Abstract
The number of genetically modified mouse lines has been increasing exponentially in the past few decades. In order to safeguard them from accidental loss and genetic drifting, to reduce animal housing cost, and to efficiently distribute them around the world, it is important to cryopreserve these valuable genetic resources. Preimplantation-stage embryos from thousands of mouse lines have been cryopreserved during the past two to three decades. Although reliable, this method requires several hundreds of embryos, which demands a sizable breeding colony, to safely preserve each line. This requirement imposes significant delay and financial burden for the archiving effort. Sperm cryopreservation is now emerging as the leading method for storing and distributing mouse lines, largely due to the recent finding that addition of a reducing agent, monothioglycerol, into the cryoprotectant can significantly increase the in vitro fertilization (IVF) rate in many mouse strains, including the most widely used C57BL/6 strain. This method is quick, inexpensive, and requires only two breeding age male mice, but it still remains tricky and strain-dependent. A small change in experimental conditions can lead to significant variations in the outcome. In this chapter, we describe in detail our sperm cryopreservation, IVF, and oviduct transfer procedures for storing and reviving genetically modified mouse lines.
1. Introduction
During the past three decades, the number of genetically modified mouse models for biomedical research has been growing at an exponential rate. Currently, the worldwide knockout mouse project (www.komp.org) is in the process of generating at least one null mouse line for essentially every mouse gene. Given the high cost of maintaining live animals and the fact that not all mouse lines are actively being used for research at a given time, it is important to cryopreserve some of these valuable animal models. The method for cryopreserving mouse embryos was first developed in the early 1970s by two independent laboratories (Whittingham et al., 1972; Wilmut, 1972). Since then, thousands of mouse lines have been successfully cryopreserved as preimplantation-stage embryos in centralized animal resource centers, such as Jackson Laboratory (JAX), and in various core facilities, as well as individual research laboratories (Mazur et al., 2008).
Generally speaking, cryopreservation of preimplantation-stage mouse embryos is efficient and reliable for banking mouse lines. Live mice can be recovered through the well-established embryo transfer procedures. However, one major drawback for embryo cryopreservation is that usually several hundreds of preimplantation-stage embryos are needed for safely cryopreserving a mouse line, and unfortunately many genetically modified mouse lines do not respond to superovulation well for yielding large number of embryos. Therefore, a relatively large breeding colony needs to be established first, which is not only time consuming, but also imposes significant financial burden up front (Mazur et al., 2008). Because of the limitation on animal space and research budgets, embryo cryopreservation is not likely to be able to keep pace with the large number of mouse lines that are being generated each year. Cryopreservation of the male gametes, spermatozoa, can potentially overcome this problem because each suitable male can yield enough sperm to reconstitute a large number of offspring. The cryopreservation of human and live stock semen has been a common practice for several decades, but mouse sperm has remained difficult to freeze for quite some time. The introduction of 18% raffinose and 3% skim milk as a cryoprotective agent (CPA) is a significant breakthrough for mouse sperm cryopreservation (Kaneko et al., 2006; Nakagata, 2000), although its success is still heavily dependent on the genetic background of the mice. Unfortunately, its efficiency is unacceptable for many important inbred strains, including the most popular mouse strain, C57BL/6 (Nakagata and Takeshima, 1993; Nishizono et al., 2004; Songsasen and Leibo, 1997; Sztein et al., 2000). Assisted reproductive techniques, such as partial zona dissection (PZD) (Anzai et al., 2006; Kawase et al., 2002; Nakagata et al., 1997) and intracytoplasmic sperm injection (ICSI) (Kimura and Yanagimachi, 1995; Sakamoto et al., 2005; Szczygiel et al., 2002a), are often required for recovering the lines. Various attempts have been made to improve the IVF rate from cryopreserved inbred sperm (Bath, 2003; Liu et al., 2009; Ostermeier et al., 2008; Suzuki-Migishima et al., 2009; Szczygiel et al., 2002b; Taguma et al., 2009; Takeo et al., 2008), but the method that attracted the most attention is the discovery that inclusion of a reducing agent, more specifically monothioglycerol (MTG), in the freezing medium can substantially increase the postthaw IVF rate in many inbred strains including the C57BL/6 strain (Ostermeier et al., 2008). This methodological advancement together with the Jackson Laboratory’s efforts to educate (JAX Cryopreservation Workshop) and assist (JAX Sperm Cryopreservation Kit) has facilitated the rapid adoption of sperm cryopreservation as the preferred method for archiving and distributing genetically modified mice.
The mouse sperm cryopreservation method is simple, fast, and does not require sophisticated instrumentation, such as a controlled rate freezer, but this does not mean it is trivial. Small variations in experimental conditions, such as timing, temperature, and concentration, can dramatically change the outcome. The successfulness of sperm freezing experiments is commonly measured by the efficiency of thawed sperm in reconstituting the mouse lines through IVF and subsequent embryo transfer procedures. Therefore, in this chapter, we describe the methods for sperm cryopreservation, IVF, and embryo transfer used in our laboratories, which are largely based on the protocols developed by the Jackson Laboratory.
2. Sperm Cryopreservation
2.1. Preparing cryoprotective agent
Add 70 ml ultra pure water into a flask and heat to 37–50 °C on a stirrer/hot plate. Slowly add 18 g raffinose pentahydrate (Sigma Cat#: R-7630). Stir with a magnetic stir bar till the solution becomes clear.
Add 3 g of Difco dry skim milk (BD Diagnostics, Cat#: 232100) and stir till the dry milk is dissolved. Add more ultra pure water to bring the final volume to 100 ml.
Centrifuge at 13,000 rpm in a Beckman Coulter JA25.50 rotor for 60 min at 4 °C. Filter the supernatant through a sterile 0.22 µm filter unit (Millipore, Cat#: SCGPUO2RE).
Divide the filtered CPA into ten 15 ml disposable conical tubes, each containing 10 ml. Aliquoted CPA can be stored in a −80 °C freezer for 6 months. Once thawed, the CPA can be stored at 4 °C for 1 week.
