Abstract
Background:
This study aimed to investigate the beneficial effects of angiotensin receptor blockers (ARBs) on markers of endothelial function in patients with early stage of diabetic nephropathy (DN).
Methods:
This cross-sectional study was conducted on 32 participants with IDDM from January 2010 until May 2011 in Isfahan, Iran. The participants were candidate for receiving ARBs or angiotensin-converting enzyme inhibitors (ACEIs) to decrease microalbuminuria. The inclusion criteria were as follows: the age of onset of insulin-dependent diabetes mellitus (IDDM)less than 15 years; normal glomerular filtration rate (GFR); normal blood pressure; normal cardiovascular examination; negative urine culture, receiving no medications except insulin. Microalbuminuria was measured in two fasting urine samples with a sampling interval of at least 1–2 months by ELISA method. Patients with two abnormal results were included. Microalbumin to creatinin ratio equal to or more than 30 mg/gm was considered abnormal. The fasting blood samples to determine serum nitric oxide (NO) and vascular cell adhesion molecule (VCAM) were obtained at the time 0 (before starting the study), and after 2 months of receiving ARBmedication. Valsartan tablet (Diovan, Novartis Company) with a dose of 1 mg/kg/day up to 80 mg/day in a single dose was administered.
Results:
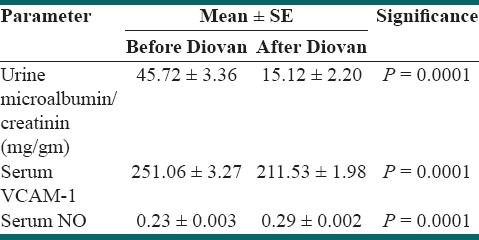
Urine microalbumin to creatinin ratio after valsartan consumption was lower than microalbumin level before the medication, P < 0.05. After valsartan consumption, serum VCAM-1 level reduced and NO level increased significantly, P < 0.05.
Conclusion:
Angiotensin receptor blockers may reduce VCAM-1 and microalbuminuria and may increase NO levels in early stages of DN. Thus administration of ARBs might be considered even in early stages of DN.
Keywords: Angiotensin receptor blocker, diabetic nephropathy, endothelial dysfunction, valsartan
INTRODUCTION
Diabetic nephropathy (DN) is a major cause of end-stage renal disease (ESRD) affecting nearby 20%–30% of diabetic patients worldwide.[1–3] Therefore, preventing DN as a serious microvascular complication of IDDM to reduce the risk of ESRD is a clinical priority.
Mechanisms by which kidney glomerular, interstitial, and vascular functions are injured consist of inflammation, oxidative stress, endothelial dysfunction, and accelerated fibrosis.[4] Endothelium dysfunction that has been described in DM consists of impairment in many aspects of endothelial functions including anti-inflammatory, antiproliferative, and vasodilatation.[1,5,6] In vessels, a balance between vasodilatation and vasoconstriction is achieved by normal endothelial function.[4] Vascular inflammation is a result of combining damage in vasomotor response, augmenting cell proliferation, increasing platelet aggregation, and vascular permeability.[2] Furthermore, endothelial dysfunction has been reported as the early sign of atherosclerosis and atherogenesis.[4,7] The renin–angiotensin–aldosteron system (RAAS) has a main role in the progression of DN.[8] Inhibition of the renin-angiotensin system (RAAS) may be effective in preventing DN through amending all above mentioned complications.[4] Findings from several studies in animal models and subsequent clinical trials in DN showed that systematic use of RAAS blocking agents including angiotensin converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) would reduce the risk of ESRD.[1,5] Long-lasting prescription of ACEIs (Enalapril) and or ARBs (Olmesarten) has been demonstrated to ameliorate microalbuminuria in patients with insulin-dependent and independent diabetes mellitus (NIDDM and IDDM).[9,10]
However, there is limited evidence about effects of blocking RAS system on endothelial dysfunction in young IDDM patients with microalbuminuria and early stages of DN, i.e., glomerular filtration rate (GFR) equals or more than 90 ml/min/1.73 m2 and normal blood pressure.[11]
This study aimed to investigate the beneficial effects of 2 months oral treatment of ARB on markers of endothelial function such as nitric oxide (NO) and vascular cell adhesion molecule (VCAM) in young IDDM patients with microalbuminuria and normal renal function.
