Abstract
Background
Exercise oscillatory ventilation (EOV) is a non-invasive parameter that potently predicts outcomes in systolic heart failure (HF). However, mechanistic insights into EOV have been limited by the absence of studies relating EOV to invasive hemodynamic measurements and blood gases performed during exercise.
Methods and Results
56 patients with systolic HF (age 59±2 years [mean±SEM], left ventricular ejection fraction 30±1%) and 19 age-matched controls were studied with incremental cardiopulmonary exercise testing. Fick cardiac outputs, filling pressures, and arterial blood gases were measured at one-minute intervals during exercise. EOV was detected in 45% of HF (HF+EOV) patients and in none of the controls. The HF+EOV group did not differ from the HF patients without EOV (HF-EOV) in age, gender, BMI, LVEF, or etiology of HF. Univariate predictors of the presence of EOV in HF, among measurements performed during exercise, included higher right atrial pressure and pulmonary capillary wedge pressure and lower cardiac index (CI), but not PaCO2 or PaO2. Multivariate logistic regression identified that low exercise CI is the strongest predictor of EOV (odds ratio 1.39, for each 1.0 L/min/m2 decrement in CI, 95% confidence interval 1.14–1.70, P=0.001). Among HF patients with EOV, exercise CI was inversely related to EOV cycle length (R=−0.71) and amplitude (R=−0.60), both P<0.001. EOV cycle length and amplitude decreased proportionate to increases in CI in 11 HF+EOV subjects treated with 12 weeks of sildenafil.
Conclusions
EOV is closely related to reduced CI and elevated filling pressures during exercise and may be an important surrogate for exercise-induced hemodynamic impairment in HF patients.
Clinical Trials Registration
ClinicalTrials.gov number NCT00309790.
Keywords: Heart failure, exercise, ventilation, cardiac output
INTRODUCTION
Periodic breathing (PB), the cyclic variation of ventilation with a period of approximately 1 minute at rest, has been a recognized feature of systolic heart failure (HF) for almost 200 years.1, 2 More recently, cyclic fluctuations in minute ventilation during exercise, termed exercise oscillatory ventilation (EOV), have been observed in 19–51% 3–8 of patients with HF. EOV has emerged as a potent independent risk factor for adverse prognosis in HF that is additive to traditional echocardiographic and metabolic indices of clinical risk.3, 5, 7–10
Despite the clear association between EOV and outcomes in chronic HF, there is limited data regarding the mechanistic basis for EOV. Theoretical models to explain PB at rest implicate instability in the feedback systems controlling ventilation.11–15 Ventilation is regulated through the feedback loop between pulmonary gas exchanging capillaries and chemoreceptors in the carotid bodies (peripheral) and the medulla (central) that respond to O2 and CO2 levels in blood.11–16 As with any feedback control system, instability and oscillations in ventilation in HF may arise from: 1) delay in information transfer (i.e. increased circulation time due to reduced cardiac index);8, 14 2) increase in controller gain (i.e. increased chemosensitivity);16, 17 or reduction in system damping (i.e. baroreflex impairment).18 Increased chemosensitivity triggers a cycle of hyperventilation-induced reduction in PaCO2 until the apnea threshold is approached; then hypoventilation until PaCO2 rises and hyperventilation resumes. Over-stimulation of the ventilatory control center by pulmonary congestion19, 20 has also been postulated to contribute to PB at rest in HF.
Putative mechanisms involved in the etiology of PB during exercise (i.e. EOV) have been largely extrapolated from studies of PB at rest and during sleep.8, 21, 22 Metabolic abnormalities in skeletal muscle due to HF may also lead to enhanced ergoreflex during exercise,23 increasing central chemosensitivity and EOV. In the one study that examined both PB during sleep and EOV in the same patients, however, only one third of patients with either PB at rest or EOV had both conditions. The lack of overlap between PB during exercise and during sleep in this study indicates potentially distinct pathophysiologic mechanisms mediating PB at rest, during sleep, and during exertion.3 To date, however, simultaneous measurements of ventilatory drive (i.e. PaCO2), systemic oxygenation (PaO2), circulatory delay (i.e. CI), and pulmonary congestion (i.e. pulmonary capillary wedge pressure) have not been performed during exercise and related to EOV.
In this study, we hypothesized that EOV is primarily related to impaired ability to augment CI during exercise with resultant circulatory delay causing instability in the ventilatory control feedback loop. To test this hypothesis we measured right heart hemodynamics, arterial blood gases, and ventilatory response patterns at rest and throughout exercise in patients with systolic HF and in matched controls. We associated amplitudes and cycle lengths of oscillations with ventilatory gas exchange parameters and exercise hemodynamic measurements in HF patients with EOV (HF+EOV) and without EOV (HF-EOV). Finally, we assessed whether previously demonstrated improvements in CI with administration of the phosphodiesterase 5-(PDE5) inhibitor, sildenafil,24 would reduce oscillatory cycle length and amplitude in individuals who underwent cardiopulmonary exercise testing (CPET) before and after 12 weeks of treatment.
METHODS
Patient population and study design
Consecutive patients who underwent CPET with invasive hemodynamic monitoring at Massachusetts General Hospital, had left ventricular ejection fraction (LVEF) < 0.40, and chronic NYHA class II–IV symptoms despite standard therapy were included in the study population. Exclusion criteria consisted of the following: 1) incomplete pulmonary arterial catheter pressure measurements; 2) documented intracardiac shunting; or 3) the presence of a pulmonary mechanical limitation to exercise as defined by VE/(forced expiratory volume in 1 second [FEV1] × 35) > 0.7 at the anaerobic threshold.25 Fifty-three percent of the LV dysfunction subjects (n=30) participated in a previously reported 12-week randomized, double-blind clinical trial of treatment with sildenafil (N=15) or placebo (N=15).24 The control group was included in order to determine the extent to which hemodynamic and blood gas measurements in LVSD subjects with and without EOV differed from age-matched controls. Controls consisted of subjects referred for CPET to evaluate dyspnea on exertion during the same period of time as the LVSD group. Controls were required to have normal left ventricular function and normal exercise capacity as reflected by a peak VO2 greater than 80% of that predicted on the basis of age, gender, and height. Subjects meeting these inclusion criteria who were similar in age to the LVSD subjects composed the control cohort.
Cardiopulmonary exercise testing
All patients underwent placement of a pulmonary arterial catheter via the internal jugular vein and placement of a systemic arterial catheter via the radial artery. First-pass radionuclide ventriculography of both ventricles was performed immediately prior to cycle ergometry testing as previously described.24 Subjects then underwent maximum incremental upright cycle ergometry CPET (5–15 Watt/min continuous ramp after an initial 3 minute period of unloaded exercise, MedGraphics, St. Paul, MN) with simultaneous hemodynamic monitoring (Witt Biomedical Inc, Melbourne, FL) as previously described.24, 26 In subjects enrolled in the clinical trial of sildenafil treatment,24 CPET was performed at baseline and after 12 weeks of treatment with sildenafil or placebo. Right atrial pressure (RAP), pulmonary arterial pressure (PAP), pulmonary capillary wedge pressure (PCWP), and mean systemic arterial pressure (MAP) were measured in the upright position, at end-expiration, while patients were seated on the cycle, at rest, and at one-minute intervals during exercise. Fick cardiac outputs (CO) were also determined at one minute intervals throughout exercise by measuring O2 uptake (VO2) and simultaneous radial arterial and pulmonary arterial O2 content to calculate the arteriovenous O2 difference (C(a-v)O2). Peak VO2 was defined as the highest O2 uptake, averaged over 30 seconds, during the last minute of symptom-limited exercise, as previously described.26
Ventilatory gas exchange
To account for oscillations in gas exchange variables, we averaged breath-by-breath gas exchange variables over 1 minute for comparison with other variables collected each minute during exercise (i.e. PCWP and blood gases). Ventilatory efficiency (VE/VCO2 slope) was calculated by previously defined techniques27 using breath-by-breath data. A subject was considered to have EOV, if they had three or more consecutive, regular oscillations in ventilation (VE) during exercise, with VE oscillation amplitude ≥ 25% of average VE,28, 29 persisting for ≥ 60% of exercise duration.30 Oscillatory cycle length was measured as the average time from nadir-to-nadir for each respective oscillation. Oscillatory amplitude was measured as the difference between the peak VE of the oscillation and the average of the VE of the two surrounding nadirs (Figure 1), as previously described. 3
Figure 1.
