Abstract
Background:
Electroconvulsive therapy (ECT) is one of the most efficacious treatment for major depressive disorder (MDD), it is also used as a rapid and efficacious treatment for other psychiatric disorders, especially treatment resistant ones. The cognitive impairment is one of the most important side effects of ECT. This study examined the Memoral herbal efficacy in prevention of ECT-induced memory impairment.
Methods:
In a randomized clinical trial, 70 patients with mood disorders who were candidates for ECT enrolled in either Memoral or Control group, and received either Memoral or placebo. The memory was assessed by Addenbrook Cognitive Examination (ACE), and the findings were analyzed by ANOVA under SPSS18.
Results:
The Memoral group patients showed significantly higher total ACE scores than placebo group (P < 0.001). The scores of attention and orientation, verbal fluency and memory subscales not only never decreased during the study in Memoral group, but also increased. There was no significant difference between these scores of Memoral and placebo groups for the subscales of language and visuospacial ability.
Conclusion:
The Memoral herbal is an efficacious and safe choice in prevention of ECT- induced cognitive impairment.
Keywords: Cognitive impairment, ECT, Memoral herbal, memory
INTRODUCTION
Electroconvulsive therapy (ECT) is one of the most efficacious treatment of major depressive disorder (MDD). It is used as a rapid and efficacious treatment in emergent and/or treatment resistant forms of other psychiatric disorders.[1] The cognitive aspects which are influenced immediately after ECT are: orientation, information processing, retrograde and anterograde amnesia, visuospatial capacity and word finding.[2]
Some studies have been conducted in order to prevent these cognitive impairments. Donepezil in a case report study[3] Galantamine in study without control group,[4] a glucocorticoid antagonist (CORT108997) in another study,[5] thyroid hormones,[6] antihypertensive,[7] poropofol,[8,9] physosigmine,[10] naloxone,[11] piracetam[12,13] showed some benefits in preventing ECT-induced cognitive impairment; but many of those have not enough reliability because were not statistically significant, or were case reports,[3] unblanded, uncontrolled[4] or on animals.[5] The other studies did not show efficacy of the used drugs in prevention of memory impairments.[14,15]
Considering these non sufficient and controversial data, more structured studies with control group on human samples are necessary. So, we chose the so-called Memoral herbal for our this preventive new study, which has never been used for this propse.
The Memoral herbal capsules, each contains 360 mg of Boswellia oleo-gum resin and 36 mg of Zimgiber rhizome. The most important constituent of Boswellia is gum resin 60%, mosilage 20-23% and essence 5-9%, which contains a-b-Thujon, p-cymen and linanol. Boswellia Serrata has Boswellic acid and acetyl- 11- keto-beta boswellic acid too, which are responsible for many therapeutic effect. The volatile oils in Boswellia dilate the vasculator of the brain thus can increase the blood passage through the brain. Boswellia extract has been demonstrated by a battery of rigorous tests to have anti-inflammatory effect. Boswellic acid has memory enhancing and anti-dementia properties.[16] The mechanism by which Boswellia can improve memory is through its anti-inflammatory effect on the brain.[16]
The aim of this study is to compare Memoral herbal with placebo in prevention of ECT-induced cognitive impairments.
METHODS
Study design
This study is a randomized and controlled clinical trial which was done during 9 months of 2011 at Noor hospital (Isfahan, Iran). The studied population was inpatients with mood disorder who were candidates for ECT. The sample size was 70 patients, based on 80% power and 95% confidence interval. Each consequent patient placed randomly in intervention (Memoral) or control (placebo) group, i.e., 35 patients in each group. The inclusion criteria were: 15-65ys/o, diagnosis of major depressive disorder (MDD) or bipolar disorder (BD) according to the impression of therapist (academic member) documented in the patient file, prescribed at least 4 ECT sessions by the therapist and informed consent for participation. Exclusion criteria were: Mental retardation (MR) or dementia as documented in hospital file by psychiatrist, discontinuation of ECT before fourth sessions, discontinuation of participation before the end of study, and arising delirium during the course of study. The ethical issues of this study was approved by ethical committee of Behavioral Sciences Research Center (BSRC) of Isfahan University of Medical Sciences (IUMS), Iran. The study process registered at Iranian Registering of Clinical Trials office, with IRCT ID: IRCT201102072232N2.
