Abstract
Phorbol 12-myristate 13-acetate (PMA) increased 1,25(OH)2D3-induced human 25 hydroxyvitamin D-24 hydroxylase (hCYP24A1) gene expression and vitamin D receptor (VDR) binding to the hCYP24A1 promoter. It did not alter transient receptor potential cation channel, subfamily V, member 6 (TRPV6) expression, VDR binding to the TRPV6 promoter, or VDR binding to a crude chromatin preparation. PMA activated Extracellular signal-Regulated Kinases (ERK) 1/2 and p38 mitogen activated protein kinases (MAPK) and inhibiting these kinases reduced 1,25(OH)2D3-induced and PMA-enhanced hCYP24A1 promoter activity. Mithramycin A inhibits Specific Protein (Sp) family member binding to DNA and reduced 1,25(OH)2D3-induced and PMA-enhanced hCYP24A1 promoter activity. Sp1 or Sp3 siRNA knockdown reduced 1,25(OH)2D3-regulated hCYP24A1 promoter activity but only Sp3 siRNA reduced PMA-enhanced hCYP24A1 promoter activity. PMA increased MAPK-dependent Sp3 phosphorylation, Sp3-VDR interactions, and Sp3 binding to the hCYP24A1 promoter. These data suggest that MAPK signaling contributes to 1,25(OH)2D3-induced and PMA-enhanced CYP24A1 gene transcription by modulating Sp3 function.
Keywords: Sp3, CYP24A1, transcription, vitamin D
1. Introduction
The active form of vitamin D3, 1,25 dihydroxyvitamin D3 (1,25(OH)2D3) plays many important roles in the intestine, including regulating calcium absorption efficiency (Bronner et al., 1986), regulating intestinal immune responses (Cantorna et al., 2000; Froicu et al., 2003), and working as a chemopreventive agent against colorectal cancer (Fedirko et al., 2009; Fichera et al., 2007). While it is well established that 1,25(OH)2D3 regulates biological events by binding to the vitamin D receptor (VDR) and stimulating gene transcription, the effect of vitamin D on these physiological functions depends on a balance between 1,25(OH)2D3 synthesis and its subsequent degradation in target cells. 1,25(OH)2D3 is synthesized from the precursor 25(OH)D3 by the enzyme 25-hydroxyvitamin D3 1α-hydroxylase (CYP27B1) while its degradation is regulated by the enzyme 25-hydroxyvitamin D3 24-hydroxylase (CYP24A1) (Jones et al., 2011). 1,25(OH)2D3 is the most potent inducer of CYP24A1 gene expression and the CYP24A1 gene is the most responsive of all known 1,25(OH)2D3 target genes (Dwivedi et al., 2002). This potent induction forms a natural feedback for controlling cellular 1,25(OH)2D3 levels and action (Ly et al., 1999; Miller et al., 1995; St-Arnaud et al., 2000).
We and others have shown that phorbol 12-myristate 13-acetate (PMA) enhances 1,25(OH)2D3-mediated CYP24A1 gene expression (Armbrecht et al., 1993; Barletta et al., 2004; Chen et al., 1993; Jiang and Fleet, 2011; Nutchey et al., 2005) and several mechanisms have been proposed to contribute to this effect. Barletta et al. found that PMA enhances vitamin D action on both a full-length human CYP24A1 promoter (−5500 bp) or a minimal promoter that contained just the proximal Vitamin D responsive element (VDRE) from the human CYP24A1 promoter (Barletta et al., 2004). This suggests that PMA directly influences VDR function. Mutation of an Ets binding site (EBS) adjacent to the proximal VDRE in the rat CYP24A1 promoter reduced the impact of PMA on 1,25(OH)2D3–mediated transcription by 50% in HEK-293T cells (Nutchey et al., 2005) but not in differentiated Caco-2 cells (Jiang and Fleet, 2011). Finally, mutation of a VSE site in the rat (Nutchey et al., 2005) or human (Jiang and Fleet, 2011) CYP24A1 promoter reduces 1,25(OH)2D3 induced gene expression by 50% and significantly reduced the PMA-1,25(OH)2D3 synergy. Thus, several DNA binding sites contribute to the impact of PMA on 1,25(OH)2D3-mediated CYP24A1 gene expression. Here we have conducted studies to identify additional DNA regulatory elements that contribute to the enhancing effect of PMA on 1,25(OH)2D3 regulated human CYP24A1 gene regulation in intestinal epithelial cells, a primary target for vitamin D action.
A promising candidate site for mediating the PMA effect on vitamin D-regulated expression of the CYP24A1 gene is the GC-box. Several GC-boxes have been identified within the human CYP24A1 promoter (Chen and DeLuca, 1995) and deletion/mutation studies demonstrate that a GC-box at −114/-101 on the rat CYP24A1 promoter (Gao et al., 2004) and three GC-boxes between −548 and −294 bp of human CYP24A1 promoter (Tashiro et al., 2007) contribute to regulation of the CYP24A1 promoter by vitamin D. Specific protein 1 (Sp1) is a transcription factor which binds to GC boxes to regulate gene transcription (Courey et al., 1989; Kadonaga et al., 1987). While Sp family members Sp1, Sp3 and Sp4 can bind to the GC box (Kolell and Crawford, 2002) with similar affinity (Li et al., 2004), only Sp1 and Sp3 are expressed in the intestine (Hagen et al., 1992; Saffer et al., 1991; Supp et al., 1996) and are candidate proteins for binding to the GC-box of the CYP24A1 promoter within this tissue.
Our research shows that in the Caco-2 intestinal epithelial cell line PMA enhances 1,25(OH)2D3-mediated regulation of the CYP24A1 gene but not the TRPV6 gene. Enhancement of 1,25(OH)2D3-mediated CYP24A1 gene expression is accompanied by increased binding of VDR to the CYP24A1 proximal promoter region. Our data show that both Sp1 and Sp3 contribute to 1,25(OH)2D3-induced CYP24A1 gene transcription. In particular, we found that PMA causes ERK1/2 mediated phosphorylation of Sp3 that increased the interaction of Sp3 with VDR and with the CYP24A1 promoter. Collectively, this suggests a novel role for Sp3 as a modulator of vitamin D-mediated CYP24A1 gene regulation.
2. Experimental Procedures
2.1 Reagents
1α, 25-Dihydroxyvitamin D3 (1,25(OH)2D3) was obtained from BioMol International (Plymouth Meeting, PA) and dissolved in ethanol. Phorbol-12-myristate-13-acetate (PMA), Go6976 (classic PKC inhibitor), U0126 (specific inhibitor for MEK1/2), SB202190 (p38 kinase inhibitor) (all obtained from EMD Biosciences, Inc., San Diego, CA) and Mithramycin A (Sigma-Aldrich, St. Louis, MO) were dissolved in dimethyl sulfoxide (DMSO). Antibodies against total and phosphorylated ERK1/2 as well as total and phosphorylated p38 were obtained from Cell Signaling Technology, Inc. (Beverly, MA). Antibodies against VDR were from Santa Cruz Biotechnology (Santa Cruz, CA, sc-1008 for ChIP assay) and Affinity Bioreagents (Golden, CO, 9A7 for Western blots). The Sp3 antibody was purchased from Santa Cruz Biotechnology. An antibody against phosphoserine residues was purchased from Sigma-Aldrich, Inc.
The human CYP24A1 promoter region from −298 to +74 was amplified from human genomic DNA by PCR with primers P1, 5’GCTAGCTTCGAAGCACACCCGGTGAA3’ and P2, 5’CTCGAGGCTGGAGCACGGGGAGGT3’ and then cloned upstream from the firefly luciferase reporter gene in the pGL3 basic vector to create the −298 to +74 bp hCYP24A1-luciferase construct. The PCR3.1-hVDR vector has been described previously (Shao et al., 2001). The pRL-null renilla luciferase plasmid was from Promega (Madison, WI).
2.2 Cell Culture and Treatment Conditions
The parental line of the human colonic adenocarcinoma cell line Caco-2 was cultured as described elsewhere (Fleet et al., 2002). Experiments were carried out at two different cell stages: 90% confluent (proliferating) and 11 day post-confluent Caco-2 cells (differentiated). Differentiation was confirmed by observing high levels of expression of sucrose-isomaltase and calbindin D9k mRNA, two markers of enterocyte differentiation (Wang et al., 2004). Treatments were prepared with DMEM with 0% FBS media for protein studies and DMEM plus 5% FBS media for mRNA studies.
Cells were pretreated with either vehicle (0.002% DMSO) or 100 nM PMA for 5 min (RNA, Western, immunoprecipitation, ChIP, chromatin studies) or 10 min (siRNA, reporter gene studies). Afterwards the cells were treated with either vehicle (0.01% ethanol) or 10 nM 1,25(OH)2D3 in the absence of PMA for various times (RNA and ChIP assay, 2 h; chromatin assay, 1 h; reporter gene assay and siRNA studies, 4 h).
2.3 RNA Isolation and Analysis
RNA was isolated using the TriReagent method (Molecular Research Center, Inc., Cincinnati, OH). Total RNA (2 µg) was reverse transcribed to make cDNA and analyzed for CYP24A1, TRPV6, calbindin D9k, sucrose, and VDR mRNA by real-time PCR using primers and conditions previously described by our group (Cui et al., 2009b; Klopot et al., 2006; Wang et al., 2004). Primers to detect Sp1 mRNA expression were sense 5’-GCACAAACGTACACACACAGG-3’ and anti-sense 5’-GGTGGTAATAAGGGCTGAAGG-3’. Primers to detect Sp3 mRNA expression were sense 5’-AAGTGACCACCTTGCCAAAC-3’ and anti-sense 5’-CCTATCCCTGAAACAGAACC-3’.
2.4 VDR Association with Chromatin
Differentiated (15 d) Caco-2 cells treated with 10 nM 1,25(OH)2D3 or vehicle for 1 h were used to obtain soluble and chromatin fractions as described previously (Ismail et al., 2004). The volume of chromatin and soluble fraction obtained for each treatment condition was equalized and VDR and were analyzed by Western blot analysis as described below.