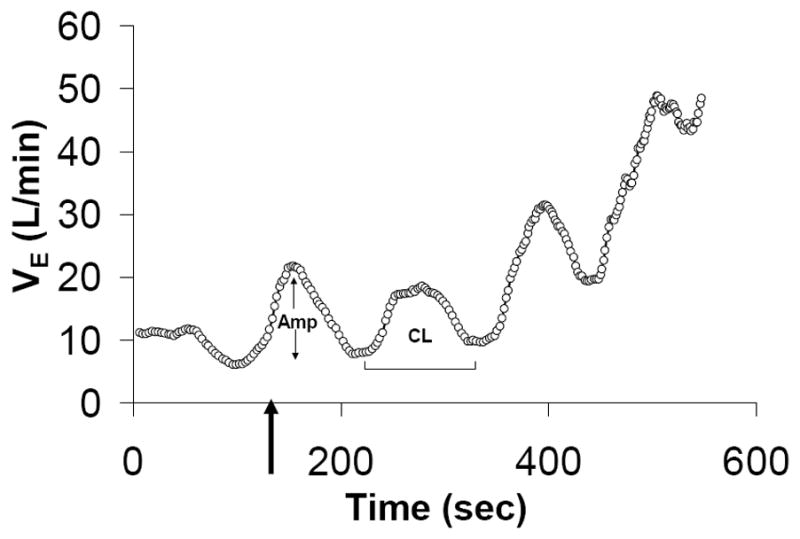
Exercise oscillatory ventilation was defined as ≥3 cyclical fluctuations of minute ventilation (VE) during exercise with an amplitude >25% of the mean trough-to-trough VE during that interval. Cycle Length (CL), and Amplitude (Amp).
Statistical methods
The STATA 10.0 software package (StataCorp LP, College Station, TX) was used for statistical analysis. For our primary hypothesis that exercise cardiac index differed between LVSD patients with and without EOV, based on exercise hemodynamic measurements from previous studies of patients with LVSD in our laboratory24, 27 we used a CV=0.24 for exercise cardiac index . We anticipated a 40% incidence of EOV within our LVSD population based on previous studies in similar populations.3–8 We therefore determined that a 56-subject study would give us 85% power to detect a clinically meaningful 20% difference in exercise cardiac index between groups. The Wilk-Shapiro test was used to assess the normality of distribution of the data. All continuous, normally-distributed measurements are presented as the mean±SEM. Categorical data are reported as percentages. Group baseline characteristics were compared using either the Student t test, Pearson’s chi square, or Fisher’s exact test, as appropriate. For clinical characteristics, comparisons between groups for continuous variables were performed using unpaired two-sample t tests or the Wilcoxon signed rank test.
Pearson or Spearman correlation coefficients were calculated, based on whether or not the data was either normally or not normally distributed, respectively. Relationships between EOV and other rest and exercise clinical variables were assessed by univariate and multivariate logistic regression. One-way ANOVA was usedto assess the effect of treatment on differences in the change in continuous variables measured at baseline and at 12 weeksof study drug treatment. The sildenafil trial was registered (ClinicalTrials.govnumber NCT00309790) and IRB approved, and separate IRB approval was obtained to study patients outside of the sildenafil trial. The authors had full access to the data and take responsibility for its integrity and for the manuscript as written.
RESULTS
Population characteristics
Baseline characteristics for HF subjects (N = 56), stratified according to the presence or absence of EOV, and control subjects (N=19), are reported in Table 1. All patients surpassed their ventilatory anaerobic thresholds and lactate thresholds during exercise. Peak exercise capacity (pVO2) in the HF group as a whole (12.4±0.5 ml/kg/min), was markedly less than that observed in age-matched controls (23.9±1.6 ml/kg/min, p<0.001).
Table 1.
Clinical characteristics of heart failure patients stratified by EOV and control subjects.
| Characteristic | Heart Failure (n=56) | HF+EOV (n=25) | HF-EOV (n=31) | Control (n=19) |
|---|---|---|---|---|
| Age – Years | 59±2 | 56±3 | 62 ± 2 | 60±3 |
| Male Sex - % | 80 | 81 | 79 | 79 |
| Body Mass Index | 28.2±0.8 | 29.2±1.2 | 27.3±1.0 | 27.5±0.8 |
| Primary Cause of Heart Failure; ischemic | 55§ | 64 | 48 | 0 |
| Heart Failure Pharmacotherapy | ||||
| Diuretic – Loop | 86§ | 96* | 81 | 5 |
| ACE Inhibitor or ARB | 80§ | 88 | 74 | 37 |
| β-Adrenergic Receptor Antagonist | 91§ | 100 | 90 | 21 |
| Spironolactone – Aldo Blocker | 54§ | 56 | 52 | 0 |
| Digoxin | 50§ | 56 | 45 | 0 |
| Cardiac Resynchronization Therapy - % | 23§ | 28 | 19 | 0 |
| Right Ventricular Ejection Fraction (rest) | 38±1§ | 34±2* | 41±2 | 56±1 |
| Left Ventricular Ejection Fraction (rest) | 30±1§ | 29±1 | 31±1 | 68±1 |
| Peak VO2 – ml/kg/min | 12.4±0.5§ | 11.3±0.6* | 13.2±0.8 | 23.9±1.6 |
| Respiratory Exchange Ratio | 1.16±0.02 | 1.18±0.03 | 1.14±0.03 | 1.11±0.024 |
| FEV1 | 2.27±0.11§ | 2.33±0.16 | 2.23±0.15 | 3.36±0.11 |
| VE/VCO2 | 38.8§ | 41.6 | 36.6 | 32.7 |
| PaCO2 (rest) | 37.4±0.7 | 37.6±0.9 | 37.2±1.1 | 35.0±1.4 |
| PaO2 (rest) | 88.5±1.7§ | 89.8±2.1 | 87.4±2.6 | 97.5±3.0 |
| pH (rest) | 7.45±0.01 | 7.46±0.01 | 7.45±0.01 | 7.44±0.01 |
| Hemoglobin (g/dL) | 12.9±0.4 | 13.1±0.3 | 12.8±0.4 | 13.1±0.3 |
Indicates P<0.05 for comparison of HF+EOV and HF-EOV and
indicates P<0.05 for comparison of HF and controls.
EOV was present in 45% of the HF patients (HF+EOV) but in none of the control subjects. HF+EOV patients did not differ from HF patients without EOV (HF-EOV) in age, gender, BMI, medication exposures, FEV1, or LVEF, but HF+EOV patients did have lower RV ejection fractions than HF-EOV patients (Table 1). During exercise, HF+EOV patients achieved lower peak VO2 than HF-EOV patients and had a trend toward higher VE/VCO2 slopes (Table 1).
