SUMMARY
Vitamin A and its metabolite, retinoic acid (RA), have recently been implicated in the regulation of immune homeostasis via the peripheral induction of regulatory T cells. Here we show that RA is also required to elicit proinflammatory CD4+ helper T cell responses to infection and mucosal vaccination. Retinoic acid receptor alpha (RARα) is the critical mediator of these effects. Strikingly, antagonism of RAR signaling and deficiency in RARα(Rara−/−) results in a cell autonomous CD4+ T cell activation defect. Altogether, these findings reveal a fundamental role for the RA/RARα axis in the development of both regulatory and inflammatory arms of adaptive immunity and establish nutritional status as a broad regulator of adaptive T cell responses.
INTRODUCTION
A growing body of literature suggests that nutrient status and metabolism can strongly influence the initiation, homing capacity and polarity of CD4+ T cell responses (Fox et al., 2005). For instance, retinoic acid (RA), a Vitamin A metabolite, was shown to upregulate the mucosal homing receptors, CCR9 and integrin alpha4beta7 (α4β7) on activated T and B lymphocytes (Iwata et al., 2004; Mora et al., 2006). We and others also previously showed that RA from dendritic cells (DC) and macrophages in the gut and associated lymphoid tissues (GALT) can synergize with transforming growth factor beta (TGF-β) to induce Foxp3 in naïve CD4+ T cells stimulated in vitro, endowing them with features that mimic thymic derived Foxp3+ T regulatory cells (Treg) (Benson et al., 2007; Coombes et al., 2007; Denning et al., 2007; Elias et al., 2008; Mucida et al., 2007; Mucida et al., 2009; Sun et al., 2007). Owing to the capacity of GALT APCs to synthesize RA, vitamin A metabolism has been posited to facilitate oral-antigen induced Treg cell induction in vivo (Coombes et al., 2007; Mucida et al., 2005; Sun et al., 2007). Conversely, RA inhibited production of T helper type-17 (TH-17) cells induced by IL-6 and TGF-β (Elias et al., 2008; Mucida et al., 2007). Reinforcing the perception that RA is anti-inflammatory, RA in concert with TGF- β was also demonstrated to inhibit the capacity of effector/memory CD4+ T cell to produce cytokine (Hill et al., 2008). Overall, the regulatory aspects of RA in vitro synchronize with the paradigm of mucosal tissues as hypo-responsive environments during steady state conditions (Elias et al., 2008; Hill et al., 2008; Maynard et al., 2009). However in the event of pathogenic exposure, overcoming these regulatory hurdles is essential, and how RA is integrated into this framework is unclear.
Vitamin A insufficiency affects approximately 250 million people worldwide and increases the likelihood of childhood mortality to common lung and gastrointestinal infection (Sommer, 2008; Underwood, 2004). Although enhanced susceptibility to such infections has been attributed, in part, to impaired epithelial barrier protection (Biesalski and Nohr, 2004; Stephensen, 2001), immunological defects may also underlie dysregulated responses to mucosal pathogens when vitamin A stores are low. In this regard, studies have suggested that vitamin A metabolites support the differentiation and functional maturation of innate immune cells (Cerwenka et al., 2000; Lawson and Berliner, 1999; Stephensen, 2001). However, it is still unclear how vitamin A metabolites contribute to T cell activation in vivo during infection and immunization.
To date, the in situ regulation of mucosal CD4+ T cell responses via retinoids during infection or inflammation has not been investigated. Gaining an understanding of the metabolites that control vitamin A dependent immunity and the relevant signaling pathways invoked is critical to resolving the paradox of why retinoids are immunosuppressive in some contexts, yet vital for host protective immunity. In this study, we demonstrate that RA is central for the induction of effector CD4+ T cell responses during infection and vaccination. Further, we show a cell intrinsic involvement of the RA receptor, RARα, in T cell activation and identify a heretofore-unappreciated role for the RA/RARα axis in driving T cell immunity. Combined with our previous findings, we propose that this axis mediates both regulatory and inflammatory circuits of the immune response.
RESULTS
Retinoic acid mediated signaling occurs during systemic inflammation
To begin to address the paradoxical effects of vitamin A metabolites in immunity, we evaluated the expression of signature homing markers influenced by retinoic acid during oral infection with Toxoplasma gondii (T. gondii), This parasite induces a strong inflammation and a robust systemic TH-1 response (Gazzinelli et al., 1992; Suzuki et al., 1988). We inoculated mice with 10 cysts of the type II avirulent T. gondii strain, ME-49, and examined CD4+ T cells, which are the dominant responding T cell subset, during the acute stage of infection. Accordingly, a large proportion of activated cells within the small intestinal lamina propria (Lp), the draining mesenteric lymph nodes (mln), and spleen (Sp) expressed the transcription factor, T-bet, which mediates interferon gamma (IFN-γ) production (Figure 1A). Expression of the mucosal homing molecules, α4β7 and CCR9, indicative of retinoic acid signaling (Iwata et al., 2004; Svensson et al., 2008), were also observed on a proportion of activated CD4+ T cells in each of these tissues (Figure 1A and data not shown). Unexpectedly, expression of these markers was mainly confined to T-bet+ cells (Figure 1A). To determine whether α4β7 induction was unique to the oral route of infection with T. gondii we inoculated animals intraperitoneally (IP), and noted again a large proportion of activated CD4+ T cells expressing α4β7, most of which coexpressed T-bet (Figure 1B). These results suggested that RA signaling is sustained and occurs systemically during inflammatory responses.
Figure 1. Vitamin A metabolite dependent signaling is sustained and systemic during T. Gondii infection.
(A) C57BL/6 mice were infected per-orally with 10 bradyzoite cysts of ME-49 clone C1. On day 8 post-infection (p.i.), single cell suspensions prepared from the spleen (Sp), mesenteric lymph nodes (mln) and small intestinal lamina propria (Lp), were stained with fluorochrome labeled antibodies and assessed for α4β7 and T-bet expression by flow cytometry. Dot plots are gated on Foxp3− CD44hi CD62Llo CD4+ T cells and representative of 3 mice per group. (B) C57BL/6 mice were infected intraperitoneally with 10 bradyzoite cysts of ME-49 clone C1. On day 8 p.i., single cell suspensions prepared from the Sp were stained and assessed as described in α4β7 and T-bet expression (C) Ctl and VAI mice were infected per-orally with T. gondii. On day 8 single cell suspensions were stained for α4β7. Bar graphs summarize the average frequency of Foxp3− CD44hi CD4+ T cells expressing α4β7, n = 3–4 mice per group. (D) Ctl and VAI mice were infected intraperitoneally with T. gondii and assessed as described in part C. n = 6–8 mice. For C and D, error bars illustrate the standard deviation (s.d.). Statistical comparisons were performed using the unpaired Student’s t test ***, P < 0.001, ns = not significant. Results are representative of 3 independent experiments.
An alternate scenario compatible with these findings is that signaling molecules, other than retinoic acid, contributed to α4β7 expression in response to infection. To address this possibility, we employed a natural model of diet-induced, vitamin A insufficiency (VAI) in which mice no longer received vitamin A in their diet starting at day 14.5 in utero (Smith et al., 1987). At 10 weeks of age, these mice, which displayed normal gut histology and thymic T cell development (data not shown), were deficient in vitamin A and its derivative metabolites as evidenced by diminished α4β7 expression on CD44hi CD4+ T cells (Figure S1). Significantly, 8 days post infection with T. gondii, α4β7 remained virtually absent on activated CD4+ T cells in VAI mice, strongly suggesting that its upregulation was strictly dependent on RA during both steady-state and inflammatory conditions (Figures 1C-1D). Thus, RA signaling is sustained during infection and exerts a systemic influence on CD4+ T cells regardless of the route of infection, an indication that vitamin A metabolism may imprint features that exceed homing potential during infection.