2.5 Western Blot Analysis
Differentiated Caco-2 cells were changed to serum free media overnight prior to treatments. For ERK1/2 analysis, samples were harvested immediately after a 5 minute PMA treatment. For p38 kinase analysis, medium was exchanged with serum-free DMEM containing 0.002% DMSO and cell were harvested 1 hour after PMA treatment. Cells extracts were prepared and analyzed for total and phosphorylated ERK1/2 as published previously (Cui et al., 2009b). Samples were also analyzed for p38 kinase using a 1:1000 dilution of primary antibody against total or phosphorylated p38 kinase antibodies.
2.6 Bioinformatic Search for Sp1 sites in the human CYP24A1 promoter
The sequence of the human CYP24A1 promoter from region −298 to +74 bp was obtained from Ensembl (www.ensembl.org) and a search for Sp1 sites on this sequence was performed using the web-based programs ConSite (Sandelin et al., 2004) and Transfac (Matys et al., 2003). Only sites identified as having a 100% match to the Sp1 positional-weight matrix were considered (Thiesen and Bach, 1990).
2.7 Transient transfection and reporter gene assays
Caco-2 cells were seeded into 24-well plates and transfected using Liopofectamine Plus (Invitrogen Corp., Carlsbad, California) following the manufacturer’s protocol. Differentiated cells were transfected using a 1:10:10 ratio of DNA:Plus reagent:Lipofectamine. Cells in each well were transfected with 300 ng −298 to +74 bp hCYP24A1-luciferase construct, 5 ng pCR3.1-hVDR plasmid, and 8 ng pRL-null renilla luciferase plasmid then incubated for 15 hours. Afterwards cells were pre-treated with 0, 1, 5 µM Mithramycin A for 30 min followed by 100 nM PMA or vehicle for 10 minutes. After removing PMA, cells were treated for 4 hours with 10 nM 1,25(OH)2D3 or vehicle in the presence or absence of Mithramycin A. After treatment, cells were collected and luciferase activity in the samples was determined using the Dual-Luciferase Reporter Assay System (Promega Corp., Madison, WI).
2.8 siRNA interference
Small interfering RNAs (siRNA) specific for Sp1 (ON-TARGETplus siRNA J-026959-08-0005) or Sp3 (ON-TARGETplus siRNA J-023096-07-0005) and a control non-targeting siRNA (D-001210-03-05) were purchased from Dharmacon (Lafayette, CO). Caco-2 cells were seeded in antibiotic free media. When cells were 30–50% confluent, they were transfected with siRNA at a final concentration of 100 nM using oligofectamine (Invitrogen). For mRNA quantification, cells were harvested 48 h after siRNA transfection. For the immunoblot of Sp3, cells were harvested 72 h after siRNA transfection. For reporter gene assays, 48 h after siRNA transfection, cells were transfected with −298 to +74 bp hCYP24A1-luciferase construct, pCR3.1-hVDR plasmid, and pRL-null renilla luciferase plasmid as described above. 15 hours after transfection, cells were pre-treated with 100 nM PMA or vehicle for 10 min followed by 4 hours treatment with vehicle or 10 nM 1,25(OH)2D3 treatment in the absence of PMA. Cells were then harvested for assessment of luciferase activity.
2.9 Sp3 Immunoprecipitation and VDR-Sp3 Co-immunoprecipitation
Proliferating Caco-2 cells were changed to 0% FBS DMEM medium 3 hours before treatment. Cells were pre-treated with 10 µM U0126 or vehicle for 30 min followed by treatment with 100 nM PMA or vehicle in 5% FBS DMEM for the last 5 min. Cells were washed in PBS twice then lysed in a buffer containing 20 mM Tris, 150 mM NaCl, 20 mM NaVO4, 0.5% Nonidet p40 plus protease inhibitor cocktails (Roche, Indianapolis, IN). Cell debris was removed by centrifugation at 12,000 RPM for 20 min at 4°C. Proteins in the cell lysate (200 µg) were immunoprecipitated with 2 µg Sp3 antibody overnight at 4°C. Immune complexes were collected with a 50% slurry of protein G for 1 h. The protein G-immunocomplex was washed three times and proteins were released in sample loading buffer containing 50 mM Tris-HCl, 2% SDS, 10% glycerol, 1% β-mercaptoethanol, 12.5 mM EDTA, 0.02% bromophenol blue. Proteins were resolved on 7.5% SDS-PAGE gels, transferred to PVDF membranes and probed with specific antibodies for phospho-serine, Sp3, or VDR. Antigen-antibody complexes were detected using the ECL Plus Western blotting detection reagents (Pierce, Rockford, IL).
2.10 Chromatin Immunoprecipitation
Differentiated Caco-2 cells were pretreated with either vehicle or 100 nM PMA for 5 minutes and then incubated with 10 nM 1,25(OH)2D3 or vehicle for 2 hours in the absence of PMA. For studies examining Sp3 binding to the CYP24A1 promoter, 90% confluent Caco-2 cells were treated with 100 nM PMA or vehicle for 30 minutes and cells were harvested.
Chromatin immunoprecipitation was performed as we have described previously (Cui et al., 2009b). Cells were fixed with 1.5% formaldehyde at room temperature for 15 minutes, neutralized with 0.125 M glycine, washed with ice-cold PBS twice, and scraped from dishes. Cell nuclei were extracted and sonicated to produce chromatin fragments from 200 bp to 1000 bp. The sonicated extract was diluted with buffer (16.7 mM Tris-HCl, pH 8.1; 150 mM NaCl; 0.01% SDS; 1.1% Triton X-100; 1.2 mM EDTA) and pre-cleared for 1 h with 50% slurry of Fastflow protein G (Amersham Biosciences, Piscataway, NJ) at 4°C. All immunoprecipitations were performed overnight with 2 µg rabbit IgG (Upstate, Charlottesville, VA), VDR, RXR, or Sp3 antibody at 4°C. Immune complexes were collected with 50% slurry of protein G for 1 h at 4°C. The immunocomplexes were washed and the cross-links were reversed overnight in 1% SDS, 0.1 M NaHCO3, and 0.2 M NaCl at 65 °C. DNA fragments were purified using QIAGEN QIAquick Spin Kits (Valencia, CA) and then subjected to real-time PCR using primers reported in the literature to amplify fragments of the proximal human CYP24A1 promoter region (−252 bp to −51 bp) (Meyer et al., 2006).
2.11 Statistics
All quantitative data is expressed as the mean ± the standard error of the mean (SEM). RNA and reporter gene studies were conducted with triplicate observations in each experiment and each experiment was repeated three times. Western blot, immunoprecipitation, and ChIP assays were conducted with single or duplicate observations in each experiment and each experiment was repeated three times. Prior to analysis, data was tested for fitting the normal distribution. If the data was not normally distributed, a log transformation was performed. Statistical analysis of the data was performed by ANOVA followed by Fisher’s protected LSD (p<0.05). P-values less than 0.05 were considered statistically significant.
3. Results
3.1 PMA enhances 1,25(OH)2D3-induced CYP24A1 but not TRPV6 gene expression in differentiated Caco-2 cells
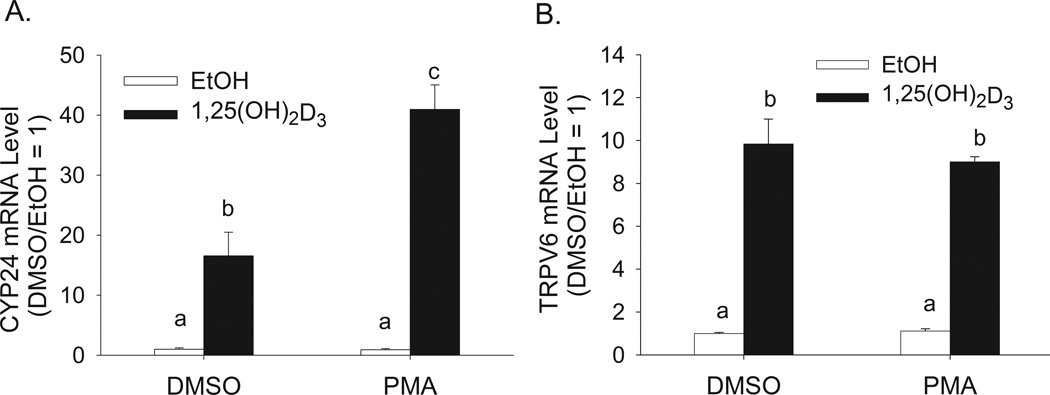
Previous studies have shown that PMA potentiates the effect of 1,25(OH)2D3 on CYP24A1 mRNA expression in rat intestinal epithelial cells (Koyama et al., 1994), renal cells (Armbrecht et al., 1993) and differentiated Caco-2 cells (Jiang and Fleet, 2011). However, it is not clear if the effect of PMA extends to other 1,25(OH)2D3 target genes. In the differentiated enterocytes of the small intestine, TRPV6 is a highly responsive vitamin D target gene (Cui et al., 2009b; Meyer et al., 2007; Song et al., 2003). Pretreatment of differentiated Caco-2 cells with 100 nM PMA for 5 min enhanced 1,25(OH)2D3-induced CYP24A1 mRNA expression by 180% (Figure 1A). In contrast, PMA treatment had no effect on 1,25(OH)2D3-mediated induction of TRPV6 mRNA (Figure 1B).
Figure 1. Effect of PMA on 1,25(OH)2D3-regulated expression of CYP24A1 and TRPV6 mRNA in differentiated Caco-2 cells.
Differentiated Caco-2 cells were pretreated with vehicle (DMSO) or 100 nM PMA for 5 min followed by treatment with vehicle (EtOH) or 10 nM 1,25(OH)2D3 for an additional 2 h in the absence of PMA. Total RNA was collected and analyzed by real time RT-PCR for (A) CYP24A1 mRNA and (B) TRPV6 mRNA. Data are expressed as the mean±sem (n=4). Bars with different letter superscripts are significantly different from one another (p < 0.05, Fisher’s protected LSD). Data in each panel are representative of three independent experiments.
