Abstract
We describe an outbreak of sudden health problems in workers at a Danish grass seed plant after exposure to a particularly dusty lot of grass seeds. The seeds are called problematic seeds. The association between development of organic dust toxic syndrome (ODTS) and the handling of grass seeds causing exposure was assessed in a four-step model: (i) identification of exposure source, (ii) characterization of the emission of bioaerosols from the problematic and reference seeds, (iii) personal and stationary exposure measurement at the plant and (iv) repeated health examinations. The grass seeds were identified as the exposure source; the emissions of some bioaerosol components were up to 107 times higher from the problematic seeds than from reference seeds. Cleaning of the seeds was not enough to sufficiently reduce the high emission from the problematic seeds. Emission in terms of dust was 3.4 times as high from the problematic cleaned seeds as from cleaned reference seeds. The personal exposure reached 3 × 105 endotoxin units m−3, 1 × 106 colony-forming units (cfu) of thermophilic actinomycetes m−3, 8 × 105 cfu of Aspergillus fumigatus m−3 and 9 × 106 hyphal fragments m−3. Several workers working with the problematic seeds had symptoms consistent with ODTS. The most severe symptoms were found for the workers performing the tasks causing highest exposure. Respiratory airway protection proved efficient to avoid development of ODTS. Work with reference seeds did not cause workers to develop ODTS. Exposure was during work with the problematic seeds higher than suggested occupational exposure limits but lower than in studies where researchers for some minutes have repeated a single task expected to cause ODTS. In this study, many different bioaerosol components were measured during a whole working day. We cannot know, whether it is the combination of different bioaerosol components or a single component which is responsible for the development of ODTS. In conclusion, workers developed specific health symptoms due to the high bioaerosol exposure and were diagnosed with ODTS. Exposure to high concentrations of endotoxin, actinomycetes, fungi, hyphal fragments, β-glucan, and A. fumigatus occurred when working with a dusty lot of grass seed. Suspicion should be elicited by seeds stored without being properly dried and by seeds producing more dust than usually.
Keywords: endotoxin, hyphal fragments, (1 → 3)-β-D-glucan, ODTS, toxic alveolitis
INTRODUCTION
Grass seed is an important crop in Danish agriculture and Denmark is a leading exporter of grass seeds accounting for 40% of the total grass seed production in the European Union (The Danish Seed Council, 2010). However, little is known about health risks associated with transporting, cleaning, and packaging grass seed. It is expectable that airway symptoms would be frequent in this kind of work as it is in several other occupations in which there is also exposure to bioaerosols (e.g. Sigsgaard et al., 1994; Vogelzang et al., 1999; Von Essen et al., 2007). Endotoxin from bacteria is one of the microbial components which can be present in high concentrations in bioaerosols. Endotoxin has been shown to correlate with acute lung function changes, accelerated lung function decline, work-related and chronic respiratory symptoms, and airway hyper-responsiveness (Rylander et al., 1985; Smid et al., 1992). Exposure to fungi in bioaerosols has been related to symptoms in the eyes and nose and to cough (Melbostad and Eduard, 2001) as well as to asthma symptoms and work-related respiratory symptoms (Eduard et al., 2004; Schlünssen et al., 2011).
Organic dust toxic syndrome (ODTS) is related to exposure to very high concentrations of bioaerosol components. ODTS is also known as inhalation fever or toxic alveolitis, and it is an acute, non-allergic, self-limiting illness characterized by flu-like symptoms: fever, chills, chest tightness, shortness of breath, dry cough, myalgia, and general fatigue. Onset is typically within a few hours after exposure to organic dust and duration of symptoms is often <24 h but may last 5–7 days (Rask-Andersen, 1989; Seifert et al., 2003). Occupational endotoxin exposure seems to increase the risk for ODTS (Basinas et al., 2011) and increased time of exposure seems to increase the risk for ODTS (Brinton et al., 1987). Exposure to very high concentrations of dust, fungi, and bacteria has been found at a compost plant where a worker developed ODTS or hypersensitivity pneumonitis (HP) (Weber et al., 1993). However, in most studies of ODTS exposure to bioaerosol components have not been measured (Brinton et al., 1987; Rask-Andersen, 1989; May et al., 1990); instead of exposure the content of, for example, fungi in the organic material expected to cause ODTS has been measured in some studies (Boehmer et al., 2009).
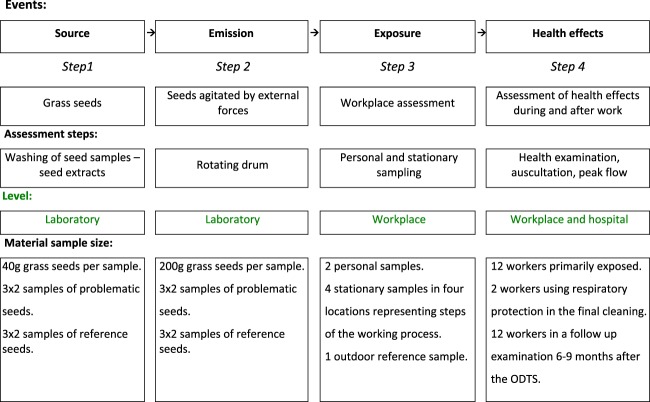
ODTS is difficult to measure retrospectively because the symptoms are similar to flu symptoms and because onset is typically a few hours after exposure has occurred and people for these reasons do not necessarily relate the symptoms to an earlier exposure at work. We herein present the first well-documented outbreak of ODTS in a grass seed cleaning facility in Denmark, supplemented with a thorough characterization of the exposure causing ODTS and of seeds causing ODTS versus reference seeds. We used an extended version of a conceptual approach to characterize grass seeds for inhalation exposure risk and associated health effects (Madsen et al., 2006). The concept included in this study a Step 1 used for hazard identification, a Step 2 used to characterize the emission of bioaerosols from the problematic grass seeds and reference seeds, a Step 3 with personal and stationary exposure measurements at the grass seed plant during an entire work day, and a Step 4 with repeated health examinations (Fig. 1).
Fig. 1.
The conceptual approach used to characterize grass seeds for inhalation exposure risk assessment and associated health effects.
MATERIALS AND METHODS
Description of plant I and plant II
Plant I receives seeds of grass and clover for cleaning and packaging. Grass seeds were unloaded from lorries inside the plant and mechanically transported into the centre of the plant through a closed system. In an intake hall, where the seeds are either loaded through tubes into open, wooden boxes, which are then covered with a cardboard lid, or transported by a closed conveyor belt to a silo and later transported to the cleaning machines. The boxes with seed are carried by a forklift and emptied 3–4 m above the floor into cleaning machines with a system of large, moving or rotating sieves. The seeds were then moved to the main part of the machines and placed on the floor close to the operator; dust was emitted as these machines were partly open. The cleaned seeds were again loaded into boxes and transported by forklift to another hall, where they are packaged into bags or wooden boxes covered with a lid. Finally, they are loaded through open gates on to lorries to be sold.
