Abstract
Purpose
With the rising incidence of melanoma, more patients are undergoing surveillance for disease recurrence. Our purpose was to study levels of proteins that might be secreted in the blood of patients with metastatic melanoma that can be used for monitoring these individuals.
Methods
Genome-wide gene expression data were used to identify abundantly expressed genes in melanoma cells that encode for proteins likely to be present in the blood of cancer patients based on high expression levels in tumors. ELISA assays were employed to measure proteins in plasma of 216 individuals; 108 metastatic melanoma patients and 108 age- and gender-matched patients with resected stage I/II disease split into equal-sized training and test cohorts.
Results
Levels of seven markers, CEACAM, ICAM-1, osteopontin, MIA, GDF-15, TIMP-1 and S100B, were higher in patients with unresected stage IV disease than in patients with resected stage I/II disease. 81% of the stage I/II patients in the training set had no marker elevation, whereas 69% of the stage IV patients had elevation of at least one marker (p<0.0001). Receiver operating characteristic curves for the markers in combination in these two patient populations had an AUC of 0.79 in the training set, 0.8 in the test set. A CART model developed in the training set further improved the AUC in the test set to 0.898.
Conclusions
Plasma markers, particularly when assessed in combination, can be used to monitor patients for disease recurrence and can compliment currently used LDH and imaging studies; prospective validation is warranted.
Introduction
Melanoma is the 5th most common cancer in men and the 7th in women, and comprises 4% of all cancers in the United States 1. It is estimated that 68,700 people in the USA were diagnosed with melanoma in 2009, of which 8,650 will have died 1. The mortality rate from melanoma has risen as well, albeit less so, possibly reflecting earlier detection and improved surgical excision 2. The rate of metastatic relapse among patients with early stage melanoma varies depending on key prognostic factors, which make up the current AJCC system; Breslow depth, presence of ulceration, mitotic rate and lymph node involvement 3. The likelihood of death from stage I-III melanoma is between 10% and 80%, depending on these prognostic variables. For example, a patient with stage IA disease has a 10% chance of death from melanoma at 15 years, a patient with stage IIA disease has a 30-40% chance of death from metastatic melanoma, and a patient with stage IIIC disease has approximately a 70% chance of death from melanoma 3. Within each staging group, we have no means of determining who will relapse, who will remain disease-free, and when they will relapse; therefore patients are monitored for recurrence by physical examinations, blood tests and imaging studies.
There is no clear consensus regarding selection and timing of laboratory and imaging studies when following patients with resected melanoma. The National Comprehensive Cancer Network (NCCN) recommends imaging for stage I patients only if they have symptoms. For stage II patients, a chest X-ray is optional and for stage IIB and IIC and III, CT scans, PET scans and MRI are recommended as clinically indicated (www.NCCN.org); however, these indications are not defined. Other imaging modalities that have been recommended for staging, but are not necessarily widely used, include PET scans and ultrasound to assess lymph node involvement 4-5. The frequency of laboratory tests and physical examinations is similarly controversial. For stage II disease the NCCN recommends an LDH (lactic dehydrogenase) and CBC (complete blood count) every 6-12 months, and a history and physical are recommended every 3-12 months. Surveillance patterns adopted in other countries are also highly variable. For example, the United Kingdom guidelines for surveillance of stage IIB, IIC or III melanoma include chest X-ray, liver ultrasound or CT scan of the chest, abdomen and pelvis, LDH, liver function tests and CBC at baseline and frequent clinical follow-up thereafter, but do not recommend subsequent imaging 6. The consensus-based German guidelines recommend lymph node sonography and serum S100β as part of the routine surveillance every 3-6 months for thicker primary melanomas, and if these studies detect regional lymph node involvement, whole body imaging is recommended 4. The European Society of Medical Oncology has specific guidelines for frequency of clinical examinations, but has no consensus regarding blood tests or imaging techniques 7. The Sydney Melanoma Group recommends scheduled follow-up, but do not make recommendations on blood tests or imaging 8.
The variability in recommendations likely reflects financial considerations and medical environment 9. Costs can be quite considerable, particularly when imaging studies are included. Given the rapidly increasing rate of diagnosis of primary melanoma, routine surveillance with imaging studies is becoming more costly to society, and cheaper blood tests, which are both sensitive and specific, to screen patients for early detection of metastatic disease can reduce the frequency of imaging studies.
In other malignancies, tumor markers have been shown to be useful in surveillance of patients at risk for metastatic relapse, and might be used instead of periodic imaging in asymptomatic patients. This practice is widely incorporated in the management of resected colon, prostate, liver, ovarian, testicular cancer and other malignancies. In melanoma, serum LDH is the only blood-based biomarker in clinical use, and elevated serum LDH occasionally leads to imaging studies that reveal metastatic relapse 10. However, the sensitivity and specificity for LDH as a predictor of metastatic relapse is low; in a study in which 373 patients were followed for metastatic relapse, LDH was the sole indicator of recurrence in only one patient 10, and additional blood based biomarkers are clearly needed to supplement history, clinical exam, routine blood work and serial chest X-rays in this setting.
