Abstract
Toll-like receptors (TLRs) recognize microbial pathogens and trigger an immune response, but their regulation by neuropeptides such as vasoactive intestinal peptide (VIP) during Pseudomonas aeruginosa corneal infection remains unexplored. Therefore, C57BL/6 (B6) mice were injected intraperitoneally with VIP and mRNA, protein and immunostaining assays done. After VIP treatment PCR array and real-time RT-PCR demonstrated that pro-inflammatory TLRs (Chuk, IRAK1, TLR1, 4, 6, 8, 9 and TRAF6) were down-regulated, while anti-inflammatory TLRs (SIGIRR and ST2) were up-regulated. ELISA showed that VIP modestly down-regulated phosphorylated IKKα, but up-regulated ST2 almost 2 fold. SIGIRR also was up-regulated, while TLR4 immunostaining was reduced in cornea; all confirming the mRNA data. To determine if VIP effects were cAMP dependent, mice were injected with siRNA for type 7 adenylate cyclase (AC7) with or without VIP treatment. After silencing AC7, changes in mRNA levels of TLR1, TRAF6 and ST2 were seen and unchanged with addition of VIP, indicating their regulation was cAMP dependent. In contrast, changes were seen in mRNA levels of Chuk, IRAK1, 2, TLR4, 9 and SIGIRR following AC7 silencing alone, which were modified by VIP addition, indicating their cAMP independence. In vitro studies tested the effects of VIP on TLR regulation in macrophages and Langerhans cells. VIP down-regulated mRNA expression of pro-inflammatory, while up-regulating anti-inflammatory TLRs in both cell types. Collectively, the data provide evidence that VIP down-regulates pro- and up-regulates anti-inflammatory TLRs, that this regulation is both cAMP dependent and independent, and involves immune cell types found in the infected cornea.
Keywords: Bacteria, cornea, VIP, TLRs, cAMP
Introduction
Keratitis induced by Pseudomonas aeruginosa (P. aeruginosa) progresses rapidly and is characterized by a suppurative stromal infiltrate with a marked mucopurulent exudate. Other untoward characteristics include inflammatory epithelial edema, ocular pain and redness, stromal ulceration, and often, decreased vision (1). Experimentally, infection with P. aeruginosa leads to corneal perforation in strains of mice such as C57BL/6 (B6, susceptible), while less severe disease is seen in similarly challenged BALB/c (resistant) animals (2). In contrast, treatment of B6 mice with vasoactive intestinal peptide (VIP) promotes better disease outcome after infection with P. aeruginosa, mainly through regulation of cytokine production and subsequent alteration of the host inflammatory cell response (3). Recent studies from this laboratory have provided further information regarding the anti-inflammatory effects of VIP and have shown that it modulates keratitis through regulation of growth factors, angiogenic molecules and beta defensins in the infected cornea, contributing to healing (4). However, the role of VIP in the regulation of Toll-like receptors (TLRs) has not been well-defined, despite the fact that other studies have demonstrated that TLRs play an important role in ocular immune defense (5). They direct the ocular immune response by differentially regulating a variety of events, including bacterial killing, polymorphonuclear leukocyte (PMN) infiltration and cytokine expression at both mRNA and protein levels (6-9). In particular, TLR4, a specific receptor for LPS contained in the outer membrane of various Gram-negative bacteria, is required for host resistance against P. aeruginosa in the infected cornea (8). In fact, upon activation by P. aeruginosa, both TLR4 and TLR5 on corneal macrophages have been shown to regulate P. aeruginosa keratitis through MyD88-dependent as well as independent pathways (10). Therefore, it is important to understand which TLRs are regulated by VIP and the mechanisms involved during microbial keratitis. In this regard, studies have suggested that VIP can signal through cAMP dependent or independent pathways (11), but whether this is important in VIP regulation of TLRs in other diseases (12) or bacterial keratitis, is not known.
Thus, the studies described herein investigated the expression of TLR signaling pathways in P. aeruginosa infected corneas with or without VIP treatment to determine whether it regulates their expression to favor disease resolution. Our data provide evidence that VIP treatment down-regulates pro-inflammatory, while up-regulating anti-inflammatory TLRs at both mRNA and protein levels in the cornea after P. aeruginosa infection and that mechanistically, two transduction pathways, cAMP-dependent and independent, are involved. Furthermore, in vitro studies suggest that at least two cell types in the cornea may be responsive, in that VIP reduced pro- and increased anti-inflammatory TLRs in both LPS stimulated elicited peritoneal macrophages and XS52 (Langerhans) cells.
Materials and Methods
Infection
Eight-week-old female B6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were anesthetized with ethyl ether, and placed beneath a stereoscopic microscope at x40 magnification. The cornea of the left eye was wounded (13) and a 5 μl-aliquot containing 1.0 × 106 CFU of P. aeruginosa (American Type Culture Collection, strain 19660, Manassas, VA) was topically delivered. Animals were treated humanely and in compliance with the Association for Research in Vision and Ophthalmology Statement on the Use of Animals in Ophthalmic and Vision Research.
VIP treatment
Treatment with synthetic VIP has been described before (3) at a dosage reported to be efficacious in a lethal endotoxemia model (14). In brief, using that dosage, B6 mice received daily i.p. injections of VIP (5 nM in 100 μl sterile phosphate buffered saline, PBS) at 1 day before infection (day= -1) through a maximum of 7 days p.i. Control mice were injected similarly with sterile PBS (100 μl).
RNA interference
Use of small interfering RNA (siRNA) has been described in previous work from this laboratory (15). For the current studies, siRNA for AC7 or an appropriate scrambled control (Santa Cruz Biotechnology, Santa Cruz, California) was injected subconjunctivally (5 μl per mouse at a concentration of 8 μM) into the left eye of B6 mice (n = 5/group/time) 1 day before infection with or without VIP treatment (described above). AC7 was then topically applied onto the infected corneas (5 μl/mouse/time at a concentration of 4 μM) once on the day of infection and twice at 1 and 3 days p.i. Corneas from scrambled control, silenced AC7 or silenced AC7 plus VIP treated (n=5/group/time) mice were individually collected at 5 days p.i. and processed for RT-PCR (described below). Using a similar assay, the efficacy of silencing AC7 in cornea (n=5/group/time) was confirmed by real-time RT-PCR (5 days p.i.).
Real-time RT-PCR
Total RNA was isolated from individual corneas (n=5/group/time) or cells using RNA-Stat 60 (Invitrogen, Carlsbad, CA), per the manufacturer's recommendations and quantitated spectrophotometrically (260 nm). One microgram of total RNA was reverse transcribed using Moloney murine leukemia virus (MMLV) reverse transcriptase. The 20 μl reaction mixture contained 200 U of MMLV-reverse transcriptase, 10 U of RNasin, 500 ng of oligo (dT) primers, 10 mM deoxyribonucleotide triphosphate (dNTP), 100 mM DTT, and MMLV reaction buffer (Invitrogen, Carlsbad, CA). cDNA was amplified using SYBR Green Master Mix (SA Bioscience, Frederick, MD), per the manufacture's recommendation. Briefly, the 20 μl reaction system contained: 10 μl of SYBR Green PCR Master Mix, 0.5 μM primers, 2 μl of cDNA (diluted 1/10) in diethyl pyrocarbonate water. All primer sets for the PCR array were purchased as a 96-well plate (RT2 Profiler™ Toll-like Receptor Signaling Pathway PCR Array; SABiosciences Corporation, Frederick, MD). The individual primer sets were designed using PrimerQuest (Integrated DNA Technologies, Cambridge, MA). Sequences of primers for β-actin, AC7, Chuk, IRAK1, IRAK2, TLR1, 4, 6, 8, 9, TRAF6, SIGIRR and ST2 are shown (Table 1).
Table 1.