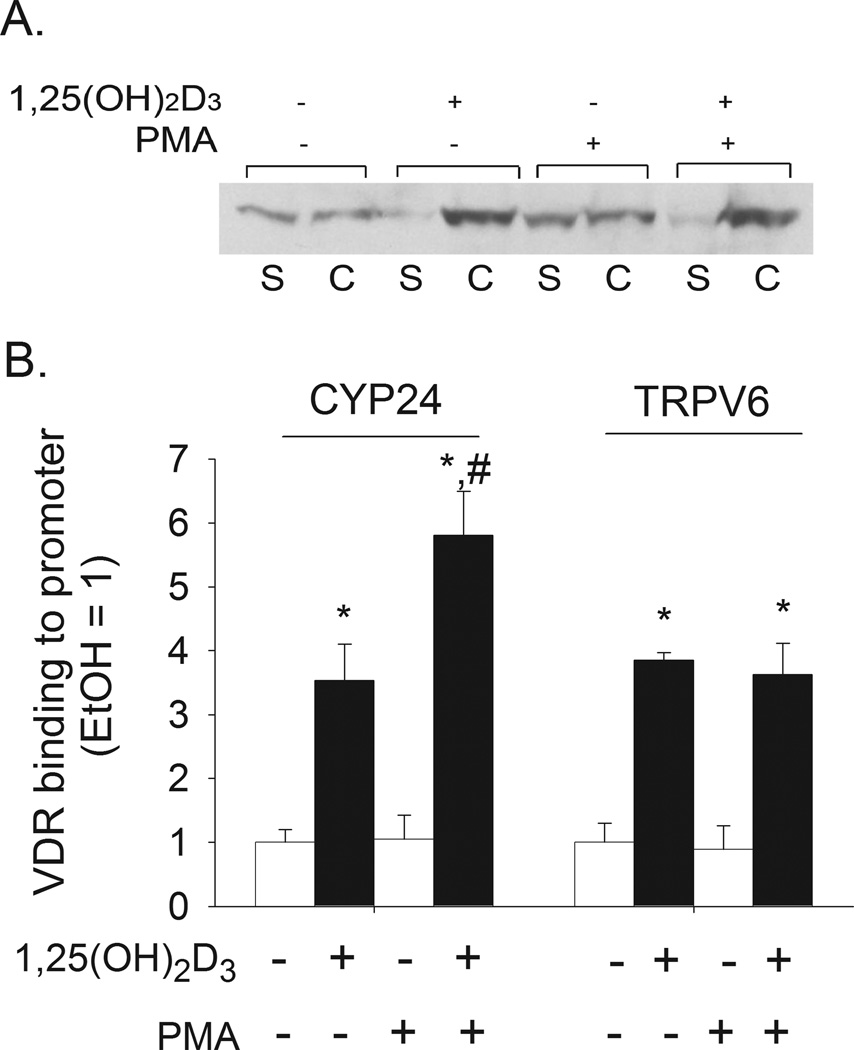
1,25(OH)2D3-mediated gene transcription follows ligand-activated VDR heterodimerization with RXR and binding of the VDR-RXR complex to VDREs in DNA. VDR was found in both the crude chromatin and soluble fractions of differentiated Caco-2 cells and 1,25(OH)2D3 treatment induced a redistribution of VDR to the chromatin fraction (Figure 2A), consistent with ligand-induced DNA binding. When cells were preincubated with PMA prior to 1,25(OH)2D3 treatment, ligand-induced association of VDR with chromatin was not changed (Figure 2A).
Figure 2. Effect of PMA on 1,25(OH)2D3-induced recruitment of VDR to chromatin, the CYP24A1 promoter, or the TRPV6 promoter in differentiated Caco-2 cells.
Cells were preincubated with 100 nM PMA or vehicle for 5 min, after which cells were treated for 1 h (panel A) or 2 h (panel B) with 10 nM 1,25(OH)2D3 or vehicle in the absence of PMA. (A) Crude chromatin association assay. VDR was detected in soluble (“S”) and chromatin (“C”) fractions by Western Blot analysis. (B) Quantitative PCR-ChIP analysis for association of VDR with the CYP24A1 and TRPV6 promoters. Bars in panel B represent the mean±sem (n = 3 replicates per treatment). * p < 0.05 versus the vehicle control group; # p < 0.05 versus 1,25(OH)2D3 only group.
Using a ChIP assay we confirmed that 1,25(OH)2D3 treatment enhanced VDR binding to known VDRE containing regions in both the CYP24A1 promoter (−252 to −52 bp) and the TRPV6 gene promoter (−4.3 kb). However, PMA increased VDR binding to the CYP24A1 promoter by 67% but had no effect on VDR binding to the TRPV6 promoter (Figure 2 B).
3.2 ERK1/2 and p38 pathways are activated by PMA and these pathways contribute to both 1,25(OH)2D3-induced and PMA enhanced human CYP24A1 promoter activity
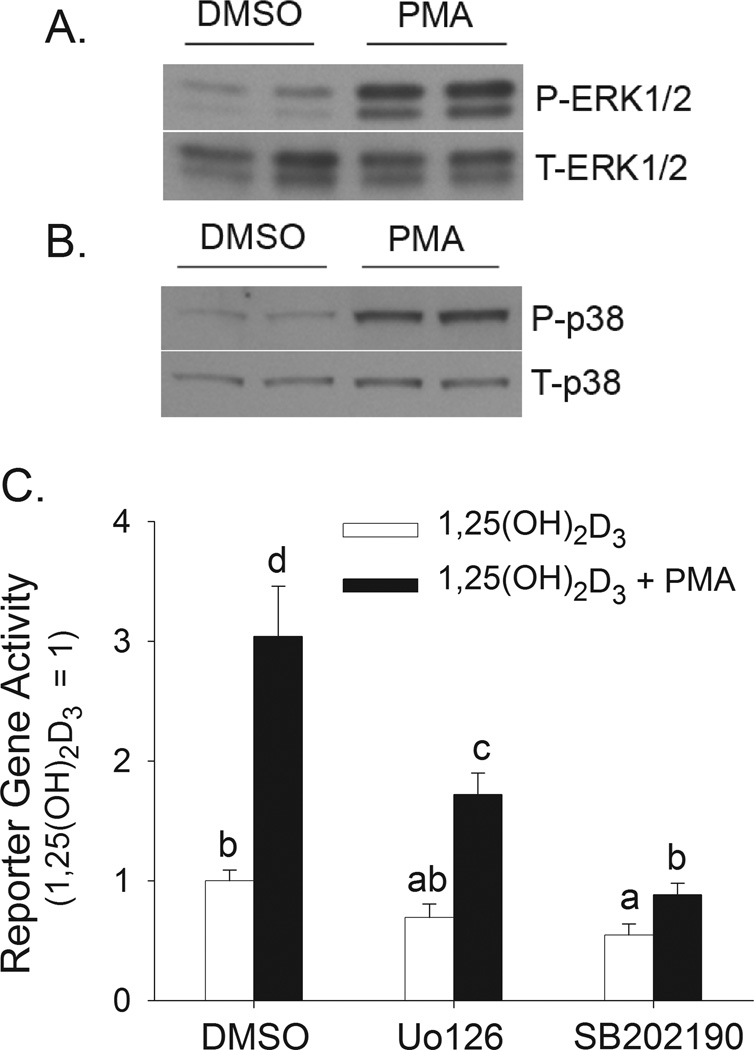
PMA is a classic PKC activator but can also activate ERK1/2 and p38 kinase (Almog and Naor, 2008; Nomura et al., 2007). In differentiated Caco-2 cells with a quiescent kinase signaling status, short-term PMA treatment was a potent activator of both ERK1/2 (Figure 3A) and p38 kinase (Figure 3B). Others have shown that differentiated Caco-2 cells expression the α, γ, and δ isoforms of p38 kinase (Vachon et al., 2002). To determine the role of ERK1/2 and p38 kinase activity in 1,25(OH)2D3-induced and PMA-enhanced human CYP24A1 promoter activity, differentiated Caco-2 cells were transfected with a −298 to +74 bp human CYP24A1 promoter-luciferase construct. PMA treatment enhanced 1,25(OH)2D3-induced CYP24A1 promoter activity by 200% (Figure 3C). MEK1/2/5 inhibition with U0126 and p38α kinase inhibition with SB202190 (English and Cobb, 2002) reduced 1,25(OH)2D3-induced human CYP24A1 promoter activity by 40 and 45%, respectively (Figure 3C). These inhibitors also reduced the enhancing effect of PMA on 1,25(OH)2D3-induced CYP24A1 promoter activity (PMA effect on 1,25(OH)2D3-action in the absence of inhibitors = 3-fold; in presence of U0126 = 2.5-fold; in presence of SB202190 = 1.6-fold).
Figure 3. Contribution of PMA-induced activation of ERK1/2 and p38 kinase to 1,25(OH)2D3-induced hCYP24A1 promoter activity in differentiated Caco-2 cells.
(A,B) Differentiated Caco-2 cells were treated with vehicle (DMSO) or 100 nM PMA for 5 min. Cells were collected immediately (for ERK1/2 analysis) or 1 h after 5 min PMA treatment (for p38 kinase analysis). (A) Total (T-ERK1/2) and phospho-ERK1/2 (P-ERK1/2) levels. (B) Total (T-p38) and phospho-p38 kinase (P-p38) levels. Data are representative of three independent experiments. Two samples were analyzed per treatment. (C) Differentiated Caco-2 cells were transfected with a −298 to +74 bp human CYP24A1 promoter-luciferase construct. After transfection, cells were preincubated with inhibitors of MEK 1/2 (10 µM U0126) or p38 kinase (8 µM SB202190) for 30 minutes in the presence or absence of 100 nM PMA for the last 10 min. Inhibitor co-treatment continued with 10 nM 1,25(OH)2D3 or ethanol vehicle for an additional 4h in the absence of PMA. Data were expressed relative to the 1,25(OH)2D3-induced expression of the reporter gene (calculated as 1,25(OH)2D3/vehicle = 100) (mean±sem, n=4). The values with different letter superscripts are significantly different from one another (p < 0.05, Fisher’s protected LSD). Data are representative of three independent experiments.
