Abstract
Objective:
Patients with COPD consistently express a desire to discuss end-of-life care with clinicians, but these discussions rarely occur. We assessed whether an intervention using patient-specific feedback about preferences for discussing end-of-life care would improve the occurrence and quality of communication between patients with COPD and their clinicians.
Methods:
We performed a cluster-randomized trial of clinicians and patients from the outpatient clinics at the Veterans Affairs Puget Sound Health Care System. Using self-reported questionnaires, we assessed patients’ preferences for communication, life-sustaining therapy, and experiences at the end of life. The intervention clinicians and patients received a one-page patient-specific feedback form, based on questionnaire responses, to stimulate conversations. The control group completed questionnaires but did not receive feedback. Patient-reported occurrence and quality of end-of-life communication (QOC) were assessed within 2 weeks of a targeted visit. Intention-to-treat regression analyses were performed with generalized estimating equations to account for clustering of patients within clinicians.
Results:
Ninety-two clinicians contributed 376 patients. Patients in the intervention arm reported nearly a threefold higher rate of discussions about end-of-life care (unadjusted, 30% vs 11%; P < .001). Baseline end-of-life communication was poor (intervention group QOC score, 23.3; 95% CI, 19.9-26.8; control QOC score, 19.2; 95% CI, 15.9-22.4). Patients in the intervention arm reported higher-quality end-of-life communication that was statistically significant, although the overall improvement was small (Cohen effect size, 0.21).
Conclusions:
A one-page patient-specific feedback form about preferences for end-of-life care and communication improved the occurrence and quality of communication from patients’ perspectives.
Trial registry:
ClinicalTrials.gov; No.: NCT00106080; URL: www.clinicaltrials.gov
In the United States, COPD is the leading cause of respiratory-related deaths and is the third overall leading cause of death.1 COPD often causes a progressive decline in quality of life and places patients at risk for acute respiratory failure that can require intensive care. Currently, there is no consensus on when discussions about preferences for end-of-life care should take place with these patients. The American Thoracic Society/European Respiratory Society guidelines on COPD recommend these discussions occur for patients with advanced disease while they are clinically stable.2 Studies show the majority of patients with COPD would like to discuss their preferences for end-of-life care with clinicians, yet less than one-third have done so.3,4 Studies examining patients dying with COPD or lung cancer have shown that patients with COPD receive more resource-intensive care that is consistent with preservation of life, including greater number of ICU days, and less focus on palliation of symptoms than patients with lung cancer.5 This focus on preservation of life occurs despite evidence that patients with COPD have similar preferences for end-of-life care as patients with lung cancer, and most state they prefer care focused on comfort.6,7
Most interventions designed to improve communication about end-of-life care have been disappointing. The Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT), a multicenter randomized trial, was designed but failed to improve the quality of communication and delivery of end-of-life care in the inpatient setting. More recently, experiential skills-building communication training has been shown to improve clinicians’ skills at communication about end-of-life care.8,9 However, these efforts are expensive, time-consuming, and have not yet been shown to improve patient-assessed outcomes. A randomized trial of elderly hospitalized patients showed that advance care planning improved satisfaction of care for patients and families, reduced intensity of care at the end of life, and reduced stress, anxiety, and depression in families of deceased patients.10 However, the planning sessions required an additional visit by a trained facilitator and took a median of 60 min. These studies suggest that interventions to improve communication about end-of-life care may improve patient and family outcomes and reduced intensity of care at the end of life, but practical ways to implement these interventions in the outpatient setting are lacking.
When clinicians do talk with patients with COPD about end-of-life care, these conversations often occur in response to acute deterioration when patients, family, and clinicians are coping with balancing the immediate need for medical care and the potential burdens and outcomes of care.11‐13 Yet, patients with COPD receive most of their care in the outpatient setting where discussions about end-of-life care could occur without concomitant stressors, such as acute dyspnea and anxiety. We sought to improve the occurrence and quality of end-of-life communication with a simple communication intervention using a patient-specific feedback form designed to facilitate communication about end-of-life care between patients with COPD and their clinicians in the outpatient setting.
Materials and Methods
Study Design
We performed a clustered-randomized trial of clinicians and their patients. The unit of randomization was at the clinician level with patients clustered by clinician. Patients and clinicians were enrolled from January 2004 to November 2007 at two Veterans Affairs (VA) facilities: a university-affiliated tertiary referral medical center and a predominantly nonteaching outpatient facility. All participants provided informed consent. The study protocol was approved by the institutional review board of the University of Washington (#24803).
