Abstract
Glutamate-cysteine ligase (GCL) is the rate-limiting step in glutathione synthesis. The enzyme is a hetero-dimer composed of a catalytic subunit GCLC and a modifier subunit GCLM.
Objective
We generated apo E−/− mice deficient in GCLM (apoE−/−/Gclm−/−) and transgenic mice that over-express GCLC specifically in macrophages (apoE−/−/Gclc-Tg) to test the hypothesis that significantly altering the availability of glutathione has a measurable impact on both the initiation and progression of atherosclerosis.
Methods and Results
Atherosclerotic plaque size and composition were measured in the innominate artery in chow-fed male and female mice at 20, 30, 40 and 50 weeks of age and in the aortic sinus at 40 or 50 weeks of age. The apoE−/−/Gclm−/− mice more rapidly developed complex lesions while the apoE−/−/Gclc-Tg mice had reduced lesion development as compared to the littermate apo E−/− control mice. Transplant of bone marrow from the apoE−/−/Gclm−/− and apoE−/−/Gclc-Tg mice into apo E−/− mice with established lesions also stimulated or inhibited further lesion development at 30 weeks post-transplant.
Conclusions
Gain and loss of function in the capacity to synthesize glutathione especially in macrophages has reciprocal effects on the initiation and progression of atherosclerosis at multiple sites in apo E−/− mice.
Keywords: Atherosclerosis, macrophages, glutathione, apo E−/−
Introduction
Reduced glutathione (GSH) is a tripeptide thiol (glutamate-cysteine-glycine) that is made by most mammalian cells at up to mM concentrations. It is a major endogenous antioxidant and enzyme cofactor1 and participates in diverse cellular processes2. The enzyme glutamate-cysteine ligase (GCL) is the rate-limiting step in the GSH synthetic pathway. GCL is a hetero-dimer composed of a heavy catalytic subunit (GCLC, 72.8 kDa) and a lighter modifier subunit (GCLM, 30.8 kDa)2. The two subunits are products of separate genes in both mice and humans3–5. The catalytic efficiency is dramatically increased when the two subunits interact6 although GCLC has catalytic activity in the absence of GCLM6, 7. Knock out of the GCLC gene is embryonic lethal, while GCLC heterozygote mice exhibit a 20% reduction in GSH levels8. Reduced GCLC expression and activity has been associated with diabetes mellitus, inflammatory lung diseases, AIDS, and aging9–11.
The promoter/enhancer region of both the GCLC and GCLM genes contain a consensus antioxidant response element (ARE) and in response to changes in the cellular redox status, GCLC and GCLM expression are increased by the activation and binding of the transcription factor Nrf-2 to the ARE12, 13. We have previously shown that mouse macrophages have increased expression of both GCLC and GCLM when treated with oxidized LDL or homocysteine in vitro and that this increase in expression is mediated by binding of Nrf-2 to the ARE in the promoter for both genes13, 14. Increased GCLC and GCLM expression have also been reported in a variety of different cell types following treatment with other electrophiles15, 16.
Depletion of GSH may play a pivotal role in cardiovascular disease. For example, polymorphisms in the 5' flanking region of the GCLC and GCLM genes are associated with an increased risk of myocardial infarction and endothelial dysfunction17, 18. Pharmacological manipulation of GSH levels modestly alters early atherosclerotic lesion development in hyperlipidemic mice19. The GSH content of the aorta is reduced prior to and during lesion development in mice20 and GSH dependent antioxidant enzyme expression is reduced as lesions progress21. However, the effects of genetically altering GSH levels on advanced lesion development and composition have not been previously reported. Thus, in this study we have evaluated the effects of GCLM deficiency that causes a dramatic systemic reduction in GSH availability22 and increased GCLC expression specifically in macrophages to test the hypothesis that alterations in glutathione availability especially in macrophages will have an impact on the development and composition of advanced atherosclerotic lesions in the innominate artery and aortic sinus in chow-fed apo E−/− mice between 20 and 50 weeks of age.
Materials and methods
Animals
Mice doubly deficient in apo E and Gclm (apoE−/−/Gclm−/−) were generated by breeding Gclm−/− mice on a C57Bl/6 background22 with apoE−/− mice on a C57Bl/6 background and backcrossing for more than 6 generations. Genotypes were confirmed by PCR and littermate apoE−/− mice were used as wild-type controls. Mice that over-express GCLC specifically in macrophages were generated using a construct that contained a 342 bp fragment of the CD68 gene promoter in addition to the CD68 first intron, the 1920 bp full length coding sequence for GCLC and the bovine growth hormone polyadenylation signal. Clones were screened for correct orientation and then sequenced. CBA/B6 eggs were microinjected with purified DNA and then transferred into pseudo-pregnant foster mothers using standard techniques. Transgene expression was measured using real-time quantitative PCR and founder lines were mated with C57Bl/6 mice. The Gclc-Tg mice were crossed with apoE−/− mice on a C57Bl/6 background (but derived from a separate breeding colony of apo E−/− mice than those that were crossed with the Gclm−/− mice) to generate apoE−/−/Gclc-Tg mice. Genotypes were confirmed by PCR and littermate apoE−/− mice were used as controls. Western blotting was also used to verify over-expression of GCLC in thioglycollate elicited peritoneal macrophages isolated from the transgenic and littermate control mice (figure 1, on-line data supplement). All of the mice included in this study were fed standard mouse chow. All animal procedures were approved by the University of Washington Institutional Animal Care and Use Committee.
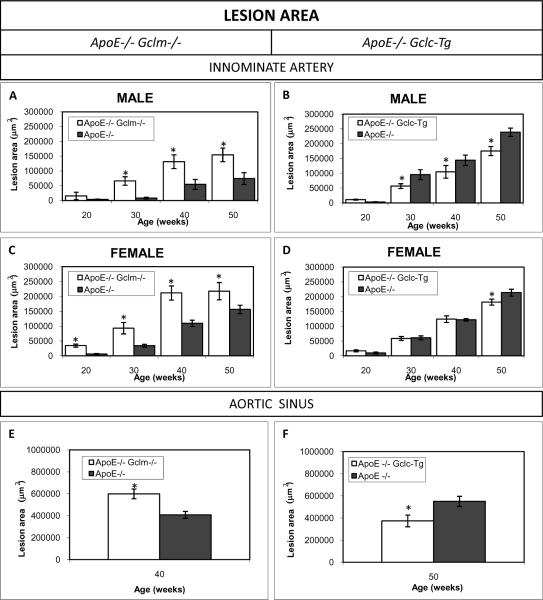
Figure 1. Average Lesion Area in the Innominate Artery and Aortic Sinus.