METHODS
We randomly assigned 32 eligible IDDM patients with confirmed microalbuminuria in this cross-sectional study from January 2010 until May 2011 in Isfahan, Iran. To select the participants, the medical file of 270 IDDM patients who had been referred to Isfahan Endocrine and Metabolic Research Centre was re-evaluated. Among them, 32 eligible patients who were candidate for receiving ARBs or ACEIs and met the inclusion criteria were recruited. The inclusion criteria were as follows: the age of onset of IDDM less than 15 years (children onset disease); GFR equals or more than 90 ml/min/1.73 m2; normal blood pressure at three consecutive blood pressure measurements (less than 95% for age and height and gender); normal cardiovascular examination (approved by a fixed cardiologist); negative urine culture, receiving no medications except insulin.
Microalbuminuria was measured in two fasting urine samples with a sampling interval of at least 1–2 months.[12] The patients who had two abnormal results were recruited in the study. Microalbumin to creatinin ratio equaled to or more than 30 mg/gm was considered abnormal. Regarding abnormal microalbuminuria, the selected patients were candidate to receive ARBs or ACEIs as a part of approved management to treat microalbuminuria.
The fasting urine samples for measuring microalbumin and creatinin were used when the blood sugar was in the acceptable range (fasting blood sugar less than 140 mg/dl and trace or negative urine dipstick results for glucose). Microalbumin was measured by ELISA method on the fasting first morning urine sample. The blood samples to determine serum NO and VCAM were obtained at the time 0 (before starting the study) and after 2 months of receiving the medication. Valsartan tablet (Diovan, angiotensin receptor blocker from Novartis Company) with a dose of 1 mg/kg/day up to 80 mg/day in a single dose was administered. This medication was selected because of extended half-life and ease of administration.
The serum level of nitrite (stable NO metabolite) was measured using a colorimetric assay kit (Cayman, USA) based on Griess reaction, as previously described.[13] For nitrite measurement, briefly, after pouring serum into wells, sulphanilamide solution was added to all experimental samples, and after incubation, N-1-naphtylethylenediamine dihydrochloride solution was added. Then, absorbance was measured by a microreader in 540° nm wavelength. The serum levels of VCAM were quantified by ELISA kit (Bendermed, UK) according to the manufacturer's instruction.
In addition to the above-mentioned factors (endothelial markers), total cholesterol, triglyceride, HDL, LDL, and hemoglobin A1C were determined in the fasting state.
Written informed consent from the participants was obtained before enrolment in the study. The study was approved by institutional review boards and carried out in accordance with declaration of Helsinki guidelines.
Statistical analysis
The data are reported as the mean ± SE. A statistical software package, SPSS (version 16), was used to perform statistical analysis. The data were tested for normality and homogeneity of variance. Paired student's t-test was used to assess the significance of any change within groups. Statistical significance was accepted at P < 0.05.
RESULTS
Thirty two patients with child-onset IDDM were recruited. The mean of age was 12.65 ± 0.38 years. Male to female ratio was ½. Mean of height was 160.16 ± 10.24 cm. Mean of body weight was 60.46 ± 13.54 kg. Means of serum triglyceride and cholesterol levels were 98.25 ± 8.08 (SE) mg/dl and 155.90 ± 5.76 (SE) mg/dl, respectively. Mean serum levels of HDL and LDL were 49.03 ± 2.24 (SE) mg/dl and 89.46 ± 3.83 (SE) mg/dl, respectively. Mean of HBA1C before and after prescribing the medication were 7.84 ± 0.35 (SE) and 7.01 ± 0.78 (SE), respectively; P > 0.05. Urine microalbumin to creatinin ratio after valsartan consumption was lower than microalbumin level before the medication, P = 0.0001 [Table 1]. After valsartan consumption, serum VCAM-1 level reduced and NO level increased significantly [Table 2]. Microalbumin levels were positively correlated with VCAM (before valsartan); r = 0.340, P = 0.04.
Table 1.
Characteristics of the participants
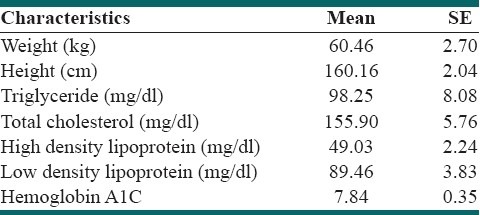
Table 2.