A number of blood-based biomarkers have been studied that have been shown to be associated with disease burden and/or prognosis. For example, blood levels of VEGF-R3, endostatin, VEGF and bFGF have been shown to be elevated in patients with metastatic melanoma compared with healthy controls 11-12. Elevated levels of serum S100β are associated with disease relapse, presumably due to shedding of S100β by tumor cells 13. Secretory CSE1L/CAS levels are elevated in patients with metastatic melanoma compared with healthy individuals 14, and numerous other published studies report elevation of levels of individual secreted proteins in the blood of patients with metastatic melanoma. We therefore embarked on a study to assess plasma levels of a number of proteins abundantly expressed in melanoma cells with the goal of developing a sensitive multiplex assay that can be used to monitor patients with resected melanoma at risk for disease relapse.
Materials and Methods
Patient cohorts
One hundred and eight patients with metastatic melanoma with at least 2 cm measurable disease at the time of blood draw were included in the study. Plasma was taken from 108 patients with stage I/II resected melanoma for comparison, matched by age and gender. 71 patients (66%) had stage I disease and 37 (34%) had stage II disease. Patients with stage III disease were excluded from these early stage studies due to concern that presence of occult metastases, particularly in lymphatic tissues, might affect our results. The cohort was divided serially into training and test cohorts by assigning every other patient to the test cohort. Plasma from healthy donors, defined as individuals who were not diagnosed with cancer, matched by age and gender to the two melanoma cohorts was also assayed to determine biologically “normal” values for the plasma markers. The patient demographics are detailed in Table 1.
Table 1.
Patient demographics
| Resected stage I/II disease (N=108) |
Unresected stage IV disease (N=108) |
Healthy volunteers (N=108) |
P value | |
|---|---|---|---|---|
| Age (mean +/− SD) |
60.48 +/− 12.6 | 60.57 +/− 12.3 | 59.8 +/− 12.7 | P = 0.97 |
| Gender (male) |
70 (64.8%) | 70 (64.8%) | 70 (64.8%) | P = 1 |
| Race – (Caucasian) |
108 (100%) | 108 (100%) | 108 (100%) | P = 1 |
Marker selection
We interrogated our gene expression data derived from cDNA hybridization of 45 short term melanoma cell cultures to NimbleGen human whole genome expression microarrays. We identified a number of abundantly expressed genes (hybridization intensity >10,000) genes that encode secreted proteins, proteins that are found on the cell plasma membrane or the extra-cellular matrix, that could be present in the blood of melanoma patients. We then conducted literature searches and selected candidates that have a known association with cancer. Markers studied further include VEGF-A, β-catenin, LIF, TIMP-3, TIMP-2, MMP-1, S-100B, Progranulin, Fibronectin, HSPA8, HDGF, IGFBP5, TIMP-1, Macrophage Migration Inhibitory Factor (MIF), CEA-related Cell Adhesion Molecule (CEACAM), Interleukin-8 (IL-8), osteopontin (OPN), Melanoma Inhibitory Activity (MIA), Intercellular Adhesion Molecule 1 (ICAM-1), Growth Differentiation Factor 15 (GDF-15) and S100B. The level of each marker was measured by ELISA assays in a subset of plasma samples to verify reproducibility and to ascertain whether differences were seen between plasma from melanoma patients and healthy volunteers. CEACAM, ICAM-1, OPN, MIA, GDF-15, TIMP-1 and S100B were selected for further analysis.
Specimen handling
Plasma and serum samples were acquired with informed consent according to a protocol approved by Yale University Institutional Review Board. Blood was collected in tubes containing EDTA, spun at 1,500 rpm for 15 minutes at 10°C in a bench top centrifuge, then aliquoted and stored at −80°C until used.
ELISA Assays
Commercially available ELISA kits were used to assess markers levels following the manufacturers’ instructions. Plasma samples were diluted 1:40 to measure MIA levels (Roche Applied Science, Indianapolis, IN, Catalog #11976826001) and ICAM-1 levels (R&D Systems, Minneapolis, MN, Quantikine® Immunoassay, Catalog #DCD540), 1:25 to measure OPN (R&D Systems, Quantikine® Immunoassay, Catalog #DOST00), and 1:100 to measure TIMP-1 (R&D Systems, Quantikine® Immunoassay, Catalog #DTM100). Undiluted plasma samples were used to measure S100B levels (Alpco, Salem, New Hampshire, Catalog #48-S1BHU-E01). Plasma levels of CEACAM and GDF-15 were assessed at a 1:80 dilution with the DuoSet® ELISA Development Kit (R&D Systems, Catalog #DY2244 and #DY957) employing Immuno 96 MicroWell Plates (Nunc, Catalog #446612). The plates were blocked with 1% BSA Fraction V and 0.2% Albumin (both from Sigma-Aldrich, St Louis, MO, USA). All incubations, with the exception of the substrate solution (KPL, Catalog #53-00-01), were carried out on a horizontal orbital shaker, 100 rpm. MIA absorbance was measured at 405nm, S100B at 490nm and all other markers at 450nm on a spectrophotometer. Concentrations of all markers except for S100B are presented in ng/ml. Concentrations of S100B are presented in pg/ml.