Participants
Subjects included clinicians and patients with COPD. Both physician and nonphysician clinicians were included from the primary care and chest clinics. Patients were required to have COPD as defined by the GOLD (Global Initiative for Chronic Obstructive Lung Disease) criteria14 and identify a participating clinician being primarily responsible for their COPD care. Patients were excluded for cognitive dysfunction, language barriers, or severe psychiatric disorders.
Randomization
We used stratified random sampling procedures to assign clinicians to either the intervention or control group. Study investigators and staff administering outcome measures were blinded to treatment assignment.
Data Collection
All patients self-completed baseline instruments with research assistants available to assist with completion of questionnaires. Baseline information included: (1) the Quality of Communication (QOC) questionnaire,11,15 (2) the Preferences for Dying and Death questionnaire,16,17 (3) the St. George Respiratory Questionnaire,18 (4) preferences for communication about end-of-life care and patient-specific barriers and facilitators to this communication,19,20 (5) preferences for life-sustaining treatments,19,20 and (6) socio-demographic information.
Intervention Content
The theoretical foundation for this intervention was social cognitive theory, and the intervention was designed to increase the self-efficacy of clinicians and patients for discussing end-of-life care.21‐23 The intervention was incorporated into usual clinical visits. We generated an individualized one-page patient-specific feedback form that was distributed to clinicians who were randomized to the intervention and to their patients (Fig 1). The patient-specific feedback form was generated using an automated, computerized process that selected the patient’s self-reported responses, including perspectives about whether their physician would know the types of care they desired, their desire for communication about advance care planning, patient-specific barriers and facilitators to communication about end-of-life care, preferences for CPR and mechanical ventilation, and the severity of their airflow limitation. We provided the patient-specific highest-ranked barrier and facilitator to end-of-life communication along with an introductory sentence that clinicians could use to help lower the threshold to initiate conversations. Last, we provided the patient’s three most important preferences for end-of-life experiences.
Figure 1.
Example of clinician patient-specific feedback form.
Timing of Intervention Delivery
For patients in the intervention group, we mailed the one-page patient-specific feedback form to patients to review and share with their surrogate(s). On the day of an ordinarily scheduled clinic visit, we provided the patient-specific feedback form to clinicians and patients in the intervention group without endorsements to use during the clinic visit. Neither physicians nor patients in the control group received the patient-specific feedback forms at any time.
Outcome Measures
All patients were surveyed at 2 weeks after the targeted clinic visit, and study staff members contacting patients were blinded to treatment group assignment. The previously validated quality of end-of-life communication score was our primary outcome measure (QOC). The QOC ranges between 0 and 100, with higher scores indicating better communication.11,15 As secondary outcomes, we assessed whether patients reported the occurrence of discussions about end-of-life preferences between themselves and either their clinician or their surrogate.
Analyses
We used the principle of “intention to treat” for all analyses. Because we randomized clinicians rather than patients, we choose a priori to adjust for baseline patient characteristics as our primary analysis as well as to account for clustering by clinicians. We chose those clinical characteristics based on whether the factor might influence clinicians’ likelihood of having discussions about end-of-life care. We adjusted our primary analyses for baseline end-of-life communication scale, age, log age, smoking status, and FEV1. For the patients who were lost to follow-up, or those who had missing covariate information, we imputed these values with the “imputation using chained equations” program in STATA 10 (StataCorp). To demonstrate the effects of missing data, we examined both unimputed and imputed results for each model. The effect of the intervention was assessed using a cross-sectional time-series generalized estimating equations regression, with an exchangeable covariance matrix. All models accounted for the clustering of patients within clinician.