Frozen CPA can be thawed by submerging the tubes in a 37 °C water bath. Before use, α-monothioglycerol (MTG, Sigma, Cat#: M6145) should be added at a final concentration of 477 µM.
2.2. Preparing cryopreservation straws
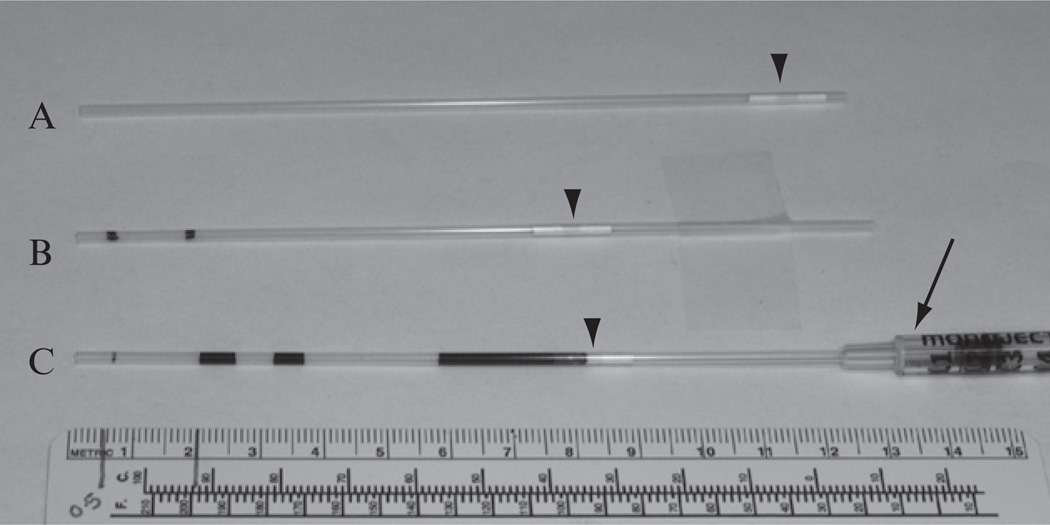
The 133-mm-long plastic straws from IMV Technologies, Inc. (Maple Grove, MN, Cat#: AAA201) are used for sperm cryopreservation. Before use, the cotton/PVA plug near one end (Fig. 4.1A) of the straw should be pushed inward till it is ~4.5 cm from the end, using a thin plastic rod (Piston Plastique from IMV, Ref. No. 7296). This 4.5 cm of space is necessary for labeling and sealing the straw. It is important to use labels and pens that can withstand long-term storage in liquid nitrogen (LN2), such as the Brady labels (Brady Serve, Cat#: LAT-PTL 19-427).
At approximately 0.5 and 2.0 cm from the open end of the straw, draw two lines with a marker (Fig. 4.1B). Insert the plugged end of the straw into a 1 cc disposable syringe (Kendall, Ref. No. 8881501400), so that liquid can be drawn in or expelled out of the straw by pulling or pushing the plunger of the syringe, respectively (Fig. 4.1C).
Draw CPA/MTG solution into the straw until the solution reaches the 2.0 cm line. Then, draw a segment of air into the straw till the air reaches the 2.0 cm line. Place these straws on a clean surface until ready for loading sperm sample.
Figure 4.1.
Preparing and loading cryopreservation straws. (A) A picture of a cryopreservation straw manufactured by IMV Technologies, Inc. (B) The cotton/PVA plug (arrow head) is pushed inward till it is ~4.5 cm from the end. Then, two lines are drawn using a marker on the straw, which are ~0.5 and ~2.0 cm from the open end, respectively. (C) Loading of CPA and sperm samples. A dark-colored solution is used in this example for easy visualization. Using the attached plastic syringe (arrow), a 2.0-cm-long segment of CPA is first drawn, and then a 2.0-cm-long segment of air is drawn into the straw. Next, two 0.5-cm-long segments of sperm samples, separated by a 0.5 cm segment of air, are drawn. Finally, more air is drawn into the straw till the first segment of CPA wets the cotton/PVA plug. The straw is now ready for sealing. Each 0.5 cm segment of sperm sample corresponds to 10 µl of sample, which is enough for one IVF dish.
2.3. Collecting and loading sperm samples
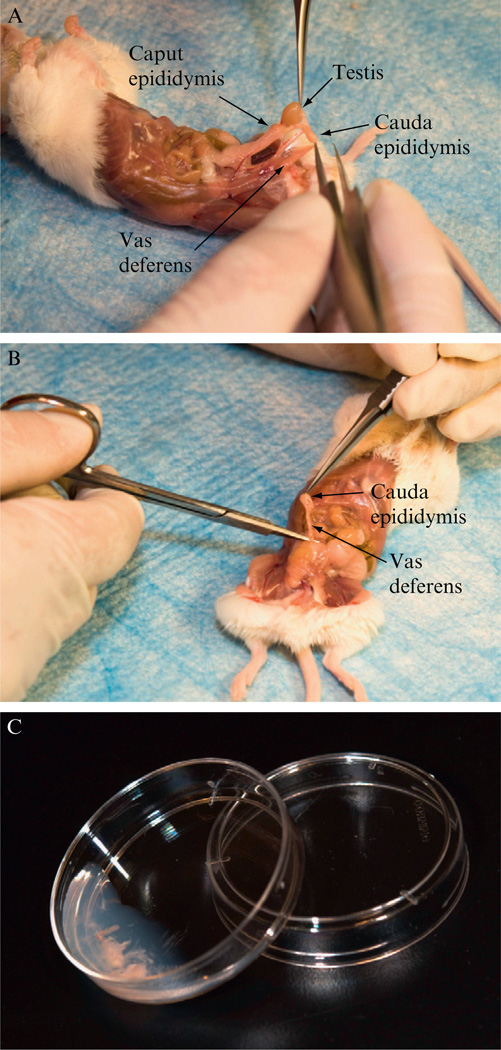
Euthanize two male mice with proven breeding track records, preferably 2–6-month-old and that have been housed alone for 1 week. Quickly open the abdominal skin and body wall with a pair of scissors. As shown in Fig. 4.2, locate and expose the internal reproductive organs. Dissect out the cauda epididymis and vas deferens using a pair of watchmaker’s No. 5 forceps (Roboz Surgical Instrument Co., Cat#: RS-4905) and a pair of dissecting scissors (Roboz, Cat#: RS-5882). Try to eliminate as much associated adipose, vascular, and other tissues as possible.