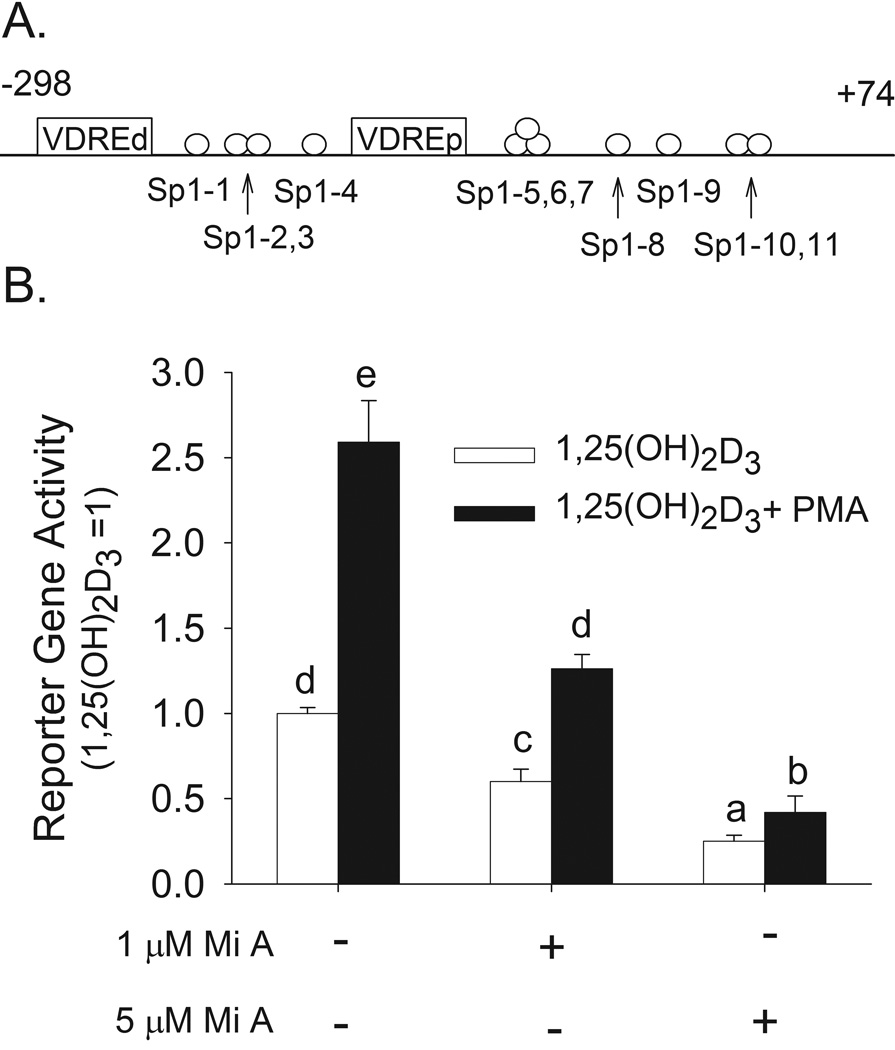
3.3 A role for Sp1 and Sp3 in 1,25(OH)2D3-induced and PMA-enhanced −298 to +74 bp human CYP24A1 promoter activity in Caco-2 cells
Eleven potential Sp1 sites were identified in the −298 to +74 bp region of the human CYP24A1 promoter (Figure 4A). Alignment of the human and rat CYP24A1 promoters at a 70% conservation level cutoff and a 70% transcription factor score threshold showed that the sites Sp1-5, 6, 7 and Sp1-9 are evolutionarily conserved. Mithramycin A is a competitive inhibitor of Sp1 and Sp3 binding to GC-boxes in DNA (Ray et al., 1989). This agent caused a dose-dependent decrease in 1,25(OH)2D3-induced hCYP24A1 promoter activity (by 40% at 1 µM and 75% at 5 µM) and on the enhancing effect of PMA (by 31% at 1 µM and 58% at 5 µM) (Figure 4B).
Figure 4. Competitive inhibition of Sp family member binding to DNA with Mithramycin A reduces 1,25(OH)2D3-induced and PMA-enhanced human CYP24A1 promoter activity in differentiated Caco-2 cells.
(A) Putative Sp1 sites identified in the −298 to +74 bp region of the human CYP24A1 promoter. (B) Differentiated Caco-2 cells were transfected with a −298 to +74 bp human CYP24A1 promoter-luciferase construct. 15 h later cells were preincubated with Mithramycin A (Mi A, 1 or 5 µM) for 30 minutes in the presence or absence of PMA (100 nM) for the last 10 min. PMA was removed and Mithramycin treatment was continued in the presence of 10 nM 1,25(OH)2D3 or ethanol vehicle for an additional 4 h. Data were expressed relative to the 1,25(OH)2D3-induced expression of the reporter gene (calculated as 1,25(OH)2D3/vehicle = 100) (mean±sem, n=4). The values with different letter superscripts are significantly different from one another (p < 0.05, Fisher’s protected LSD). Data in panel B are representative of three independent experiments.
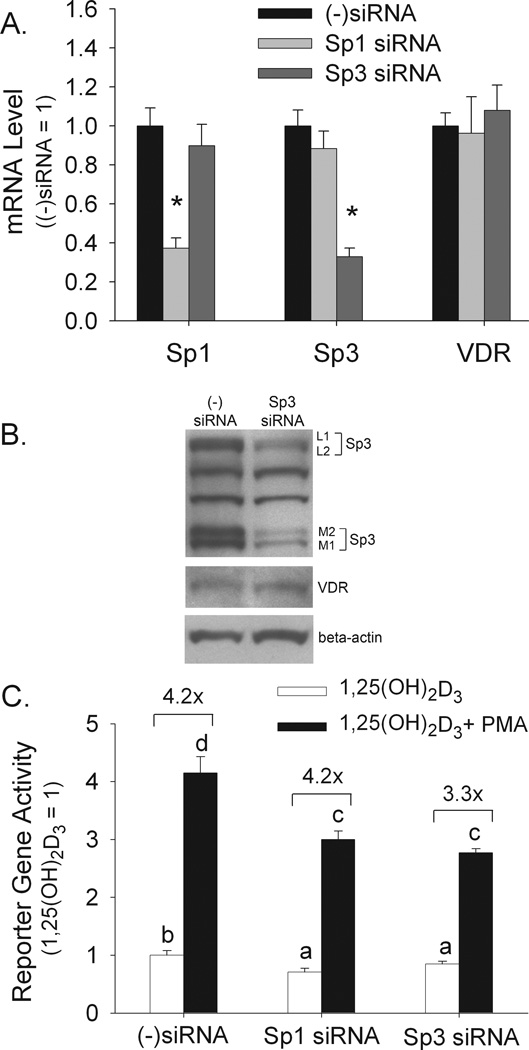
Because Mithramycin A can competitively inhibit both Sp1 and Sp3 binding to the GC-box (Ray et al., 1989) we used siRNA interference to determine the individual impact of these Sp transcription factors on 1,25(OH)2D3-mediated and PMA-enhanced regulation of the CYP24A1 promoter. Sp1 and Sp3 siRNA reduced their respective transcripts by 65–75% without influencing the transcript for the other Sp family member or the VDR (Figure 5A). We subsequently confirmed that Sp3 siRNA reduced the protein levels of all four Sp3 isoforms: two long versions (L1 and L2) and two short versions (M1 and M2) (Sapetschnig et al., 2004) (Figure 5B). Both Sp1 and Sp3 knockdown reduced 1,25(OH)2D3-mediated CYP24A1 promoter activity (by 30% and 15%, respectively, p<0.05) as well as the absolute level of CYP24A1 promoter activity after combined 1,25(OH)2D3 and PMA treatment (Figure 5C). However, while Sp1 knockdown did not influence the fold enhancement due to PMA (scambled siRNA control and Sp1 siRNA both = 4.2-fold increased vitamin D-induced reporter gene activity with PMA, Figure 5C), Sp3 siRNA reduced the PMA enhancing effect by 28% (from 315% above control in scrambled siRNA control to 228% above control in the Sp3 siRNA group, Figure 5C). These results suggest that while both Sp1 and Sp3 contribute to 1,25(OH)2D3-induced CYP24A1 promoter activity, Sp3 function is necessary for a maximal enhancing effect of PMA on 1,25(OH)2D3-induced activation of the CYP24A1 promoter. Because of this, the impact of Sp3 was studied further.
Figure 5. Effect of Sp3 or Sp1 knockdown on 1,25(OH)2D3-induced and PMA-enhanced CYP24A1 promoter activity.
Proliferating Caco-2 cells were transfected with one of three siRNAs: scrambled ((−)siRNA), Sp1, or Sp3. (A) PCR analysis of Sp1, Sp3 and VDR mRNA expression 48 h after siRNA transfection. Data were normalized to the expression level observed in the scrambled siRNA group (mean±sem, n=4). * = significantly different than the other groups within a gene group (p < 0.05). (B) Western blot analysis on whole cell extracts 72 h after Sp3 siRNA transfection. Samples were analyzed for Sp3, VDR or beta actin protein levels. Four Sp3 isoforms were observed: L1, L2, M1 and M2. (C) CYP24A1 reporter gene activity. 48 h after siRNA treatment cells were transfected with a CYP24A1 promoter-luciferase reporter gene. 15 h later cells were preincubated with PMA (100 nM, 10 min), followed by treatment with 1,25(OH)2D3 or ethanol vehicle (10 nM, 4 h in the absence of PMA). Reporter gene data were expressed relative to the 1,25(OH)2D3-induced expression of the reporter gene (calculated as 1,25(OH)2D3/vehicle = 1) (mean±sem, n=4). The values with different letter superscripts are significantly different from one another (p < 0.05, Fisher’s protected LSD). Data in each panel are representative of three independent experiments.