Results
Patient and Clinician Characteristics
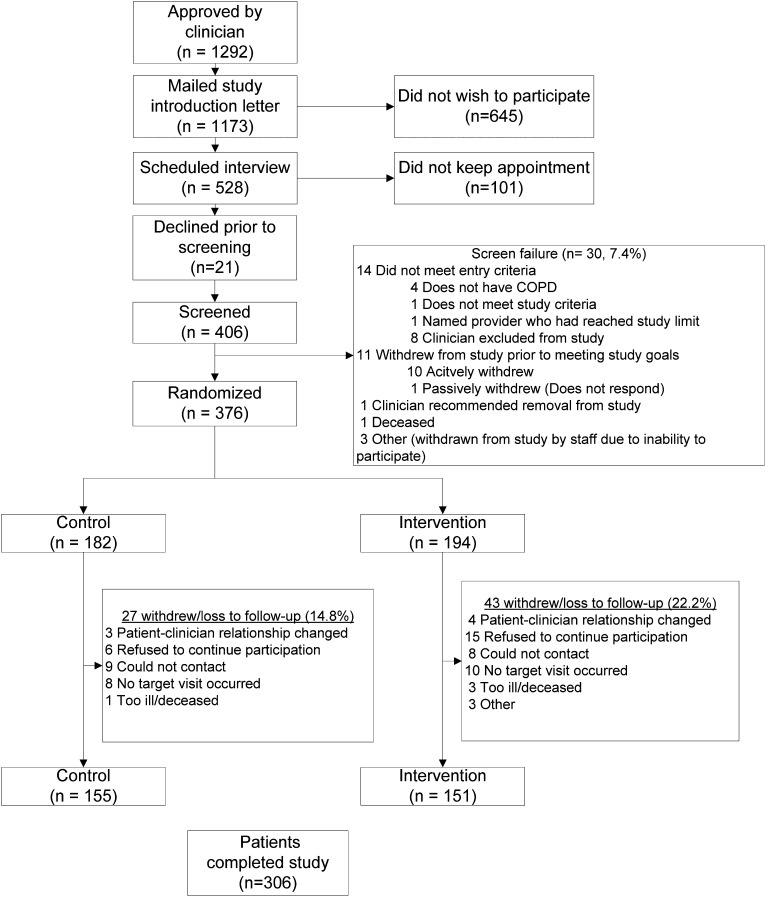
Of the 152 clinicians who were willing to participate, 92 were able to contribute patients and were included in the study. Clinicians were primarily staff clinicians (64.1%) and included physicians (69.6%) and independent midlevel practitioners (30.4%). Of the 92 clinicians, 66.3% practiced in the general internal medicine clinics and/or women’s clinic, 26.1% in the pulmonary clinic, and 7.6% in the geriatric clinics. These clinicians contributed 376 patients: 182 to the control group and 194 to the intervention group. As seen in Figure 2, a total of 306 patients completed the study (155 control group, 151 intervention group). Clinician and patient characteristics are presented in Table 1. Patients in the two arms differed in regard to a few baseline clinical characteristics (Table 1). A greater proportion of the control group reported at-risk drinking as measured by an Alcohol Use Disorders Identification Test-Alcohol Consumption Questions (AUDIT-C) score > 4 (24.7% vs 13.6%, P = .01), and a greater proportion of the intervention group reported hypertension (68.9% vs 55.5%, P = .001). There were no differences in baseline patient characteristics with respect to whether patients completed the study (n = 306, 81.4%) or were lost to follow-up (n = 70, 18.6%) (data not shown). Patient ratings of the burden of study participation were the same in both groups (Table 1).
Figure 2.
Patient enrollment and completion.
Table 1.
—Clinician and Patient Characteristics by Group Unadjusted for Clustering and Covariates
| Characteristic | Intervention | Control | P Value |
| Clinician characteristics | n = 42 | n = 50 | |
| Sex, male | 50.0 | 44.0 | .57 |
| Clinic | |||
| Geriatric | 7.1 | 8.0 | .88 |
| Primary care/internal medicine | 64.3 | 68.0 | … |
| Pulmonary | 28.6 | 24.0 | … |
| Clinician degree | |||
| MD | 69.1 | 70.0 | .75 |
| Nurse (CNS or NP) | 26.2 | 28.0 | … |
| PA | 4.8 | 2.0 | … |
| Division (Seattle) | 85.7 | 86.0 | .97 |
| Clinician type | |||
| Fellow | 19.1 | 22.0 | .59 |
| Resident first year | 2.4 | 6.0 | … |
| Resident second year | 2.4 | 8.0 | … |
| Resident third year | 4.8 | 6.0 | … |
| Staff | 71.4 | 58.0 | … |
| Race (self-reported), white | 87.5 | 64.4 | .01 |
| Self-reported patient characteristics | n = 194 | n = 182 | |
| Age at baseline | 69.4 (10.0) | 69.4 (10.0) | .96 |
| Male | 97.9 | 96.2 | .30 |
| Smoking status | |||
| Never smoked | 3.9 | 3.1 | .17 |
| Past smoker | 73.2 | 65.0 | … |
| Current smoker | 22.9 | 31.9 | … |
| White | 85.3 | 87.0 | .66 |
| Married | 46.7 | 48.8 | .70 |
| More than high school education | 58.3 | 59.2 | .88 |
| Elevated AUDIT-C score | 13.6 | 24.7 | .01 |
| AUDIT-C score | 1.4 (2.2) | 2.0 (2.5) | .05 |
| MHI 5 score | 24.0 (4.8) | 23.5 (5.1) | .38 |
| Prebronchodilator FEV1 | 1.5 (0.6) | 1.6 (0.7) | .13 |
| Prebronchodilator FEV1 % predicted | 45.7 (18.3) | 48.5 (20.7) | .16 |
| Postbronchodilator FEV1 | 1.6 (0.7) | 1.7 (0.7) | .14 |
| Postbronchodilator FEV1 % predicted | 48.6 (19.5) | 51.7 (21.1) | .14 |
| Prebronchodilator FVC | 2.8 (0.9) | 2.8 (0.9) | .66 |
| Postbronchodilator FVC | 2.9 (0.9) | 2.9 (0.9) | .92 |
| Count of coexisting illness | 7.3 (3.1) | 6.9 (3.0) | .29 |
| SGRQ total | 49.2 (17.4) | 50.5 (18.0) | .51 |
| SGRQ activity | 67.2 (21.3) | 65.1 (22.1) | .38 |