Mucosal and systemic CD4+ T cell immunity is impaired in the absence of vitamin A metabolites
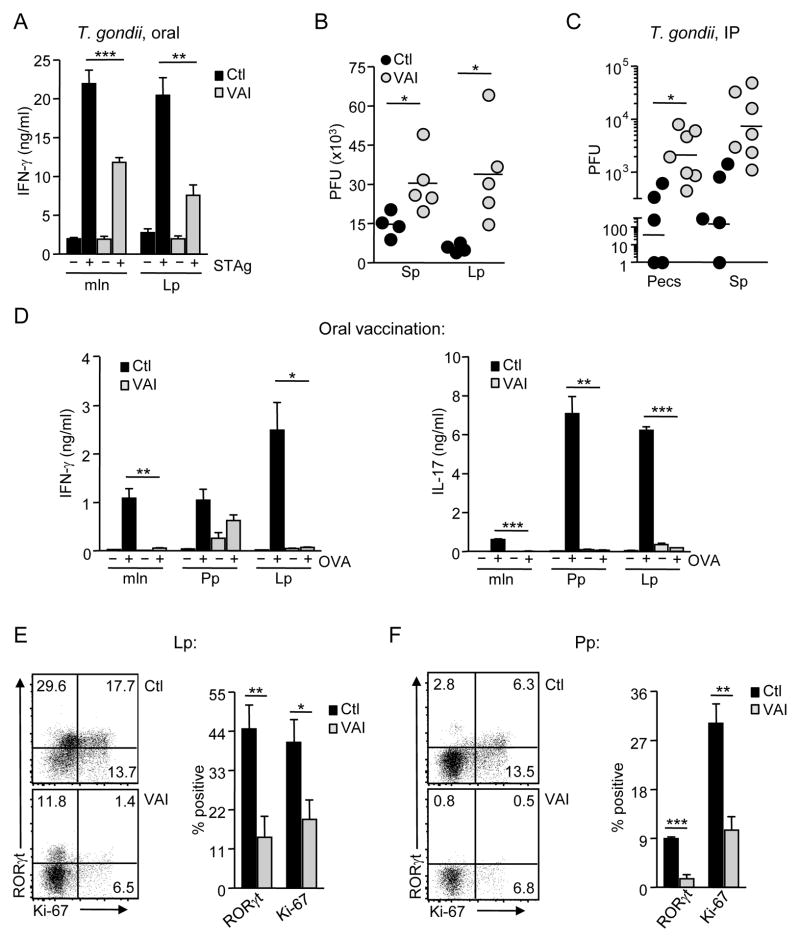
To explore the functional consequence of vitamin A insufficiency during infection, we examined the acute-phase TH-1 response in VAI mice following oral inoculation with T. gondii. To this end, we measured IFN-γ in supernatants upon in vitro restimulation of T cells with soluble T. gondii antigen (STAg). Strikingly, T cells enriched from the mln, Lp, as well as the Sp of infected VAI mice produced significantly smaller amounts of IFN-γ than their control counterparts upon recall (Figure 2A). The reduction in TH-1 responsiveness was reflected in the enhanced parasite burden observed both systemically (Sp) and in the Lp of VAI mice (Figure 2B). IP infection with T. gondii yielded similar findings, in which, CD4+ T cells removed from VAI animals and rechallenged with STAg produced lower levels of IFN-γ on a per cell basis (mean fluorescence intensity, MFI = 1800±380 in VAI versus 5300±870 for Ctl, p < 0.003). We also noted enhanced parasite burdens in the peritoneal exudates (Pecs) and Sp of these animals (Figure 2C), emphasizing that impaired TH-1 immunity to T. gondii during vitamin A insufficiency is not solely a consequence of defective responsiveness in the GALT. These data suggest a role for vitamin A metabolites in the capacity of CD4+ T cells to acquire effector function during infection.
Figure 2. TH-1 and TH-17 immune responses are impaired in the absence of vitamin A metabolites.
(A–B) Ctl and VAI mice were infected per-orally with T. gondii (A) On day 8 p.i., pooled tissue suspensions were enriched for T cells and cultured with irradiated BMDC +/− soluble T. gondii antigen (STAg) for 48 hr. IFN-γ was measured in triplicate supernatants by ELISA. n = 4–5 mice per group. (B) Parasite burdens in Sp and Lp of individual mice were determined by plaque assay. Results are expressed as plaque forming units (PFU). Each dot represents an individual mouse (C) Ctl and VAI mice were infected intraperitoneally with T. gondii. Parasite burden was assessed day 8 p.i. n = 6–8 mice per group. (D–F) Ctl and VAI mice were immunized orally with a mixture of OVA and the mutant E. coli labile toxin, LT(R129G), once per week. On day 14, pooled cell suspensions from mln, Peyer’s patches (Pp) and Lp were cultured with BMDC infected with recombinant vaccinia virus expressing OVA (iDC) for 72 hours. Supernatant triplicates were assayed for IFN-γ and IL-17 by ELISA. n = 3–4 mice per group. (E-F) Lp (E) and Pp (F) suspensions from individual mice were assessed for intracellular RORγ(t) and Ki-67 by flow cytometry. Representative dot plots from Lp and Pp of WT and VAI mice gated on viable Foxp3− CD4+ T cells are shown. Bar graphs summarize the frequency of Ki-67+ and RORγ(t)+. Error bars in A and D depict the standard error of the mean (s.e.m); error bars in E and F depict the s.d. * P < 0.05; ** P < 0.01; *** P < 0.001.
T. gondii is capable of infecting all nucleated cells and regulates adaptive immunity through multiple pathways. Therefore, we also assessed the influence of vitamin A metabolism on mucosal immunity in a non-infectious system with a model antigen. To this end, we orally vaccinated mice with a mixture of OVA and the mucosal adjuvant, LT (R129G), a non-toxic mutant of the heat-labile enterotoxin of Eschericia coli. This regimen induces a TH-1 and robust TH-17 response (Hall et al., 2008); the latter is distinguished by expression of the transcription factor, RORγ(t), in T cells and production of interlukin-17 (IL-17) (Ivanov et al., 2006). After two rounds of vaccination, we detected abundant IFN-γ and IL-17 in the supernatants of in vitro restimulated Lp and Peyer’s patch (Pp) cells from control mice, while less was detected from the mln (Figure 2D). Coincident with IL-17 protein, flow cytometric analysis revealed substantial expression of RORγ(t) in Foxp3− CD4+ T cells from the Lp (44.8±6.4%) and Pp (9.3±0.4%) (Figures 2E-2F). Yet, GALT cells from VAI mice secreted only marginal amounts of IFN-γ and IL-17 (Figure 2D). This reduction in IL-17 corresponded with diminished RORγ(t) expression in both the Lp (13.7±5.9%) and Pp (1.7±0.8%). Moreover, the frequency of CD4+ T cells expressing the nuclear proliferation antigen, Ki-67 (Gerdes et al., 1983), was significantly reduced, indicating decreased T cell activation/proliferation in response to vaccination in VAI mice (Figures 2E-2F). Collectively, these results suggest that vitamin A is critical for optimal T cell responses.
RA restores CD4+ T cell immunity in the absence of vitamin A
Impaired TH-1 and TH-17 responses in VAI mice could also emerge from a developmental defect in these animals, and not reflect a genuine role for vitamin A metabolites on TH-1 and TH-17 development (Mora et al., 2008; Ziouzenkova et al., 2007). Vitamin A metabolism produces several derivatives with signaling capacity in the host. RA, in particular, has been shown previously to induce strong effects on a variety of immune cell populations in vitro (Geissmann et al., 2003; Mora et al., 2008) and in vitamin A-replete settings in vivo (Mucida et al., 2007; Xiao et al., 2008). However, the effect of RA on T cell immunity in the absence of other vitamin A metabolites, which may exert confounding influences, has never been interrogated. To test if RA could restore protective immunity in vivo, we treated VAI mice with RA every other day. We then innoculated them orally with T. gondii on day 5 of treatment. In contrast to vehicle treated animals, T. gondii infected VAI mice that received short-term RA displayed a striking recovery in ex vivo T cell IFN-γ responses to STAg, both in the Sp and Lp (Figure 3A). Assessment of intracelular IFN-γ further revealed that Lp CD4+ T cells responded as potently as their control counterparts to antigen restimulation (Figure 3B). Importantly, this rebound culminated in functional immunity to toxoplasmosis, enhancing parasite clearance so that parasite burdens mirrored those observed in control infected animals (Figures 3C-3D). We next examined the effect of RA on TH-17 responses via oral vaccination with LT(R129G) and OVA. Significantly, Lp Foxp3− CD4+ T cells from RA treated animals regained their capacity to produce IL-17, in some instances to a degree that exceeded what was observed in control animals (Figure 3E). This capacity was coupled to a rebound in RORγ(t) expression and markedly enhanced T cell proliferation, as measured by intracellular Ki-67 (Figures 3E-3F). The efficacy of short-term treatment with RA, argues that rather than a developmental deficit causing impaired CD4+ T cell immunity in VAI mice, this metabolite provides essential signals to mediate TH-1 and TH-17 responses.
