A photoluminescence probe ARC-1185, possessing both high affinity towards basophilic protein kinases (PKs) and microsecond-scale luminescence lifetime when associated with a kinase, was used for the mapping of ARC-1185/PK complexes in living cells with time-gated luminescence microscopy.
Protein kinases (PKs) play a key role in signalling pathways that regulate cellular functions. The aberrant activity of PKs due to mutation, altered expression or dysregulation is characteristic of many diseases, especially cancer.1 Therefore, sensitive, selective and reliable methods for monitoring localization and activity of PKs in living cells would be useful for understanding complex signalling cascades and for explaining the roles of kinases in human diseases.
Both substrate-based and activity-based probes have been used to map the activity of enzymes (especially proteases) in living cells and animals.2,3,4 Numerous examples of genetically encoded protein- and small molecule-based fluorescence probes for monitoring PK activity have also been reported.5 Fluorescent probes possessing optical properties that are dependent on their fast, reversible association with a target PK6,7 have a distinct advantage compared to irreversible activity-based probes in that they can be used to continuously monitor PK activity.
We have described responsive luminescence probes (ARC-Lum probes) for PKs.8 These probes that are based on conjugates of adenosine analogues and arginine-rich peptides (ARCs) act as bisubstrate inhibitors of PKs.9 ARC-Lum probes incorporate a thiophene or a selenophene containing moiety and a fluorophore conjugated to the ε-amino group of a C-terminal lysine residue of the peptide fragment (Figure 1). In the complex with a PK, ARC-Lum probes emit long lifetime (τ = 19 – 266 μs) luminescence at the emission wavelengths of the fluorescent label if the complex is illuminated at the excitation wavelength of the thiophene or selenophene containing donors (λex < 380 nm). Free ARC-Lum probes in a buffer solution do not possess long lifetime luminescence properties. ARC-Lum probes are cell plasma membrane permeable, stable in intracellular milieu, bind to PKs with high affinity (Kd < 100 pM)10 and hence are suitable for imaging of PKs in living cells.
Figure 1.
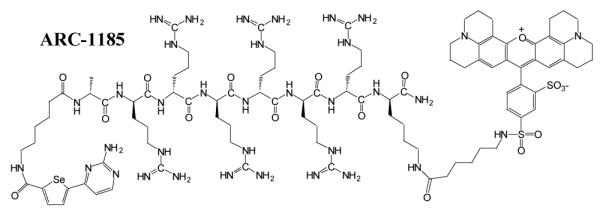
Structure of ARC-1185
The enhancement of the luminescence signal upon binding of the probe to the active form of target kinase enables highly sensitive, time-gated luminescence (TGL)-based imaging of PK localization . With TGL microscopy, a short pulse of light is used to excite the sample and the detector is switched on after a brief delay (> 100 ns) when short-lifetime (~ns) fluorescence has decayed. The possibility of parallel measurement of the intensity of steady state fluorescence (FL) of the probe via direct excitation of its fluorescent label makes the probe suitable for ratiometric measurements alleviating many of the problems characteristic for luminescence measurements in cells.11
Herein, we describe the results of ARC-Lum probe-based imaging studies in living cells using a TGL microscope with epi-illumination and wide-field detection. We show that an ARC-Lum probe that is taken up by MDCKII cells specifically associates with active kinases resulting in a long lifetime signal that is well separable from the fluorescence background of the cells.
In cells, ARC-based probes have to compete with ATP present at millimolar concentration for binding to target PKs. Therefore, high affinity of the probe is needed to achieve substantial binding of the probe to the kinases. ARC-1185 is a new compound that was designed to have higher selectivity towards PKAc than previous ARC-Lum probes, and to possess stronger luminescence signal in the complex with PKAc. To establish the affinity of the probe we titrated ARC-1185 (Figure 1) against fixed concentration (2 nM) of various PKs in a biochemical assay (Figure 2). Illumination of ARC-1185 in complex with a PK with near-UV light (λex < 380 nm) leads to phosphorescence of the selenophene-containing aromatic system followed by Förster-type energy transfer to the fluorescence dye Texas Red12 [optical characteristics for Texas Red dye12: λex(max) = 591 nm, λem(max) = 610 nm, ε = 85,400 M−1 cm−1] and generation of long lifetime (τ > 20 μs) signal at emission wavelengths of the fluorescent dye. Using a luminescence plate reader, the complexes of ARC-Lum probe with different kinases were excited with a flash of the xenon lamp at 337 (50) nm, thereafter the signal decay curves were recorded and luminescence lifetimes calculated for the complexes (Table 1). The dissociation constants KD for binding of ARC-1185 to various PKs ranged in the region of 0.2 nM < KD < 40 nM (Table 1). The most intense photoluminescence signal and the highest affinity was measured for the complex of ARC-1185 with PKAc (Table 1 and Figure 2).