| SGRQ impact | 37.0 (17.2) | 39.8 (19.1) | .16 |
| SGRQ symptoms | 55.1 (24.4) | 57.6 (21.9) | .31 |
| Burden of study participation | 1.1 (2.1) | 1.0 (1.9) | .61 |
| Comorbid conditions | |||
| Hypertension | 68.9 | 55.5 | .01 |
| Stroke | 11.7 | 9.8 | .57 |
| Depression | 37.8 | 37.2 | .91 |
| PTSD | 23.3 | 21.3 | .66 |
| Diabetes | 27.2 | 24.4 | .55 |
| Pneumonia | 43.3 | 44.5 | .83 |
| CVD-CABG | |||
| Previous heart attack | 18.3 | 20.7 | .57 |
| Angina | 23.9 | 27.4 | .45 |
| Congestive heart failure | 12.8 | 17.7 | .20 |
| Coronary artery disease | 19.4 | 18.3 | .79 |
| CVD (excluding hypertension) | 42.7 | 38.9 | .48 |
| Cardiac revascularization | 21.9 | 21.0 | .84 |
Data are presented as % or mean (SD). AUDIT-C = Alcohol Use Disorders Identification Test-Alcohol Consumption Questions; CABG = coronary artery bypass graft; CVD = cardiovascular disease; MHI = Mental Health Inventory; PTSD = posttraumatic stress disorder; SGRQ = St. George Respiratory Questionnaire.
Primary Outcome: Effect of Intervention on Quality of Communication
On a scale measured from 0 to 100, where 100 represents the best possible communication, in nonadjusted analyses, baseline quality of communication in both treatment and control groups was poor (intervention group QOC score, 23.3; 95% CI, 19.9-26.8; and control QOC score, 19.2; 95% CI, 15.9-22.4). At follow-up, the quality of communication remained relatively poor for both groups (intervention group QOC score, 34.0; 95% CI, 28.5-39.4; and control QOC score, 25.5; 95% CI, 20.4-30.5), with some improvement in both groups but a larger improvement in the intervention group. To assess the effect of the intervention, using our a priori primary analytic plan with an intention-to-treat approach, prespecified covariates, and a prespecified imputation method, we found that the intervention was associated with a statistically significant improvement in the quality of communication (difference, 5.74 points; P = .03; Cohen effect size, 0.21) (Table 2).
Table 2.
—Effect of Intervention on End-of-Life Quality of Communication and Discussions About End-of-Life Care Among Patients, Clinicians, and Surrogates (n = 376 Patients)
| Outcome | Unadjusted Intervention Group |
Unadjusted Control Group |
Primary Analysisa | P Value | ||
| Pre-visit (n = 194) | Post-visit (n = 194) | Pre-visit (n = 182) | Post-visit (n = 182) | |||
| Primary outcome | ||||||
| Improvement in scores on the QOC about End-of-Life Care Scale (range 0-100) | 23.3 (19.9-26.8) | 34.0 (28.5-39.4) | 19.2 (15.9-22.4) | 25.5 (20.4-30.5) | … | … |
| Change post-visit minus pre-visit QOC about End-of-Life Care Scale | 10.7 points | 6.3 points | 5.7 pointsb | .03 | ||
| Secondary outcomes | ||||||
| Responsec | ||||||
| Discussions with their clinicians about treatment preferences at their last clinic visit | N/A | 35.2% (24.7-45.7) | N/A | 15.9% (7.5-24.4) | 18.6%d | < .001 |
| Having had discussions about treatment preferences with their clinician | 22.3% (16.2-28.4) | 60.3% (43.0-77.6) | 17.4% (10.5-24.3) | 30.8% (5.3-56.3) | 27.4%d | < .001 |
| Discussions with their surrogate since last contact with study staff | N/A | 53.6% (37.4-69.8) | N/A | 45.2% (34.5-55.8) | 6.9%d | .29 |
| Discussions with their surrogate | 62.9% (47.2-78.6) | 86.2% (74.3-98.1) | 62.5% (45.9-79.2) | 75.2% (58.5-91.9) | 10.9%d | < .01 |
N/A = not applicable; QOC = quality of communication.
