Abstract
Most sporadic endometrial cancers (ECs) can be histologically classified as endometrioid, serous, or clear cell. Each histotype has a distinct natural history, clinical behavior, and genetic etiology. Endometrioid ECs have an overall favorable prognosis. They are typified by high frequency genomic alterations affecting PIK3CA, PIK3R1, PTEN, KRAS, FGFR2, ARID1A (BAF250a), and CTNNB1 (β-catenin), as well as epigenetic silencing of MLH1 resulting in microsatellite instability. Serous and clear cell ECs are clinically aggressive tumors that are rare at presentation but account for a disproportionate fraction of all endometrial cancer deaths. Serous ECs tend to be aneuploid and are typified by frequent genomic alterations affecting TP53 (p53), PPP2R1A, HER-2/ERBB2, PIK3CA, and PTEN; additionally, they display dysregulation of E-cadherin, p16, cyclin E, and BAF250a. The genetic etiology of clear cell ECs resembles that of serous ECs, but it remains relatively poorly defined. A detailed discussion of the characteristic patterns of genomic alterations that distinguish the three major histotypes of endometrial cancer is reviewed herein.
Keywords: endometrial, cancer, genomics, genetics, sporadic
Introduction
Endometrial cancer (EC) is the sixth most commonly diagnosed cancer among women worldwide, causing ~74,000 deaths in 2008.1 Most ECs are sporadic but 2%–5% are familial. Familial EC is linked to germline mutations in the mismatch repair genes MLH1, MSH2, MSH6, or PMS2, and to certain germline deletions in EPCAM, in families with Lynch syndrome (reviewed by Meyer et al),2 or to germline mutations in PTEN associated with Cowden Syndrome.2–4
ECs can be classified into a number of distinct histological subtypes. Endometrioid, serous, and clear cell ECs represent the three major histological subtypes, each with a distinct natural history, genetic etiology, and associated clinical outcome.5,6 Other rare histological subtypes of EC include carcinosarcomas, also known as malignant mixed Müllerian tumors, mucinous carcinomas, squamous cell carcinomas, and transitional cell carcinomas.7,8 In the clinical setting, endometrial tumors can be comprised of a single histology or an admixture of two or more distinct histotypes, in which each component represents at least 10% of the tumor volume (reviewed by Acharya et al).7 In cases of mixed histology, clinical treatment is generally based on the most aggressive component (reviewed by Acharya et al).7 This review will focus on endometrioid, serous, and clear cell ECs because, collectively, they comprise the majority of endometrial carcinomas.
Endometrioid ECs (EECs) represent the most common histological subtype at presentation. They are estrogen-dependent tumors that may be preceded by hyperplasia, atypical hyperplasia, and endometrial intraepithelial neoplasia, a premalignant outgrowth from hormonally-induced, benign endometrial hyperplasia.9–11 Epidemiological risk factors leading to unopposed estrogen exposure including obesity, nulliparity, early age at menarche, late age at menopause, and unopposed estrogen therapy in post-menopausal women, are established risk factors for EEC (reviewed by Mahboubi et al).12 Most EECs are low-grade (G1 or G2) tumors that are diagnosed at an early stage, before extra-uterine spread.6 Consequently, surgical intervention is curative in many cases, and contributes to an overall favorable prognosis for EEC, as evidenced by a 5-year relative survival rate of ~90%.6 However, the prognosis is markedly less favorable for advanced stage disease and high-grade (G3) EECs.5,6,13
Serous and clear cell ECs are high-grade, estrogen-independent tumors that generally arise from the atrophic endometrium in postmenopausal women, although there are examples of serous EC in a non-atrophic background.14 They have no known epidemiological risk factors other than increasing age. Serous ECs can be preceded by precancerous cells that exhibit a “p53 signature,” endometrial glandular dysplasia (EmGD), and endometrial intra-epithelial carcinoma (EIC).15–22 Serous EC is frequently diagnosed at an advanced stage and has a significantly poorer prognosis than EEC, with an overall 5-year relative survival rate of only 44%.6 Clear cell EmGD has been suggested to precede clear cell EC.23 The overall 5-year relative survival rate for clear cell ECs is 65%, intermediate to serous and EECs.6 Together, serous and clear cell tumors represent only ~13% of diagnosed tumors, but they contribute disproportionately to mortality and account for more than half of all deaths from EC.13,24 Even when corrected for stage, patients with serous and clear cell EC have a much worse prognosis than those with EEC, pointing to differences in the underlying biology of these subtypes.13
Historically, most genetic studies have focused on EEC. There have been few systematic studies of serous EC, and even fewer on clear cell EC. Thus, the genetic etiology of the most clinically aggressive subtypes remains relatively poorly defined. Nonetheless, it is clear that there are important genetic distinctions between the three subtypes, both from mutational analyses and gene expression profiling.25 In this review, we will highlight the somatic genetic alterations that distinguish sporadic endometrioid and non-endometrioid (serous and clear cell) tumors.
The genetic etiology of endometrioid endometrial cancers
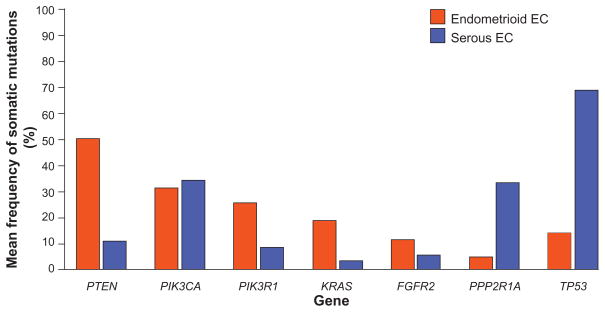
EECs are typified by frequent microsatellite instability (MSI), and somatic alterations within the PI3K pathway, the MAPK pathway, CTNNB1(β-Catenin), and ARID1A (BAF250a) (Table 1 and Figure 1).
Table 1.
Frequency range of genomic and proteomic aberrations among endometrioid and non-endometrioid endometrial cancers
| Tumor characteristic | Frequency (range)
|
References | |
|---|---|---|---|
| EECs (%) | NEECs (%) | ||
| Aneuploidy | 10–50 | 70–95 | 116,118–120 |
| MSI+ | 20–23 | 15 | 26–28,30 |
| AKT1 mutation | 2–3 | 13 | 53,199 |
| ARID1A mutation | 40 | 0 | 113 |
| BRAF mutation | 0–23 | 11 | 88,92 |
| CDKN2A mutation | 10–30 | 44* | 163–165 |
| CTNNB1 mutation | 2–45 | 0** | 52,103,110 |
| FBXW7 mutation | 2–16 | 0 | 154,155 |
| FGFR2 mutation | 5–16 | 2–3 | 100,101 |
| KRAS mutation | 8–43 | 2 | 57,80,88 |
| PIK3CA mutation | 20–52 | 33 | 57,62 |
| PIK3R1 mutation | 21–43 | 12 | 52,58 |
| PPP2R1A mutation | 3–7 | 17–41 | 133–135 |
| PTEN mutation | 26–79 | 13–19 | 57,198 |
| TP53 mutation | 5–20 | 53–90 | 60,122,125,129 |
| CCNE1 amplification | 5 | 42 | 154 |
| ERBB2 amplification | 1–63 | 17–42 | 137,138,200 |
| E-Cadherin negative expression | 5–53 | 62–88 | 173,174,176 |
| Claudin-3 positive expression | 38 | 74 | 167 |
| Claudin-4 positive expression | 9 | 63 | 167 |
| p16 positive expression | 5–38 | 63–100 | 160,161 |
Notes:
Based on an analysis of twelve tumors;
based on an analysis of nine tumors.
Figure 1.
Mean frequency of somatic mutations in cancer genes in endometrioid and serous ECs. The data were derived for genes that have been evaluated in at least 40 tumors of each subtype: FGFR2,52,100,101 KRAS,38,52,53,57,63,77,78,80,87,88,90,92,100,102,105 PIK3CA,52,57 PIK3R1,52,58 PPP2R1A,133–135 PTEN,52,53,56,57,59,60,62,72,102,196–198 TP53.52,60,80,92,122,128,129,131,196
Microsatellite instability (MSI)
A MSI phenotype is marked by a high frequency of mutations at sites of short nucleotide repeats (microsatellites) within the genome. MSI is the result of unrepaired errors that arise during DNA replication. It is detectable in ~20% of unselected endometrial tumors,26–28 and is more frequent among EECs than non-EECs (NEECs).29,30 In sporadic endometrial tumors, MSI-positivity reflects an increased mutation rate resulting from somatic alterations in DNA mismatch repair genes. Most presumed sporadic, MSI-positive EECs are associated with epigenetic silencing of MLH1, via promoter hypermethylation.31–34 This occurs early in EEC progression; MLH1 promoter hypermethylation has been documented in 3% of complex endometrial hyperplasias, and 33% of atypical hyperplasias.29
A smaller fraction of MSI-positive EECs have somatic mutations in MSH6,30,33 or loss of MSH2 protein expression.35,36 Somatic mutations in MSH3 have also been described in sporadic EC but, because they occur within a mononucleotide repeat tract, it has been suggested that they may be a consequence, rather than a cause, of defective mismatch repair.37 Likewise, certain MSH6 mutations have recently been proposed to occur secondarily to MSI.38
MLH1 and other mismatch repair genes are among the so-called “caretaker genes” that normally function to preserve genomic stability; loss of their function leads to the accumulation of mutations in other target genes that drive tumorigenesis.39 A number of target genes have been described in EC, although it is worth noting that most studies do not state whether the tumors occurred sporadically or in the context of Lynch syndrome. Within MSI-high EECs, the presence of somatic mutations involving simple nucleotide repeats in BHD (13%),40 BAX (29%–53%),33,41–43 IGFIIR (14%–21%),33,41 TGFβ-RII (10%–37%),38,41,44,45 E2F4 (21%),33 MLH3 (21%),42 MSH3 (14%–33%),37,41,42 MHS6 (7%–36%),33,38,41,42 CDC25C (7%),42 DNAPKcs (34%),46 RAD50 (17%),46 MRE11 (15%–50%),46,47 ATR (14%–15%),42,46 BRCA1 (15%),46 CtIP (12%),46 CHK1 (7%–28%),41,42 and MCPH1 (12%),46 implicates these genes as targets of MSI and potential drivers of MSI-positive endometrial tumorigenesis. Many of these genes, including ATR, are involved in the DNA damage response. MSI-associated truncating mutations in ATR are loss-of-function mutations that are significantly associated with both disease-free survival and overall survival in multivariate analyses.48,49
Early-stage EECs with and without MSI exhibit distinct gene expression profiles.50 It has been suggested that this might be either a direct effect of their differing MSI status, or alternatively, it might result from differences in the global methylation status of MSI+ and MSI− tumor subgroups, and therefore be indirectly associated with MSI caused by MLH1 hypermethylation.50
The PI3K pathway
The most frequently altered biochemical pathway in EECs is the PI3K-PTEN-AKT signal transduction pathway, which regulates numerous cellular processes including proliferation, growth, and survival.51 In the most comprehensive evaluation of PI3K pathway alterations in EECs to date, more than 80% of tumors had one or more somatic alterations affecting the pathway.52 These alterations consist of high frequency mutations in PIK3R1 (p85α), PIK3CA (p110α), and PTEN; PIK3CA amplification (7%–33% of EECs); PTEN promoter methylation or loss of PTEN expression; as well as rare mutations in AKT1 (2%) and PIK3R2 (p85β) (5%).52–64 In EEC, an additional level of dysregulation of mTOR is achieved by loss of expression of TSC2 and LKB1, which have been documented in 13% and 21% of EECs, respectively.65
The interplay between the various PI3K pathway alterations in EECs is complex. PIK3R1 and PIK3CA mutations are generally mutually exclusive, suggesting functional redundancy.52,58 In contrast, PTEN mutations frequently coexist, and can functionally cooperate, with PIK3R1 and PIK3CA mutations.52,54,58 Although PTEN is an important regulator of the PI3K-AKT pathway, it also has PI3K-independent functions. For example, PTEN plays an important role in the maintenance of genomic integrity.66 Recent work revealed that PTEN-deficient EC cell lines are sensitive to PARP inhibitors, pointing to a potential Achille’s heel for targeted therapy in EC.67
Endometrial tumors have a tissue specific pattern of PIK3CA mutations, with a significantly higher frequency of mutations in the ABD and C2 domains of p110α than any other tumor type that has been comprehensively evaluated.52,57,62 The reason for this tissue specificity is unclear but it is intriguing that p85α, which binds the ABD and C2 domains of p110α, is somatically mutated at high frequency in EC but only rarely in other tumors. Together, these observations suggest that disrupting the p85α-p110α interaction may confer a tissue specific selective advantage in endometrial tumorigenesis.