Nucleotide sequence of the primers used in PCR amplification
| Gene | Primer Sequence | Sense |
|---|---|---|
| b-actin | 5’-GATTACTGCTCTGGCTCCTAGC-3′ | F |
| 5’-GACTCATCGTACTCCTGCTTGC-3’ | R | |
| AC7 | 5’ -TGGGCTTGCCTCATGGTACTA-3’ | F |
| 5’ -ACCACAAAGACGACAAACAGG -3’ | R | |
| Chuk | 5’-GTCAGGACCGTGTTCTCAAGG-3’ | F |
| 5’ -GCTTCTTTGATGTTACTGAGGGC-3’ | R | |
| IRAK1 | 5’-CAGAACCACCACAGATCATCATC-3’ | F |
| 5 ′-GGCTATCCAAGACCCCTTCTTC-3’ | R | |
| IRAK2 | 5’ -GGAGTGAAGCAGATGTCGTCCAAGC-3’ | F |
| 5’ -GCATCTGAGGCAGAGCTGCATCTCT-3’ | R | |
| TLR1 | 5 ′-TCTTCGGCACGTTAGCACTG-3’ | F |
| 5’-CCAAACCGATCGTAGTGCTGA-3’ | R | |
| TLR4 | 5’-CGCTTTCACCTCTGCCTTCACTACAG-3’ | F |
| 5’-ACACTACCACAATAACCTTCCGGCTC-3’ | R | |
| TLR6 | 5’-CAACCTTATTGAATGTGACCCTCCAGC-3’ | F |
| 5’-TCATCTCAGCAAACACCGAGTATAGCG-3’ | R | |
| TLR8 | 5’ -CACGTGTGACATAAGTGATTTTCG-3’ | F |
| 5’ -TTTGATCCCCAGGATTGGAA-3’ | R | |
| TLR9 | 5’-AGCTCAACCTGTCCTTCAATTACCGC-3’ | F |
| 5’-ATGCCGTTCATGTTCAGCTCCTGC-3’ | R | |
| TRAF6 | 5’ -GCGAGAGATTCTTTCCCTGAC-3’ | F |
| 5’-TGTATTAACCTGGCACTTCTGG-3’ | R | |
| SIGIRR | 5’ -GTGGCTGAAAGATGGTCTGGCATTG-3’ | F |
| 5’ -CAGGTGAAGGTTCCATAGTCCTCTGC-3’ | R | |
| ST2 | 5’ -TGACGGCCACCAGATCATTCACAG-3’ | F |
| 5’ -TGACGGCCACCAGATCATTCACAG-3’ | R |
Quantitative real-time RT-PCR was performed using the MyiQ single color real-time RT-PCR detection system (Bio-Rad). Optimal conditions for PCR amplification of cDNA were established using routine methods (16). Relative mRNA levels were calculated after normalization to β-actin.
ELISA
Protein levels for TLR associated molecules were tested by ELISA. Corneas from PBS- and VIP-treated B6 mice were individually collected (n = 5/group/time) from normal uninfected and infected mice at 1 and 7 days p.i. For total Chuk (IKKα) and phosphorylated IKKα (Cell Signaling Technology, Danvers, MA), corneas were homogenized in 0.5 ml lysis buffer (Cell Signaling Technology) with 1mM PMSF (Sigma, St. Louis, MO) and protease inhibitor (1 tablet/10 ml, Roche, Indianapolis, IN). For ST2 (R&D, Minneapolis, MN), corneas were homogenized in 0.5 ml of PBS with 0.1% Tween 20 and protease inhibitor (as above). All samples were centrifuged at 13,000 rpm for 5 min and an aliquot of each supernatant was assayed in duplicate for total IKKα, phosphorylated IKKα and ST2 per the manufacturer's instructions.
Western blot
Corneas (n = 5/group/time) were collected from PBS- and VIP-treated normal uninfected and infected B6 mice at 1 and 5 days p.i. Pooled corneas (n = 5/group) were lysed in 250 μl radio immunoprecipitation assay (RIPA) lysis buffer (Santa Cruz Biotechnology) and homogenized for 15 min. Tissue debris was pelleted by centrifugation for 10 min at 12,000 rpm, and protein concentration of the supernatant was determined by bicinchoninic acid (Bio-Rad) protein assay. Supernatants were separated on 10% SDS-PAGE, after loading 80 μg of sample to each lane; as a control, recombinant murine SIGIRR (15 ng, R&D) was similarly loaded. The electrophoretically separated material was transferred to a supported nitrocellulose membrane (Bio-Rad), and blocked in a 5% solution of nonfat dry milk prepared in 1X PBS and 0.05% Tween 20. Blots were incubated with primary goat anti-mouse SIGIRR Ab (R&D Systems) diluted in PBS overnight (4°C), washed three times for 10 min each with TBST, detected with HRP-conjugated secondary Ab (R&D Systems) diluted 1/1000 in PBS containing 5% nonfat milk, and developed using the ECL method (PerkinElmer, Waltham, MA) per the manufacturer's protocol. The blot was scanned on a FlurorChem E imaging system (Cell Biosciences, Santa Clara, CA) and band relative Integrated Density Value (IDV) was analyzed by AlphaView software (Cell Biosciences).
Immunofluorescent staining
Normal, uninfected and infected eyes were enucleated from VIP and PBS treated B6 mice (n = 5/group/time) at 1 and 5 days p.i. Tissue was immersed in 1X Dulbecco's PBS (Mediatech, Inc., Herndon, VA), embedded in Tissue-Tek OCT compound (Miles, Elkhart, IN) and frozen in liquid nitrogen. Ten μm thick sections were cut, mounted to polylysine-coated glass slides, and incubated in a moist chamber at 37 °C overnight. After a 2 min acetone fixation, nonspecific staining was blocked with 10 mM sodium phosphate buffer containing 2.5% bovine serum albumin and donkey IgG (1:100) for 30 min at room temperature. For immunostaining, sections were incubated for 1 h each with goat anti-mouse TLR4 (Santa Cruz Biotechnology), followed by an Alexa Fluor 594 conjugated donkey anti-goat secondary Ab (1:1500, Invitrogen). Sections were incubated for 2 min with SYTOX Green nuclear acid stain (1:20,000 Lonza, Walkersville, MD). Controls were similarly treated, but the primary Ab was replaced with the same host IgG. Finally, sections were visualized (TLR4 positive staining = red ) and digital images captured with a Leica TCS SP2 confocal laser scanning microscope (Leica Microsystems, Bannockburn, IL).
Cell isolation, culture and treatment
Peritoneal macrophages were elicited and isolated from B6 mice as described before (3). Briefly, cells were induced into the peritoneal cavity by i.p. injection of 1.0 ml of 3% Brewer's thioglycollate medium (Becton Dickinson, Sparks, MD) 5 days before harvest (n=12 mice). Cells were collected by peritoneal lavage with DMEM, stained with trypan blue (1:1), and viable cells (>95%) counted using a hemacytometer. After a differential cell count, cells were collected, pooled, and 3 × 106 macrophages/well (5 wells/treatment) were seeded into 6-well plates. Non-adherent cells were removed 24 h later and isolated macrophages were used for in vitro stimulation assays described below. Mouse XS52 (Langerhans) cells were cultured in complete RPMI (with 10% fetal calf serum) (both from Gibco, Grand Island, NY) supplemented with 0.5 ng/mL murine recombinant granulocyte macrophage–colony-stimulating factor (GM-CSF) (Gibco) as detailed before (6). These cells (capable of presenting protein antigen to primed CD4+ T cells) were derived from newborn BALB/c mouse epidermis as stable long-term cells lines (dendritic shaped, CD45+/E-cadherin+ and B7-1-) (17). After differential cell count, 3 × 106 XS52 (Langerhans) cells/well (5 wells/treatment) were seeded into 6-well plates. Both cell types were treated with 1 μg ultrapure LPS (E. coli 01111:B4 strain; which only activates the TLR4 pathway; Invivogen, San Diego, CA) plus or minus VIP (10-9M). For both macrophage and XS52 (Langerhans) cell experiments, which were repeated similarly once (n=2 experiments for each cell type), cells from each well were collected and mRNA extracted and assayed in duplicate by real-time RT-PCR (described above) and the data analyzed statistically.
Langerhans cell staining and quantitation
Normal, uninfected corneas and at 1 and 3 days p.i. were harvested and placed in 0.02 mol/L EDTA, pH 7.2 for 1 h at 37 °C for epithelial removal and Langerhans cell staining. Epithelial sheets were fixed in cacodylate-buffered formaldehyde for 20 min at 4 °C, washed 4 times with cold 0.1 mol/L cacodylate buffer, and incubated in ADPase substrate solution containing ADPase buffer, 2% lead nitrate, and ADP (5 mg/mL; Sigma) for 15 min at 37 °C. Sheets were washed 4 times with trismaleate buffer (pH 7.2), developed for 5 min in a 1:10 ammonium sulfide solution, washed again 3 times with buffer, mounted onto slides with glycerol, flattened and coverslipped (18). Epithelial sheets were observed and photographed with a Zeiss Axiophot microscope with Axiocam digital imagery (Carl Zeiss, Morgan Instruments, Cincinnati, OH) at 375X and the number of cells quantitated per field (n = 12; field size = 270 μm2) by two of us blinded as to treatment. Staining of Langerhans cells for ADPase activity has been done before and shown to provide data similar to immunostaining these cells with surface markers such as DEC205 (18).
