Abstract
Background
Hydroxyapatite (HA) coatings composed with bisphosphonates (BPs) which have high mineral-binding affinities have been confirmed to successfully enhance implant stability. However, few previous studies focused on HA coatings composed with low-affinity BPs or on systemic effects of locally released BPs.
Methods
In this long-term study, we developed two kinds of BP-HA composite coatings using either high-affinity BP (alendronate, ALN) or low-affinity BP (risedronate, RIS). Thirty-six rabbits were divided into three groups according to different coating applications (group I: HA, group II: ALN-HA, and group III: RIS-HA). Implants were inserted into the proximal region of the medullary cavity of the left tibiay. At insertion, 2 × 108 wear particles were injected around implants to induce a peri-implant high bone turnover environment. Both local (left tibias) and systemic (right tibias and lumbar vertebrae) inhibitory effect on bone resorption were compared, including bone-implant integration, bone architecture, bone mineral density (BMD), implant stability, and serum levels of bone turnover markers.
Results
The results indicated that ALN-HA composite coating, which could induce higher bone-implant contact (BIC) ratio, bone mass augmentation, BMD, and implant stability in the peri-implant region, was more potent on peri-implant bone, while RIS-HA composite coating, which had significant systemic effect, was more potent on non-peri-implant bone, especially lumbar vertebrae.
Conclusions
It is instructive and meaningful to further clinical studies that we could choose different BP-HA composite coatings according to the patient’s condition.
Background
As the most effective surgical procedure for treating severe arthritis and other joint-related diseases, the number of total joint arthroplasty (TJA) increases steadily every year. Thus wear debris-induced aseptic loosening and subsequent revision surgery may be unavoidable, especially for young patients.
Considerable studies have focused on preventing aseptic loosening and enhancing implant stability in revision surgeries. BPs are a promising class of widely used drugs for implant stability due to their inhibitory effects on osteoclasts [1-6].
BPs have a P-C-P structure and two side-chains (R1 and R2). Their mineral-binding affinities are mainly influenced by R1 side-chain. In addition, R2 side-chain, three-dimensional (3D) conformation, and the orientation of the nitrogen also play a role [3]. Nancollas et al.[7] established a rank order of their mineral- binding affinities: zoledronate (ZOL) > ALN > ibandronate > RIS > etidronate > clodronate. The anti-resorptive potency is mainly influenced by R2 side-chain, and the rank order is: ZOL > RIS > ibandronate > ALN > pamidronate, which closely matches the order of potency on inhibiting farnesyl pyrophosphate synthase (FPPS) [3,8-10].
Due to the different affinities and anti-resorptive potencies, systemically administrated high-affinity BPs (ALN and ZOL) are effective on vertebral fractures, while low-affinity BPs (RIS) are effective on all fracture types, especially non-vertebral fractures [3,11]. The positive effects of BPs on bone-implant integration have been widely studied by being administrated either systemically (oral, intravenous or subcutaneous) [12-15] or locally (peri-implant injection or composite coating delivery) [1,2,4,5,16-22].
Peri-prosthetic high bone turnover is the major pathological response to aseptic loosening, so local BPs treatment may be preferable for local high bone turnover-related diseases. Digestive ulcer [23] and osteonecrosis of the jaw [24] are the major side effects caused by BPs treatment. High oral or intravenous dose is considered to be the major cause of these side effects. Because of the mineral-binding selectivity [25] and poor oral bioavailability [8] of BPs, most BPs will be consumed by non-peri-implant bone which has the highest turnover rate [25], and be excreted via urine [8]. Thus, patients have to take more BPs than their bone really need if they take BPs systemically. Oppositely, if BPs are administrated locally, the unnecessary consumption can be avoided, and we do not need to worry about their poor oral bioavailability. Therefore, lower drug dose than systemic treatment will be enough, and dose-depended side effects may be avoided. However, some problems should be avoided if BPs are administrated locally that local high drug concentration and massive bone compaction may impair bone-implant integration [26,27].
Recently, some studies focused on the BP-HA composite coatings to prevent aseptic loosening. Suratwala et al.[4] developed a ZOL-HA composite coating, which enhanced the peri-implant bone quality and implant stability in rats with aseptic loosening. However, as we know, revision patients are averagely older than the primary patients that they have higher incidence rate of systemic osteopenia or osteoporosis. Therefore, in addition to enhancing implant stability, it is better for the composite coatings to have systemic effects of bone mass augmentation and BMD increment which may benefit the recovery for revision patients. However, none of previous studies investigated or compared the systemic effects of locally released high-/low-affinity BPs.
In this long-term study, we developed two kinds of BP-HA composite coatings using either high-affinity BP (ALN) or low-affinity BP (RIS) in rabbits with peri-implant high bone turnover rate. Both local and systemic inhibitory effects on bone resorption were compared. We hypothesised that the ALN-HA composite coating is more effective on peri-implant bone while RIS-HA composite coating has more obvious systemic effects and is more effective on non-peri-implant bone.
Methods
Experimental design
Thirty-six male New Zealand white rabbits (3.00 ± 0.20 kg) were divided into three groups. The implant was inserted into the proximal region of the medullary cavity of the left tibia, and particles were injected around the implant to induce a peri-implant high bone turnover environment. In group I (n = 12), HA-coated implants were not composed with BPs. In group II (n = 12), HA-coated implants were composed with ALN. In group III (n = 12), HA-coated implants were composed with RIS. The study lasted for 24 weeks, and every 12 weeks six animals from each group were killed.