Markers of endothelial function before and after valsartan (Diovan) consumption
DISCUSSION
In this study, the response of endothelia dysfunction and microalbuminuria to ARBs in normotensive young IDDM patients was evaluated. We demonstrated that an 8-week course of administrating valsartan (ARBs) in IDDM patients before attaining overt stages of DN was able to recover endothelial dysfunction by increasing NO and diminishing VCAM levels. In addition, the level of microalbuminuria was decreased after 8 weeks of receiving valsartan. Although at the end of the study a minority of patients still had abnormal amounts of microalbumin to creatinine ratio, the difference between the values before and after valsartan was significant. While numerous studies have assessed the effect of RAS inhibition on DN, most of them evaluated only microalbuminuria as an alternate to DN in patients with impaired renal function.[14] The results of Collaborative Study Group's captopril trial have emphasized on the protective role of RAS inhibition in patients with DN and renal failure.[15] Nonetheless, the benefit of RAS inhibition by ACEIs was not approved in patients with normal GFR by this group. The results of DIRECT program did not support the preventive effects of candesartan (ARBs) in reducing microalbuminuria in IDDM patients with a low vascular burden.[16] Mauer et al. showed that early administration of RAS blocking agents did not reduce DN progression.[17] However, the results of a cohort study by Bakris et al. showed that telmisartan was more effective than losartan in reducing microalbuminuria in type 2 diabetes.[18]
In fact, in DN endothelium dysfunction occurs in both great and small arteries leading to cardiovascular and renal diseases.[19] In the capillary and arteriolar endothelium, dysfunction leads to insulin resistance, impaired fibrinolysis, microalbuminuria, dyslipidemia, and even hypertension.[20,21] Therefore, microalbuminuria was supposed to be a herald sign of early stages of DN.[5,6] Furthermore, its presence has been introduced is an important predictor of endothelial dysfunction in DN regardless of the degree of renal impairment.[22] Whereas microalbuminuria has been known as one of the most important markers of initiation of end organ damage, reducing NO bioactivity and increasing oxygen free radicals are the early indicators of endothelial dysfunction.[4,23] The balance between vasodilators and vasoconstrictors largely is maintained by NO. In addition to being a key player for the vasodilator effects, NO has been known as second messenger for actions of many growth factors, hormones, and coagulation factors besides to its inhibitory effects on adhering VCAM-1 and ICAM-1 to the arterial wall.[24–27] Higher production of prostanoid vasoconstrictors and increased oxidative degradation of NO have been reported as early mechanisms of diabetes-induced endothelial dysfunction.[28,29] Furthermore, asymmetric dimethylarginine (ADMA) accumulation in diabetes may impair endothelial vasodilator dysfunction.[30] The results of Calver et al.'s study revealed that patients with IDDM had a lower forearm blood flow response to locally infused L-NMMA (an inhibitor of endogenous NO synthesis) and SNP (an exogenous donor of NO).[31] In our patients, serum NO levels rose dramatically after consuming valsartan. Irrespective of short-course therapy, increasing NO levels were significant.
In addition to microalbuminuria, cell adhesion molecules (such as VCAM-1 and ICAM-1) that mediate adhesion and relocation of leukocyte into the arterial wall, have been reported as markers of endothelial dysfunction.[32,33] These markers are widely affected by angiotensin II (AG II) through inducing cytokine release.[34,35] Vascular cell adhesion molecule-1 has been known as independent predictors of atherosclerosis in diabetes. Romuk et al. demonstrated increased levels of VCAM-1 on type 2 but not in type 1diabetes.[7] The lowering effect of ACEI but not ARBs on VCAM-1 level in non-diabetic hypertensive patients was described by Jilma et al.[36]
A double-blind placebo-controlled study on a small sample size of hypercholestrolemic normotensive volunteers, revealed diminishing c-VCAM-1 levels after consuming either ARBs or ACEIs.[37] Gasic and colleagues demonstrated the lowering effect of ACEIs (fosinopril) on VCAM-1 levels in type 2 diabetes (NIDDM).[38] The unique characteristic of ARBs in blocking AT1 receptor promotes using these types of drugs to reduce proteinuria, microalbuminuria, and renal dysfunction in type 2 diabetes (NIDDM).[39–41] While angiotensin II increases VCAM-1 levels, NO down regulates its amounts at cellular level.[42] Irrespective of Romuk et al.'s study, we revealed lowering effects of ARBs on VCAM levels in IDDM patients. Although microalbumin level was correlated with VCAM before administering valsartan, this correlation was not achieved after finishing the short-course evaluation.