Plant II is a similar, recently closed facility but as it was still functional, it was selected for the final cleaning of the specific lot of seeds (in the following called problematic seeds) in an isolated operation. In this plant, the cleaning machines were newer.
Tasks in the grass seed facility
During the day where the problematic seeds were received the 12 employees (called worker 1 to 12) worked with reference seeds or the problematic seeds. During the day of exposure measurement, the two workers, called workers 1b and 2b, worked with the problematic seeds. All 12 workers each carried out one or more of the following tasks:
Lorry drivers stood beside the lorry when it was emptied by dumping grass seeds into a transport screw in the floor and again when the lorry was loaded.
Receiving and depositing the seeds in boxes; the worker stood close to big boxes on the floor being filled with seeds from a pipe. Exhaust ventilation was present around the box. Visible dust was released from the falling seeds.
Driving fork lifts with boxes with seed to be emptied into the cleaning machines and to the packaging facility. The cabins of the forklift trucks were closed, but air intake to the cabin was without a dust filter.
Packaging: seeds that have been cleaned were emptied into big bags or boxes by an automatized machine operated by a worker standing some meters away.
All workers had been employed at the grass seed plant for at least 3 years.
Health examination of employees
After only a few hours of unloading and cleaning of the lot of problematic grass seeds 5 out of 12 workers at plant I began to experience airway symptoms and flu-like symptoms along with malaise. All workers were males. Cleaning was stopped the following morning as workers 3 and 4 were brought to the hospital due to respiratory symptoms. The problematic grass seed was reported to produce much more visible dust than normal grass seed. According to the seed company, the problematic seeds had been stored on the farm without being sufficiently dried before storage.
All 10 employees at work during the time the problematic seeds were handled as well as the two truck drivers (workers 11 and 12) transporting the seeds were offered a general health examination at the hospital. The examination included a complete medical history, auscultation, spirometry (Dry ‘Vitalograph ®’, measuring forced expiratory volume in one second (FEV1), forced vital capacity (FVC), FEV1/FVC, and peak expiratory flow), blood sample (leukocyte count, C-reactive protein [CRP], total IgE, and specific IgE against specific moulds (Penicillium chrysogenum, Cladosporium herbarum, Aspergillus fumigatus, and Alternaria alternata; ImmunoCAP®; Phadia, Uppsala, Sweden), grasses, and a standard mix of airborne allergens (Phadiatop®; Phadia). Chest X-ray scans were only performed on workers with symptoms to avoid unnecessary radiation.
After the first health examination, the problematic seeds were cleaned at plant II by two selected workers (workers 1 and 2 and in the final cleaning called workers 1b and 2b). They were supplied with a powered air-purifying respirator with a face shield and helmet (Compact Air mod. Junior A, filter P3, North®; Honeywell, USA) and were examined at the beginning and end of the first work day as well as after 17 days, when cleaning of the problematic seeds was finished. These examinations included health anamnesis, auscultation, inspection of mucosal membranes of the nose, throat and eyes, and spirometry. Furthermore, these two workers were instructed in monitoring expiratory peak flow value (PF) during work and at home, at least six times a day, during the 11 working days the cleaning of the problematic lot was on going. Worker 1b, who failed to use the respiratory airway protection correctly, had an extra examination after 11 days.
After 6–9 months, all 12 workers went through a follow-up examination.
Seeds used for exposure analysis
Four sacks of grass seeds (tall fescue, Festuca arundinaceae) were obtained from the seed company for analysis: Two sacks of the problematic seeds, one uncleaned and one cleaned, and two sacks of reference seeds, one uncleaned and one cleaned. Three samples of 5 g of each kind of seed were weighed before and after drying in an oven (105°C, 18 h) in order to measure the water content. The moisture content of the grass seeds was between 6.1 and 7.3%.
Inhalation exposure risk assessment
Total amount of microbial components in seeds (Step 1).
A subsample of 40 g was taken from each of the four kinds of grass seed samples and shaken (250 r.p.m.) in isotonic water for 15 min on ice (washing) (Fig. 1). The suspensions of seed extracts were used for quantifying colony-forming units (cfu) of fungi and actinomycetes and also for endotoxin analysis. The experiment was performed in triplicate.
Agitation of seeds in a rotating drum (Step 2).
Emission of dust from each of the four kinds of grass seed samples was determined according to the European standard EN 15051 (CEN—European Committee for Standardization, 2006) using a rotating drum (HSE Rotating Drum Dustiness Tester model EDT38L; JS Holdings, Hertfordshire, UK) (Fig. 1). Bulk samples weighing 200 g (≈270 ml) were used, as suggested in MDHS 81 (Health and Safety Laboratory, 1996). The drum was 0.3 m in diameter and equipped with eight internal longitudinal vanes, which lifted the sample and subsequently let it fall at a rate of 4 r.p.m. for 1 min. An air current created by a vacuum pump (flow rate = 38 l min− 1; mean velocity 0.1 m s− 1 [Pensis et al., 2010]) acted on the suspended material causing dust to be released and collected on a polycarbonate filter at the end of the drum. Each filter was cut into two equal pieces, one piece used for endotoxin analysis, and the other for quantification of microorganisms. Data are presented as concentrations (unit per milligram dust) (Table 2) and as dustiness (amount aerosolised per kilogram grass seeds) (Table 3). The experiment was performed in triplicate.
Table 2.
Concentrations (amount mg− 1 dust) of endotoxin and microorganisms in dust aerosolised from grass seeds (n = 3) and amount of aerosolised dust in the rotating drum (Step 2).
| Source | Dust (mg) | Endotoxin (EU) | Mesophilic fungi (cfu) | Aspergillus fumigatus (cfu) | Thermophilic actinomycetes (cfu) | Mesophilic actinomycetes (cfu) |
| Problematic seeds, uncleaned | 72.4a | 1.05 × 104b | 1.32 × 106a | 4.14 × 105a | 6.22 × 106a | 1.45 × 107a |
| Problematic seeds, cleaned | 9.9c | 1.28 × 104a | 3.03 × 105b | 1.38 × 105b | 7.58 × 105b | 3.79 × 106b |
| Reference seeds, uncleaned | 14.5b | 2.27 × 103c | 8.28 × 103c | 2.61 × 101c | 1.9d | 3.82 × 104b |
| Reference seeds, cleaned | 2.9d | 5.49 × 102d | 1.12 × 104c | 9.31 × 101c | 1.45 × 103c | 3.01 × 104b |
Numbers in the same column followed by the same letter are not significantly different (P > 0.05).
Table 3.