Statistical Analyses
Matlab (Natick, MA) software was used for the principal components analyses. JMP version 5 was used to perform data analysis (SAS Institute Inc., Cary, NC). Associations with clinical parameters were assessed by paired t-tests. Area under the Receiver Operating Curve was calculated with metastatic versus early stage disease as an endpoint. A multivariate model was built using the Classification and Regression Trees (CART) algorithm (Breiman, et al. 1964) in the R statistical package rpart. Ten-fold cross-validation was employed to choose the best tree size in the training set and the results were validated with the independent test set.
Results
Assay reproducibility
Representative samples were run in duplicate to verify assay reproducibility. All plates contained replicates for 10 samples, which were used to normalize results across different plates. For all seven markers used in our analyses, replicate samples were highly correlated, with R values >0.9. The ranges of protein levels for the six markers in the stage I/II and IV patients were: CEACAM 7-246 ng/ml, mean 36 +/− 28; ICAM-1 91-1100 ng/ml, mean 282 +/− 175; OPN ng/ml 2-270, mean 28 +/− 38; MIA ng/ml 0-207, mean 8 +/− 20; GDF-15 0-123 ng/ml, mean 3 +/− 9; TIMP-1 0-540 ng/ml, mean 126 +/− 66 and S100B 0-4124 pg/ml, mean 394.
Comparison between marker levels in patients with resected stage I/II melanoma and age and gender matched individuals with measurable stage IV disease
ELISA assays for the seven markers were run on plasma samples from the training cohort of 54 age and gender matched individuals with resected stage I/II disease and patients with at least 2 cm of radiographically measurable stage IV disease (using unidimensional measurements). As shown in Table 2, paired t-tests of continuous ELISA measurements show that marker levels are higher in patients with measurable metastatic disease than in patients with resected, early stage disease in both the training and the test sets, with the exception of GDF-15, for which the differences in the test set were not statistically significant. Differences between these patient subsets were highest for ICAM-1, OPN and TIMP-1. Examples of marker distribution in these two patient groups in the two cohorts are shown in the means plots in Figure 1 for ICAM-1 and OPN. Distribution of the other markers is shown in Supplemental Figures 1-5.
Table 2.
Differences in marker level between resected stage I/II patients and patients with unresected stage IV disease
| Marker |
t statistic (training) |
P value (training) |
t statistic (test) |
P value (test) |
|---|---|---|---|---|
| CEACAM | 2.8 | 0.0058 | 2 | 0.05 |
| ICAM-1 | 3.9 | 0.0001 | 4.4 | <0.0001 |
| OPN | 4.4 | <0.0001 | 5.2 | <0.0001 |
| MIA | 3 | 0.003 | 2.5 | 0.03 |
| GDF-15 | 2 | 0.05 | 1.2 | 0.2 |
| TIMP-1 | 3.6 | 0.0004 | 3.7 | 0.0004 |
| S100B | 3 | 0.0037 | 4.8 | <0.0001 |
Figure 1.
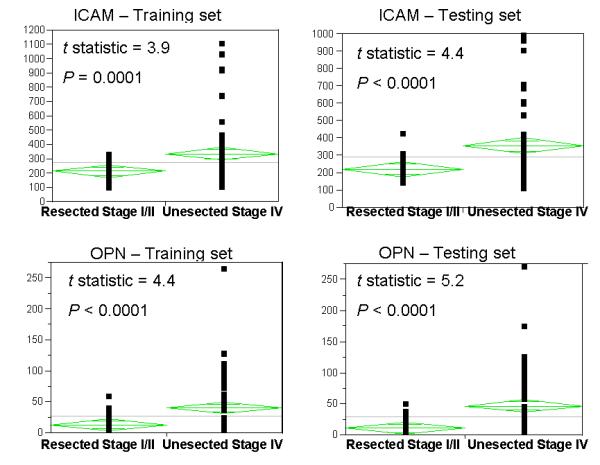
Means plots showing distribution on levels of ICAM-1 and OPN in the training and test sets of patients with resected stage I/II melanoma and age and gender matched patients unresected stage IV melanoma.
Associations between marker level and age and gender
Associations between marker level and age and gender were analyzed by t-tests, binarizing age using a cut-point of 60. None of the seven markers were associated with age or gender.