Particles and implants
Commercial pure ultra-high molecular weight polyethylene (UHMWPE) particles (average 1.74 ± 1.43 μm, range 0.05-11.06 μm) were obtained from the manufacturer (Ceridust VP 3610, Clariant, Germany). Detailed parameters have been described by von Knoch et al.[28]. Before injection, the particles were tested using a quantitative limulus amebocyte lysate assay to assure that the endotoxin level was lower than 0.25 EU/mL.
Titanium alloy rods (Ti6Al4V, 2.5-mm diameter and 45-mm length) were plasma spray coated with HA (coating thickness = 30 μm, Ca/P = 1.67, Biomaterial Centre of Sichuan University, Sichuan, China).
To prepare the BP-HA composite coatings, 100-μg of ALN or 50-μg of RIS (potency is equivalent to 100-μg of ALN) was dissolved in 100-μL of distilled water at 60 °C. The solution was evenly dropped onto the surface of an HA coating using a micropipette, and the coating was entirely covered by the solution. Because 100-μL of solution could not be absorbed by the HA coating at once (see Additional file 1), we rotated the implant (90 degrees) every 5 minutes until there was no dripping on the implant, and then the implant was dried at 50 °C for 24 h and sterilised with gamma irradiation. Therefore, the homogenous distribution of the BP solution on the implant surface could be guaranteed.
The doses were referred to previous studies done by other authors [15] and us (in press). Under these low initial doses, peri-implant drug concentration could keep at a low level for a long period that locally released BPs could inhibit osteoclast activity without inhibiting osteoblast activity [4-6]. 100-μg/50-μg of locally administrated ALN/RIS in a rabbit equates approximately to 50-mg/25-mg of orally administrated ALN/RIS in an adult, which are lower than their weekly doses.
Animals and surgery
Animals were anaesthetised with an intramuscular injection of ketamine (35 mg/kg) and xylazine (5 mg/kg). After an incision was made to the left knee joint, a 2.5-mm diameter bone tunnel was drilled through the tibia plateau into the medullary cavity. Then 2 × 108 UHMWPE particles (suspended in 200-μL of saline) were injected around implants to induce a high bone turnover environment. Buprenorphine (0.04 mg/kg) was administered subcutaneously every 6–12 h for two days after surgery for postoperative pain control. Unrestricted activity was allowed after surgery. Antibiotics were administered 1 day preoperatively and 3 days postoperatively. On days 14 and 4 before being killed, the animals received tetracycline (25 mg/kg) injections to label the newly formed bone.
After sacrifice, bilateral tibias and lumbar vertebrae (L2 and L3) were retrieved and fixed in 70% ethanol for 7 days. After fixation of the tibias, serial cross-sections (Figure 1) were cut using a low speed saw (IsoMet, Buehler LTD, Lake Bluff, IL). An 8-mm-thick cross-section (section A) was cut 5-mm below the proximal epiphyseal growth plate for dynamic histomorphometric evaluation, and a 7-mm-thick cross-section (section B) was cut for micro-CT scanning and push-out test. The protocol was approved by our Institutional Animal Care and Use Committee.
Figure 1.
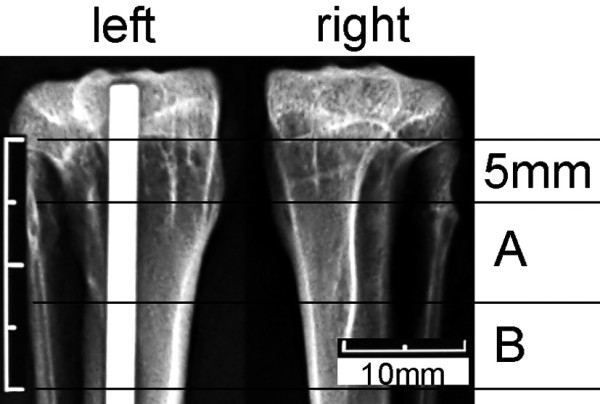
A radiograph of bilateral tibias (group I) retrieved at week 12 postoperatively. Levels (A and B) of the consecutive cross-section specimens.
Histomorphometry
Dynamic bone histomorphometry. Section A of bilateral tibias (Figure 1) and the L2 vertebrae were embedded in methylmethacrylate and two serial 15-μm thick slices of each section were made using a hard tissue microtome (Leica SP1600, Leica, Nussloch, Germany). They were processed for toluidine blue staining and fluorescence microscopy. The parameters were expressed in accordance with the American Society of Bone and Mineral Research (ASBMR) nomenclature, viz., bone formation rate (BFR/BV) and mineral apposition rate (MAR).
Static bone histomorphometry. The BIC was defined as the ratio of the implant surface covered with bone, and it was measured using toluidine blue-stained slices. Section B of bilateral tibias (Figure 1) and the L3 vertebrae were scanned using an eXplore Locus SP micro-CT system (GE Healthcare, Healthcare, Milwaukee, WI). The scanning protocol was set at 80 kV and 80 μA with an exposure time of 3000 ms and the resolution of 21 × 21 × 21 μm. The scanning protocol was referred to previous studies [19,20], the manual instruction of the micro-CT system, and our previous study (in press). This protocol can minimise the influence of scanning artifacts induced by metal implants. The image data were analysed using GEHC MicroView software to measure BMD and static bone histomorphometric parameters, viz., bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and structure model index (SMI). Micro-CT was used to measure the static histomorphometric parameters because it is based on 3D imaging, which can reduce measurement errors.