Difference between intervention group and control group at post-visit after adjustment for baseline characteristics.
Adjusted for baseline QOC about End-of-Life Care Scale: age, log age, current smoker, past smoker, FEV1, and clustering of patients within clinician.
Percent improvement in the proportion of patients reporting.
Adjusted for whether patient at baseline had reported previously having discussions (if appropriate), age, log age, current smoker, past smoker, FEV1, and clustering of patients within clinician.
In addition, our results were generally consistent regardless of whether we imputed missing data or adjusted for baseline patient characteristics. For example, accounting for baseline QOC scores and clustering by clinician, but without adjustment for covariates or imputation, we found a statistically significant improvement in the quality of communication (difference, 6.95 points for intervention vs control; P = .01). Similarly, in models that only adjusted for baseline QOC scores and were clustered by clinician, imputation of missing data had a small effect on the magnitude of the intervention (difference, 6.14 points; P for intervention vs control = .02). Furthermore, restricting to only those individuals with complete data and adjusting for prespecified variables, the effect of the intervention on improving quality of communication was similar to our primary analysis; however, the effect was no longer statistically significant (difference, 4.85 points; P for intervention vs control = 0.11). Among participants in the control arm using our prespecified modeling approach noted here, we found no significant improvement in the QOC scores between participant’s pre-visit and post-visit QOC scores (P = .23).
Secondary Outcome Measures
Effect of Intervention on Occurrence of Patient Communication With Clinicians and Surrogates:
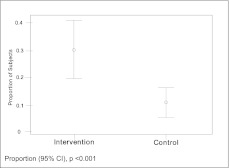
Participants frequently reported that they would like to discuss end-of-life care preferences with their clinician (67.7%); however, at baseline only a minority reported having had a previous discussion (14.6%). Since their last clinic visit, patients in the intervention arm reported nearly threefold higher likelihood of having an end-of-life discussion with an absolute difference of 18.6% (P < .001) (Fig 3). Using similar modeling approaches as described here, we found regardless of modeling approach that participants in the intervention arm were significantly more likely to report having “ever had” discussions with their clinicians (range of absolute difference between intervention and control, 23.6%-28.2%; P < .0001 for all models) (primary analysis results shown in Table 2). When framed in the context of “since the last time we talked,” the intervention had a small but nonsignificant effect on discussion between patients and surrogates (range of absolute differences, 5%-8%; P > .05 for all models) (primary analysis results shown in Table 2). Participants in the intervention arm, however, reported having had discussions with surrogates about the types of treatments they wanted at a significantly higher rate than control subjects (range absolute differences, 10%-12% higher; P < .01 for all models) (primary analysis results shown in Table 2).
Figure 3.
Effect of intervention on patient reported discussions about treatment preferences at their last clinic visit.
Assessment of Individual Items of the QOC Score:
Last, we were interested whether individual items on the QOC score were significantly higher as the result of the intervention and examined the individual items of the end-of-life quality of communication scale after the intervention, controlling for the patient assessment of this item prior to the intervention (Table 3). On average, discussions about patients’ feelings about getting sicker and asking about spiritual or religious beliefs received statistically higher ratings. On the other hand, topics that focused on the details of getting sicker, prognosis, what dying might be like, involving family in discussions, and asking about things important to the patient did not improve.
Table 3.
—Effect of Intervention on the Individual Components of the End-of-Life Quality of Communication Scale
| Itema | No. | Intervention Post QOC (mean, 95% CI) | Control Post QOC (mean, 95% CI) | Post QOC Difference (Intervention − Control) mean, 95% CI | P Value |
| Talking about your feelings about getting sicker | 243 | 47.0 (41.2, 52.7) | 34.2 (29.4, 39.0) | 12.8 (5.2, 20.4) | .001 |
| Talking about details if you got sicker | 242 | 37.7 (32.0, 43.4) | 33.3 (28.5, 38.0) | 4.5 (−3.1, 12.0) | .25 |
| Talking about how long you have to live | 252 | 22.9 (17.2, 28.5) | 15.6 (9.9, 21.3) | 7.2 (−0.8, 15.3) | .08 |
| Talking to you about what dying might be like | 252 | 11.1 (6.8, 15.4) | 8.4 (4.2, 12.5) | 2.8 (−3.3, 8.8) | .38 |
| Involving you in discussions about your care | 239 | 37.9 (30.0, 45.8) | 33.3 (25.6, 41.0) | 4.6 (−6.5, 15.8) | .42 |
| Asking you about important things in your life | 246 | 41.4 (32.9, 50.0) | 43.2 (34.6, 51.7) | 1.8 (−10.4, 13.9) | .78 |
| Asking about spiritual, religious beliefs | 253 | 18.0 (12.5, 23.5) | 8.5 (3.1, 13.9) | 9.4 (1.7, 17.2) | .02 |
Statistical comparisons adjusted for baseline rating for QOC item, age, log age, current smoker, past smoker, FEV1, and clustering of patients within clinician. See Table 2 legend for expansion of abbreviation.