PTEN mutation is one of the earliest known events in the genesis of EEC, occurring in 20%–27% of endometrial hyperplasias,68,69 and in 55% of endometrial intraepithelial neoplasias.70 PTEN mutations are believed to precede mismatch repair defects in the progression of sporadic EECs.71 In contrast to PTEN, PIK3CA mutations are rare in complex atypical hyperplasia, and appear to be later events in the progression of EEC.72
The RAS-RAF-MEK-ERK pathway
The RAS family of oncogenes are frequently activated in a variety of human cancers. RAS proteins mediate signal transduction via both the RAF-MEK-ERK and PI3K-PTEN-AKT pathways, and thus regulate numerous processes including cell proliferation and cell survival.73
Somatic mutations in KRAS were first described in EC over two decades ago, and were subsequently found to be significantly more frequent in EEC than in serous EC.74–80 On average, KRAS is mutated in 18% of EECs compared with 3% of serous ECs (Figure 1).81 KRAS mutations occur early in the genesis of EECs, having been documented in atypical endometrial hyperplasia.82–84 However, MSI appears to precede KRAS mutation in the progression of EEC.82
In EC, KRAS mutations can coexist with mutations in PTEN, PIK3CA, and PIK3R1, suggesting that KRAS mutations are not functionally redundant with PI3K pathway mutations.52,57,58,85 This is supported by the results of a recent comprehensive genomics and proteomics analysis of the RAS-RAF-MEK-ERK and PI3K-PTEN-AKT pathways in EC in which Cheung et al showed that KRAS mutations were associated with increased phosphorylation of MEK1/2, ERK1/2, and p38MAPK.52 Oda et al have also shown functional synergy between mutant KRAS and mutant PIK3CA in the transformation of HMLE cells.85 Finally, a conditional mouse model of EC in which PTEN was ablated and KRAS was activated in the reproductive tract, showed an acceleration in the development of EC as compared to mice with only a single lesion.86
In contrast to KRAS mutations, somatic mutations in codons 11 and 15 of BRAF, the sites of hotspot mutations in other cancers, are infrequent in EECs,38,87–91 and are mutually exclusive with KRAS mutations and hypermethylation of RASSF1A.89 The overall BRAF mutation frequency in ECs is 1%.81 Only one study noted a high frequency (21%) of BRAF mutations in EC.92 It has been suggested that this high frequency of mutations might reflect ethnic differences between study populations,91 although this has not yet been verified.
RASSF1A is a multifunctional tumor suppressor that has been implicated in the regulation of numerous cellular processes and pathways, including the RAS signal transduction pathway.93 Hypermethylation of the RASSF1A promoter is frequent in EECs (62%–74%) and correlates with reduced expression of RASSF1A.94–96 RASSF1A promoter methylation has been documented in histologically normal tissue adjacent to EEC, and in complex hyperplasia with and without atypia.95,97
In EECs, methylation of the RASSF1A promoter is significantly associated with advanced stage disease.94 RASSF1A promoter hypermethylation is significantly more frequent in microsatellite unstable tumors than in microsatellite stable tumors, leading to the proposal that this reflects an underlying methylator phenotype that targets the MLH1 mismatch repair gene and other genes, including RASSF1A.89 RASSF1A methylation is also more frequent in tumors lacking a KRAS mutation than in tumors with mutant KRAS (38% vs 14%), although the difference did not achieve statistical significance.89
Several other genes that modulate the activity of the RAS-RAF-MAPK pathway are also subjected to aberrant methylation in EECs. These include RASSF2A, HDAB2IP, BLU, SPROUTY-2, and RSP6KA6 (RSK4).94,98,99
FGFR2
Somatic mutations in the FGFR2 receptor tyrosine kinase have been described in 12% of EECs.52,100,101 FGFR2 mutations are mutually exclusive with KRAS mutations, indicating functional redundancy, whereas most (77%) FGFR2-mutant ECs are PTEN-mutant.102 In EC, the vast majority of FGFR2 mutations are missense mutations within the extracellular, transmembrane, and kinase domains of the protein. Codon 252 (S252) forms a prominent mutation hotspot within a region of the extracellular domain that mediates ligand binding.101 The S252W mutant is oncogenic and accounts for ~41% of all mutations reported in EC to date.81,100 EC cell lines that harbor the FGFR2-S252W mutant appear to be dependent upon expression of the mutant protein for their survival.100,102 Importantly, EC cell lines with an activating mutation in FGFR2 are more sensitive to killing by PD173074, a pan-FGFR inhibitor, than FGFR2-wildtype EC cell lines, thus pointing to mutant FGFR2 as a potential therapeutic target.100,102
CTNNB1(β-Catenin)
CTNNB1 encodes β-catenin, an integral member of the canonical WNT signaling pathway. Somatic mutations in CTNNB1 and stabilization of β-catenin are common features of EEC.103,104 CTNNB1 mutations occur in up to 45% of EECs; they have not been found in NEECs, but only a small number of tumors have been evaluated (Table 1). Similarly, nuclear expression of β-catenin has been observed in 31%–47% of EECs, compared with 0%–3% of NEECs.104 A significant correlation between β-catenin accumulation and CTNNB1 mutations has been noted (P < 0.0001).105 Dysregulation of CTNNB1/β-catenin occurs early in the pathogenesis of EEC; it has been observed in atypical hyperplasias, in the squamous component of complex endometrial hyperplasia with atypia, and in endometrial intraepithelial neoplasia.106–110
ARID1A (BAF250a)
ARID1A is a recently described tumor suppressor gene that encodes BAF250a, a component of the SWI/SNF chromatin-remodeling complex.111 Dysregulation of ARID1A and BAF250a has been implicated in a large fraction of EECs. Loss of BAF250a expression has been observed by immunohistochemistry (IHC) in 26%–29% of low-grade (G1 or G2) EECs, and in 39% of high-grade (G3) EECs.112,113 Consistent with this observation, somatic mutations in ARID1A were detected among 40% of low-grade EECs; 50% of mutated tumors showed loss of BAF250a expression.113
The genetic etiology of serous and clear cell ECs
In contrast to EECs, serous ECs are often aneuploid,114–120 and are typified by frequent stabilization or mutation of p53, overexpression of cyclin E and ERBB2, p16 dysregulation, mutations in PPP2R1A, and a moderate frequency of alterations within the PI3K pathway (Table 1 and Figure 1).
TP53 (p53)
The most frequently altered cancer gene in serous EC is the TP53 tumor suppressor gene. In early landmark studies, 80%–86% of serous tumors showed positive immunostaining for p53, and 53%–90% of tumors had somatic TP53 mutations.80,121–125 TP53/p53 aberrations occur very early in the genesis of serous EC. They are present in morphologically benign endometrial glands or epithelium adjacent to serous EC, the so-called “p53 signature”, as well as in EmGD, and EIC.18,22,122,124 In 50% of uteri with coexisting “p53 signatures,” EmGD, EIC, and serous EC, identical p53 mutations were observed in all four entities.22 An increasing frequency of TP53 mutations has also been noted between the normal endometrium (0%), EmGD (43%), EIC (72%), and serous-EC (96%).18 These observations, coupled with detailed pathologic descriptions of “p53 signatures,” EmGD, and EIC, present a new model for the evolution of serous EC. This model posits a transition from the normal resting epithelium, to latent precancerous “p53 signatures,” to precancerous EmGD, to EIC, and finally to serous EC.18,22
In contrast to serous ECs, EECs have a significantly lower overall incidence of p53 positivity (3%–52%) and TP53 mutation (12%–23%).80,92,123,124,126–129 The incidence of TP53 mutations is greater in high-grade (G3) EECs than in low-grade EECs (43% of G3, 8% of G2, 0% of G1).80 However the incidence of p53 positivity and TP53 mutation in high-grade EECs is still subject to interpretation, as some of the reported high-grade EEC cases may actually be serous EC, due to the occasional histological ambiguity between these two subtypes.130 The frequency of TP53 mutations in clear cell EC has not been well defined although one study noted mutations in 9% of tumors.131
PPP2R1A
The PP2A serine-threonine phosphatase is a trimeric holoenzyme composed of a catalytic subunit (PP2Ac; subunit C), a scaffolding subunit (PR65; subunit A) and one of a number of variable regulatory (B) subunits (reviewed by Eichhorn et al).132 The scaffolding subunits are encoded by PPP2R1A (PR65α) or PPP2R1B (PR65β). They contain 15 HEAT (Huntington/elongation/A-subunit/TOR) motifs; HEAT motifs 2–7 mediate binding to the regulatory subunits, whereas HEAT motifs 11–15 mediate binding to the catalytic subunit of the holoenzyme.
Somatic mutations in PPP2R1A (PR65α) occur at very high frequency (17%–41%) in serous EC.133–135 In contrast, PPP2R1A is infrequently (5%–7%) mutated in EECs.133–135 It remains to be determined whether PPP2R1A is mutated in pure clear cell ECs; only five primary tumors of this subtype have been sequenced and no mutations were detected.133,134
Resequencing of PPP2R1A in ECs has thus far been confined to exons 5 and 6, based on earlier observations that PPP2R1A mutations in ovarian cancer localized exclusively within these two exons.135 Interestingly, the distribution of PPP2R1A mutations within exons 5 and 6 differs between endometrial and ovarian cancers. The majority (72%, 18 of 25 mutations) of mutations in ovarian cancer involve codons 182 and 183 whereas the majority of mutations in EC (77%, 30 of 39) involve codons 179, 256, and 257. The significance of this tissue-specific difference is currently unclear but has been suggested to possibly reflect different underlying mechanisms of mutagenesis, or perhaps tissue-specific functional effects.135 Only a small number of mutations in PPP2R1A have been described in EECs, but it is noteworthy that they were more frequent in codons 182/183 than in codons 256/257.133–135
The mechanism whereby PPP2R1A mutations contribute to tumorigenesis is currently unclear. The clustering of mutations to the 5th and 7th HEAT motifs of PR65α which interface with the regulatory subunits of PP2A, has led to speculation that the mutant scaffolding proteins might have an impaired interaction with the regulatory subunits, thus resulting in altered substrate recognition and/or altered phosphatase activity.133,135 Because the majority of PPP2R1A mutations are heterozygous, and PP2A has been ascribed tumor suppressor properties (reviewed by Eichhorn et al),132 it has been proposed that mutant PPP2R1A might function either as a haploinsufficient tumor suppressor gene, or by exerting a dominant negative effect on the protein encoded by retained wild type allele.135
HER-2/ERBB2
Protein overexpression and genomic amplification of the HER-2/ERBB2 receptor tyrosine kinase are significantly more frequent among serous ECs than among EECs.136–138 In serous EC, overexpression of HER-2/ERBB2 by IHC has been noted in 17%–80% of cases.119,136,137,139–147 HER-2/ERBB2 amplification, determined by FISH, has been noted in 17%–68% of serous tumors that overexpress the protein,141,148 and in 17%–42% of serous tumors overall.136,138,143,148 A number of factors have been suggested to account for the inter-study variability in the frequency of HER-2/ERBB2 overexpression, including the small number of samples in some studies, differences in study populations, and variability in IHC, including inconsistencies in scoring HER-2/ERBB2 positivity.141
Several studies have observed correlations between HER-2/ERBB2 status and clinicopathological characteristics of serous ECs. HER-2/ERBB2-overexpressing serous ECs were associated with significantly shorter survival times (overall, 2-year, and 5-year) than HER-2/ERBB2-negative serous ECs, suggesting that HER-2/ERBB2 overexpression may be of prognostic value.136,141,142,145 Higher frequencies of HER-2/ERBB2 overexpression and amplification have been noted in serous ECs from African Americans compared with Caucasians, although the basis for this difference remains unexplained.140,149 Finally, two studies noted that patients with HER-2/ERBB2-positive serous ECs were more likely to have had a personal history of breast cancer than those who were HER-2/ERBB2-negative.141,144 The role of HER-2/ERBB2 perturbations in clear cell EC remains poorly defined due to the limited number of tumors analyzed.