Push-out test
Following micro-CT scanning, the biomechanical properties of section B of the left tibias were analysed using an Autograph AGS-J (SHIMADZU, Japan) universal test machine. The whole specimen was placed vertically on a metal jig with a central opening, and the implant was pushed out of the bone. A preload of 2 N defined the start of the test, and the pushing speed was 0.5 mm/min. During the pushing period, load–displacement curves were generated. The detailed protocol was implemented according to the studies by Dhert et al.[29] and Jakobsen et al.[16]. The maximum force (MF) was defined as the peak value of the load–displacement curve, and apparent shear stiffness (ASS) and total energy absorption (TEA) were defined as the slope of the linear section of the curve and the area under the curve until failure, respectively.
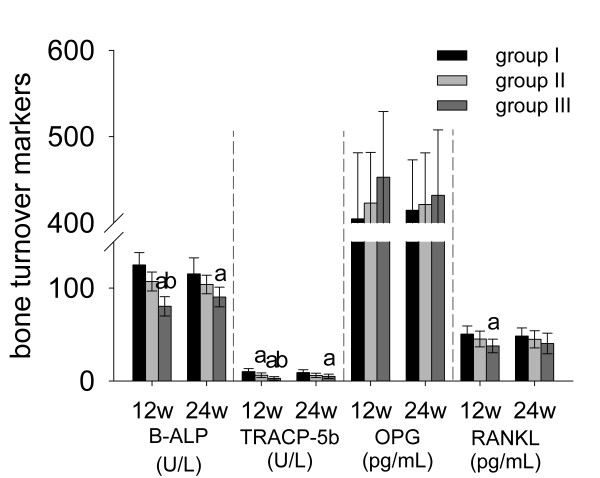
Bone turnover markers
Serum levels of B-ALP (ADL, USA), TRACP-5b (SBA Sciences, Finland), OPG and RANKL (R&D systems, USA) were measured with enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturers' instructions at weeks 12 and 24 post-operatively. The intra- and inter-assay CVs for each assay were lower than 7.6% and 5.3% (B-ALP), 8.0% and 6.5% (TRACP-5b), and 6.7% and 5.5% (OPG and RANKL), respectively. All the samples were tested in duplicate.
Statistical analysis
One-way ANOVA and Student-Newman-Keuls (SNK-q) tests were performed for multiple comparisons among all groups. A p value less than 0.05 was considered significant, and data were expressed as means ± standard deviations (SDs).
Results
No complications occurred during the experimental period.
Bone-implant contact
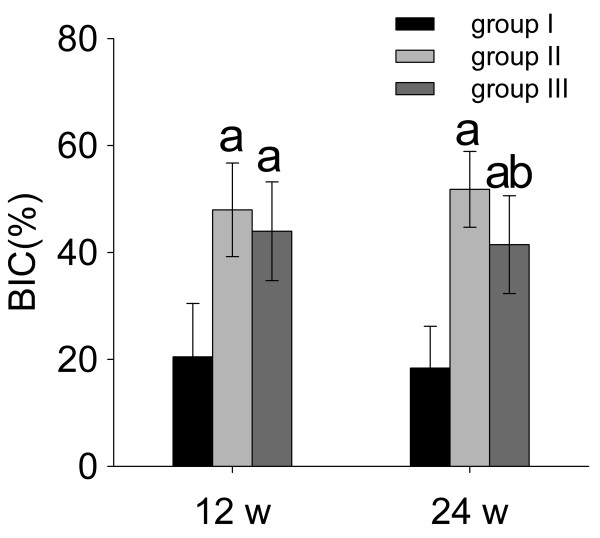
As shown in Figure 2, both ALN- and RIS-HA composite coatings could increase BIC ratios significantly at each time point (p < 0.05). At week 24, ALN (BIC = 51.82 ± 7.09%) showed stronger osteo-integrating effect compared to RIS (BIC = 41.46 ± 9.15%, p < 0.05).
Figure 2.
Bone-implant contact curves. a: significant vs. group I, b: significant vs. group II. Data were expressed as means ± SDs. BIC: bone-implant contact.
Bone histomorphometry
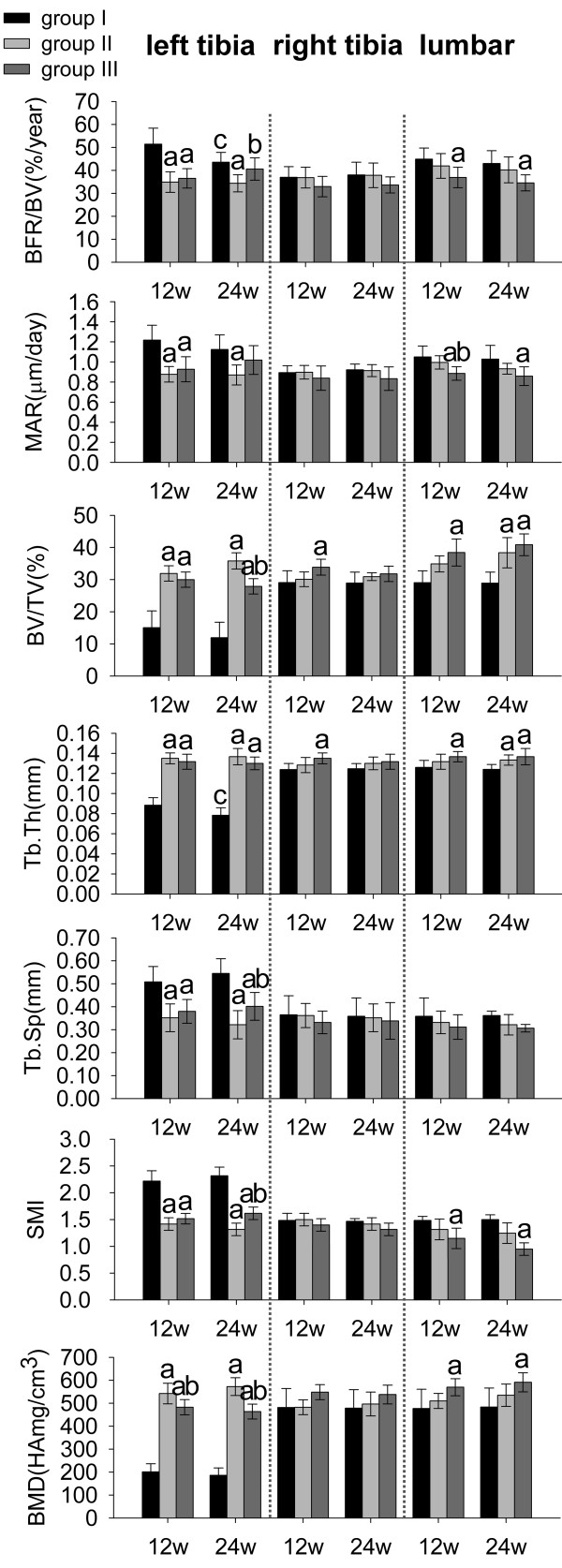
Dynamic bone histomorphometry. As shown in Figure 3, both ALN and RIS reduced peri-implant (left tibia) BFR/BV (−32% and −29%) and MAR (−28% and −24%) significantly at week 12. However, only ALN reduced peri-implant BFR/BV (−21%) and MAR (−22%) significantly at week 24 (p < 0.05). Although RIS treatment resulted in the lowest BFR/BV and MAR in right tibias, it showed no significant differences between three groups. Only RIS reduced BFR/BV (−18% at week 12 and −20% at week 24) and MAR (−15% at week 12 and −17% at week 24) significantly in lumbar vertebrae (p < 0.05).