Figure 3. Retinoic acid is required for CD4+ T cell immunity.
(A–C) Ctl and VAI mice treated with RA or vehicle were infected orally with T. gondii and evaluated on day 8 p.i. n = 3 mice per group. (A) Pooled cell suspensions from Sp or Lp were enriched for T cells and cultured with irradiated BMDC +/- STAg for 48 hr. Bar graphs present the average amount of IFN-γ in duplicate or triplicate supernatants. (B) Lp samples were incubated for 14 hrs with STAg, and analyzed for intracellular IFN-γ via flow cytometry. Stacked histograms are gated on viable, Foxp3− CD4+ T cells. (C) Parasite burden in Sp and Lp of individual mice was measured by PFU. (D) Visualization of parasite localization in duodenal-jejunal sections of individual mice orally infected with T. gondii on day 9 p.i. (E–F) Ctl and VAI mice treated with RA or vehicle were immunized orally with a mixture of OVA and LT(R129G) on day 0 and 4. n = 3 mice per group. (E) On day 7, suspensions pooled from Lp were enriched for T cells and cultured with SpDC +/− OVA for 14 hr and examined for intracellular IL-17 and IFN-γ. Contour plots are gated on viable, Foxp3− CD4+ T cells. (F) Lp cells from individual mice were analyzed for intracellular RORγ(t) and Ki-67 by flow cytometry. Bar graphs depict Ki-67 and RORγt as a frequency of Foxp3− CD4+ T cells. (A-F), RA treated groups received 250μg RA intraperitoneally 5 days prior to infection or vaccine and every other day thence until takedown. Error bars in A depict the s.e.m. Error bars in C and F depict the s.d. * P < 0.05; ** P < 0.01; *** P < 0.001; ns = not significant. Results are representative of 2 (E-F) or 3 (A-D) independent experiments.
RARα regulates CD4+ T cell immunity and homeostasis
RA signals through several families of nuclear hormone receptors. The best characterized are RA receptors (RAR) α, β and γ, which transcriptionally regulate gene expression in partnership with retinoid X receptors (Chambon, 1996). Previously, TGF-β was shown to induce RARα expression (Schambach et al., 2007). Accordingly, we and others also showed that this receptor was required for RA to enhance Treg cell differentiation in vitro (Hill et al., 2008; Nolting et al., 2009). Using real time quantitative PCR, we observed that naïve CD4+ T cells had high mRNA expression of RARα, while RARγ was less expressed and RARβ was not detectable (ND) (Figure 4A). To deduce whether RARα played a role in RA directed immunity, we orally vaccinated RARα deficient (Rara−/−) mice with LT (R129G) and OVA. Unlike VAI mice, which after 16 weeks begin to succumb to a wasting disease, these animals display no overt impairments as adults (Lufkin et al., 1993). Notably, this vaccine regimen was unable to induce an antigen specific TH-17 response in mice on this mixed 129 background (data not shown) (Chapellier et al., 2002). Nevertheless, relative to WT littermates, CD4+ T cells from Rara−/− mice expressed attenuated levels of the TH-1 driving transcription factor, T-bet, after vaccination and secreted virtually no IFN-γ upon antigen recall (data not shown and Figure 4B), Thus, abrogation of RARα impaired CD4+ T cell immunity.
Figure 4. The RA/RARα signaling axis regulates CD4+ T cell immunity and homeostasis.
(A) mRNA from sort-purified naïve CD4+ T cells (Foxp3− CD25− CD44lo) was assessed for Rara, Rarb and Rarg via quantitative RT-PCR and normalized to the housekeeping gene, hypoxanthine phosphoribosyltransferase. ND = not detected. (B) Rara−/ − and littermate control WT mice were immunized orally with a mixture of OVA and the mutant E. coli labile toxin, LT(R129G), once per week. On day 21, suspensions pooled from Sp were enriched for T cells and cultured with BMDC infected with recombinant vaccinia virus expressing OVA (iDC) for 72 hours. IFN-γ was quantified in triplicate supernatants. Results are representative of 2 independent experiments. (C) Lp cell suspensions from VAI, Rara−/− and their respective control counterparts were assessed for Foxp3+ Treg via flow cytometry. Results are expressed as a proportion of viable TCRβ+ CD4+ T cells. (DE) Ctl, VAI and VAI DEREG mice immunized orally with a mixture of OVA and LT(R129G) on days 0 and 4 were treated with 1μg of diphtheria toxin 72 and 24 hrs prior to vaccination and every subsequent 48 hrs through termination of experiment. (D) On day 7, suspensions from individual mice were assessed for intracellular Foxp3 and Ki-67 by flow cytometry. Data are gated on CD4+ T cells. Each dot represents an individual mouse. (E) Suspensions pooled from the Lp were enriched for T cells and cultured with purified SpDC +/− OVA for 14 hr, then assessed for intracellular IL-17 and IFN-γ via flow cytometry. Contour plots are gated on viable Foxp3− CD4+ T cells. n = 3–4 mice per group (F) Summary of the absolute number of Foxp3+ and Foxp3− CD4+ T cells in the Lp of mice. (G) Vehicle or RA treated mice were orally infected with T. gondii or vaccinated as described in Figure 3. A summary of the frequency of Lp Treg ± s.d. as a proportion of viable CD4+ T cells is shown. n = 3 mice per group. Data are representative of 2–3 experiments. Error bars in A and B depict the s.d. ** P < 0.01; *** P < 0.001; ns = not significant.
We demonstrated previously that RAR antagonism inhibited mucosal DC-induced Treg generation in vitro (Coombes et al., 2007; Mucida et al., 2007; Sun et al., 2007). Consistent with this finding, Treg induction in response to antigen feeding was abrogated in VAI mice (Figures S3A-S3C). In spite of the inability to generate GALT Treg cells, we noted a paradoxical increase in the frequency of thymically derived Treg cells within the Lp of these mice (Figure 4C). We observed a similar increase in the frequency of Lp Treg cells in Rara−/− mice (Hill et al., 2008). However, thymic production of Treg remained normal in both of these animals (Figures S3D-S3E and data not shown). Thus, upon loss of RA or RARα mediated signaling, impaired GALT Treg generation is coupled with an aberrant increase in the frequency of thymically derived Lp Treg cells. Based on their potential to raise the threshold of immune activation (Hall et al., 2008), we assessed whether the relative increase in thymically derived Lp Treg cells contributed to impaired CD4+ T cell responses. We established VAI DEREG mice (Lahl et al., 2007), in which Treg cells could be selectively depleted upon injection of diphtheria toxin (DT). Oral vaccination in conjunction with DT treatment readily depleted Treg cells and restored Foxp3− CD4+ T cell proliferation in the Lp (Figure 4D). However, TH-1 and TH-17 cells remained undetectable in VAI mice after this treatment (Figure 4E). These data suggest that impaired immune responses upon loss of vitamin A dependent signaling are not caused by an enhanced frequency of Treg cells.
The increase in the frequency of Lp Treg cells revealed that GALT T cell homeostasis was perturbed in both Rara−/− and VAI mice. Quantification of CD4+T cell subsets indicated that in both these animals, the increase in Lp Treg frequency was due to a 2 to 4 fold selective reduction in the number of Foxp3− CD4+ T cells within the Lp (Figure 4F). Importantly, treating VAI mice with RA during T. gondii infection and vaccination restored GALT T cell equilibrium in addition to TH responses (Figure 4G). These findings demonstrate that deficiency in RARα alone can recapitulate the effects of vitamin A insufficiency on T cell homeostasis. Moreover, they identify a crucial contribution of RA/RARα signaling to vitamin A dependent CD4+ T cell homeostasis and protective immunity.