Intervention
The study was illustrated to patients and an informed consent form was signed by each patient or his/her first degree related person who was supporting him/her (In cases the patient was unable to decide). Each patient received three capsules (TID) of either Memoral or placebo from the day before first ECT session until 2 months after the last session of ECT.[16] Memoral and placebo capsules were produced by Goldaru pharmaceutical manufactory (Isfahan, Iran). The capsules were labeled by only “A” or “B” brand and were taken to patients by a nurse. Both patients and nurse were blind to the Memoral or placebo capsules.
Assessment
The cognition of patients was evaluated by Addonbrokes Cognitive Examination (ACE). This scale was developed through extension of mini mental status examination (MMSE) memory, language and visuospacial ability subscales, and adding verbal fluency subscale to it.[17] It is translated to Persian and validated by Pouretemad H.R.(2006). Its sensitivity and specificity for screening normal persons from mild cognitive impairment (MCI) is 93% and 91% , respectively; for screening alzheimer patients from MCI patients is 73% and 93%, respectively, and for screening normal persons from Alzheimer patients is 100% and 96%, respectively. It has five subscales: attention and orientation, memory, language, verbal fluency, visuospatial ability. Also it has the capacity for scoring based on the past so-called MMSE scale.
It takes approximately 15 minutes to fill the questionnaire. The patients’ cognitive function was assessed at four occasions: before the first ECT session, before the 4th ECT session, 1 month and 2 months after the last ECT session, by a psychologist.[18] The probable side effects of Memoral were also evaluated at those occasions, using a checklist. The psychologist was blind for Memoral or placebo using by each patient.
The findings were analyzed by Repeated Measure of Analysis of Variance (ANOVA) and Pearson Chi-Square, using SPSS18.
RESULTS
Of 70 patients, two patients from Memoral group and five patients from placebo group were excluded because of premature discontinuation of ECT and discharge from the hospital, causing disruption of participation. So 33 patients in Memoral group, and 30 patients in placebo group remained and there was not any significant difference between two groups (P = 0.95). Considering educational levels, there was not any significant difference between two groups (P = 0.18): including 6 (9.5%) patients had primary school studies, 23 (36.5%) guidance school, 24 (38.1%) high school, 10 (15.9%) had university studies. The frequency of patients’ diagnosis was: 26 (41.3) patients with BD manic or mixed episode, 13 (20.6%) patients BD depressive episode, 24 (38.1%) patients with MDD. There was not any significant difference between Memoral and placebo group about diagnoses distribution (P = 0.09). Also there was no significant difference between the number of ECT sessions in two groups (Pv=0.95%); included 2 (3.2%) patients received five ECT sessions,25 (39.7%) received six sessions, four (6.3%) received seven sessions, 17 (26.9%) received eight sessions, two (3.2%) patients received nine sessions, 8 (12.7%) received 10 sessions, two (3.2%) patients received 11 sessions, two (3.2%) patients received 12 sessions, and one (1.6%) patient received 14 ECT sessions. The mean energy of ECT used in Memoral group was 25.8 joule in Memoral group, and 25.9 joule in placebo group (P = 0.98).The mean age of patients in memoral group was 33.8 ys/o, and 32.8 ys/o in placebo group, with no significant difference between them (P = 0.17).We did not find any important side effects that could exclude patients from the study, and there was not any significant difference between two groups for this issue (P = 0.30).
There was not any significant difference between two groups between total score of ACE of Memoral group (76) and placebo group (79.4) before intervention (P = 0.32). Also, there was not any significant difference between two groups for the scores of attention and orientation subscales (P = 0.64), memory (P = 0.75), verbal fluency (Pv = 0.35), language (P = 0.11), and MMSE subscales (P = 0.40) before intervention.
Table 1 shows the mean± SD of the total score of ACE and the scores of ACE subscales in Memoral and placebo groups at four assessment occasions, i.e., before intervention, before the 4th ECT session, 1 month and 2 months after the last ECT sessions [Figure 1].
Table 1.