Figure 3.
Histomorphometric parameters and bone mineral density. a: significant vs. group I, b: significant vs. group II, c: within-group significant vs. week 12. Data were expressed as means ± SDs. BFR/BV: bone formation rate, MAR: mineral apposition rate, BV/TV: bone volume fraction, Tb.Th: trabecular thickness, Tb.Sp: trabecular separation, SMI: structure model index.
Static bone histomorphometry. As shown in Figure 3, both ALN and RIS treatment resulted in significantly higher peri-implant BV/TV (+112% and +99% at week 12, +199% and +133% at week 24, respectively) and Tb.Th (+53% and +50% at week 12, +76% and +67% at week 24, respectively) and lower Tb.Sp (−31% and −25% at week 12, -42% and −27% at week 24, respectively) and SMI (−36% and −32% at week 12, -43% and −30% at week 24, respectively) compared to the control group at each time point (p < 0.05), and ALN was more effective than RIS. However, ALN did not have significant effect in right tibias. Compared to the other groups, RIS treatment resulted in the highest BV/TV and Tb.Th and the lowest Tb.Sp and SMI in right tibias and lumbar vertebrae at each time point. However, the effect of RIS became insignificant in right tibias at week 24.
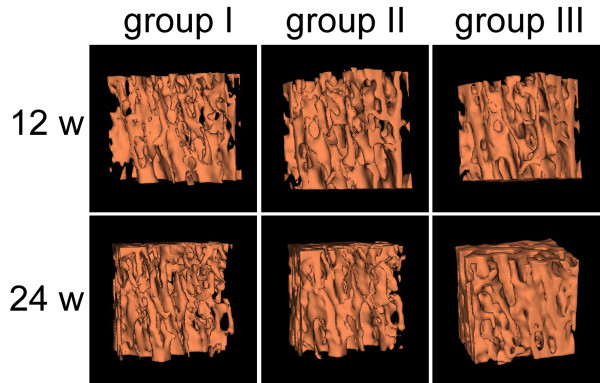
Micro-CT scanning (Figure 4) showed obvious lumbar vertebral bone mass augmentation in RIS-treated animals, and these trabecular bones tended to be plate-like in structure. However, ALN did not have the equivalent effect on lumbar vertebrae.
Figure 4.
Bone architecture of L3 vertebrae reconstructed by micro-CT at weeks 12 and 24 postoperatively. RIS could induce higher lumbar vertebral bone mass augmentation compared to ALN.
Bone mineral density
As shown in Figure 3, both ALN and RIS treatment resulted in significant peri-implant BMD augmentation, and ALN was more effective than RIS (+12% at week 12 and +24% at week 24, p < 0.05). Although RIS treatment resulted in the highest BMD augmentation in right tibias, the effect of neither ALN nor RIS was significant. In lumbar vertebrae, only RIS treatment resulted in significant higher BMD augmentation than the control group (p < 0.05).
Implant stability
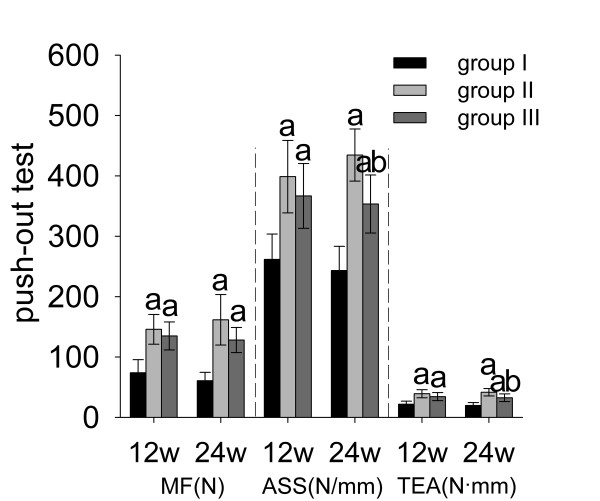
As shown in Figure 5, both ALN and RIS improved implant stability significantly at each time point (p < 0.05). It was more obvious in ALN-treated animals at week 24 that ALN treatment resulted in higher ASS (+19%) and TEA (+13%) at week 24 compared to RIS treatment (p < 0.05).