Place all four dissected vas deferentia and epididymides (two from each mouse) into a 35 mm culture dish containing 0.7 ml of 37 °C CPA/MTG. Under a stereomicroscope with transmitted light source, use a pair of watchmaker’s forceps and a 26-gauge hypodermic needle to tear open the cauda epididymis by piercing the tissue several times. Semen inside each vas deferens can be expelled by gently pressing the tubule with the needle to slowly “walk” the semen out. For maximum sperm quality, the above two steps should be completed within 10 min.
Gently tap and swirl the dish for approximately 30 s and then leave the dish tilted as shown in Fig. 4.2C for 10 min at room temperature to allow sperm to swim out of the tissues. The dish should remain covered with a lid to minimize medium evaporation.
Assess the concentration and motility of the sperm under a high-magnification stereomicroscope. Discard the larger pieces of tissues and gently swirl the dish to mix the sperm suspension evenly.
Dip the open end of the straws prepared in Section 2.2 into the sperm suspension, and slowly draw in the sample until reaching the 0.5 cm mark. Then lift the straw and draw in air until reaching the 0.5 cm mark. A second aliquot, or even more aliquots, of sperm sample can be drawn into the straw using the same method. Each 0.5-cm-long segment of sample corresponds to ~10 µl, which is enough for one fertilization dish (see Section 4.2).
After finishing loading the sample, air is drawn into the straw until the first segment of CPA wets the cotton/PVA plug (Fig. 4.1C). Dry the outside of the straw with Kimwipes. Seal the open end of the straw using a heat sealer (American International Electric Inc., Model AIE—310 HD). Gently detach the straw from the syringe, and then seal the plugged end.
Figure 4.2.
Collection of mouse sperm. (A) Exposed male internal reproductive organs, showing the testis, cauda epididymis, caput epididymis, and vas deferens. (B) Removing of the cauda epididymis and a portion of the vas deferens using scissors and forceps. (C) A sperm collection dish with torn epididymis and squeezed vas deferens in CPA.
2.4. Freezing sperm samples
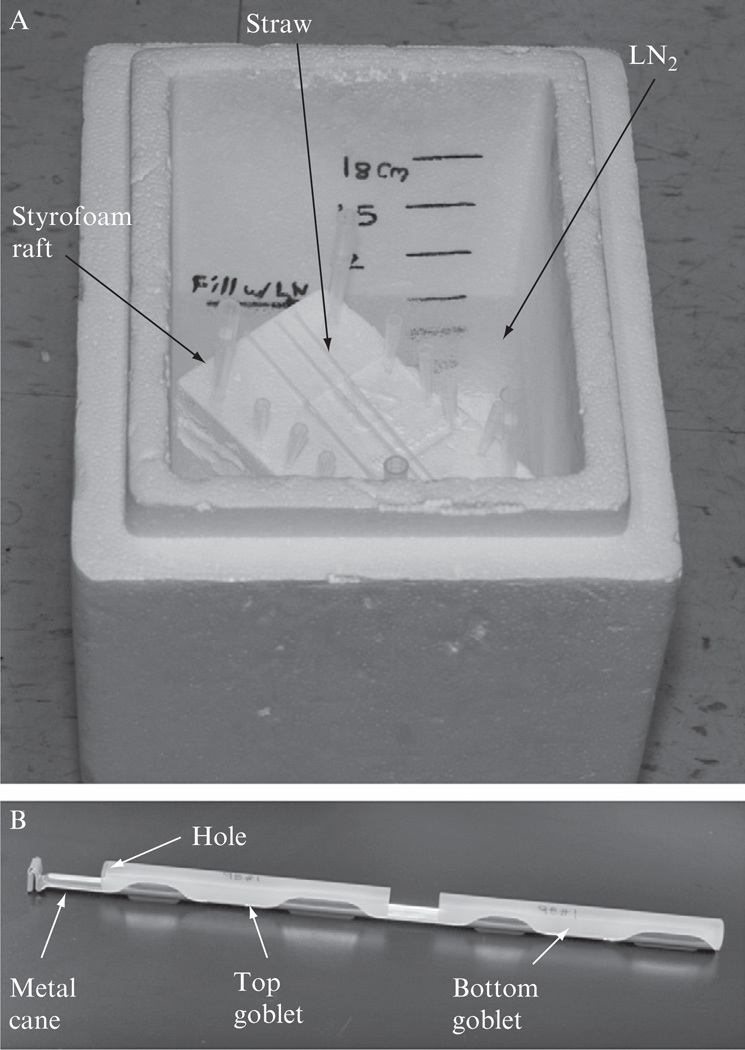
Dedicated LN2 freezing box, ThermoSafe (Thermo Fisher, Cat#: 11-676-13) is available, but we still use a medium sized Styrofoam box. Before each use, we fill the box with about 9-cm-deep LN2, and then place a 3.5-cm-thick Styrofoam board into the box, which should float on the LN2 surface (Fig. 4.3A).
Lay the straws loaded with sperm on top of the Styrofoam raft (Fig. 4.3A). Cover the box with the matching Styrofoam lid and leave the box undisturbed for 10 min. Any straws that accidentally contacted LN2 prior to the 10 min of gradual cooling should be discarded.
Open the lid of the Styrofoam box and quickly plunge the straws into the LN2. Dip the metal cane with attached plastic goblet (Visoture Rond 13 mm Blanc, IMV, Ref. No. 006420) into the LN2, and quickly transfer the frozen straws into the goblet using a pair of long forceps. To prevent the straws from falling out, a second goblet is inserted in the up-side-down orientation into the cane. As indicated in Fig. 4.3B, holes should be made in the closed end of the goblet to allow air to come out and LN2 enter the goblets during storage. It is noteworthy that cassettes (Zanders Medical Supplies, Cat#: 16980/0601) specifically made for holding straws are available. Sealed straws can be loaded into these cassettes before placing on the Styrofoam raft for cooling.
