Structured Abstract
Objectives
To evaluate the relationship of age with symptoms and interference with daily function and QOL during RT.
Design
A prospective observational study.
Setting
A university-based radiation oncology department.
Participants
903 cancer patients who received radiation therapy (RT). The mean age was 61 yrs (18-92) and 41% were ≥ 65 yrs.
Measurements
A symptom inventory was administered pre- and post-RT. Patients rated 10 symptoms and their interference with daily function and QOL on a Likert scale from 0 (not present) to 10 (as bad as possible). A total symptom score was calculated by adding the ratings of individual symptoms. T-tests, Pearson correlation coefficients, and mixed modeling were used to investigate relationships between symptoms and their interference with daily function and QOL.
Results
For older and younger patients, the total symptom score worsened during RT (p's < .001). There were no differences in the change in total symptom burden and interference with QOL between older and younger patients during RT. After RT, although younger patients reported significantly worse pain (p = .03), nausea (p <.01), and sleep disturbance (p <.01), symptom interference with walking was more severe in older patients (p = .01). Mixed modeling showed that older age (p=<.001), time of survey (after RT, p<.001), and age*time interaction (p<.001) increased the likelihood of reporting that symptoms interfered with walking.
Conclusion
The prevalence of symptoms was similar for older and younger patients during RT. Older patients are more likely to report that symptoms interfere with walking after RT.
Keywords: elderly, cancer, symptoms, radiotherapy
Introduction
Cancer and its treatment produce multiple physical and psychological symptoms that can interfere with daily function and quality of life.1 Older patients with underlying comorbidities or lower baseline functional status experience a different symptom profile resulting from cancer and its treatment than their younger counterparts. These symptoms can lead to delays or early discontinuation of cancer therapies, especially in older patients who may have a reduced tolerance for side effects from treatment.2
Several studies have assessed the impact of overall symptom burden on the quality of life (QOL) of cancer patients undergoing radiation therapy (RT). In one study, 63% of lung cancer patients receiving chemoradiation suffered from moderate-to-severe levels of multiple symptoms by the end of treatment.3 Investigators at the University of Rochester characterized the longitudinal course of common symptoms in 1,129 consecutive patients during RT.4 In 419 patients, all symptoms significantly increased in frequency during the 5-week study period. Only 13% of RT patients reported no fatigue.5
Few studies have evaluated the differential impact of symptoms during RT for cancer on daily function in older patients compared to younger patients. There is little information from large patient cohorts about when individual symptoms begin to appear in the context of cancer treatment, how symptoms compare to each other in frequency and severity, and whether individual symptoms worsen or improve during RT in older patients compared to their younger counterparts. In this study, we utilized a standardized validated symptom inventory (SI)4, 5 to assess whether the prevalence and severity of multiple symptoms and their interference with daily function and QOL in patients undergoing RT differs with age. The primary objectives were to determine the association of age on the prevalence and the pattern of change in specific symptoms during and after RT and on the interference of symptoms with daily responsibilities and QOL.
Materials and Methods
Patients
The sample consisted of 903 consecutive cancer patients beginning RT at the James P. Wilmot Cancer Center (JPWCC). Older patients were classified as those aged 65 years and over. All radiation oncology patients who were not receiving concurrent chemotherapy were asked to fill out a SI before and after RT. Patients who completed the SI in the first week and last week of RT were included in the analysis. Patients received their first RT treatment between January 3, 2001 and March 4, 2004.
Procedures
The procedures utilized in this study have been described in previous research.4, 5 All patients completed a patient authorization form allowing the information obtained in the SI to be stored in a database that is maintained by the JPWCC Behavioral Medicine Unit for research purposes. Permission for this study was obtained from the University of Rochester Research Subjects Review Board.
Measures
Demographics
Demographic and relevant medical information was obtained from a review of patient medical records. This information included age, sex, race, cancer type, days of RT, and RT dose.
Symptom inventory (SI)
The SI is a list of 10 symptoms (fatigue, sleep disturbance, drowsiness, pain, nausea, feeling distressed [upset], shortness of breath, memory trouble, loss of appetite, vomiting)6. The 1 -page questionnaire, which takes approximately 5 minutes to complete, consists of a series of scales on which the presence and severity of each symptom is rated when it was at its worst during the prior five days. Patients filled out the appropriate circle on an 11-point horizontal scale anchored by 0 = —Not Present” and 10 = —As Bad As You Can Imagine.” The presence of a symptom was defined as a score of at least 1 “on the 11-point SI scale. Severity of each symptom was measured by this score which reflects the highest intensity of symptom over the prior 5 days as reported by the patient. Patients also rated the interference of symptoms (in total) on 5 measures (work, mood, activity, walking, and relationships) and overall QOL using the same scales as described for the individual symptoms.