Statistics
Two-way analysis of variance (ANOVA) followed by the Bonferroni post-test was used for analysis of the data shown in Figures 1, 2 and 7 (Prism 3.0, GraphPad Software Inc., La Jolla, CA). One-way ANOVA was used followed by Tukey's post-test for the data shown in Figures 4, 5 and 6 (SPSS, SPSS Inc, Chicago, IL). For each test, differences were considered significant at p < 0.05 and represented as the mean ± the standard error of the mean (S.E.M.). Each experiment was performed at least twice and combined data are shown, except for Figures 5 and 6, in which representative data from a single similar experiment are provided. Infection of mice, confocal microscopy and Langerhans cell quantitative studies were done in blinded fashion.
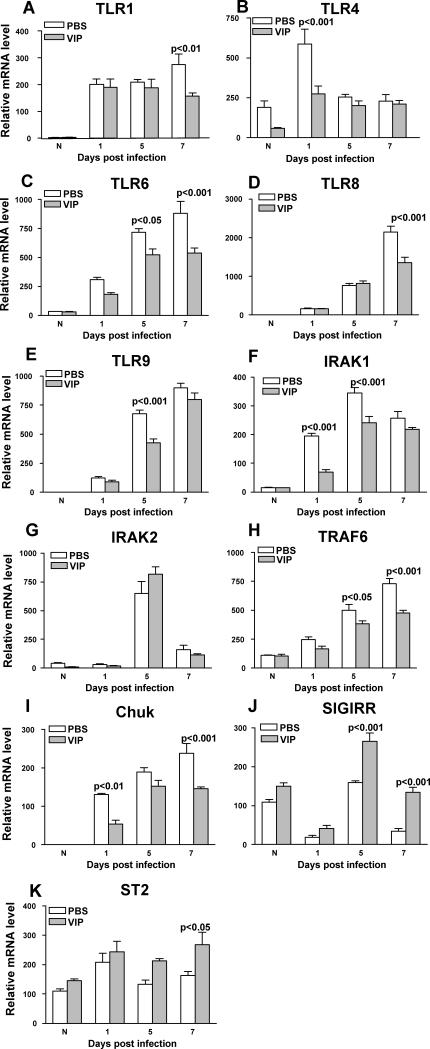
Figure 1.
RT-PCR for mRNA expression. VIP vs PBS treatment decreased mRNA levels for: TLR1 (A) at 7 days p.i.; TLR4 (B) at 1 day p.i.; TLR6 (C) at 5 and 7 days p.i.; TLR8 (D) at 7 days p.i.; TLR9 (E) at 5 days p.i.; IRAK1 (F) at 1 and 5 days p.i.; TRAF6 (H) at 5 and 7 days p.i.; and Chuk (I) at 1 and 7 days p.i. VIP vs PBS treatment increased mRNA levels for: SIGIRR (J) at 5 and 7 days p.i.; and ST2 (K) at 7 days p.i. No significant change was seen for IRAK 2 (G); nor were changes seen at other times tested for all of the assays.
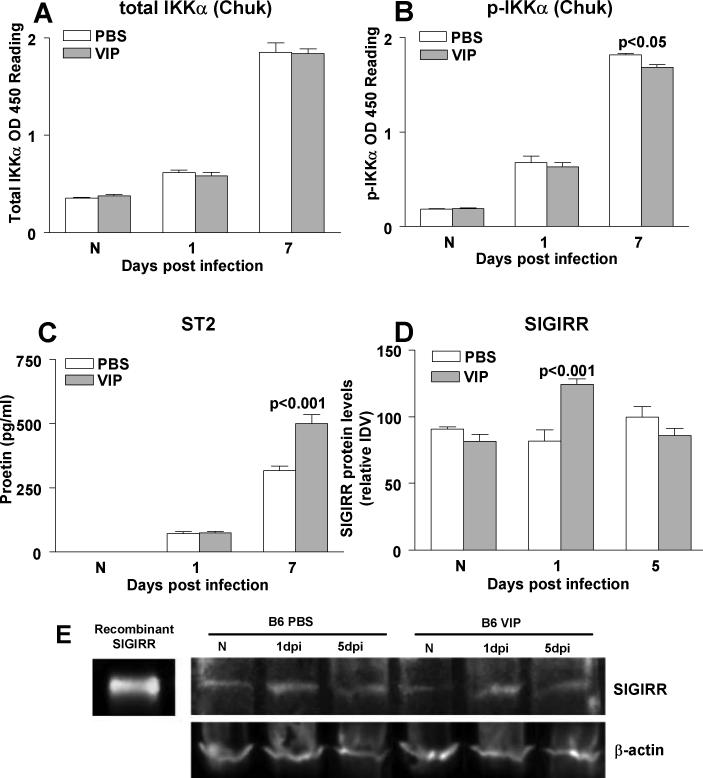
Figure 2.
ELISA. VIP vs PBS treatment had no effect on total IKKα (A) in normal (N) or infected cornea, but significantly, although slight, reduced phosphorylated IKKα (B) protein levels at 7 days p.i, with no effect in normal (N) or at 1 day p.i. ST2 (C) protein levels were significantly increased by VIP, but only at 7 days p.i. Western blot of SIGIRR (E) protein levels in the uninfected, normal (N) and infected cornea (1 and 5 days p.i.) of PBS vs VIP treated mice. Equivalent protein loaded (80 μg/lane). Lanes: 1, murine recombinant SIGIRR (15 ng); 2, PBS treated normal (N) cornea; 3, PBS treated cornea at 1 day p.i.; 4, PBS treated cornea at 5 days p.i.; 5, VIP treated normal (N) cornea; 6, VIP treated cornea at 1 day p.i.; 7, VIP treated cornea at 5 days p.i. (D) The intensity of bands was quantitated and normalized to the β-actin control. Data, expressed as the mean ± SEM integrated density values (IDV) at each time point, are significant only at 1 day p.i.
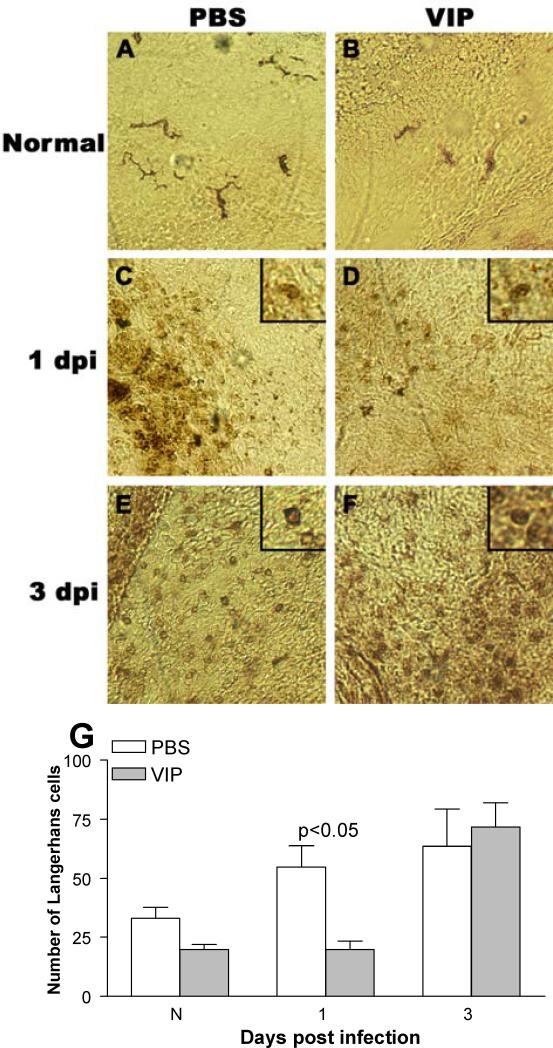
Figure 7.
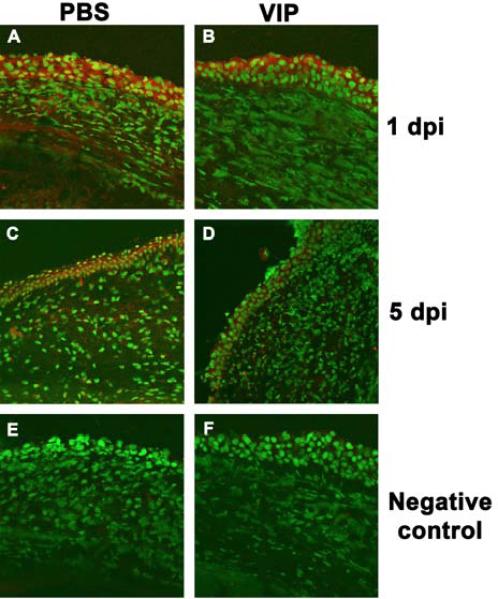
Staining and quantitation of Langerhans cells. In the normal, uninfected eye (conjunctiva/peripheral cornea) dendritic-shaped ADPase positive Langerhans cells appeared similar in number after VIP (B) vs PBS (A). At 1 day p.i., rounded ADPase positive cells in the peripheral cornea appeared decreased after VIP (D) vs PBS (C) treatment. At 3 days p.i. cells qualitatively appeared similar after PBS (E) or VIP (F) treatment. Quantitation confirmed these findings (G). (A-F at 375 X magnification, field size = 270 μm2).