Figure 5.
Push-out test. a: significant vs. group I, b: significant vs. group II. Data were expressed as means ± SDs. MF: maximum force, ASS: apparent shear stiffness, TEA: total energy absorption.
Bone turnover markers
As shown in Figure 6, serum levels of both B-ALP (−36% at week 12 and −22% at week 24) and TRACP-5b (−77% at week 12 and −45% at week 24) reduced significantly in group III at each time point and it was more obvious at week 12 than week 24. However, ALN could only reduce TRACP-5b (−38%) significantly at week 12 (p < 0.05). Serum levels of OPG and RANKL showed no significant differences between three groups except the significantly reduced serum level of RANKL (−25%) by RIS treatment at week 12.
Figure 6.
The level of bone turnover makers measured by ELISA. a: significant vs. group I, b: significant vs. group II. Data were expressed as means ± SDs.
Discussion
Locally released BP from either ALN- or RIS-HA composite coating could induce peri-implant bone mass augmentation, BMD increment, and the improvement of bone architecture, which significantly improved the bone-implant integration (BIC increment), at each time point (Figures 2, 3 (left tibia) and 4).
Early bone-implant integration that can provide a sealed interface is important to implant stability because it can inhibit the migration of debris and cytokines [13,30]. In addition, implant stability is also influenced by peri-implant bone architecture. Architecturally compromised trabecular bone will transmit a lesser load to the cortical bone. Thus, stress will concentrate at the bone-implant interface, which ultimately results in implant loosening. Rod-like trabecular bone (SMI = 3) had poorer biomechanical properties than plate-like trabecular bone (SMI = 0). After the particle stimulation, SMI of the peri-implant bone was higher than 2.0 (Figure 3, SMI, left tibia, group I). However, locally released BP from BP-HA composite coatings significantly reduced SMI to almost 1.5 (Figure 3, SMI, left tibia, groups II and III) and led to higher implant stability, which was confirmed by the results of push-out test (Figure 5).
It was obvious that ALN was more potent than RIS on peri-implant bone due to their different mineral-binding affinities [8], especially at week 24. ALN has high mineral-binding affinity and intermediate inhibitory potency on FPPS. It can keep a stable concentration in peri-implant bone and will hardly be delivered far away. Therefore, previous studies [1,2,4,12-20,31,32] on enhancing bone-implant integration almost focused on such high-affinity BPs. Oppositely, RIS has low mineral-binding affinity and high inhibitory effect on FPPS [7]. Such low-affinity BPs will be delivered to the entire osteocyte network through the canalicular compartment by extracellular fluid [3] that effective peri-implant drug concentration may not be sustained for a long lime. Therefore it is used for treating non-vertebral fractures but not for enhancing bone-implant integration [3,11].
However, as we know, revision patients are averagely older than the primary patients that they have a higher incidence rate of systemic osteopenia or osteoporosis. Therefore, in addition to enhancing implant stability, it is better for the composite coatings to have systemic effects of bone mass augmentation and BMD increment which may benefit the recovery of revision patients. However, the HA coating composed with high-affinity BPs may not fit the needs as Jakobsen et al.[16] confirmed that locally released ALN did not have systemic effects. Thus, we should re-consider the possibility of using low-affinity BPs as coating materials.
The histomorphometric and BMD results of the contralateral tibias and lumbar vertebrae (Figure 3) indicated that RIS-HA composite coating could induce bone mass augmentation and BMD increment significantly in non-peri-implant region, especially lumbar vertebrae. Oppositely, ALN had very limited systemic effects. This difference suggests that low-affinity BPs are more systemically effective than high-affinity BPs. Additionally, the variations of serum cytokine levels can reflect their systemic effects more directly and precisely compared to histomorphometric parameters. The significant lower serum concentrations of B-ALP, TRACP-5b and RANKL in group III than in groups I and II at week 12 also supported our histomorphometric results (Figure 6). The effects of RIS were more pronounced in lumbar vertebrae than contralateral tibias (Figure 3), because BPs tend to bind with the bone which has the highest turnover rate [25]. Kimmel et al.[33] confirmed that lumbar vertebrae and proximal humerus have the highest turnover rates.
Local BP administration (BP-HA composite coating) is the best way for enhancing implant stability and preventing aseptic loosening. Because of the binding selectivity of BPs, they will almost concentrate in the bone with the highest turnover rate, such as the lumbar vertebrae, but not in the peri-implant bone if they are administrated systemically. The drug potency is then diminished. Especially in the osteoporosis patients who are usually in the state of systemic high bone turnover, BP is more likely to be consumed by non-peri-implant bone. Additionally, local administration has many advantages such as lower initial dosage, higher peri-implant drug concentration and fewer side effects compared to systemic administration.
For inhibiting peri-implant bone resorption, low-affinity BPs have some limitations that peri-implant drug concentration may not sustain for a long time and their effects on peri-implant bone will become weaker than high-affinity BPs. In our study, it is obvious that BIC, BV/TV, Tb.Sp, SMI, ASS, and TEA in group III became significantly worse than in group II at week 24, and BFR/BV and MAR showed no significant differences compared to the control group (Figures 23 (left tibia) and 5). Therefore, low-affinity BP alone is not suitable for revision patients who suffer from osteoporosis simultaneously. Composing HA with both high- and low-affinity BPs may be feasible. Recently, Abtahi et al.[21] composed both high- (pamidronate) and low-affinity (ibandronate) BPs to the fibrinogen matrix as the coating material, and each oral implant was then screwed into the upper jaw in five patients. The results indicated that this composite coating could successfully improve implant stability. However, they did not explain why they composed two kinds of BPs, and they did not compare it with single BP composed coatings.