Statistical Analyses
Analytical methods included the calculation of descriptive statistics to assess patient demographics and symptoms. Two-sample t-tests were used to evaluate age differences (Young vs. Old) for the Pre-RT and Post-RT responses, and the change scores, Post-RT – Pre-RT. Symptom severity was represented by calculating the mean score of each symptom. Because patients were asked to rate each symptom at its worst over the prior 5 days, this score represents the highest intensity of each symptom. Total symptom burden was calculated by adding the mean scores of each of the 10 symptoms. Pearson correlations were utilized to assess the relationship between total symptom burden and interferences. Chi-square tests were used to assess differences in proportions of young and old patients with symptoms and interferences. All tests for significant differences were performed at the 0.05 level of significance.
The above comparisons treated age as a dichotomous variable. To evaluate age as a continuous variable, mixed modeling was employed to provide detailed information about age, time (pre- versus post-RT), and gender on each symptom and interference. We noted nonlinear age effects and therefore entered age into the model through cubic basis splines with three degrees of freedom.7 A cubic spline is a nonparametric regression method based on subdividing age, and fitting a cubic polynomial into the data in each of the sections with continuity constraints between the polynomial fits.7,8 The results of the analyses were converted to graphs to display symptom/interference score versus age, broken down by gender and time.
Results
Patients
A total of 903 patients provided complete data at baseline and during the last week of RT. Table 1 shows the descriptive data on patient characteristics for the total sample and by age group (i.e., younger versus older patients). The median age of the sample was 60.8 years. Of the 903 patients, 368 (40.7%) were aged 65 years or older. Among the most frequent diagnoses for which patients were receiving RT were breast carcinoma, 27.3%; genitourinary carcinoma, 19.2%; and lung carcinoma, 12.1%. Older patients were more likely to be female (p<.001) and were less likely to have breast cancer as their diagnosis (p<.001). There were no significant differences in number of days or dose of RT between younger and older patients.
Table 1. Patient and Disease Characteristics.
| Total | Young | Older | P value | |
|---|---|---|---|---|
| Age-median and range (yrs) | 60.7 (18-92) | 51.7 (18-64) | 74.1 (65-92) | NA |
| <65 | 59.3% | |||
| >=65 | 40.7% | |||
| Gender | <0.01 | |||
| Female | 49.7% | 43.7% | 58.4% | |
| Male | 50.3% | 56.3% | 41.6% | |
| Race | 0.19 | |||
| White | 90.3% | 89.5% | 92.0% | |
| African American | 6.9% | 8.0% | 4.78% | |
| Diagnosis | ||||