The PI3K pathway
Somatic alterations in the PI3K pathway are significantly less frequent in serous EC than EEC. Nonetheless, the combined frequency of PI3K pathway alterations in serous EC is appreciable (39%), resulting from mutations in PTEN (13%), PIK3CA (35%), and PIK3R1 (8%).57,58,150,151 Compared to serous ECs, clear cell ECs do not show a statistically significant difference in the mutation frequency of PTEN (5%), PIK3CA (30%), and PIK3R1 (20%), although only a small number of clear cell tumors have been analyzed.57,58 Overall, 35% of clear cell ECs had a PI3K pathway mutation in one series.58
The spectrum of PIK3R1 mutations in NEECs differs somewhat from that of EECs.58 Most PIK3R1 mutants found in NEECs are truncation mutations, which preferentially co-occur with PIK3CA mutations and are currently of unknown functional significance. This is in contrast to PIK3R1 mutations in EECs, which tend to be small in-frame deletions that are mutually exclusive with PIK3CA mutations and, in some cases, have an impaired ability to inhibit AKT activation.52,58
ARID1A (BAF250a)
Loss of BAF250a expression has recently been reported in 18% of serous ECs and in 26% of clear cell ECs.112 The frequency of BAF250a loss is significantly lower in serous ECs than in high-grade endometrioid carcinomas (P < 0.001).112 No mutations in ARID1A have been reported in either serous or clear cell ECs but only a limited number of these tumors have been sequenced.113 Given the strong correlation between mutations in ARID1A and loss of BAF250a expression in other tumors,113 it seems likely that ARID1A mutations are also present within NEECs.
CCNE (cyclin E)
High levels of cyclin E, measured by IHC staining, have been reported in 51%–80% of poorly differentiated ECs, compared with 31%–45% of well- to moderately-differentiated ECs; in some studies, this difference attained statistical significance.152–154 Cyclin E overexpression is also statistically significantly more frequent among NEECs than EECs (54.5% vs 27.5%; P = 0.035).154
There are at least two underlying molecular mechanisms that account for high levels of cyclin E in EC. The first mechanism is amplification of CCNE, which is present in 16% of ECs overall, and in 30% of ECs that over express cyclin E.154 The second mechanism is loss-of-function mutations within the FBXW7/CDC4/hAGO tumor suppressor gene.154,155 FBXW7 encodes the substrate recognition component of an SCF-ubiquitin ligase complex that targets cyclin E for ubiquitin-mediated proteosomal degradation.156,157 Somatic mutations within FBXW7 have been reported at variable frequency among endometrial carcinomas. Two studies found FBXW7 mutations only rarely (~3%) in endometrial carcinomas, though neither specified the histology of tumors analyzed for mutations.100,154 In contrast, Suehiro et al identified a high frequency of FBXW7 mutations in EECs (46.8%).158 Spruck et al reported a moderate frequency (16%) of FBXW7 mutations in endometrial tumors that had elevated levels of cyclin E or phosphorylated cyclin E, although the tumor histotype was not specified.155 Thus, the frequency of FBXW7 mutations in NEECs remains to be elucidated.
CDKN2A (p16)
The CDKN2A/p16 tumor suppressor is a negative regulator of G1/S cell cycle progression. Recent studies on large tumor panels have revealed that serous ECs nearly uniformly show strong diffuse staining of p16, indicative of high expression.159–162 This is in stark contrast to the weak focal staining of endometrioid tumors of all grades. Though the prognostic significance and molecular basis for p16 overexpression has yet to be determined, it has been suggested that p16 expression may serve as a potent biomarker that might be useful in the molecular classification of ECs, particularly for high-grade tumors.161–163 In addition, CDKN2A is mutated in 10%–28% of EECs compared with 44% of NEECs, although the latter observation is based on a small sample size.163–165
Claudins and other cellular adhesion proteins
In 2005, Santin et al noted differential expression of numerous genes by microarray analysis between primary short-term cultures of serous ECs and normal endometrial cells, including several genes that regulate cell adhesion.166 Among these genes, claudins-3 and -4, which encode cell adhesion proteins present at tight junctions, were upregulated in serous EC. RT-PCR confirmed the upregulation of claudin-3 (8-fold) and claudin-4 (12-fold) in serous cultures compared with normal endometrial cell cultures. Immunohistochemistry for claudin-4 on corresponding primary tumor specimens, as well as a small number of additional serous tumors, revealed stronger staining for claudin-4 in serous EC compared with normal endometrial cells. In a subsequent study of a large number of endometrial tumors, Konecny et al showed that positive immunohistochemical staining for claudins-3 and -4 is significantly more frequent among serous (78% and 56%) and clear cell (61% and 44%) ECs than among EECs (38% and 9%).167 In multivariate analyses, claudin expression was not a significant independent prognostic indicator. In contrast, Sobel et al failed to find an association between claudin-3 and -4 levels and histotype of EC in a small series of tumors.168 One possible reason for the variability between studies might be the differences in sample sizes. The observation that claudins-3 and -4 are upregulated in serous EC holds promise for the development of targeted therapy, since Clostridium perfringens enterotoxin (CPE) targets claudins-3 and -4 and causes cytolysis upon binding.169
The original study that uncovered upregulation of claudins-3 and -4 in serous EC, compared with normal endometrium, also noted upregulation of several other genes that encode cell adhesion proteins, including L1CAM (L1 cellular adhesion molecule), and EpCAM (Epithelial Cell Adhesion Molecule).166 The observation of L1CAM upregulation was consistent with an earlier report that immunohistochemical expression of L1CAM was more frequent among serous ECs than EECs (75% vs 16%), although the number of serous tumors evaluated was small.170 The upregulation of EpCAM expression in serous EC has also been verified immunohistochemically; in one study, EpCAM staining was shown to be significantly higher in serous ECs than in normal endometrium.171 Serous EC cell lines that were positive for EpCAM were sensitive to MT201 (adecatumumab), a human monoclonal antibody against EpCAM, suggesting that high EpCAM levels may represent a druggable target for serous ECs.171 As discussed below, E-cadherin, another cell adhesion molecule, has a well-established role in serous EC.
E-cadherin
The CDH1 tumor suppressor gene encodes E-cadherin, a calcium-dependent cell adhesion molecule. Loss of E-cadherin expression is a characteristic feature of the epithelial to mesenchymal transition.172 Negative or reduced expression of E-cadherin has been described in ECs, and is significantly more frequent among serous and clear cell endo-metrial tumors than among EECs [83% vs 53%; P = 0.002],173 [62% vs 5%; P < 0.001],174 [87.1% vs 50%; P = 0.001],175 [75% vs 43%; P = 0.04].176 In stage I–III EC, multivariate Cox regression analysis showed that high E-cadherin expression was associated with decreased overall mortality, and was statistically significantly associated with decreases in EC mortality, disease progression, and extra pelvic recurrence.177 In a recent multicenter Phase II trial (GOG-119) that examined prognostic factors in stage IV or recurrent ECs, high expression of E-cadherin was associated with longer median survival, and reduced risks of disease progression and death.178
The molecular mechanisms accounting for reduced E-cadherin expression in EC are not fully elucidated. Somatic mutations in CDH1 are rare in EC.179 Loss of heterozygosity encompassing the gene has been reported at higher frequency in NEECs than EECs (57% vs 22.5%).176 CDH1 promoter hypermethylation is also common among ECs (21%–40%) but does not always correlate with reduced protein expression.176,180,181
Other mechanisms that may contribute to decreased E-cadherin expression in EC include dysregulation of certain transcriptional repressors of E-cadherin. Transcriptional repressors of E-cadherin include SNAI1 (Snail), SNAI2 (Slug), ZEB1, HMGA2, and TWIST.182 In stage IC EECs, each of these repressors is significantly overexpressed at the mRNA level, compared with normal endometrium, with a tendency towards associated lower E-cadherin levels, although this was not statistically significant.182 Other studies have reported a statistically significant inverse correlation between Snail and E-cadherin expression in metastatic EECs.183 Similarly, an inverse correlation between ZEB1 expression and E-cadherin expression has been noted in EC cell lines.184
Therapeutic targets for EC
Uncovering the genetic etiology of EC has provided not only new insights into the biology of the disease, but has also revealed molecular alterations that may be exploited for targeted therapy (Table 2).
Table 2.
Ongoing clinical trials of targeted therapies for endometrial cancer (ClinicalTrials.gov)
| Inhibitor | Molecular target | Phase | Monotherapy/combination therapy | Trial identifier |
|---|---|---|---|---|
| Temsirolimus | mTOR | II | Combination | NCT01010126 |
| Temsirolimus | mTOR | II | Combination | NCT00977574 |
| Temsirolimus | mTOR | I | Combination | NCT00982631 |
| Ridaforolimus | mTOR | I | Combination | NCT01256268 |
| Everolimus | mTOR | I | Combination | NCT00703807 |
| MK2206 | AKT | II | Monotherapy | NCT01307631 |
| BKM120 | PI3K | II | Monotherapy | NCT01397877 |
| XL147 | PI3K | II | Monotherapy | NCT01013324 |
| XL147 | PI3K | I | Combination | NCT00756847 |
| GDC-0980 | PI3K | II | Monotherapy | NCT01455493 |
| BKM120 | Pan-PI3K | II | Monotherapy | NCT01289041 |
| DS-7423 | PI3K/mTOR | I | Monotherapy | NCT01364844 |
| BEZ235 | PI3K/mTOR | II | Monotherapy | NCT01290406 |
| PF-04691502/PF-05212384 | PI3K-mTOR/PI3K-mTOR | II | Combination | NCT01420081 |
| XL147/MSC1936369B | PI3K/MEK | I | Combination | NCT01357330 |
| GSK1120212/GSK2110183 | MEK/AKT | I | Combination | NCT01476137 |
| MSC1936369B/SAR245409 | MEK/PI3K-mTOR | I | Combination | NCT01390818 |
| FP-1039 | FGF | II | Monotherapy | NCT01244438 |
| Trastuzumab | HER-2 | II | Combination | NCT01367002 |
| ARRY-380 | HER-2 | I | Monotherapy | NCT00650572 |
| BIBF 1120 | VEGFR/FGFR/PDGFR | II | Monotherapy | NCT01225887 |
| TKI258 | RTKs | II | Monotherapy | NCT01379534 |
| Sunitinib or temsirolimus | RTKs/mTOR | II | Monotherapy | NCT01396408 |
| GSK2636771 | PTEN-deficiency | I/IIa | Monotherapy | NCT01458067 |
| ARRY-382 | CSF-1 receptor | I | Monotherapy | NCT01316822 |
| RO4929097/temsirolimus | Gamma-secretase/mTOR | I | Combination | NCT01198184 |
| Olaparib | PARP | I | Combination | NCT01237067 |
The frequent mutational disruption of the PI3K-PTEN-AKT axis in EEC prompted clinical trials of drugs that target this pathway. Among these agents are the mTOR inhibitors temsirolimus (CC1-779), everolimus (RAD001), and ridaforolimus (AP23573). Encouraging results of a Phase II trial of temsirolimus in chemotherapy-naïve and chemotherapy-treated patients, with recurrent or metastatic EC, have recently been reported.185 In the chemotherapy-naïve group, 14% of evaluable patients had a partial response and 69% had stable disease (mean duration 9.7 months; range 2.1 to 14.6 months). In the chemotherapy-treated group, partial responses were achieved in 4% of patients, while 48% of patients had stable disease (mean duration 3.8 months; range 2.4 to 23.2 months). Interestingly, the PTEN mutational status of archival tumor tissue was not predictive of response but, as noted in the study, this may not reflect the mutational status of the recurrent tumor.185 A Phase II trial of everolimus in previously treated, progressive, or recurrent EEC also reported encouraging results.186 Although there were no cases of complete or partial response, 43% of evaluable patients had stable disease at the time of first evaluation (8 weeks); the confirmed clinical benefit rate was 21% at 20 weeks. No significant molecular correlates of response were detected.187 The interim results of a Phase II trial of ridaforolimus (AP23573) as a single agent in advanced EC patients with disease progression, revealed that 33% of evaluable patients had clinical benefit response, including two partial responses, one of which was serous EC.188 Importantly, preclinical studies have shown that inhibition of mTOR can lead to activation of MAPK, while pharmacological inhibition of MAPK enhances the anti-tumor effect of rapamycin, both in vitro and in vivo.189 These observations have led to clinical trials using combinatorial approaches to target both the PI3K and MEK pathways in EC and other cancers.