RARα regulates T cell activation
Although RA mediated enhancement of protective immunity could involve multiple cellular targets, expression of RARα in naïve CD4+ T cells, combined with impaired T helper immunity in Rara−/− mice, suggested a potential cell intrinsic requirement for this receptor in T cell responses. To address this possibility, we utilized an APC-free system and stimulated isolated naïve WT or Rara−/− CD4+ CD62Lhi T cells under TH-1 and TH-17 polarizing conditions, in vitro. After 48hrs in culture, relative to WT T cells, we detected significant reductions of IFN-γ and IL-17 in supernatants from TH-1 and TH-17 polarized RARα deficient T cells (Figure 5A). However, these cultures contained significantly fewer cells than WT cultures (data not shown), indicating that differences in cytokine levels were potentially secondary to impaired cell proliferation. Underscoring this, T cells lacking RARα failed to proliferate as efficiently as their WT counterparts upon polyclonal T cell receptor (TCR) activation with anti-CD3 (Figure 5B and Table I). This defect persisted in the presence of co-stimulation and upon exogenous provision of IL-2 (IV), suggesting that the loss of RARα may cause an activation and or survival defect. Nevertheless, the cells that had undergone proliferation were as capable as WT T cells to produce the canonical cytokines IFN-γ and IL-17 during TH-1, TH-17 polarization, respectively (Figure S3). In regard to activation, after 16 hrs of stimulation, there was less upregulation of the activation markers: CD69, CD25 and the iron transferrin receptor, CD71, on Rara−/− T cells (Figure 5C). Since the expression of CD71 was recently shown to depend on the mammalian target of rapamycin kinase (mTOR) activation (Zheng et al., 2007), an essential promoter of cell growth via protein translation (Wullschleger et al., 2006), we measured the phosphorylation of the ribosomal protein S6 (pS6), a downstream target of the mTOR complex 1 (mTORC1), as a readout for mTOR activity. In accord with decreased CD71, pS6 was significantly diminished in these cells (Figure 5D). To further evaluate the effects of RA/RARα activation on mTOR activation, we examined phosphorylation of AKT on S473, a target of the mTOR complex 2 (mTORC2) in T cells (Zhang et al.). Strikingly, we observed decreased phosphorylation of AKT S473 (Figure 5E). Together, these findings suggest that impaired immune responses in the absence of RA/RARα signals may directly affect T cell activation (Figure S3).
Figure 5. Role of RARα signaling for T cell activation.
(A) CD4+ CD62Lhi T cells purified from Sp and lymph nodes of Rara−/− and littermate WT mice were activated with plate-bound α-CD3 + soluble α-CD28, in TH-1 or TH-17 polarizing conditions for 48 hrs. IFN-γ and IL-17 ± s.e.m. in culture supernatants were measured by ELISA. ***, P < 0.001 (B) CD4+ T cells were plated for 48 hrs in the following conditions: I. unstimulated II. α-CD3 III. α-CD3 + α-CD28 IV. α-CD3 + α-CD28 + IL-2. After 48 hrs, cells were rested overnight in IL-2 and assayed for CFSE intensity by flow cytometric analysis. Histograms are gated on viable CD4+ T cells. (C-D) CD4+ CD62Lhi T cells were isolated and activated for 16 hrs. (C) Assessment of CD69, CD25, and CD71. Shaded histograms = unstimulated, black lines = stimulated with α-CD3 + α-CD28 + IL-2. Numbers indicate the mean fluorescence intensity. (D) Assessment of phosphorylated ribosomal protein S6 (pS6) in: I. unstimulated II. α-CD3 IV. α-CD3 + α-CD28 + IL-2 conditions (E) CD4+ T cells (5x106) were stimulated with plate-bound α-CD3 (2μg/ml) and/or α-CD28 (10μg/ml) for the indicated times and then lysed. Total cell lysates were immunoblotted for phosphorylated (Ser473) and total Akt.
Table I.
Impaired proliferation of Rara−/− T cells upon activation
| % (cell divided) | WT | Rara−/− | Prolif Index | WT | Rara−/− |
|---|---|---|---|---|---|
| II | 51 | 8 | II | 2.2 | 1.5 |
| III | 74.8 | 15.8 | III | 3.4 | 2.1 |
| IV | 76.4 | 15.4 | IV | 3.5 | 2.2 |
The percent of cells that underwent proliferation and calculated proliferation index are indicated.
TCR engagement results in the activation of multiple signaling pathways, which promote naïve T cell transition into effector T cells. To begin to address whether RARα imposes global effects on TCR pathways, we assessed proximal activation events after anti-CD3 crosslinking. We found that phosphorylation of the Syk family protein tyrosine kinase member, ZAP70 (Iwashima et al., 1994), and the transmembrane adaptor protein, LAT (Zhang et al., 2000), were similar in the absence of Rara compared to WT T cells (Figure 6A and data not shown), suggesting that proximal TCR signaling remained intact.
Figure 6. Loss of basal RARα signaling impairs responsiveness to TCR/CD3 engagement.
(A-C) CD4+ T cells (7x106) were stimulated with plate-bound α-CD3 (2μg/ml) for the indicated times and then lysed. (A) Total cell lysates (TCL) were immunoblotted for phosphorylated (Y493) and total ZAP-70. (B) PLC-γ1 was immunoprecipitated from TCL. Tyrosine phosphorylation was assessed by immunoblotting with 4G10. ERK1/2 activation was evaluated from TCL. (C-E) Ca2+ mobilization, cells are gated on total CD4+ T cells. (C) Rara−/− (grey line), and WT mice (black line). (D-E) Vehicle treated (black line) versus LE540 (dashed line, 2.5mM) treated cells. Histograms depict the median ratio of DAPI-A/Indo-1-A of Ca2+ fluxing cells as a function of time (seconds). (C-D) Addition of biotinylated α-CD3 (10μg/ml) (1) and crosslinking, streptavidin (20mg/ml) (2). (E) Black arrow denotes addition of ionomycin (iono).
Phosphorylation of LAT induces the recruitment and stabilization of a signaling complex comprised of multiple critical mediators of TCR signal transduction, including Phospholipase C gamma-1 (PLCγ1) (Balagopalan et al.). PLCγ1 catalyses hydrolysis of the membrane phospholipid phosphatidylinositol 4,5-biphosphate into the second messengers, inositol triphosphate (IP3), required for Ca2+ mobilization, and diacylglycerol (DAG) (Rhee, 2001). DAG contributes to activation of the Ras-Raf-ERK-1/2 pathway via the DAG sensitive guanine nucleotide exchange factor, RAS_GDP. Intriguingly, phosphorylation of PLC-γ1 was reduced in Rara−/− CD4+ T cells (Figure 6B). Consistent with this observation, we noted reduced phosphorylation of the mitogen-activated protein kinases, ERK-1/2 (Figure 6B). Although we observed variability in the degree to which the activation of these proteins was reduced between experiments, defective signaling was a common feature of Rara−/− CD4+ T cells.
In line with the impaired PLC-γ1 phosphorylation, Ca2+ mobilization was also dramatically reduced in Rara−/− CD4+ T cells relative to WT counterparts upon anti-CD3 crosslinking (Figure 6C). As RA and its metabolic precursors are constitutively present in serum (Kane et al., 2008a; Kane et al., 2008b), we could not exclude the possibility that that deficiency in Rara may result in a long-lasting metabolic defect that also impairs T cell activation. As such, we also incubated WT cells with the pan-RAR antagonist, LE540, prior to T cell activation. Treatment with this antagonist recapitulated the Rara−/− phenotype, manifesting in impaired Ca2+ mobilization upon anti-CD3 crosslinking (Figure 6D). Supporting the idea that RARα exerts a function downstream of the TCR/CD3 complex, Ca2+ mobilization was unaffected in LE540 treated cells stimulated with the Ca2+ ionophore, ionomycin (Liu and Hermann, 1978) (Figure 6E). These findings imply that transient blockade of RARα signaling is sufficient to impede signal transduction events upon TCR recognition. Together, these data reveal an unexpected role for RARα in regulating signaling pathways downstream of TCR engagement.