Relationship between EOV and resting hemodynamic measurements
Resting hemodynamic measurements for all subjects are shown in Table 2. Compared to control subjects, the HF group as a whole had elevated RAP, PAP, and PCWP. HF subjects also had reduced resting CI and a compensatory increase in arterio-venous oxygen concentration [C(a-v)O2], compared to control subjects.
Table 2.
Hemodynamic values measured at rest.
| Hemodynamic Variable | HF | HF+EOV | HF-EOV | Control |
|---|---|---|---|---|
| Heart Rate, beats/min | 75±2 | 76±3 | 74±3 | 72±3 |
| Systolic BP | 123±3§ | 118±5 | 126±4 | 153±5†‡ |
| Diastolic BP | 67±2§ | 68±3 | 66±3 | 78±2†‡ |
| MAP, mmHg | 86±2§ | 85±3 | 86±2 | 103±2†‡ |
| RAP, mmHg | 6±1§ | 7±1 | 5±1 | 3±0† |
| PAP, mmHg | 27±1§ | 31±2* | 24±1 | 15±1†‡ |
| PCWP, mmHg | 15±1§ | 19±2* | 13±1 | 6±1†‡ |
| Stroke Volume, mL | 50.7±2.1§ | 44.4±3.1* | 55.7±2.5 | 70±4†‡ |
| C(a-v)O2, mLO2/dL | 7.8±0.2§ | 8.7±0.4* | 7.2±0.2 | 6.5±0.4† |
| Cardiac Index, L/min-m2 | 1.85±0.06§ | 1.62±0.09* | 2.03±0.08 | 2.47±0.15†‡ |
MAP indicates mean systemic arterial pressure; RAP mean right atrial pressure; PAP mean pulmonary arterial pressure; TPG transpulmonary gradient; PVR pulmonary vascular resistance; SVR systemic vascular resistance; C(a-v)O2 Indicates arterio-venous difference in oxygen content (i.e. oxygen extraction).
Indicates P<0.05 for comparison of HF+EOV and HF-EOV,
indicates P<0.05 for EOV vs. controls,
indicates P<0.05 for comparison of HF-EOV and controls, and
indicates P<0.05 for comparison of HF and controls.
Comparison of HF+EOV and HF-EOV groups indicated that presence of EOV was associated with a greater degree of hemodynamic impairment than was its absence. Among HF patients, unvariate predictors of the presence of EOV included higher PAP, higher PCWP and lower CI (see Table 2, Supplemental Table 1 contains a complete list of univariate predictors of EOV). Resting CI was markedly less in HF+EOV subjects (1.62±0.09 L/min/m2) than in HF-EOV subjects (2.03±0.08;P=0.001).
In contrast, the resting PaCO2 “setpoint”, which is indicative of respiratory drive, did not differ between HF+EOV and HF-EOV groups (Table 1). PaO2 and pH also did not differ between HF+EOV and HF-EOV groups (Table 1).
Relationship between EOV and physiologic parameters measured during exercise
Hemodynamic measurements derived during each minute of a standardized period of six minutes (for which all subjects were able to exercise) are displayed in Figure 2. Hemodynamic measurements were averaged over the 6-minute period for comparison between groups. Controls demonstrated consistently lower exercise RAP (denoted as xRAP), xPAP, and xPCWP, and a higher xCI and systemic blood pressures during exercise compared to both HF+EOV and HF-EOV patients (Table 3).
Figure 2.
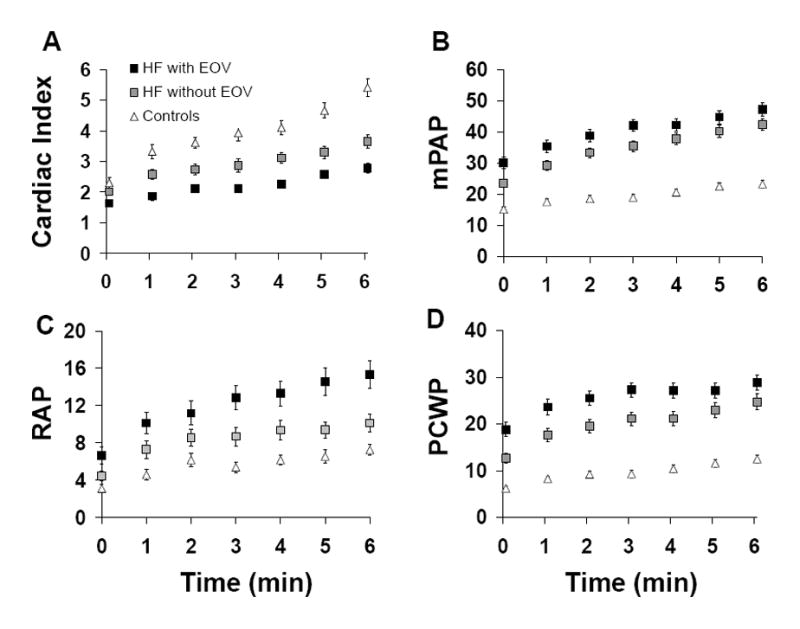
Mean ± SEM hemodynamic measurements at rest (time 0), and during the first 6 minutes of exercise in HF patients with and without exercise oscillatory ventilation (EOV) and in controls. (Panel A) cardiac indices; (Panel B) mean pulmonary arterial pressures (PAP); (Panel C) right atrial pressures (RAP); (Panel D) pulmonary capillary wedge pressures (PCWP).
Table 3.
Averages of hemodynamic and blood gas values measured each minute for 6 minutes of exercise.
| HF | HF+EOV | HF-EOV | Control | |
|---|---|---|---|---|
| HR (bpm) | 88±1 | 90±4 | 86 ± 3 | 84±4 |
| SBP (mmHg) | 135±4§ | 126±5* | 143 ± 5 | 167±5†‡ |
| DBP (mmHg) | 71±2§ | 71±3 | 72 ± 2 | 80±2†‡ |
| MAP (mmHg) | 93±2§ | 89±3 | 95 ± 3 | 109±2†‡ |
| RAP, mmHg | 11±1§ | 13±1* | 9 ± 1 | 6±1†‡ |
| PAP, mmHg | 39±1§ | 42±2* | 36 ± 2 | 20±1†‡ |
| PCWP (mmHg) | 24±1§ | 26±2* | 21 ± 1 | 10±1†‡ |
| CI (L/min/m2) | 2.68±0.10§ | 2.28±0.10* | 3.01 ± 0.13 | 4.18±0.21†‡ |
| Ca-vO2 (mmHg) | 11.1±0.3§ | 12.0±0.4* | 10.3 ± 0.3 | 9.4±0.7† |
| PaO2 (mmHg) | 91±3 | 96±3 | 87 ± 3 | 100±4‡ |
| PaCO2 (mmHg) | 37±1 | 36±1 | 38 ± 1 | 36±2 |
| pH (units) | 7.45±0.01 | 7.46±0.01* | 7.43 ± 0.01 | 7.43±0.01 |
| VE (L/min) | 21.9±0.5 | 21.2±0.2 | 22.7 ± 0.3 | 23.6±0.3 |
| RR (breaths/min) | 26±1 | 27±1 | 26 ± 1 | 25±0 |
Indicates P<0.05 for comparison of HF+EOV and HF-EOV,
indicates P<0.05 for EOV vs. controls,
indicates P<0.05 for comparison of HF-EOV and controls, and
indicates P<0.05 for comparison of HF and controls.