ApoE−/−/Gclm−/− Mice (A) Male mice at 20 weeks (apoE−/−/Gclm−/− n=5, apoE−/−/Gclm+/+ n=17), 30 weeks (apoE−/−/Gclm−/− n=8, apoE−/−/Gclm+/+ n=13), 40 weeks (apoE−/−/Gclm−/− n=7, apoE−/−/Gclm+/+ n=10) and 50 weeks (apoE−/−/Gclm−/− n=9, apoE−/−/Gclm+/+ n=4); (C) female mice at 20 weeks (apoE−/−/Gclm−/− n=5, apoE−/−/Gclm+/+ n=24), 30 weeks (apoE−/−/Gclm−/− n=8, apoE−/−/Gclm+/+ n=14), 40 weeks (apoE−/−/Gclm−/− n=6, apoE−/−/Gclm+/+ n=10) and 50 weeks (apoE−/−/Gclm−/− n=5, apoE−/−/Gclm+/+ n=20). (E) Aortic sinus of female mice at 40 weeks of age (apoE−/−/Gclm−/− n=5, apoE−/−/Gclm+/+ n= 5)
ApoE−/−/Gclc-Tg Mice. (B) Male mice at 20 weeks (apoE−/−/Gclc-Tg n=11, apoE−/−/Gclc-WT n=12), 30 weeks (apoE−/−/Gclc-Tg n=7, apoE−/−/Gclc-WT n=6), 40 weeks (apoE−/−/Gclc-Tg n=6, apoE−/−/Gclc-WT n=11) and 50 weeks (apoE−/−/Gclc-Tg n=7 apoE−/−/Gclc-WT n=13); (D) Female mice at 20 weeks (apoE−/−/Gclc-Tg n=14, apoE−/−/Gclc-WT n=15), 30 weeks (apoE−/−/Gclc-Tg n=15, apoE−/−/Gclc-WT n=12), 40 weeks (apoE−/−/Gclc-Tg n=7, apoE−/−/Gclc-WT n=7) and 50 weeks (apoE−/−/Gclc-Tg n=8, apoE−/−/Gclc-WT n=18); (F) Aortic sinus of male mice at 50 weeks of age (apoE−/−/Gclc-Tg n=5, apoE−/−/Gclc-WT n=5). Data are presented as means ± SE, *p<0.05 vs controls.
Genotyping mice by PCR
DNA was isolated from mouse tail snips using the DNeasy kit (Quiagen). The mutant and the wild-type allele for GCLM and the GCLC transgene were amplified by PCR. The targeted allele for GCLM was identified by the following primer: forward 5'- CAGTTTGAGGGGACGACGACA-3'. The wild type allele was identified by the forward primer 5'-GCCCGCTCGCCATCTCTC-3'. Both alleles shared the reverse primer 5'-GTTGAGCAGGTTCCCGGCT-3'. Analysis of the GCLC transgene used the following primers: forward 5'- TTCTCGGCTCTGTGAATGACA -3'; reverse 5'-CAGCCCTCTCTTGGAAAGGAGG-3'.
Bone marrow transplantation
To determine whether a reduced or increased GSH content in macrophages would influence the progression of established atherosclerotic lesions, we performed bone marrow transplantion (BMT) with bone marrow from 8 week old male apoE−/−, apoE−/−/Gclm−/−, or apoE−/−/Gclc-Tg mice transplanted into irradiated female apoE−/− mice (the recipient mice were all obtained from the same breeding colony as the apo E−/− mice used to generate the apo E−/− GCLCTg mice). One hundred and twenty, 20-week-old apoE−/− females received a dose of 950 rad whole-body x-ray irradiation. The irradiated recipients were divided into three groups that were injected with 1×107 bone marrow cells from the apoE−/−, apoE−/−/Gclm−/− or apoE−/−/Gclc-Tg male mice by tail vein injection. Atherosclerosis development and composition were analyzed at 20 and 30 weeks after BMT.
Mouse perfusion and dissection
At 20, 30, 40 and 50 weeks of age (40 or 50 weeks in the BMT studies), blood was collected via the retro-orbital sinus and the animals were euthanized by pharmacological overdose of a xylazine and ketamine mix (IP). The mice were first perfused with 20 ml of PBS and then with 20 ml of fixative (4% paraformaldehyde) at physiological pressure via the left ventricle. The heart with the aortic root and the thoracic aorta and its branching vessels were dissected out intact. The ascending aorta and carotid arteries were separated from the heart and the heart was further fixed in 1% paraformaldehyde and then paraffin embedded. The innominate arteries were dissected, processed and paraffin embedded. Five micron thick sections from each of the paraffin blocks were generated and every fifth section was stained with a modified Movat's pentachrome stain23, 24.
Plaque size and composition analysis
The cross sectional lesion area was determined in each Movat's stained section using computer assisted morphometric analysis (Image Pro, Media Cybernetics, Silver Spring, MD). We also tabulated the frequency of features of plaque composition in each Movat's stained section. These included the following: thin fibrous cap (defined as < 3 cell layers), large necrotic core (defined as occupying >50% of the volume of the plaque), intra-plaque hemorrhage (defined as the presence of red blood cells), medial enlargement/erosion (defined as the replacement of the normal media by plaque components), calcification, presence of foam cells, presence of chondrocyte-like cells, and lateral xanthomas (defined as the presence of aggregates of macrophage-derived foam cells situated on the lateral margins of the plaques). These parameters were recorded as binary outcomes and the frequency per lesion for each animal was determined. The total number of cells in the lesions was determined by counting nuclei in each section and the number was then normalized to the area of the lesion. All data collection was done with the operator blinded to the treatment groups.
Immunohistochemistry
Paraffin-embedded sections of the innominate artery were de-paraffinized and rehydrated. The endogenous peroxidase activity was blocked by incubation with Peroxoblock (Invitrogen). Smooth muscle cells (SMCs) and macrophages were detected using mouse anti-smooth muscle actin (Dako), and rat anti-Mac2 (Pharmingen) according to the manufacturer's protocols. Negative controls included an irrelevant antibody and omission of the secondary antibody.
Isolation and stimulation of mouse peritoneal macrophages
Macrophages were collected from the peritoneum of the apo E−/−/gclm−/−, apoE−/−/Gclc-Tg, and apo E−/− mice four days after i.p. injection of 2 ml of 4% thioglycollate. For details of the culturing of the peritoneal macrophages, see the on-line data supplement.
Caspase 9 activity assay
Thioglycollate elicted peritoneal macrophages from the apo E−/−/gclm−/−, apoE−/−/Gclc-Tg, and apo E−/− mice were grown in 6 well plates at a density of 6 × 104 cells/cm2 and treated with 10mM acrolein for 6h. Cells were also treated with 0.5mM staurosporine as a positive control for caspase activation. Caspase 9 activity was measured in 3 repeated experiments with the Caspase-9 LEHD-AFC fluorimetric assay kit (Biovision, Mountainview, CA) according to the manufacturer's protocol.
Measurement of cellular and tissue GSH content
The total glutathione content (nmoles/mg protein of GSH+GSSG) in the peritoneal macrophages, liver, kidney, spleen and lung was determined in a 96-well fluorescent microtiter plate assay as described previously25, 26. For details of this assay, see the on-line data supplement.
Measurement of plasma cholesterol and triglycerides levels
Total plasma cholesterol and triglyceride levels were measured using commercially available kits (Sigma). Lipoprotein cholesterol profiles were generated by FPLC as previously described27 (figures 4 and 5 on-line data supplement).
