The field of brain imaging has benefited from enormous technological advances over the past 25 years. There is rapid progress in the application of magnetic resonance imaging (MRI) for functional imaging (fMRI) and positron emission tomography (PET) for biochemical imaging.
Imaging techniques enable us to assess the properties of brain tissue and to obtain information of how the brain works across scales from the systems level to the molecular level. These assessments and imaging modalities include:
(i) Brain morphology and tissue composition: computerized axial tomography (CAT) and MRI.
(ii) Electrical and magnetic signals which result from the communication between cells and hence can be used to assess regional brain activation: electroencephalography (EEG) and magnetoencephalography (MEG).
(iii) Biochemical components of neurotransmission which provide information on neuronal activity and communication: PET, single-photon emission computed tomography (SPECT), and magnetic resonance spectroscopy (MRS).
(iv) Physiological processes which provide information on tissue energy requirements and cerebral blood flow and hence can be used to assess regional brain function: fMRI, PET, SPECT, dynamic CAT.
The sensitivity and specificity for different parameters defines the unique properties as well as the limitations of each imaging modality. For applied research it is thus important to compare one with another with respect to spatial resolution, temporal resolution, sensitivity, and biochemical specificity (Table 1).
Table 1.
Comparisons among the most commonly used functional and/or biochemical imaging modalities
| Imaging instrument | Temporal resolution | Spatial resolution | Sensitivity |
|---|---|---|---|
| MEG | 1 msec | 5 mm | |
| EEG | 1 msec | 10–15 mm | |
| MRI | 3–5 sec | 1.0–1.5 mm | 10−3 molar (mM) |
| PET* | 45 sec | 4 mm | 10−12 molar (pM) |
| SPECT | >60 sec | 6–8 mm | 10−12 molar |
For PET the temporal resolution shown corresponds to that obtained when using H215O to measure cerebral blood flow; otherwise, for dynamic studies measures can be made at 15- to 30-sec intervals (provided there is adequate counts).
fMRI has the highest spatial resolution of the imaging technologies that are used for functional mapping of the human brain (1–3). The resolution is significantly better than that of PET and does not require ionizing radiation. This is advantageous because the regional representation in cortex involves areas that are smaller than the current resolution of most PET scanners, and multiple studies can be done in the same subject without the limitation of radiation exposure (Fig. 1). However, compared with PET, the technique is limited by the lack of absolute quantitation. It is generally believed that the activation signal which is generated from fMRI is due to differences in magnetic properties of oxygenated versus deoxygenated hemoglobin (BOLD contrast). During activation of a brain region, there is an excess of arterial blood delivered into the area with concomitant changes in the ratio of deoxyhemoglobin to oxyhemoglobin. It has still not been clarified whether this relative excess of oxyhemoglobin in the activated region is due to perfusion of nonactivated adjacent areas or to excess oxygenation in the activated area secondary to anaerobic glycolysis. However, because functional processes in brain occur at the millisecond range, whereas hemodynamic changes are slower, fMRI is limited in its ability to sequence the temporal patterns of serial and/or parallel activation. In this respect MEG and EEG are superior in that they enable investigators to assess the temporal displacement of activation signals as they propagate in brain on the order of a few milliseconds (review in ref. 4). A current challenge is to combine the fMRI with MEG and/or EEG to produce maps with millimeter spatial, and millisecond temporal, resolution across the entire human brain. Such a development seems likely in the next several years, and would be unique in its ability to assess brain function at a system level.
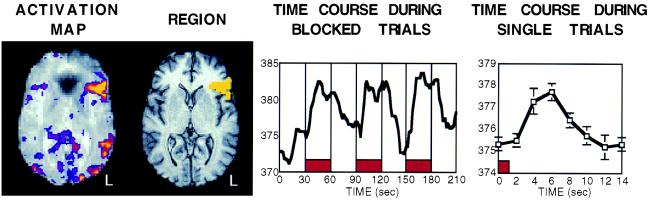
Figure 1.
fMRI activation images obtained with a visually guided generation task. The leftmost figure shows a horizontal section of an activation map. Areas of increased activation (BOLD contrast) are shown in brighter colors overlaid on top of a T2* weighted image. Robust activations are seen in visual cortex and in left prefrontal cortex. The image second to the left shows a region (in yellow) that was defined around significantly activated voxels in left prefrontal cortex. The next image shows the time course of this region’s activity during periods where multiple trials of the word-generation task were performed during 30-sec blocks (placement of blocks shown in red). Note the consistent and sustained signal increase that occurs shortly after the onset of the word-generation task. The rightmost image shows what happens in the same left prefrontal region when the time course is examined during a different paradigm involving separated individual trials of the task. Within such a paradigm, a small but reliable signal increase was observed in relation to the onset of individual trials of the task (placement of the averaged 1.5-sec trial shown in red). Courtesy of Massachusetts General Hospital.