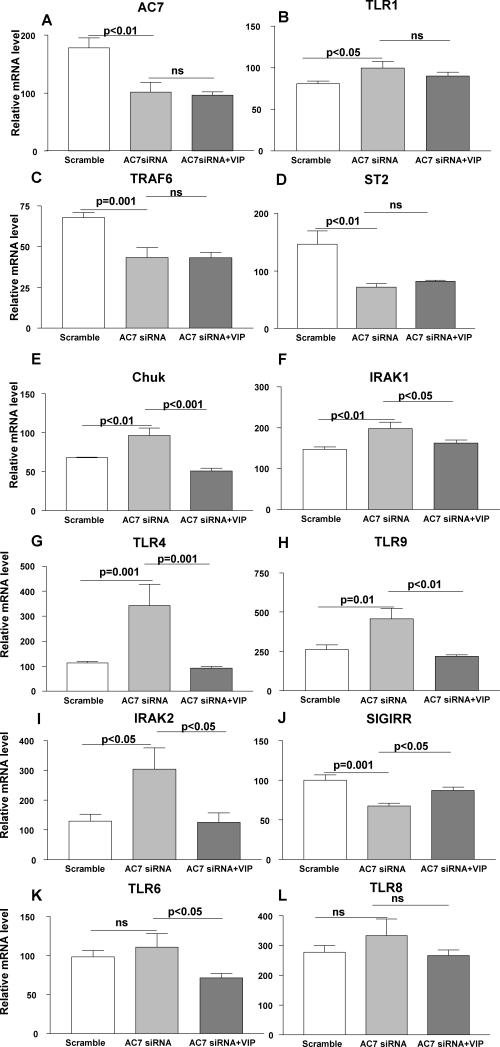
Figure 4.
When tested at 5 days p.i., RT-PCR confirmed that silencing AC7 vs scrambled control treatment significantly lowered AC7 mRNA levels (A) and silencing together with VIP treatment did not reverse the effect. When tested at 5 days p.i., silencing AC7 increased mRNA levels of TLR1 (B), while TRAF6 (C) and ST2 (D) were decreased. Silencing together with VIP injection did not change the effect of silencing for any of the above mentioned molecules. In contrast, Chuk (E), IRAK1 (F), TLR4 (G), TLR9 (H) and IRAK2 (I) mRNA levels were increased after silencing AC7 vs scrambled control treatment. Silencing together with VIP injection decreased mRNA expression of each of these molecules significantly. SIGIRR mRNA levels (J) were decreased after silencing AC7, but together with VIP treatment, mRNA levels increased over silencing alone. No significant changes in mRNA levels of TLR6 (K) or TLR8 (L) were seen with AC7 siRNA treatment, but together with VIP treatment, mRNA levels were reduced only for TLR6.
Figure 5.
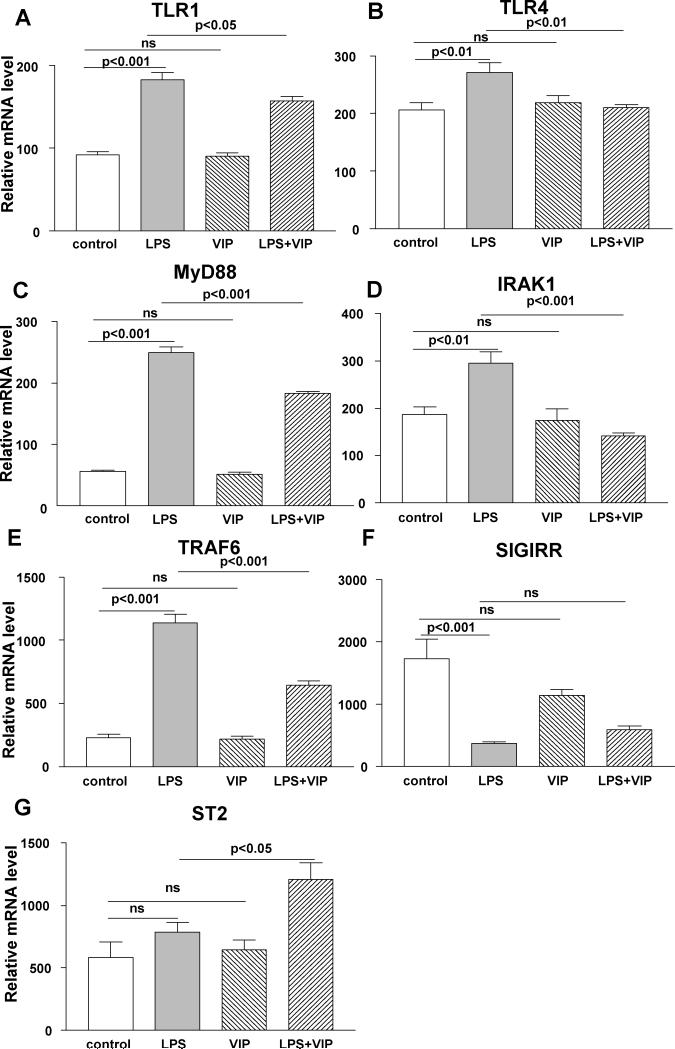
In vitro studies using macrophages. Stimulation of macrophages with ultrapure LPS vs media control significantly increased mRNA levels of TLR1 (A), 4 (B), MyD88 (C), IRAK1 (D), TRAF6 (E), decreased SIGIRR (F) and had no effect on ST2 (G). When compared with LPS alone, VIP plus LPS treatment significantly decreased mRNA expression of TLR1 (A), 4 (B), MyD88 (C), IRAK1 (D), and TRAF6 (E), but increased ST2 (G) with no change in SIGIRR (F). VIP alone had no significant effect on any of the molecules tested when compared with media control.
Figure 6.
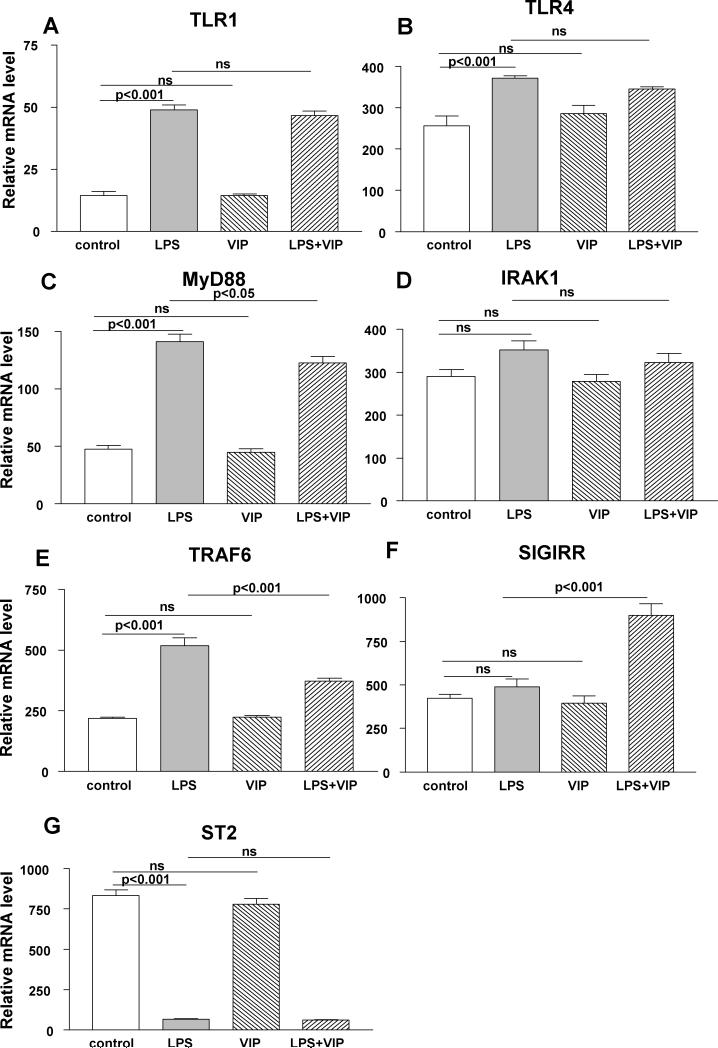
In vitro studies using XS52 (Langerhans) cells. Stimulation of these cells with ultrapure LPS vs media control significantly increased mRNA levels of TLR1 (A), TLR4 (B), MyD88 (C) and TRAF6 (E), decreased ST2 (G) and had no effect on IRAK1 (D) and SIGIRR (F). When compared with LPS alone, VIP plus LPS treatment significantly decreased MyD88 (C), and TRAF6 (E), increased SIGIRR (F) and had no significant effect on TLR1 (A), TLR4 (B), IRAK1 (D) or ST2 (G). Cultured cells treated with VIP alone, did not differ from media treated controls for any of the molecules tested.