The mechanism by which BPs released from either pure HA in vitro or bone in vivo are crucial to further studies on composite HA coatings. In addition to different mineral-binding affinities, the release duration of BPs also depends on the bone remodelling activity that BPs will be consumed more rapidly in the sites with high turnover rates. Thus, for further evaluating peri-implant concentration and the release duration of BP-HA composite coatings, detecting drug concentration in plasma or bone is meaningful. Tanzer et al.[1] used a spectrophotometric method [1,34] to investigate the in vitro release characteristic of ZOL from pure HA but it cannot precisely reflect the release duration in vivo, because in addition to chemical desorption, osteoclastic resorption also plays an important role. Legay et al.[35] developed a highly sensitive radioimmunoassay (RIA) method to detect ZOL concentration, and the limit of quantification was 0.4 ng/mL in plasma and 5 ng/mL in urine, respectively. Yun et al.[36] developed a high-performance liquid chromatography (HPLC) method to detect ALN concentration, and the limit of quantification was 1 ng/mL in plasma. These methods are not sensitive enough for our study because the doses of ALN and RIS were 100-μg and 50-μg, respectively, per implant, that the drug concentration was too low to detect, especially in plasma. Recently, Stadelmann et al.[22] developed a novel mathematic-based modelling method for evaluating peri-implant BP concentration and bone density. Using this method, they found the optimal coating dose of ZOL per implant. All these efforts mentioned above will help us further realise the characteristic of BP-HA composite coatings.
The results of this study are instructive and meaningful to further clinical studies that we could choose different BP-HA composite coatings according to the patient’s condition. In brief: 1) In primary TJA patients without systemic high bone turnover-related diseases (osteopenia or osteoporosis), we can prefer ordinary non-BP-composed prostheses; 2) In primary TJA patients with systemic high bone turnover-related diseases, we can prefer low-affinity BP-composed prostheses. Their significant systemic effects may cooperate with the BP which is systemically administrated. The systemic bone mass augmentation and BMD increment can result in earlier movement and shorter recovery time for patients; 3) In revision patients without systemic high bone turnover-related diseases, we can prefer high-affinity BP-composed prostheses which can significantly reduce peri-implant high bone turnover rate and improve implant stability due to the stable peri-implant drug concentration and long release duration; 4) In revision patients with systemic high bone turnover-related diseases, the HA coating composed with both high- and low-affinity BPs may be preferable, in addition to improving implant stability, it can result in systemic bone mass augmentation and BMD increment.
Nevertheless, our study has some limitations. Firstly, the homogenous distribution of the BP solution on the implant surface could be guaranteed due to the implant size and the BP solution volume in our study. However, in further studies, if the implant is bigger or the solution amount is less, the homogenous distribution may not be guaranteed. Therefore, other loading methods like spraying may be possible if the drug amount can be controlled at the same time. Secondly, we compared local and systemic inhibitory effects on bone resorption only between high- and low-affinity BPs. In further studies, an additional group that the HA coating is composed with both high- and low-affinity BPs should be added and an appropriate ratio of high-/low-affinity BPs should be investigated. Finally, the animal model used in this study was to induce peri-implant (local) high bone turnover. In further studies, these composite coatings should be studied in osteoporosis models which have systemic high bone turnover rate.
Conclusions
In this long-term study, we developed two kinds of BP-HA composite coatings using either high- or low-affinity BPs in rabbits. The results indicated that ALN-HA composite coating was more effective on peri-implant bone, while RIS-HA composite coating was more effective on non-peri-implant bone, especially lumbar vertebrae. It is instructive and meaningful to further clinical studies that we could choose different BP-HA composite coatings according to the patient’s condition.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SN and XC participated in the experimental design, surgery process, histomorphometric examinations and push-out tests. YZ was responsible for animal care and performed the ELISA assays and statistical analysis. This study and its design were conceived by QZ, JZ, and PZ, who coordinated the project, participated in the experimental design. All authors contributed to the drafting of the manuscript, and have read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
BP-HA composite coating. The top figure shows the HA-coated implant which has not been loaded, and we can see the HA coating is dry. After loading with 100-μL of BP solution, as shown in the bottom figure, the implant surface is entirely covered with the solution. We rotated the implants (90 degrees) every 5 minutes until there was no dripping (arrow) on the implant surface.
Contributor Information
Shun Niu, Email: rococelly@yahoo.com.cn.
Xiaorui Cao, Email: caoxr528@sohu.com.
Yan Zhang, Email: zhangyanhappy@yahoo.com.cn.
Qingsheng Zhu, Email: xijingbone@yahoo.com.cn.
Jinyu Zhu, Email: xijingbone@yahoo.com.cn.
Ping Zhen, Email: zhenping@yahoo.cn.
Acknowledgments
The authors wish to thank Dr. Ernst Krendlinger and Dr. Veronika Stegmair (Clariant Produkte, Germany) for their generous contribution of Ceridust VP 3610 particles.