3.4 PMA induced Sp3 phosphorylation increases Sp3 interactions with VDR and the CYP24A1 promoter
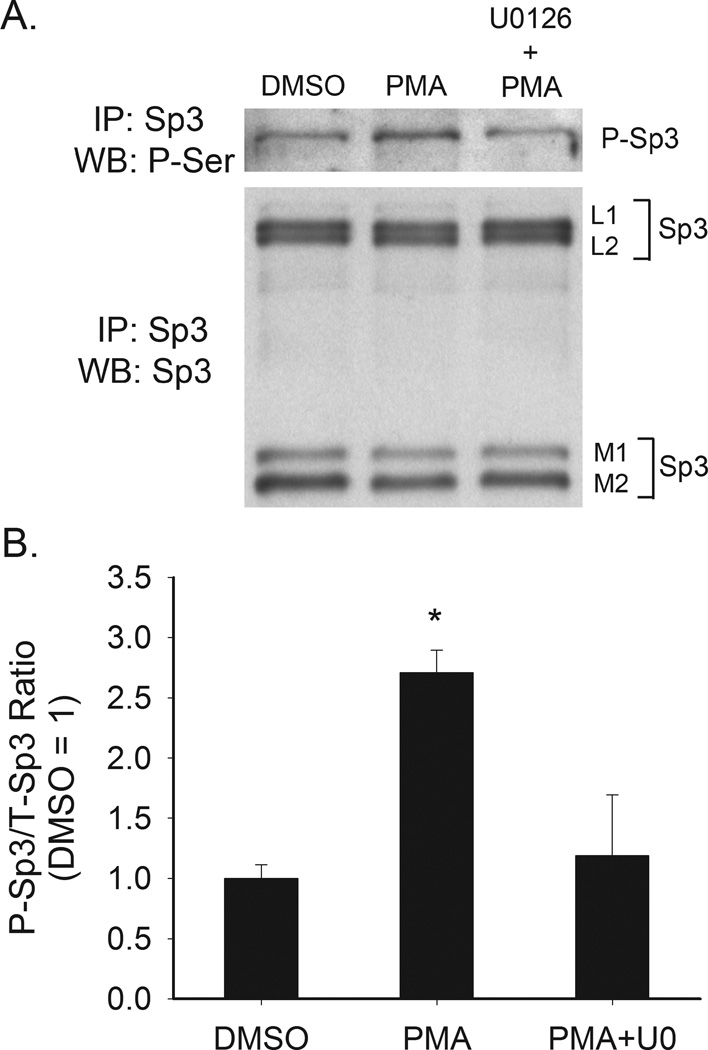
No antibody is commercially available to detect the phosphorylated form of Sp3. As a result we examined the impact of PMA on phosphorylation of Sp3 using immunoprecipitation of Sp3 followed by Western blot analysis with an anti phospho-serine antibody. Only the L1 isoform of Sp3 was phosphorylated in Caco-2 cells (Figure 6A). Phosphorylation of this isoform was increased by 170% after PMA treatment and this effect was blocked by pretreatment with the MEK1 inhibitor U0126 (Figure 6).
Figure 6. Effect of PMA on Sp3 serine phosphorylation in proliferating Caco-2 cells.
Differentiated Caco-2 cell were treated with vehicle (DMSO) or PMA (5 min, 100 nM) and then harvested. An additional group of cells were pretreated with U0126 (10 µM, 30 min) prior to PMA treatment. Sp3 was immunoprecipitated from the cell extracts and examined by Western blot analysis for total Sp3 level (L1, L2, M1 and M2 are Sp3 isoforms) and for phospho-serine. (A) Representative Western blot. The phospho-serine band corresponds to the L1 Sp3 isoform, (B) Quantitative data from three repeat experiments. Bands were quantified by densitometry and phospho-serine labeled Sp3 (L1) was normalized to the total amount of Sp3 L1 isoform present. Data are expressed as mean±sem (n=3) of the fold change above non-treated levels. * Significantly different from the other two groups (p < 0.05, Fisher’s protected LSD).
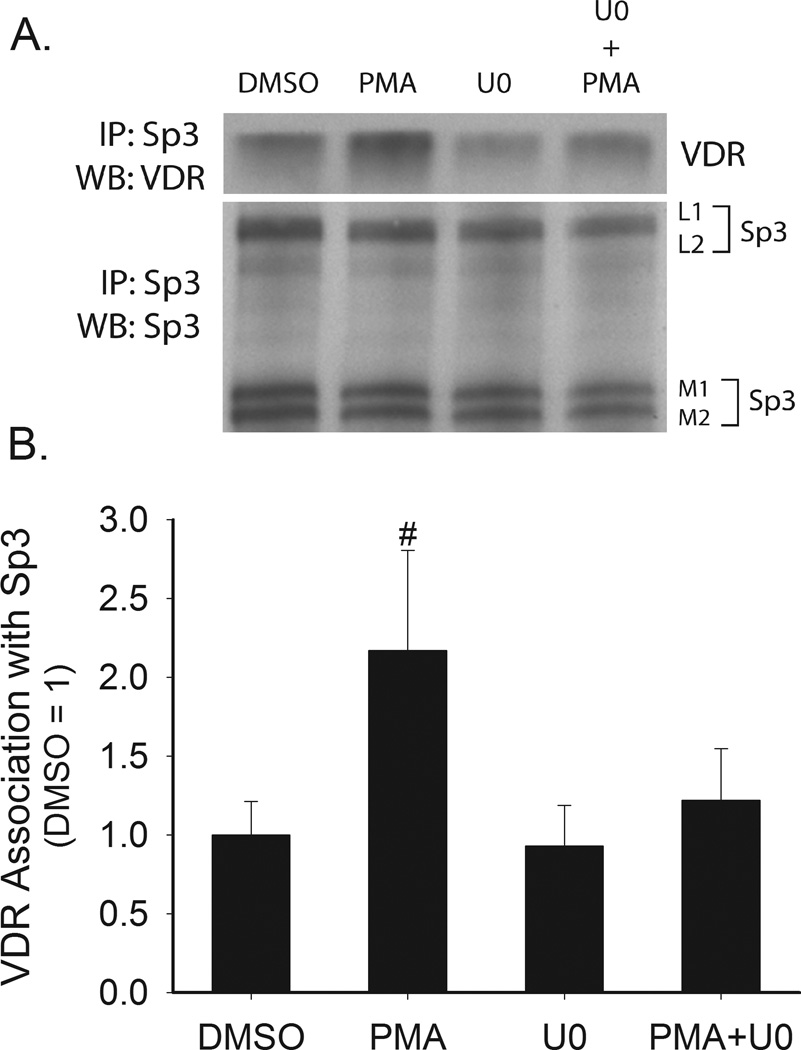
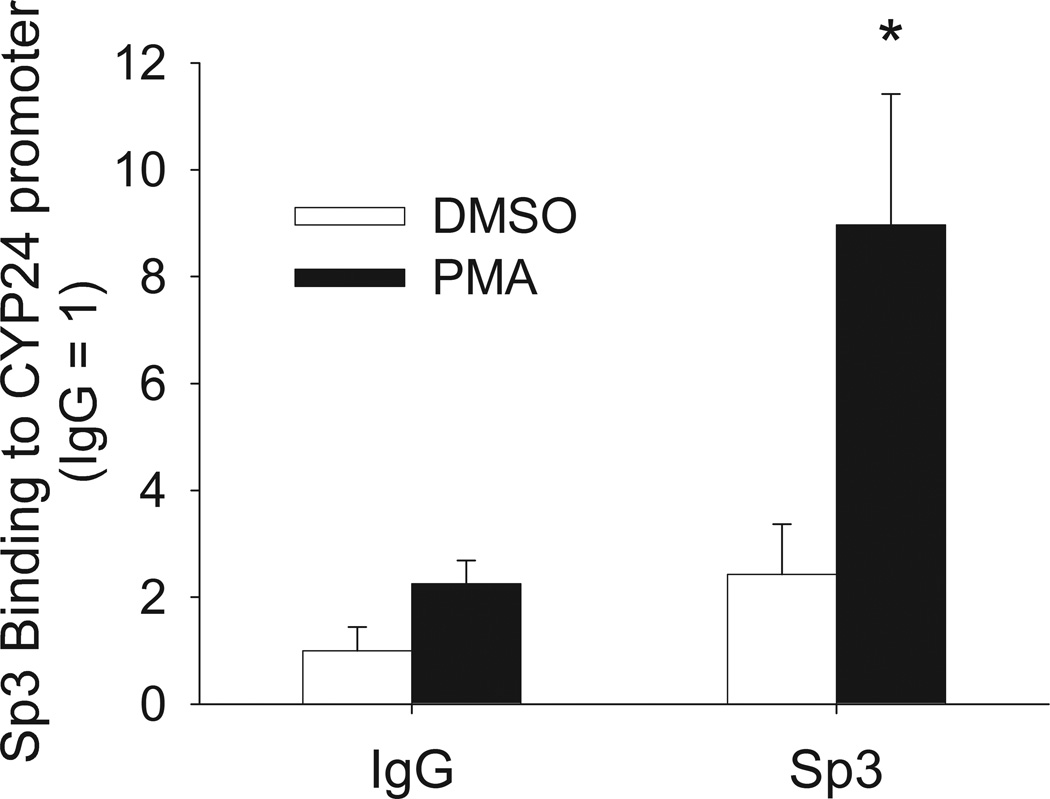
Using co-immunoprecipitation we found that VDR could be pulled-down in an Sp3 immunoprecipitation in the presence or absence of 1,25(OH)2D3 (data obtained in the absence of vitamin D is presented in Figure 7). PMA treatment enhanced the VDR-Sp3 interaction and the enhancing effect of PMA treatment was blocked by the MEK1/2/5 inhibitor U0126 (Figure 7). By using a ChIP assay we found that Sp3 can bind to human CYP24A1 promoter and that PMA treatment increased Sp3 binding to the promoter by 270% (Figure 8).
Figure 7. Effect of PMA on the interaction between Sp3 and VDR in Caco-2 cells.
Proliferating Caco-2 cells were pretreated with U0126 (10 µM,, 30 min) followed by treatment with 100 nM PMA for 5 min. Whole cell extracts were collected and Sp3 was immunoprecipitated. Immunoprecipitated proteins were analyzed by Western blot analysis for Sp3 and VDR (A) Representative Western blot. (B) Quantitative data from four repeat experiments. Data are expressed as mean±sem (n=4) of the fold change above non-treated levels. # represents significant different from all other groups (p < 0.05).
Figure 8. Effect of PMA treatment on recruitment of Sp3 to the CYP24A1 promoter.
Proliferating Caco-2 cells were treated with 100 nM PMA or vehicle for 30 min. ChIP assays were performed with 2 µg rabbit IgG or anti-Sp3 antibody. ChIP enriched DNA was amplified with primers recognizing the proximal CYP24A1 promoter (−252 to −52 bp). Quantitative PCR from three independent experiments was conducted and the result is reported as the mean+sem, n = 3. * Significantly different from all other groups (p < 0.05, Fisher’s protected LSD).