Among HF patients, exercise filling pressures were greater in the HF+EOV group than in the HF-EOV group while the reverse was true for SBP (Table 3). HF+EOV patients had 25% lower xCI compared to HF-EOV (P<0.001). In parallel with the reduced ability to increase CI, HF+EOV patients had significantly higher compensatory O2 extraction (xC(a-v)O2). There was a modest difference in xpH detected between the HF+EOV and HF-EOV groups (Table 3). However, in contrast to the differences in hemodynamic values during exercise between the HF+EOV and HF-EOV groups, the two groups did not differ in total minute ventilation (xVE), respiratory rate (xRR), PaO2 (xPaO2) or PaCO2 (xPaCO2) during exercise (Figure 3 and Table 3).
Figure 3.
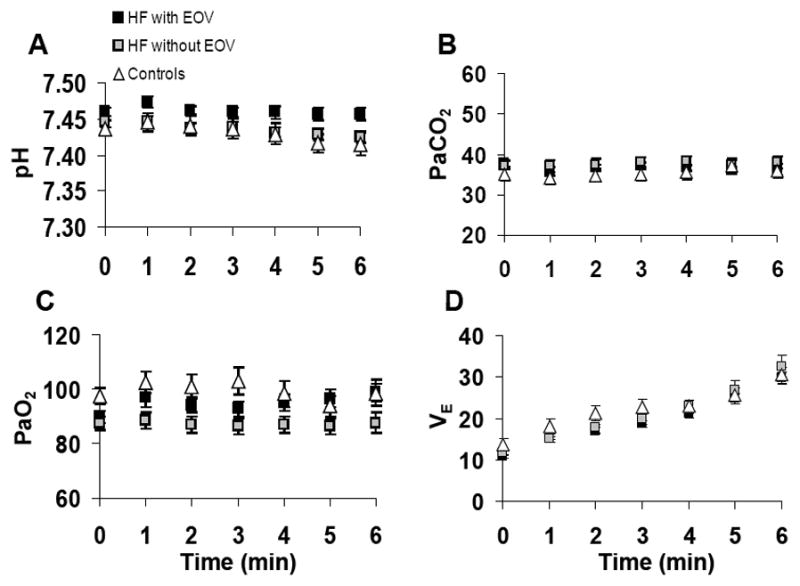
Mean ± SEM arterial blood gas and minute ventilation values at rest (time 0), and during the first 6 minutes of exercise in HF patients with and without exercise oscillatory ventilation (EOV) and in controls. (Panel A) arterial pH; (Panel B) arterial PaCO2; (Panel C) arterial PaO2; (Panel D) minute ventilation (VE).
When each of the univariate predictors of EOV (Supplemental Table 1) was combined into a multivariate analysis, only xCI was retained as a predictor of EOV (odds ratio 1.39 for each 1.0 L/min/m2 decrement in CI, 95% confidence interval 1.14–1.70, P=0.001). ROC analysis was also performed to determine if measurement of xCI was of incremental value to the measurement of resting CI for predicting EOV. For resting CI and xCI, the areas under the curve for predicting the presence of EOV were 0.65 and 0.79, respectively, with a significant increment in the C statistic (P=0.02) with measurement of exercise CI (Supplemental Figure 1). Taken together, these findings indicate that EOV in HF is more closely related to impaired exercise hemodynamics than to indicators of ventilatory drive.
Amplitude and duration of oscillatory ventilations
In HF+EOV subjects, the average oscillatory amplitude was 7.5±0.7 L/min, with an average oscillatory cycle length of 67±5 seconds. The amplitude and duration of oscillations were inversely related to xCI (R=−0.60 and R=−0.71, respectively, both P≤0.001) (Figure 4). HF+EOV subjects with EOV cycle length < 1 minute had a significantly greater xCI that HF+EOV subjects with an EOV cycle length > 1 minute (2.6±0.2 vs. 2.0±0.1 L/min/m2, P=0.009).
Figure 4.
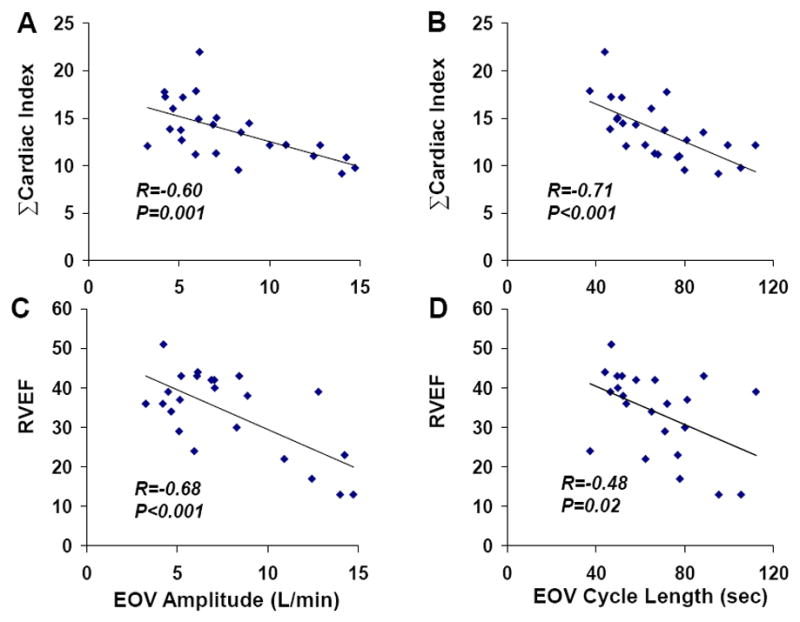
Correlations between EOV parameters and cardiac performance; EOV amplitude is inversely related to cumulative cardiac index during exercise (ΣCI, Panel A), and right ventricular ejection fraction (RVEF, Panel C). EOV cycle length is also inversely related to cumulative cardiac index during exercise (ΣCI, Panel B), and right ventricular ejection fraction (RVEF, Panel D).
EOV amplitude and cycle length were also inversely related to right ventricular ejection fraction (R=−0.68 and R=−0.48, both P<0.05, Figure 4). An EOV cycle length > 1 minute was associated with an average RVEF of 29%, compared to 41% in the HF-EOV group (P=0.003). The correlation between resting RVEF and cardiac index at rest (R=0.41, P=0.051) was of borderline significance, but resting RVEF was correlated with xCI (R=0.59, P=0.003). In contrast, we did not observe a correlation between resting LVEF and cardiac index either at rest (R=0.08, P=70) or with exercise (R=0.11, P=0.61). There was no association between oscillatory amplitude or duration with LVEF or with measures of gas exchange, including PaO2 and PaCO2 (Figure 5).
Figure 5.