Questions are scaled 0 to 10 with 0 as the worst you could imagine and 10 as the best you could imagine.
Discussion
This relatively simple intervention using a patient-specific feedback form increased the occurrence of patient-reported discussions about end-of-life care between patients, surrogates, and their clinicians. The intervention also improved the quality of communication about end-of-life care, although the improvement was modest as assessed by the Cohen effect size. The intervention was designed to incorporate patient-centered preferences for communication about end-of-life care and promote this communication between patients, surrogates, and clinicians. To enhance translation into practice, the intervention was designed to fit within the context of an outpatient clinic and was designed not to require additional clinic visits to discuss advance care planning. Our results suggest that providing patient-specific preferences in a one-page feedback form is an effective intervention to improve the occurrence of advanced care planning discussions in the outpatient setting. However, our results suggest that even though these discussions occurred more frequently and were rated as higher quality by patients, improvements in the overall quality of end-of-life communication were modest.
SUPPORT demonstrated that, in comparison with patients with lung cancer, patients with COPD received care more often directed at prolongation of life despite having similar preferences for end-of-life care.7 A comparison among US veterans in the last 6 months of life found that patients with COPD were much more likely to be admitted to the ICU and have significantly greater lengths of stay than patients with lung cancer.5 Moreover, there was dramatic geographic variation in ICU use among patients with COPD that was not present among patients with lung cancer, suggesting a lack of consensus about treating patients with COPD at the end of life. Among patients with cancer, communication about end-of-life care has been associated with decreased life-sustaining treatments at the end of life, increased quality of life at the end of life, and no increase in symptoms of anxiety or depression.24,25 Improving the occurrence of these conversations is an important initial step to help preserve patient autonomy and improve the quality of end-of-life care.
This study also confirms the results of others demonstrating poor quality of communication about end-of-life care between patients with COPD and their clinicians.3,26 Topics that patients consistently noted as being absent from discussions included asking about religious beliefs, talking about feelings about getting sicker, what dying might be like, and talking about how long they may have to live. Future interventions will likely need to not only integrate processes to improve the frequency with which these concepts are discussed but also focus on improving how clinicians communicate these difficult concepts and topics. Although our simple intervention can increase the quality and occurrence of communication about end-of-life care, major improvements in the quality of this communication will likely require experiential skill-building training for clinicians.8
One reason that communication about end-of-life care among patients with COPD may be particularly difficult is the overall uncertainty of life expectancy.7 Unlike diagnoses such as cancer, prognostication for patients with COPD is difficult. For example, 5 days prior to death, the SUPPORT prognostic model predicted that 50% of patients would be alive at 6 month.7 Among patients with very severe impairment as assessed by BODE (BMI, airflow obstruction, dyspnea, and exercise capacity index) scores, 50% of patients were still alive at 3 years.27 Given the uncertainty about prognosis and the chronicity of this disease, developing and teaching effective communication skills regarding advance care planning for COPD represent an important challenge and a useful paradigm for chronic, life-limiting illness with uncertainty regarding prognosis.
There are some important limitations to our study. First, there was a slightly higher dropout in the intervention group (22% vs 15%, P = .6). It is possible that the content of the intervention made the intervention patients uncomfortable, and, therefore, they withdrew at a greater rate. However, we asked the same questions of both intervention and control groups, and participants rated the burden of the study the same in both the intervention and control groups. A possible explanation is that study staff had more contact with the intervention group by virtue of the additional contact at the time of the target clinic visit to give the patient-specific feedback, thus allowing an additional opportunity for patients to withdraw. Second, this study was performed exclusively within a VA facility, potentially limiting the generalizability for implementation in systems outside the VA. Third, although we did not exclude women, the few women included in this study may limit inferences for women. Fourth, the study was designed to assess whether patients had conversations at one target clinic visit. We did not continue to assess whether new or additional conversations were stimulated by the intervention. Future studies should evaluate the sustainability of an intervention to change clinicians’ behavior about discussing end-of-life care. Fifth, because of the nature of the content of the questionnaires and our desire to approximate clinical practice, patients were not asked to complete questions that were left unanswered. This produced missing data in a number of our measures. However, comparison of unimputed intention-to-treat model with imputed models produced similar results. Sixth, the clustered randomized design increased the risk of differences in distributions of baseline patient characteristics. We used adjusted analyses to help address these differences. Finally, we did not follow these individuals to assess whether the intervention had effects on the actual delivery of care, such as the number of ICU visits or referrals to palliative care services.