Results
PCR array and real-time RT-PCR
To determine the effects of VIP treatment on regulation of TLRs in cornea during bacterial keratitis, mRNA levels of 84 TLR related genes were profiled by PCR array at 3 days p.i. VIP treatment decreased expression (greater than three-fold) of 21 genes compared with PBS treated controls and these are listed in Table 2. RT-PCR was used to confirm the array data and to test TLR4 (8) and IRAK1 (19-20) that are important in Pseudomonas-induced infections, but that were not changed on the PCR array greater than three-fold: TLR4 (-1.11) and IRAK1 (-2.9). Negative regulators of TLRs (ST2 and SIGIRR), not included on the PCR array, shown before to promote resistance to P. aeruginosa keratitis (7, 9) also were tested. VIP significantly down-regulated mRNA expression for TLR1 (Fig. 1A) only at 7 days p.i. (p<0.01). However, when compared with PBS treatment, VIP decreased mRNA levels of TLR4 (Fig. 1B) only at 1 day after infection (p<0.001). VIP also down-regulated mRNA levels for TLR6 (Fig. 1C) at 5 and 7 days p.i. (p<0.05, p<0.001); for TLR8 (Fig. 1D) only at 7 days p.i. (p<0.001); and for TLR9 (Fig. 1E) only at 5 days p.i. (p<0.001). VIP- vs PBS- treatment also decreased mRNA expression of TLR adaptor molecule IRAK1 (Fig. 1F) at 1 and 5 days p.i. (p<0.001, p<0.001), but no differences were seen between groups for IRAK2 (Fig. 1G). TRAF6 (Fig. 1H) which interacts with IRAK1 (19) also was reduced at the mRNA level by VIP vs PBS treatment at 5 and 7 days p.i. (p<0.05, p<0.001). mRNA levels of Chuk (Fig. 1I), a down-stream molecule of the IRAK-TRAF6 signaling pathway, which regulates NF-κB expression (19), also was down-regulated by VIP at 1 and 7 days p.i. (p<0.01, p<0.001). In contrast, VIP treatment increased SIGIRR (Fig. 1J) mRNA levels at 5 and 7 days p.i. (p<0.001 for both). However, levels in both groups decreased at 1 day p.i. ST2 mRNA levels (Fig. 1K) also were increased by VIP treatment at all times tested, but was significant only 7 days p.i. (p<0.05).
Table 2.
Selected TLRs from RT2 Profiler™ PCR array
| Genes | Fold Difference |
|---|---|
| VIP/PBS | |
| Casp8 | -3.45 |
| Chuk | -11.78 |
| Csf2 | -8.27 |
| Csf3 | -5.42 |
| Fos | -18.74 |
| Hmgb1 | -13.72 |
| Hspd1 | -8.10 |
| Il1a | -3.70 |
| Il6 | -3.01 |
| Irak2 | -5.27 |
| Myd88 | -3.58 |
| Ripk2 | -3.31 |
| Ticam2 | -3.05 |
| Tlr1 | -11.78 |
| Tlr6 | -6.36 |
| Tlr8 | -7.77 |
| Tlr9 | -4.40 |
| Tnfaip3 | -14.20 |
| Tnfrsf1a | -3.24 |
| Traf6 | -7.61 |
| Ube2n | -5.49 |
ELISA and Western blot
ELISA and/or Western blot were used to selectively confirm the mRNA data for total IKKα (Chuk), phosphorylated IKKα (p-IKKα), ST2 and SIGIRR and data are shown in Figure 2 A-E. For total IKKα Fig. 2A), there was no significant difference between PBS and VIP treatment at all times tested. However, when compared with PBS, VIP treatment slightly down-regulated p-IKKα protein expression (Fig. 2B) only at 7 days p.i. (p<0.05). In contrast, protein levels of ST2 (Fig. 2C) were significantly enhanced by VIP- vs PBS- treatment at 7 days p.i. (p<0.001), not different at 1 day p.i. and not detectable in either normal sample. SIGIRR protein (Fig. 2D, E) was constitutively expressed similarly in normal PBS vs VIP treated mouse cornea. At 1 day p.i., greater levels of SIGIRR (p<0.001) were observed after VIP treatment, with no difference between the two groups at 5 days after infection.
TLR4 immunostaining
Immunohistochemistry was used to spatially localize TLR4 (red staining) in the cornea of VIP- vs PBS- treated B6 mice at 1 and 5 days p.i. (Fig. 3A-F). At 1 day p.i., reduced TLR4 staining was seen after VIP (Fig. 3B) vs PBS (Fig. 3A) treatment in corneal epithelium and stroma. At 5 days p.i., VIP vs PBS treatment further reduced TLR4 epithelial and stromal staining (compare Fig. 3D and C). Controls (Fig. 3E and F) in which primary antibody was substituted with species-specific IgG were negative after PBS (Fig. 3E) or VIP (Fig. 3F) treatment at 1 or 5 (data not shown) days p.i.
Figure 3.
TLR4 immunostaining. At 1 and 5 days p.i., TLR4 staining (red) was less intense in the epithelium and stroma after VIP (B, D) vs PBS (A,C) treatment. PBS (E) and VIP (F) treated controls (1 day p.i.) in which the primary Ab was replaced by IgG, were negative for TLR4 immunostaining. Magnification = x100
Silencing AC7
To determine whether the anti-inflammatory effect of VIP on TLRs was cAMP dependent, siRNA was used in vivo to knockdown AC7, selected because among the ten mammalian adenyl cyclase isotypes, it is highly expressed in the immune system (21). When tested at 5 days p.i., RT-PCR confirmed that silencing AC7 vs scrambled control treatment significantly lowered AC7 mRNA levels (Fig. 4A, p<0.01) and that silencing together with VIP treatment did not reverse the effect. Furthermore, at this time, silencing AC7 increased mRNA levels of TLR1 (Fig. 4B), while TRAF6 (Fig. 4C) and ST2 (Fig. 4D) were decreased (p<0.05, p=0.001, p<0.01). Silencing together with VIP injection did not change the effect, indicating that VIP regulation of these TLRs is cAMP dependent. Pro-inflammatory TLRs including Chuk (Fig. 4E), IRAK1 (Fig. 4F), TLR4 (Fig. 4G), TLR9 (Fig. 4H), IRAK2 (Fig. 4I) mRNA levels were increased after silencing vs scrambled control treatment (p<0.01, p<0.01, p=0.001, p=0.01, p<0.05); anti-inflammatory TLR, SIGIRR (Fig. 4J) levels were decreased (p=0.001). Silencing together with VIP injection changed the effect (Chuk: p<0.001; IRAK1: p<0.05; TLR4: p=0.001; TLR9: p<0.01; IRAK2: p<0.05; SIGIRR p<0.05) indicating that VIP regulation of these molecules is cAMP independent. No significant changes in mRNA levels of TLR6 (Fig. 4K) were seen with AC7 siRNA treatment, but VIP together with silencing decreased mRNA levels for TLR6 (p<0.05), suggesting the possibility of its regulation by another adenyl cyclase isoform (22). No effects were seen for TLR8 (Fig. 4L) suggesting it is independent of AC7 and VIP regulation.
In vitro studies: macrophages
To provide some indication of which cells may be involved in VIP regulation of TLRs, peritoneal exudate macrophages and XS52 (Langerhans) cells were tested in an in vitro assay. Ultrapure LPS (only activates the TLR4 pathway) vs media control significantly increased TLR1 (Fig. 5A, p<0.001), TLR4 (Fig. 5B, p<0.01), MyD88 (Fig. 5C, p<0.001), IRAK1 (Fig. 5D, p<0.01) and TRAF6 (Fig. 5E, p<0.001) with no effect on ST2 (Fig. 5G) mRNA. However, SIGIRR mRNA (Fig. 5F, p<0.001) was decreased after LPS vs media control stimulation. LPS plus VIP vs LPS alone significantly decreased mRNA levels of TLR1 (Fig. 5A, p<0.05), TLR4 (Fig. 5B, p<0.01), MyD88 (Fig. 5C, p<0.001), IRAK1 (Fig. 5D, p<0.001), and TRAF6 (Fig. 5E, p<0.001), but increased ST2 (Fig. 5G, p<0.05); no difference was seen for SIGIRR (Fig. 5F). Cultured cells treated with VIP alone, showed no significant difference when compared with media treated controls for any of the molecules tested.