Dustiness of grass seeds (n = 3) in terms of numbers of microorganisms and amount of endotoxin and dust aerosolised during 1 min of rotation in a rotating drum (amount aerosolised kg− 1 grass seed) (Step 2).
| Source | Dust (mg) | Endotoxin (EU) | Mesophilic fungi (cfu) | Aspergillus fumigates(cfu) | Thermophilic actinomycetes (cfu) | Mesophilic actinomycetes (cfu) |
| Problematic seeds, uncleaned | 362a | 3.79 × 106a | 4.77 × 108a | 1.50 × 108a | 2.25 × 109a | 5.25 × 109a |
| Problematic seeds, cleaned | 49.5c | 6.41 × 105b | 1.50 × 107b | 6.82 × 106b | 3.75 × 107b | 1.88 × 108b |
| Reference seeds, uncleaned | 72.5b | 1.62 × 105c | 6.36 × 105c | 2.03 × 103c | 1.57 × 102d | 2.77 × 106c |
| Reference seeds, cleaned | 14.5d | 7.78 × 103d | 1.62 × 105d | 1.35 × 103c | 2.10 × 104c | 4.36 × 105d |
Numbers in the same column followed by the same letter are not significantly different (P > 0.05).
Personal and stationary sampling of inhalable aerosols (Step 3).
Personal dust monitoring was conducted using GSP inhalable samplers (CIS by BGI, INC Waltham, MA, USA) from 7 am to 3 pm in May 2009 (Fig. 1). Each worker carried two GSP samplers (flow rate 3.5 l min− 1; Apex pumps, Castella, England), one mounted with a Teflon filter (Millipore, Copenhagen, Denmark, pore size 1 μm) for gravimetric and endotoxin analysis and one with a polycarbonate filter (pore size 1 μm; GE Water & Process Technologies, Trevose, PA, USA) for quantification of microorganisms and pollen. While personal dust monitoring was being performed, we recorded the different tasks being carried out by the two workers. Results are presented as time-weighted averages.
Inhalable bioaerosols were sampled by stationary GSP samplers for ∼7 h in four working areas representing different steps in the seed treatment process. In addition, an outdoor reference was made upwind from the plant.
Gravimetric analysis.
The mass of the dust collected on the GSP and rotating drum filters was determined by weighing the filters before and after dust sampling. Before weighing, the filters used for collecting the dust were equilibrated at constant air temperature and humidity for 20–24 h (22°C and 50% relative humidity ± 5%).
Extraction of dust.
The dust for endotoxin analysis was extracted in 10.0 ml pyrogen-free water with 0.05% Tween 20 by orbital shaking (300 r.p.m.) at room temperature for 60 min and centrifuging (1000 g) for 15 min. The supernatant was stored at −80°C. The dust on polycarbonate filters for quantification of microorganisms was extracted in 10.0 ml sterile 0.05% Tween 80 and 0.85% NaCl aqueous solution by shaking for a 15-min period (500 r.p.m.) at room temperature.
Determination of endotoxin and ß-glucan by the Limulus method.
The supernatant was analysed (in duplicate) for endotoxin using the kinetic Limulus amoebocyte lysate test (Kinetic-QCL endotoxin kit; BioWhittaker, Walkersville, Maryland, USA) with β-glucan blocker. A standard curve obtained from an Escherichia coli O55:B5 reference endotoxin was used to determine the concentrations in terms of endotoxin units (EU) (10.0 EU ≈ 1.0 ng). All samples were diluted at least 10 times before the endotoxin analysis was performed. The limit of detection was 0.05 EU ml− 1. The data are presented as EU m− 3 air, as EU mg− 1 dust or as EU kg− 1 seeds.
Airborne β-glucan was extracted using 0.3 M NaOH for 60 min. After extraction, β-glucan was quantified in duplicate using the kinetic Fungitic G Test (Seikagaku Co., Tokyo, Japan). We used a standard curve ranging from 4.0 to 100 pg ml− 1. The data are presented as nanogram per cubic metre air.
Quantification of microorganisms (CAMNEA).
Microorganisms were quantified using a modified CAMNEA method (Palmgren et al., 1986). The number of fungi culturable on dichloran glycerol agar (DG18 agar; Oxoid, Basingstoke, England) at 25°C was counted after 3 and 7 days of incubation. In addition, DG18 agar plates were incubated at 45°C to quantify culturable A. fumigatus. Mesophilic actinomycetes and thermophilic actinomycetes (55°C) were after 3 and 7 days of incubation quantified on 10 and 100% nutrient agar (Oxoid) with actidione (cycloheximide; 50 mg l− 1), respectively.
The total numbers of bacteria, fungal spores, hyphal fragments, and grass pollen were determined in samples from Step 3 after staining in 20 ppm acridine orange (Merck, Hellerup, Denmark) in acetate buffer for 30 s with subsequent filtration through a polycarbonate filter (0.4 μm; Nuclepore, Cambridge, MA, USA). Counts were made at a magnification of 1250 times in 40 randomly chosen fields using epifluorescence microscopy (Orthoplan; Leitz, Wetzlar, Germany). The number of fungal spores is called ‘total fungi’ and the number of bacterial cells is called ‘total bacteria’. Total fungi, total bacteria, hyphal fragments, and grass pollen are expressed as number per cubic metre air. The detection limit was 1.6 × 104 units m−3 air.
Statistical analysis
Statistical analyses were performed with SAS (Version 8e; SAS Institute, Cary, NC, USA). Analysis of variance (t-test, PROC ANOVA) was used on the lognormal distributed data to test the significance of the differences between the seed samples with regard to concentrations of the different microorganisms, dust, and endotoxin.
RESULTS
Inhalation exposure risk assessment
Grass seed extracts (Step 1).
The total amount of endotoxin and microorganisms in seed extracts (Step 1) are presented in Table 1. The concentrations of all components are significantly higher in the extracts from problematic seeds than in reference seeds also after cleaning of the problematic seeds. More endotoxin was aerosolised from uncleaned seeds than from cleaned seeds both when considering problematic and reference seeds. In addition, endotoxin and microorganisms were aerosolised in significantly higher amounts from the cleaned problematic seeds than from uncleaned reference seeds.
Table 1.
Concentrations (amount kg− 1 seed) of endotoxin and microorganisms in grass seed extracts (Step 1).
| Source | Endotoxin (EU) | Mesophilic fungi (cfu) | Aspergillus fumigatus (cfu) | Thermophilic actinomycetes (cfu) | Mesophilic actinomycetes (cfu) |
| Problematic seeds, uncleaned | 2.63 × 109a | 2.00 × 1010a | 4.50 × 109a | 1.20 × 1011a | 2.85 × 101a1 |
| Problematic seeds, cleaned | 1.71 × 109b | 1.18 × 1010a | 3.10 × 109a | 1.35 × 1011a | 4.36 × 1010b |
| Reference seeds, uncleaned | 8.02 × 107c | 2.70 × 107c | 9.00 × 103b | 3.60 × 105b | 1.80 × 106d |
| Reference seeds, cleaned | 1.48 × 107d | 4.10 × 108b | 9.20 × 103b | 9.00 × 103c | 4.50 × 107c |
Numbers in the same column followed by the same letter are not significantly different (P > 0.05).