Figure 2.

Titration of different protein kinases (● PKAc, ∎ ROCKII, ▴ MSK1, ▾ PKB ◆ PKCδ, × Pim1; all at 2 nM concentration) with the luminescence probe ARC-1185 [λex = 337(50) nm, λem = 630(40) nm, delay time 50 μs, integration time 150 μs]. The lines represent nonlinear least squares fits to the data according to Supplementary Eq.1.
Table 1.
Luminescence intensities, luminescence lifetimes and dissociation constants for complexes of ARC-1185 with various protein kinases
| Kinase | NLIa | τ (μs)b | KD (nM) |
|---|---|---|---|
| No kinase | ~0.0005 | < 5 | - |
| PKAc | 1.00 | 80.5±1.8 | <0.2 |
| ROCKII | 0.53 | 35.5± 1.5 | 2.2±0.9 |
| MSK1 | 0.44 | 47.7± 1.5 | 10.1±3.5 |
| AKT3 | 0.50 | 36.5± 1.3 | 38.8±4.4 |
| PKCδ | 0.46 | 47.8± 1.9 | 20.2±5.0 |
| Pim1 | 0.03 | 24.8± 1.2 | 9.1±4.8 |
NLI (normalized luminescence intensity): integrated luminescence intensity of the ARC-1185/PK complex (delay time 50 μs, integration time 150 μs) normalized to that of ARC-1185/PKAc complex;
τ : luminescence lifetime of the ARC-1185/PK complex.
Intracellular delivery of ARC-1185 was monitored with FL microscopy and the localization of ARC-1185/kinase complex was visualized using a TGL microscope.13,14 With this system, pulsed UV light (LED, λex = 365 nm) was used to excite the selenophene-containing donor moiety of ARC-1185, and emission of light in red spectral region [λem = 610(75) nm] was measured after a delay of 10 μs with an intensified charge coupled device (ICCD) camera. This measurement regime effectively eliminated short-lived fluorescence signals of cells and direct excitation of the Texas Red fluorescent dye, enabling imaging of ARC-Lum/PK complexes with high signal-to-background ratio. The sensitivity of the microscope could be adjusted by varying either the number of pulse/detection cycles integrated during a single camera frame or by varying the ICCD gain level. A complete description of the instrumental setup used to acquire all images reported in this study is provided in Supplementary.
The applicability of ARC-1185 for mapping ARC-1185/PK complexes was studied in MDCKII cells. The cells were incubated in growth medium containing 10 μM ARC-1185 for 1 h, thereafter the cells were washed with PBS and imaged. FL images revealed intensive uptake of the probe in all cells with stronger signal intensities from the nuclei (Figure 3, left panel). Comparison of FL and TGL images (Figure 3) pointed to approximate co-localization of the ARC-1185 probe (FL microscopy; Figure 3, left panel) and ARC-1185/PK complexes (TGL microscopy, Figure 3, right panel). As controls, the application of both an ARC-based fluorescent probe (ARC-Fluo) possessing no long lifetime luminescence properties and a nona-D-arginine peptide labelled with Texas Red revealed cellular uptake of the compounds according to FL microscopy setup but no signal was detected in the TGL mode (Supplementary Figure 1).
Figure 3.
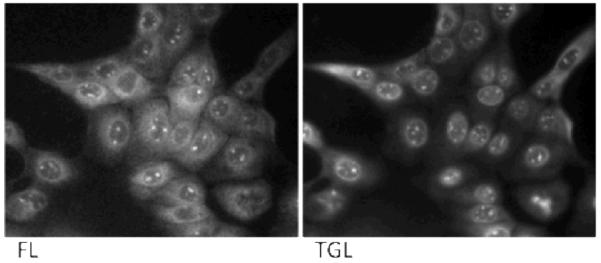
Luminescence imaging of MDCKII cells using ARC-1185 (10 μM, 1 h) with microscopy in steady state fluorescence [FL, λex = 545 (30) nm, λem = 605 (15) nm] and time-gated luminescence [TGL, λex = 365 nm, λem = 610(75) nm, delay time 10 μs] modes. Representative of experiments repeated three times with similar results.