In vitro studies: XS52 (Langerhans) cells
Similar LPS vs media control treatment of XS52 (Langerhans) cells significantly increased mRNA levels of TLR1 (Fig. 6A, p<0.001), TLR4 (Fig. 6B, p<0.001), MyD88 (Fig. 6C, p<0.001), and TRAF6 (Fig. 6E, p<0.001), but decreased ST2 (Fig. 6G, p<0.001). IRAK1 (Fig. 6D) and SIGIRR (Fig. 6F) mRNA levels were unchanged after LPS vs media control stimulation. LPS plus VIP vs LPS alone significantly decreased mRNA levels for MyD88 (Fig. 6C, p<0.05) and TRAF6 (Fig. 6E, p<0.001), but increased SIGIRR (Fig. 6F, p<0.001) mRNA expression. LPS plus VIP vs LPS alone did not significantly change mRNA levels for TLR1 (Fig. 6A), TLR4 (Fig. 6 B), IRAK1 (Fig. 6D) or ST2 (Fig. 6G). Cultured cells treated with VIP alone, did not differ from media treated controls for any of the molecules tested.
Langerhans cell detection and quantitation
In normal, uninfected eyes, dendritic-shaped Langerhans cells were detected in the peripheral cornea/conjunctiva of PBS (Fig. 7A) and VIP treated (Fig. 7B) mice, and quantitatively, were slightly decreased (not significantly) after VIP treatment (Fig. 7G). At 1 day p.i., rounded, motile Langerhans cells appeared decreased in the conjunctiva and peripheral cornea of VIP (Fig. 7D) vs PBS (Fig. 7C) treated mice and quantitation showed the difference was significant (p<0.05, Fig. 7G). By 3 days p.i., cell number appeared similar in PBS (Fig. 7E) and VIP (Fig. 7F) treated mice and when quantitated, no difference was seen between the two groups (Fig. 7G).
Discussion
VIP regulates a wide variety of immune reactions and among its numerous anti-inflammatory functions, participates in the maintenance of immune homeostasis (23, 24). Previously, this laboratory has shown that in the cornea of B6 mice infected with P. aeruginosa, VIP treatment regulates cytokine production through up-regulation of VIP receptor 1 on inflammatory cells, leading to less stromal destruction and prevention of corneal perforation (3). Recent work has provided further evidence that similar VIP treatment of B6 mice also has an indirect effect in that it modulates growth factors, angiogenic molecules and defensins in the infected cornea and that these in turn promote healing (4). In addition, other studies have shown that VIP down-regulates pro-inflammatory TLRs and related molecules in several non-ocular disease models (12). TLRs, important in innate immunity, are critical for recognition of conserved pathogen-associated molecular patterns (PAMPs) through TLR/IL-1R expressed on the surface of various cell types to trigger cytokine/chemokine production (25, 26). In this regard, previous studies from this laboratory and other investigators have shown that TLR1 (27), TLR3 (28), TLR4 (8) and TLR9 (6) play important roles in the pathogenesis of experimentally induced keratitis. However, VIP regulation of TLRs during these diseases has not been characterized. Thus, the current study, using a PCR array to profile 84 TLR related genes, provided an overview of the ability of VIP to provide master regulation of TLRs and signaling pathway molecules in bacterial keratitis. Twenty-one genes were down-regulated three-fold or more by VIP and several of them were selected for further confirmation by RT-PCR. Results indicated that VIP reduced pro-inflammatory TLRs and related molecules (TLR1, 4, 6, 8, 9, IRAK1, TRAF6 and Chuk) and agreed well with the array data. The array did not contain two anti-inflammatory TLRs important in promoting the resistance response of BALB/c mice to P. aeruginosa: SIGIRR (9) and ST2 (7), but when tested by RT-PCR, these genes, in general, were up-regulated by VIP treatment. Immunohistochemistry data further confirmed that VIP decreased TLR4 protein expression in both epithelial and stromal layers of the cornea. These results also are consistent with previous studies showing that VIP inhibits or even reverses TLR2 (29), TLR3 (30) and TLR4 (31, 32) expression or their stimulated signaling pathways in other non-ocular disease models.
On ligand-binding, TLRs recruit adaptor molecules such as IRAK and TRAF6 to their intracellular signaling domain, leading to the activation of several kinases, as well as the transcription factor, NF-κB, thereby directly up-regulating immune-response genes (33). In the current study, VIP treatment in vivo, down-regulated IRAK1 and TRAF6 mRNA levels, which is consistent with other reports using synovial fibroblasts isolated from patients with rheumatoid arthritis, where LPS stimulation together with VIP treatment vs LPS alone, inhibited IRAK1 and TRAF6 mRNA and phosphorylated protein expression (31), thereby acting as a negative regulator of TLR4 signaling.
Down-stream of IRAK-TRAF6 signaling, another important signaling complex, the IKK complex, is found and is composed of IKKα, IKKβ and IKKγ (34). The current in vivo study showed that VIP inhibited Chuk/IKKα mRNA expression, but only modestly decreased its activity, compatible with previous in vitro studies showing that VIP directly inhibited IKKα activity in human monocytes (35) and microglia cells (36). In microglial cells, VIP inhibition of IKKα, reduced Aβ induced neurodegeneration by indirectly inhibiting the production of numerous inflammatory and neurotoxic molecules (36). Furthermore, loss or decreased IKKα also has been suggested to negatively regulate NFκB and subsequent gene expression, by inhibiting its translocation to the nucleus and thus down-regulating inflammation (37).
In contrast to other TLRs, SIGIRR (38) and ST2 (39), members of the TLR−IL-1R superfamily, act as negative regulators of IL-1 and LPS signaling. Previous studies from this laboratory have found that SIGIRR (9) and ST2 (7) promote natural resistance of BALB/c vs B6 mice against P. aeruginosa keratitis and that antibody depletion of SIGIRR increased disease in BALB/c mice. However, whether VIP treatment regulates expression of either molecule in susceptible B6 mice was not tested. The current study showed that in vivo treatment of infected B6 mice with VIP increased (but not significantly) SIGIRR mRNA levels over PBS treated-controls in normal uninfected eyes and that at 1 day p.i. levels dropped in both groups, but remained higher with VIP treatment. In previous work from this laboratory (9), comparing the expression of SIGIRR in BALB/c vs B6 mice after P. aeruginosa infection, we also observed a decrease in mRNA expression in both groups at 12 h after infection (and in B6 mice at 1 day p.i., similar to the current study) which increased in both mouse strains later in disease. SIGIRR protein levels decreased in the two mouse strains at 1 day p.i. and increased later in disease, most significantly in BALB/c mice (9). After in vivo LPS challenge, similar consumption of SIGIRR mRNA (protein not tested) was shown by Wald (38) and he suggested that this may reflect its functional involvement in decreasing inflammation. We cited this possibility as well, but did not prove it functionally (9). The current study suggests that a decrease in SIGIRR protein is not required for functionality as we did not observe SIGIRR consumption at the protein level in B6 mice treated with VIP, rather, protein levels were increased at 1 day p.i. These data are supported by other studies, in which overexpression of SIGIRR (adenoviral vector expressing murine SIGIRR) has been shown to ameliorate LPS-induced acute lung injury in mice (40); and its overexpression in human macrophages and dendritic cells down-regulated TLR-induced cytokines, while its knockdown increased cytokine production following TLR stimulation (41). In contrast, ST2 protein is increased later (7 days p.i.) in disease, suggesting that after VIP treatment, the molecules could act disparately in time to lessen disease or participate in corneal healing.
Previous studies on the immunomodulatory properties of VIP led to identification of two pathways: one cAMP dependent and the other cAMP independent (11). However, whether VIP regulation of TLRs is cAMP dependent or independent had not been tested. To address this issue, we silenced AC7 using siRNA, as among the ten adenyl cyclases, it is highly expressed in the immune system (21). Data showed that VIP regulation of TLR1, TRAF6 and ST2 was cAMP dependent because silencing together with VIP treatment did not change the effects of silencing alone. These data agree with previous similarly designed in vitro studies in which a PKA inhibitor was used to block up-stream cAMP pathways (42, 43). Data from those studies concluded that VIP regulation of both a pro-inflammatory cytokine TNF-α (42) and NO (43) are cAMP dependent. In contrast, the current study showed that regulation of Chuk, IRAK1, 2, TLR4, 9 and SIGIRR are cAMP independent, as silencing together with VIP treatment changed the effects of silencing AC7 alone. These data are consistent with in vitro studies showing that VIP can function to regulate molecules such as NF-κB in a cAMP independent manner (35). Unexpectedly, AC7 siRNA vs scrambled control treatment did not induce significant changes in TLR6, but when silencing was combined with VIP treatment, TLR6 levels were lowered, suggesting the possibility that other adenyl cyclase isoforms (22) may participate in TLR regulation by VIP.
