worse prognosis in patients with stage II pancreatic ductal adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2010;19:2598–2604. doi: 10.1158/1055-9965.EPI-10-0405. [DOI] [PubMed] [Google Scholar]
- 157.Sung B, Jhurani S, Ahn KS, et al. Zerumbone down-regulates chemokine receptor CXCR4 expression leading to inhibition of CXCL12-induced invasion of breast and pancreatic tumor cells. Cancer Res. 2008;68:8938–8944. doi: 10.1158/0008-5472.CAN-08-2155. [DOI] [PubMed] [Google Scholar]
- 158.Wente MN, Mayer C, Gaida MM, et al. CXCL14 expression and potential function in pancreatic cancer. Cancer Lett. 2008;259:209–217. doi: 10.1016/j.canlet.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 159.Wente MN, Gaida MM, Mayer C, et al. Expression and potential function of the CXC chemokine CXCL16 in pancreatic ductal adenocarcinoma. Int J Oncol. 2008;33:297–308. [PubMed] [Google Scholar]
- 160.Singh S, Srivastava SK, Bhardwaj A, Owen LB, Singh AP. CXCL12-CXCR4 signalling axis confers gemcitabine resistance to pancreatic cancer cells: a novel target for therapy. Br J Cancer. 2010;103:1671–1679. doi: 10.1038/sj.bjc.6605968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Okudaira K, Tsuzuki Y, Hokari R, et al. Effects of intratumoral injection of CCL2 on monocyte-endothelial cell interactions in mouse pancreatic cancer. Microcirculation. 2007;14:241–251. doi: 10.1080/10739680601139393. [DOI] [PubMed] [Google Scholar]
- 162.Turnquist HR, Lin X, Ashour AE, et al. CCL21 induces extensive intratumoral immune cell infiltration and specific anti-tumor cellular immunity. Int J Oncol. 2007;30:631–639. [PubMed] [Google Scholar]