Emissions from grass seeds (Step 2).
Each grass seed sample was rotated in a drum for 1 min (Step 2) and the concentrations of endotoxin and microorganisms in the released dust are presented in Table 2 and the amounts aerosolised per kilogram seed are termed dustiness and are presented in Table 3.
Significantly higher concentrations of all microbial components were found in the dust from the problematic seeds than in dust from the reference seeds (P < 0.0001 for all components) (Table 2). From the uncleaned problematic seeds, five times as much dust was aerosolised as from the uncleaned reference seeds. Even after cleaning, 3.4 times as much dust was aerosolised from the problematic seeds as from the cleaned reference seeds (Table 3). It is notable that especially the thermotolerant fungus A. fumigatus and thermophilic actinomycetes were aerosolised from the problematic uncleaned seeds in very high amounts compared to the corresponding reference seeds (Table 3).
Exposure at plant II (Step 3).
Personal and stationary exposure measured during working hours at plant II and an outdoor reference measurement are presented in Table 4 and Table 5. Personal exposures to all microorganisms and endotoxin exceeded suggested occupational exposure limits (OELs). Stationary measurements showed high concentrations in all areas at the plant, but the seed reception (S1) and the seed silo area (S2) were the most problematic working areas concerning exposure to dust and endotoxin.
Table 4.
Exposure (twa m− 3 air) to dust, endotoxin and bacteria (Step 3).
| Position (s) or worker | Measurement | Dust (mg) | Endotoxin (EU) | Total bacteria (number) | Thermophilic actinomycetes (cfu) | Mesophilic actinomycetes (cfu) |
| Worker 1b, position S1, S4 and in a trucka | Personal | 4.15 | 3.22× 105 | 1.25 × 108 | 1.19× 106 | 1.26 × 108 |
| Worker 2b, position S4 and in a truck | Personal | 1.52 | 1.40× 105 | 1.08 × 108 | 1.05× 106 | 2.45 × 107 |
| Seed reception (S1) | Stationary | 1.49 | 7.44× 105 | 8.44 × 107 | 7.83× 105 | 3.43 × 107 |
| Seed silo (S2) | Stationary | 9.10 | 1.34× 105 | 1.58 × 108 | 9.05× 106 | 3.05 × 108 |
| Seed cleaning (S3) | Stationary | 1.05 | 4.25× 104 | 4.06 × 107 | 1.53× 106 | 1.93 × 107 |
| Packaging of cleaned seeds (S4) | Stationary | 0.26 | 2.39× 104 | 2.08 × 107 | 1.29× 106 | 6.80 × 106 |
| Outdoor reference | Stationary | 0.011 | 2.35 | Bd | 9.74 × 102 | 3.82 × 103 |
Bd, below detection level; Twa, time-weighted average. Concentrations higher than suggested exposure limits (OELs) are in bold. For dust, it is concentrations larger than 3 mg m−3 air (Arbejdstilsynet, 2007); for endotoxin, it is concentrations larger than 90 EU m−3 (Health Council of the Netherlands, 2010) and for cfu of thermophilic actinomycetes, it is concentrations larger than 2 × 104 m−3 (Dutkiewicz et al., 1994). For cfu of mesophilic actinomycetes, no suggested TLV is available.
Areas the workers reported to be occupied in.
Table 5.
Exposure (twa m− 3) to fungi, fungal components and grass pollen (Step 3).
| Position (S) or worker | Measurement | Total fungi (number) | Hyphal fragments (number) | ß-glucan (ng) | Mesophilic fungi (cfu) | Aspergillus fumigatus (cfu) | Grass pollen (number) |
| Worker 1b, position S1, S4 and in a truck | Personal | 2.46× 107 | 8.69 × 106 | 4.84 × 103 | 7.95 × 106 | 8.09 × 105 | 1.45 × 105 |
| Worker 2b, position S4 and in a truck | Personal | 1.10× 107 | 3.05 × 106 | 2.82 × 103 | 1.62 × 106 | 4.09 × 105 | 2.93 × 105 |
| Seed reception (S1) | Stationary | 7.17× 106 | 8.52 × 105 | 1.62 × 103 | 1.84 × 106 | 5.02 × 106 | 2.62 × 104 |
| Seed silo (S2) | Stationary | 7.01× 106 | 1.09 × 106 | 2.22 × 103 | 1.61 × 106 | 6.79 × 106 | 8.11 × 104 |
| Seed cleaning (S3) | Stationary | 3.01× 106 | 4.33 × 105 | 1.66 × 103 | 1.05 × 106 | 4.12 × 105 | 1.38 × 104 |
| Packaging of cleaned seeds (S4) | Stationary | 2.13× 106 | 3.64 × 105 | 1.97 × 103 | 6.9 × 105 | 3.20 × 105 | 5.12 × 103 |
| Outdoor reference | Stationary | Bd | Bd | 1.81 × 101 | 9.74 × 102 | 2.44 × 102 | Bd |
Bd, below detection level; twa, time-weighted average. Suggested OEL only available for fungal spores: concentrations larger than 105 spores m−3 air are in bold according to a suggested OEL (Eduard, 2009).
Health reports and medical examination
Ten people (workers 1–10) worked at the plant the first day the problematic seeds in addition to reference seeds were handled in the facility. Two drivers (workers 11 and 12) transported the problematic seeds. All 12 underwent a health examination, and seven workers allowed a blood sample to be taken. Workers 1 and 2 are mentioned as workers 1b and 2b in Table 6 when involved in the final cleaning of the seeds.
Table 6.