Providing high-quality care is predicated not only on understanding patients’ preferences for care but also on attempting to align those preferences with actual delivery of health care. We have demonstrated that a relatively simple intervention can engage patients, surrogates, and clinicians to significantly increase the number of conversations about end-of-life care, but additional studies and more in-depth interventions are likely needed to further improve the quality of the communication that occurs between patients and clinicians. Current health-care training often neglects the development of high-quality communication skills, leaving trainees to learn by experience.28,29 Health-care training curricula need to be expanded to include formal and experiential skill-building education about how to communicate difficult topics, including how to prepare patients and their families for decisions about end-of-life care. In the meantime, a relatively simple patient-specific feedback form can increase the quantity and quality of communication about end-of-life care for patients with COPD.
Acknowledgments
Author contributions: The authors take responsibility and vouch for the completeness and accuracy of the data and analyses. Dr Au is the guarantor of the entire manuscript.
Dr Au: contributed to conception, hypotheses delineation, and design of the study; acquisition of the data or the analysis and interpretation; and writing the article or substantial involvement in its revision prior to submission.
Mr Udris: contributed to conception, hypotheses delineation, and design of the study; acquisition of the data or the analysis and interpretation; and writing the article or substantial involvement in its revision prior to submission.
Dr Engelberg: contributed to conception, hypotheses delineation, and design of the study; acquisition of the data or the analysis and interpretation; and writing the article or substantial involvement in its revision prior to submission.
Dr Diehr: contributed to conception, hypotheses delineation, and design of the study; acquisition of the data or the analysis and interpretation; and writing the article or substantial involvement in its revision prior to submission.
Dr Bryson: contributed to acquisition of the data or the analysis and interpretation; and writing the article or substantial involvement in its revision prior to submission.
Dr Reinke: contributed to acquisition of the data or the analysis and interpretation; and writing the article or substantial involvement in its revision prior to submission.
Dr Curtis: contributed to conception, hypotheses delineation, and design of the study; acquisition of the data or the analysis and interpretation; and writing the article or substantial involvement in its revision prior to submission.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Au is a research consultant for Bosch Inc and was on the medical advisory board for Nexcura. He has additional research support from Gilead Sciences. Mr Udris and Drs Engelberg, Diehr, Bryson, Reinke, and Curtis have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The authors designed this study and collected and analyzed all data independent of the sponsor, who had no role in the design, implementation, analysis, interpretation, and reporting of this study. The views expressed in this manuscript are those of the authors and do not necessarily represent the opinions of the Department of Veterans Affairs.
Abbreviations
- AUDIT-C
Alcohol Use Disorders Identification Test-Alcohol Consumption Questions
- QOC
quality of communication
- SUPPORT
Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments
- VA
Veterans Affairs
Footnotes
Funding/Support: This study was funded by the Department of Veterans Affairs [Grant IIR-02-292]. Dr Au was funded during the trial period by a Veterans Affairs Health Services Research and Development Career Development Award. Dr Curtis was funded by a K24 Award from that National Heart Lung and Blood Institute [Grant K24 HL068593].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970-2002. JAMA. 2005;294(10):1255–1259. doi: 10.1001/jama.294.10.1255. [DOI] [PubMed] [Google Scholar]
- 2.Standards for the Diagnosis and Management of Patients with COPD 2004. American Thoracic Society Web site. http://www.thoracic.org/clinical/copd-guidelines/index.php. Accessed July 14 2008.