The recent identification of activating FGFR2 mutations in a subset of EECs, and subsequent preclinical studies, have highlighted mutant FGFR2 as a potentially druggable target. A Phase II clinical trial to access safety, tolerability, and pharmacokinetics of FP-1039, a soluble fusion protein designed to bind FGFR ligands, for patients with metastatic or locally advanced EC and a somatic FGFR2S252W or FGFR2P243R mutation, is currently recruiting patients (NCT01244438). A Phase II study (NCT01379534) evaluating the efficacy of TKI258 (dovitinib), a multitargeted receptor tyrosine kinase inhibitor, for treatment of FGFR2-mutated or -wildtype, advanced or metastatic EC, is also recruiting patients.
Recent preclinical evidence has indicated that PTEN deficiency sensitizes EC cells to PARP inhibitors.67 Encouragingly, a recent case report of an EC patient with a PTEN-deficient, presumed BRCA-intact, metastatic endometrioid endometrial tumor reported clinical benefit following treatment with olaparib, a PARP inhibitor.190 NCI clinical trial #NCT01237067 is a study for refractory or recurrent women’s cancers, including EC, which is designed to determine the safety and efficacy of olaparib, a PARP inhibitor, in combination with carboplatin.
HER-2 amplification and overexpression in NEECs also presents a druggable target. Individual case reports have documented clinical responses in advanced or recurrent EC patients following treatment with trastuzumab, an anti-HER-2 monoclonal antibody.191,192 However, a Phase II trial of trastuzumab in a small cohort of recurrent or advanced stage, HER2-positive EC patients documented stable disease in 40% of evaluable cases, with no reports of partial or complete response.193
Additional targeted therapies being evaluated for the treatment of EC include pharmacological inhibitors of VEGF, HIF1a, EphA2, and EGFR, as reviewed in detail elsewhere.8,194
Conclusion and future prospects
In conclusion, our understanding of the genetic etiology of EECs and serous ECs has advanced considerably over the past 20 years, and reflects a large body of research on individual genes, gene families, and pathways, as reviewed herein. As for most cancers, the rate-limiting step in dissecting the genetic alterations that underlie EC has been the availability of sufficiently high-resolution genomic technologies.195 However, within the past 5 years, the development and implementation of so-called next generation sequencing has resulted in a massive paradigm shift in cancer genomics because it provides the tools to systematically interrogate cancer genomes, exomes, and transcriptomes, nucleotide by nucleotide, for somatic alterations in gene sequence, structure, and copy number.195
The Cancer Genome Atlas is currently conducting large-scale, integrated genomic and epigenomic analyses of low-grade EECs, high-grade EECs, and serous ECs using massively parallel sequencing and other high resolution genomic and epigenomic approaches. The resulting catalogs of somatic alterations are eagerly awaited because they will reveal, for the first time, the most comprehensive view of the genomic, transcriptomic, and epigenomic landscape of ECs. This will provide a solid foundation for future studies to determine whether the altered genes are relevant to the biology and clinical management of women with EC.
Acknowledgments
This review was funded by the Intramural Program of the National Human Genome Research Institute at NIH (DWB). We thank our colleagues for critical reading of the manuscript. We apologize to those authors whose work we could not cite, due to space limitations.
Footnotes
This is an Open Access article which permits unrestricted noncommercial use, provided the original work is properly cited.
Disclosure
The authors have no conflicts of interest in this work.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Meyer LA, Broaddus RR, Lu KH. Endometrial cancer and Lynch syndrome: clinical and pathologic considerations. Cancer Control. 2009;16(1):14–22. doi: 10.1177/107327480901600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pilarski R, Eng C. Will the real Cowden syndrome please stand up (again)? Expanding mutational and clinical spectra of the PTEN hamartoma tumour syndrome. J Med Genet. 2004;41(5):323–326. doi: 10.1136/jmg.2004.018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kempers MJ, Kuiper RP, Ockeloen CW, et al. Risk of colorectal and endometrial cancers in EPCAM deletion-positive Lynch syndrome: a cohort study. Lancet Oncol. 2011;12(1):49–55. doi: 10.1016/S1470-2045(10)70265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Creasman WT, Odicino F, Maisonneuve P, et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95 (Suppl 1):S105–S143. doi: 10.1016/S0020-7292(06)60031-3. [DOI] [PubMed] [Google Scholar]
- 6.Ries LA, Young JL, Keel GE, Eisner MP, Lin YD, Horner MJ. SEER Program, NIH Pub No 076215. Bethesda, MD: National Cancer Institute; 2007. SEER Survival Monograph: Cancer Survival Among Adults: US. SEER Program, 1988–2001. Patient and Tumor Characteristics. [Google Scholar]
- 7.Acharya S, Hensley ML, Montag AC, Fleming GF. Rare uterine cancers. Lancet Oncol. 2005;6(12):961–971. doi: 10.1016/S1470-2045(05)70463-0. [DOI] [PubMed] [Google Scholar]
- 8.Dedes KJ, Wetterskog D, Ashworth A, Kaye SB, Reis-Filho JS. Emerging therapeutic targets in endometrial cancer. Nat Rev Clin Oncol. 2011;8(5):261–271. doi: 10.1038/nrclinonc.2010.216. [DOI] [PubMed] [Google Scholar]
- 9.Mutter GL, Boynton KA, Faquin WC, Ruiz RE, Jovanovic AS. Allelotype mapping of unstable microsatellites establishes direct lineage continuity between endometrial precancers and cancer. Cancer Res. 1996;56(19):4483–4486. [PubMed] [Google Scholar]
- 10.Mutter GL, Baak JP, Crum CP, Richart RM, Ferenczy A, Faquin WC. Endometrial precancer diagnosis by histopathology, clonal analysis, and computerized morphometry. J Pathol. 2000;190(4):462–469. doi: 10.1002/(SICI)1096-9896(200003)190:4<462::AID-PATH590>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 11.Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer. 1985;56(2):403–412. doi: 10.1002/1097-0142(19850715)56:2<403::aid-cncr2820560233>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 12.Mahboubi E, Eyler N, Wynder EL. Epidemiology of cancer of the endometrium. Clin Obstet Gynecol. 1982;25(1):5–17. doi: 10.1097/00003081-198203000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton CA, Cheung MK, Osann K, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer. 2006;94(5):642–646. doi: 10.1038/sj.bjc.6603012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng W, Xiang L, Fadare O, Kong B. A proposed model for endometrial serous carcinogenesis. Am J Surg Pathol. 2011;35(1):e1–e14. doi: 10.1097/PAS.0b013e318202772e. [DOI] [PubMed] [Google Scholar]
- 15.Ambros RA, Sherman ME, Zahn CM, Bitterman P, Kurman RJ. Endometrial intraepithelial carcinoma: a distinctive lesion specifically associated with tumors displaying serous differentiation. Hum Pathol. 1995;26(11):1260–1267. doi: 10.1016/0046-8177(95)90203-1. [DOI] [PubMed] [Google Scholar]
- 16.Sherman ME, Bitterman P, Rosenshein NB, Delgado G, Kurman RJ. Uterine serous carcinoma. A morphologically diverse neoplasm with unifying clinicopathologic features. Am J Surg Pathol. 1992;16(6):600–610. doi: 10.1097/00000478-199206000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Spiegel GW. Endometrial carcinoma in situ in postmenopausal women. Am J Surg Pathol. 1995;19(4):417–432. doi: 10.1097/00000478-199504000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Jia L, Liu Y, Yi X, et al. Endometrial glandular dysplasia with frequent p53 gene mutation: a genetic evidence supporting its precancer nature for endometrial serous carcinoma. Clin Cancer Res. 2008;14(8):2263–2269. doi: 10.1158/1078-0432.CCR-07-4837. [DOI] [PubMed] [Google Scholar]
- 19.Liang SX, Chambers SK, Cheng L, Zhang S, Zhou Y, Zheng W. Endometrial glandular dysplasia: a putative precursor lesion of uterine papillary serous carcinoma. Part II: molecular features. Int J Surg Pathol. 2004;12(4):319–331. doi: 10.1177/106689690401200405. [DOI] [PubMed] [Google Scholar]
- 20.Zheng W, Liang SX, Yi X, Ulukus EC, Davis JR, Chambers SK. Occurrence of endometrial glandular dysplasia precedes uterine papillary serous carcinoma. Int J Gynecol Pathol. 2007;26(1):38–52. doi: 10.1097/01.pgp.0000228138.56222.4e. [DOI] [PubMed] [Google Scholar]
- 21.Zheng W, Liang SX, Yu H, Rutherford T, Chambers SK, Schwartz PE. Endometrial glandular dysplasia: a newly defined precursor lesion of uterine papillary serous carcinoma. Part I: morphologic features. Int J Surg Pathol. 2004;12(3):207–223. doi: 10.1177/106689690401200302. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Liang SX, Jia L, et al. Molecular identification of “latent precancers” for endometrial serous carcinoma in benign-appearing endometrium. Am J Pathol. 2009;174(6):2000–2006. doi: 10.2353/ajpath.2009.081085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fadare O, Liang SX, Ulukus EC, Chambers SK, Zheng W. Precursors of endometrial clear cell carcinoma. Am J Surg Pathol. 2006;30(12):1519–1530. doi: 10.1097/01.pas.0000213296.88778.db. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton CA, Kapp DS, Chan JK. Clinical aspects of uterine papillary serous carcinoma. Curr Opin Obstet Gynecol. 2008;20(1):26–33. doi: 10.1097/GCO.0b013e3282f2b10d. [DOI] [PubMed] [Google Scholar]
- 25.Risinger JI, Maxwell GL, Chandramouli GV, et al. Microarray analysis reveals distinct gene expression profiles among different histologic types of endometrial cancer. Cancer Res. 2003;63(1):6–11. [PubMed] [Google Scholar]
- 26.Duggan BD, Felix JC, Muderspach LI, Tourgeman D, Zheng J, Shibata D. Microsatellite instability in sporadic endometrial carcinoma. J Natl Cancer Inst. 1994;86(16):1216–1221. doi: 10.1093/jnci/86.16.1216. [DOI] [PubMed] [Google Scholar]
- 27.Burks RT, Kessis TD, Cho KR, Hedrick L. Microsatellite instability in endometrial carcinoma. Oncogene. 1994;9(4):1163–1166. [PubMed] [Google Scholar]
- 28.Kobayashi K, Sagae S, Kudo R, Saito H, Koi S, Nakamura Y. Microsatellite instability in endometrial carcinomas: frequent replication errors in tumors of early onset and/or of poorly differentiated type. Genes Chromosomes Cancer. 1995;14(2):128–132. doi: 10.1002/gcc.2870140207. [DOI] [PubMed] [Google Scholar]