Demographic data and health symptoms of grass seed workers (Step 4)
| Worker | Respiratory protection | Age | Years employed | Smoker | Problematic seedsa | Dry cough | Mucosal irritation | Shortness of breath | Chest tightness | Fatigue | Flu like | Fever | Durationb | X-ray scan | Spirometry | Auscultation | IgE | ODTS |
| 1 | − | 39 | 3 | + | + | + | + | + | + | − | + | + | 1 week | N | N | N | Negative | Yes |
| 1b | (+) | 39 | 3 | + | + | + | + | + | − | − | (+) | − | 1 day | n.p. | N | N | n.p. | Possible |
| 2 | − | 52 | >5 | − | ((+)) | − | − | − | − | − | − | − | 0 | n.p. | N | N | Negative | − |
| 2b | + | 52 | >5 | − | + | − | − | − | − | − | − | − | 0 | n.p. | N | N | n.p. | − |
| 3 | − | 45 | >5 | − | + | + | + | + | + | − | + | + | 1 week | Possible infiltrate | N | N | Negative | Yes |
| 4 | − | 41 | >5 | + | + | + | + | + | + | + | (+) | + | 1 week | N | N | N | Negative | Yes |
| 5 | − | 59 | >5 | − | (+) | + | − | + | + | − | + | + | 3 days | N | n.p. | N | Negative | Yes |
| 6 | − | 45 | >5 | − | (+) | − | − | − | (+) | − | (+) | − | 1 day | n.p. | N | N | n.p. | Possible |
| 7 | − | 47 | >5 | − | (+) | − | − | + | + | − | − | − | 1 day | N | n.p. | N | Negative | Possible |
| 8 | − | 39 | >5 | − | (+) | − | − | − | − | − | − | − | 0 | n.p. | N | N | n.p. | − |
| 9 | − | 58 | >5 | − | ((+)) | − | − | − | − | − | − | − | 0 | n.p. | N | N | Negative | − |
| 10 | − | 35 | 5 | + | ((+)) | − | − | − | − | − | − | − | 0 | n.p. | N | N | n.p. | − |
| 11 | − | 58 | >5 | − | ((+)) | − | − | − | − | − | − | − | 0 | n.p. | n.p. | N | n.p. | − |
| 12 | − | 34 | 4 | − | (+) | + | + | − | (+) | − | − | − | 3 days | n.p. | N | N | n.p. | Possible |
+, Yes; (+), partly; ((+)), very limited; −, no; N, normal; n.p., prescribed, but not performed due to worker’s wish. Workers number 1 and 2 are also included as workers 1b and 2b as they, after primary exposure, later performed final cleaning of seeds. Fever considered ‘suspected’, if felt by the worker but not measured.
Work with problematic seeds.
Duration of any symptom of ODTS.
Seven workers showed respiratory symptoms few hours after working with the problematic seeds and four of them had a fever and six had flu-like symptoms (Table 6). All 12 workers had normal lung function values, leukocyte counts, CRP values and auscultation and in none of the seven workers that were tested were found raised levels of IgE. The found symptoms were consistent with those of ODTS. On five workers, chest X-ray scans were performed. In one worker, clearly exposed and diagnosed with ODTS, suspicion of a minor infiltrate was found; the remaining four had normal chest X-rays.
The workers (workers 1, 1b, 3, 4, 5, and 12) were those receiving the seeds (position 1, Tables 4 and 5), loading them into a silo or boxes (position 2, Tables 4 and 5) and those operating the cleaning machines (position 3, Table 6). These were also the workers with most pronounced symptoms. Four workers (workers 2, 9, 10, and 11) at work at the time the problematic seeds were received did not work with or near the problematic seeds, but worked with reference seeds and none of them had any health complaints suggestive of airway disease (Table 6).
Workers 1 and 2 (called 1b and 2b), involved in the final cleaning at plant II, had normal auscultation findings and spirometry values before, during, and after the period of work and they had no work-related changes in peak flow values. During the 2 days, worker 1b did not use personal airway protection properly, and he developed symptoms similar to those during the first exposure and compatible with ODTS. However, PF was unchanged and an extra health examination showed normal auscultation and spirometry values.
Follow-up
At follow-up, 6–9 months after work with the problematic seeds, none of the 12 workers had any respiratory symptoms nor did they report symptoms of ODTS in the period in between. The seven workers with symptoms had a general health examination which was normal in all cases.
DISCUSSION
In this study, we show that workers at a grass seed plant can be exposed to very high concentrations of different bioaerosol components. Workers were diagnosed with ODTS. The investigation was prompted by health complaints by employees at a grass seed plant. All workers showing symptoms had an obvious exposure to dust from a problematic seed lot. Health examination was almost normal (lung auscultation, spirometry, and X-ray scan) even in workers who experienced clear symptoms and subjective difficulty in breathing. This is in accordance with the definition of ODTS (Seifert et al., 2003). There was a close association between exposure to the problematic seeds and onset of symptoms and after exposure symptoms waned also in accordance with the diagnosis of ODTS. Those at work when the problematic seeds were handled but not working near these seeds showed no symptoms. The severity of complaints from the workers was associated with the degree of exposure, with position 1 (receiving the seeds) and position 2 (loading the seeds into a silo or boxes) being places with high exposure (Tables 4 and 5).
During the final cleaning, worker 1b in Table 6, using airway protection while cleaning the seeds, generally had no complaints but twice showed symptoms of ODTS after a few hours when not using the protection properly. Worker 2b used airway protection correctly at all times during the final cleaning and showed no symptoms. According to Seifert et al. (2003), efforts to prevent ODTS should focus on avoiding exposure, which is why the correct use of airway protection is of importance. Investigations of the effects of respiratory devices (disposable particulate respirators or particle-filtering half masks) during exposure to organic dust found that they reduced symptoms in short-term exposures both for healthy participants (Dosman et al., 2000) and for participants already suffering from a dust-related disease (Müller-Wening and Repp, 1989; Müller-Wening and Neuhauss, 1998).
Exposure to a wide variety of microorganisms has been suggested or found to be responsible for more severe diseases, for example, HP, which may lead to chronic lung damage (Selman et al., 2010). Actinomycetes (Bourke et al., 2001) and A. fumigatus (Selman et al., 2010) have been mention as causing HP, and we found A. fumigatus and actinomycetes to be released in very high quantities from the problematic seeds. The health examinations do, however, not indicate further health complications, and findings of the follow-up examination also gave no reasons to suspect chronic changes.