4. Discussion
CYP24A1 is the cytochrome P450 component of the enzyme 25-hydroxyvitamin D3-24-hydroxylase that catalyzes the conversion of 1,25(OH)2D3 into a 24-hydroxylated product and which constitutes the degradation of the vitamin D molecule (Jones et al., 2011). Transcription of the CYP24A1 gene is strongly up-regulated by 1,25(OH)2D3 (Kerry et al., 1996) but various kinase pathways can modulate vitamin D-mediated CYP24A1 gene regulation, e.g. MAP kinases like ERK1/2/5 (Cui et al., 2009b; Dwivedi et al., 2002) and protein kinase C (PKC) (Armbrecht et al., 2001; Koyama et al., 1994). Previous studies found that 1,25(OH)2D3-regulated expression of CYP24A1 mRNA or CYP24A1 promoter-reporter genes was enhanced by phorbol ester treatment in kidney cells (Barletta et al., 2004; Chen et al., 1993; Nutchey et al., 2005) and in intestinal epithelial cell lines (Armbrecht et al., 1993; Armbrecht et al., 2001; Jiang and Fleet, 2011).
Here we extend these earlier observations in several important ways. First, we make our initial observations in differentiated cultures of Caco-2 cells that are a model for the mature, calcium-transporting cells of the small intestine (Fleet and Wood, 1999) and that have low basal activity of many signaling pathways and therefore permit a clearer evaluation of PMA-induced signaling events on CYP24A1 gene transcription (Cui et al., 2009a; Cui et al., 2009b). Second, we directly demonstrate that the impact of PMA and vitamin D mediated gene activation does not affect all vitamin D target genes. While both the CYP24A1 and TRPV6 genes are strongly induced by 1,25(OH)2D3 treatment in differentiated Caco-2 cells (Figure 1) (Fleet et al., 2002), only the induction of the CYP24A1 gene is enhanced by PMA treatment. This enhancement is accompanied by increased binding of the VDR to the CYP24A1 promoter (Figure 2B). In contrast, neither 1,25(OH)2D3-induced binding of VDR to the TRPV6 promoter nor binding to a crude chromatin fraction is altered by PMA pretreatment. This suggests that the enhancing effect of PMA on 1,25(OH)2D3-induced gene expression is due to unique DNA sequences that permit improved recruitment of VDR to, or stability of VDR on, the CYP24A1 promoter.
Several mechanisms have been proposed to account for the enhancing effect of PMA on vitamin D-mediated CYP24A1 gene expression and our research extends that repertoire. Earlier studies showed that activation of classical protein kinase C isoforms, ERK1/2, and JNK (Nutchey et al., 2005) work in part through events occurring at DNA binding sites within −298 bp of the transcription start site (Barletta et al., 2004; Nutchey et al., 2005), i.e. the classical VDRE (Barletta et al., 2004), an Ets-1 binding site (EBS) downstream from the proximal VDRE (Nutchey et al., 2005), and a VSE (vitamin D stimulatory element) located between the proximal and distal VDREs (Jiang and Fleet, 2011; Nutchey et al., 2005) in the CYP24A1 gene promoter. Our findings add to this foundation by demonstrating the importance of the transcription factors Sp1 and Sp3 in 1,25(OH)2D3-induced and PMA-enhanced activation of the human CYP24A1 gene.
Sp1 and Sp3 are ubiquitously expressed transcription factors that bind to GC-boxes with similar affinity (Hagen et al., 1992; Saffer et al., 1991; Supp et al., 1996). We identified 11 putative GC-boxes in −298 to +74 bp regions of human CYP24A1 promoter and then used competitive inhibition of Sp protein binding to these sites with mithramycin A to show that one or more of these GC-boxes are important for both 1,25(OH)2D3 mediated and PMA enhanced CYP24A1 promoter activity (Figure 4B). Recent studies have shown that p38 MAPK regulates gene expression through phosphorylation of Sp1 on threonine 453 and 739 residues leading to enhanced Sp1 binding to DNA (D'Addario et al., 2002; D'Addario et al., 2006; Moon et al., 2006). Because of this, our initial hypothesis was that Sp1 is the downstream target of PMA activated p38 kinase. However, while Sp1 knockdown reduced 1,25(OH)2D3-mediated CYP24A1 gene transcription it did not reduce the enhancing effect of PMA in differentiated Caco-2 cells (Figure 5C). Dwivedi et al (Dwivedi et al., 2010) previously reported that Sp1 contributes to 1,25(OH)2D3 mediated CYP24A1 gene transcription in HEK-293T human embryonic kidney cells by binding to a putative GC-box at −113 to −105 bp of the rat CYP24A1 promoter; this site corresponds to the cluster of GC-boxes labeled Sp1-5, 6, and 7 that we predicted in the human CYP24A1 promoter (Figure 4A). They found that Sp1 effects on the rat CYP24A1 promoter are dependent upon PKCζ mediated phosphorylation of Sp1. Although PKCζ is present in Caco-2 cells (Fichera et al., 2007; Han et al., 1998), PKCζ is an atypical PKC isoform that is not activated by PMA (Newton, 2001; Simboli-Campbell et al., 1994) or 1,25(OH)2D3 (Simboli-Campbell et al., 1994) and so our data showing a lack of Sp1 involvement in the PMA enhanced activation of the CYP24A1 gene are not in conflict with Dwivedi et al..
Synergism between nuclear receptors and Sp1 appears to be common (Schule et al., 1988) and may account for the impact of Sp1 knockdown on vitamin D-mediated CYP24A1 gene regulation. Several nuclear receptors can form ternary complexes with Sp1 and the GC-box on promoters (Fleming et al., 2006; Husmann et al., 2000; Meissner et al., 2004; Owen et al., 1998; Pipaon et al., 1999; Schule et al., 1988; Strahle et al., 1988) and Sp1-VDR interactions are important for the regulation of the p27 and p45 genes (Cheng et al., 2006; Huang and Hung, 2006). Other studies conducted using reporter-gene constructs assembled from individual DNA binding sites suggest that a GC-box in close proximity to a VDRE can facilitate 1,25(OH)2D3 regulated gene expression (Liu and Freedman, 1994). Our data are consistent with these earlier findings.
In contrast to the effect of Sp1 knockdown, Sp3 siRNA reduced both 1,25(OH)2D3-induced and PMA-enhanced CYP24A1 transcriptional activity in Caco-2 cells (Figure 5C). Sp3 is phosphorylated by ERK1/2 on serine 73 and this is critical for its ability to regulate gene expression (Bakovic et al., 2003; Pages, 2007). Consistent with this, we found that PMA treatment increases phospho-ERK1/2 MAPK levels leading to phosphorylation of the L1 form of Sp3 that contains this critical serine site but not the truncated Sp3 forms lacking the N-terminal region where serine 73 resides (Figure 6). Similar to earlier studies by Bakovic et al. (Bakovic et al., 2003) who used EMSA to show that Sp3 phosphorylation increased binding of Sp3 to regions in the CTP: phosphocholine cytidylyltransferase (CT) alpha gene, we found that PMA-mediated phorphorylation of Sp3 in differentiated Caco-2 cells increased Sp3 binding to the proximal CYP24A1 promoter (Figure 8). Basal CYP24A1 expression is not enhanced by PMA alone but requires treatment with 1,25(OH)2D3 for regulation of the CYP24A1 gene (e.g. Figure 1A). This suggests the impact of Sp3 in PMA-treated Caco-2 cells is to influence the association of VDR with the CYP24A1 promoter. Co-immunoprecipitation confirmed PMA could enhance Sp3-VDR associations (Figure 7) and when coupled to our ChIP data in Figure 2, this suggests that Sp3-VDR interactions stabilize VDR binding to the CYP24A1 promoter.
Conclusions
Our data show that the enhancing effect of PMA on 1,25(OH)2D3-induced gene expression is limited to the CYP24A1 gene. These data support a model whereby signaling events activated by PMA enhance VDR binding to CYP24A1 promoter. A candidate factor mediating this effect is Sp3, a target of ERK1/2 phosphorylation that interacts with VDR and can bind to the CYP24A1 promoter. In addition, Sp1 modulates 1,25(OH)2D3-induced CYP24A1 gene expression through a mechanism that is not modulated by signaling pathways activated by PMA.
Highlights.
Phorbol 12-myristate 13-acetate (PMA) enhances 1,25(OH)2D3-induced vitamin D receptor binding to the human 25 hydroxyvitamin D-24 hydroxylase (hCYP24A1) gene promoter and increases hCYP24A1 gene expression.
Other vitamin D responsive genes and VDR binding sites on DNA are not influenced by PMA.
Specific protein (Sp) family members Sp1 and Sp3 are involved in 1,25(OH)2D3-induced and PMA-enhanced hCYP24A1 gene expression
PMA increased Sp3 phosphorylation, Sp3-VDR interactions, and Sp3 binding to the hCYP24A1 promoter.
The effect of PMA on Sp3 is mitogen activated protein kinases-dependent.
Acknowledgments
This work was supported by NIH grant R01 DK054111 to JCF.