- 29.Esteller M, Catasus L, Matias-Guiu X, et al. hMLH1 promoter hypermethylation is an early event in human endometrial tumorigenesis. Am J Pathol. 1999;155(5):1767–1772. doi: 10.1016/S0002-9440(10)65492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodfellow PJ, Buttin BM, Herzog TJ, et al. Prevalence of defective DNA mismatch repair and MSH6 mutation in an unselected series of endometrial cancers. Proc Natl Acad Sci U S A. 2003;100(10):5908–5913. doi: 10.1073/pnas.1030231100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esteller M, Levine R, Baylin SB, Ellenson LH, Herman JG. MLH1 promoter hypermethylation is associated with the microsatellite instability phenotype in sporadic endometrial carcinomas. Oncogene. 1998;17(18):2413–2417. doi: 10.1038/sj.onc.1202178. [DOI] [PubMed] [Google Scholar]
- 32.Simpkins SB, Bocker T, Swisher EM, et al. MLH1 promoter methylation and gene silencing is the primary cause of microsatellite instability in sporadic endometrial cancers. Hum Mol Genet. 1999;8(4):661–666. doi: 10.1093/hmg/8.4.661. [DOI] [PubMed] [Google Scholar]
- 33.Gurin CC, Federici MG, Kang L, Boyd J. Causes and consequences of microsatellite instability in endometrial carcinoma. Cancer Res. 1999;59(2):462–466. [PubMed] [Google Scholar]
- 34.Salvesen HB, MacDonald N, Ryan A, et al. Methylation of hMLH1 in a population-based series of endometrial carcinomas. Clin Cancer Res. 2000;6(9):3607–3613. [PubMed] [Google Scholar]
- 35.Chiaravalli AM, Furlan D, Facco C, et al. Immunohistochemical pattern of hMSH2/hMLH1 in familial and sporadic colorectal, gastric, endometrial and ovarian carcinomas with instability in microsatellite sequences. Virchows Arch. 2001;438(1):39–48. doi: 10.1007/s004280000325. [DOI] [PubMed] [Google Scholar]
- 36.Hardisson D, Moreno-Bueno G, Sanchez L, et al. Tissue microarray immunohistochemical expression analysis of mismatch repair (hMLH1 and hMSH2 genes) in endometrial carcinoma and atypical endometrial hyperplasia: relationship with microsatellite instability. Mod Pathol. 2003;16(11):1148–1158. doi: 10.1097/01.MP.0000095646.70007.6A. [DOI] [PubMed] [Google Scholar]
- 37.Swisher EM, Mutch DG, Herzog TJ, et al. Analysis of MSH3 in endometrial cancers with defective DNA mismatch repair. J Soc Gynecol Investig. 1998;5(4):210–216. doi: 10.1016/s1071-5576(98)00016-1. [DOI] [PubMed] [Google Scholar]
- 38.Kawaguchi M, Yanokura M, Banno K, et al. Analysis of candidate target genes for mononucleotide repeat mutation in microsatellite instability-high (MSI-H) endometrial cancer. Int J Oncol. 2009;35(5):977–982. doi: 10.3892/ijo_00000411. [DOI] [PubMed] [Google Scholar]
- 39.Kinzler KW, Vogelstein B. Cancer-susceptibility genes. Gatekeepers and caretakers. Nature. 1997;386(6627):761–763. doi: 10.1038/386761a0. [DOI] [PubMed] [Google Scholar]
- 40.Fujii H, Jiang W, Matsumoto T, et al. Birt-Hogg-Dube gene mutations in human endometrial carcinomas with microsatellite instability. J Pathol. 2006;209(3):328–335. doi: 10.1002/path.1992. [DOI] [PubMed] [Google Scholar]
- 41.Furlan D, Casati B, Cerutti R, et al. Genetic progression in sporadic endometrial and gastrointestinal cancers with high microsatellite instability. J Pathol. 2002;197(5):603–609. doi: 10.1002/path.1162. [DOI] [PubMed] [Google Scholar]
- 42.Vassileva V, Millar A, Briollais L, Chapman W, Bapat B. Genes involved in DNA repair are mutational targets in endometrial cancers with microsatellite instability. Cancer Res. 2002;62(14):4095–4099. [PubMed] [Google Scholar]
- 43.Catasus L, Matias-Guiu X, Machin P, Munoz J, Prat J. BAX somatic frameshift mutations in endometrioid adenocarcinomas of the endometrium: evidence for a tumor progression role in endometrial carcinomas with microsatellite instability. Lab Invest. 1998;78(11):1439–1444. [PubMed] [Google Scholar]
- 44.Sakaguchi J, Kyo S, Kanaya T, et al. Aberrant expression and mutations of TGF-beta receptor type II gene in endometrial cancer. Gynecol Oncol. 2005;98(3):427–433. doi: 10.1016/j.ygyno.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 45.Myeroff LL, Parsons R, Kim SJ, et al. A transforming growth factor beta receptor type II gene mutation common in colon and gastric but rare in endometrial cancers with microsatellite instability. Cancer Res. 1995;55(23):5545–5547. [PubMed] [Google Scholar]
- 46.Bilbao C, Ramirez R, Rodriguez G, et al. Double strand break repair components are frequent targets of microsatellite instability in endometrial cancer. Eur J Cancer. 2010;46(15):2821–2827. doi: 10.1016/j.ejca.2010.06.116. [DOI] [PubMed] [Google Scholar]
- 47.Giannini G, Rinaldi C, Ristori E, et al. Mutations of an intronic repeat induce impaired MRE11 expression in primary human cancer with microsatellite instability. Oncogene. 2004;23(15):2640–2647. doi: 10.1038/sj.onc.1207409. [DOI] [PubMed] [Google Scholar]
- 48.Zighelboim I, Schmidt AP, Gao F, et al. ATR mutation in endometrioid endometrial cancer is associated with poor clinical outcomes. J Clin Oncol. 2009;27(19):3091–3096. doi: 10.1200/JCO.2008.19.9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewis KA, Mullany S, Thomas B, et al. Heterozygous ATR mutations in mismatch repair-deficient cancer cells have functional significance. Cancer Res. 2005;65(16):7091–7095. doi: 10.1158/0008-5472.CAN-05-1019. [DOI] [PubMed] [Google Scholar]
- 50.Risinger JI, Maxwell GL, Chandramouli GV, et al. Gene expression profiling of microsatellite unstable and microsatellite stable endometrial cancers indicates distinct pathways of aberrant signaling. Cancer Res. 2005;65(12):5031–5037. doi: 10.1158/0008-5472.CAN-04-0850. [DOI] [PubMed] [Google Scholar]
- 51.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9(8):550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 52.Cheung LW, Hennessy BT, Li J, et al. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer Discov. 2011;1(2):170–185. doi: 10.1158/2159-8290.CD-11-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shoji K, Oda K, Nakagawa S, et al. The oncogenic mutation in the pleckstrin homology domain of AKT1 in endometrial carcinomas. Br J Cancer. 2009;101(1):145–148. doi: 10.1038/sj.bjc.6605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oda K, Stokoe D, Taketani Y, McCormick F. High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer Res. 2005;65(23):10669–10673. doi: 10.1158/0008-5472.CAN-05-2620. [DOI] [PubMed] [Google Scholar]
- 55.Risinger JI, Hayes AK, Berchuck A, Barrett JC. PTEN/MMAC1 mutations in endometrial cancers. Cancer Res. 1997;57(21):4736–4738. [PubMed] [Google Scholar]
- 56.Tashiro H, Blazes MS, Wu R, et al. Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res. 1997;57(18):3935–3940. [PubMed] [Google Scholar]
- 57.Rudd ML, Price JC, Fogoros S, et al. A unique spectrum of somatic PIK3CA (p110alpha) mutations within primary endometrial carcinomas. Clin Cancer Res. 2011;17(6):1331–1340. doi: 10.1158/1078-0432.CCR-10-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Urick ME, Rudd ML, Godwin AK, Sgroi D, Merino M, Bell DW. PIK3R1 (p85{alpha}) is somatically mutated at high frequency in primary endometrial cancer. Cancer Res. 2011;71(12):4061–4067. doi: 10.1158/0008-5472.CAN-11-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salvesen HB, Stefansson I, Kalvenes MB, Das S, Akslen LA. Loss of PTEN expression is associated with metastatic disease in patients with endometrial carcinoma. Cancer. 2002;94(8):2185–2191. doi: 10.1002/cncr.10434. [DOI] [PubMed] [Google Scholar]
- 60.Dutt A, Salvesen HB, Greulich H, Sellers WR, Beroukhim R, Meyerson M. Somatic mutations are present in all members of the AKT family in endometrial carcinoma. Br J Cancer. 2009;101(7):1218–1219. doi: 10.1038/sj.bjc.6605301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peiffer SL, Herzog TJ, Tribune DJ, Mutch DG, Gersell DJ, Goodfellow PJ. Allelic loss of sequences from the long arm of chromosome 10 and replication errors in endometrial cancers. Cancer Res. 1995;55(9):1922–1926. [PubMed] [Google Scholar]
- 62.Konopka B, Janiec-Jankowska A, Kwiatkowska E, et al. PIK3CA mutations and amplification in endometrioid endometrial carcinomas: relation to other genetic defects and clinicopathologic status of the tumors. Hum Pathol. 2011;42(11):1710–1719. doi: 10.1016/j.humpath.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 63.Kang S, Seo SS, Chang HJ, Yoo CW, Park SY, Dong SM. Mutual exclusiveness between PIK3CA and KRAS mutations in endometrial carcinoma. Int J Gynecol Cancer. 2008;18(6):1339–1343. doi: 10.1111/j.1525-1438.2007.01172.x. [DOI] [PubMed] [Google Scholar]
- 64.Miyake T, Yoshino K, Enomoto T, et al. PIK3CA gene mutations and amplifications in uterine cancers, identified by methods that avoid confounding by PIK3CA pseudogene sequences. Cancer Lett. 2008;261(1):120–126. doi: 10.1016/j.canlet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 65.Lu KH, Wu W, Dave B, et al. Loss of tuberous sclerosis complex-2 function and activation of mammalian target of rapamycin signaling in endometrial carcinoma. Clin Cancer Res. 2008;14(9):2543–2550. doi: 10.1158/1078-0432.CCR-07-0321. [DOI] [PubMed] [Google Scholar]
- 66.Shen WH, Balajee AS, Wang J, et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128(1):157–170. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 67.Dedes KJ, Wetterskog D, Mendes-Pereira AM, et al. PTEN deficiency in endometrioid endometrial adenocarcinomas predicts sensitivity to PARP inhibitors. Sci Transl Med. 2010;2(53):53ra75. doi: 10.1126/scitranslmed.3001538. [DOI] [PubMed] [Google Scholar]
- 68.Maxwell GL, Risinger JI, Gumbs C, et al. Mutation of the PTEN tumor suppressor gene in endometrial hyperplasias. Cancer Res. 1998;58(12):2500–2503. [PubMed] [Google Scholar]
- 69.Levine RL, Cargile CB, Blazes MS, van Rees B, Kurman RJ, Ellenson LH. PTEN mutations and microsatellite instability in complex atypical hyperplasia, a precursor lesion to uterine endometrioid carcinoma. Cancer Res. 1998;58(15):3254–3258. [PubMed] [Google Scholar]
- 70.Mutter GL, Lin MC, Fitzgerald JT, et al. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst. 2000;92(11):924–930. doi: 10.1093/jnci/92.11.924. [DOI] [PubMed] [Google Scholar]
- 71.Zhou XP, Kuismanen S, Nystrom-Lahti M, Peltomaki P, Eng C. Distinct PTEN mutational spectra in hereditary non-polyposis colon cancer syndrome-related endometrial carcinomas compared to sporadic microsatellite unstable tumors. Hum Mol Genet. 2002;11(4):445–450. doi: 10.1093/hmg/11.4.445. [DOI] [PubMed] [Google Scholar]
- 72.Hayes MP, Wang H, Espinal-Witter R, et al. PIK3CA and PTEN mutations in uterine endometrioid carcinoma and complex atypical hyperplasia. Clin Cancer Res. 2006;12(20 Pt 1):5932–5935. doi: 10.1158/1078-0432.CCR-06-1375. [DOI] [PubMed] [Google Scholar]
- 73.Castellano E, Downward J. RAS Interaction with PI3K: More than just another effector pathway. Genes Cancer. 2011;2(3):261–274. doi: 10.1177/1947601911408079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lester DR, Cauchi MN. Point mutations at codon 12 of C-K-ras in human endometrial carcinomas. Cancer Lett. 1990;51(1):7–10. doi: 10.1016/0304-3835(90)90223-k. [DOI] [PubMed] [Google Scholar]