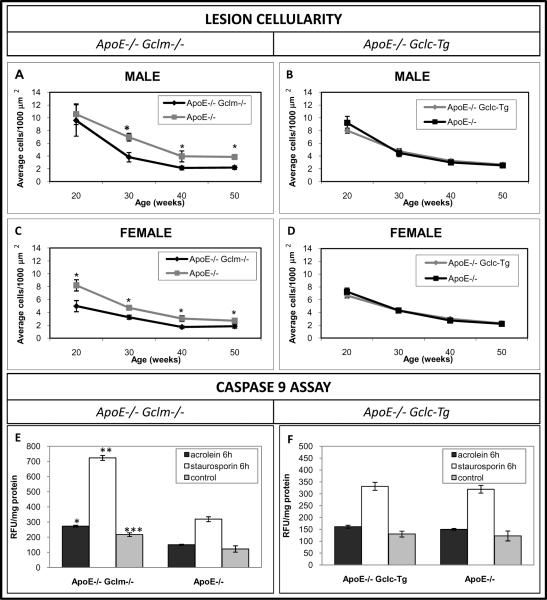
Figure 4. Total Cell Content of the Lesions in the Innominate Arteries and Caspase-9 Activity in Peritoneal Macrophages.
Total # nuclei/1000 μm2 cross sectional area in lesions in the innominate artery of both the male (A,C) and female (B,D) mice. Sample sizes were identical to those listed in figure 1. Caspase 9 activity (E,F) was evaluated in thioglycollate-elicited peritoneal macrophages after 6h treatment with acrolein or staurosporin (n= 3 mice/group). *p<0.05 vs littermate controls. Data are presented as means ± SE.
Statistical analysis
All data were expressed as mean ± SE. Significant differences between means in serum cholesterol and triglycerides and lesion size were determined by using the Student's two-tailed t test and by ANOVA for multiple comparisons. Frequency measures and non-normally distributed data were analyzed using the Mann-Whitney test. Values of p < 0.05 were considered statistically significant.
Results
Tissue Content of Total GSH
The levels of total GSH were measured in liver, spleen, kidney, lung, and in thioglycollate-elicted peritoneal macrophages from apoE−/−/Gclm−/−, apoE−/−/Gclc-Tg and control apo E−/− mice (table 1). There were radically reduced levels of GSH (>80%) in the liver, kidney, spleen, lung, and macrophages from the apoE−/−/Gclm−/− as compared with the control apoE−/− mice. The level of GSH in macrophages of the apoE−/−/Gclc-Tg mice was about 3 fold higher as compared with macrophages from the control apoE−/− mice. There were no significant differences in the GSH content of the liver, kidney, spleen, and lung from the transgenic and control apo E−/− mice.
Table 1.
Tissue Glutathione Content.
|
|
|||
|---|---|---|---|
| ApoE−/−/Gclm−/− | ApoE−/−/Gclc-Tg | ApoE−/−+ | |
| GSH (nmoles/mg protein) | |||
| Macrophages | 1.10 ± 0.20* | 21.57 ± 4.53* | 6.67 ± 1.39 |
| Liver | 8.70 ± 0.85* | 58.49 ± 6.33 | 64.82 ± 20.42 |
| Spleen | 2.72 ± 0.34* | 12.06 ± 0.01 | 12.61 ± 0.78 |
| Kidney | 0.28 ± 0.02* | 1.29 ± 0.01 | 1.21 ± 0.81 |
| Lung | 2.55 ± 0.54* | 17.64 ± 2.46 | 15.17 ± 1.06 |
Data are presented as means ± SE
p<0.05 vs apoE −/− mice.
Littermate controls for apoE−/−/Gclc-Tg mice. n= 3 mice/group.
Initiation and Progression of Atherosclerotic Plaques
The average cross sectional area of atherosclerotic plaque in the innominate artery was evaluated in both female and male mice at 20, 30, 40 and 50 weeks of age. In comparison to the sex- and age-matched littermate apo E−/− controls, the apoE−/−/Gclm−/− mice more rapidly developed larger and more complex lesions. In the females the difference was already significant at 20 weeks of age, while in the males the difference between groups was significant starting at 30 weeks of age (fig 1 a, c). In contrast, the apoE−/−/Gclc-Tg mice developed smaller lesions than the litter-mate controls (fig 1 b, d). The decrease of lesion area in the males was significant at 30, 40 and 50 weeks of age (fig 1b). The apoE−/−/Gclc-Tg female mice had a significant reduction in lesion area only at 50 weeks of age (fig 1d). To make sure that the effects of the GCLM deficiency or GCLC transgene expression was not site specific, we also measured the average lesion area in the aortic sinus of apoE−/−/Gclm−/− female mice at 40 weeks of age and apoE−/−/Gclc-Tg male mice at 50 weeks of age. Again, there were significantly larger lesions in the sinus of the apoE−/−/Gclm−/− mice than the corresponding littermate control mice (fig 1e) and significantly smaller lesions in the apoE−/−/Gclc-Tg mice than the corresponding control mice (fig 1f).
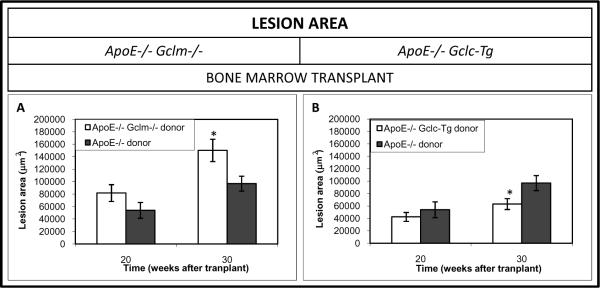
Effects of Bone Marrow Transplantation on the Progression of Established Atherosclerotic Plaques
In order to determine whether the reduced or increased availability of GSH in macrophages contributes to the acceleration or the reduction in the progression of established lesions, we conducted bone marrow transplantation studies. There was about a 60% increase in lesion area in the apoE−/− mice transplanted with bone marrow from the apoE−/−/Gclm−/− mice as compared to apoE−/− mice transplanted with apoE−/− bone marrow at 30 weeks post-transplant (fig 2a). The rate of progression of the lesions was reduced by 35% at 30 weeks post-transplant when bone marrow from apoE−/−/Gclc-Tg mice was transplanted into the apoE−/− recipients (figure 2b).
Figure 2. Average Lesion Area in the Innominate Artery Following Bone marrow Transplant.
ApoE−/−/Gclm−/− Mice. A) Lesion area in female apoE−/− mice following bone marrow transplantation at 20 weeks (apoE−/−/Gclm−/− donor, apo E−/− recipients n=15, apoE−/−/Gclm+/+ donor, apo E−/− recipients n =14), and 30 weeks (apoE−/−/Gclm−/− donor, apo E−/− recipients n=13, apoE−/−/Gclm+/+ donor, apo E−/− recipients n=8).