The mean ± SD of the total score of ACE and the scores of ACE subscales in Memoral and placebo groups at four assessment occasions: before intervention, before 4th ECT session, 1 month and 2 months after last ECT sessions
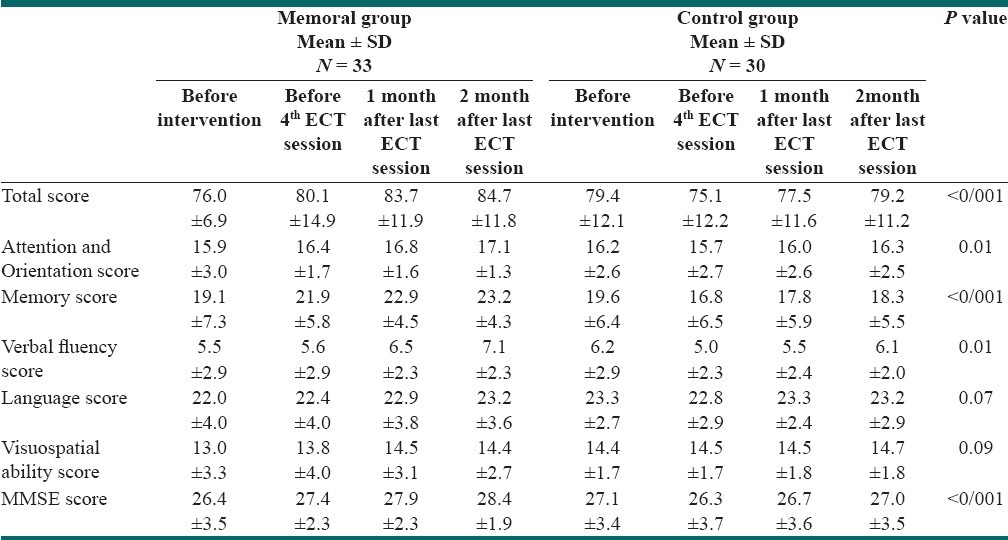
Figure 1.
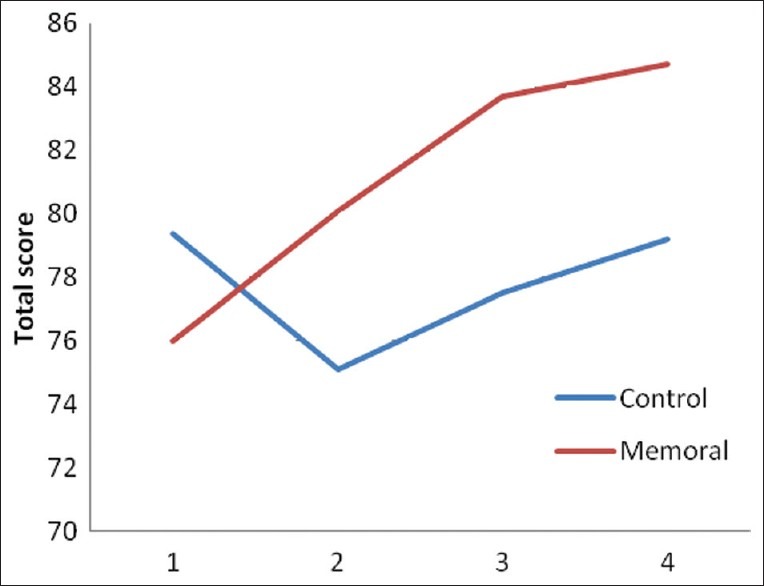
The mean of the total score of ACE in Memoral and placebo groups at four assessment occasions :before intervention, before 4th ECT session, 1month and 2months after last ECT sessions
Repeated measure of ANOVA showed that the total score of ACE and the scores of attention and orientation subscale, memory, verbal fluency and MMSE subscales were significantly higher in Memoral group than in placebo group after intervention, and also were uprising, unlike placebo group [Table 1, Figure 2]. But the scores of the language, and visuospatial ability subscales did not show any significant difference between two groups, although these were uprising in Memoral group, unlike in placebo group [Table 1]. The paired comparison of each subscales in two groups also showed significant difference at each stage.
Figure 2.