There are no internationally accepted threshold limit values (TLVs) or OELs for endotoxin, actinomycetes, and fungal spores. Suggested TLVs or calculated ‘no effect values’ for inhalable or ‘total endotoxin’ exposure are between 30 and 2000 EU m− 3 (Haglind and Rylander, 1984; Rylander et al., 1985; Kennedy et al., 1987; Castellan et al., 1987; Smid et al., 1992; Smid, 1993; Michel, 1997; Rylander, 1997; Donham and Cumro, 1999; Donham et al., 2000; Health Council of the Netherlands, 2010). A review of the literature on human challenge experiments suggests a no-effect level for endotoxin exposure in relation to development of toxic pneumonitis of 200 ng endotoxin m−3 (≈2000 EU m− 3) (Rylander, 1997). Based on a review study, it has been suggested that an exposure level of 105 fungal spores per cubic metre air would be an appropriate baseline for an OEL for spores from various fungi (Eduard, 2009). Exposures larger than 2 × 104 cfu of thermophilic actinomycetes m−3 have been suggested as a TLV (Dutkiewicz et al., 1994). All stationary and personal measurements of exposure were higher than the highest suggested limit of 200 ng m− 3 for endotoxin, higher than the 105 fungal spores m−3 and higher than the 2 × 104 cfu of thermophilic actinomycetes m−3. As these suggested OELs all are well below the measurements at the grass seed plants and below what has been measured in two other environments where workers developed ODTS—at a compost plant (Weber et al., 1993) and at farms (Malmberg et al., 1993)—keeping below the OELs is expected to protect the workers from developing ODTS. In other environments where plant materials such as seeds are handled, very different exposure levels to endotoxin have been found (reviewed in Madsen et al., 2009). High endotoxin exposure has been found in cucumber and tomato nurseries (median = 320 EU m− 3, max = 4020 EU m− 3) (Madsen et al., 2009), grain farming (GM = 5900 EU m− 3) (Halstensen et al., 2007) and cotton mills (median = 438 ng m− 3, max = 6936 ng m− 3) (Simpson et al., 1999), and a grass seed processing plant (78 000 EU m− 3) (Smit et al., 2006) but the exposure at the Danish grass seed plant was higher. The exposure of grass seed workers to β-glucan was also higher than earlier found for compost workers (median = 0.79 ng m− 3, max = 14.45 ng m− 3) (Hryhorczuk et al., 2001).
In a study of the effect of various airborne particles on asthma severity, hyphal fragments caused the most consistently significant effects on the parameters used to define asthma severity (Delfino et al., 1997). Most studies, however, do not include hyphal fragments, but Halstensen et al, (2007) argue that hyphal fragments should be included in microbial analyses as they contain many of the same properties as fungal spores. In this study, the exposure to hyphal fragments was up to 8.7 × 106 fragments m−3, which appears high when compared with other studies (Pady and Kramer, 1960; Delfino et al., 1997; Tendal and Madsen, 2011). Also the exposure to grass pollen was high and it greatly exceeded what is usually found in outdoor air (Sommer and Rasmussen, 2009; Tendal and Madsen, 2011).
This investigation clearly demonstrates that this particular lot of grass seeds produced much more dust than normal, comparable seeds. Simulated handling of grass seeds (Step 2) caused an aerosolisation of ∼5 times as much dust, 23 times as much endotoxin, 750 times as many mesophilic fungi, 104 times as many A. fumigatus and 107 times as many thermophilic actinomycetes as reference seeds. In studies on a farm (Malmberg et al., 1993) and a compost plant (Weber et al., 1993), exposure events, which had led to ODTS, were reconstructed. The mean spore (bacterial, fungal, and actinomycetes) concentration during a 5- to 15-min measurement was 1.3 × 1010 spores m− 3, which was 110 times higher than the concentration measured during exposure on reference farms for 1–2 h (Malmberg et al., 1993). During a 20-min exposure assessment during a worst case scenario at a compost plant, the exposure to dust was 149 mg m− 3, the exposure to fungal spores was 3.7 × 109 spores m− 3 and the exposure to endotoxin ∼2 × 104 EU m− 3 (Weber et al., 1993). On the grass seed plant where we measured during a whole working day (Step 3), we found that handling of the seeds causing ODTS led to a corresponding exposure of 1.3 × 108 bacterial (including actinomycetes) cells plus fungal spores m−3. Thus, a lower level of exposure to fungi or fungi plus bacteria than at the farms and a lower concentration of fungal spores and dust than at the compost plant seem to cause ODTS for the grass seed workers. However, the very high exposure to endotoxin of 3.2 × 105 EU m− 3 is probably also involved in the development of ODTS. Furthermore, our exposure measurement was performed during a whole working day and not only during working processes expected to cause the highest exposures. The stationary measurements in the different areas of the plant revealed different exposure levels but all can be considered as high.
Field exposure measurements (Step 3) reflects a combination of the contribution from the handled seeds, how and for how long time the seeds are handled and the admixture of bioaerosols from neighbouring areas while measurements using the rotating drum (Step 2) reflects the concentrations that can during certain conditions be aerosolised from the seeds. Step 1 shows that the seeds are the source of the bioaerosol exposure, and the low outdoor reference measurement shows that there is no notable outdoor contribution to the high indoor exposure—outdoor air will dilute the exposure. The outdoor reference exposure to endotoxin and ß-glucan was on the same level as other outdoor reference measurements of endotoxin (Madsen, 2006a) and ß-glucan (Madsen et al., 2011). Step 2 shows that the exposure from handling cleaned problematic seeds (Table 3) is significantly reduced in comparison with handling uncleaned seeds. However, the bioaerosol contribution from cleaned problematic seeds is high and cleaning of problematic seeds is not enough to reduce the exposure to the level caused by work with reference seeds.
Comparison of concentrations of microorganisms in Step 1 versus Step 2 (Table 1 versus Tables 2 and 3) shows that only a small fraction of the present microorganisms are aerosolised during mechanical handling for 1 min. This is in accordance with what is seen for straw and wood chips (Madsen et al., 2006) and indicates that repeated exposure will occur if seeds are handled repeatedly. The dustiness (Step 2) of uncleaned problematic seeds in terms of all the studied microorganisms and endotoxin was higher than what has earlier been measured for wood chips and straw (Madsen et al., 2004). The concentration of endotoxin in extracts from the problematic seeds was higher than what has earlier been seen in grass seed extracts; the concentration in reference seed extract (Step 1) is at the level of what has earlier been found for grass seed extracts (Smit et al., 2006).
In most environments where organic material is handled, personal measurements of exposure to bioaerosol and dust show higher levels than stationary measurements (Ogden et al., 1993; Madsen, 2006b; Madsen et al., 2009). However, at the grass seed plant exposure to dust and some bacteria at the seed silo area (S2) was higher than personal exposure. This may be because some of the automatic processes generated dust.
CONCLUSION
Exposure to very high concentrations of bioaerosol components can occur when working with grass seeds. The high exposure to bioaerosols and symptoms of grass seed workers was in accordance with the diagnosis of ODTS. Up to 107 times as many microorganisms were aerosolised from the seeds causing ODTS as from reference seeds. Suspicion should be elicited by seeds producing more dust than usually or not having been sufficiently dried, and precautions should be taken.
The conceptual approach used in this study proved to be good for both hazard identification and for determination of the influence of handling problematic seeds versus reference seeds on the exposure and health effects. The exposure assessment and health examination showed that it was likely the simultaneous exposure to high levels of many different bioaerosol components that caused the development of ODTS. This serves to emphasize the importance of measuring a wide range of bioaerosol components in studies of ODTS. If we had measured only one component as, for example, endotoxin, actinomycetes or fungi, we could have been misled to conclude that it was this one component which caused the ODTS. But we cannot know, whether it is the combination of different bioaerosol components or a single component which is responsible for the development of ODTS.