Associations between marker level and stage in patients with resected stage I/II disease
Using 1 mm as a cut-point, we studied the association between Breslow depth in the stage I/II patients and levels of the seven markers. No association was found between Breslow depth and any of the seven plasma markers (P>0.05 for all). 66% of the patients with early stage, resected melanoma had stage I disease and 34% had stage II disease. By unpaired t-tests, no differences were found in levels of any of the seven markers between stage I and stage II patients (P>0.6 for all markers).
Sample clustering
Given that patients with stage IV disease clearly have a greater likelihood of having more than one marker elevated, we conducted principal components analyses to asses the combined effects of continuous marker levels in our patients and healthy individuals. As shown in Figure 2, stage IV patients clearly tend to cluster together on the two-dimensional plot, whereas patients with resected stage I/II disease and healthy individuals clearly tend to cluster together.
Figure 2.
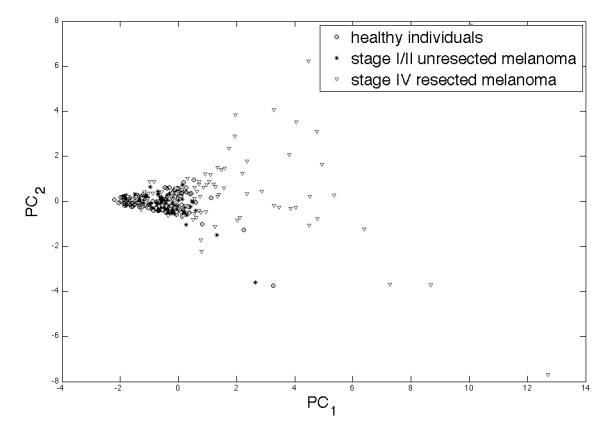
Principal components analysis for all 7 markers in patients with stage I/II resected melanoma, patients with unresected stage IV melanoma and age and gender matched healthy individuals showing clear separation of the many of the stage IV patients from the healthy individuals and patients with resected, early stage disease.
Generation of cut-points for high versus low marker level
Protein concentration measured by ELISA yield continuous variables. In order for an assay to be clinically useful, definitions of “normal” versus “abnormal” (or “high” versus “low”) need to be determined. We therefore collected plasma samples on healthy individuals, defined as people who have never had a diagnosis of malignancy, matched by age and gender to the training and test sets. Elevated levels for each marker were defined as those above the 95th percentile level for this control group. The cut-points were 40.17 for CEACAM, 292.8 for ICAM-1, 57.08 for OPN, 7.8 for MIA, 3.72 for GDF-15 148.7 for TIMP-1 and 598 for S100B.
Marker combinations
Given that none of the individual markers fully distinguishes the resected stage I/II patients from the unresected stage IV patients, we assessed the distribution of positive markers in our training and test sets. As shown in Figure 3, in the training set 38 (70%) patients with stage IV disease had elevation of one or more markers, and 16 (30%) had no marker elevation. In the test set, there were 42 (78%) and 12 (22%) stage IV patients, respectively. Among the patients with resected stage I/II disease, 13 (24%) had elevation of one or more marker in the training set, whereas 41 (76%) had no marker elevation. In the test set 10 patients (19%) had elevation of one marker and 44 (81%) had no marker elevation.
Figure 3.
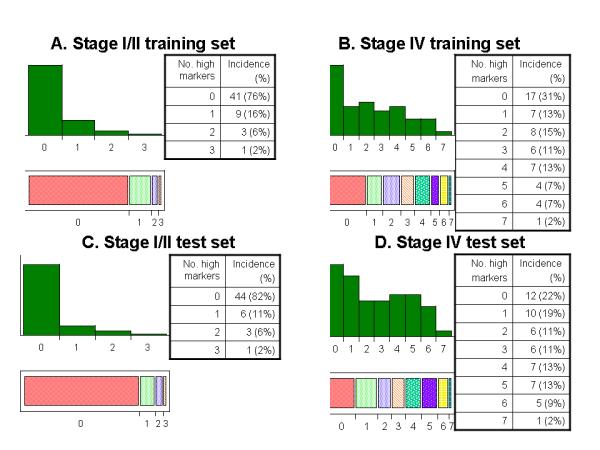
Distribution of number of markers with high levels in training set (panels A and B) and test set (panels C and D) showing clear differences between patients with stage I/II resected disease and stage IV unresected disease.
Sensitivity and specificity
The degree of sensitivity and specificity of our markers for determining whether an individual has resected early stage disease versus unresected stage IV disease depend on cutpoints for determining “abnormal” versus “normal”. When considering high levels of one or more markers in our assay an abnormal result, the sensitivity of this assay in the training and test cohorts in combination is 74%, the specificity 79% and the positive and negative predictive rates are 78% and 75%, respectively. When considering high levels of two or more markers in our assay an abnormal result, the sensitivity of this assay in all the patients is 57.4%, the specificity 94.4% and the positive and negative predictive rates are 89% and 70%, respectively.