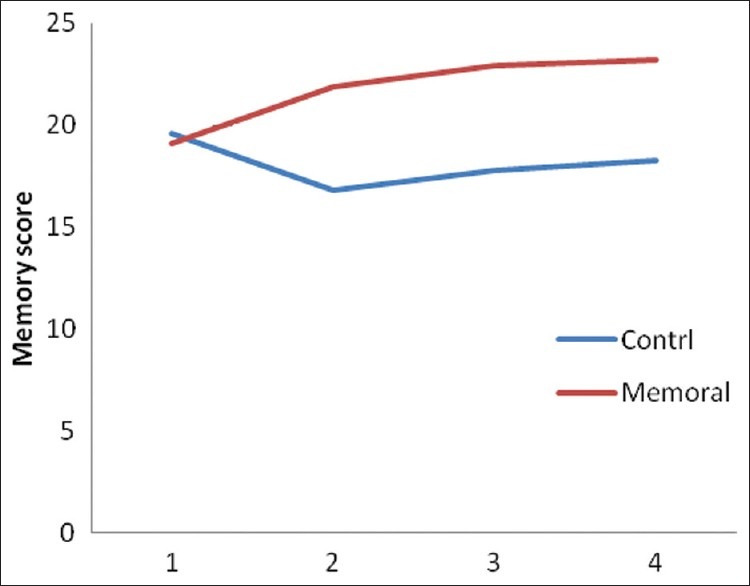
The mean of the memory subscale score of ACE in Memoral and placebo groups at four assessment occasions: before intervention, before 4th ECT session, 1month and 2 months after last ECT sessions
DISCUSSION
This study assessed the efficacy of Memoral herbal on prevention of ECT-induced cognitive impairments, using ACE scale. The finding showed improvement of total score of ACE in Memoral group. The total score of ACE and the subscales of memory, attention and orientation, verbal fluency and MMSE in Memoral group were significantly higher than in placebo, and did not declined by ECT unlike in placebo group. The noticeable finding was the largest amount of difference for memory subscale in this intervention [Table 1, Figure 2].
This study showed that Memoral herbal prevents ECT-induced memory impairment more rapidly and more impressively than other drugs used in previous similar studies.[3–13] Unlike previous studies[3–13] in this intervention, we assessed cognitive status in more detailed and itemized description. So these data may have more reliability and scrupulosity than previous findings, and may somehow interpret the inconsistency of some previous findings.[12–15]. The sample size of this study is another precedence of this study over previous ones.[4,5]
A limitation of our work is the focusing study on mood disorder patients. Other limitation is renunciation of assessing immediately after last ECT session cognitive status. This was some deal because of the blinding goals and the independence of researchers from therapists.
The future studies on prevention of ECT-induced cognitive impairments may comprise Memoral herbal with other drugs in a research, and on patients with other diagnoses.
CONCLUSIONS
Our work showed the effective prevention of ECT-induct cognitive impairment by using Memoral herbal. The cognitive status of patients not only declined, but also improved. The memory, attention and orientation, verbal fluency, and MMSE of patients showed improvement by Memoral use.
ACKNOWLEDGEMENT
The authors thanks the Goldaru Pharmaceutical Manufactory, especially the Dr. Ghafghazi, for their supports by providing Memoral and placebo capsules, Behavioral Sciences Research Center (BSRC) of Isfahan University of Medical Sciences (IUMS) because of their supports for this research all staffs of psychiatric wards and ECT unit of Noor Hospital of IUMS especially Mrs. Zomorodian, because without their good collaboration we couldn’t do this work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sadock BJ, Sadock VA. Kaplan and Sadock's Synopsis of psychiatry. 10th ed. Philadelphia: Lippincott Williams and Wilkins; 2007. pp. 1117–24. [Google Scholar]
- 2.Sadock BJ, Sadock VA, Ruiz P. Kaplan and Sadock's, Comprehensive Textbook of psychiatry. 9th ed. Philadelphia: Lippincott Williams and Wilkins; 2009. pp. 3285–300. [Google Scholar]
- 3.Rao NP, Palaniyappan P, Chandur J, Venkatasubramanian G, Gangadhar BN. Successful use of donepezil in treatment of cognitive impairment caused by maintenance electroconvulsive therapy: A case report. J ECT. 2009;25:216–8. doi: 10.1097/YCT.0b013e3181926ada. [DOI] [PubMed] [Google Scholar]