In conclusion, we demonstrated that the increment of NO and decrement of VCAm-1 occurred after an 8-week course of consuming valsartan in IDDM patients. Recruiting normotensive diabetic patients with normal GFR allowed us to show the effect of ARBs in modulating endothelial function besides to decreasing microalbuminuria in the absence of hypertension and kidney failure.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bayraktutan U. Free radicals, diabetes and endothelial dysfunction. Diabetes Obes Metab. 2002;4:224–38. doi: 10.1046/j.1463-1326.2002.00184.x. [DOI] [PubMed] [Google Scholar]
- 2.Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: Molecular mechanisms and therapeutic interventions. Clin Sci(Lond) 2007;112:375–84. doi: 10.1042/CS20060247. [DOI] [PubMed] [Google Scholar]
- 3.Locatelli F, Canaud B, Eckardt KU, Stenvinkel P, Wanner C, Zoccali C. The importance of diabetic nephropathy in current nephrological practice. Nephrol Dial Transplant. 2003;18:1716–25. doi: 10.1093/ndt/gfg288. [DOI] [PubMed] [Google Scholar]
- 4.de Haro Miralles J, Martinez-Aguilar E, Florez A, Varela C, Bleda S, Acin F. Nitric oxide: Link between endothelial dysfunction and inflammation in patients with peripheral arterial disease of the lower limbs. Interact Cardiovasc Thorac Surg. 2009;9:107–12. doi: 10.1510/icvts.2008.196428. [DOI] [PubMed] [Google Scholar]
- 5.Cohen RA. Dysfunction of vascular endothelium in diabetes mellitus. Circulation. 1993;87(Suppl. V):V67–76. [Google Scholar]
- 6.Pieper GM, Siebeneich W, Moore-Hilton G, Roza AM. Reversal by L-arginine of a dysfunctional arginine/nitric oxide pathway in endothelium of the genetic diabetic BB rat. Diabetologia. 1997;40:910–5. doi: 10.1007/s001250050767. [DOI] [PubMed] [Google Scholar]
- 7.Romuk E, Jagosz J, Skrzep-Poloczek B, Wojciechowska C, Strojek K, Sędek L, et al. Evaluation of VCAM-1 and PAI-1 concentration in diabetes mellitus patients. Experimental and Clinical Diabetology. 2008;8:85–8. [Google Scholar]
- 8.Ruggenenti P, Cravedi P, Remuzzi G. The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat Rev Nephrol. 2010;6:319–30. doi: 10.1038/nrneph.2010.58. [DOI] [PubMed] [Google Scholar]
- 9.Salardi S, Balsamo C, Zucchini S, Maltoni G, Scipione M, Rollo A, et al. High rate of regression from micro-macroalbuminuria to normoalbuminuria in children and adolescents with type 1 diabetes treated or not with enalapril: The influence of HDL cholesterol. Diabetes Care. 2011;34:424–9. doi: 10.2337/dc10-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haller H, Ito S, Izzo JL, Januszewicz A, Katayama S, Menne J, et al. Olmesartan for the Delay or Prevention of Microalbuminuria in Type 2 Diabetes. N Engl J Med. 2011;364:907–17. doi: 10.1056/NEJMoa1007994. [DOI] [PubMed] [Google Scholar]
- 11.Levey SA, Eckardt KU, Tsukamuto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 12.Czekalski S. How to diagnose and how to interpret microalbuminuria in the diabetic patient. Nephrol Dial Transplant. 1996;11:1509–11. [PubMed] [Google Scholar]
- 13.Dirsch MV, Stuppner H, Vollmar MA. The Griess Assay: Suitable for a bio-guided fractionation of anti-inflammatory plant extracts? Planta Med. 1998;64:423–6. doi: 10.1055/s-2006-957473. [DOI] [PubMed] [Google Scholar]
- 14.Perkins BA, Aiello LP, Krolewski AS. Diabetes complications and the renin-angiotensin system. N Engl J Med. 2009;361:83–5. doi: 10.1056/NEJMe0904293. [DOI] [PubMed] [Google Scholar]