Receiver operating characteristic curves
To produce a single measure of sensitivity and specificity in determining metastatic versus early stage, resected disease, we generated receiver operating characteristic curves. As shown in Figures 4a and 4b, the area under these curves (AUC) was 0.84 in the training set and 0.84 in the test set as well when using a linear combination of dichotomized scores (high=1, low=0) for the seven markers.
Figure 4.
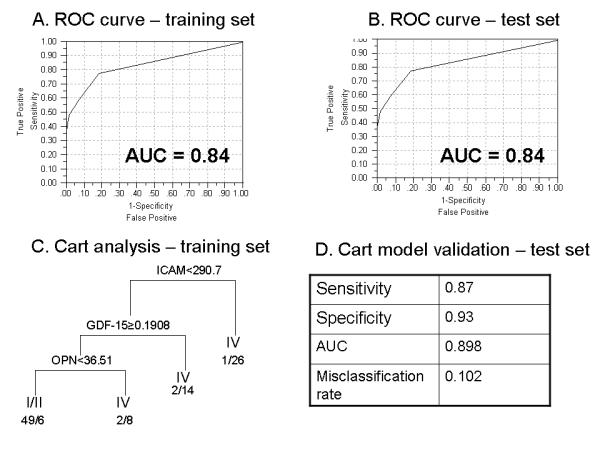
Receiver operating characteristic curves for the training set (A) and the test set (B) showing an area under the curve of 0.84 for both sets. Classification and Regression Tree (CART) analysis of the training set generating a model that uses three variables (ICAM-1, GDF-15 and OPN) to differentiate between patients with resected stage I/II disease and unresected stage IV disease (C). The model was validated in the test set with high sensitivity, specificity and AUC and a low misclassification rate (D).
CART analysis for variable reduction
With the ultimate goal of cost containment, we attempted to reduce the number of variables used. A CART analysis of the training set showed that patients who had levels of OPN<36.51, GDF-15≥0.1908 and ICAM-1<290.7 tend to have resected, early stage disease rather than stage IV disease, as shown in Figure 4, panel C. In the test set, this model resulted in superior separation of these two groups of patients with a sensitivity of 0.87, a specificity of 0.93, an AUC of 0.898 and a misclassification rate of 0.102, as shown in Figure 4, panel D.
Comparison between plasma based ELISA assays and routine LDH testing
Our purpose was to develop a blood-based assay to compliment routine laboratory and imaging tests recommended by the NCCN for monitoring patients with early stage melanoma. None of the patients with stage I/II disease had abnormalities on imaging. Of the stage I/II disease patients, 4% had abnormalities in their LDH at the time the blood was drawn. Of the metastatic patients, 38% had abnormalities in their LDH, indicating that our assay is much more sensitive, but less specific LDH in screening early stage patients for metastatic relapse.
Discussion
The purpose of this work was to initiate development of a multiplex, plasma-based protein biomarker panel to differentiate between patients with resected melanoma and melanoma patients with unresected disease. To achieve our goal, we screened a number of abundantly expressed candidate genes identified in early passage melanoma cell strains which encoded for secreted proteins, plasma membrane proteins, and extra-cellular matrix proteins known to be associated with cancer, as these were felt to be more likely to found in the plasma of patients with unresected disease. Seven proteins were found to have higher levels in unresected stage IV patients compared with age and gender matched patients with resected, early stage disease. Among the stage I/II patients, levels of none of these markers was associated with stage or Breslow depth, indicating that they might require a larger burden of unresected disease than primary melanomas to result in elevation in the plasma at the time of diagnosis, and thus might have value for monitoring patients. In combination, these proteins were superior in discriminating between the two groups of patients (resected stage I/II and unresected stage IV) than each protein alone, and the proteins were clearly superior to serum LDH in differentiating between these patient populations. A more compact model containing a subset of these variables (ICAM-1, GDF-15 and OPN) resulted in an improved misclassification rate. Our findings were validated in a separate test set.
The biological basis for finding these specific proteins in the blood of our metastatic melanoma patients varied from marker to marker. CEACAM (CEA-related Cell Adhesion Molecule) expression has been shown to markedly enhance melanoma cell invasion and migration, and the molecule is expressed in melanoma tumor-stroma interface of invading melanoma masses 15. It is therefore likely that CEACAM would be secreted into the blood system in melanoma patients. ICAM-1 (intercellular adhesion molecule) is expressed on the surface of melanoma cells, and interacts with polymorphonuclear leukocytes, which facilitate melanoma cell extravasation through the endothelium and into the circulation 16. ICAM-1 is therefore likely to be associated with hematogenous melanoma dissemination. In mouse models, osteopontin (OPN) levels are increased during the progression from primary to metastatic melanoma 17. In patient tissue samples, OPN is associated with melanoma progression 18-19. Serum levels of MIA (melanoma-inhibiting activity) have been shown by others to be associated with melanoma progression, as reviewed 20. GDF-15 (growth differentiation factor 15, also known as Macrophase Inhibitory Cytokine-1) has been shown to be up-regulated in advanced melanoma tumors in a number of reports 21. Given that this is a secreted protein, its utility as a plasma marker for metastatic melanoma has been suggested by Boyle et al 22. TIMP-1 (tissue inhibitor of metalloproteinase-1) has been shown in a small series to be elevated in patients with stage IV melanoma when compared with healthy controls and patients with thin primary melanomas 23. S100B is expressed on over 90% of melanoma cells and has been shown to be elevated in blood of patients with metastatic melanoma 13.