DISCUSSION
Although insufficient stores of vitamin A have long been linked to impaired immunity to pathogens, the role of vitamin A metabolism in the regulation of CD4+ T cell responses remains poorly understood. In our current study, we reveal that the retinoic acid/RARα signaling axis is essential for adaptive CD4+ T cell immunity. Specifically, we demonstrate that mucosal TH-1 and TH-17 responses to oral infection and vaccination are compromised upon loss of vitamin A. These impairments are unlikely to be manifestations of a developmental defect propagated upon loss of vitamin A, as RA rapidly restores mucosal TH-1 and TH-17 responses. This finding, in particular, indicates that this metabolite is the cardinal mediator of vitamin A dependent immunity in vivo. Strikingly, genetic ablation of Rara is sufficient to recapitulate the phenotype of VAI mice, both at steady-state and during infection. Furthermore, T cells lacking Rara or subjected to RA receptor antagonism display early TCR activation defects and proliferate less efficiently in response to T cell stimulation. Thus, the RA/RARα axis controls the fate of adaptive immunity, at least in part, via cell autonomous effects on CD4+ T cells and reveals one potential explanation for the broad control of this pathway over various T cell fates.
RA has been proposed to serve as a switch factor in the induction of regulatory versus inflammatory T cells. However, our data demonstrate that RA is a physiological mediator of not just GALT Treg induction, but also inflammatory TH-1 and TH-17 responses. Although we attribute these defects to a role of RA/RARα in activation, as discussed below, we do not discount the possibility that indirect effects of RA in the milieu can also affect adaptive T cell responses. Importantly, the majority of CD4+ T cells that displayed an RA signature (based on α4β7), co-expressed T-bet, the transcription factor required for TH-1 commitment (Szabo et al., 2000). Further, in vivo add-back experiments demonstrated that RA was capable of restoring TH-1 responses in VAI mice. These data are somewhat in conflict with previous reports that have suggested that RA is a negative regulator of TH-1 inflammation (Cui et al., 2000). For instance, VAI mice produced abnormally high levels of IFN-γ during infection with the nematode, Trichinella Spiralis and failed to elicit a proper and robust TH-2 response (Carman et al., 1992). In this system, RA was shown to decrease IFN-γ production when added to in vitro restimulated cell suspensions and was, hence, characterized as a suppressor of TH-1 responses (Cantorna et al., 1994). However, this type of “add-back” experiment is difficult to interpret, especially when considering that the cells treated in culture were likely of a heterogeneous activation status. In this regard, RA was shown to be able to inhibit effector/memory T cell cytokine production (Hill et al., 2008). Integrating these data into a working model suggests that RA signaling is potentially biphasic - driving T cell activation/differentiation during the early stages of an immune response, but regulating the amplitude of effector responses at later stages. This could be a particularly effective strategy to allow proper initiation of immune responses while minimizing tissue damage.
TH-17 cells are elicited via the actions of multiple cytokines, including TGF-β, and any combination of IL-6, IL-21, IL-23 and/or IL-1 (Korn et al., 2009). We found that diminished vitamin A prevented the acquisition of a robust TH-17 response in vivo. The ability of RA to restore TH-17 responses in VAI mice initially appears discordant with other studies that have reported negative effects of RA on IL-17 production in vitro (Elias et al., 2008; Mucida et al., 2007) and in certain animal models of autoimmune disease (Xiao et al., 2008). However, in systems that have scrutinized the effects of RA at low doses (Wang et al.), and in conjunction with microbial stimuli, such as TLR5 ligands (Uematsu et al., 2008), TH-17 generation was shown to be unaffected or enhanced, respectively. Thus, in physiological settings and microbial rich environments, RA can potentially amplify the inflammatory tone of the mucosal environment. In this regard RA may act in a comparable fashion to TGF-β on T cell differentiation, leading to tolerance or immunopathology depending on the presence of inflammatory stimuli (Zhou et al., 2008). Lending further support for a role of RA in the generation of TH-17, several recent studies showed that TH-17 cells were virtually ablated in the Pp and Lp of VAI mice during steady-state (Cha et al.; Wang et al.). In one of these studies, the authors attributed this finding to impeded migration of these cells into the gut; yet, TH-17 cells were not increased elsewhere (Wang et al.). In another study, the deficit in TH-17 was noted in young mice on a vitamin A deficient diet, before the defect in T cell homing capacity to mucosal sites should have set in (Cha et al.). Taken together, physiological concentrations of retinoids appear to sustain TH-17 development and maintenance.
Rara−/− mice complemented VAI mice on multiple levels. First, the number of Lp effector T cells was reduced in both animals compared to their control counterparts (~3 fold for VAI; ~2 fold for Rara−/−). These findings suggest that during homeostatic activation, RA/RARα signaling is critical for the upregulation of homing receptors. Remarkably, transient treatment with RA restored both T cell equilibrium and the CD4+ T cell response within the Lp of VAI mice upon challenge. Since RA restored both of these parameters, it is difficult to dissect the contribution of homing versus activation to the rescue of immune responses in this tissue. However, despite defects in gastrointestinal homing, VAI mice that were infected systemically with T. gondii also mounted a markedly impaired TH-1 response. This outcome could be the product of both direct and indirect actions of RA/RARα signaling on T lymphocytes.
As evidence that this pathway can function directly through T cells, we demonstrate that RARα mediates signal transduction events downstream of the TCR that govern T cell activation. Recent findings indicate a role for nutrient metabolism in T cell activation. For instance, vitamin D/vitamin D receptor (VDR) signaling was shown to promote the proliferation of human T cells in response to TCR stimulation via the induction of PLCγ (von Essen et al.). The DNA binding capacity of VDR presumably mediates this induction via transcription. RARα is also recognized to regulate gene expression in the same fashion (Chambon, 1996). As RA, as well as other retinoids, are present in the serum and tissues of mice (Kane et al., 2008a; Kane et al., 2008b), it is intriguing to speculate that these compounds exert constitutive effects on the phosphorylation status, localization and/or conformation of RARα in T cells, which may in turn regulate proteins involved in T cell signal transduction pathways. Short-term incubation (< 1 hr) with a pan-RAR antagonist impaired T cell Ca2+ mobilization in a manner similar to that observed in Rara−/− T cells. One explanation for this finding is that transcriptional modification via RARα regulates the expression or activity of a mediator of T cell activation. Alternatively, RARα may potentially facilitate TCR dependent signal transduction through extranuclear activity. For example, in a neuroblastoma cell line, RARα was described to interact with the p85 subunit of phosphoinositide 3-kinsase in an RA dependent manner (Masia et al., 2007).
Although we identify a novel function for RA/RARα signaling in T cells, RARα may also affect the function of APC, including DC. For instance, RARα ligands were shown to synergize with inflammatory mediators to enhance the activation of human Langerhans cell-type dendritic cells (Geissmann et al., 2003). Thus, it is possible that altered APC function also contributes to impaired adaptive immune responses in VAI and Rara−/− mice. In this regard, we noted a specific defect in the capacity of LpDC from VAI mice to produce IL-6, while other proinflammatory mediators, such as TNF-α and IL-12/23p40 remained intact (Figure S4). This finding is consistent with the reduction of TH-17 cells in VAI mice during steady-state conditions, as previously reported (Cha et al.; Wang et al.), and in response to oral vaccination, as reported in our study. RA signaling may also influence other cell types that can shape T cell responses. Recently, RA was revealed to promote TGF-β activation in non-hematopoietic follicular DC (Suzuki et al.). This effect may extend to other cell types of stromal/mesenchymal origin as well as DC and potentially contributes further to the concomitant loss of both Foxp3+ Treg and TH-17 induction in VAI mice. Thus, RA/RARα signaling may converge on both innate and adaptive arms of immunity.
In summary, the GI tract must be able to tolerate constant exposure to food antigen and commensals, while maintaining the capacity to rapidly respond to encounters with pathogen. These conflicting pressures confront the host immune system defending the GI tract with a unique challenge. One would predict that the most judicious strategy to respond to this spectrum of recurring challenges would involve a conserved pathway that can readily adjust to environmental cues. Here we identify the RA/RARα signaling pathway as fitting this mode of host control, promoting Treg generation and likely tolerance during steady-state conditions, while adaptive T cell responses in the face of pathogen. As such, we propose that RA regulates adaptive immunity in a manner that is symmetrical to TGF-β where accompanying signals dictate whether a response ultimately becomes regulatory in nature or inflammatory. An important consideration is that adaptive immune responses often involve multiple waves of antigen presenting cell recruitment. Based on the systemic RA mediated signals that we observe during infection, it will be interesting to examine how newly recruited APC contribute to the RA/RARα signaling axis during inflammation. Finally, the requirement of RARα for T cell activation suggests that this pathway may have evolved early with the development of adaptive CD4+ T cell responses to coordinate host protection.