ARC-type inhibitors rich in D-arginine residues bind with high affinity to basophilic PKs that mostly belong to the AGC group,15 but also to some other groups (e.g., PIM kinases). Several basophilic kinases (incl. PKA) catalyze phosphorylation of nuclear proteins with important physiological roles (CREB, histones, etc.).16,17 Thus the bright spot-like structures in the nuclei may point to regions (speckles) rich in active PKs18. To demonstrate that the punctuate staining mode of ARC-1185 in the nuclei visualized with TGL microscopy is not resulting from non-specific binding of the arginine-rich probe to negatively-charged nucleic acids, biochemical titration of the ARC-Lum probe with different DNA and RNA samples was carried out. Although good binding of ARC-1185 to salmon sperm DNA was detected by change in fluorescence anisotropy (KD = 100 nM; Supplementary Figure 2, left), the association of DNA with the probe did not result in a significant long lifetime luminescence signal (Supplementary Figure 2, right).
Approximate co-localization of intensity profiles of FL and TGL signals in some regions of cells points to the situation when most of ARC-1185 is associated with binding PKs. Furthermore, as active cellular kinases compete for binding to the ARC-Lum probe the implication of each active PK to the intensity profile of the TGL image is a combination of the (local) concentration of the PKs and their affinity towards the probe, molar brightness of the complexes of the PKs with the probe, and other factors.
To further demonstrate that the observed TGL signal was due to the association of ARC-1185 with PKs, MDCKII cells were incubated with ARC-1185 (10 μM) in the presence of forskolin (25 μM) and IBMX (100 μM). The latter compounds increase cellular cAMP concentration leading to activation of PKA and liberation of free PKAc that associates with ARC-1185. Real-time monitoring of the TGL signal was started immediately without removal of the incubation solution (time-lapse microscopy), and it was continued for the following 120 minutes. The TGL signal was detectable both in the cytoplasm and nucleus of forskolin-activated cells already after 3 min of incubation (Figure 4, Supplementary Figure 3 and 4), whereas the signal appeared in non-activated cells after 10-15 min. After 30 - 60 min of incubation the localization profiles of luminescence intensity in non-activated and activated cells were similar. The earlier onset of TGL signal in forskolin-activated cells suggests that the signal results from specific binding of ARC-1185 to PKAc. The similarity in luminescence phenotypes seen in forskolin-activated and non-activated cells after longer incubation times suggests that a substantial portion of the signal results from the interaction of the probe with other than PKAc basophilic PKs or the affinity of ARC-1185 towards PKAc is high enough to outcompete the regulatory subunit of PKA from the holoenzyme bound to cellular membranes, resulting in relocation of the ARC-1185/PKAc complex to the nucleus. Alternatively, recent research has pointed to the presence a resident pool of PKA holoenzyme19 or a spotty localization of free PKAc18 in the nuclei of cells. PKAc from both sources may associate with ARC-1185 and give a TGL signal.
Figure 4.
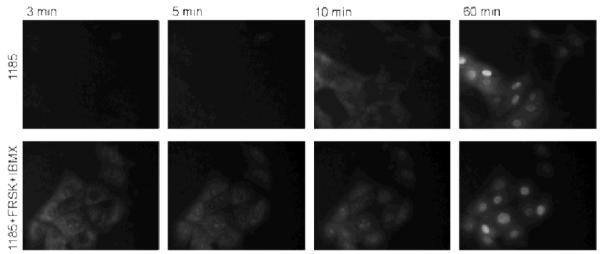
Formation of the TGL signal in MDCKII cells at 3, 5, 10, and 60 min after starting the incubation with ARC-1185 (10 μm). Upper row, non-activated cells; lower row, PKA-activated (forskolin and IBMX) cells, as monitored with TGL microscopy [λex = 365 nm, λem = 610(75) nm, delay time = 10 μs]. Representative of experiments repeated three times with similar results.
Further research with the application of ARC-Lum probes with altered affinity or selectivity profiles would clarify the role of different PKs in the generation of TGL signal in live cells. Also, the use of different cells possessing different PK expression and activity profiles and their activation mechanisms will establish the usefulness of the novel ARC-Lum probes for signal transduction research.