Abbreviations used
- 1,25(OH)2D3
1,25 dihydroxyvitamin D3
- 25(OH)D3
25 hydroxyvitamin D3
- CYP24A1
25-hydroxyvitamin D, 24-hydroxylase
- CYP27B1
1 alpha hydroxylase
- TRPV6
transient receptor potential cation channel, subfamily V, member 6
- Sp1
specific protein 1
- Sp3
specific protein 3
- VDR
vitamin D receptor
- MAPK
mitogen activated protein kinase
- MEK
MAP kinase/Erk kinase kinase
- ERK
extracellular regulated kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Citations
- Almog T, Naor Z. Mitogen activated protein kinases (MAPKs) as regulators of spermatogenesis and spermatozoa functions. Molecular and Cellular Endocrinology. 2008;282:39–44. doi: 10.1016/j.mce.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Armbrecht HJ, Boltz MA, Hodam TL, Kumar VB. Differential responsiveness of intestinal epithelial cells to 1,25- dihydroxyvitamin D3--role of protein kinase C. J. Endocrinol. 2001;169:145–151. doi: 10.1677/joe.0.1690145. [DOI] [PubMed] [Google Scholar]
- Armbrecht HJ, Hodam TL, Boltz MA, Chen ML. Phorbol ester markedly increases the sensitivity of intestinal epithelial cells to 1,25-dihydroxyvitamin D3. FEBS Lett. 1993;327:13–16. doi: 10.1016/0014-5793(93)81028-x. [DOI] [PubMed] [Google Scholar]
- Bakovic M, Waite K, Vance DE. Oncogenic Ha-Ras transformation modulates the transcription of the CTP : Phosphocholine cytidylyltransferase alpha gene via p42/44(MAPK) and transcription factor Sp3. Journal of Biological Chemistry. 2003;278:14753–14761. doi: 10.1074/jbc.M300162200. [DOI] [PubMed] [Google Scholar]
- Barletta F, Dhawan P, Christakos S. Integration of hormone signaling in the regulation of human 25(OH)D3 24-hydroxylase transcription. Am J Physiol Endocrinol Metab. 2004;286:E598–E608. doi: 10.1152/ajpendo.00214.2003. [DOI] [PubMed] [Google Scholar]
- Bronner F, Pansu D, Stein WD. An analysis of intestinal calcium transport across the rat intestine. Am J Physiol. 1986;250:G561–G569. doi: 10.1152/ajpgi.1986.250.5.G561. [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. Journal of Nutrition. 2000;130:2648–2652. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- Chen K-S, DeLuca HF. Cloning of the human 1 alpha, 25-dihydroxyvitamin D3 24-hydroxylase gene promoter and identification of two vitamin D-responsive elements. Biochim. Biophys. Acta. 1995;1263:1–9. doi: 10.1016/0167-4781(95)00060-t. [DOI] [PubMed] [Google Scholar]
- Chen ML, Boltz MA, Armbrecht HJ. Effects of 1,25-dihydroxyvitamin D3 and phorbol ester on 25- hydroxyvitamin D3 24-hydroxylase cytochrome P450 messenger ribonucleic acid levels in primary cultures of rat renal cells. Endocrinology. 1993;132:1782–1788. doi: 10.1210/endo.132.4.7681765. [DOI] [PubMed] [Google Scholar]
- Cheng HT, Chen JY, Huang YC, Chang HC, Hung WC. Functional role of VDR in the activation of p27Kip1 by the VDR/Sp1 complex. J Cell Biochem. 2006;98:1450–1456. doi: 10.1002/jcb.20780. [DOI] [PubMed] [Google Scholar]
- Courey AJ, Holtzman DA, Jackson SP, Tjian R. Synergistic Activation by the Glutamine-Rich Domains of Human Transcription Factor Sp1. Cell. 1989;59:827–836. doi: 10.1016/0092-8674(89)90606-5. [DOI] [PubMed] [Google Scholar]
- Cui M, Klopot A, Jiang Y, Fleet JC. The effect of differentiation on 1,25 dihydroxyvitamin D-mediated gene expression in the enterocyte-like cell line, Caco-2. J Cell Physiol. 2009a;218:113–121. doi: 10.1002/jcp.21574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Zhao Y, Hance KW, Shao A, Wood RJ, Fleet JC. Effects of MAPK signaling on 1,25-dihydroxyvitamin D-mediated CYP24 gene expression in the enterocyte-like cell line, Caco-2. J Cell Physiol. 2009b;219:132–142. doi: 10.1002/jcp.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Addario M, Arora PD, Ellen RP, McCulloch CAG. Interaction of p38 and Sp1 in a mechanical force-induced, beta(1), integrin-mediated transcriptional circuit that regulates the actin-binding protein filamin-A. Journal of Biological Chemistry. 2002;277:47541–47550. doi: 10.1074/jbc.M207681200. [DOI] [PubMed] [Google Scholar]
- D'Addario M, Arora PD, McCulloch CA. Role of p38 in stress activation of Sp1. Gene. 2006;379:51–61. doi: 10.1016/j.gene.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Dwivedi PP, Gao XH, Tan JCT, Evdokiou A, Ferrante A, Morris HA, May BK, Hii CST. A role for the phosphatidylinositol 3-kinase - protein kinase C zeta-Sp1 pathway in the 1,25-dihydroxyvitamin D3 induction of the 25-hydroxyvitamin D3 24-hydroxylase gene in human kidney cells. Cellular Signalling. 2010;22:543–552. doi: 10.1016/j.cellsig.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Dwivedi PP, Hii CST, Ferrante A, Tan J, Der CJ, Omdahl JL, Morris HA, May BK. Role of MAP kinases in the 1,25-dihydroxyvitamin D3-induced transactivation of the rat cytochrome P450C24 (CYP24) promoter - Specific functions for ERK1/ERK2 and ERK5. Journal of Biological Chemistry. 2002;277:29643–29653. doi: 10.1074/jbc.M204561200. [DOI] [PubMed] [Google Scholar]
- English JM, Cobb MH. Pharmacological inhibitors of MAPK pathways. Trends Pharmacol Sci. 2002;23:40–45. doi: 10.1016/s0165-6147(00)01865-4. [DOI] [PubMed] [Google Scholar]
- Fedirko V, Bostick RM, Flanders WD, Long Q, Sidelnikov E, Shaukat A, Daniel CR, Rutherford RE, Woodard JJ. Effects of Vitamin D and Calcium on Proliferation and Differentiation In Normal Colon Mucosa: a Randomized Clinical Trial. Cancer Epidemiology Biomarkers & Prevention. 2009;18:2933–2941. doi: 10.1158/1055-9965.EPI-09-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichera A, Little N, Dougherty U, Mustafi R, Cerda S, Li YC, Delgado J, Arora A, Campbell LK, Joseph L, Hart J, Noffsinger A, Bissonnette M. A vitamin D analogue inhibits colonic carcinogenesis in the AOM/DSS model. J Surg Res. 2007;142:239–245. doi: 10.1016/j.jss.2007.02.038. [DOI] [PubMed] [Google Scholar]
- Fleet JC, Eksir F, Hance KW, Wood RJ. Vitamin D-inducible calcium transport and gene expression in three Caco-2 cell lines. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2002;283:G618–G625. doi: 10.1152/ajpgi.00269.2001. [DOI] [PubMed] [Google Scholar]
- Fleet JC, Wood RJ. Specific 1,25(OH)2 D3-mediated regulation of transcellular calcium transport in Caco-2 cells. Am J Physiol. 1999;276:G958–G964. doi: 10.1152/ajpgi.1999.276.4.G958. [DOI] [PubMed] [Google Scholar]
- Fleming JGW, Spencer TE, Safe SH, Bazer FW. Estrogen regulates transcription of the ovine oxytocin receptor gene through GC-rich SP1 promoter elements. Endocrinology. 2006;147:899–911. doi: 10.1210/en.2005-1120. [DOI] [PubMed] [Google Scholar]
- Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Molecular Endocrinology. 2003;17:2386–2392. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- Gao XH, Dwivedi PP, Omdahl JL, Morris HA, May BK. Calcitonin stimulates expression of the rat 25-hydroxyvitamin D3-24-hydroxylase (CYP24) promoter in HEK-293 cells expressing calcitonin receptor: identification of signaling pathways. Journal of Molecular Endocrinology. 2004;32:87–98. doi: 10.1677/jme.0.0320087. [DOI] [PubMed] [Google Scholar]
- Hagen G, Muller S, Beato M, Suske G. Cloning by Recognition Site Screening of 2 Novel Gt Box Binding-Proteins - a Family of Sp1 Related Genes. Nucleic Acids Research. 1992;20:5519–5525. doi: 10.1093/nar/20.21.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han OH, Li GD, Sumpio BE, Basson MD. Strain induces Caco-2 intestinal epithelial proliferation and differentiation via PKC and tyrosine kinase signals. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1998;275:G534–G541. doi: 10.1152/ajpgi.1998.275.3.G534. [DOI] [PubMed] [Google Scholar]
- Huang YC, Hung WC. 1,25-Dihydroxyvitamin D3 transcriptionally represses p45(Skp2) expression via the Sp1 sites in human prostate cancer cells. Journal of Cellular Physiology. 2006;209:363–369. doi: 10.1002/jcp.20741. [DOI] [PubMed] [Google Scholar]
- Husmann M, Dragneva Y, Romahn E, Jehnichen P. Nuclear receptors modulate the interaction of Sp1 and GC-rich DNA via ternary complex formation. Biochemical Journal. 2000;352:763–772. [PMC free article] [PubMed] [Google Scholar]
- Ismail A, Nguyen CV, Ahene A, Fleet JC, Uskokovic MR, Peleg S. Effect of Cellular Environment on the Selective Activation of the Vitamin D Receptor by 1α,25-dihydroxyvitamin D3 and its Analog 1α-fluoro-16-ene-20-epi-23-ene-26,27-bishomo-25-hydroxyvitamin D3 (Ro-26-9228) Mol Endocrinol. 2004;18:874–887. doi: 10.1210/me.2003-0310. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Fleet JC. Effect of phorbol 12-myristate 13-acetate activated signaling pathways on 1alpha, 25 dihydroxyvitamin D3 regulated human 25-hydroxyvitamin D3 24-hydroxylase gene expression in differentiated Caco-2 cells. J Cell Biochem. 2011 doi: 10.1002/jcb.24028. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G, Prosser DE, Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): Its important role in the degradation of vitamin-D. Arch Biochem Biophys. 2011 doi: 10.1016/j.abb.2011.11.003. In Press. [DOI] [PubMed] [Google Scholar]