References
- Tanzer M, Karabasz D, Krygier JJ, Cohen R, Bobyn JD. Bone Augmentation around and within Porous Implants by Local Bisphosphonate Elution. Clin Orthop Relat Res. 2005;441:30–39. doi: 10.1097/01.blo.0000194728.62996.2d. [DOI] [PubMed] [Google Scholar]
- Peter B, Gauthier O, Laïb S, Bujoli B, Guicheux J, Janvier P, van Lenthe GH, Müller R, Zambelli PY, Bouler JM, Pioletti DP. Local delivery of bisphosphonate from coated orthopedic implants increases implants mechanical stability in osteoporotic rats. J Biomed Mater Res A. 2006;76:133–143. doi: 10.1002/jbm.a.30456. [DOI] [PubMed] [Google Scholar]
- Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19:733–759. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- Suratwala SJ, Cho SK, van Raalte JJ, Park SH, Seo SW, Chang SS, Gardner TR, Lee FY. Enhancement of periprosthetic bone quality with topical hydroxyapatite-bisphosphonate composite. J Bone Joint Surg. 2008;90:2189–2196. doi: 10.2106/JBJS.G.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucheux C, Verron E, Soueidan A, Josse S, Arshad MD, Janvier P, Pilet P, Bouler JM, Bujoli B, Guicheux J. Controlled release of bisphosphonate from a calcium phosphate biomaterial inhibits osteoclastic resorption in vitro. J Biomed Mater Res A. 2009;89:46–56. doi: 10.1002/jbm.a.31989. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Chen SM, Chen CH, Wang CK, Wang GJ, Chang JK, Ho ML. The effect of the local delivery of alendronate on human adipose-derived stem cell-based bone regeneration. Biomaterials. 2010;31:8674–8683. doi: 10.1016/j.biomaterials.2010.07.096. [DOI] [PubMed] [Google Scholar]
- Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, Wu W, Mangood A, Russell RG, Ebetino FH. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone. 2006;38:617–627. doi: 10.1016/j.bone.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Lin JH. Bisphosphonates: a review of their pharmacokinetic properties. Bone. 1996;18:75–85. doi: 10.1016/8756-3282(95)00445-9. [DOI] [PubMed] [Google Scholar]
- Ebetino FH, Francis MD, Rogers MJ. Mechanisms of action of etidronate and other bisphosphonates. Rev Contemp Pharmacother. 1998;9:233–243. [Google Scholar]
- Luckman SP, Coxon FP, Ebetino FH, Russell RG, Rogers MJ. Hetero-cycle containing bisphosphonates cause apoptosis and inhibit bone resorption by preventing protein prenylation: evidence from structure-activity relationships in J774 macrophages. J Bone Miner Res. 1998;13:1668–1678. doi: 10.1359/jbmr.1998.13.11.1668. [DOI] [PubMed] [Google Scholar]
- Cranney A, Wells GA, Yetisir E, Adami S, Cooper C, Delmas PD, Miller PD, Papapoulos S, Reginster JY, Sambrook PN, Silverman S, Siris E, Adachi JD. Ibandronate for the prevention of nonvertebral fractures: a pooled analysis of individual patient data. Osteoporos Int. 2009;20:291–297. doi: 10.1007/s00198-008-0653-8. [DOI] [PubMed] [Google Scholar]
- Iwase M, Kim KJ, Kobayashi Y, Itoh M, Itoh T. A novel bisphosphonate inhibits inflammatory bone resorption in a rat osteolysis model with continuous infusion of polyethylene particles. J Orthop Res. 2002;20:499–505. doi: 10.1016/S0736-0266(01)00155-3. [DOI] [PubMed] [Google Scholar]
- Millett PJ, Allen MJ, Bostrom MP. Effects of alendronate on particle-induced osteolysis in a rat model. J Bone Joint Surg. 2002;84:236–249. doi: 10.2106/00004623-200202000-00011. [DOI] [PubMed] [Google Scholar]
- Wise LM, Waldman SD, Kasra M, Cheung R, Binnington A, Kandel RA, White LM, Grynpas MD. Effect of zoledronate on bone quality in the treatment of aseptic loosening of hip arthroplasty in the dog. Calcif Tissue Int. 2005;77:367–375. doi: 10.1007/s00223-005-0062-3. [DOI] [PubMed] [Google Scholar]
- von Knoch F, Eckhardt C, Alabre CI, Schneider E, Rubash HE, Shanbhag AS. Anabolic effects of bisphosphonates on peri-implant bone stock. Biomaterials. 2007;28:3549–3559. doi: 10.1016/j.biomaterials.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Jakobsen T, Kold S, Bechtold JE, Elmengaard B, Søballe K. Local alendronate increases fixation of implants inserted with bone compaction: 12-week canine study. J Orthop Res. 2007;25:432–441. doi: 10.1002/jor.20276. [DOI] [PubMed] [Google Scholar]
- Peter B, Pioletti DP, Laïb S, Bujoli B, Pilet P, Janvier P, Guicheux J, Zambelli PY, Bouler JM, Gauthier O. Calcium phosphate drug delivery system: influence of local zoledronate release on bone implant osteointegration. Bone. 2005;36:52–60. doi: 10.1016/j.bone.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Stadelmann VA, Gauthier O, Terrier A, Bouler JM, Pioletti DP. Implants delivering bisphosphonate locally increase periprosthetic bone density in an osteoporotic sheep model. A pilot study. Eur Cell Mater. 2008;16:10–16. doi: 10.22203/ecm.v016a02. [DOI] [PubMed] [Google Scholar]
- Gao Y, Luo E, Hu J, Xue J, Zhu S, Li J. Effect of combined local treatment with zoledronic acid and basic fibroblast growth factor on implant fixation in ovariectomized rats. Bone. 2009;44:225–232. doi: 10.1016/j.bone.2008.10.054. [DOI] [PubMed] [Google Scholar]