Brain neurotransmission is dependent on biochemical processes that occur at very low concentrations, typically in the nanomolar to the picomolar range. This is the reason for the superiority of nuclear medicine technologies, since they have the highest sensitivity of all currently available imaging techniques (see ref. 5 for review and ref. 6 for commentary). The high sensitivity is given by the use of radiotracers that bind selectively to molecules in tissue. PET or SPECT technologies are used for the measurement of molecular targets such as receptors, transporters, and the enzymes that are involved in the synthesis and metabolism of neurotransmitters (reviewed in ref. 7). The high sensitivity of PET and SPECT allows the use of radiolabeled compounds at concentrations that are devoid of pharmacological effect and hence do not perturb the system. The use of radiotracers that are tailored for selective labeling of a molecule enables investigators to achieve highly selective specificity with these techniques. For example, they can be used for selective imaging of transporters, which are presynaptic molecules in the nerve terminal, and receptors, which are mostly located in postsynatic cells, despite the fact that these two molecules are 20–50 nm apart. It can also be used to label enzymes located in different cellular populations (Fig. 2). Biochemical specificity is important not only to investigate disease processes but also to identify targets for pharmacological agents.
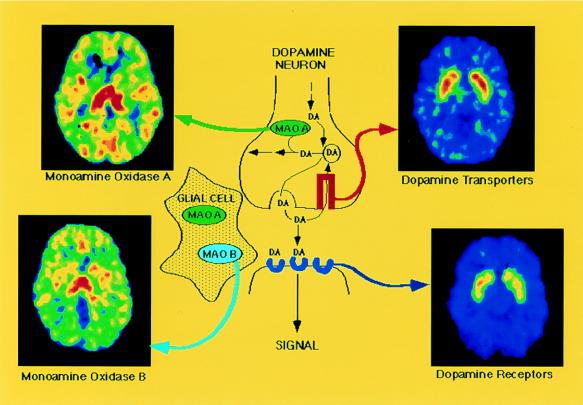
Figure 2.
Diagram of the dopamine (DA) synapse along with the PET images for different molecular targets: DA D2 receptors are imaged with [11C]raclopride, DA transporters are imaged with d-threo-[11C]methylphenidate, monoamine oxidase A is imaged with [11C]clorgyline, and monoamine oxidase B (located in glial cells) is imaged with deuterium-substituted [11C]deprenyl. Courtesy of Brookhaven National Laboratory.
Finally, the labeling of drugs with positron emitters such as carbon (11C) does not change the pharmacological properties. PET can therefore be uniquely used to investigate drug distribution and biochemical interactions in the human brain. This approach has a major potential in drug development. Because studies are performed in awake human subjects it is possible to assess the relation between the biochemical interactions of the drug and the temporal sequence for drug-induced behavioral effects.
It is 25 years since the first papers describing PET, MRI, and CAT were published; since then dramatic advances in computation, instrumentation, and radiopharmaceutical development have occurred that have enabled investigators to probe the morphological, physiological, and biochemical aspects of brain function and age and disease-related disturbances. We can predict that future technological developments will continue to occur along with our ability to pose ever more sophisticated questions of how the brain works. We have now opened the brain for observation. The challenge for the years ahead is to learn how to see it work.
ABBREVIATIONS
- MRI
magnetic resonance imaging
- fMRI
functional MRI
- PET
positron emission tomography
- CAT
computerized axial tomography
- EEG
electroencephalography
- MEG
magnetoencephalography
- SPECT
single-photon emission computed tomography
References
- 1.Bandettini P A, Wong E C, Hinks R S, Tikofsky R S, Hyde J S. Magn Reson Med. 1992;25:390–397. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- 2.Kwong K K, Belliveau J W, Chesler D A, Goldberg I E, Weisskoff R M, Poncelet B P, Kennedy D N, Hoppel B E, Cohen M S, Turner R, Cheng H-M, Brady T J, Rosen B R. Proc Natl Acad Sci USA. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogawa S, Tank D W, Menon R, Ellermann J M, Kim S-G, Merkle H, Ugurbil K. Proc Natl Acad Sci USA. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lounasmaa O V, Hämäläinen M, Hari R, Salmelin R. Proc Nat Acad Sci USA. 1996;93:8809–8815. doi: 10.1073/pnas.93.17.8809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phelps M E, Mazziotta J C, Schelbert H. Positron Emission Tomography and Autoradiography: Principles and Applications for the Brain and Heart. New York: Raven; 1986. [Google Scholar]
- 6.Jones T. Eur J Nucl Med. 1996;23:207–211. doi: 10.1007/BF01731847. [DOI] [PubMed] [Google Scholar]
- 7.Volkow N D, Fowler J S, Gatley J S, Logan J, Wang G-J, Ding Y-S, Dewey S L. J Nucl Med. 1996;37:1242–1256. [PubMed] [Google Scholar]