| Breast | 27.3% | 34.4% | 17.1% | <0.01 |
| Genitourinary Tract | 19.2% | 11.6% | 30.4% | <0.01 |
| Lung | 12.0% | 9.5% | 15.8% | <0.01 |
| Brain & Peripheral Nervous System | 9.9% | 12.3% | 6.5% | <0.01 |
| Alimentary Tract | 8.1% | 6.7% | 10.1% | 0.07 |
| Hematologic | 8.1% | 10.3% | 4.9% | <0.0 |
| Head & Neck | 6.2% | 6.2% | 6.3% | 0.96 |
| Soft-Tissue Sarcoma | 3.3% | 4.5% | 1.6% | 0.02 |
| Bone & Cartilaginous | 1.6% | 1.9% | 1.4% | 0.56 |
| Skin | 1.5% | 0.4% | 3.3% | <0.01 |
| Gynecologic | 0.9% | 1.3% | 0.8% | 0.48 |
| Unknown Primary | 0.9% | 0.4% | 1.6% | 0.05 |
| Melanoma | 0.4% | 0.6% | 0.3% | 0.52 |
| RT characteristics | ||||
| Total Gy—median and range | 57.6 (30-168) | 57.2 (30-168) | 57.6 (30-161) | 0.95 |
| Total days treated—median and range | 28.0 (10-48) | 28.5 (10-42) | 28.0 (10-48) | 0.95 |
Baseline prevalence and severity of symptoms and their interference with daily function and QOL
Total sample
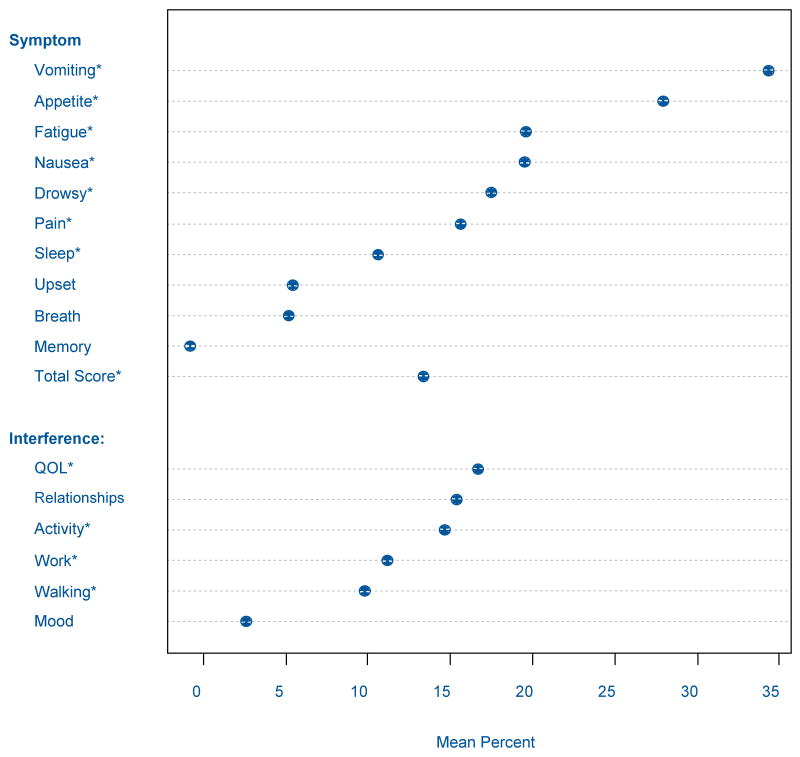
At the beginning of RT, 83.8% of the total sample experienced at least one of the 10 symptoms (Figure 1). The most frequent symptoms were drowsiness (62.3%), fatigue (60.9%), sleep disturbance (50.8%), and distress (50.3%). The most severe symptoms included fatigue (mean score 2.70, 95% CI: 2.52-2.89), drowsiness (mean score 2.34, 95% CI: 2.10-2.57), and sleep disturbance (mean score 1.88, 95% CI: 1.71-2.05).
Figure 1. Mean Percent Increase During Radiation Therapy of Symptom Severity and Their Interference with QOL and Daily Life Responsibilities.
In the total sample, 66.3% of patients reported that their symptoms interfered with at least one domain prior to RT. The most frequently affected was working (53.1%), general activities (51.8%), and mood (52.2%). Fifty-two percent of patients reported that symptoms interfered with overall QOL. Interference with working was the most intense (mean score 2.33, 95% CI: 2.13-2.54) followed by interference with general activity (mean score 2.18, 95% CI: 1.99-2.37).
Age-related differences in symptom prevalence and severity and their interference with daily function and QOL before RT
The most prevalent symptoms at baseline among both the younger and older patients were fatigue, drowsiness, sleep disturbance, and distress (Table 2). Sleep disturbance, distress, and pain were significantly less prevalent among the older patients compared to the younger patients (p's<.01), while shortness of breath was significantly more prevalent among older patients before RT (p=.04). More younger patients reported that symptoms interfered with mood (p=.02) and working (p=.03). Older patients were more likely to report that their symptoms interfered with walking, although this result was not statistically significant (44.3% versus 38.5%, p=.09).
Table 2. Prevalence of Symptoms and their Interference with Daily Life Responsibilities During RT.