Figure 4.3.
(A) Cooling of loaded straws in a Styrofoam box. The Styrofoam box must be covered with a matching lid (not shown) during the cooling period. (B) A metal cane with attached plastic goblets. After freezing, the straws are placed inside the bottom goblet, and then the top goblet is sliding down to completely enclose the straws, to prevent the straws from falling out. Holes are made on the closed end of the top goblet to let air out and LN2 into the goblet.
3. Storing and Shipping Frozen Sperm Samples
The straws can be maintained in a LN2 freezer for long-term storage. For each strain, the straws should be divided into two different canes and stored in two different LN2 freezers preferably at two separate locations. Ideally, one set of samples is stored in your facility for easy access, while the other set is stored some distance away to reduce the possibility of losing all samples due to unpredictable disasters. It is highly recommended to store the second set of samples in a public or commercial repository, such as KamTech, Inc. (Gaithersburg, MD), JAX (Bar Harbor, ME), or MMRRC. Such repositories have reliable monitoring system, emergency power and supplies, and catastrophe-prevention measures to safeguard your valuable samples.
Frozen sperm can be shipped around the world for reconstitution of mouse lines through IVF or artificial insemination (AI). It is strongly advised to keep the frozen sperm at LN2 temperature at all times, even during transportation. Because LN2 is a regulated hazardous material for transportation, an LN2 dry shipper (Fig. 4.4) is often used for shipping cryopreserved samples. Dry shippers will not spill LN2 if tipped over during transportation, but they can maintain the LN2 temperature for up to 2 weeks if charged correctly. It is important to follow the manufacturer’s instruction when filling the dry shipper with LN2. Protective gloves and a face shield should be worn to prevent accidental injuries. The absorbent material in the dry shippers should be fully saturated with LN2, but free flowing LN2 should be poured out of the dry shipper before loading and shipping samples. When loading and unloading the dry shipper, it is critical to minimize the exposure of straws to non-LN2 temperature. The lid/stopper of the dry shipper should be securely closed and fastened with a zip tie. Avoid wraping and boxing the shipper. Ideally it should be shipped in its matching shipping container (Fig. 4.4).
Figure 4.4.
A photograph of an LN2 dry shipper and its matching container for shipping.
4. In Vitro Fertilization
4.1. Preparing egg donor mice
Three days prior to the scheduled IVF procedure, inject (i.p.) the egg donor mice with pregnant mare serum gonadotropin (PMSG, Sigma # G-4877). The number of females needed is dependent on the genetic background, age, as well as how many offspring are needed. For some strains, newly weaned young mice (19–24 days) are best, but for some other strains, mature females (6–8 weeks) are more consistent. The optimal dose of PMSG also varies depending on strain, age, and body weights, but in most cases 5 IU dissolved in 0.1 ml physiological saline (0.9% NaCl) is a good starting concentration. Avoid repeated thaw and freeze of the PMSG solution. Typically 5–15 usable eggs can be expected from each superovulated female. We routinely inject PMSG late in the afternoon (6–8 p.m.) to avoid coming to work too early on the day of IVF.
On the day before the scheduled IVF procedure, 46–48 h after the PMSG injection, inject (i.p.) the same females with 5 IU of human chorionic gonadotropin (hCG, Sigma # C-1063) dissolved in 0.1 ml physiological saline.
4.2. Preparing culture and IVF dishes
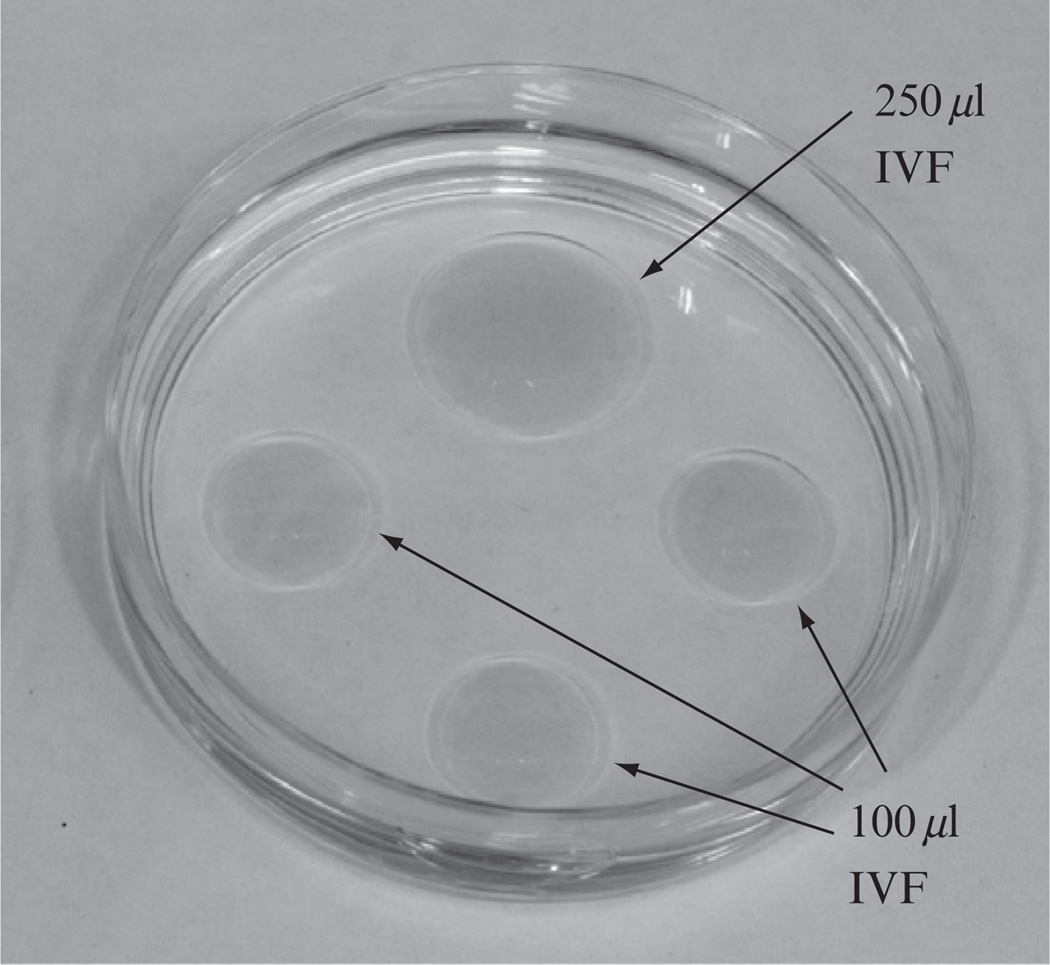
Because IVF procedures usually begin early in the morning (13 h after hCG injection) and it takes time for the media, under mineral oil, to fully equilibrate with the gas contents in the incubator, we normally set up the culture and IVF dishes on the day before the scheduled IVF. Each IVF dish (Fig. 4.5) is made by adding one 250 µl and three 100 µl drops of Cook’s IVF medium (Cooks Cat#: K-RVFE-50) into a 60 mm dish, and then quickly covering the media drops with embryo-tested mineral oil (Sigma Cat#: M8410). Each egg collection dish is made by adding 400 µl of IVF medium into a 35 mm tissue culture dish and then quickly covering the medium drop with mineral oil. All dishes should be placed in a 37 °C incubator with 5% CO2, 5% O2, and 90% N2 (Robert Oxygen premixed gas, R0133502001) until use.