ApoE−/−/Gclc-Tg Mice. B) Lesion area in female apoE−/− mice following bone marrow transplantation at 20 weeks post-transplant (apoE−/−/Gclc-Tg donor, apo E−/− recipients n=15, apoE−/−/Gclc-WT donor, apo E−/− recipients n=14), and 30 weeks post-transplant (apoE−/−/Gclc-Tg donor, apo E−/− recipients n=13, apoE−/−/Gclc-WT, apo E−/− recipients donor n=8). Data are presented as means ± SE, *p<0.05 vs controls.
All recipient apo E−/− mice were from the same breeding colony as the apo E−/− mice used to generate the apo E−/−/Gclc-Tg mice.
Plaque Composition
We have previously described the age-related changes in lesion characteristics in the advanced lesions in the innominate arteries of chow-fed apo E−/− mice28. In order to determine the effects of GCLM deficiency and of GCLC transgene expression in macrophages on the same developmental changes in plaque composition, the frequency of these features were tabulated in the Movat's stained sections from each mouse. At 20 weeks of age there were no differences in lesion composition between the apoE−/−/Gclm−/− and apoE−/− male mice. However, by 30 weeks the apoE−/−/Gclm−/− male mice had a higher frequency of thin fibrous caps, large necrotic cores, cholesterol clefts, lateral xanthomas and foam cells, intra-plaque hemorrhage and chondrocytes-like cells (table 1 on-line data supplement). In the apoE−/−/Gclm−/− female mice an increased frequency of most of these features was already evident by 20 weeks of age and there continued to be higher frequencies of a large necrotic core and cholesterol clefts at both 30 and 40 weeks of age and intra-plaque hemorrhage at 30 weeks of age in the apoE−/−/Gclm−/− female mice. There were no differences in the frequency of plaque calcification in either the male or female mice at any time points (table 1, on-line data supplement). The transplanted mice were also analyzed for features of plaque composition. At 20 weeks post-transplant the mice transplanted with the apoE−/−/Gclm−/− bone marrow had a higher frequency of thin fibrous caps and chondrocyte like cells compared to the controls. At 30 weeks post-transplant there was a higher frequency of cholesterol clefts, lateral xanthoma and calcification in the experimental group compared to the control (table 2, on-line data supplement).
Table 2.
Total Plasma Cholesterol and Triglycerides.
| ApoE−/−/Gclc−/− | ApoE−/− | ApoE−/−/Gclc-Tg | ApoE−/− | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| male | female | male | female | male | female | male | female | |
|
| ||||||||
| Cholesterol (mg/dl) | ||||||||
|
| ||||||||
| 20 weeks | 492.4±59.1 | 371.3±20.9 | 546.6±34.2 | 412.4±5.9 | 590.4±34.4 | 511.9±24.9 | 537.2±20.5 | 446.5±8.0 |
|
| ||||||||
| 30 weeks | 563.6±63.4 | 377.1±41.7 | 535.3±29.5 | 433.4±23.8 | 655.2±36.3 | 608.3±25.9 | 585.8±14.2 | 499.7±19.8 |
|
| ||||||||
| 40 weeks | 523.9±38.4 | 388.5±18.2 | 619.9±29.4 | 456.3±81.4 | 648.2±66.7 | 594.7±38.2 | 614.0±25.0 | 508.5±36.3 |
|
| ||||||||
| 50 weeks | 605.9±56.7 | 457.2±44.1 | 640.9±49.5 | 496.7±23.2 | 634.0±29.5 | 593.5±26.9 | 631.0±25.3 | 558.2±22.1 |
|
| ||||||||
| Triglycerides (mg/dl) | ||||||||
|
| ||||||||
| 20 weeks | 582.6±94.4 | 408.5±59.8 | 551.4±93.0 | 342.2±38.8 | 626.0±52.4 | 399.1±31.6 | 551.4±34.2 | 410.4±36.3 |
|
| ||||||||
| 30 weeks | 446.9±52.3 | 299.5±22.1 | 494.3±36.9 | 243.7±30.3 | 688.2±86.3 | 506.6±55.0 | 512.7±55.1 | 469.7±10.5 |
|
| ||||||||
| 40 weeks | 474.2±47.6 | 251.0±41.9 | 503.6±47.9 | 363.9±24.3 | 523.9±38.4 | 395.5±20.1 | 497.9±45.6 | 424.8±26.0 |
|
| ||||||||
| 50 weeks | 412.4±24.0 | 305.9±35.1 | 446.4±65.9 | 327.6±29.7 | 386.4±74.6 | 206.2±65.9 | 467.7±75.2 | 357.6±41.3 |
Data are presented as the means ± SE.
Sample sizes are identical to those listed for figure 1.
There were no consistent differences at any time point or in either gender for any of the features of plaque composition between the apoE−/−/Gclc-Tg and apoE−/− mice. For example, at 20 weeks of age there were no differences while by 30 weeks both the male and female apoE−/−/Gclc-Tg mice had a lower frequency of thin fibrous caps. At 30 and 50 weeks of age, the apoE−/−/Gclc-Tg males had a higher frequency of plaques containing foam cells, while there was no significant difference in the females. In the female apoE−/−/Gclc-Tg mice at 40 and 50 weeks of age there was an increased frequency of extra-cellular cholesterol clefts. The lesions from the mice transplanted with bone marrow from the apoE−/−/Gclc-Tg mice were also analyzed for changes in plaque composition. There were few consistent differences for both time points (table 2, on-line data supplement). At 20 weeks post transplant, the lesions in the apoE−/− recipients had a higher frequency of foam cells, and a reduced frequency of chondrocyte-like cells and calcification as compared to the control mice. At 30 weeks post transplant there was also an increased frequency of foam cells and a reduced frequency of large necrotic cores and chondrocyte-like cells (table 2, on-line data supplement).
Immunohistochemistry
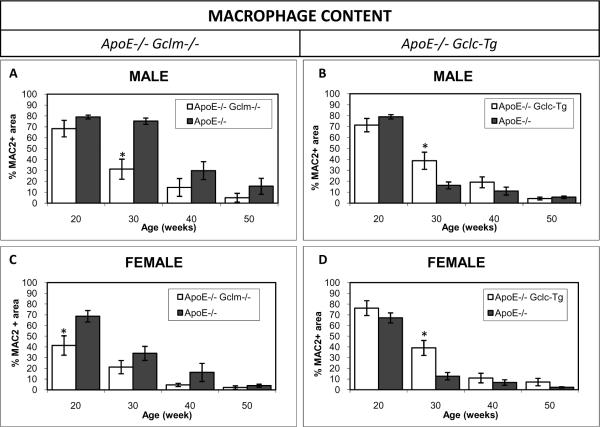
The presence of macrophages and smooth muscle cells in the plaques was confirmed by immunoperoxidase staining for the macrophage-specific marker Mac2 and for smooth muscle actin. There were fewer macrophages in the lesions of the apoE−/−/Gclm−/− male mice as compared to age matched littermate controls at 30 weeks of age. (fig 3a). The number of cells staining positive for smooth muscle actin increased with formation of the fibrous cap in the apoE−/−/Gclm−/− male mice at 20 weeks of age (fig 2 on-line data supplement). The lesions in the apoE−/−/Gclc-Tg mice had more macrophage staining as compared to age matched controls at 30 weeks of age in both male and female mice (fig 3b,d). The smooth muscle actin content of the lesions in the apoE−/−/Gclc-Tg was higher in the males at 20 weeks of age (fig 2 on-line data supplement).