While a number of published studies have assessed our blood-based biomarkers for surveillance of melanoma recurrence, individual markers are associated with high misclassification rates. S100B has perhaps been the most widely studied and has been validated as a single biomarker in samples from a large multi-center randomized clinical trial 13. Rangel et al. showed that elevated OPN levels in tumors are associated with metastatic relapse, but plasma OPN levels in metastatic and primary melanoma have not been studied 18. Elevated levels of MIA were found in peripheral blood mononuclear cells in 26.8% of samples from stage I/II patients and 86.% of patients with clinically evident, untreated stage IV disease 27. The stage IV cohort in this study, however, only included 13 patients with clinically evident disease. Both OPN and MIA have been shown to be associated with metastases in uveal melanoma 28. Yamada et al showed increasing elevation in levels of ICAM-1 in two patients whose melanoma metastasized 29. Levels of TIMP-1 were elevated in plasma of a small sample or 19 stage IV patients compared to stage I-III patients 23. We did not find similar studies on the other biomarkers in our panel. In our study, these markers in combination had a stronger association with metastatic disease than any single marker. The CART analysis resulted in a more compact model with improved sensitivity and specificity.
To the best of our knowledge, this is the first published study that assesses these markers in combination. As is the case with other clinically used biomarker studies, multiplex analysis of our biomarker panel improved the error rate for predicting metastatic disease. The area under the receiver operating curve for our biomarker panel was 0.84 in both the training and test set, and 0.898 in the reduced variable model, which compares well to that of other clinically used assays, such as the Oncotype Dx model, which is used for predicting relapse in breast cancer 24.
The sensitivity of our combined marker set of seven variables was 74% and 87% using the reduced variable model. Ideally, none of the stage I/II patients would have elevated marker levels. Given that we are proposing a screening test that can be used to select out individuals who need surveillance imaging, however, such a test, once validated, could still eliminate unnecessary scans in asymptomatic patients with normal blood work and normal marker levels. Our test, however, compares favorably to use of serum LDH in melanoma, and is in the ballpark of other clinically used assays for cancer surveillance. For example, in a large study of patients with resected early stage colon cancer under surveillance in the United Kingdom, CEA was associated with a sensitivity of 64% in identifying metastatic disease 25. In patients with resected ovarian cancer, serum CA-125 was 23.3% sensitive in identifying disease recurrence 26. Ongoing and future studies will focus on including other secreted proteins to increase the sensitivity of our assay while preserving or improving specificity.
Plasma markers associated with metastatic disease can be useful in identifying patients who might benefit from early intervention. There are a number of lines of evidence to suggest that early detection of metastases and resection of oligometastases can result in improved survival 30-32. Most of these series assess the benefit of metastatectomy compared to historical controls, although one phase III trial showed benefit to aggressive surgical resection 32. Other studies suggest that survival rates for patients with stage IV disease that has been resected (called stage IV NED for stage IV with No Evidence of Disease) have improved over the past years, suggesting that active radiographic surveillance and immediate intervention might improve survival in patients whose disease metastasizes 33. In patients with unresectable metastases, the likelihood of response to systemic therapy is sometimes inversely related to disease burden, although it is unclear whether this is directly related to overall survival 34. Nonetheless, early detection of metastases can result in treatment of patients with a better performance status and less symptoms, and as newer, more effective therapies for metastatic disease become available, tools for early detection of metastases and subsequent early intervention are likely to be highly useful. This is supported by a recent study demonstrating improved overall survival in metastatic melanoma in the United States compared to Australia; the difference in overall survival was attributable to more active surveillance in the United States and early detection of metastases 35.
In summary, we have identified a group of markers that are elevated in unresected metastatic melanoma compared to individuals with resected stage I/II disease. Particularly when used in combination, these markers can be used to monitor patients for disease recurrence and might be useful for complimenting surveillance imaging for resected stage I-III patients, or increasing the interval between imaging studies, especially in high risk stage IIC and III patients. Prospective validation of these findings in an independent cohort of stage I-III patients with resected melanoma at high risk for disease recurrence is warranted.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
In this work we employed genome-wide expression arrays to identify abundantly expressed genes in melanoma tumors. Using computational tools we selected candidate genes that encode for proteins that are likely to be present in the blood of cancer patients and subsequently studied these proteins as markers of disease burden in a retrospective training and test cohort of melanoma patients with resected, low disease burden melanoma and patients with unresected, measurable disease. These pre-clinical ELISA studies require prospective validation, and can result in development of a clinical assay to monitor patients with resected melanoma at high risk for disease recurrence.