Previous studies from this laboratory have shown the importance of both macrophages (44) and Langerhans cells (18) in Pseudomonas keratitis. In this regard, VIP functions as a macrophage deactivating factor and has been shown to down-regulate pro-inflammatory cytokine expression (IL-1β and MIP-2) after LPS (not ultrapure) stimulation in murine peritoneal macrophages (3). It also down-regulates pro-inflammatory cytokine production in epidermal Langerhans cells (45), but its role in TLR regulation has not been characterized in these types of cells. Data from the current study showed that in peritoneal macrophages and XS52 (Langerhans) cells, ultrapure LPS stimulation up-regulated TLR1 and TLR4, but VIP plus LPS, down-regulated both TLRs only in macrophages. These data agree with others who have shown that stimulation of murine macrophages with LPS (not ultrapure) and VIP vs LPS alone resulted in less TLR4 expression at the transcriptional level (32), but no similar data is available for XS52 (Langerhans) cells, nor why they respond dissimilarly to macrophages. Regarding TLR1, since others have shown that cytokines such as IFN-γ can increase TLR1 expression in murine monocytes (46), we similarly tested for IFN-γ after ultrapure LPS stimulation of both cell types and found elevated levels which also were decreased by treatment with LPS plus VIP (data not shown). With similar treatment, TLR4 downstream signaling molecules also were down-regulated in macrophages including MD88, IRAK1 and TRAF6, while in XS52 (Langerhans) cells only MyD88 and TRAF6 were down-regulated. The current study also showed that LPS stimulation together with VIP vs LPS treatment alone, increased ST2 mRNA expression in macrophages, but in XS52 (Langerhans) cells only SIGIRR was increased. These data suggest that VIP disparately regulates TLR on these cells and may potentially reflect the kinetics of pathogen encounter (ocular surface vs stroma) after infection in the eye. Despite differential regulation of these cells, it is clear that VIP together with LPS reduces pro-inflammatory, while promoting anti-inflammatory TLR expression. VIP treatment in vivo also reduces Langerhans cell centripetal migration and thus decreased the number of cells in the infected cornea at 1 day p.i. Others also have shown that VIP treatment inhibits mouse dendritic cell migration to adjacent lymph nodes (47), limiting infection. All of this is consistent with the anti-inflammatory effects of VIP and with previous studies from this laboratory which showed that the presence of Langerhans cells in the cornea (before infection) exacerbates subsequent disease (18).
Overall, this study provides evidence that in vivo treatment with VIP down-regulates pro- and elevates anti-inflammatory TLRs and related signaling molecule expression via cAMP dependent and independent pathways and regulates Langerhans cell number and migration in the infected cornea. In vitro studies using ultrapure LPS which only activates the TLR4 pathway, confirm the in vivo data and provide evidence that TLR expression in macrophages and XS52 (Langerhans) cells are differentially regulated by VIP to improve disease outcome.
Acknowledgments
This investigation was supported by grants R01 EY002986, EY016058 and P30 EY004068 from the National Eye Institute, the National Institutes of Health, Bethesda, MD
Abbreviations used in this paper
- P. aeruginosa
Pseudomonas aeruginosa
- VIP
vasoactive intestinal peptide
- AC7
adenylate cyclase 7
- IRAK
interleukin-1 receptor-associated kinase
- TRAF
TNF receptor associated factors
- SIGIRR
Single Ig IL-1-related receptor
- Chuk/IKK-α
conserved helix-loop-helix ubiquitous kinase/Inhibitor of nuclear factor kappa-B kinase subunit alpha
References
- 1.Hazlett LD. Corneal response to Pseudomonas aeruginosa infection. Prog Retin Eye Res. 2004;23:1–30. doi: 10.1016/j.preteyeres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Hazlett LD, McClellan S, Kwon B, Barrett R. Increased severity of Pseudomonas aeruginosa corneal infection in strains of mice designated as Th1 versus Th2 responsive. Invest Ophthalmol Vis Sci. 2000;41:805–810. [PubMed] [Google Scholar]
- 3.Szliter EA, Lighvani S, Barrett RP, Hazlett LD. Vasoactive intestinal peptide balances pro- and anti-inflammatory cytokines in the Pseudomonas aeruginosa-infected cornea and protects against corneal perforation. J Immunol. 2007;178:1105–1114. doi: 10.4049/jimmunol.178.2.1105. [DOI] [PubMed] [Google Scholar]
- 4.Jiang X, McClellan SA, Barrett RP, Berger EA, Zhang Y, Hazlett LD. VIP and growth factors in the infected cornea. Invest Ophthalmol Vis Sci. 2011;52:6154–6161. doi: 10.1167/iovs.10-6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu FS, Hazlett LD. Toll-like receptors and the eye. Invest Ophthalmol Vis Sci. 2006;47:1255–1263. doi: 10.1167/iovs.05-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang X, Barrett RP, McClellan SA, Hazlett LD. Silencing Toll-like receptor-9 in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2005;46:4209–4216. doi: 10.1167/iovs.05-0185. [DOI] [PubMed] [Google Scholar]
- 7.Huang X, Du W, Barrett RP, Hazlett LD. ST2 is essential for Th2 responsiveness and resistance to pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2007;48:4626–4633. doi: 10.1167/iovs.07-0316. [DOI] [PubMed] [Google Scholar]
- 8.Huang X, Du W, McClellan SA, Barrett RP, Hazlett LD. TLR4 is required for host resistance in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2006;47:4910–4916. doi: 10.1167/iovs.06-0537. [DOI] [PubMed] [Google Scholar]
- 9.Huang X, Hazlett LD, Du W, Barrett RP. SIGIRR promotes resistance against Pseudomonas aeruginosa keratitis by down-regulating type-1 immunity and IL-1R1 and TLR4 signaling. J Immunol. 2006;177:548–556. doi: 10.4049/jimmunol.177.1.548. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Karmakar M, Roy S, Ramadan RT, Williams SR, Howell S, Shive CL, Han Y, Stopford CM, Rietsch A, Pearlman E. TLR4 and TLR5 on corneal macrophages regulate Pseudomonas aeruginosa keratitis by signaling through MyD88-dependent and -independent pathways. J Immunol. 2010;185:4272–4283. doi: 10.4049/jimmunol.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chorny A, Gonzalez-Rey E, Varela N, Robledo G, Delgado M. Signaling mechanisms of vasoactive intestinal peptide in inflammatory conditions. Regul Pept. 2006;137:67–74. doi: 10.1016/j.regpep.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Gomariz RP, Arranz A, Juarranz Y, Gutierrez-Canas I, Garcia-Gomez M, Leceta J, Martinez C. Regulation of TLR expression, a new perspective for the role of VIP in immunity. Peptides. 2007;28:1825–1832. doi: 10.1016/j.peptides.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Rudner XL, Kernacki KA, Barrett RP, Hazlett LD. Prolonged elevation of IL-1 in Pseudomonas aeruginosa ocular infection regulates macrophage-inflammatory protein-2 production, polymorphonuclear neutrophil persistence, and corneal perforation. J Immunol. 2000;164:6576–6582. doi: 10.4049/jimmunol.164.12.6576. [DOI] [PubMed] [Google Scholar]
- 14.Delgado M, Martinez C, Pozo D, Calvo JR, Leceta J, Ganea D, Gomariz RP. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activation polypeptide (PACAP) protect mice from lethal endotoxemia through the inhibition of TNF-alpha and IL-6. J Immunol. 1999;162:1200–1205. [PubMed] [Google Scholar]
- 15.Wu M, McClellan SA, Barrett RP, Hazlett LD. Beta-defensin-2 promotes resistance against infection with P. aeruginosa. J Immunol. 2009;182:1609–1616. doi: 10.4049/jimmunol.182.3.1609. [DOI] [PubMed] [Google Scholar]
- 16.Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 17.Xu S, Ariizumi K, Caceres-Dittmar G, Edelbaum D, Hashimoto K, Bergestresser PR, Takashima A. Successive generation of antigen-presenting, dendritic cell lines from murine epidermis. J Immunol. 1995;154:2697–2705. [PubMed] [Google Scholar]