METHODS
Mice
B6.SJL (CD45.1) and OTII mice were purchased from Taconic Farms. Foxp3eGFP and DEREG (Lahl et al., 2007) mice were bred in house. Rara−/− mice were a generous Gift of Dr Pierre Chambon (Chapellier et al., 2002) and obtained from Christophe Benoist (Harvard Medical School). All mice were bred and maintained under pathogen-free conditions at an American Association for the Accreditation of Laboratory Animal Care accredited animal facility at the NIAID and housed in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals under an animal study proposal approved by the NIAID Animal Care and Use Committee. For each experiment, mice were gender, aged and/or littermate matched. All mice were used between 8 and 13 weeks of age.
Diet Studies
Vitamin A deficient (TD.09838) and sufficient (20,000 IU vitamin A/kg, TD.09839) diets were purchased from Harlan Teklad Diets. At day 14.5 of gestation, pregnant females were administered either Vitamin A deficient or sufficient diet and maintained on diet until weaning of litter. Upon weaning, females were returned to standard Harlan chow, while weanlings were maintained on special diet until use. Diet was replaced in feed hopper every 3–4 days to prevent degradation. For breeding, females were rested on a standard Harlan chow diet for at least 2 weeks prior to re-mating. After 3 birth cycles under the vitamin A deficient diet, females were retired.
Tissue preparation
Cells from Sp, mln, and Lp were prepared as described previously(Sun et al., 2007), with the following modifications for Lp digestion: Minced tissue was further digested with serum free RPMI 1640 containing: 25mM HEPES, 50 μM β-mercaptoethanol, 100 μg/ml liberase TI (Roche) and 500 μg/ml DNase I (Sigma-Aldrich) - by continuous stirring at 37°C for 28 min. Digested tissue was then immediately diluted in RPMI-1640 medium containing: 3% FCS, 100μg/ml Penn/Strep, 25 mM HEPES and 50 mM β-mercaptoethanol, and serially mashed through 70- and 40-μm cell strainers (BD Biosciences). Cell suspensions were spun down and resuspended in complete RPMI-1640 medium containing: 10% FBS, 100μg/ml Penn/Strep, 25mM HEPES, 2mM L-glutamine, 1 mM Na Pyruvate (cellgro), 1X MEM nonessential amino acids (MEM) (cellgro) and 50 mM of β-ME – prior to use. Pp were treated with RPMI-1640 medium containing: 3% FCS, 100μg/ml Penn/Strep, 25 mM HEPES, 50 mM β-ME, 5mM EDTA, and 145 mg/ml of DTT for 20 min in an incubator at 37ºC/5% CO2. They were then washed up-and-down several times with a 1ml pipette, incubated in serum free RPMI-1640 containing: 25mM HEPES, 50 mM β-ME, liberase CI (100 mg/ml) or liberase Tl (50 mg/ml) and 150 mg/ml DNase I, minced, and digested for 20 min prior to serially smashing through 70- and 40-μm cell strainers.
Flow cytometry
Single-cell suspensions were incubated in ice-cold HBSS with 1/100 α-mouse CD16/32 (eBioscience), 0.2mg/ml purified Rat IgG (Jackson Immunoresearch) and stained with fluorochrome-conjugated antibodies against any combination of the following surface antigens: TCR-β (H57-597), CD4 (RM4-5), CD8α (53-6.7), CD25 (PC61.5), CD44 (IM7), CD62L (MEL-14), CD103 (2E7) and α4β7 (DATK32) – for 15min on ice. All antibodies were purchased from eBioscience.
T cell phenotype
Cell suspensions were stained with 1/500 LIVE/DEAD Fixable Blue Dead cell stain kit (Invitrogen) in tandem to exclude dead cells. For examination of transcription factors and cellular proliferation, cells were subsequently treated with the Foxp3 staining kit (eBioscience) in accord with the manufacturer’s instructions and stained for 30 min on ice with with fluorochrome-conjugated antibodies against: Ki-67 (B56, BD Pharmingen), Foxp3 (FJK-16s), T-bet (eBio4B10) and/or RORγ(t) (AFKJS-9) or isotype controls: mouse IgG1 (BD Pharmingen) rat IgG2a (eBR2a), mouse IgG1 (clone P3) - in 1/100 α-mouse CD16/32 and 0.2mg/ml purified Rat IgG. Unless indicated, antibodies were purchased from eBioscience. All cell acquisition was performed using an LSRII machine with FACSDiVa software (BD Biosciences). Data were analyzed using FlowJo software (TreeStar). To calculate absolute numbers, the fraction of a particular subset to singlet-gated, total living cells was multiplied by the total cellularity of the tissue based on trypan blue exclusion.
CFSE labeling of T cells
Cells were labeled at a final density of 1x107cell/ml in 1μM CFSE (Vybrant™ CFDA SE Cell Tracer Kit, Invitrogen) dissolved in HBSS (Mediatech Inc.) for 7 min at 37°C. Labeling was quenched by washing cells 2X in complete RPMI supplemented with 30% FBS. Cells were resuspended in ice-cold PBS for transfer in in vivo experiments or complete RPMI for in vitro assays.
Parasite and Infection Protocol
The parental ME-49 type II strain (ATCC no. 50840) (American Type Culture Collection, Manassas, VA, USA) of T. gondii was electroporated with RFP and selected for red fluorescence. ME-49 clone C1 was established and passed through mice. To obtain tissue cysts, brains were removed from C57BL/6 mice that were inoculated with three cysts by gavage 1–2 months prior and homogenized in 1 ml of phosphate buffer saline (pH 7.2). Cysts were counted on the basis of 2 aliquots of 20 μl. Diet study mice were infected orally with 10 cysts at 9.5–11.5 wks of age. STAg was prepared as previously described (Grunvald et al., 1996).
Parasite Burden
Human fibroblast (Hs27; ATCC no. CRL-1634) cultures were used to quantify parasite burden as described previously (Pfefferkorn and Pfefferkorn, 1976) and (Roos et al., 1994). In brief, titrations of single-cell tissue suspensions (1x104 up to 1x106 cells) were added onto confluent fibroblast monolayers cultured in DMEM supplemented with 100μg/ml Penn/Strep and 10% FBS (in 24-well plates). Plaques were detected by fluorescence using an Axiovert 40 inverted microscope (Zeiss) outfitted with an RFP filter. Titration results are reported in Plaque Forming Units (PFUs).
Vaccine Protocol
For vaccination, mice were orally inoculated with an isotonic bicarbonate buffer. 10min later, mice were gavaged with a mixture of 1mg of OVA and 20 μg of the mutant form of E. coli LT (R129G) prepared in the same buffer. For reconstitution experiments with RA, mice were vaccinated again on day 4. Immune responses were then assessed on day 8. For all other experiments, mice were vaccinated once per week and immune responses were assessed one week post-challenge.
In vivo RA reconstitution
250μg of all-trans-RA (Sigma Aldrich), resuspended in 30μl of Biotechnology Performance Certified DMSO (Sigma Aldrich) was administered intraperitoneally to vitamin A insufficient mice every other day. 24hrs after the 3rd injection, mice were infected with T. gondii or vaccinated. Injection of RA continued until takedown of the mice. Mice not receiving RA, received DMSO vehicle instead. RA was stored at -80°C in pure DMSO in amber ependorf tubes. Aliquots were one use only.