Figure 4.5.
A photograph of a typical IVF dish, which contains one 250 µl and three 100 µl drops of IVF media cover under mineral oil.
4.3. Thawing cryopreserved sperm
Take the sperm-containing straw out of the LN2 freezer using a pair of forceps, and immediately submerge the straw into a 37 °C water bath until all ice inside the straw disappears (20–40 s). Dry the straw with Kimwipes.
Cut the straw through the middle of the cotton/PVA plug using a pair of sharp scissors. Then, cut the unplugged (open) end of the straw near the sealed tip. Disinfect the open end with an alcohol wipe and then leave in air for a couple of minutes to allow the alcohol to evaporate.
Dip the disinfected end into the 250 µl drop in the IVF dish (Fig. 4.5), and then using a thin plastic rod (Piston Plastique from IMV, Ref. No. 7296), slowly push the cotton/PVA plug inward to dispense one segment of sperm sample (~10 µl) into the IVF medium. The other segment(s) of sperm sample contained in the same straw can be dispensed into other IVF dish(s) for fertilizing different batch(es) of eggs.
Incubate the IVF dishes in a 37 °C incubator containing 5% O2, 5% CO2, and 90% N2 for ~60 min before adding eggs for fertilization.
4.4. Collecting eggs and IVF
Thirteen hours after hCG injection (see Section 4.1), euthanize the superovulated females and open the abdominal cavity using a pair of scissors. Dissect out both oviducts from each female using a pair of watchmaker’s forceps and dissecting scissors. To avoid damaging the oviducts, the ovary as well as a small piece of uterine horn can also be dissected.
Place the dissected oviducts into the 35 mm dish containing 400 µl of IVF medium prepared in Section 4.2. Using the fine forceps to hold the oviduct in place, and then use a 29-gauge needle to tear open the ampulla, which is the swollen and translucent segment of the oviducts. The cumulus mass, which usually contains several eggs surrounded by follicular cells, will extrude spontaneously into the media. Cumulus masses from up to five mice can be collected into the same dish, and each oviduct should be discarded immediate after processing.
Using a P1000 pipetman setting at 40–50 µl, carefully aspirate the cumulus masses with minimum volume of media and transfer them into the IVF medium drop that already contains the capacitated sperm (see Section 4.3). Alternatively, the eggs can be released directly from the ampulla into the IVF medium drop containing the incubated sperm.
Incubate the sperm and egg mix for 4–6 h in the incubator to allow fertilization to take place. During this incubation, the IVF dish can be taken out of the incubator briefly for monitoring the IVF process under a microscope, but avoid leaving the dishes outside the incubator for more than a few minutes. The eggs should be moving or spinning around because of the swimming of the bound sperm.
After the incubation, the cumulus masses usually dissemble and the follicle cells are no longer attached to the eggs. Therefore, individual eggs can be picked up and transferred using a mouth-controlled pipette (Fig. 4.6A), which is assembled by connecting the mouthpiece and rubber aspirator tubing (Sigma, Cat#: A5177) through a filtered 1000 µl pipette tip to a pulled long-tipped glass Pasteur pipette. Wash the eggs through two of the 100 µl drops of IVF media to eliminate debris and extra sperm, and leave them in the last (third) drop of medium.
Return the IVF dish into a 37 °C incubator with 5% CO2 for culturing overnight.
Figure 4.6.
(A) A photograph of a mouth pipette for transferring embryos. It is assembled by connecting a pulled long glass Pasteur pipette through a filtered 1000 µl pipette tip and a rubber tubing to a plastic mouthpiece. (B) Microphotograph of the tip of the embryo transfer pipette with loaded embryos and air bubbles.
5. Oviduct Transfer
On the day of IVF, select female Swiss Webster mice (Taconic Farm) with swollen and reddish genital areas, which indicates they are in estrus, and pair them up with vasectomized male mice for mating.
Check the mice for vaginal plugs the next morning. Mice with visible plugs can be used in Step 4 as surrogate mothers.
Examine the IVF dish using a stereomicroscope. Count the number of embryos that have reached the two-cell stage of development, and transfer them into an M2 drop covered under mineral oil.
Inject (i.p.) 2.5% Avertin solution at a dose of 0.017 ml/g of body weight to anesthetize the surrogate mothers. The Avertin solution is made by first dissolving 5.0 g of 2,2,2-tribromoethanol (Aldrich, Milwaukee, WI) in 5 ml tert-amyl alcohol (Aldrich), and then adding 195 ml of isotonic saline (0.9% NaCl solution). Aliquoted Avertin solution can be stored at −20 °C in the dark.