- 3.Curtis JR, Engelberg RA, Nielsen EL, Au DH, Patrick DL. Patient-physician communication about end-of-life care for patients with severe COPD. Eur Respir J. 2004;24(2):200–205. doi: 10.1183/09031936.04.00010104. [DOI] [PubMed] [Google Scholar]
- 4.Heffner JE, Fahy B, Barbieri C. Advance directive education during pulmonary rehabilitation. Chest. 1996;109(2):373–379. doi: 10.1378/chest.109.2.373. [DOI] [PubMed] [Google Scholar]
- 5.Au DH, Udris EM, Fihn SD, McDonell MB, Curtis JR. Differences in health care utilization at the end of life among patients with chronic obstructive pulmonary disease and patients with lung cancer. Arch Intern Med. 2006;166(3):326–331. doi: 10.1001/archinte.166.3.326. [DOI] [PubMed] [Google Scholar]
- 6.Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax. 2000;55(12):1000–1006. doi: 10.1136/thorax.55.12.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claessens MT, Lynn J, Zhong Z, et al. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments Dying with lung cancer or chronic obstructive pulmonary disease: insights from SUPPORT. J Am Geriatr Soc. 2000;48(suppl 5):S146–S153. doi: 10.1111/j.1532-5415.2000.tb03124.x. [DOI] [PubMed] [Google Scholar]
- 8.Fallowfield L, Jenkins V, Farewell V, Saul J, Duffy A, Eves R. Efficacy of a Cancer Research UK communication skills training model for oncologists: a randomised controlled trial. Lancet. 2002;359(9307):650–656. doi: 10.1016/S0140-6736(02)07810-8. [DOI] [PubMed] [Google Scholar]
- 9.Back AL, Arnold RM, Baile WF, et al. Efficacy of communication skills training for giving bad news and discussing transitions to palliative care. Arch Intern Med. 2007;167(5):453–460. doi: 10.1001/archinte.167.5.453. [DOI] [PubMed] [Google Scholar]
- 10.Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345. doi: 10.1136/bmj.c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtis JR. Palliative and end-of-life care for patients with severe COPD. Eur Respir J. 2008;32(3):796–803. doi: 10.1183/09031936.00126107. [DOI] [PubMed] [Google Scholar]
- 12.Heffner JE, Fahy B, Hilling L, Barbieri C. Attitudes regarding advance directives among patients in pulmonary rehabilitation. Am J Respir Crit Care Med. 1996;154(6 pt 1):1735–1740. doi: 10.1164/ajrccm.154.6.8970363. [DOI] [PubMed] [Google Scholar]
- 13.Heffner JE, Fahy B, Hilling L, Barbieri C. Outcomes of advance directive education of pulmonary rehabilitation patients. Am J Respir Crit Care Med. 1997;155(3):1055–1059. doi: 10.1164/ajrccm.155.3.9116986. [DOI] [PubMed] [Google Scholar]
- 14.Global Initiative for Chronic Obstructive Pulmonary Disease: Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: NHLBI/WHO workshop report. Updated 2003. http://www.goldcopd.com.
- 15.Engelberg R, Downey L, Curtis JR. Psychometric characteristics of a quality of communication questionnaire assessing communication about end-of-life care. J Palliat Med. 2006;9(5):1086–1098. doi: 10.1089/jpm.2006.9.1086. [DOI] [PubMed] [Google Scholar]
- 16.Downey L, Engelberg RA, Curtis JR, Lafferty WE, Patrick DL. Shared priorities for the end-of-life period. J Pain Symptom Manage. 2009;37(2):175–188. doi: 10.1016/j.jpainsymman.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engelberg RA, Patrick DL, Curtis JR. Correspondence between patients’ preferences and surrogates’ understandings for dying and death. J Pain Symptom Manage. 2005;30(6):498–509. doi: 10.1016/j.jpainsymman.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 19.Knauft E, Nielsen EL, Engelberg RA, Patrick DL, Curtis JR. Barriers and facilitators to end-of-life care communication for patients with COPD. Chest. 2005;127(6):2188–2196. doi: 10.1378/chest.127.6.2188. [DOI] [PubMed] [Google Scholar]
- 20.Stapleton RD, Nielsen EL, Engelberg RA, Patrick DL, Curtis JR. Association of depression and life-sustaining treatment preferences in patients with COPD. Chest. 2005;127(1):328–334. doi: 10.1378/chest.127.1.328. [DOI] [PubMed] [Google Scholar]
- 21.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 22.Bandura A. Human agency in social cognitive theory. Am Psychol. 1989;44(9):1175–1184. doi: 10.1037/0003-066x.44.9.1175. [DOI] [PubMed] [Google Scholar]
- 23.Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 24.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med. 2009;169(5):480–488. doi: 10.1001/archinternmed.2008.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reinke LF, Slatore CG, Uman J, et al. Patient-clinician communication about end-of-life care topics: is anyone talking to patients with chronic obstructive pulmonary disease? J Palliat Med. 2011;14(8):923–928. doi: 10.1089/jpm.2010.0509. [DOI] [PubMed] [Google Scholar]
- 27.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 28.Billings ME, Engelberg R, Curtis JR, Block S, Sullivan AM. Determinants of medical students’ perceived preparation to perform end-of-life care, quality of end-of-life care education, and attitudes toward end-of-life care. J Palliat Med. 2010;13(3):319–326. doi: 10.1089/jpm.2009.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Billings ME, Curtis JR, Engelberg RA. Medicine residents’ self-perceived competence in end-of-life care. Acad Med. 2009;84(11):1533–1539. doi: 10.1097/ACM.0b013e3181bbb490. [DOI] [PMC free article] [PubMed] [Google Scholar]