- 75.Enomoto T, Inoue M, Perantoni AO, Terakawa N, Tanizawa O, Rice JM. K-ras activation in neoplasms of the human female reproductive tract. Cancer Res. 1990;50(19):6139–6145. [PubMed] [Google Scholar]
- 76.Boyd J, Risinger JI. Analysis of oncogene alterations in human endometrial carcinoma: prevalence of ras mutations. Mol Carcinog. 1991;4(3):189–195. doi: 10.1002/mc.2940040305. [DOI] [PubMed] [Google Scholar]
- 77.Sato S, Ito K, Ozawa N, Yajima A, Sasano H. Analysis of point mutations at codon 12 of K-ras in human endometrial carcinoma and cervical adenocarcinoma by dot blot hybridization and polymerase chain reaction. Tohoku J Exp Med. 1991;165(2):131–136. doi: 10.1620/tjem.165.131. [DOI] [PubMed] [Google Scholar]
- 78.Caduff RF, Johnston CM, Frank TS. Mutations of the Ki-ras oncogene in carcinoma of the endometrium. Am J Pathol. 1995;146(1):182–188. [PMC free article] [PubMed] [Google Scholar]
- 79.Jones MW, Kounelis S, Hsu C, et al. Prognostic value of p53 and K-ras-2 topographic genotyping in endometrial carcinoma: a clinicopathologic and molecular comparison. Int J Gynecol Pathol. 1997;16(4):354–360. doi: 10.1097/00004347-199710000-00010. [DOI] [PubMed] [Google Scholar]
- 80.Lax SF, Kendall B, Tashiro H, Slebos RJ, Hedrick L. The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer. 2000;88(4):814–824. [PubMed] [Google Scholar]
- 81.Forbes SA, Bhamra G, Bamford S, et al. The Catalogue of Somatic Mutations in Cancer (COSMIC) Curr Protoc Hum Genet. 2008;Chapter 10(Unit 10.11) doi: 10.1002/0471142905.hg1011s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cohn DE, Mutch DG, Herzog TJ, et al. Genotypic and phenotypic progression in endometrial tumorigenesis: determining when defects in DNA mismatch repair and KRAS2 occur. Genes Chromosomes Cancer. 2001;32(4):295–301. doi: 10.1002/gcc.1194. [DOI] [PubMed] [Google Scholar]
- 83.Mutter GL, Wada H, Faquin WC, Enomoto T. K-ras mutations appear in the premalignant phase of both microsatellite stable and unstable endometrial carcinogenesis. Mol Pathol. 1999;52(5):257–262. doi: 10.1136/mp.52.5.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Enomoto T, Inoue M, Perantoni AO, et al. K-ras activation in premalignant and malignant epithelial lesions of the human uterus. Cancer Res. 1991;51(19):5308–5314. [PubMed] [Google Scholar]
- 85.Oda K, Okada J, Timmerman L, et al. PIK3CA cooperates with other phosphatidylinositol 3′-kinase pathway mutations to effect oncogenic transformation. Cancer Res. 2008;68(19):8127–8136. doi: 10.1158/0008-5472.CAN-08-0755. [DOI] [PubMed] [Google Scholar]
- 86.Kim TH, Wang J, Lee KY, et al. The synergistic effect of conditional Pten loss and oncogenic K-ras mutation on endometrial cancer development occurs via decreased progesterone receptor action. J Oncol. 2010;2010:139087. doi: 10.1155/2010/139087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mutch DG, Powell MA, Mallon MA, Goodfellow PJ. RAS/RAF mutation and defective DNA mismatch repair in endometrial cancers. Am J Obstet Gynecol. 2004;190(4):935–942. doi: 10.1016/j.ajog.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 88.Pappa KI, Choleza M, Markaki S, et al. Consistent absence of BRAF mutations in cervical and endometrial cancer despite KRAS mutation status. Gynecol Oncol. 2006;100(3):596–600. doi: 10.1016/j.ygyno.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 89.Kang S, Lee JM, Jeon ES, et al. RASSF1A hypermethylation and its inverse correlation with BRAF and/or KRAS mutations in MSI-associated endometrial carcinoma. Int J Cancer. 2006;119(6):1316–1321. doi: 10.1002/ijc.21991. [DOI] [PubMed] [Google Scholar]
- 90.Mizumoto Y, Kyo S, Mori N, et al. Activation of ERK1/2 occurs independently of KRAS or BRAF status in endometrial cancer and is associated with favorable prognosis. Cancer Sci. 2007;98(5):652–658. doi: 10.1111/j.1349-7006.2007.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moreno-Bueno G, Sanchez-Estevez C, Palacios J, Hardisson D, Shiozawa T. Low frequency of BRAF mutations in endometrial and in cervical carcinomas. Clin Cancer Res. 2006;12(12):3865. doi: 10.1158/1078-0432.CCR-06-0284. [DOI] [PubMed] [Google Scholar]
- 92.Feng YZ, Shiozawa T, Miyamoto T, et al. BRAF mutation in endometrial carcinoma and hyperplasia: correlation with KRAS and p53 mutations and mismatch repair protein expression. Clin Cancer Res. 2005;11(17):6133–6138. doi: 10.1158/1078-0432.CCR-04-2670. [DOI] [PubMed] [Google Scholar]
- 93.Richter AM, Pfeifer GP, Dammann RH. The RASSF proteins in cancer; from epigenetic silencing to functional characterization. Biochim Biophys Acta. 2009;1796(2):114–128. doi: 10.1016/j.bbcan.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 94.Liao X, Siu MK, Chan KY, et al. Hypermethylation of RAS effector related genes and DNA methyltransferase 1 expression in endometrial carcinogenesis. Int J Cancer. 2008;123(2):296–302. doi: 10.1002/ijc.23494. [DOI] [PubMed] [Google Scholar]
- 95.Arafa M, Kridelka F, Mathias V, et al. High frequency of RASSF1A and RARb2 gene promoter methylation in morphologically normal endometrium adjacent to endometrioid adenocarcinoma. Histopathology. 2008;53(5):525–532. doi: 10.1111/j.1365-2559.2008.03147.x. [DOI] [PubMed] [Google Scholar]
- 96.Pallares J, Velasco A, Eritja N, et al. Promoter hypermethylation and reduced expression of RASSF1A are frequent molecular alterations of endometrial carcinoma. Mod Pathol. 2008;21(6):691–699. doi: 10.1038/modpathol.2008.38. [DOI] [PubMed] [Google Scholar]
- 97.Nieminen TT, Gylling A, Abdel-Rahman WM, et al. Molecular analysis of endometrial tumorigenesis: importance of complex hyperplasia regardless of atypia. Clin Cancer Res. 2009;15(18):5772–5783. doi: 10.1158/1078-0432.CCR-09-0506. [DOI] [PubMed] [Google Scholar]
- 98.Dewdney SB, Rimel BJ, Thaker PH, et al. Aberrant methylation of the X-linked ribosomal S6 kinase RPS6KA6 (RSK4) in endometrial cancers. Clin Cancer Res. 2011;17(8):2120–2129. doi: 10.1158/1078-0432.CCR-10-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Velasco A, Pallares J, Santacana M, et al. Promoter hypermethylation and expression of sprouty 2 in endometrial carcinoma. Hum Pathol. 2011;42(2):185–193. doi: 10.1016/j.humpath.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 100.Dutt A, Salvesen HB, Chen TH, et al. Drug-sensitive FGFR2 mutations in endometrial carcinoma. Proc Natl Acad Sci U S A. 2008;105(25):8713–8717. doi: 10.1073/pnas.0803379105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pollock PM, Gartside MG, Dejeza LC, et al. Frequent activating FGFR2 mutations in endometrial carcinomas parallel germline mutations associated with craniosynostosis and skeletal dysplasia syndromes. Oncogene. 2007;26(50):7158–7162. doi: 10.1038/sj.onc.1210529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Byron SA, Gartside MG, Wellens CL, et al. Inhibition of activated fibroblast growth factor receptor 2 in endometrial cancer cells induces cell death despite PTEN abrogation. Cancer Res. 2008;68(17):6902–6907. doi: 10.1158/0008-5472.CAN-08-0770. [DOI] [PubMed] [Google Scholar]
- 103.Machin P, Catasus L, Pons C, Munoz J, Matias-Guiu X, Prat J. CTNNB1 mutations and beta-catenin expression in endometrial carcinomas. Hum Pathol. 2002;33(2):206–212. doi: 10.1053/hupa.2002.30723. [DOI] [PubMed] [Google Scholar]
- 104.Schlosshauer PW, Ellenson LH, Soslow RA. Beta-catenin and E-cadherin expression patterns in high-grade endometrial carcinoma are associated with histological subtype. Mod Pathol. 2002;15(10):1032–1037. doi: 10.1097/01.MP.0000028573.34289.04. [DOI] [PubMed] [Google Scholar]
- 105.Moreno-Bueno G, Hardisson D, Sanchez C, et al. Abnormalities of the APC/beta-catenin pathway in endometrial cancer. Oncogene. 2002;21(52):7981–7990. doi: 10.1038/sj.onc.1205924. [DOI] [PubMed] [Google Scholar]
- 106.Ashihara K, Saito T, Mizumoto H, Nishimura M, Tanaka R, Kudo R. Mutation of beta-catenin gene in endometrial cancer but not in associated hyperplasia. Med Electron Microsc. 2002;35(1):9–15. doi: 10.1007/s007950200001. [DOI] [PubMed] [Google Scholar]
- 107.Saegusa M, Hashimura M, Yoshida T, Okayasu I. Beta-catenin mutations and aberrant nuclear expression during endometrial tumorigenesis. Br J Cancer. 2001;84(2):209–217. doi: 10.1054/bjoc.2000.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brachtel EF, Sanchez-Estevez C, Moreno-Bueno G, Prat J, Palacios J, Oliva E. Distinct molecular alterations in complex endometrial hyperplasia (CEH) with and without immature squamous metaplasia (squamous morules) Am J Surg Pathol. 2005;29(10):1322–1329. doi: 10.1097/01.pas.0000171001.87599.e2. [DOI] [PubMed] [Google Scholar]
- 109.Norimatsu Y, Moriya T, Kobayashi TK, et al. Immunohistochemical expression of PTEN and beta-catenin for endometrial intra-epithelial neoplasia in Japanese women. Ann Diagn Pathol. 2007;11(2):103–108. doi: 10.1016/j.anndiagpath.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 110.Mirabelli-Primdahl L, Gryfe R, Kim H, et al. Beta-catenin mutations are specific for colorectal carcinomas with microsatellite instability but occur in endometrial carcinomas irrespective of mutator pathway. Cancer Res. 1999;59(14):3346–3351. [PubMed] [Google Scholar]
- 111.Guan B, Wang TL, Shih IM. ARID1A, a factor that promotes formation of SWI/SNF-mediated chromatin remodeling, is a tumor suppressor in gynecologic cancers. Cancer Res. 2011;71(21):6718–6727. doi: 10.1158/0008-5472.CAN-11-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wiegand KC, Lee AF, Al-Agha OM, et al. Loss of BAF250a (ARID1A) is frequent in high-grade endometrial carcinomas. J Pathol. 2011;224(3):328–333. doi: 10.1002/path.2911. [DOI] [PubMed] [Google Scholar]
- 113.Guan B, Mao TL, Panuganti PK, et al. Mutation and loss of expression of ARID1A in uterine low-grade endometrioid carcinoma. Am J Surg Pathol. 2011;35(5):625–632. doi: 10.1097/PAS.0b013e318212782a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Micci F, Teixeira MR, Haugom L, Kristensen G, Abeler VM, Heim S. Genomic aberrations in carcinomas of the uterine corpus. Genes Chromosomes Cancer. 2004;40(3):229–246. doi: 10.1002/gcc.20038. [DOI] [PubMed] [Google Scholar]
- 115.Kato DT, Ferry JA, Goodman A, et al. Uterine papillary serous carcinoma (UPSC): a clinicopathologic study of 30 cases. Gynecol Oncol. 1995;59(3):384–389. doi: 10.1006/gyno.1995.9957. [DOI] [PubMed] [Google Scholar]