In conclusion, the work demonstrates that the responsive nonmetal photoluminescence probe ARC-1185 with a wider PK activity profile is applicable for monitoring and mapping of formation of ARC-1185/PK complexes and their localization in living cells with TGL microscopy. In complex with a PK, the probe possesses microsecond-scale luminescence lifetime after pulsed excitation with UV-light. Such probes are of interest for high-content analysis of cellular responses to different extracellular and intracellular stimuli, but also for signal transduction studies. Highly specific ARC-Lum probes for spying cellular activity of specific kinases are currently under development.
Supplementary Material
Acknowledgments
This work was supported by grants from the Estonian Ministry of Education and Sciences (SF0180121s08) and the Estonian Science Foundation (8230 and 8419) and the U.S. National Institutes of Health (R01GM081030).
Footnotes
Electronic Supplementary Information (ESI) available : Experimental details and further characterization. See DOI: 10.1039/b000000x/
Notes and references
- 1.Schwartz PA, Murray BW. Bioorg. Chem. 2011;39:192. doi: 10.1016/j.bioorg.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Van Noorden CJF. J. Histochem. Cytochem. 2010;58:481. doi: 10.1369/jhc.2010.955518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edgington LE, Verdoes M, Bogyo M. Curr. Opin. Chem. Biol. 2011;15:798. doi: 10.1016/j.cbpa.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Razgulin A, Maand N, Rao J. Chem. Soc. Rev. 2011;40:4186. doi: 10.1039/c1cs15035a. [DOI] [PubMed] [Google Scholar]
- 5.Newman RH, Fosbrink MD, Zhang J. Chem. Rev. 2011;111:3614. doi: 10.1021/cr100002u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C-S, Poenie M. J. Biol. Chem. 1993;268:15812. [PubMed] [Google Scholar]
- 7.Dupont JL, Janoshazi A, Bellahcene M, Mykita S, de Barry J. Eur. J. Neurosci. 2000;12:215. doi: 10.1046/j.1460-9568.2000.00906.x. [DOI] [PubMed] [Google Scholar]
- 8.Enkvist E, Vaasa A, Kasari M, Kriisa M, Ivan T, Ligi K, Raidaru G, Uri A. ACS Chem. Biol. 2011;6:1052. doi: 10.1021/cb200120v. [DOI] [PubMed] [Google Scholar]
- 9.Enkvist E, Lavõgina D, Raidaru G, Vaasa A, Viil I, Lust M, Viht K, Uri A. J. Med. Chem. 2006;49:7150. doi: 10.1021/jm0605942. [DOI] [PubMed] [Google Scholar]
- 10.Kasari M, Padrik P, Vaasa A, Saar K, Leppik K, Soplepmann J, Uri A. Anal. Biochem. 2012;422:79. doi: 10.1016/j.ab.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 11.Lemke EA, Schultz C. Nat. Chem. Biol. 2011;7:480. doi: 10.1038/nchembio.620. [DOI] [PubMed] [Google Scholar]
- 12.Lefevre C, Kang HC, Haugland RP, Malekzadeh N, Arttamangkul S, Haugland RP. Bioconjug. Chem. 1996;7:482. doi: 10.1021/bc960034p. [DOI] [PubMed] [Google Scholar]
- 13.Gahlaut N, Miller LW. Cytometry A. 2010;77:1113. doi: 10.1002/cyto.a.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajapakse HE, Gahlaut N, Mohandessi S, Yu D, Turner JR, Miller LW. Proc. Natl. Acad. Sci. USA. 2010;107:13582. doi: 10.1073/pnas.1002025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. Science. 2002;298:1912. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 16.Baek SH. Mol. Cell. 2011;42:274. doi: 10.1016/j.molcel.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Johannessen M, Moens U. Front Biosci. 2007;12:1814. doi: 10.2741/2190. [DOI] [PubMed] [Google Scholar]
- 18.Kvissel AK, Ørstavik S, Eikvar S, Brede G, Jahnsen T, Collas P, Akusjärvi G, Skålhegg BS. Exp. Cell Res. 2007;313:2795. doi: 10.1016/j.yexcr.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Sample V, DiPilato LM, Yang JH, Ni Q, Saucerman JJ, Zhang J. Nat. Chem. Biol. 2012;8:375. doi: 10.1038/nchembio.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