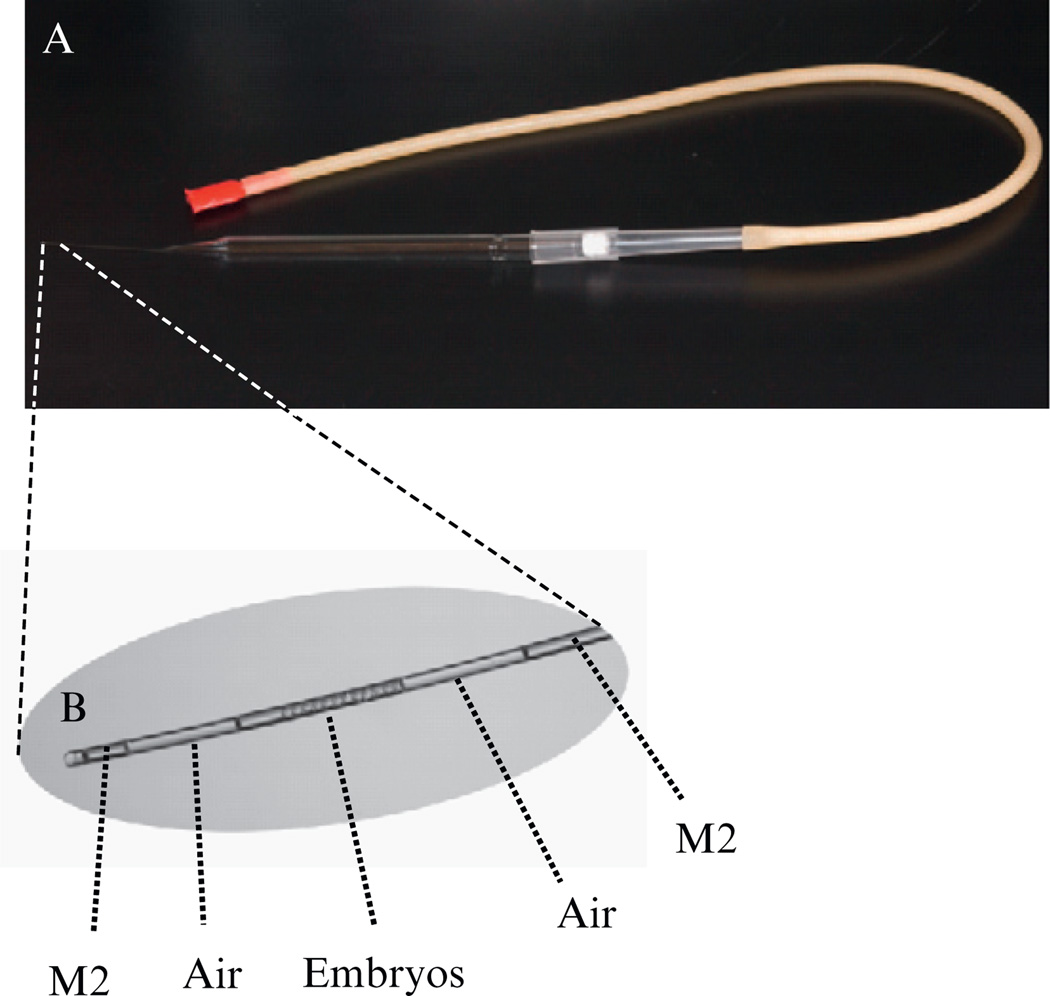
Load 10 two-cell stage embryos into the mouth operated transfer pipette. As shown in Fig. 4.6B, two air bubbles are sucked into the transfer pipette to mark the boundaries of the suspended embryos.
Check the depth of anesthesia at ~5 min after Avertin injection by gently pinching one of the hind paws. A supplemental dose of Avertin, usually a third of the initial dose, should be injected if the animal can still respond to this gentle pinch.
Remove the hair from a generous area on the dorsal lumbar back using a small hair clipper. Disinfect the clipped area by alternating applications of Betadine (Medline Industries, Mundelein, IL) and 70% alcohol.
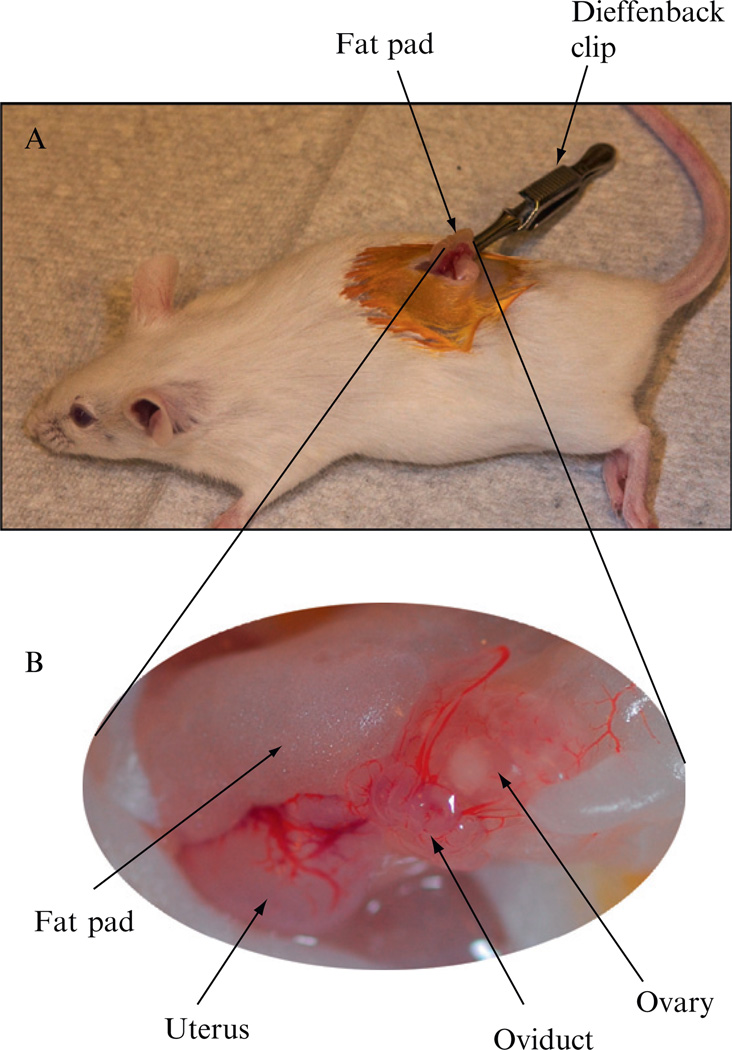
Cut a dorsal midline incision (~1 cm long) in the cleaned skin area using a pair of sterilized scissors (Fig. 4.7).Move the skin incision to either side of the para-lumbar using a pair of iris forceps (Roboz Surgical Instrument Co., Cat#: RS-5130) to find the ovary and associated fat pad, which are slightly paler than the surrounding internal organs.