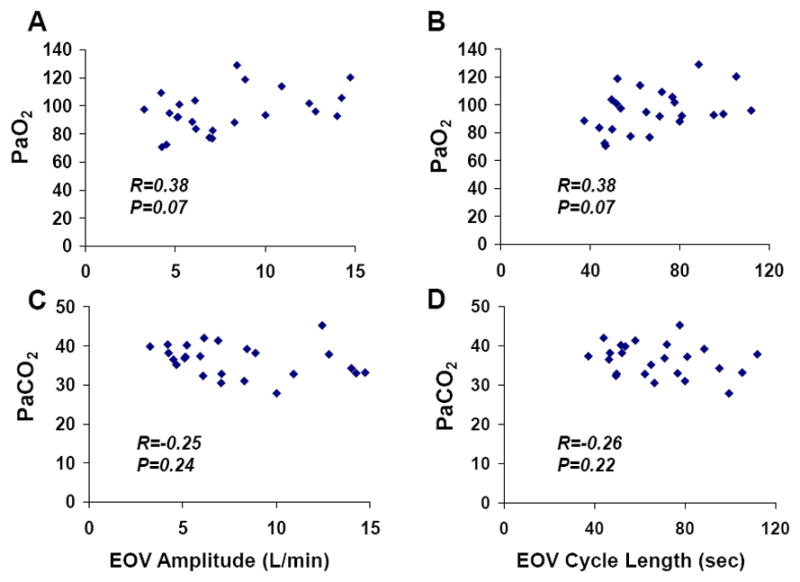
Correlations between EOV parameters and arterial blood gases; EOV amplitude was not related to cumulative PaO2 (ΣCI, Panel A) or PaCO2 (Panel C) during exercise. EOV cycle length was also not related to PaO2 (Panel B) or PaCO2 (Panel D).
In our study, at peak exercise, those subjects in whom oscillations were clearly no longer present at peak exercise (N=9, see Supplemental Figure 2) had higher peak CI (3.9±0.3 L/m/m2) than those in whom oscillations persisted throughout exercise (N=16, CI=3.0±0.1, P=0.036). Furthermore, patients in whom oscillations extinguished as they approached peak exercise demonstrated shorter EOV cycle length (55±4sec vs. 76±5sec, P=0.006) and lower EOV amplitude (6.0±0.7L/min vs. 8.9±0.9L/min, P=0.019) during the early standardized portion of exercise.
Effect of sildenafil on EOV characteristics
EOV was present in 18 of the 30 patients included in a prior study of sildenafil for the treatment of secondary pulmonary hypertension in systolic HF.24 Twelve weeks of sildenafil treatment reduced EOV cycle length (70±8 to 57±4 seconds) and oscillatory amplitude (7.8±1.3 to 6.3±1.4 L, P < 0.05 by ANOVA with repeated measures for both comparisons) in the setting of increasing exercise CI by 18±6% as previously reported.24 Changes in oscillatory cycle length and amplitude after sildenafil treatment were inversely related to changes in exercise CI (R=−0.72 and R=−0.64, respectively, both P<0.01). The improvement in oscillatory cycle length and amplitude following treatment with sildenafil in one subject with particularly prominent oscillatory amplitudes and cycle lengths and another with more modest oscillatory ventilation are shown in Figure 6. In both patients oscillations appear to diminish during the final portion of exercise after sildenafil treatment.
Figure 6.
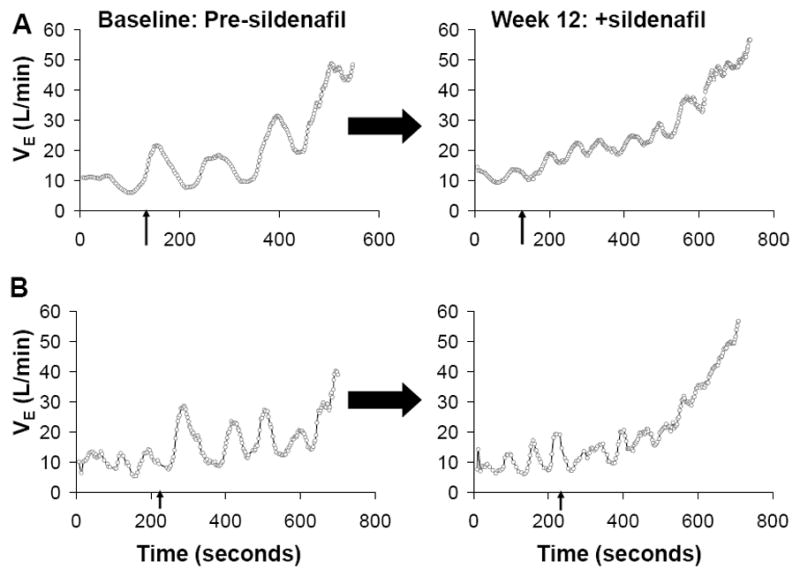
Effect of sildenafil treatment on EOV amplitude and cycle length. Graphical depiction of EOV in two representative patients indicates that amplitude and cycle length of ventilatory oscillations are reduced by treatment with sildenafil. Arrows on the X axis denote onset of exercise. Following sildenafil treatment, EOV amplitude and cycle length diminish and oscillations resolve during late exercise.
DISCUSSION
EOV is a non-invasive parameter that is easily measurable during submaximum exercise and purports a poor prognosis. We found that the presence of EOV signals hemodynamic impairment during exercise, as evidenced by increased right and left sided cardiac filling pressures and reduced systolic blood pressure and CI. Amplitude and cycle length of oscillations were also directly related to degree of impairment in exercise CI. A pharmacologic intervention that improved exercise CI in HF reduced the amplitude and cycle length of oscillations proportionate to increases in CI. These findings, along with the lack of relationship between the presence of EOV and PaCO2 or PaO2 during exercise, support the notion of a hemodynamic basis for EOV in HF.
The prevalence of EOV in our cohort (45%) is higher than that reported in cohorts with less advanced HF (i.e. 19–30%),3, 6–8 but it is highly consistent with the prevalence of EOV reported by Sun and colleagues (51%) in a cohort with exercise capacity similar to our HF subjects (peak VO2 =12 ml/kg/min vs. 12.6 ml/kg/min in our cohort).5 Like other investigators, we found that EOV was not related to age, gender, or LVEF7, 29 but was associated with reduced peak VO2 and a trend toward increased VE/VCO2 slope.5, 7, 8
Characterization of the relationship between hemodynamic measurements, blood gases, and EOV in HF patients has been limited by the technical challenges associated with hemodynamic and blood gas measurements during exercise, and limited normative data on exercise hemodynamic values.31 Our study is the first to relate EOV to serial measurements of circulatory delay (i.e. cardiac index), ventilatory drive (i.e. PaCO2), cardiac filling pressures (i.e. PCWP), and systemic oxygenation (i.e. PaO2) during exercise.
Relationship between EOV and Circulatory Delay
The role of prolonged circulation time in contributing to periodic breathing at rest has been debated for over 50 years.32, 33 Several studies characterizing PB have suggested a significant role of circulatory delay in PB generation,34, 35 while others argue against this possibility.36, 37 Proponents of the circulatory delay hypothesis for PB believe that time-delay in transit of blood between lungs and chemoreceptors results in imprecise control of respiration, with under- and over-shooting of the target ventilatory responses.34
Agostini et al. speculated that circulatory delay is unlikely to play a major role in determining EOV, because EOV persists during incremental exercise, with unchanged periodicity, despite an expected exercise-induced increase in CI.36 However, none of the aforementioned studies directly measured CI during exercise. We found that patients with EOV do not augment CI normally, and in fact their CI during early exercise was only 2.2 L/min/m2, markedly lower than that of the HF-EOV patients (3.0 L/min/m2), and controls (4.2 L/min/m2). The near-maximum predicted oxygen extraction during early exercise in HF+EOV further points to heightened reliance on oxygen extraction to increase VO2 in the setting of inability to augment CI normally.