- 116.Konski AA, Domenico D, Irving D, et al. Clinicopathologic correlation of DNA flow cytometric content analysis (DFCA), surgical staging, and estrogen/progesterone receptor status in endometrial adenocarcinoma. Am J Clin Oncol. 1996;19(2):164–168. doi: 10.1097/00000421-199604000-00015. [DOI] [PubMed] [Google Scholar]
- 117.Newbury R, Schuerch C, Goodspeed N, Fanning J, Glidewell O, Evans M. DNA content as a prognostic factor in endometrial carcinoma. Obstet Gynecol. 1990;76(2):251–257. [PubMed] [Google Scholar]
- 118.Pradhan M, Abeler VM, Danielsen HE, Trope CG, Risberg BA. Image cytometry DNA ploidy correlates with histological subtypes in endometrial carcinomas. Mod Pathol. 2006;19(9):1227–1235. doi: 10.1038/modpathol.3800641. [DOI] [PubMed] [Google Scholar]
- 119.Prat J, Oliva E, Lerma E, Vaquero M, Matias-Guiu X. Uterine papillary serous adenocarcinoma. A 10-case study of p53 and c-erbB-2 expression and DNA content. Cancer. 1994;74(6):1778–1783. doi: 10.1002/1097-0142(19940915)74:6<1778::aid-cncr2820740621>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 120.Rosenberg P, Wingren S, Simonsen E, Stal O, Risberg B, Nordenskjold B. Flow cytometric measurements of DNA index and S-phase on paraffin-embedded early stage endometrial cancer: an important prognostic indicator. Gynecol Oncol. 1989;35(1):50–54. doi: 10.1016/0090-8258(89)90010-3. [DOI] [PubMed] [Google Scholar]
- 121.Moll UM, Chalas E, Auguste M, Meaney D, Chumas J. Uterine papillary serous carcinoma evolves via a p53-driven pathway. Hum Pathol. 1996;27(12):1295–1300. doi: 10.1016/s0046-8177(96)90340-8. [DOI] [PubMed] [Google Scholar]
- 122.Tashiro H, Isacson C, Levine R, Kurman RJ, Cho KR, Hedrick L. p53 gene mutations are common in uterine serous carcinoma and occur early in their pathogenesis. Am J Pathol. 1997;150(1):177–185. [PMC free article] [PubMed] [Google Scholar]
- 123.Ambros RA, Sheehan CE, Kallakury BV, et al. MDM2 and p53 protein expression in the histologic subtypes of endometrial carcinoma. Mod Pathol. 1996;9(12):1165–1169. [PubMed] [Google Scholar]
- 124.Sherman ME, Bur ME, Kurman RJ. p53 in endometrial cancer and its putative precursors: evidence for diverse pathways of tumorigenesis. Hum Pathol. 1995;26(11):1268–1274. doi: 10.1016/0046-8177(95)90204-x. [DOI] [PubMed] [Google Scholar]
- 125.Kovalev S, Marchenko ND, Gugliotta BG, Chalas E, Chumas J, Moll UM. Loss of p53 function in uterine papillary serous carcinoma. Hum Pathol. 1998;29(6):613–619. doi: 10.1016/s0046-8177(98)80012-9. [DOI] [PubMed] [Google Scholar]
- 126.Kohler MF, Berchuck A, Davidoff AM, et al. Overexpression and mutation of p53 in endometrial carcinoma. Cancer Res. 1992;52(6):1622–1627. [PubMed] [Google Scholar]
- 127.Geisler JP, Geisler HE, Wiemann MC, Zhou Z, Miller GA, Crabtree W. p53 expression as a prognostic indicator of 5-year survival in endometrial cancer. Gynecol Oncol. 1999;74(3):468–471. doi: 10.1006/gyno.1999.5482. [DOI] [PubMed] [Google Scholar]
- 128.Sakuragi N, Watari H, Ebina Y, et al. Functional analysis of p53 gene and the prognostic impact of dominant-negative p53 mutation in endometrial cancer. Int J Cancer. 2005;116(4):514–519. doi: 10.1002/ijc.21097. [DOI] [PubMed] [Google Scholar]
- 129.Saffari B, Bernstein L, Hong DC, et al. Association of p53 mutations and a codon 72 single nucleotide polymorphism with lower overall survival and responsiveness to adjuvant radiotherapy in endometrioid endometrial carcinomas. Int J Gynecol Cancer. 2005;15(5):952–963. doi: 10.1111/j.1525-1438.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 130.Garg K, Leitao MM, Jr, Wynveen CA, et al. p53 overexpression in morphologically ambiguous endometrial carcinomas correlates with adverse clinical outcomes. Mod Pathol. 2010;23(1):80–92. doi: 10.1038/modpathol.2009.153. [DOI] [PubMed] [Google Scholar]
- 131.An HJ, Logani S, Isacson C, Ellenson LH. Molecular characterization of uterine clear cell carcinoma. Mod Pathol. 2004;17(5):530–537. doi: 10.1038/modpathol.3800057. [DOI] [PubMed] [Google Scholar]
- 132.Eichhorn PJ, Creyghton MP, Bernards R. Protein phosphatase 2A regulatory subunits and cancer. Biochim Biophys Acta. 2009;1795(1):1–15. doi: 10.1016/j.bbcan.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 133.McConechy MK, Anglesio MS, Kalloger SE, et al. Subtype-specific mutation of PPP2R1A in endometrial and ovarian carcinomas. J Pathol. 2011;223(5):567–573. doi: 10.1002/path.2848. [DOI] [PubMed] [Google Scholar]
- 134.Nagendra DC, Burke J, 3rd, Maxwell GL, Risinger JI. PPP2R1A mutations are common in the serous type of endometrial cancer. Mol Carcinog. 2011 doi: 10.1002/mc.20850. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 135.Shih IeM, Panuganti PK, Kuo KT, et al. Somatic mutations of PPP2R1A in ovarian and uterine carcinomas. Am J Pathol. 2011;178(4):1442–1447. doi: 10.1016/j.ajpath.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Morrison C, Zanagnolo V, Ramirez N, et al. HER-2 is an independent prognostic factor in endometrial cancer: association with outcome in a large cohort of surgically staged patients. J Clin Oncol. 2006;24(15):2376–2385. doi: 10.1200/JCO.2005.03.4827. [DOI] [PubMed] [Google Scholar]
- 137.Engelsen IB, Stefansson IM, Beroukhim R, et al. HER-2/neu expression is associated with high tumor cell proliferation and aggressive phenotype in a population based patient series of endometrial carcinomas. Int J Oncol. 2008;32(2):307–316. [PubMed] [Google Scholar]
- 138.Konecny GE, Santos L, Winterhoff B, et al. HER2 gene amplification and EGFR expression in a large cohort of surgically staged patients with nonendometrioid (type II) endometrial cancer. Br J Cancer. 2009;100(1):89–95. doi: 10.1038/sj.bjc.6604814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Santin AD, Bellone S, Gokden M, et al. Overexpression of HER-2/neu in uterine serous papillary cancer. Clin Cancer Res. 2002;8(5):1271–1279. [PubMed] [Google Scholar]
- 140.Santin AD, Bellone S, Siegel ER, et al. Racial differences in the overexpression of epidermal growth factor type II receptor (HER2/neu): a major prognostic indicator in uterine serous papillary cancer. Am J Obstet Gynecol. 2005;192(3):813–818. doi: 10.1016/j.ajog.2004.10.605. [DOI] [PubMed] [Google Scholar]
- 141.Slomovitz BM, Broaddus RR, Burke TW, et al. Her-2/neu overexpression and amplification in uterine papillary serous carcinoma. J Clin Oncol. 2004;22(15):3126–3132. doi: 10.1200/JCO.2004.11.154. [DOI] [PubMed] [Google Scholar]
- 142.Diaz-Montes TP, Ji H, Smith Sehdev AE, et al. Clinical significance of Her-2/neu overexpression in uterine serous carcinoma. Gynecol Oncol. 2006;100(1):139–144. doi: 10.1016/j.ygyno.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 143.Grushko TA, Filiaci VL, Mundt AJ, Ridderstrale K, Olopade OI, Fleming GF. An exploratory analysis of HER-2 amplification and overexpression in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2008;108(1):3–9. doi: 10.1016/j.ygyno.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Odicino FE, Bignotti E, Rossi E, et al. HER-2/neu overexpression and amplification in uterine serous papillary carcinoma: comparative analysis of immunohistochemistry, real-time reverse transcription-polymerase chain reaction, and fluorescence in situ hybridization. Int J Gynecol Cancer. 2008;18(1):14–21. doi: 10.1111/j.1525-1438.2007.00946.x. [DOI] [PubMed] [Google Scholar]
- 145.Ren Y, Wang H, Zhou X, et al. Clinicopathological characteristics and her-2/neu status in chinese patients with uterine papillary serous carcinoma. ISRN Obstet Gynecol. 2011;2011:575327. doi: 10.5402/2011/575327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Halperin R, Zehavi S, Habler L, Hadas E, Bukovsky I, Schneider D. Comparative immunohistochemical study of endometrioid and serous papillary carcinoma of endometrium. Eur J Gynaecol Oncol. 2001;22(2):122–126. [PubMed] [Google Scholar]
- 147.Singh P, Smith CL, Cheetham G, Dodd TJ, Davy ML. Serous carcinoma of the uterus-determination of HER-2/neu status using immunohistochemistry, chromogenic in situ hybridization, and quantitative polymerase chain reaction techniques: its significance and clinical correlation. Int J Gynecol Cancer. 2008;18(6):1344–1351. doi: 10.1111/j.1525-1438.2007.01181.x. [DOI] [PubMed] [Google Scholar]
- 148.Santin AD, Bellone S, Van Stedum S, et al. Determination of HER2/ neu status in uterine serous papillary carcinoma: Comparative analysis of immunohistochemistry and fluorescence in situ hybridization. Gynecol Oncol. 2005;98(1):24–30. doi: 10.1016/j.ygyno.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 149.Santin AD, Bellone S, Van Stedum S, et al. Amplification of c-erbB2 oncogene: a major prognostic indicator in uterine serous papillary carcinoma. Cancer. 2005;104(7):1391–1397. doi: 10.1002/cncr.21308. [DOI] [PubMed] [Google Scholar]
- 150.Hayes MP, Douglas W, Ellenson LH. Molecular alterations of EGFR and PIK3CA in uterine serous carcinoma. Gynecol Oncol. 2009;113(3):370–373. doi: 10.1016/j.ygyno.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Catasus L, Gallardo A, Cuatrecasas M, Prat J. Concomitant PI3K-AKT and p53 alterations in endometrial carcinomas are associated with poor prognosis. Mod Pathol. 2009;22(4):522–529. doi: 10.1038/modpathol.2009.5. [DOI] [PubMed] [Google Scholar]
- 152.Milde-Langosch K, Bamberger AM, Goemann C, et al. Expression of cell-cycle regulatory proteins in endometrial carcinomas: correlations with hormone receptor status and clinicopathologic parameters. J Cancer Res Clin Oncol. 2001;127(9):537–544. doi: 10.1007/s004320100256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kato N, Watanabe J, Jobo T, et al. Immunohistochemical expression of cyclin E in endometrial adenocarcinoma (endometrioid type) and its clinicopathological significance. J Cancer Res Clin Oncol. 2003;129(4):222–226. doi: 10.1007/s00432-003-0426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cassia R, Moreno-Bueno G, Rodriguez-Perales S, Hardisson D, Cigudosa JC, Palacios J. Cyclin E gene (CCNE) amplification and hCDC4 mutations in endometrial carcinoma. J Pathol. 2003;201(4):589–595. doi: 10.1002/path.1474. [DOI] [PubMed] [Google Scholar]
- 155.Spruck CH, Strohmaier H, Sangfelt O, et al. hCDC4 gene mutations in endometrial cancer. Cancer Res. 2002;62(16):4535–4539. [PubMed] [Google Scholar]
- 156.Moberg KH, Bell DW, Wahrer DC, Haber DA, Hariharan IK. Archipelago regulates Cyclin E levels in Drosophila and is mutated in human cancer cell lines. Nature. 2001;413(6853):311–316. doi: 10.1038/35095068. [DOI] [PubMed] [Google Scholar]
- 157.Strohmaier H, Spruck CH, Kaiser P, Won KA, Sangfelt O, Reed SI. Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature. 2001;413(6853):316–322. doi: 10.1038/35095076. [DOI] [PubMed] [Google Scholar]