Intracellular cytokine staining
Tissues were harvested from infected or vaccinated mice, pooled by group, and enriched for T cells by incubating with CD90.2 selecting magnetic beads (Miltenyi) in accord with the manufacturer’s protocol. T cells were obtained by running the labeled suspension through an Automacs (Miltenyi) on the program Posseld2. For T. gondii, T cells (2.5x105) were incubated with irradiated BMDCs (5x104) ± STAg (5μg/ml). For vaccine, T cells (2.5x105) were incubated with SpDC (5x104) ± OVA (100μg/ml) (Worthington). Cells were cultured for 14 hrs at 37°C, 5% CO2 in 250μl/well in 96-well flat bottom plates. Brefeldin A (GolgiPlug, BD Biosciences) was added for the final 7 hrs of culture. Cells were washed with FACS buffer and stained with 1/500 LIVE/DEAD Fixable Blue Dead cell stain kit in HBSS on ice for 15min. After washing with FACS buffer, cells were fixed with 1.6% paraformaldehyde (Electron Microscopy Sciences) for 20 min at room temp. For intracellular cytokine staining, cells were stained with fluorochrome-conjugated antibodies against TCR-β, CD4, CD8α , IFN-γ (XMG1.2), IL-17A (eBio17B7), Foxp3, T-bet, or isotype controls: rat IgG1, rat IgG2a, and mouse IgG1 (clone P3) in the presence of CD16/32 and 0.2mg/ml purified Rat IgG for 45 min on ice in FACS buffer containing 0.5% saponin (SigmaAldrich).
ELISA
Tissues were harvested from infected or vaccinated mice, pooled by group, and enriched for T cells as described above. For T. gondii, T cells (2.5x105) were incubated with irradiated day-6 BMDC (5x104) ± STAg (5μg/ml) in 250μl/well in 96-well round bottom plates for 48 hrs at 37°C, 5% CO2. For vaccine, T cells (2.5x105) were incubated with day-7 BMDC (5x104) that had been infected or not for 12hrs with recombinant vaccinia virus expressing OVA (MOI 10:1) for 72hrs at 37°C, 5% CO2. Upon harvest of supernatants, IFN-γ and IL-17A was quantitated with the DuoSet ELISA system (R&D Systems). In initial vaccine experiments, pooled suspensions were not T cell enriched, but rather cultured at 5x105 cells to 1x105 infected or uninfected BMDC.
Real-time quantitative PCR
Naïve CD4+ T cells were flow cytometrically purified from splenic and peripheral LN of 2 mice/assay. RNA was then extracted with RNAeasy columns (QIAGEN) and analyzed by quantitative RT-PCR according to the manufacturer's instructions using primers for murine Rara, Rarb, and Rarg (QIAGEN).
CD4+ T cell activation/ polarization assays
Cells were extracted from the Sp and secondary LNs of RARα−/− or littermate WT mice and enriched for CD4+ CD62Lhi T cells using the CD4+ CD62Lhi T cell isolation kit II from Miltenyi in accord with the manufacturer’s protocol. Cells were then CFSE labeled as described above, plated in complete media at 3.38x105 cells/well into 96-well flat bottom plates, and stimulated with 1μg/ml of plate-bound αCD3ε (BD Pharmingen, 145-2C11, coated over night in 100μl at 4°C) + soluble αCD28 (BD Pharmingen, 37.51) + 50U of recombinant hIL-2 (Peprotech). To assess activation, cells were harvested after 18hrs and stained for CD4, CD25, CD69 (H1.2F3), and CD71 (R17217) in HBSS containing 1/500 of the LIVE/DEAD Fixable Blue Dead cell stain kit, 1/100 CD16/32, and 0.2mg/ml purified Rat IgG for 15 min on ice. Expression of markers was compared to unstimulated cells. To assess polarization, cells were further cultured in TH-1 or TH-17 (no hIL-2) biased conditions, which included: purified α-IL-4 (11B11) + 10ng/ml of recombinant mIL-12; or purified α-IL-4 + α-IFN-γ (R4-6A2) + 20ng/ml recombinant mIL-6 + 0.75ng/ml recombinant hTGF-β, respectively. All cytokines, except hIL-2, were purchased from R&D Systems. 48hrs post-activation, half the supernatant from each well was removed and measured for the cytokines (IFN-γ and IL-17) by ELISA. Complete media, including polarizing cytokines + blocking antibodies were replenished as cells were removed from αCD3ε and transferred into 96-well round bottom plates for 24hrs. CFSE proliferation was then measured.
Ca2+ mobilization assay
Ca2+ flux measurements were performed as previously described (Laky and Fowlkes, 2007). Briefly, cells pooled from PLN and Sp of WT and RARa−/− mice were resuspended in loading buffer (HBSS with CaCl2 and MgSO4, 10mM HEPES and 1%FBS) at a density of 1x107 cells/ml in 3mM Probenecid (Invitrogen). 1ml of cells was then labeled with 9ml of indo mix containing (50ml of 1mM indo-1 AM + 113ml of FBS + 25 ml of 20% pluronic (Invitrogen)) at 30°C for 40 min, then stained with cell surface markers. In a separate condition, LE540 (2.5μM) or DMSO was added to WT cells during these steps.
Immunoblotting and Reagents
Cells were lysed in PBS containing 1% Triton X-100 and 0.05% SDS with protease and phosphatase inhbitors. Lysates were immunoprecipitated with the indicated antibodies and immune complexes captured with protein A (Santa Cruz). Protein complexes were resolved on SDS-PAGE gels and transferred to nitrocellulose membranes for immunoblot analysis. Lysates were immunoblotted with the following antibodies: anti-phosphotyrosine (4G10) and anti-PLC-γ (Millipore), anti-phospho ERK1/2, anti-ERK, anti-phosphoSer473Akt, anti-Akt, anti-phosphoTyr493 ZAP-70 and anti-ZAP-70 (Cell Signaling Technology).
Statistical Analysis
Groups were compared with Prism software (GraphPad) using the unpaired or paired Student's t test.
Supplementary Material
HIGHLIGHTS.
TH-1 and TH-17 immunity are abrogated in the absence of vitamin A metabolites
Retinoic acid rescues TH-1 and TH-17 immune responses in the absence of vitamin A
RA receptor alpha is a dominant mediator of CD4+ T cell immunity and homeostasis
RA receptor driven signals influence TCR signaling pathways
Acknowledgments
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health and the Office of Dietary Supplements. We thank Dr. Kevin Holmes, David Stephany, and the NIAID sorting facility. We thank Kim Beacht and the Comparative Medicine Branch animal for animal handling and technical assistance. We thank Drs. Karen Laky and David Hildeman for experimental advice. Finally, we thank Drs. Remy Bosselut and Alan Sher for critical reading of the manuscript.