Figure 3. Macrophage Content of the Lesions in the Innominate Artery.
Area of Mac 2 staining in lesions in the innominate artery as a percentage of the total area of the lesion for males (A, B), and females (C, D). Sample sizes were identical to those listed in figure 1. Data shown are the means ± SE. * p<0.05 vs littermate control.
Reduced Lesion Cellularity and Activation of Caspase 9
There was a reduced overall cellularity of the lesions (total # nuclei/1000 μm2) in both the male and female apoE−/−/Gclm−/− mice as compared to the littermate controls at all time points (fig 4a,c). There were no differences in the total cellularity of the lesions in both the male and female apoE−/−/Gclc-Tg mice as compared to the littermate controls at any time point (fig 4b,d). To help explain the reduced macrophage content and lesion cellularity in the apoE−/−/Gclm−/− mice, we investigated whether peritoneal macrophages from the apoE−/−/Gclm−/− mice were more susceptible to apoptosis induced by the pro-oxidant acrolein and whether the macrophages from the apoE−/−/Gclc-Tg mice were less susceptible than macrophages from apoE−/− mice. There was an increase in caspase 9 activity (1.8 fold) in the macrophages of the apoE−/−/Gclm−/− mice following treatment with acrolein. The cells were also more susceptible to staurosporine (2.2 fold increase) and when cultured in standard media they showed a higher baseline caspase 9 activity (~ 1.8 fold increase) than the macrophages from the control apo E−/− mice (fig 4e). There was no difference in caspase 9 activity in the peritoneal macrophages from the apoE−/−/Gclc-Tg mice after treatment with acrolein as compared to macrophages from the apoE−/− mice (figure 4f).
Plasma Cholesterol and Triglycerides Levels
There were no differences in the total plasma cholesterol levels between the age matched apoE−/−/Gclm−/− and apoE−/− or the apoE−/−/Gclc-Tg and control apo E−/− mice (table 2). There were also no differences in total plasma triglycerides. However, the analysis of the lipoprotein cholesterol profiles by FPLC showed a significant difference between the apoE−/−/Gclm−/− and apoE−/− mice at 20 weeks of age with the majority of the cholesterol in the VLDL fraction in apoE−/−/Gclm−/− mice with corresponding reductions in LDL and HDL cholesterol. These differences disappeared in the apoE−/−/Gclm−/− mice at 50 weeks of age (fig 4, on-line data supplement). In contrast, there were no differences in the lipoprotein cholesterol profiles in the apo E−/− mice 20 weeks following transplant with bone marrow from the apoE−/−/Gclm−/− mice (fig 5, on-line data supplement) suggesting that the absence of GCLM in macrophages did not account for the altered profile seen in the apoE−/−/Gclm−/− mice at 20 weeks of age.
Discussion
An association between glutathione metabolism and cardiovascular disease has been previously established17, 18, 29–33 and experimental studies have provided further support for this association20, 21, 34–36. For example, Rosenblat et al., previously demonstrated that manipulation of GSH levels in very young apo E−/− mice by pharmacological interventions modestly increased or protected against early fatty streak development in the aortic sinus19. Our current data is consistent with these previous observations and with previous in vitro and in vivo studies that show that increased GSH levels have anti-atherosclerotic effects14, 15, 37, 38. Importantly, this study is the first to show that increasing or decreasing endogenous antioxidant levels in macrophages is sufficient to have a measurable impact on established atherosclerosis.
The apoE−/−/Gclm−/− and apoE−/−/Gclc-Tg colonies were generated several years apart and were originally derived from separate apo E−/− breeding stocks. Thus, with the exception of the bone marrow transplant studies where the recipient mice were all from the same colony, all comparisons were made with the separate littermate control apo E−/− mice. However, there were a few consistencies between the groups. For example, there were more dramatic effects of the reduced availability of GSH in the females from the apoE−/−/Gclm−/− mice and more modest effects of increased GSH in the females from the apoE−/−/Gclc-Tg mice. It is currently unclear why there were gender dependent differences although it is well known that female mice on a C57Bl/6 background develop larger lesions39, 40. Estrogen also plays a role in repressing the expression of ARE dependent genes41. These observations reinforce the point that it is important to evaluate lesions in both sexes and at multiple sites42.
The up to 8 fold increase in lesion area in the innominate artery of the apoE−/−/Gclm−/− mice is a much larger increase than observed with most other genetic or pharmacological interventions reported so far for apo E−/− mice. This may reflect the fundamental role of GSH in many cellular processes. The reduced availability of GSH in the apoE−/−/Gclm−/− mice had the most dramatic effect at 20 and 30 weeks of age, a point at which the plaques were rapidly progressing43. Initiation and progression of atherosclerotic lesions in mice is largely dependent on cellular influx and proliferation coupled with lipid uptake and connective tissue deposition. This suggests that GSH may play an essential role in regulating these processes37, 44–47. It is surprising however that the larger lesions in the apoE−/−/Gclm−/− mice at 20 and 30 weeks of age also exhibited characteristics of more advanced lesions normally seen in the apo E−/− mice at 40 or more weeks of age43. Thus, the currently unknown processes that lead to formation of a thin fibrous cap, a large central necrotic core and intra-plaque hemorrhage in mice are also all accelerated with the reduction of GSH.
The more rapid formation of the necrotic core for example, may involve an increase in foam cell death due to increased oxidative stress48. To address this possibility, we originally attempted to measure cell death in the plaques in all of the mice. Unfortunately, in keeping with previous reports, we could not get consistent data using the TUNEL assay or staining for activated caspases with formalin fixed and paraffin embedded tissue49, 50. Thus, we compared the temporal changes in the overall cellularity of the plaques and in the macrophage and SMC content. There were fewer macrophages in the male mice at 30 weeks of age and a reduced number of total cells in the lesions from all of the apoE−/−/Gclm−/− mice and more macrophages in the lesions from some of the apoE−/−/Gclc-Tg mice suggesting that the differences in the GSH levels may have contributed to controlling the influx of monocytes/macrophages and/or the turnover of the cells. This is in keeping with previous in vitro studies that have shown that depletion of GSH makes vascular cells more susceptible to pro-oxidant induced cytotoxicity48, 51–53. It is also consistent with studies showing that alterations in macrophage glutathione content affects NFkB activation and expression of pro-inflammatory cytokines that stimulate adhesion molecule expression by endothelial cells and thus recruitment of monocytes/macrophages into developing lesions54–56.
However, protection from oxidant induced cytotoxicity may not have contributed significantly to the modestly reduced atherosclerosis in the apoE−/−/Gclc-Tg mice as there were no differences in the cellularity of the plaques or in the caspase 9 activity in the peritoneal macrophages. This suggests that there may be minimal oxidative stress in chow-fed apo E−/− mice. It is conceivable that there would have been more dramatic protective effects had we fed the mice a high fat diet and/or induced diabetes or uremia to elevate the levels of oxidative stress. The athero-protective effect of increasing GSH in macrophages may also be due to the role that GSH plays in maintaining redox dependent signal transduction11, 57 and protein glutathionylation58, 59, or to indirect effects mediated by other antioxidants such as alpha lipoic acid60.