The much lower dustiness of reference seeds and the absence of health symptoms when only reference seeds were handled show that handling of normal grass seeds may not cause health problems. However, it is not known how often the plant receives problematic grass seeds and a more comprehensive investigation of the exposure and general health of employees in the grass seed industry is still lacking and indeed of relevance.
In this study, personal respiratory airway protection proved efficient to avoid development of ODTS and should always be at hand in facilities handling grass seed. It should be used consistently at all stages when handling seeds which seem to be dustier than usually. All direct handling of the problematic seeds seemed to imply a serious exposure even after cleaning. Thus, precautions should also be taken when handling the cleaned problematic seeds and it should also be considered whether such seeds might pose a risk to the consumer.
Acknowledgments
We want to thank Margit Wagtberg Frederiksen and Signe H. Nielsen for highly qualified technical assistance. Furthermore, we thank the National Research Centre for the Working Environment for financial support.
References
- Arbejdstilsynet. At-vejledning. Grænseværdier for stoffer og materialer. 2007 [The Danish Working Authority. Work Place Exposure Limits], Arbejdstilsynet. p. 1–85. [Google Scholar]
- Basinas I, Schlünssen V, Heederik D, et al. Sensitisation to common allergens and respiratory symptoms in endotoxin exposed workers: a pooled analysis. Occup Environ Med. 2011 doi: 10.1136/oem.2011.065169. doi:10.1136/oem.2011.065169. [DOI] [PubMed] [Google Scholar]
- Boehmer TK, Jones TS, Ghosh TS, et al. Cluster of presumed organic dust toxic syndrome cases among urban landscape workers-Colorado, 2007. Am J Ind Med. 2009;52:534–8. doi: 10.1002/ajim.20699. [DOI] [PubMed] [Google Scholar]
- Bourke SJ, Dalphin JC, Boyd G, et al. Hypersensitivity pneumonitis: current concepts. Eur Respir J Suppl. 2001;32:81s–92s. [PubMed] [Google Scholar]
- Brinton WT, Vastbinder EE, Greene JW, et al. An outbreak of organic dust toxic syndrome in a college fraternity. JAMA. 1987;258:1210–2. [PubMed] [Google Scholar]
- Castellan RM, Olenchock SA, Kinsley KB, et al. Inhaled endotoxin and decreased spirometric values. An exposure-response relation for cotton dust. N Engl J Med. 1987;317:605–10. doi: 10.1056/NEJM198709033171005. [DOI] [PubMed] [Google Scholar]
- CEN—European Committee for Standardization. Workplace atmospheres—measurement of the dustiness of bulk materials—requirements and reference test methods. 2006. EN 15051. Brussels, Belgium: European Committee for Standardization. [Google Scholar]
- Delfino RJ, Zeiger RS, Seltzer JM, et al. The effect of outdoor fungal spore concentrations on daily asthma severity. Environ Health Perspect. 1997;105:622–35. doi: 10.1289/ehp.97105622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donham K, Cumro D. Setting maximum dust exposure levels for people and animals in livestock facilities. 1999 Proceedings of the International Symposium on Dust Control in Animal Production Facilities 93–111. [Google Scholar]
- Donham KJ, Cumro D, Reynolds SJ, et al. Dose-response relationships between occupational aerosol exposures and cross-shift declines of lung function in poultry workers: recommendations for exposure limits. J Occup Environ Med. 2000;42:260–9. doi: 10.1097/00043764-200003000-00006. [DOI] [PubMed] [Google Scholar]
- Dosman JA, Senthilselvan A, Kirychuk SP, et al. Positive human health effects of wearing a respirator in a swine barn. Chest. 2000;118:852–60. doi: 10.1378/chest.118.3.852. [DOI] [PubMed] [Google Scholar]
- Dutkiewicz J, Pomorski ZJH, Sitkowska J, et al. Airborne microorganisms and endotoxin in animal houses. Grana. 1994;33:85–90. [Google Scholar]
- Eduard W. Fungal spores: a critical review of the toxicological and epidemiological evidence as a basis for occupational exposure limit setting. Crit Rev Toxicol. 2009;39:799–864. doi: 10.3109/10408440903307333. [DOI] [PubMed] [Google Scholar]
- Eduard W, Douwes J, Omenaas E, et al. Do farming exposures cause or prevent asthma? Results from a study of adult Norwegian farmers. Thorax. 2004;59:381–6. doi: 10.1136/thx.2004.013326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglind P, Rylander R. Exposure to cotton dust in an experimental cardroom. Br J Ind Med. 1984;41:340–5. doi: 10.1136/oem.41.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstensen AS, Nordby K-C, Wouters IM, et al. Determinants of microbial exposure in grain farming. Ann Occup Hyg. 2007;51:581–92. doi: 10.1093/annhyg/mem038. [DOI] [PubMed] [Google Scholar]
- Health and Safety Laboratory. MDHS 81-methods for the determination of hazardous substances—dustiness of powders and materials. Sudbury, UK: Health and Safety Executive, HSE Books; 1996. [Google Scholar]
- Health Council of the Netherlands. Endotoxins: health based recommended exposure limit. The Hague, The Netherlands: Health Council of the Netherlands: 2010. [Google Scholar]
- Hryhorczuk D, Curtis L, Scheff P, et al. Bioaerosol emmissions from a suburban yard waste composting facility. Ann Agric Environ Med. 2001;8:177–85. [PubMed] [Google Scholar]
- Kennedy SM, Christiani DC, Eisen EA, et al. Cotton dust and endotoxin exposure-response relationships in cotton textile workers. Am Rev Respir Dis. 1987;135:194–200. doi: 10.1164/arrd.1987.135.1.194. [DOI] [PubMed] [Google Scholar]
- Madsen AM. Airborne endotoxin in different background environments and seasons. Ann Agric Environ Med. 2006a;13:81–6. [PubMed] [Google Scholar]
- Madsen AM. Exposure to airborne microbial components in autumn and spring during work at Danish biofuel plants. Ann Occup Hyg. 2006b;50:821–31. doi: 10.1093/annhyg/mel052. [DOI] [PubMed] [Google Scholar]
- Madsen AM, Frederiksen MW, Allermann L, et al. (1 → 3)-b-D-glucan in different background environments and seasons. Aerobiologia. 2011;27:173–9. [Google Scholar]