- 15.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–62. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 16.Bilous R, Chaturvedi N, Sjølie AK, Fuller J, Klein R, Orchard T, et al. Effect of candesartan on microalbuminuria and albumin excretion rate in diabetes. Ann Intern Med. 2009;151:11–20. doi: 10.7326/0003-4819-151-1-200907070-00120. [DOI] [PubMed] [Google Scholar]
- 17.Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361:40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakris G, Burgess E, Weir M, Davidai G, Koval S. Telmisartan is more effective than losartan in reducing proteinuria in patients with diabetic nephropathy: Comparing telmisartan's and losartan's effects on proteinuria. Kidney Int. 2008;74:364–9. doi: 10.1038/ki.2008.204. [DOI] [PubMed] [Google Scholar]
- 19.Meigs BJ, Hu BF, Rifai N, Manson EJ. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA. 2004;291:1978–86. doi: 10.1001/jama.291.16.1978. [DOI] [PubMed] [Google Scholar]
- 20.Pinkney JH, Stehouwer CD, Coppack SW, Yudkin JS. Endothelial dysfunction: Cause of the insulin resistance syndrome. Diabetes. 1997;46:S9–13. doi: 10.2337/diab.46.2.s9. [DOI] [PubMed] [Google Scholar]
- 21.Clark MG, Wallis MG, Barrett EJ, Vincent MA, Richards SM, Clerk LH, et al. Blood flow and muscle metabolism: A focus on insulin action. Am J Physiol Endocrinol Metab. 2003;284:E241–58. doi: 10.1152/ajpendo.00408.2002. [DOI] [PubMed] [Google Scholar]
- 22.Yilmaz MI, Saglam M, Qureshi AR, Carrero JJ, Caglar K, Eyileten T, et al. Endothelial dysfunction in type-2 diabetics with early diabetic nephropathy is associated with low circulating adiponectin. Nephrol Dial Transplant. 2008;23:1621–7. doi: 10.1093/ndt/gfm828. [DOI] [PubMed] [Google Scholar]
- 23.Stehouwer CD, Nauta JJ, Zeldenrust GC, Hackeng WH, Donker AJ, den Ottolander GJ. Urinary albumin excretion, cardiovascular disease, and endothelial dysfunction in non-insulin-dependent diabetes mellitus. Lancet. 1992;340:319–23. doi: 10.1016/0140-6736(92)91401-s. [DOI] [PubMed] [Google Scholar]
- 24.Aizawa JK, Shoemaker K, Overend TJ, Petrella RJ. Metabolic syndrome, endothelial function and lifestyle modification. DiabVasc Dis Res. 2009;6:181–9. doi: 10.1177/1479164109336375. [DOI] [PubMed] [Google Scholar]
- 25.Tsao PS, McEvoy LM, Drexler H, Butcher EC, Cooke JP. Enhanced endothelial adhesiveness in hypercholesterolemia is attenuated by L-arginine. Circulation. 1994;89:2176–82. doi: 10.1161/01.cir.89.5.2176. [DOI] [PubMed] [Google Scholar]
- 26.De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr, et al. Nitric oxide decreases cytokine-induced endothelial activation nitric oxide selectively reduces endothelial expression of adhesion molecules and pro-inflammatory cytokines. J Clin Invest. 1995;96:60–8. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudic RD, Sessa WC. Nitric oxide in endothelial dysfunction and vascular remodeling: Clinical correlates and experimental links. Am J Hum Genet. 1999;64:673–7. doi: 10.1086/302304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tesfamariam B, Cohen RA. Free radicals mediate endothelial cell dysfunction caused by elevated glucose. Am J Physiol. 1992;263:H321–6. doi: 10.1152/ajpheart.1992.263.2.H321. [DOI] [PubMed] [Google Scholar]
- 29.Tesfamariam B, Brown ML, Cohen RA. 15-Hydroxyeicosatetraenoic acid and diabetic endothelial dysfunction in rabbit aorta. J Cardiovasc Pharmacol. 1995;25:748–55. doi: 10.1097/00005344-199505000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Lin YK, Ito A, Asagami T, Tsao SP, Adimoolam S, Kimoto M, et al. Impaired nitric oxide synthase pathway in diabetes mellitus: Role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation. 2002;106:987–92. doi: 10.1161/01.cir.0000027109.14149.67. [DOI] [PubMed] [Google Scholar]