| Symptom | Pre-RT | Post-RT | Change | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Young % | Old % | p value | Young % | Old % | p value | Young % | Old % | p value | |
| Fatigue | 68.8 | 69.1 | 0.92 | 80.5 | 82.4 | 0.48 | 11.7 | 13.2 | 0.75 |
| Drowsiness | 60.5 | 64.7 | 0.34 | 69.9 | 76.7 | 0.10 | 9.4 | 12.0 | 0.39 |
| Sleep | 57.1 | 41.7 | <0.01 | 62.7 | 55.8 | 0.04 | 5.7 | 14.0 | 0.02 |
| Distress | 54.5 | 44.2 | <0.01 | 58.1 | 49.6 | 0.01 | 3.7 | 5.4 | 0.86 |
| Pain | 45.9 | 35.4 | <0.01 | 55.4 | 49.3 | 0.07 | 9.5 | 13.9 | 0.20 |
| Memory | 46.4 | 40.1 | 0.08 | 47.3 | 46.3 | 0.78 | 0.9 | 6.3 | 0.07 |
| Appetite | 33.1 | 36.6 | 0.28 | 41.4 | 47.0 | 0.10 | 8.3 | 10.4 | 0.55 |
| Breath | 33.3 | 40.1 | 0.04 | 36.2 | 40.9 | 0.15 | 2.8 | 0.9 | 0.42 |
| Nausea | 24.5 | 21.0 | 0.23 | 32.6 | 25.3 | 0.02 | 8.1 | 4.3 | 0.22 |
| Vomiting | 10.7 | 8.5 | 0.28 | 14.5 | 12.5 | 0.48 | 3.8 | 4.1 | 0.71 |
| Any symptom | 78.5 | 74.5 | 0.10 | 84.9 | 84.8 | 0.47 | 6.3 | 10.3 | 0.20 |
| 2 or more symptoms | 60.6 | 57.6 | 0.40 | 70.3 | 67.1 | 0.34 | 9.7 | 9.5 | 0.95 |
| Activity | 53.2 | 49.7 | 0.32 | 66.8 | 62.4 | 0.18 | 13.6 | 12.7 | 0.72 |
| Mood | 54.7 | 46.7 | 0.02 | 60.5 | 53.5 | 0.04 | 5.9 | 6.9 | 0.98 |
| Working | 56.1 | 48.7 | 0.03 | 65.8 | 62.3 | 0.29 | 9.7 | 13.6 | 0.38 |
| Relationships | 39.8 | 33.2 | 0.05 | 47.9 | 39.7 | 0.02 | 8.2 | 6.5 | 0.57 |
| Walking | 38.5 | 44.3 | 0.09 | 46.0 | 53.1 | 0.04 | 7.5 | 8.8 | 0.76 |
| QOL | 54.2 | 49.1 | 0.15 | 63.8 | 58.8 | 0.13 | 9.7 | 9.6 | 0.92 |
| Any interference | 68.8 | 62.6 | 0.07 | 76.2 | 75.0 | 0.68 | 7.5 | 12.4 | 0.21 |
| 2 or more interferences | 51.4 | 44.3 | 0.04 | 61.9 | 55.4 | 0.06 | 10.3 | 11.1 | 0.84 |
Multiple symptoms were reported by all patients. The younger patients had an average of 3.7 symptoms (median=4, range 0-9) and 3.0 interferences (median=3, range 0-6) at baseline. The older patients had an average of 3.4 symptoms (median=3, range 0-9) and 2.7 interferences (median=2, range 0-6). Although there were no differences in prevalence of patients who reported 2 or more symptoms prior to RT, younger patients were more likely than older patients to report 2 or more interferences (51.4% vs 44.3%, p=.04)
Younger patients reported a significantly higher intensity of sleep disturbance (2.20 versus 1.43, p<.01), distress (1.80 versus 1.42, p=.02), and vomiting (0.43 versus 0.23, p=.02). At baseline, the only significant difference in interference was that older patients reported higher interference of symptoms with walking (1.97 versus 1.57, p=.04).
Changes in prevalence and severity of symptoms and their interference with daily function and QOL during RT
Total sample
The total symptom score significantly worsened (12.6 versus 14.3, p<.01) during RT as did scores of all interferences. There were significant positive correlations between change in total symptom score and all interferences (all p values <.001).
Age-related differences in prevalence and severity of symptoms and their interference with daily function and QOL after RT
After RT, more younger patients had sleep disturbance (p=.04), distress (p=.01), and nausea (p=.02) than older patients. Younger patients also had greater interference of symptoms with mood (p=.04) and relationships (p=.02). More older patients reported interference of symptoms with walking after RT (53.1% versus 46.0%, p=.04).
After RT, younger patients rated sleep disturbance (2.30 versus 1.76, p<.01), pain (2.00 versus 1.64, p=.03), and nausea (1.06 versus 0.73, p<.01) as more intense than older patients. Older patients reported more severe interference of symptoms with walking after RT (2.19 versus 1.70, p=.01).