- Gao Y, Zou S, Liu X, Bao C, Hu J. The effect of surface immobilized bisphosphonates on the fixation of hydroxyapatite-coated titanium implants in ovariectomized rats. Biomaterials. 2009;30:1790–1796. doi: 10.1016/j.biomaterials.2008.12.025. [DOI] [PubMed] [Google Scholar]
- Abtahi J, Tengvall P, Aspenberg P. Bisphosphonate coating might improve fixation of dental implants in the maxilla: a pilot study. Int J Oral Maxillofac Surg. 2010;39:673–677. doi: 10.1016/j.ijom.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Stadelmann VA, Terrier A, Gauthier O, Bouler JM, Pioletti DP. Prediction of bone density around orthopedic implants delivering bisphosphonate. J Biomech. 2009;42:1206–1211. doi: 10.1016/j.jbiomech.2009.03.024. [DOI] [PubMed] [Google Scholar]
- Elliott SN, McKnight W, Davies NM, MacNaughton WK, Wallace JL. Alendronate induces gastric injury and delays ulcer healing in rodents. Life Sci. 1998;62:77–91. doi: 10.1016/s0024-3205(97)01040-0. [DOI] [PubMed] [Google Scholar]
- Rizzoli R, Burlet N, Cahall D, Delmas PD, Eriksen EF, Felsenberg D, Grbic J, Jontell M, Landesberg R, Laslop A, Wollenhaupt M, Papapoulos S, Sezer O, Sprafka M, Reginster JY. Osteonecrosis of the jaw and bisphosphonate treatment for osteoporosis. Bone. 2008;42:841–847. doi: 10.1016/j.bone.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Gonnelli S, Cepollaro C, Pondrelli C, Martini S, Montagnani A, Monaco R. Gennari: Bone turnover and the response to alendronate treatment in postmenopausal osteoporosis. Calcif Tissue Int. 1999;65:359–364. doi: 10.1007/s002239900713. [DOI] [PubMed] [Google Scholar]
- Back DA, Pauly S, Rommel L, Haas NP, Schmidmaier G, Wildemann B, Greiner SH. Effect of local zoledronate on implant osseointegration in a rat model. BMC Musculoskelet Disord. 2012;22(13):42. doi: 10.1186/1471-2474-13-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen T, Baas J, Bechtold JE, Elmengaard B, Søballe K. Soaking morselized allograft in bisphosphonate can impair implant fixation. Clin Orthop Relat Res. 2007;463:195–201. doi: 10.1097/BLO.0b013e31813c6696. [DOI] [PubMed] [Google Scholar]
- von Knoch M, Sprecher C, Barden B, Saxler G, Löer F, Wimmer M. Size and shape of commercially available polyethylene particles for in-vitro and in-vivo-experiments. Z Orthop Ihre Grenzgeb. 2004;142:366–370. doi: 10.1055/s-2004-822589. [DOI] [PubMed] [Google Scholar]
- Dhert WJ, Verheyen CC, Braak LH, de Wijn JR, Klein CP, de Groot K, Rozing PM. A finite element analysis of the push-out test: influence of test conditions. J Biomed Mater Res. 1992;26:119–130. doi: 10.1002/jbm.820260111. [DOI] [PubMed] [Google Scholar]
- Rahbek O, Overgaard S, Lind M, Bendix K, Bunger C, Søballe K. Sealing effect of hydroxyapatite coating on peri-implant migration of particles. An experimental study in dogs. J Bone Joint Surg. 2001;83:441–447. doi: 10.1302/0301-620X.83B3.10667. [DOI] [PubMed] [Google Scholar]
- Perilli E, Le V, Ma B, Salmon P, Reynolds K, Fazzalari NL. Detecting early bone changes using in vivo micro-CT in ovariectomized, zoledronic acid-treated, and sham-operated rats. Osteoporos Int. 2010;21:1371–1382. doi: 10.1007/s00198-009-1082-z. [DOI] [PubMed] [Google Scholar]
- Wermelin K, Suska F, Tengvall P, Thomsen P, Aspenberg P. Stainless steel screws coated with bisphosphonates gave stronger fixation and more surrounding bone. Histomorphometry in rats. Bone. 2008;42:365–371. doi: 10.1016/j.bone.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Kimmel DB, Jee WS. A quantitative histologic study of bone turnover in young adult beagles. Anat Rec. 1982;203:31–45. doi: 10.1002/ar.1092030104. [DOI] [PubMed] [Google Scholar]
- Kuljanin J, Janković I, Nedeljković J, Prstojević D, Marinković V. Spectrophotometric determination of alendronate in pharmaceutical formulations via complex formation with Fe(III) ions. J Pharm Biomed Anal. 2002;28:1215–1220. doi: 10.1016/S0731-7085(02)00021-3. [DOI] [PubMed] [Google Scholar]
- Legay F, Gauron S, Deckert F, Gosset G, Pfaar U, Ravera C, Wiegand H, Schran H. Development and validation of a highly sensitive RIA for zoledronic acid, a new potent heterocyclic bisphosphonate, in human serum, plasma and urine. J Pharm Biomed Anal. 2002;30:897–911. doi: 10.1016/S0731-7085(02)00218-2. [DOI] [PubMed] [Google Scholar]
- Yun MH, Kwon KI. High-performance liquid chromatography method for determining alendronate sodium in human plasma by detecting fluorescence: application to a pharmacokinetic study in humans. J Pharm Biomed Anal. 2006;40:168–172. doi: 10.1016/j.jpba.2005.06.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
BP-HA composite coating. The top figure shows the HA-coated implant which has not been loaded, and we can see the HA coating is dry. After loading with 100-μL of BP solution, as shown in the bottom figure, the implant surface is entirely covered with the solution. We rotated the implants (90 degrees) every 5 minutes until there was no dripping (arrow) on the implant surface.