Make a small incision in the muscle body wall and then extending the cut to 5–10 cm long using the back of the scissors’ blades. Using a pair of iris forceps grasp the fat pad and carefully pull out the fat pad, the ovary, the oviduct, and a segment of the uterine horn through the incision. Clamp the fat pad with a Dieffenbach clip (Roboz, Cat#: RS-7422) to hold the organs in place (Fig. 4.7).
Under a dissecting microscope, find the ampulla, which is the swollen and translucent segment of the oviducts, and usually can be found a few millimeters from the infundebulum. Use a 26-gauge needle to punch a hole in the oviduct wall between the ampulla and the ovary.
Insert the tip of the transfer pipette into the hole with the pipette opening pointing toward the ampulla. Gently blow into the mouthpiece to expel the two air bubbles and the embryos sandwiched between them. The presence of two air bubbles in the ampulla is a good indication that all embryos have been successfully implanted.
Carefully push the reproductive organs back into the abdominal cavity, and sew the abdominal wall incision with a cruciate suture utilizing absorbable 5-0 Vicryl suture (Ethicon, Sommerville, NJ). Using a 1 cc disposable syringe with an attached 26-gauge needle, drop one or two drops of 0.25% Bupivacaine solution, which is a long-lasting local anesthetic, on the muscle at the surgical site. Finally, close the skin incision with two stainless steel surgical wound clips (Roboz, Cat#: RS-9260).
To aid the recovery, place the mice in a cage warmed by a circulating water blanket (GayMar Industries, Orchard Park, NY) or a carefully adjusted infra-red heating lamp (Bel-art Products, Pequannock, NJ).
After the mice wake up and start to move around, usually 30–60 min post-Avertin injection, return them to the animal room.
Remove the wound clip after 10–14 days, and pups will be born about 19 days post embryo transfer surgery. The offspring can be weaned, ear-tagged, and tail-biopsied when they are 18–21 days old.
Figure 4.7.
(A) A photograph of a recipient foster mother during embryo transfer procedures. The reproductive organs were pulled out through an incision on the back. A Dieffenbach clip is clamped on the fat pad to hold the reproductive organs in place. (B) Magnified view of the reproductive organs.
6. Conclusions
Mouse line cryopreservation is not only important for saving animal space and research budget but also for safeguarding important strains against accidental loss due to diseases, contamination, human errors in screening and breeding colonies, and catastrophes such as fire, flood, and earthquake. Frozen embryos retain their genomes at a specific time point and hence prevent genetic drifting caused by long-term breeding and propagation. Cryopreservation of preimplantation embryos has been used during the past few decades for successfully archiving thousands of valuable mouse lines. Now, sperm cryopreservation is emerging as the leading method due to recent improvements in the efficiency and reliability of freezing and IVF procedures. The main advantage of sperm cryopreservation is that it requires much less “front end” investment in labor and animal space (Mazur et al., 2008). Usually, two breeding age males can yield enough sperm to reconstitute a large number of offspring. The procedure itself is also simpler, faster, and does not require expensive instruments, such as a controlled rate freezer. However, sperm are haploid cells and therefore only half of the genetic material from the mouse is preserved. The thawed sperm must fertilize an egg, which brings in the other half of the genetic material, in order to reconstitute the mouse line. This could be problematic if the phenotypes of interest are dependent on multiple chromosomal loci or on an extensive breeding scheme. Currently, the efficiencies for sperm cryopreservation and IVF still varies dependent on the mouse strains and on the hands of the researchers. However, these disadvantages do not seem to prevent the rapid adoption of sperm cryopreservation for maintaining and distributing mouse lines. This can be partially attributed to the fact that a number of assisted reproduction techniques, such as PZD and ICSI, can help to recover mouse lines using imperfectly cryopreserved sperm. If fact, even freeze dried dead sperm have been used to recover mouse lines (Wakayama and Yanagimachi, 1998). Lastly, the method for mouse sperm cryopreservation is continually being improved (Liu et al., 2009; Suzuki-Migishima et al., 2009), which will undoubtedly make it more robust, reliable, and playing an even greater role in future archiving and distribution of genetically modified mouse lines.
ACKNOWLEDGMENT
This work was supported by the Intramural Research Program of NIH, NHLBI and NEI.