Age-related changes in prevalence and severity of symptoms and their interference with daily function and QOL over RT
Among both younger and older patients, the prevalence of most symptoms increased significantly during RT (Table 2). The prevalence of memory difficulties significantly increased for older patients (p<.01), but not for younger patients. The prevalence of sleep disturbance increased more for older patients over the course of RT than younger patients (p=.02). The prevalence of patients reporting any interference increased more for older patients than for younger patients when using 70 as the age cut-off (change of 15.1% versus 6.9%, p=.03).
Both younger and older patients had an increase in severity of scores for fatigue and loss of appetite over RT. After RT, older patients also reported more sleep disturbance (1.76 versus 1.43, p=.03) and distress (1.71 versus 1.42, p=.03). There were statistically significant increases in severity of symptom interference with general activity (2.52 versus 2.15, p=.01) and QOL (2.40 versus 2.02, p<.01) among the younger patients. Older patients had an increase in severity of distress while severity of distress symptoms did not change for younger patients (0.23 versus −.06, p=.046) (Table 3).
Table 3. Symptom and Interference Severity During Radiation Therapy (RT).
| Pre-RT Scores | Post-RT Scores | Change | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Symptom | Young | Old | p value | Young | Old | p value | Young | Old | p value |
| Fatigue | 2.74 | 2.64 | 0.57 | 3.23 | 3.23 | 0.98 | 0.47 | 0.54 | 0.69 |
| Sleep | 2.20 | 1.43 | <.01 | 2.30 | 1.76 | <.01 | 0.10 | 0.29 | 0.27 |
| Distress | 1.80 | 1.42 | 0.02 | 1.77 | 1.71 | 0.72 | −0.06 | 0.23 | 0.05 |
| Pain | 1.71 | 1.45 | 0.13 | 2.00 | 1.64 | 0.03 | 0.27 | 0.18 | 0.58 |
| Memory | 1.30 | 1.34 | 0.76 | 1.23 | 1.42 | 0.19 | −0.06 | 0.05 | 0.27 |
| Appetite | 1.22 | 1.39 | 0.31 | 1.53 | 1.83 | 0.09 | 0.31 | 0.44 | 0.41 |
| Breath | 1.06 | 1.31 | 0.08 | 1.16 | 1.31 | 0.31 | 0.09 | 0.01 | 0.36 |
| Nausea | 0.86 | 0.63 | 0.06 | 1.06 | 0.73 | <.01 | 0.19 | 0.08 | 0.43 |
| Vomiting | 0.43 | 0.23 | 0.02 | 0.56 | 0.34 | 0.03 | 0.12 | 0.11 | 0.90 |
| Drowsy | 2.48 | 2.16 | 0.18 | 2.81 | 2.67 | 0.59 | 0.35 | 0.60 | 0.31 |
| Total Score | 14.44 | 13.19 | 0.25 | 16.11 | 14.98 | 0.31 | 1.32 | 1.66 | 0.68 |
| Activity | 2.15 | 2.22 | 0.75 | 2.52 | 2.45 | 0.72 | 0.37 | 0.21 | 0.38 |
| Mood | 1.98 | 1.77 | 0.23 | 2.01 | 1.88 | 0.43 | 0.02 | 0.09 | 0.70 |
| Work | 2.43 | 2.20 | 0.30 | 2.62 | 2.55 | 0.73 | 0.19 | 0.29 | 0.61 |
| Relationships | 1.23 | 1.07 | 0.25 | 1.39 | 1.30 | 0.57 | 0.13 | 0.22 | 0.59 |
| Walking | 1.57 | 1.97 | 0.04 | 1.70 | 2.19 | 0.01 | 0.12 | 0.23 | 0.53 |
| QOL | 2.02 | 2.07 | 0.79 | 2.40 | 2.37 | 0.96 | 0.33 | 0.27 | 0.75 |
Association of age on symptoms and interference with daily function and QOL
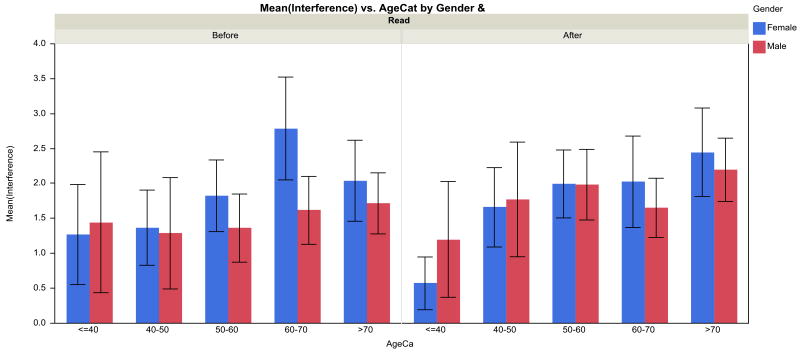
Mixed modeling of the symptoms showed significant age effects for nausea (p=.03), pain (p=.02), and sleep disturbance (p<.01), with a maximum in these symptoms at 50 years of age. Mixed modeling of the interference with walking showed a significant age effect (p<.001), age*time interaction (p<.001), gender effect (p=.048), gender*time interaction (p=.02), and time effect (p<.001). Figure 2 depicts the results graphically. Before RT, the age effect was small, while post-RT walking interference increased with age. In other words, the age effect is the most salient feature; before RT, the age effect was small, while post-RT walking interference increased with age.