- 158.Suehiro Y, Okada T, Anno K, et al. Aneuploidy predicts outcome in patients with endometrial carcinoma and is related to lack of CDH13 hypermethylation. Clin Cancer Res. 2008;14(11):3354–3361. doi: 10.1158/1078-0432.CCR-07-4609. [DOI] [PubMed] [Google Scholar]
- 159.Reid-Nicholson M, Iyengar P, Hummer AJ, Linkov I, Asher M, Soslow RA. Immunophenotypic diversity of endometrial adenocarcinomas: implications for differential diagnosis. Mod Pathol. 2006;19(8):1091–1100. doi: 10.1038/modpathol.3800620. [DOI] [PubMed] [Google Scholar]
- 160.Yemelyanova A, Ji H, Shih IeM, Wang TL, Wu LS, Ronnett BM. Utility of p16 expression for distinction of uterine serous carcinomas from endometrial endometrioid and endocervical adenocarcinomas: immunohistochemical analysis of 201 cases. Am J Surg Pathol. 2009;33(10):1504–1514. doi: 10.1097/PAS.0b013e3181ac35f5. [DOI] [PubMed] [Google Scholar]
- 161.Alkushi A, Kobel M, Kalloger SE, Gilks CB. High-grade endometrial carcinoma: serous and grade 3 endometrioid carcinomas have different immunophenotypes and outcomes. Int J Gynecol Cancer. 2010;29(4):343–350. doi: 10.1097/PGP.0b013e3181cd6552. [DOI] [PubMed] [Google Scholar]
- 162.Netzer IM, Kerner H, Litwin L, Lowenstein L, Amit A. Diagnostic implications of p16 expression in serous papillary endometrial cancer. Int J Gynecol Cancer. 2011;21(8):1441–1445. doi: 10.1097/IGC.0b013e31822eee04. [DOI] [PubMed] [Google Scholar]
- 163.Nakashima R, Fujita M, Enomoto T, et al. Alteration of p16 and p15 genes in human uterine tumours. Br J Cancer. 1999;80(3–4):458–467. doi: 10.1038/sj.bjc.6690379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Salvesen HB, Kumar R, Stefansson I, et al. Low frequency of BRAF and CDKN2A mutations in endometrial cancer. Int J Cancer. 2005;115(6):930–934. doi: 10.1002/ijc.20702. [DOI] [PubMed] [Google Scholar]
- 165.Semczuk A, Boltze C, Marzec B, Szczygielska A, Roessner A, Schneider-Stock R. p16INK4A alterations are accompanied by aberrant protein immunostaining in endometrial carcinomas. J Cancer Res Clin Oncol. 2003;129(10):589–596. doi: 10.1007/s00432-003-0482-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Santin AD, Zhan F, Cane S, et al. Gene expression fingerprint of uterine serous papillary carcinoma: identification of novel molecular markers for uterine serous cancer diagnosis and therapy. Br J Cancer. 2005;92(8):1561–1573. doi: 10.1038/sj.bjc.6602480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Konecny GE, Agarwal R, Keeney GA, et al. Claudin-3 and claudin-4 expression in serous papillary, clear-cell, and endometrioid endometrial cancer. Gynecol Oncol. 2008;109(2):263–269. doi: 10.1016/j.ygyno.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sobel G, Nemeth J, Kiss A, et al. Claudin 1 differentiates endometrioid and serous papillary endometrial adenocarcinoma. Gynecol Oncol. 2006;103(2):591–598. doi: 10.1016/j.ygyno.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 169.Katahira J, Sugiyama H, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Clostridium perfringens enterotoxin utilizes two structurally related membrane proteins as functional receptors in vivo. J Biol Chem. 1997;272(42):26652–26658. doi: 10.1074/jbc.272.42.26652. [DOI] [PubMed] [Google Scholar]
- 170.Fogel M, Gutwein P, Mechtersheimer S, et al. L1 expression as a predictor of progression and survival in patients with uterine and ovarian carcinomas. Lancet. 2003;362(9387):869–875. doi: 10.1016/S0140-6736(03)14342-5. [DOI] [PubMed] [Google Scholar]
- 171.El-Sahwi K, Bellone S, Cocco E, et al. Overexpression of EpCAM in uterine serous papillary carcinoma: implications for EpCAM-specific immunotherapy with human monoclonal antibody adecatumumab (MT201) Mol Cancer Ther. 2010;9(1):57–66. doi: 10.1158/1535-7163.MCT-09-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118(3):277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 173.Stefansson IM, Salvesen HB, Akslen LA. Prognostic impact of alterations in P-cadherin expression and related cell adhesion markers in endometrial cancer. J Clin Oncol. 2004;22(7):1242–1252. doi: 10.1200/JCO.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 174.Holcomb K, Delatorre R, Pedemonte B, McLeod C, Anderson L, Chambers J. E-cadherin expression in endometrioid, papillary serous, and clear cell carcinoma of the endometrium. Obstet Gynecol. 2002;100(6):1290–1295. doi: 10.1016/s0029-7844(02)02391-8. [DOI] [PubMed] [Google Scholar]
- 175.Scholten AN, Aliredjo R, Creutzberg CL, Smit VT. Combined E-cadherin, alpha-catenin, and beta-catenin expression is a favorable prognostic factor in endometrial carcinoma. Int J Gynecol Cancer. 2006;16(3):1379–1385. doi: 10.1111/j.1525-1438.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 176.Moreno-Bueno G, Hardisson D, Sarrio D, et al. Abnormalities of E- and P-cadherin and catenin (beta-, gamma-catenin, and p120ctn) expression in endometrial cancer and endometrial atypical hyperplasia. J Pathol. 2003;199(4):471–478. doi: 10.1002/path.1310. [DOI] [PubMed] [Google Scholar]
- 177.Mell LK, Meyer JJ, Tretiakova M, et al. Prognostic significance of E-cadherin protein expression in pathological stage I–III endometrial cancer. Clin Cancer Res. 2004;10(16):5546–5553. doi: 10.1158/1078-0432.CCR-0943-03. [DOI] [PubMed] [Google Scholar]
- 178.Singh M, Darcy KM, Brady WE, et al. Cadherins, catenins and cell cycle regulators: impact on survival in a gynecologic oncology group phase II endometrial cancer trial. Gynecol Oncol. 2011;123(2):320–328. doi: 10.1016/j.ygyno.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Risinger JI, Berchuck A, Kohler MF, Boyd J. Mutations of the E-cadherin gene in human gynecologic cancers. Nat Genet. 1994;7(1):98–102. doi: 10.1038/ng0594-98. [DOI] [PubMed] [Google Scholar]
- 180.Park JH, Lee BI, Song ES, Whang SO, Lee WY, Cho SJ. Hypermethylation of E-cadherin in endometrial carcinoma. J Gynecol Oncol. 2008;19(4):241–245. doi: 10.3802/jgo.2008.19.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yi TZ, Guo J, Zhou L, et al. Prognostic value of E-cadherin expression and CDH1 promoter methylation in patients with endometrial carcinoma. Cancer Invest. 2011;29(1):86–92. doi: 10.3109/07357907.2010.512603. [DOI] [PubMed] [Google Scholar]
- 182.Montserrat N, Mozos A, Llobet D, et al. Epithelial to mesenchymal transition in early stage endometrioid endometrial carcinoma. Hum Pathol. 2011 doi: 10.1016/j.humpath.2011.06.021. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 183.Blechschmidt K, Kremmer E, Hollweck R, et al. The E-cadherin repressor snail plays a role in tumor progression of endometrioid adenocarcinomas. Diagn Mol Pathol. 2007;16(4):222–228. doi: 10.1097/PDM.0b013e31806219ae. [DOI] [PubMed] [Google Scholar]
- 184.Singh M, Spoelstra NS, Jean A, et al. ZEB1 expression in type I vs type II endometrial cancers: a marker of aggressive disease. Mod Pathol. 2008;21(7):912–923. doi: 10.1038/modpathol.2008.82. [DOI] [PubMed] [Google Scholar]
- 185.Oza AM, Elit L, Tsao MS, et al. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. J Clin Oncol. 2011;29(24):3278–3285. doi: 10.1200/JCO.2010.34.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Slomovitz BM, Lu KH, Johnston T, et al. A phase 2 study of the oral mammalian target of rapamycin inhibitor, everolimus, in patients with recurrent endometrial carcinoma. Cancer. 2010;116(23):5415–5419. doi: 10.1002/cncr.25515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Meyer LA, Slomovitz BM, Djordjevic B, et al. The search continues: Looking for predictive biomarkers for response mTOR inhibition in endometrial cancer [abstract]. ASCO Meeting Abstracts; 2011. p. 5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Colombo N, McMeekin S, Schwartz P, et al. A phase II trial of the mTOR inhibitor AP23573 as a single agent in advanced endometrial cancer [abstract]. ASCO Meeting Abstracts; 2007. p. 5516. [Google Scholar]
- 189.Carracedo A, Ma L, Teruya-Feldstein J, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118(9):3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Forster MD, Dedes KJ, Sandhu S, et al. Treatment with olaparib in a patient with PTEN-deficient endometrioid endometrial cancer. Nat Rev Clin Oncol. 2011;8(5):302–306. doi: 10.1038/nrclinonc.2011.42. [DOI] [PubMed] [Google Scholar]
- 191.Santin AD, Bellone S, Roman JJ, McKenney JK, Pecorelli S. Trastuzumab treatment in patients with advanced or recurrent endometrial carcinoma overexpressing HER2/neu. Int J Gynaecol Obstet. 2008;102(2):128–131. doi: 10.1016/j.ijgo.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 192.Jewell E, Secord AA, Brotherton T, Berchuck A. Use of trastuzumab in the treatment of metastatic endometrial cancer. Int J Gynecol Cancer. 2006;16(3):1370–1373. doi: 10.1111/j.1525-1438.2006.00543.x. [DOI] [PubMed] [Google Scholar]
- 193.Fleming GF, Sill MW, Darcy KM, et al. Phase II trial of trastuzumab in women with advanced or recurrent, HER2-positive endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2010;116(1):15–20. doi: 10.1016/j.ygyno.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Westin SN, Broaddus RR. Personalized therapy in endometrial cancer: Challenges and opportunities. Cancer Biol Ther. 2012;13(1):1–13. doi: 10.4161/cbt.13.1.18438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bell DW. Our changing view of the genomic landscape of cancer. J Pathol. 2010;220(2):231–243. doi: 10.1002/path.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Koul A, Willen R, Bendahl PO, Nilbert M, Borg A. Distinct sets of gene alterations in endometrial carcinoma implicate alternate modes of tumorigenesis. Cancer. 2002;94(9):2369–2379. doi: 10.1002/cncr.10498. [DOI] [PubMed] [Google Scholar]
- 197.Moreno-Bueno G, Fernandez-Marcos PJ, Collado M, et al. Inactivation of the candidate tumor suppressor par-4 in endometrial cancer. Cancer Res. 2007;67(5):1927–1934. doi: 10.1158/0008-5472.CAN-06-2687. [DOI] [PubMed] [Google Scholar]
- 198.Sun H, Enomoto T, Fujita M, et al. Mutational analysis of the PTEN gene in endometrial carcinoma and hyperplasia. Am J Clin Pathol. 2001;115(1):32–38. doi: 10.1309/7JX6-B9U9-3P0R-EQNY. [DOI] [PubMed] [Google Scholar]
- 199.Cohen Y, Shalmon B, Korach J, Barshack I, Fridman E, Rechavi G. AKT1 pleckstrin homology domain E17K activating mutation in endometrial carcinoma. Gynecol Oncol. 2010;116(1):88–91. doi: 10.1016/j.ygyno.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 200.Manavi M, Bauer M, Baghestanian M, et al. Oncogenic potential of c-erbB-2 and its association with c-K-ras in premalignant and malignant lesions of the human uterine endometrium. Tumour Biol. 2001;22(5):299–309. doi: 10.1159/000050631. [DOI] [PubMed] [Google Scholar]