- Kadonaga JT, Carner KR, Masiarz FR, Tijian R. Isolation of Cdna-Encoding Transcription Factor Sp1 and Functional-Analysis of the DNA-Binding Domain. Cell. 1987;51:1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- Kerry DM, Dwivedi PP, Hahn CN, Morris HA, Omdahl JL, May BK. Transcriptional synergism between vitamin D-responsive elements in the rat 25-hydroxyvitamin D3 24-hydroxylase (CYP24) promoter. J Biol Chem. 1996;271:29715–29721. doi: 10.1074/jbc.271.47.29715. [DOI] [PubMed] [Google Scholar]
- Klopot A, Hance KW, Peleg S, Barsony J, Fleet JC. Nucleo-cytoplasmic cycling of the vitamin D receptor in the enterocyte-like cell line, Caco-2. J Cell Biochem. 2006;100:617–628. doi: 10.1002/jcb.21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolell KJ, Crawford DL. Evolution of Sp transcription factors. Molecular Biology and Evolution. 2002;19:216–222. doi: 10.1093/oxfordjournals.molbev.a004074. [DOI] [PubMed] [Google Scholar]
- Koyama H, Inaba M, Nishizawa Y, Ohno S, Morii H. Protein-Kinase-C Is Involved in 24-Hydroxylase Gene-Expression Induced by 1,25(OH)2 D3 in Rat Intestinal Epithelial-Cells. Journal of Cellular Biochemistry. 1994;55:230–240. doi: 10.1002/jcb.240550210. [DOI] [PubMed] [Google Scholar]
- Li L, He SH, Sun JM, Davie JR. Gene regulation by Sp1 and Sp3. Biochemistry and Cell Biology-Biochimie Et Biologie Cellulaire. 2004;82:460–471. doi: 10.1139/o04-045. [DOI] [PubMed] [Google Scholar]
- Liu M, Freedman LP. Transcriptional Synergism between the Vitamin-D3 Receptor and Other Nonreceptor Transcription Factors. Molecular Endocrinology. 1994;8:1593–1604. doi: 10.1210/mend.8.12.7708050. [DOI] [PubMed] [Google Scholar]
- Ly LH, Zhao ZY, Holloway L, Feldman D. Liarazole acts synergistically with 1α,25-dihydroxyvitamin D3 to inhibit growth of DU145 human prostate cancer cells by blocking 24-hydroxylase activity. Endocrinology. 1999;140:2071–2076. doi: 10.1210/endo.140.5.6698. [DOI] [PubMed] [Google Scholar]
- Matys V, Fricke E, Geffers R, Gossling E, Haubrock M, Hehl R, Hornischer K, Karas D, Kel AE, Kel-Margoulis OV, Kloos DU, Land S, Lewicki-Potapov B, Michael H, Munch R, Reuter I, Rotert S, Saxel H, Scheer M, Thiele S, Wingender E. TRANSFAC (R): transcriptional regulation, from patterns to profiles. Nucleic Acids Research. 2003;31:374–378. doi: 10.1093/nar/gkg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner M, Stein M, Urbich C, Reisinger K, Suske G, Staels B, Kaufmann R, Gille J. PPAR alpha activators inhibit vascular endothelial growth factor receptor-2 expression by repressing Sp1-dependent DNA binding and transactivation. Circulation Research. 2004;94:324–332. doi: 10.1161/01.RES.0000113781.08139.81. [DOI] [PubMed] [Google Scholar]
- Meyer MB, Watanuki M, Kim S, Shevde NK, Pike JW. The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol Endocrinol. 2006;20:1447–1461. doi: 10.1210/me.2006-0031. [DOI] [PubMed] [Google Scholar]
- Meyer MB, Zella LA, Nerenz RD, Pike JW. Characterizing early events associated with the activation of target genes by 1,25-dihydroxyvitamin D3 in mouse kidney and intestine in vivo. J Biol Chem. 2007 doi: 10.1074/jbc.M703475200. [DOI] [PubMed] [Google Scholar]
- Miller GJ, Stapleton GE, Hedlund TE, Moffat KA. Vitamin D receptor expression, 24-hydroxylase activity, and inhibition of growth by 1α, 25-dihydroxyvitamin D3 in seven human prostatic carcinoma cell lines. Clin. Cancer. Res. 1995;1:997–1003. [PubMed] [Google Scholar]
- Moon SK, Choi YH, Kim CH, Choi WS. p38MAPK mediates benzyl isothiocyanate-induced p21WAF1 expression in vascular smooth muscle cells via the regulation of Sp1. Biochemical and Biophysical Research Communications. 2006;350:662–668. doi: 10.1016/j.bbrc.2006.09.092. [DOI] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: Structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chemical Reviews. 2001;101:2353–2364. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- Nomura N, Nomura M, Sugiyama K, Hamada JI. Phorbol 12-myristate 13-acetate (PMA)-induced migration of glioblastoma cells is mediated via p38MAPK/Hsp27 pathway. Biochemical Pharmacology. 2007;74:690–701. doi: 10.1016/j.bcp.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Nutchey BK, Kaplan JS, Dwivedi PP, Omdahl JL, Ferrante A, May BK, Hii CS. Molecular action of 1,25-dihydroxyvitamin D3 and phorbol ester on the activation of the rat cytochrome P450C24 (CYP24) promoter: role if MAP kinase activities and identification of an important transcription factor binding site. Biochemical Journal. 2005;389:753–762. doi: 10.1042/BJ20041947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen GI, Richer JK, Tung L, Takimoto G, Horwitz KB. Progesterone regulates transcription of the p21(WAF1) cyclin-dependent kinase inhibitor gene through Sp1 and CBP/p300. Journal of Biological Chemistry. 1998;273:10696–10701. doi: 10.1074/jbc.273.17.10696. [DOI] [PubMed] [Google Scholar]
- Pages G. Sp3-mediated VEGF regulation is dependent on phosphorylation by extra-cellular signals regulated kinases (Erk) Journal of Cellular Physiology. 2007;213:454–463. doi: 10.1002/jcp.21104. [DOI] [PubMed] [Google Scholar]
- Pipaon C, Tsai SY, Tsai MJ. COUP-TF upregulates NGFI-A gene expression through an Sp1 binding site. Molecular and Cellular Biology. 1999;19:2734–2745. doi: 10.1128/mcb.19.4.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R, Snyder RC, Thomas S, Koller CA, Miller DM. Mithramycin Blocks Protein-Binding and Function of the Sv40 Early Promoter. Journal of Clinical Investigation. 1989;83:2003–2007. doi: 10.1172/JCI114110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffer JD, Jackson SP, Annarella MB. Developmental Expression of Sp1 in the Mouse. Molecular and Cellular Biology. 1991;11:2189–2199. doi: 10.1128/mcb.11.4.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandelin A, Wasserman WW, Lenhard B. ConSite: web-based prediction of regulatory elements using cross-species comparison. Nucleic Acids Research. 2004;32:W249–W252. doi: 10.1093/nar/gkh372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapetschnig A, Koch F, Rischitor G, Mennenga T, Suske G. Complexity of translationally controlled transcription factor Sp3 isoform expression. Journal of Biological Chemistry. 2004;279:42095–42105. doi: 10.1074/jbc.M404989200. [DOI] [PubMed] [Google Scholar]
- Schule R, Muller M, Kaltschmidt C, Renkawitz R. Many Transcription Factors Interact Synergistically with Steroid-Receptors. Science. 1988;242:1418–1420. doi: 10.1126/science.3201230. [DOI] [PubMed] [Google Scholar]
- Shao A, Wood RJ, Fleet JC. Increased vitamin D receptor level enhances 1,25-dihydroxyvitamin D3- mediated gene expression and calcium transport in Caco-2 cells. J Bone Miner Res. 2001;16:615–624. doi: 10.1359/jbmr.2001.16.4.615. [DOI] [PubMed] [Google Scholar]
- Simboli-Campbell M, Gagnon AM, Franks DJ, Welsh JE. 1,25-Dihydroxyvitamin D3 Translocates Protein Kinase Cß to Nucleus and Enhances Plasma Membrane Association of Protein Kinase C-alpha in Renal Epithelial Cells. The Journal of Biological Chemistry. 1994;269:3257–3264. [PubMed] [Google Scholar]
- Song Y, Peng X, Porta A, Takanaga H, Peng JB, Hediger MA, Fleet JC, Christakos S. Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology. 2003;144:3885–3894. doi: 10.1210/en.2003-0314. [DOI] [PubMed] [Google Scholar]
- St-Arnaud R, Arabian A, Travers R, Barletta F, Raval-Pandya M, Chapin K, Depovere J, Mathieu C, Christakos S, Demay MB, Glorieux FH. Deficient mineralization of intramembranous bone in vitamin D-24-hydroxylase-ablated mice is due to elevated 1,25-dihydroxyvitamin D and not to the absence of 24,25-dihydroxyvitamin D. Endocrinology. 2000;141:2658–2666. doi: 10.1210/endo.141.7.7579. [DOI] [PubMed] [Google Scholar]
- Strahle U, Schmid W, Schutz G. Synergistic Action of the Glucocorticoid Receptor with Transcription Factors. EMBO Journal. 1988;7:3389–3395. doi: 10.1002/j.1460-2075.1988.tb03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supp DM, Witte DP, Branford WW, Smith EP, Potter SS. Sp4, a member of the Sp1-family of zinc finger transcription factors, is required for normal murine growth, viability, and male fertility. Developmental Biology. 1996;176:284–299. doi: 10.1006/dbio.1996.0134. [DOI] [PubMed] [Google Scholar]
- Tashiro K, Ishii C, Ryoji M. Role of distal upstream sequence in vitamin D-induced expression of human CYP24 gene. Biochem Biophys Res Commun. 2007;358:259–265. doi: 10.1016/j.bbrc.2007.04.103. [DOI] [PubMed] [Google Scholar]
- Thiesen HJ, Bach C. Target Detection Assay (Tda) - A Versatile Procedure to Determine Dna-Binding Sites As Demonstrated on Sp1 Protein. Nucleic Acids Research. 1990;18:3203–3209. doi: 10.1093/nar/18.11.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon PH, Harnois C, Grenier A, Dufour G, Bouchard V, Han J, Landry J, Beaulieu JF, Vezina A, Dydensborg AB, Gauthier R, Cote A, Drolet JF, Lareau F. Differentiation state-selective roles of p38 isoforms in human intestinal epithelial cell anoikis. Gastroenterology. 2002;123:1980–1991. doi: 10.1053/gast.2002.37072. [DOI] [PubMed] [Google Scholar]
- Wang L, Klopot A, Freund JN, Dowling LN, Krasinski SD, Fleet JC. Control of Differentiation-Induced Calbindin-D9k Gene Expression in Caco-2 Cells by Cdx-2 adn HNF-1α. Am J Physiol. 2004;287:G943–G953. doi: 10.1152/ajpgi.00121.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]