The current results showing that increasing the capacity of macrophages to make GSH provides some protection from lesion progression even after lesions have been established is in contrast to our recent study showing no effect on the progression of established lesions in the apo E−/− mice following dietary treatment with antioxidants61. As antioxidant supplementation in humans has also not been very effective at reducing clinical events related to cardiovascular disease62, our data suggest that increasing endogenous antioxidant production rather than supplementing with dietary antioxidants may be an effective alternative therapeutic approach for controlling the progression of atherosclerosis.
Supplementary Material
Acknowledgements
We would like to thank Jerry Ricks, Charles Mahan, Kalynn Simmons and Brent Read for their expert technical assistance in the animal dissection and histology and Warren Ladiges and Carol Ware for helping in generating the Gclm−/− and Gclc-Tg mice.
Sources of funding: This study was supported by grants from the National Heart Lung and Blood Institute (R01 HL6748, R01 HL30086) and grants from the National Institute of Environmental Health Sciences (R01 ES10849, the Center for Ecogenetics and Environmental Health P30 ES007033, and the Environmental Pathology and Toxicology Training Grant T32 ES007032).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Dalton TP, Chen Y, Schneider SN, Nebert DW, Shertzer HG. Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Radic Biol Medq. 2004;37:1511–1526. doi: 10.1016/j.freeradbiomed.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 2.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 3.Sierra-Rivera E, Dasouki M, Summar ML, Krishnamani MR, Meredith M, Rao PN, Phillips JA, 3rd, Freeman ML. Assignment of the human gene (glclr) that encodes the regulatory subunit of gamma-glutamylcysteine synthetase to chromosome 1p21. Cytogenet Cell Genet. 1996;72:252–254. doi: 10.1159/000134202. [DOI] [PubMed] [Google Scholar]
- 4.Sierra-Rivera E, Summar ML, Dasouki M, Krishnamani MR, Phillips JA, Freeman ML. Assignment of the gene (glclc) that encodes the heavy subunit of gamma-glutamylcysteine synthetase to human chromosome 6. Cytogenet Cell Genet. 1995;70:278–279. doi: 10.1159/000134051. [DOI] [PubMed] [Google Scholar]
- 5.Tsuchiya K, Mulcahy RT, Reid LL, Disteche CM, Kavanagh TJ. Mapping of the glutamate-cysteine ligase catalytic subunit gene (glclc) to human chromosome 6p12 and mouse chromosome 9d-e and of the regulatory subunit gene (glclr) to human chromosome 1p21–p22 and mouse chromosome 3h1-3. Genomics. 1995;30:630–632. doi: 10.1006/geno.1995.1293. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Shertzer HG, Schneider SN, Nebert DW, Dalton TP. Glutamate cysteine ligase catalysis: Dependence on atp and modifier subunit for regulation of tissue glutathione levels. J Biol Chem. 2005;280:33766–33774. doi: 10.1074/jbc.M504604200. [DOI] [PubMed] [Google Scholar]
- 7.Seelig GF, Simondsen RP, Meister A. Reversible dissociation of gamma-glutamylcysteine synthetase into two subunits. J Biol Chem. 1984;259:9345–9347. [PubMed] [Google Scholar]
- 8.Dalton TP, Dieter MZ, Yang Y, Shertzer HG, Nebert DW. Knockout of the mouse glutamate cysteine ligase catalytic subunit (gclc) gene: Embryonic lethal when homozygous, and proposed model for moderate glutathione deficiency when heterozygous. Biochem Biophys Res Commun. 2000;279:324–329. doi: 10.1006/bbrc.2000.3930. [DOI] [PubMed] [Google Scholar]
- 9.Bekris LM, Shephard C, Janer M, Graham J, McNeney B, Shin J, Zarghami M, Griffith W, Farin F, Kavanagh TJ, Lernmark A. Glutamate cysteine ligase catalytic subunit promoter polymorphisms and associations with type 1 diabetes age-at-onset and gad65 autoantibody levels. Exp Clin Endocrinol Diabetes. 2007;115:221–228. doi: 10.1055/s-2007-970574. [DOI] [PubMed] [Google Scholar]
- 10.Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother. 2003;57:145–155. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biswas SK, Rahman I. Environmental toxicity, redox signaling and lung inflammation: The role of glutathione. Mol Aspects Med. 2008 doi: 10.1016/j.mam.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moinova HR, Mulcahy RT. Up-regulation of the human gamma-glutamylcysteine synthetase regulatory subunit gene involves binding of nrf-2 to an electrophile responsive element. Biochem Biophys Res Commun. 1999;261:661–668. doi: 10.1006/bbrc.1999.1109. [DOI] [PubMed] [Google Scholar]
- 13.Bea F, Hudson FN, Chait A, Kavanagh TJ, Rosenfeld ME. Induction of glutathione synthesis in macrophages by oxidized low-density lipoproteins is mediated by consensus antioxidant response elements. Circ Res. 2003;92:386–393. doi: 10.1161/01.RES.0000059561.65545.16. [DOI] [PubMed] [Google Scholar]
- 14.Bea F, Hudson FN, Neff-Laford H, White CC, Kavanagh TJ, Kreuzer J, Preusch MR, Blessing E, Katus HA, Rosenfeld ME. Homocysteine stimulates antioxidant response element-mediated expression of glutamate-cysteine ligase in mouse macrophages. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortese MM, Suschek CV, Wetzel W, Kroncke KD, Kolb-Bachofen V. Zinc protects endothelial cells from hydrogen peroxide via nrf2-dependent stimulation of glutathione biosynthesis. Free Radic Biol Med. 2008;44:2002–2012. doi: 10.1016/j.freeradbiomed.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Woo AY, Waye MM, Tsui SK, Yeung ST, Cheng CH. Andrographolide up-regulates cellular-reduced glutathione level and protects cardiomyocytes against hypoxia/reoxygenation injury. J Pharmacol Exp Ther. 2008;325:226–235. doi: 10.1124/jpet.107.133918. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura S, Kugiyama K, Sugiyama S, Miyamoto S, Koide S, Fukushima H, Honda O, Yoshimura M, Ogawa H. Polymorphism in the 5'-flanking region of human glutamate-cysteine ligase modifier subunit gene is associated with myocardial infarction. Circulation. 2002;105:2968–2973. doi: 10.1161/01.cir.0000019739.66514.1e. [DOI] [PubMed] [Google Scholar]