In multivariate analysis, mean exercise CI emerged as the leading predictor of the presence of EOV in our study. This observation extends findings from studies of PB at rest. Mortara et al found that resting CI and prolonged lung-to-ear circulation time emerged as the major determinants of PB at rest.34 PB cycle length has also been related to estimated lung-to-ear circulation time and lung-to-femoral artery circulation time by other investigators.15,38 However, EOV and its cycle length and amplitude have not been previously linked to resting or exercise CI or circulation time. Ribiero and colleagues observed that administration of milrinone abolished EOV in 3 HF patients, and heart transplantation reversed EOV in two additional patients.39 While Ribiero’s findings support a central role of impaired hemodynamics in mediating EOV, exercise hemodynamics were not reported, and both PDE inhibition and cardiac transplantation have mulifactorial effects that could influence respiratory responses to exercise (i.e. modulating chemosensitivity)40, 41 beyond the hemodynamic effects of these interventions. Our findings that CI is the leading predictor of EOV in multivariate analysis extend Ribiero’s observations by linking EOV, as well as EOV cycle length and amplitude, directly to exercise CI.
The relatively constant cycle length of EOV within individuals, despite changes in CI during exercise, was evident in our study and in others.36 This is intriguing because one would think that if EOV cycle length is closely related to exercise CI in HF+EOV patients, then within individuals, as CI increases EOV cycle length would decrease. The modest rate of change in CI in our HF+EOV may also explain why cycle length remains relatively constant. Furthermore, circulatory delay must be interpreted in the context of relative ventilatory drive and metabolic changes imposed by exercise. Therefore, inadequate CI at a given exercise intensity is likely to lead to persistent oscillatory ventilation during exercise.
A subset of patients, in whom EOV extinguishes late in exercise, has been reported to have better exercise capacity29 and a more favorable prognosis than patients who continue to have oscillatory ventilation throughout exercise.7 While we focused on early, matched periods of exercise in this study, it is possible that late resolution of EOV during exercise may occur when CI increases above a certain threshold that shortens circulatory delay sufficiently to abolish EOV. EOV does appear to resolve during late exercise, following treatment with sildenafil and improvement in peak CI in the two subjects depicted in Figure 6.
Relationship between Cardiac Filling Pressures and EOV
We observed that RAP, PAP, and PCWP at rest and during exercise in HF+EOV subjects were consistently greater than those observed in HF-EOV subjects and controls (Figure 2). Elevated PCWP and pulmonary congestion stretches J-receptors, which stimulate medullary respiratory centers via vagal afferents leading to rapid shallow breathing,42 hypocapnea, and initiation of PB at rest.11, 19 Pulmonary congestion also reduces ability of lungs to damp variations in O2 and CO2 concentrations through storage of gases, particularly CO2, in the residual capacity of the lungs.14 Christie and colleagues attributed PB at rest to pulmonary congestion because they were able to induce it by occluding a pulmonary vein.43 Olson et al. described increased left atrial volumes and increased echocardiographic-estimates of resting filling pressures in HF subjects with EOV compared to matched HF controls without EOV. Our observation that RAP, PAP, and PCWP in HF+EOV subjects remain higher than those in HF-EOV subjects throughout exercise extends the findings of Christie and Olson.
EOV abates in some patients late in exercise, when PCWP would be expected to be increasing rather than decreasing, which has called the relationship between the presence of EOV and pulmonary congestion into question.36 In multivariate analysis, CI, but not PCWP, emerged as the leading predictor of the presence of EOV.
Moreover, sildenafil treatment reduced EOV cycle length and amplitude in proportion to improvements in CI, whereas sildenafil did not alter PCWP during exercise in our HF+EOV subjects treated with sildenafil. Taken together, this suggests a dominant role of CI in determining development of EOV. The interrelatedness of cardiac performance and filling pressures makes precise relative contributions of filling pressures and CI difficult to discern, but our concordant findings of reduced CI and elevated filling pressures relating to hemodynamic impairment to EOV suggests a central role of cardiac hemodynamic function in response to exercise in the genesis of EOV.
Relationship between PaCO2, PaO2, and EOV
Arterial blood gases at rest and cumulative values across the first 6 minutes of exercise revealed no relationship between rest or exercise PaCO2, PaO2 and EOV. This lack of association argues against a PaCO2 setpoint close to the apnea threshold serving as a major determinant of the presence of EOV. While PaCO2 fluctuates during EOV, with repeated sampling, one would expect to see lower cumulative PaCO2 values if heightened ventilatory drive was present in EOV. However, minute ventilation and PaCO2 at rest and during exercise did not differ in HF+EOV, HF-EOV, and control groups. Moreover, the amplitude and duration of EOV were unrelated to mean PaCO2. Our finding that PaCO2 did not differ in HF+EOV and HF-EOV groups or in controls, and that resting PaCO2 values were within the normal range, is consistent with findings by Agostini et al., who noted that patients with EOV had resting PaCO2=39±3.8 mmHg.
We did not directly measure central44 or peripheral45 chemosensitivity by systematically altering O2 and CO2 exposures and measuring VE responses. Therefore, we can not rule out potential contributions of heightened chemosensitivity to EOV in our study. However, the similar levels of resting and exercise PaCO2, PaO2, and VE among HF+EOV, HF-EOV, and controls suggest that enhancement of chemosensitivity did not play a major role in mediating EOV in our study. Ergoreflex was also not assessed, but it too would be expected to increase VE during a matched period of exercise.
Clinical Implications
EOV is an easily recognized, reproducible, non-invasive measurement than can be measured during submaximal exercise testing and, therefore, represents an attractive surrogate for exercise hemodynamics. Exercise probes the reserve capacity of the cardiovascular system, but hemodynamic measurements during exercise are challenging to perform and are not routinely available during clinical CPET. By indicating an inadequate hemodynamic response to exercise (i.e. impaired CI augmentation, increased filling pressures, and increased reliance on oxygen extraction) independent of LVEF, EOV may provide not only prognostic information but also an impetus to intensify therapy to optimize cardiac hemodynamics in HF subjects. The ability of a pharmacological intervention to alter EOV in our study also makes EOV a potential endpoint of interest for interventions expected to improve exercise hemodynamics and functional capacity in HF. Further work is needed to determine if interventions such as cardiac resynchronization, intensification of neurohormonal blockade, diuretics, or emerging HF therapies will successfully attenuate EOV.
In addition to its implications in systolic HF, EOV has recently been recognized in patients with HF with preserved left ventricular ejection fraction (HFpEF). The applicability of EOV as a potential indicator of cardiac compensation during exercise in HFpEF is particularly intriguing because of the inherent challenges in discerning contributing factors to exercise intolerance in patients with HFpEF.
Limitations
Our study has several limitations. Results were derived from a small patient cohort, and we tested multiple hypotheses regarding associations between EOV and physiologic parameters, increasing the chance of type 1 error. Our control population was limited in size (N=19) based on the infrequency with which subjects without significant cardiopulmonary disease undergo CPET with invasive hemodynamic monitoring. However, our population was extensively phenotyped, and the consistency of our results pertaining to individual hemodynamic and gas exchange parameters lend credence to our findings. We applied strict definitions of EOV to separate patients into HF+EOV and HF-EOV groups, which resulted in classifying HF subjects with low-level oscillations as non-oscillators. Optimal cut-points for the definition of EOV have yet to be established, but, based on our findings correlating cycle length and amplitude with worse cardiac function, low amplitude and cycle length oscillations are likely to be less clinically relevant. We used the gold standard Fick method to measure CI. Oscillations in VO2 are known to occur along with oscillations in VE and, therefore, may result in variable values of derived CI. However, inclusion of six measurements per patient during exercise and relatively linear increments in CI observed in our patients during exercise served to dampen potential variance introduced by oscillations in VO2.