Abbreviations used
- TH
T helper
- TH-1
type 1
- TH-17
type 17
- RA
retinoic acid
- RAR
retinoic acid receptor
- DC
dendritic cell
- Lp
lamina propria
- RALDH
retinal dehydrogenase enzyme
- VAI
vitamin A insufficient
- Treg
Foxp3+ regulatory T cells
- GALT
gut-associated lymphoid tissue
- TGF-β
transforming growth factor beta
- OVA
ovalbumin
- IFN-γ
interferon gamma
- T. gondii
Toxoplasma gondii
- mln
mesenteric lymph nodes
- Sp
spleen
- IL-17
interlukin-17
- Pp
Peyer’s patch
- DT
diphtheria toxin
- mTOR
mammalian target of rapamycin kinase
- mTORC1
mTOR complex 1
- mTORC2
mTOR complex 2
- PLCγ1
phospholipase C gamma-1
References
- Balagopalan L, Coussens NP, Sherman E, Samelson LE, Sommers CL. The LAT story: a tale of cooperativity, coordination, and choreography. Cold Spring Harb Perspect Biol. 2:a005512. doi: 10.1101/cshperspect.a005512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesalski HK, Nohr D. New aspects in vitamin a metabolism: the role of retinyl esters as systemic and local sources for retinol in mucous epithelia. J Nutr. 2004;134:3453S–3457S. doi: 10.1093/jn/134.12.3453S. [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Nashold FE, Hayes CE. In vitamin A deficiency multiple mechanisms establish a regulatory T helper cell imbalance with excess Th1 and insufficient Th2 function. J Immunol. 1994;152:1515–1522. [PubMed] [Google Scholar]
- Carman JA, Pond L, Nashold F, Wassom DL, Hayes CE. Immunity to Trichinella spiralis infection in vitamin A-deficient mice. J Exp Med. 1992;175:111–120. doi: 10.1084/jem.175.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerwenka A, Bakker AB, McClanahan T, Wagner J, Wu J, Phillips JH, Lanier LL. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12:721–727. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- Cha HR, Chang SY, Chang JH, Kim JO, Yang JY, Kim CH, Kweon MN. Downregulation of Th17 cells in the small intestine by disruption of gut flora in the absence of retinoic acid. J Immunol. 184:6799–6806. doi: 10.4049/jimmunol.0902944. [DOI] [PubMed] [Google Scholar]
- Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- Chapellier B, Mark M, Garnier JM, LeMeur M, Chambon P, Ghyselinck NB. A conditional floxed (loxP-flanked) allele for the retinoic acid receptor alpha (RARalpha) gene. Genesis. 2002;32:87–90. doi: 10.1002/gene.10071. [DOI] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-{beta}- and retinoic acid-dependent mechanism. J Exp Med. 2007 doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui D, Moldoveanu Z, Stephensen CB. High-level dietary vitamin A enhances T-helper type 2 cytokine production and secretory immunoglobulin A response to influenza A virus infection in BALB/c mice. J Nutr. 2000;130:1132–1139. doi: 10.1093/jn/130.5.1132. [DOI] [PubMed] [Google Scholar]
- Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, O'Shea JJ. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111:1013–1020. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992;149:175–180. [PubMed] [Google Scholar]
- Geissmann F, Revy P, Brousse N, Lepelletier Y, Folli C, Durandy A, Chambon P, Dy M. Retinoids regulate survival and antigen presentation by immature dendritic cells. J Exp Med. 2003;198:623–634. doi: 10.1084/jem.20030390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Grunvald E, Chiaramonte M, Hieny S, Wysocka M, Trinchieri G, Vogel SN, Gazzinelli RT, Sher A. Biochemical characterization and protein kinase C dependency of monokine-inducing activities of Toxoplasma gondii. Infect Immun. 1996;64:2010–2018. doi: 10.1128/iai.64.6.2010-2018.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JA, Bouladoux N, Sun C, Wohlfert EA, Blank RB, Zhu Q, Grigg ME, Berzofsky JA, Belkaid Y. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008 doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Iwashima M, Irving BA, van Oers NS, Chan AC, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994;263:1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Kane MA, Folias AE, Napoli JL. HPLC/UV quantitation of retinal, retinol, and retinyl esters in serum and tissues. Anal Biochem. 2008a;378:71–79. doi: 10.1016/j.ab.2008.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MA, Folias AE, Wang C, Napoli JL. Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectrometry. Anal Chem. 2008b;80:1702–1708. doi: 10.1021/ac702030f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, Hamann A, Wagner H, Huehn J, Sparwasser T. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laky K, Fowlkes BJ. Presenilins regulate alphabeta T cell development by modulating TCR signaling. J Exp Med. 2007;204:2115–2129. doi: 10.1084/jem.20070550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson ND, Berliner N. Neutrophil maturation and the role of retinoic acid. Exp Hematol. 1999;27:1355–1367. doi: 10.1016/s0301-472x(99)00085-5. [DOI] [PubMed] [Google Scholar]
- Liu C, Hermann TE. Characterization of ionomycin as a calcium ionophore. J Biol Chem. 1978;253:5892–5894. [PubMed] [Google Scholar]
- Lufkin T, Lohnes D, Mark M, Dierich A, Gorry P, Gaub MP, LeMeur M, Chambon P. High postnatal lethality and testis degeneration in retinoic acid receptor alpha mutant mice. Proc Natl Acad Sci U S A. 1993;90:7225–7229. doi: 10.1073/pnas.90.15.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masia S, Alvarez S, de Lera AR, Barettino D. Rapid, nongenomic actions of retinoic acid on phosphatidylinositol-3-kinase signaling pathway mediated by the retinoic acid receptor. Mol Endocrinol. 2007;21:2391–2402. doi: 10.1210/me.2007-0062. [DOI] [PubMed] [Google Scholar]
- Maynard CL, Hatton RD, Helms WS, Oliver JR, Stephensen CB, Weaver CT. Contrasting roles for all-trans retinoic acid in TGF-beta-mediated induction of Foxp3 and Il10 genes in developing regulatory T cells. J Exp Med. 2009;206:343–357. doi: 10.1084/jem.20080950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008 doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- Mucida D, Pino-Lagos K, Kim G, Nowak E, Benson MJ, Kronenberg M, Noelle RJ, Cheroutre H. Retinoic acid can directly promote TGF-beta-mediated Foxp3(+) Treg cell conversion of naive T cells. Immunity. 2009;30:471–472. doi: 10.1016/j.immuni.2009.03.008. author reply 472–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolting J, Daniel C, Reuter S, Stuelten C, Li P, Sucov H, Kim BG, Letterio JJ, Kretschmer K, Kim HJ, von Boehmer H. Retinoic acid can enhance conversion of naive into regulatory T cells independently of secreted cytokines. J Exp Med. 2009;206:2131–2139. doi: 10.1084/jem.20090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn ER, Pfefferkorn LC. Toxoplasma gondii: isolation and preliminary characterization of temperature-sensitive mutants. Exp Parasitol. 1976;39:365–376. doi: 10.1016/0014-4894(76)90040-0. [DOI] [PubMed] [Google Scholar]
- Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos DS, Donald RG, Morrissette NS, Moulton AL. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 1994;45:27–63. doi: 10.1016/s0091-679x(08)61845-2. [DOI] [PubMed] [Google Scholar]
- Schambach F, Schupp M, Lazar MA, Reiner SL. Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur J Immunol. 2007;37:2396–2399. doi: 10.1002/eji.200737621. [DOI] [PubMed] [Google Scholar]
- Smith SM, Levy NS, Hayes CE. Impaired immunity in vitamin A-deficient mice. J Nutr. 1987;117:857–865. doi: 10.1093/jn/117.5.857. [DOI] [PubMed] [Google Scholar]
- Sommer A. Vitamin a deficiency and clinical disease: an historical overview. J Nutr. 2008;138:1835–1839. doi: 10.1093/jn/138.10.1835. [DOI] [PubMed] [Google Scholar]
- Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007 doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Maruya M, Kawamoto S, Sitnik K, Kitamura H, Agace WW, Fagarasan S. The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut. Immunity. 33:71–83. doi: 10.1016/j.immuni.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- Svensson M, Johansson-Lindbom B, Zapata F, Jaensson E, Austenaa LM, Blomhoff R, Agace WW. Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells. Mucosal Immunol. 2008;1:38–48. doi: 10.1038/mi.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- Underwood BA. Vitamin A deficiency disorders: international efforts to control a preventable “pox”. J Nutr. 2004;134:231S–236S. doi: 10.1093/jn/134.1.231S. [DOI] [PubMed] [Google Scholar]
- von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol. 11:344–349. doi: 10.1038/ni.1851. [DOI] [PubMed] [Google Scholar]
- Wang C, Kang SG, Hogenesch H, Love PE, Kim CH. Retinoic Acid Determines the Precise Tissue Tropism of Inflammatory Th17 Cells in the Intestine. J Immunol. doi: 10.4049/jimmunol.0903942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008;181:2277–2284. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Readinger JA, Dubois W, Janka-Junttila M, Robinson R, Pruitt M, Bliskovsky V, Wu JZ, Sakakibara K, Patel J, et al. Constitutive reductions in mTOR alter cell size, immune cell development, and antibody production. Blood. doi: 10.1182/blood-2010-05-287821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Trible RP, Zhu M, Liu SK, McGlade CJ, Samelson LE. Association of Grb2, Gads, and phospholipase C-gamma 1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell angigen receptor-mediated signaling. J Biol Chem. 2000;275:23355–23361. doi: 10.1074/jbc.M000404200. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Collins SL, Lutz MA, Allen AN, Kole TP, Zarek PE, Powell JD. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J Immunol. 2007;178:2163–2170. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziouzenkova O, Orasanu G, Sharlach M, Akiyama TE, Berger JP, Viereck J, Hamilton JA, Tang G, Dolnikowski GG, Vogel S, et al. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat Med. 2007;13:695–702. doi: 10.1038/nm1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.