- 18.Koide S, Kugiyama K, Sugiyama S, Nakamura S, Fukushima H, Honda O, Yoshimura M, Ogawa H. Association of polymorphism in glutamate-cysteine ligase catalytic subunit gene with coronary vasomotor dysfunction and myocardial infarction. J Am Coll Cardiol. 2003;41:539–545. doi: 10.1016/s0735-1097(02)02866-8. [DOI] [PubMed] [Google Scholar]
- 19.Rosenblat M, Coleman R, Aviram M. Increased macrophage glutathione content reduces cell-mediated oxidation of ldl and atherosclerosis in apolipoprotein e-deficient mice. Atherosclerosis. 2002;163:17–28. doi: 10.1016/s0021-9150(01)00744-4. [DOI] [PubMed] [Google Scholar]
- 20.Biswas SK, Newby DE, Rahman I, Megson IL. Depressed glutathione synthesis precedes oxidative stress and atherogenesis in apo-e(−/−) mice. Biochem Biophys Res Commun. 2005;338:1368–1373. doi: 10.1016/j.bbrc.2005.10.098. [DOI] [PubMed] [Google Scholar]
- 21.t Hoen PA, Van der Lans CA, Van Eck M, Bijsterbosch MK, Van Berkel TJ, Twisk J. Aorta of apoe-deficient mice responds to atherogenic stimuli by a prelesional increase and subsequent decrease in the expression of antioxidant enzymes. Circ Res. 2003;93:262–269. doi: 10.1161/01.RES.0000082978.92494.B1. [DOI] [PubMed] [Google Scholar]
- 22.McConnachie LA, Mohar I, Hudson FN, Ware CB, Ladiges WC, Fernandez C, Chatterton-Kirchmeier S, White CC, Pierce RH, Kavanagh TJ. Glutamate cysteine ligase modifier subunit deficiency and gender as determinants of acetaminophen-induced hepatotoxicity in mice. Toxicol Sci. 2007;99:628–636. doi: 10.1093/toxsci/kfm165. [DOI] [PubMed] [Google Scholar]
- 23.Elbadawi A. Hexachrome modification of movat's stain. Stain Technol. 1976;51:249–253. doi: 10.3109/10520297609116713. [DOI] [PubMed] [Google Scholar]
- 24.Russell HK., Jr. A modification of movat's pentachrome stain. Arch Pathol. 1972;94:187–191. [PubMed] [Google Scholar]
- 25.Ou YC, White CC, Krejsa CM, Ponce RA, Kavanagh TJ, Faustman EM. The role of intracellular glutathione in methylmercury-induced toxicity in embryonic neuronal cells. Neurotoxicology. 1999;20:793–804. [PubMed] [Google Scholar]
- 26.White CC, Viernes H, Krejsa CM, Botta D, Kavanagh TJ. Fluorescence-based microtiter plate assay for glutamate-cysteine ligase activity. Anal Biochem. 2003;318:175–180. doi: 10.1016/s0003-2697(03)00143-x. [DOI] [PubMed] [Google Scholar]
- 27.Kirk EA, Dinauer MC, Rosen H, Chait A, Heinecke JW, LeBoeuf RC. Impaired superoxide production due to a deficiency in phagocyte nadph oxidase fails to inhibit atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2000;20:1529–1535. doi: 10.1161/01.atv.20.6.1529. [DOI] [PubMed] [Google Scholar]
- 28.Rosenfeld ME, Polinsky P, Virmani R, Kauser K, Rubanyi G, Schwartz SM. Advanced atherosclerotic lesions in the innominate artery of the apoe knockout mouse. Arterioscler Thromb Vasc Biol. 2000;20:2587–2592. doi: 10.1161/01.atv.20.12.2587. [DOI] [PubMed] [Google Scholar]
- 29.Hamanishi T, Furuta H, Kato H, Doi A, Tamai M, Shimomura H, Sakagashira S, Nishi M, Sasaki H, Sanke T, Nanjo K. Functional variants in the glutathione peroxidase-1 (gpx-1) gene are associated with increased intima-media thickness of carotid arteries and risk of macrovascular diseases in japanese type 2 diabetic patients. Diabetes. 2004;53:2455–2460. doi: 10.2337/diabetes.53.9.2455. [DOI] [PubMed] [Google Scholar]
- 30.Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, Smieja M, Cambien F, Meyer J, Lackner KJ. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med. 2003;349:1605–1613. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- 31.Dogru-Abbasoglu S, Kanbagli O, Bulur H, Babalik E, Ozturk S, Aykac-Toker G, Uysal M. Lipid peroxides and antioxidant status in serum of patients with angiographically defined coronary atherosclerosis. Clin Biochem. 1999;32:671–672. doi: 10.1016/s0009-9120(99)00076-4. [DOI] [PubMed] [Google Scholar]
- 32.Espinola-Klein C, Rupprecht HJ, Bickel C, Schnabel R, Genth-Zotz S, Torzewski M, Lackner K, Munzel T, Blankenberg S. Glutathione peroxidase-1 activity, atherosclerotic burden, and cardiovascular prognosis. Am J Cardiol. 2007;99:808–812. doi: 10.1016/j.amjcard.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 33.Winter JP, Gong Y, Grant PJ, Wild CP. Glutathione peroxidase 1 genotype is associated with an increased risk of coronary artery disease. Coron Artery Dis. 2003;14:149–153. doi: 10.1097/00019501-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Torzewski M, Ochsenhirt V, Kleschyov AL, Oelze M, Daiber A, Li H, Rossmann H, Tsimikas S, Reifenberg K, Cheng F, Lehr HA, Blankenberg S, Forstermann U, Munzel T, Lackner KJ. Deficiency of glutathione peroxidase-1 accelerates the progression of atherosclerosis in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:850–857. doi: 10.1161/01.ATV.0000258809.47285.07. [DOI] [PubMed] [Google Scholar]
- 35.Guo Z, Ran Q, Roberts LJ, 2nd, Zhou L, Richardson A, Sharan C, Wu D, Yang H. Suppression of atherogenesis by overexpression of glutathione peroxidase-4 in apolipoprotein e-deficient mice. Free Radic Biol Med. 2008;44:343–352. doi: 10.1016/j.freeradbiomed.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiao M, Kisgati M, Cholewa JM, Zhu W, Smart EJ, Sulistio MS, Asmis R. Increased expression of glutathione reductase in macrophages decreases atherosclerotic lesion formation in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:1375–1382. doi: 10.1161/ATVBAHA.107.142109. [DOI] [PubMed] [Google Scholar]
- 37.Dedoussis GV, Kaliora AC, Psarras S, Chiou A, Mylona A, Papadopoulos NG, Andrikopoulos NK. Antiatherogenic effect of pistacia lentiscus via gsh restoration and downregulation of cd36 mrna expression. Atherosclerosis. 2004;174:293–303. doi: 10.1016/j.atherosclerosis.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Rosenblat M, Aviram M. Macrophage glutathione content and glutathione peroxidase activity are inversely related to cell-mediated oxidation of ldl: In vitro and in vivo studies. Free Radic Biol Med. 1998;24:305–317. doi: 10.1016/s0891-5849(97)00231-1. [DOI] [PubMed] [Google Scholar]
- 39.Tangirala RK, Rubin EM, Palinski W. Quantitation of atherosclerosis in murine models: Correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in ldl receptor-deficient and apolipoprotein e-deficient mice. J Lipid Res. 1995;36:2320–2328. [PubMed] [Google Scholar]