Acknowledgments
This work was supported by the Yale SPORE in Skin Cancer, 1 P50 CA121974 (R. Halaban, PI) and the Milstein-Meyer Funds for Melanoma Research at Yale.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Ak I, Stokkel MP, Bergman W, Pauwels EK. Cutaneous malignant melanoma: clinical aspects, imaging modalities and treatment. Eur J Nucl Med. 2000;27:447–58. doi: 10.1007/s002590050529. [DOI] [PubMed] [Google Scholar]
- 3.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garbe C, Hauschild A, Volkenandt M, et al. Evidence and interdisciplinary consense-based German guidelines: diagnosis and surveillance of melanoma. Melanoma Res. 2007;17:393–9. doi: 10.1097/CMR.0b013e3282f05039. [DOI] [PubMed] [Google Scholar]
- 5.Ho Shon IA, Chung DK, Saw RP, Thompson JF. Imaging in cutaneous melanoma. Nucl Med Commun. 2008;29:847–76. doi: 10.1097/MNM.0b013e32830439fb. [DOI] [PubMed] [Google Scholar]
- 6.Bishop JA, Corrie PG, Evans J, et al. UK guidelines for the management of cutaneous melanoma. Br J Plast Surg. 2002;55:46–54. doi: 10.1054/bjps.2001.3745. [DOI] [PubMed] [Google Scholar]
- 7.Dummer R, Hauschild A, Jost L. Cutaneous malignant melanoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2008;19(Suppl 2):ii86–8. doi: 10.1093/annonc/mdn100. [DOI] [PubMed] [Google Scholar]
- 8.Francken AB, Accortt NA, Shaw HM, et al. Follow-up schedules after treatment for malignant melanoma. Br J Surg. 2008;95:1401–7. doi: 10.1002/bjs.6347. [DOI] [PubMed] [Google Scholar]
- 9.Grange F, Vitry F, Granel-Brocard F, et al. Variations in management of stage I to stage III cutaneous melanoma: a population-based study of clinical practices in France. Arch Dermatol. 2008;144:629–36. doi: 10.1001/archderm.144.5.629. [DOI] [PubMed] [Google Scholar]
- 10.Poo-Hwu WJ, Ariyan S, Lamb L, et al. Follow-up recommendations for patients with American Joint Committee on Cancer Stages I-III malignant melanoma. Cancer. 1999;86:2252–8. [PubMed] [Google Scholar]
- 11.Mouawad R, Spano JP, Comperat E, Capron F, Khayat D. Tumoural expression and circulating level of VEGFR-3 (Flt-4) in metastatic melanoma patients: correlation with clinical parameters and outcome. Eur J Cancer. 2009;45:1407–14. doi: 10.1016/j.ejca.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Kurschat P, Eming S, Nashan D, Krieg T, Mauch C. Early increase in serum levels of the angiogenesis-inhibitor endostatin and of basic fibroblast growth factor in melanoma patients during disease progression. Br J Dermatol. 2007;156:653–8. doi: 10.1111/j.1365-2133.2006.07724.x. [DOI] [PubMed] [Google Scholar]
- 13.Tarhini AA, Stuckert J, Lee S, Sander C, Kirkwood JM. Prognostic significance of serum S100B protein in high-risk surgically resected melanoma patients participating in Intergroup Trial ECOG 1694. J Clin Oncol. 2009;27:38–44. doi: 10.1200/JCO.2008.17.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tung MC, Tsai CS, Tung JN, et al. Higher prevalence of secretory CSE1L/CAS in sera of patients with metastatic cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:1570–7. doi: 10.1158/1055-9965.EPI-08-0948. [DOI] [PubMed] [Google Scholar]
- 15.Ebrahimnejad A, Streichert T, Nollau P, et al. CEACAM1 enhances invasion and migration of melanocytic and melanoma cells. Am J Pathol. 2004;165:1781–7. doi: 10.1016/S0002-9440(10)63433-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoskins MH, Dong C. Kinetics analysis of binding between melanoma cells and neutrophils. Mol Cell Biomech. 2006;3:79–87. [PMC free article] [PubMed] [Google Scholar]
- 17.Metge BJ, Liu S, Riker AI, Fodstad O, Samant RS, Shevde LA. Elevated osteopontin levels in metastatic melanoma correlate with epigenetic silencing of breast cancer metastasis suppressor 1. Oncology. 2010;78:75–86. doi: 10.1159/000292363. [DOI] [PubMed] [Google Scholar]
- 18.Rangel J, Nosrati M, Torabian S, et al. Osteopontin as a molecular prognostic marker for melanoma. Cancer. 2008;112:144–50. doi: 10.1002/cncr.23147. [DOI] [PubMed] [Google Scholar]