- Madsen AM, Hansen VM, Nielsen SH, et al. Exposure to dust and endotoxin of employees in cucumber and tomato nurseries. Ann Occup Hyg. 2009;53:129–38. doi: 10.1093/annhyg/men073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen AM, Kruse P, Schneider T. Characterization of microbial particle release from biomass and building material surfaces for inhalation exposure risk assessment. Ann Occup Hyg. 2006;50:175–87. doi: 10.1093/annhyg/mei057. [DOI] [PubMed] [Google Scholar]
- Madsen AM, Mårtensson L, Schneider T, et al. Microbial dustiness and particle release of different biofuels. Ann Occup Hyg. 2004;48:327–38. doi: 10.1093/annhyg/meh016. [DOI] [PubMed] [Google Scholar]
- Malmberg P, Andersen A, Rosenhall L. Exposure to microorganisms associated with allergic alveolitis and febrile reactions to mold dust in farmers. Chest. 1993;103:1202–9. doi: 10.1378/chest.103.4.1202. [DOI] [PubMed] [Google Scholar]
- May JJ, Marvel LH, Pratt DS, et al. Organic dust toxic syndrome: a follow-up study. Am J Ind Med. 1990;17:111–3. doi: 10.1002/ajim.4700170137. [DOI] [PubMed] [Google Scholar]
- Melbostad E, Eduard W. Organic dust-related respiratory and eye irritation in Norwegian farmers. Am J Ind Med. 2001;39:209–17. doi: 10.1002/1097-0274(200102)39:2<209::aid-ajim1008>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Michel O. Human challenge studies with endotoxins. Int J Occup Environ Health. 1997;( 3):18–25. [Google Scholar]
- Müller-Wening D, Neuhauss M. Protective effect of respiratory devices in farmers with occupational asthma. Eur Respir J. 1998;12:569–72. doi: 10.1183/09031936.98.12030569. [DOI] [PubMed] [Google Scholar]
- Müller-Wening D, Repp H. Investigation on the protective value of breathing masks in farmer's lung using an inhalation provocation test. Chest. 1989;95:100–5. doi: 10.1378/chest.95.1.100. [DOI] [PubMed] [Google Scholar]
- Ogden TL, Bartlett IW, Purnell CJ, et al. Dust from cotton manufacture: changing from static to personal sampling. Ann Occup Hyg. 1993;37:271–85. doi: 10.1093/annhyg/37.3.271. [DOI] [PubMed] [Google Scholar]
- Pady SM, Kramer CL. Kansas aeromycology VI: hyphal fragments. Mycol Soc Am. 1960;52:681–7. [Google Scholar]
- Palmgren U, Ström G, Blomquist G, et al. Collection of airborne micro-organisms on nuclepore filters, estimation and analysis-CAMNEA method. J Appl Bacteriol. 1986;61:401–6. doi: 10.1111/j.1365-2672.1986.tb04303.x. [DOI] [PubMed] [Google Scholar]
- Pensis I, Mareels J, Dahmann D, et al. Comparative evaluation of the dustiness of industrial minerals according to European standard EN 15051, 2006. Ann Occup Hyg. 2010;54:204–16. doi: 10.1093/annhyg/mep077. [DOI] [PubMed] [Google Scholar]
- Rask-Andersen A. Organic dust toxic syndrome among farmers. Br J Ind Med. 1989;46:233–8. doi: 10.1136/oem.46.4.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylander R. Evaluation of the risks of endotoxin exposures. Int J Occup Environ Health. 1997;( 3):32–6. [Google Scholar]
- Rylander R, Haglind P, Lundholm M. Endotoxin in cotton dust and respiratory function decrement among cotton workers in an experimental cardroom. Am Rev Respir Dis. 1985;131:209–13. doi: 10.1164/arrd.1985.131.2.209. [DOI] [PubMed] [Google Scholar]
- Schlünssen V, Madsen AM, Skov S, et al. Does the use of biomasss affect respiratory symptoms or lung function among male Danish heat and power plant workers? Occup Environ Med. 2011;68:467–73. doi: 10.1136/oem.2009.054403. [DOI] [PubMed] [Google Scholar]
- Seifert SA, Von ES, Jacobitz K, et al. Organic dust toxic syndrome: a review. J Toxicol Clin Toxicol. 2003;41:185–93. doi: 10.1081/clt-120019136. [DOI] [PubMed] [Google Scholar]
- Selman M, Lacasse Y, Pardo A, et al. Hypersensitivity pneumonitis caused by fungi. Proc Am Thoracic Soc. 2010;7:229–36. doi: 10.1513/pats.200906-041AL. [DOI] [PubMed] [Google Scholar]
- Sigsgaard T, Malmros P, Nersting L, et al. Respiratory disorders and atopy in Danish resource recovery workers. Am J Respir Crit Care Med. 1994;149:1407–12. doi: 10.1164/ajrccm.149.6.8004291. [DOI] [PubMed] [Google Scholar]
- Simpson JCG, Niven RM, Pickering CAC, et al. Comparative personal exposure to organic dust and endotoxin. Ann Occup Hyg. 1999;43:107–15. [PubMed] [Google Scholar]
- Smid T. Exposure to organic dust and respiratory disorders: an epidemiological study in the animal feed industry. Den Haag, The Netherlands: CIP gegevens Koinklijke Bibliotheek; 1993. [Google Scholar]
- Smid T, Heederik D, Houba R, et al. Dust- and endotoxin-related respiratory effects in the animal feed industry. Am Rev Respir Dis. 1992;146:1474–9. doi: 10.1164/ajrccm/146.6.1474. [DOI] [PubMed] [Google Scholar]
- Smit LAM, Wouters IM, Hobo MM, et al. Agricultural seed dust as a potential cause of organic dust toxic syndrome. Occup Environ Med. 2006;63:59–67. doi: 10.1136/oem.2005.021527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer J, Rasmussen A. Pollen- og Sporemålinger i Danmark, 2009 [in Danish] Roskilde, Denmark: Astma–Allergi forbundet; 2009. [Google Scholar]
- Tendal K, Madsen AM. Exposure to airborne microorganisms, hyphal fragments, and pollen in a field of organically grown strawberries. Aerobiologia. 2011;27:13–23. [Google Scholar]
- The Danish Seed Council. 2010 Årsberetning 2010, Brancheudvalget for Frø (in Danish). Available at http://www.seedcouncildk/Brancheudvalget_for_Froe/∼/media/seedcouncil/Aarsberetning/Beretning_2010_finalashx. [Google Scholar]
- Vogelzang PFJ, Van der Gulden JWJ, Folgering H, et al. Organic dust toxic syndrome in swine confinement farming. Am J Ind Med. 1999;35:332–4. doi: 10.1002/(sici)1097-0274(199904)35:4<332::aid-ajim2>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Von Essen S, Fryzek J, Nowakowski B, et al. Respiratory symptoms and farming practices in farmers associated with an acute febrile illness after organic dust exposure. Chest. 2007;116:1452–8. doi: 10.1378/chest.116.5.1452. [DOI] [PubMed] [Google Scholar]
- Weber S, Kullman GJ, Petsonk E, et al. Organic dust exposures from compost handling: case presentation and respiratory exposure assessment. Am J Ind Med. 1993;24:365–74. doi: 10.1002/ajim.4700240403. [DOI] [PubMed] [Google Scholar]