- 40.Paigen B, Holmes PA, Mitchell D, Albee D. Comparison of atherosclerotic lesions and hdl-lipid levels in male, female, and testosterone-treated female mice from strains c57bl/6, balb/c, and c3h. Atherosclerosis. 1987;64:215–221. doi: 10.1016/0021-9150(87)90249-8. [DOI] [PubMed] [Google Scholar]
- 41.Zhou W, Lo SC, Liu JH, Hannink M, Lubahn DB. Errbeta: A potent inhibitor of nrf2 transcriptional activity. Mol Cell Endocrinol. 2007;278:52–62. doi: 10.1016/j.mce.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 42.VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: Site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24:12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- 43.Rosenfeld ME, Averill MM, Bennett BJ, Schwartz SM. Progression and disruption of advanced atherosclerotic plaques in murine models. Curr Drug Targets. 2008;9:210–216. doi: 10.2174/138945008783755575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moon SK, Thompson LJ, Madamanchi N, Ballinger S, Papaconstantinou J, Horaist C, Runge MS, Patterson C. Aging, oxidative responses, and proliferative capacity in cultured mouse aortic smooth muscle cells. Am J Physiol Heart Circ Physiol. 2001;280:H2779–2788. doi: 10.1152/ajpheart.2001.280.6.H2779. [DOI] [PubMed] [Google Scholar]
- 45.Tyagi SC, Kumar S, Borders S. Reduction-oxidation (redox) state regulation of extracellular matrix metalloproteinases and tissue inhibitors in cardiac normal and transformed fibroblast cells. J Cell Biochem. 1996;61:139–151. doi: 10.1002/(sici)1097-4644(19960401)61:1<139::aid-jcb15>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 46.Fuhrman B, Volkova N, Aviram M. Oxidative stress increases the expression of the cd36 scavenger receptor and the cellular uptake of oxidized low-density lipoprotein in macrophages from atherosclerotic mice: Protective role of antioxidants and of paraoxonase. Atherosclerosis. 2002;161:307–316. doi: 10.1016/s0021-9150(01)00646-3. [DOI] [PubMed] [Google Scholar]
- 47.Aviram M. Macrophage foam cell formation during early atherogenesis is determined by the balance between pro-oxidants and anti-oxidants in arterial cells and blood lipoproteins. Antioxid Redox Signal. 1999;1:585–594. doi: 10.1089/ars.1999.1.4-585. [DOI] [PubMed] [Google Scholar]
- 48.Gotoh N, Graham A, Nikl E, Darley-Usmar VM. Inhibition of glutathione synthesis increases the toxicity of oxidized low-density lipoprotein to human monocytes and macrophages. Biochem J. 1993;296(Pt 1):151–154. doi: 10.1042/bj2960151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tateyama H, Tada T, Hattori H, Murase T, Li WX, Eimoto T. Effects of prefixation and fixation times on apoptosis detection by in situ end-labeling of fragmented DNA. Arch Pathol Lab Med. 1998;122:252–255. [PubMed] [Google Scholar]
- 50.Ichimura E, Fukuda T, Oyama T, Kashiwabara K, Sakurai S, Sano T, Nakajima T. Formalin fixation by boiling: Is it suitable for the tunel staining? Pathol Int. 1995;45:971–972. doi: 10.1111/j.1440-1827.1995.tb03424.x. [DOI] [PubMed] [Google Scholar]
- 51.Cho S, Hazama M, Urata Y, Goto S, Horiuchi S, Sumikawa K, Kondo T. Protective role of glutathione synthesis in response to oxidized low density lipoprotein in human vascular endothelial cells. Free Radic Biol Med. 1999;26:589–602. doi: 10.1016/s0891-5849(98)00232-9. [DOI] [PubMed] [Google Scholar]
- 52.Zhao Z, Francis CE, Welch G, Loscalzo J, Ravid K. Reduced glutathione prevents nitric oxide-induced apoptosis in vascular smooth muscle cells. Biochim Biophys Acta. 1997;1359:143–152. doi: 10.1016/s0167-4889(97)00093-1. [DOI] [PubMed] [Google Scholar]
- 53.Guo Z, Van Remmen H, Yang H, Chen X, Mele J, Vijg J, Epstein CJ, Ho YS, Richardson A. Changes in expression of antioxidant enzymes affect cell-mediated ldl oxidation and oxidized ldl-induced apoptosis in mouse aortic cells. Arterioscler Thromb Vasc Biol. 2001;21:1131–1138. doi: 10.1161/hq0701.092092. [DOI] [PubMed] [Google Scholar]
- 54.Gosset P, Wallaert B, Tonnel AB, Fourneau C. Thiol regulation of the production of tnf-alpha, il-6 and il-8 by human alveolar macrophages. Eur Respir J. 1999;14:98–105. doi: 10.1034/j.1399-3003.1999.14a17.x. [DOI] [PubMed] [Google Scholar]
- 55.Neuschwander-Tetri BA, Bellezzo JM, Britton RS, Bacon BR, Fox ES. Thiol regulation of endotoxin-induced release of tumour necrosis factor alpha from isolated rat kupffer cells. Biochem J. 1996;320(Pt 3):1005–1010. doi: 10.1042/bj3201005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parmentier M, Hirani N, Rahman I, Donaldson K, MacNee W, Antonicelli F. Regulation of lipopolysaccharide-mediated interleukin-1beta release by nacetylcysteine in thp-1 cells. Eur Respir J. 2000;16:933–939. doi: 10.1183/09031936.00.16593300. [DOI] [PubMed] [Google Scholar]
- 57.Rahman I, Biswas SK, Jimenez LA, Torres M, Forman HJ. Glutathione, stress responses, and redox signaling in lung inflammation. Antioxid Redox Signal. 2005;7:42–59. doi: 10.1089/ars.2005.7.42. [DOI] [PubMed] [Google Scholar]
- 58.Ghezzi P. Regulation of protein function by glutathionylation. Free Radic Res. 2005;39:573–580. doi: 10.1080/10715760500072172. [DOI] [PubMed] [Google Scholar]
- 59.Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A. S-glutathionylation in protein redox regulation. Free Radic Biol Med. 2007;43:883–898. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 60.Zhang WJ, Bird KE, McMillen TS, LeBoeuf RC, Hagen TM, Frei B. Dietary alpha-lipoic acid supplementation inhibits atherosclerotic lesion development in apolipoprotein e-deficient and apolipoprotein e/low-density lipoprotein receptor-deficient mice. Circulation. 2008;117:421–428. doi: 10.1161/CIRCULATIONAHA.107.725275. [DOI] [PubMed] [Google Scholar]
- 61.Averill MM, Bennett BJ, Rattazzi M, Rodmyre RM, Kirk EA, Schwartz SM, Rosenfeld ME. Neither antioxidants nor genistein inhibit the progression of established atherosclerotic lesions in older apoe deficient mice. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kris-Etherton PM, Lichtenstein AH, Howard BV, Steinberg D, Witztum JL. Antioxidant vitamin supplements and cardiovascular disease. Circulation. 2004;110:637–641. doi: 10.1161/01.CIR.0000137822.39831.F1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.