- 19.Conway C, Mitra A, Jewell R, et al. Gene expression profiling of paraffin-embedded primary melanoma using the DASL assay identifies increased osteopontin expression as predictive of reduced relapse-free survival. Clin Cancer Res. 2009;15:6939–46. doi: 10.1158/1078-0432.CCR-09-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perrotta R, Bevelacqua Y, Malaguarnera G, Paladina I, Giordano M, Malaguarnera M. Serum markers of cutaneous melanoma. Front Biosci (Elite Ed) 2010;2:1115–22. doi: 10.2741/e170. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita T, Yoneta A, Hida T. Macrophage inhibitory cytokine-1: a new player in melanoma development. J Invest Dermatol. 2009;129:262–4. doi: 10.1038/jid.2008.366. [DOI] [PubMed] [Google Scholar]
- 22.Boyle GM, Pedley J, Martyn AC, et al. Macrophage inhibitory cytokine-1 is overexpressed in malignant melanoma and is associated with tumorigenicity. J Invest Dermatol. 2009;129:383–91. doi: 10.1038/jid.2008.270. [DOI] [PubMed] [Google Scholar]
- 23.Yoshino Y, Kageshita T, Nakajima M, Funakubo M, Ihn H. Clinical relevance of serum levels of matrix metallopeptidase-2, and tissue inhibitor of metalloproteinase-1 and -2 in patients with malignant melanoma. J Dermatol. 2008;35:206–14. doi: 10.1111/j.1346-8138.2008.00446.x. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein LJ, Gray R, Badve S, et al. Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features. J Clin Oncol. 2008;26:4063–71. doi: 10.1200/JCO.2007.14.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan E, Gouvas N, Nicholls RJ, Ziprin P, Xynos E, Tekkis PP. Diagnostic precision of carcinoembryonic antigen in the detection of recurrence of colorectal cancer. Surg Oncol. 2009;18:15–24. doi: 10.1016/j.suronc.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Gadducci A, Fuso L, Cosio S, et al. Are surveillance procedures of clinical benefit for patients treated for ovarian cancer?: A retrospective Italian multicentric study. Int J Gynecol Cancer. 2009;19:367–74. doi: 10.1111/IGC.0b013e3181a1cc02. [DOI] [PubMed] [Google Scholar]
- 27.Muhlbauer M, Langenbach N, Stolz W, et al. Detection of melanoma cells in the blood of melanoma patients by melanoma-inhibitory activity (MIA) reverse transcription-PCR. Clin Cancer Res. 1999;5:1099–105. [PubMed] [Google Scholar]
- 28.Barak V, Frenkel S, Kalickman I, Maniotis AJ, Folberg R, Pe’er J. Serum markers to detect metastatic uveal melanoma. Anticancer Res. 2007;27:1897–900. [PMC free article] [PubMed] [Google Scholar]
- 29.Yamada M, Yanaba K, Takehara K, Sato S. Clinical significance of serum levels of soluble intercellular adhesion molecule-1 and soluble L-selectin in malignant melanoma. Arch Dermatol Res. 2005;297:256–60. doi: 10.1007/s00403-005-0605-5. [DOI] [PubMed] [Google Scholar]
- 30.McLoughlin JM, Zager JS, Sondak VK, Berk LB. Treatment options for limited or symptomatic metastatic melanoma. Cancer Control. 2008;15:239–47. doi: 10.1177/107327480801500307. [DOI] [PubMed] [Google Scholar]
- 31.Sanki A, Scolyer RA, Thompson JF. Surgery for melanoma metastases of the gastrointestinal tract: indications and results. Eur J Surg Oncol. 2009;35:313–9. doi: 10.1016/j.ejso.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Mosca PJ, Teicher E, Nair SP, Pockaj BA. Can surgeons improve survival in stage IV melanoma? J Surg Oncol. 2008;97:462–8. doi: 10.1002/jso.20950. [DOI] [PubMed] [Google Scholar]
- 33.Faries MB, Morton DL. Therapeutic vaccines for melanoma: current status. BioDrugs. 2005;19:247–60. doi: 10.2165/00063030-200519040-00004. [DOI] [PubMed] [Google Scholar]
- 34.Agarwala SS, Keilholz U, Gilles E, et al. LDH correlation with survival in advanced melanoma from two large, randomised trials (Oblimersen GM301 and EORTC 18951) Eur J Cancer. 2009 doi: 10.1016/j.ejca.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 35.Atkins GVL MB, Warneke CL, Carlino M, DeRose ER, Johnson MM, Brown PT, Lee M, Kefford R, Gershenwald JE. J Clin Oncol. suppl. Vol. 28. Chicago, IL: 2010. Unraveling the prognostic heterogeneity in patients with advanced melanoma between Australia (OZ) and the United States (US): Preliminary report of the PHAMOUS study; p. 15s. abstr 8516) 2010. 2010. p. J Clin Oncol 28:15s, 2010 (suppl; abstr 8516) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