- 31.Calver A, Collier J, Valiance P. Inhibition and stimulation of nitric oxide synthesis in the human forearm arterial bed of patients with insulin-dependent diabetes. J Clin Invest. 1992;90:2548–54. doi: 10.1172/JCI116149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmlund A, Hulthe J, Millgard J, Sarabi M, Kahan T, Lind L. Soluble intercellular adhesion molecule-1 is related to endothelial vasodilatory function in healthy individuals. Atherosclerosis. 2002;165:271–6. doi: 10.1016/s0021-9150(02)00234-4. [DOI] [PubMed] [Google Scholar]
- 33.Witte DR, Broekmans WM, Kardinaal AF, Klöpping-Ketelaars IA, van Poppel G, Bots ML, et al. Soluble intercellular adhesion molecule 1 and flow-mediated dilatation are related to the estimated risk of coronary heart disease independently from each other. Atherosclerosis. 2003;170:147–53. doi: 10.1016/s0021-9150(03)00253-3. [DOI] [PubMed] [Google Scholar]
- 34.Schieffer B, Luchtefeld M, Braun S, Hilfiker A, Hilfiker-Kleiner D, Drexler H. Role of NAD (P)H oxidase in angiotensin II-induced JAK/STAT signaling and cytokine induction. Circ Res. 2000;87:1195–201. doi: 10.1161/01.res.87.12.1195. [DOI] [PubMed] [Google Scholar]
- 35.Hernandez-Presa M, Bustos C, Ortego M, Tuñon J, Renedo G, Ruiz-Ortega M, et al. Angiotensin-converting enzyme inhibition prevents arterial nuclear factor-κB activation, monocyte chemoattractant protein-1 expression and macrophage infiltration in a rabbit model of early accelerated atherosclerosis. Circulation. 1997;95:1532–41. doi: 10.1161/01.cir.95.6.1532. [DOI] [PubMed] [Google Scholar]
- 36.Jilma B, Li-Saw-Hee FL, Wagner OF, Wagner OF, Beevers DG, Lip GY. Effects of enalapril and losartan on circulating adhesion molecules and monocyte chemotactic protein. Clin Sci (Lond) 2002;103:131–6. doi: 10.1042/cs1030131. [DOI] [PubMed] [Google Scholar]
- 37.Graninger M, Reiter R, Drucker C, Minar E, Jilma B. Angiotens in receptor blockade decreases markers of vascular inflammation. J Cardiovasc Pharmacol. 2004;44:335–9. doi: 10.1097/01.fjc.0000137160.76616.cc. [DOI] [PubMed] [Google Scholar]
- 38.Gasic S, Wagner OF, Fasching P, Ludwig C, Veitl M, Kapiotis S, et al. Fosinopril decreases levels of soluble vascular cell adhesion molecule-1 in borderline hypertensive type II diabetic patients with microalbuminuria. Am J Hypertens. 1999;12:217–22. doi: 10.1016/s0895-7061(98)00229-5. [DOI] [PubMed] [Google Scholar]
- 39.Barnett AH, Bain SC, Bouter P, Karlberg B, Madsbad S, Jervell J, et al. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004;351:1952–61. doi: 10.1056/NEJMoa042274. [DOI] [PubMed] [Google Scholar]
- 40.Redón J, Luque-Otero M, Martell N, Chaves FJ. POLPRI Investigators. Renin-angiotensin system gene polymorphisms: Relationship with blood pressure and microalbuminuria in telmisartan-treated hypertensive patients. Phamacogenomics J. 2005;5:14–20. doi: 10.1038/sj.tpj.6500280. [DOI] [PubMed] [Google Scholar]
- 41.Ruilope LM, Redon J, Schmieder R. Cardiovascular risk reduction by reversing endothelial dysfunction: ARBs, ACE inhibitors, or both? Expectations from The ONTARGET Trial Programme. Vasc Health Risk Manag. 2007;3:1–9. [PMC free article] [PubMed] [Google Scholar]
- 42.Tsao PS, Buitrago R, Chan JR, Cooke JP. Fluid flow inhibits endothelial adhesiveness.Nitric oxide and transcriptional regulation of VCAM-1. Circulation. 1996;94:1682–9. doi: 10.1161/01.cir.94.7.1682. [DOI] [PubMed] [Google Scholar]