- 18.Hazlett LD, McClellan SA, Rudner XL, Barrett RP. The role of Langerhans cells in Pseudomonas aeruginosa infection. Invest Ophthalmol Vis Sci. 2002;43:189–197. [PubMed] [Google Scholar]
- 19.Hacker H, Tseng PH, Karin M. Expanding TRAF function: TRAF3 as a tri-faced immune regulator. Nat Rev Immunol. 2011;11:457–468. doi: 10.1038/nri2998. [DOI] [PubMed] [Google Scholar]
- 20.Deng JC, Cheng G, Newstead MW, Zeng X, Kobayashi K, Flavell RA, Standiford TJ. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Invest. 2006;116:2532–2542. doi: 10.1172/JCI28054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duan B, Davis R, Sadat EL, Collins J, Sternweis PC, Yuan D, Jiang LI. Distinct roles of adenylyl cyclase VII in regulating the immune responses in mice. J Immunol. 2010;185:335–344. doi: 10.4049/jimmunol.0903474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song J, Duncan MJ, Li G, Chan C, Grady R, Stapleton A, Abraham SN. A novel TLR4-mediated signaling pathway leading to IL-6 responses in human bladder epithelial cells. PLoS Pathog. 2007;3:e60. doi: 10.1371/journal.ppat.0030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomariz RP, Gutierrez-Canas I, Arranz A, Carrion M, Juarranz Y, Leceta J, Martinez C. Peptides targeting Toll-like receptor signalling pathways for novel immune therapeutics. Curr Pharm Des. 2010;16:1063–1080. doi: 10.2174/138161210790963841. [DOI] [PubMed] [Google Scholar]
- 24.Smalley SG, Barrow PA, Foster N. Immunomodulation of innate immune responses by vasoactive intestinal peptide (VIP): its therapeutic potential in inflammatory disease. Clin Exp Immunol. 2009;157:225–234. doi: 10.1111/j.1365-2249.2009.03956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chtarbanova S, Imler JL. Microbial sensing by Toll receptors: a historical perspective. Arterioscler Thromb Vasc Biol. 2011;31:1734–1738. doi: 10.1161/ATVBAHA.108.179523. [DOI] [PubMed] [Google Scholar]
- 26.Medzhitov R, Janeway C., Jr. The Toll receptor family and microbial recognition. Trends Microbiol. 2000;8:452–456. doi: 10.1016/s0966-842x(00)01845-x. [DOI] [PubMed] [Google Scholar]
- 27.Yuan X, Wilhelmus KR. Toll-like receptors involved in the pathogenesis of experimental Candida albicans keratitis. Invest Ophthalmol Vis Sci. 2010;51:2094–2100. doi: 10.1167/iovs.09-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson AC, Li X, Pearlman E. MyD88 functions as a negative regulator of TLR3/TRIF-induced corneal inflammation by inhibiting activation of c-Jun N-terminal kinase. J Biol Chem. 2008;283:3988–3996. doi: 10.1074/jbc.M707264200. [DOI] [PubMed] [Google Scholar]
- 29.Foster N, Lea SR, Preshaw PM, Taylor JJ. Pivotal advance: vasoactive intestinal peptide inhibits up-regulation of human monocyte TLR2 and TLR4 by LPS and differentiation of monocytes to macrophages. J Leukoc Biol. 2007;81:893–903. doi: 10.1189/jlb.0206086. [DOI] [PubMed] [Google Scholar]
- 30.Lee H, Park K, Kim JS, Lee SJ. Vasoactive intestinal peptide inhibits toll-like receptor 3-induced nitric oxide production in Schwann cells and subsequent sensory neuronal cell death in vitro. J Neurosci Res. 2009;87:171–178. doi: 10.1002/jnr.21820. [DOI] [PubMed] [Google Scholar]
- 31.Arranz A, Gutierrez-Canas I, Carrion M, Juarranz Y, Pablos JL, Martinez C, Gomariz RP. VIP reverses the expression profiling of TLR4-stimulated signaling pathway in rheumatoid arthritis synovial fibroblasts. Mol Immunol. 2008;45:3065–3073. doi: 10.1016/j.molimm.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Arranz A, Androulidaki A, Zacharioudaki V, Martinez C, Margioris AN, Gomariz RP, Tsatsanis C. Vasoactive intestinal peptide suppresses toll-like receptor 4 expression in macrophages via Akt1 reducing their responsiveness to lipopolysaccharide. Mol Immunol. 2008;45:2970–2980. doi: 10.1016/j.molimm.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 33.Michelsen KS, Doherty TM, Shah PK, Arditi M. TLR signaling: an emerging bridge from innate immunity to atherogenesis. J Immunol. 2004;173:5901–5907. doi: 10.4049/jimmunol.173.10.5901. [DOI] [PubMed] [Google Scholar]
- 34.Israel A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb Perspect Biol. 2010;2:a000158. doi: 10.1101/cshperspect.a000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delgado M, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit nuclear factor-kappa B-dependent gene activation at multiple levels in the human monocytic cell line THP-1. J Biol Chem. 2001;276:369–380. doi: 10.1074/jbc.M006923200. [DOI] [PubMed] [Google Scholar]
- 36.Delgado M, Varela N, Gonzalez-Rey E. Vasoactive intestinal peptide protects against beta-amyloid-induced neurodegeneration by inhibiting microglia activation at multiple levels. Glia. 2008;56:1091–1103. doi: 10.1002/glia.20681. [DOI] [PubMed] [Google Scholar]
- 37.Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS. A nucleosomal function for IkappaB kinase-alpha in NF-kappaB-dependent gene expression. Nature. 2003;423:659–663. doi: 10.1038/nature01648. [DOI] [PubMed] [Google Scholar]
- 38.Wald D, Qin J, Zhao Z, Qian Y, Naramura M, Tian L, Towne J, Sims JE, Stark GR, Li X. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2003;4:920–927. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- 39.Liew FY, Xu D, Brint EK, O'Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Zhao Y, Wu X, Qian G. Enhanced expression of single immunoglobulin IL-1 receptor-related molecule ameliorates LPS-induced acute lung injury in mice. Shock. 2011;35:198–204. doi: 10.1097/SHK.0b013e3181f226f3. [DOI] [PubMed] [Google Scholar]
- 41.Drexler SK, Kong P, Inglis J, Williams RO, Garlanda C, Mantovani A, Yazdi AS, Brennan F, Feldmann M, Foxwell BM. SIGIRR/TIR-8 is an inhibitor of Toll-like receptor signaling in primary human cells and regulates inflammation in models of rheumatoid arthritis. Arthritis Rheum. 2010;62:2249–2261. doi: 10.1002/art.27517. [DOI] [PubMed] [Google Scholar]
- 42.Delgado M, Pozo D, Martinez C, Leceta J, Calvo JR, Ganea D, Gomariz RP. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit endotoxin-induced TNF-alpha production by macrophages: in vitro and in vivo studies. J Immunol. 1999;162:2358–2367. [PubMed] [Google Scholar]
- 43.Delgado M, Munoz-Elias EJ, Gomariz RP, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide prevent inducible nitric oxide synthase transcription in macrophages by inhibiting NF-kappa B and IFN regulatory factor 1 activation. J Immunol. 1999;162:4685–4696. [PubMed] [Google Scholar]
- 44.McClellan SA, Huang X, Barrett RP, van Rooijen N, Hazlett LD. Macrophages restrict Pseudomonas aeruginosa growth, regulate polymorphonuclear neutrophil influx, and balance pro- and anti-inflammatory cytokines in BALB/c mice. J Immunol. 2003;170:5219–5227. doi: 10.4049/jimmunol.170.10.5219. [DOI] [PubMed] [Google Scholar]
- 45.Kodali S, Ding W, Huang J, Seiffert K, Wagner JA, Granstein RD. Vasoactive intestinal peptide modulates Langerhans cell immune function. J Immunol. 2004;173:6082–6088. doi: 10.4049/jimmunol.173.10.6082. [DOI] [PubMed] [Google Scholar]
- 46.Krutzik SR, Ochoa MT, Sieling PA, Uematsu S, Ng YW, Legaspi A, Liu PT, Cole ST, Godowski PJ, Maeda Y, Sarno EN, Norgard MV, Brennan PJ, Akira S, Rea TH, Modlin RL. Activation and regulation of Toll-like receptors 2 and 1 in human leprosy. Nat Med. 2003;9:535–532. doi: 10.1038/nm864. [DOI] [PubMed] [Google Scholar]
- 47.Weng Y, Sun J, Wu Q, Pan J. Regulatory effects of vasoactive intestinal peptide on the migration of mature dendritic cells. J Neuroimmunol. 2007;182:48–54. doi: 10.1016/j.jneuroim.2006.09.009. [DOI] [PubMed] [Google Scholar]