REFERENCES
- Anzai M, Nishiwaki M, Yanagi M, Nakashima T, Kaneko T, Taguchi Y, Tokoro M, Shin SW, Mitani T, Kato H, Matsumoto K, Nakagata N, et al. Application of laser-assisted zona drilling to in vitro fertilization of cryopreserved mouse oocytes with spermatozoa from a subfertile transgenic mouse. J. Reprod. Dev. 2006;52(5):601–606. doi: 10.1262/jrd.18040. [DOI] [PubMed] [Google Scholar]
- Bath ML. Simple and efficient in vitro fertilization with cryopreserved C57BL/6J mouse sperm. Biol. Reprod. 2003;68(1):19–23. doi: 10.1095/biolreprod.102.007344. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Yamamura A, Ide Y, Ogi M, Yanagita T, Nakagata N. Longterm cryopreservation of mouse sperm. Theriogenology. 2006;66(5):1098–1101. doi: 10.1016/j.theriogenology.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Kawase Y, Iwata T, Ueda O, Kamada N, Tachibe T, Aoki Y, Jishage K, Suzuki H. Effect of partial incision of the zona pellucida by piezo-micromanipulator for in vitro fertilization using frozen-thawed mouse spermatozoa on the developmental rate of embryos transferred at the 2-cell stage. Biol. Reprod. 2002;66(2):381–385. doi: 10.1095/biolreprod66.2.381. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Yanagimachi R. Intracytoplasmic sperm injection in the mouse. Biol. Reprod. 1995;52(4):709–720. doi: 10.1095/biolreprod52.4.709. [DOI] [PubMed] [Google Scholar]
- Liu L, Nutter LM, Law N, McKerlie C. Sperm freezing and in vitro fertilization in three substrains of C57BL/6 mice. J.Am. Assoc. Lab. Anim. Sci. 2009;48(1):39–43. [PMC free article] [PubMed] [Google Scholar]
- Mazur P, Leibo SP, Seidel GE., Jr Cryopreservation of the germplasm of animals used in biological and medical research: Importance, impact, status, and future directions. Biol. Reprod. 2008;78(1):2–12. doi: 10.1095/biolreprod.107.064113. [DOI] [PubMed] [Google Scholar]
- Nakagata N. Cryopreservation of mouse spermatozoa. Mamm. Genome. 2000;11(7):572–576. doi: 10.1007/s003350010109. [DOI] [PubMed] [Google Scholar]
- Nakagata N, Takeshima T. Cryopreservation of mouse spermatozoa from inbred and F1 hybrid strains. Jikken Dobutsu. 1993;42(3):317–320. doi: 10.1538/expanim1978.42.3_317. [DOI] [PubMed] [Google Scholar]
- Nakagata N, Okamoto M, Ueda O, Suzuki H. Positive effect of partial zona-pellucida dissection on the in vitro fertilizing capacity of cryopreserved C57BL/6J transgenic mouse spermatozoa of low motility. Biol. Reprod. 1997;57(5):1050–1055. doi: 10.1095/biolreprod57.5.1050. [DOI] [PubMed] [Google Scholar]
- Nishizono H, Shioda M, Takeo T, Irie T, Nakagata N. Decrease of fertilizing ability of mouse spermatozoa after freezing and thawing is related to cellular injury. Biol. Reprod. 2004;71(3):973–978. doi: 10.1095/biolreprod.103.024422. [DOI] [PubMed] [Google Scholar]
- Ostermeier GC, Wiles MV, Farley JS, Taft RA. Conserving, distributing and managing genetically modified mouse lines by sperm cryopreservation. PLoS ONE. 2008;3(7):e2792. doi: 10.1371/journal.pone.0002792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto W, Kaneko T, Nakagata N. Use of frozen-thawed oocytes for efficient production of normal offspring from cryopreserved mouse spermatozoa showing low fertility. Comp. Med. 2005;55(2):136–139. [PubMed] [Google Scholar]
- Songsasen N, Leibo SP. Cryopreservation of mouse spermatozoa. II. Relationship between survival after cryopreservation and osmotic tolerance of spermatozoa from three strains of mice. Cryobiology. 1997;35(3):255–269. doi: 10.1006/cryo.1997.2047. [DOI] [PubMed] [Google Scholar]
- Suzuki-Migishima R, Hino T, Takabe M, Oda K, Migishima F, Morimoto Y, Yokoyama M. Marked improvement of fertility of cryopreserved C57BL/6J mouse sperm by depletion of Ca2þ in medium. J. Reprod. Dev. 2009;55(4):386–392. doi: 10.1262/jrd.20163. [DOI] [PubMed] [Google Scholar]
- Szczygiel MA, Kusakabe H, Yanagimachi R, Whittingham DG. Intracytoplasmic sperm injection is more efficient than in vitro fertilization for generating mouse embryos from cryopreserved spermatozoa. Biol. Reprod. 2002a;67(4):1278–1284. doi: 10.1095/biolreprod67.4.1278. [DOI] [PubMed] [Google Scholar]
- Szczygiel MA, Kusakabe H, Yanagimachi R, Whittingham DG. Separation of motile populations of spermatozoa prior to freezing is beneficial for subsequent fertilization in vitro: A study with various mouse strains. Biol. Reprod. 2002b;67(1):287–292. doi: 10.1095/biolreprod67.1.287. [DOI] [PubMed] [Google Scholar]
- Sztein JM, Farley JS, Mobraaten LE. In vitro fertilization with cryopreserved inbred mouse sperm. Biol. Reprod. 2000;63(6):1774–1780. doi: 10.1095/biolreprod63.6.1774. [DOI] [PubMed] [Google Scholar]
- Taguma K, Nakamura C, Ozaki A, Suzuki C, Hachisu A, Kobayashi K, Mochida K, Ogura A, Kaneda H, Wakana S. A practical novel method for ensuring stable capacitation of spermatozoa from cryopreserved C57BL/6J sperm suspension. Exp. Anim. 2009;58(4):395–401. doi: 10.1538/expanim.58.395. [DOI] [PubMed] [Google Scholar]
- Takeo T, Hoshii T, Kondo Y, Toyodome H, Arima H, Yamamura K, Irie T, Nakagata N. Methyl-beta-cyclodextrin improves fertilizing ability of C57BL/6 mouse sperm after freezing and thawing by facilitating cholesterol efflux from the cells. Biol. Reprod. 2008;78(3):546–551. doi: 10.1095/biolreprod.107.065359. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Yanagimachi R. Development of normal mice from oocytes injected with freeze-dried spermatozoa. Nat. Biotechnol. 1998;16(7):639–641. doi: 10.1038/nbt0798-639. [DOI] [PubMed] [Google Scholar]
- Whittingham DG, Leibo SP, Mazur P. Survival of mouse embryos frozen to _196 degrees and _269 degrees C. Science. 1972;178(59):411–414. [PubMed] [Google Scholar]
- Wilmut I. The effect of cooling rate, warming rate, cryoprotective agent and stage of development on survival of mouse embryos during freezing and thawing. Life Sci. II. 1972;11(22):1071–1079. doi: 10.1016/0024-3205(72)90215-9. [DOI] [PubMed] [Google Scholar]