Our findings should be viewed as exploratory until prospective trial data is available in which EOV is evaluated as a primary endpoint. We acknowledge that sildenafil may influence EOV through mechanisms beyond improving exercise CI. For example, theophylline, which acts as a non-selective phosphodiesterase inhibitor, has been shown to modulate chemosensitivity. It is possible that sildenafil worked through mechanisms other than improving CI.40, 41 However, the close relationship between changes in EOV cycle length and amplitude and changes in CI with sildenafil treatement suggest that this intervention influenced EOV through improvements in CI.
Additional studies are also needed to ascertain the reversibility of EOV with other HF therapeutic interventions. The lack of simultaneous echocardiographic assessment of mitral regurgitation at rest and during exercise precluded definitive assessment of the potential contribution of mitral regurgitation to exercise oscillatory ventilation. Finally, our findings regarding the close relationship between exercise hemodynamics and EOV do not exclude the contribution of alternative mechanisms unrelated to hemodynamics such as increased neural respiratory drive.
Conclusion
In patients with systolic HF, the presence of EOV indicates significant impairment in resting and exercise cardiac performance, particularly when EOV cycle length exceeds 1 minute. Our findings support a central role of cardiac hemodynamics in the development of EOV and suggest that EOV may be a surrogate for exercise hemodynamics. The presence of EOV therefore could serve as a target for therapeutic interventions that are postulated to alter exercise hemodynamics and functional capacity.
Supplementary Material
Acknowledgments
We thank the staff of the cardiopulmonary exercise laboratory for helping with data collection.
Funding Sources
The authors gratefully acknowledge support from the National Heart Lung and Blood Institute (NIH-K23HL091106, GDL), and the National Heart Lung and Blood Institute Heart Failure Network training grant (RVS, GDL), and the Heart Failure Research Innovation Fund (RMM, GDL).
Footnotes
Conflict of Interest Disclosures
None
References
- 1.Cheyne J. A case of apoplexy in which the fleshy part of the heart was converted in fat. Dublin Hosp Rep. 1818;2:216–219. [Google Scholar]
- 2.Stokes W. The Disease of the Heart and Aorta. Dublin: Hodges and Smith; 1854. [Google Scholar]
- 3.Corra U, Pistono M, Mezzani A, Braghiroli A, Giordano A, Lanfranchi P, Bosimini E, Gnemmi M, Giannuzzi P. Sleep and exertional periodic breathing in chronic heart failure: prognostic importance and interdependence. Circulation. 2006;113:44–50. doi: 10.1161/CIRCULATIONAHA.105.543173. [DOI] [PubMed] [Google Scholar]
- 4.Kremser CB, O'Toole MF, Leff AR. Oscillatory hyperventilation in severe congestive heart failure secondary to idiopathic dilated cardiomyopathy or to ischemic cardiomyopathy. Am J Cardiol. 1987;59:900–905. doi: 10.1016/0002-9149(87)91116-7. [DOI] [PubMed] [Google Scholar]
- 5.Sun XG, Hansen JE, Beshai JF, Wasserman K. Oscillatory breathing and exercise gas exchange abnormalities prognosticate early mortality and morbidity in heart failure. J Am Coll Cardiol. 55:1814–1823. doi: 10.1016/j.jacc.2009.10.075. [DOI] [PubMed] [Google Scholar]
- 6.Guazzi M, Raimondo R, Vicenzi M, Arena R, Proserpio C, Sarzi Braga S, Pedretti R. Exercise oscillatory ventilation may predict sudden cardiac death in heart failure patients. J Am Coll Cardiol. 2007;50:299–308. doi: 10.1016/j.jacc.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 7.Arena R, Myers J, Abella J, Peberdy MA, Pinkstaff S, Bensimhon D, Chase P, Guazzi M. Prognostic value of timing and duration characteristics of exercise oscillatory ventilation in patients with heart failure. J Heart Lung Transplant. 2008;27:341–347. doi: 10.1016/j.healun.2007.11.574. [DOI] [PubMed] [Google Scholar]
- 8.Leite JJ, Mansur AJ, de Freitas HF, Chizola PR, Bocchi EA, Terra-Filho M, Neder JA, Lorenzi-Filho G. Periodic breathing during incremental exercise predicts mortality in patients with chronic heart failure evaluated for cardiac transplantation. J Am Coll Cardiol. 2003;41:2175–2181. doi: 10.1016/s0735-1097(03)00460-1. [DOI] [PubMed] [Google Scholar]
- 9.Corra U, Mezzani A, Giordano A, Bosimini E, Giannuzzi P. Exercise haemodynamic variables rather than ventilatory efficiency indexes contribute to risk assessment in chronic heart failure patients treated with carvedilol. Eur Heart J. 2009;30:3000–3006. doi: 10.1093/eurheartj/ehp138. [DOI] [PubMed] [Google Scholar]
- 10.Guazzi M, Dickstein K, Vicenzi M, Arena R. Six-minute walk test and cardiopulmonary exercise testing in patients with chronic heart failure: a comparative analysis on clinical and prognostic insights. Circ Heart Fail. 2009;2:549–555. doi: 10.1161/CIRCHEARTFAILURE.109.881326. [DOI] [PubMed] [Google Scholar]
- 11.Cherniack NS, Longobardo GS. Cheyne-Stokes breathing. An instability in physiologic control. N Engl J Med. 1973;288:952–957. doi: 10.1056/NEJM197305032881810. [DOI] [PubMed] [Google Scholar]
- 12.Khoo MC, Kronauer RE, Strohl KP, Slutsky AS. Factors inducing periodic breathing in humans: a general model. J Appl Physiol. 1982;53:644–659. doi: 10.1152/jappl.1982.53.3.644. [DOI] [PubMed] [Google Scholar]
- 13.Ponikowski P, Anker SD, Chua TP, Francis D, Banasiak W, Poole-Wilson PA, Coats AJ, Piepoli M. Oscillatory breathing patterns during wakefulness in patients with chronic heart failure: clinical implications and role of augmented peripheral chemosensitivity. Circulation. 1999;100:2418–2424. doi: 10.1161/01.cir.100.24.2418. [DOI] [PubMed] [Google Scholar]
- 14.Dowell AR, Buckley CE, 3rd, Cohen R, Whalen RE, Sieker HO. Cheyne-Stokes respiration. A review of clinical manifestations and critique of physiological mechanisms. Arch Intern Med. 1971;127:712–726. doi: 10.1001/archinte.127.4.712. [DOI] [PubMed] [Google Scholar]
- 15.Millar TW, Hanly PJ, Hunt B, Frais M, Kryger MH. The entrainment of low frequency breathing periodicity. Chest. 1990;98:1143–1148. doi: 10.1378/chest.98.5.1143. [DOI] [PubMed] [Google Scholar]
- 16.Ponikowski P, Chua TP, Anker SD, Francis DP, Doehner W, Banasiak W, Poole-Wilson PA, Piepoli MF, Coats AJ. Peripheral chemoreceptor hypersensitivity: an ominous sign in patients with chronic heart failure. Circulation. 2001;104:544–549. doi: 10.1161/hc3101.093699. [DOI] [PubMed] [Google Scholar]
- 17.Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N Engl J Med. 1999;341:949–954. doi: 10.1056/NEJM199909233411304. [DOI] [PubMed] [Google Scholar]