- 4.Matthews JD, Blais M, Park L, Welch C, Baity M, Murakami J, et al. The impact of galantamine on cognition and mood during electroconvulsive therapy: A pilot study. J Psychiatr Res. 2008;42:526–31. doi: 10.1016/j.jpsychires.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Andrade C, Shaikh SA, Narayan L, Blasey C, Belanoff J. Administration of a selective glucocorticoid antagonist attenuates electroconvulsive shock-induced retrograde amnesia. J Neural Transm. 2012;119:337–44. doi: 10.1007/s00702-011-0712-8. [DOI] [PubMed] [Google Scholar]
- 6.Tremont G, Stern RA. Minimizing the cognitive effects of lithium therapy and electroconvulsive therapy using thyroid hormone. Int J Neuropsychopharmacol. 2000;3:175–86. doi: 10.1017/S1461145700001838. [DOI] [PubMed] [Google Scholar]
- 7.Kamath S, Andrade C, Faruqi S, Venkataraman BV, Naga Rani MA, Candade VS. Evaluation of pre-ECS antihypertensive drug administration in the attenuation of ECS-induced retrograde amnesia. Convuls Ther. 1997;13:185–95. [PubMed] [Google Scholar]
- 8.Butterfield NN, Graf P, Macleod BA, Ries CR, Zis AP. Propofol reduces cognitive impairment after electroconvulsive therapy. J ECT. 2004;20:3–9. doi: 10.1097/00124509-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Luo J, Min S, Wei K, Li P, Dong J, Liu YF. Propofol protects against impairment of learning-memory and imbalance of hippocampal Glu/GABA induced by electroconvulsive shock in depressed rats. J Anesth. 2011;25:657–65. doi: 10.1007/s00540-011-1199-z. [DOI] [PubMed] [Google Scholar]
- 10.Levin Y, Elizur A, Korezyn AD. Physostigmine improves ECT-induced memory disturbances. Neurology. 1987;37:871–5. doi: 10.1212/wnl.37.5.871. [DOI] [PubMed] [Google Scholar]
- 11.Prudic J, Fitzsimons L, Nobler MS, Saceim HA. Naloxan in the prevention of the adverse cognitive effects of ECT: A within-subject, placebo controlled study. Neuropsychopharmacology. 1999;21:285–93. doi: 10.1016/S0893-133X(99)00015-9. [DOI] [PubMed] [Google Scholar]
- 12.Tiurenkov IN, Bagmetov MN, Epishina VV, Borodkina LE, Voronkov AV. Comparative evaluation of neuroprotective activity of phenibut and piracetam under experimental cerebral ischemia in rats. Eksp Klin Farmakol. 2006;69:19–22. [PubMed] [Google Scholar]
- 13.Ostrovskaia RU, Trofimov SS, Tsybina NM, Gudasheva TA, Skoldinov AP. Antagonism of piracetam with prolin in relation to mnestic effects. Biull Eksp Biol Med. 1985;99:311–4. [PubMed] [Google Scholar]
- 14.Mindus P, Cronholm B, Levander SE. Does piracetam counteract the ECT-induced memory dysfunctions in depressed patients? Acta psychiatr Scand. 1975;51:319–26. doi: 10.1111/j.1600-0447.1975.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 15.Hakkarainen H, Hakamies L. Piracetam in the treatment of post concussional Syndrome. A double-blind study. Eur Neurol. 1978;17:50–5. doi: 10.1159/000114922. [DOI] [PubMed] [Google Scholar]
- 16.Association of Producers of Herbal Medicines (A.P.H.M.P) Iranian Licensed Herbal Medicines. 2009 May;:181. [Google Scholar]
- 17.Pouretemad HR, Khatibi A, Ganjavi A, Shams J, Zarei M. Validation of addenbrookes cognitive examination (ACE) in a persion-speaking population. Dement Geriatr Cogn Disord. 2009;28:343–7. doi: 10.1159/000252772. [DOI] [PubMed] [Google Scholar]
- 18.Porter RJ, Douglas K, Knight RG. Monitoring of cognitive effects during a course of electroconvulsive therapy: Recommendations for clinical practice. J ECT. 2008;24:25–34. doi: 10.1097/YCT.0b013e31815d9627. [DOI] [PubMed] [Google Scholar]