- 18.Osterziel KJ, Hanlein D, Willenbrock R, Eichhorn C, Luft F, Dietz R. Baroreflex sensitivity and cardiovascular mortality in patients with mild to moderate heart failure. Br Heart J. 1995;73:517–522. doi: 10.1136/hrt.73.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solin P, Bergin P, Richardson M, Kaye DM, Walters EH, Naughton MT. Influence of pulmonary capillary wedge pressure on central apnea in heart failure. Circulation. 1999;99:1574–1579. doi: 10.1161/01.cir.99.12.1574. [DOI] [PubMed] [Google Scholar]
- 20.Lorenzi-Filho G, Azevedo ER, Parker JD, Bradley TD. Relationship of carbon dioxide tension in arterial blood to pulmonary wedge pressure in heart failure. Eur Respir J. 2002;19:37–40. doi: 10.1183/09031936.02.00214502. [DOI] [PubMed] [Google Scholar]
- 21.Naughton M, Benard D, Tam A, Rutherford R, Bradley TD. Role of hyperventilation in the pathogenesis of central sleep apneas in patients with congestive heart failure. Am Rev Respir Dis. 1993;148:330–338. doi: 10.1164/ajrccm/148.2.330. [DOI] [PubMed] [Google Scholar]
- 22.Andreas S, Hagenah G, Moller C, Werner GS, Kreuzer H. Cheyne-Stokes respiration and prognosis in congestive heart failure. Am J Cardiol. 1996;78:1260–1264. doi: 10.1016/s0002-9149(96)00608-x. [DOI] [PubMed] [Google Scholar]
- 23.Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P, Coats AJ. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation. 1996;93:940–952. doi: 10.1161/01.cir.93.5.940. [DOI] [PubMed] [Google Scholar]
- 24.Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 25.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129:S49–55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]
- 26.Lewis GD, Lachmann J, Camuso J, Lepore JJ, Shin J, Martinovic ME, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation. 2007;115:59–66. doi: 10.1161/CIRCULATIONAHA.106.626226. [DOI] [PubMed] [Google Scholar]
- 27.Lewis GD, Shah RV, Pappagianopolas PP, Systrom DM, Semigran MJ. Determinants of ventilatory efficiency in heart failure: the role of right ventricular performance and pulmonary vascular tone. Circ Heart Fail. 2008;1:227–233. doi: 10.1161/CIRCHEARTFAILURE.108.785501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ben-Dov I, Sietsema KE, Casaburi R, Wasserman K. Evidence that circulatory oscillations accompany ventilatory oscillations during exercise in patients with heart failure. Am Rev Respir Dis. 1992;145:776–781. doi: 10.1164/ajrccm/145.4_Pt_1.776. [DOI] [PubMed] [Google Scholar]
- 29.Olson LJ, Arruda-Olson AM, Somers VK, Scott CG, Johnson BD. Exercise oscillatory ventilation: instability of breathing control associated with advanced heart failure. Chest. 2008;133:474–481. doi: 10.1378/chest.07-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corra U, Giordano A, Bosimini E, Mezzani A, Piepoli M, Coats AJ, Giannuzzi P. Oscillatory ventilation during exercise in patients with chronic heart failure: clinical correlates and prognostic implications. Chest. 2002;121:1572–1580. doi: 10.1378/chest.121.5.1572. [DOI] [PubMed] [Google Scholar]
- 31.Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J. 2009;34:888–894. doi: 10.1183/09031936.00145608. [DOI] [PubMed] [Google Scholar]
- 32.Crowell JW, Guyton AC, Moore JW. Basic oscillating mechanism of Cheyne-Stokes breathing. Am J Physiol. 1956;187:395–398. doi: 10.1152/ajplegacy.1956.187.2.395. [DOI] [PubMed] [Google Scholar]
- 33.Douglas CG, Haldane JS. The causes of periodic or Cheyne-Stokes breathing. J Physiol. 1909;38:401–419. doi: 10.1113/jphysiol.1909.sp001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mortara A, Sleight P, Pinna GD, Maestri R, Capomolla S, Febo O, La Rovere MT, Cobelli F. Association between hemodynamic impairment and Cheyne-Stokes respiration and periodic breathing in chronic stable congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1999;84:900–904. doi: 10.1016/s0002-9149(99)00462-2. [DOI] [PubMed] [Google Scholar]
- 35.Hall MJ, Xie A, Rutherford R, Ando S, Floras JS, Bradley TD. Cycle length of periodic breathing in patients with and without heart failure. Am J Respir Crit Care Med. 1996;154:376–381. doi: 10.1164/ajrccm.154.2.8756809. [DOI] [PubMed] [Google Scholar]
- 36.Agostoni P, Apostolo A, Albert RK. Mechanisms of periodic breathing during exercise in patients with chronic heart failure. Chest. 2008;133:197–203. doi: 10.1378/chest.07-1439. [DOI] [PubMed] [Google Scholar]
- 37.Mansfield DR, Solin P, Roebuck T, Bergin P, Kaye DM, Naughton MT. The effect of successful heart transplant treatment of heart failure on central sleep apnea. Chest. 2003;124:1675–1681. doi: 10.1378/chest.124.5.1675. [DOI] [PubMed] [Google Scholar]
- 38.Lange RL, Hecht HH. The mechanism of Cheyne-Stokes respiration. J Clin Invest. 1962;41:42–52. doi: 10.1172/JCI104465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ribeiro JP, Knutzen A, Rocco MB, Hartley LH, Colucci WS. Periodic breathing during exercise in severe heart failure. Reversal with milrinone or cardiac transplantation. Chest. 1987;92:555–556. doi: 10.1378/chest.92.3.555. [DOI] [PubMed] [Google Scholar]
- 40.Javaheri S, Parker TJ, Wexler L, Liming JD, Lindower P, Roselle GA. Effect of theophylline on sleep-disordered breathing in heart failure. N Engl J Med. 1996;335:562–567. doi: 10.1056/NEJM199608223350805. [DOI] [PubMed] [Google Scholar]
- 41.Andreas S, Reiter H, Luthje L, Delekat A, Grunewald RW, Hasenfuss G, Somers VK. Differential effects of theophylline on sympathetic excitation, hemodynamics, and breathing in congestive heart failure. Circulation. 2004;110:2157–2162. doi: 10.1161/01.CIR.0000144356.39262.16. [DOI] [PubMed] [Google Scholar]
- 42.Roberts AM, Bhattacharya J, Schultz HD, Coleridge HM, Coleridge JC. Stimulation of pulmonary vagal afferent C-fibers by lung edema in dogs. Circ Res. 1986;58:512–522. doi: 10.1161/01.res.58.4.512. [DOI] [PubMed] [Google Scholar]
- 43.Christie RV, Hayward GW. Periodic changes in respiratory depth, produced by changes in the lung. J Physiol. 1943;102:88–94. doi: 10.1113/jphysiol.1943.sp004018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chua TP, Clark AL, Amadi AA, Coats AJ. Relation between chemosensitivity and the ventilatory response to exercise in chronic heart failure. Journal of the American College of Cardiology. 1996;27:650–657. doi: 10.1016/0735-1097(95)00523-4. [DOI] [PubMed] [Google Scholar]
- 45.Chua TP, Ponikowski P, Webb-Peploe K, Harrington D, Anker SD, Piepoli M, Coats AJ. Clinical characteristics of chronic heart failure patients with an augmented peripheral chemoreflex. European heart journal. 1997;18:480–486. doi: 10.1093/oxfordjournals.eurheartj.a015269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