Figure 2. Interference of symptoms with walking as related to RT (before versus after), by age and gender.
Discussion
In a large sample of consecutive patients receiving RT for cancer, older patients have a similar symptom burden profile before and after RT compared to younger patients. However, a higher prevalence of older patients reported greater interference of symptoms with walking after RT and severity of distress increased more for older patients over the course of RT. This study is one of the first studies to report differences in prevalence and severity of symptoms between older and younger patients receiving RT for cancer.
At any given time during the patient's diagnosis or treatment course, symptom burden consists of the subjective perception of the patient's overall impressions of cancer and treatment burden.9 In this study, 90% of older patients reported a symptom and 75% of older patients reported that symptoms interfered with daily function or QOL after RT. The high prevalence of symptoms and their interference with QOL in elders with cancer has been described in other studies. Given et al. reported that symptoms such as insomnia, fatigue and pain had a consistent and significant effect on QOL unrelated to the type of cancer, treatment, stage of disease or comorbid conditions in 826 elderly patients with cancer.10 High fatigue and pain levels are associated with interference with QOL and functioning in elderly lung cancer11, 12 and breast cancer patients.13 Our study adds to the literature by evaluating symptoms, in a large cohort of patients, over the course of a single treatment modality, RT.
Younger and older patients have similarities in symptom prevalence and the change in symptom prevalence over the course of RT. Fatigue, drowsiness, and pain were very common and severe, and increased during RT for both younger and older patients. Other studies have reported that 70% to 100% of patients undergoing treatment experience cancer-related fatigue (CRF).2, 14 CRF is usually more intense and more disruptive than other etiologies of fatigue and is not relieved by rest.15, 16 The National Comprehensive Cancer Network Guidelines (NCCN) reports that a score of 4 or more on a 10 point scale for fatigue or other symptoms should trigger a comprehensive intervention.17 Our study showed that the mean scores of symptoms and their interferences were in the —mild” range has described by the NCCN. A systematic review of CRF found that most studies reported no relationship between age and level of fatigue.18 Most studies have evaluated fatigue at a single point in time rather than longitudinally in relationship with cancer treatment. Ahlberg et al. found that CRF increased in women with uterine cancer who were undergoing RT (mean age 66, range 37 to 84).19 Cleeland et al. found that fatigue increased longitudinally in patients receiving concurrent chemo-radiotherapy for lung cancer.3 It has been reported that 50% to 90% of older adults with cancer experience pain.2 Even with this high prevalence, few studies have specifically examined cancer pain in the elderly or addressed the issue of differences related to age.16, 20
A higher prevalence of younger patients had sleep problems and nausea after RT compared to older patients. Other studies have shown that older patients with cancer may be less likely to experience or report these symptoms.21, 22 Younger patients also reported greater interference of symptoms with working and relationships which may be due to a higher level of day-to-day responsibilities. In this study, older age was significantly associated with greater interference of symptoms with walking. Although several studies have reported interference of cancer symptoms with physical functioning, these studies have been small and heterogeneous in the timing of survey administration as related to treatment, and they have not evaluated age differences in outcomes.12, 15, 23 In one study, Gift et al. showed that in elderly patients with lung cancer, dyspnea and fatigue interfered with physical activity in approximately 50% of patients.12 Cancer treatments such as chemotherapy and hormonal therapy have been associated with adverse physical performance in elders. For example, prostate cancer patients receiving androgen deprivation therapy have a high prevalence of falls and physical performance deficits.24-26
This study suggests that clinicians should assess not only symptoms but also their effects on daily functioning and physical performance. Although certain symptoms are more common and more severe in younger patients, the interference of symptoms with function may be more prevalent and severe in older patients. Interference of symptoms with function can lead to a higher level of distress in older patients. This study showed that the severity of distress increased more during RT for older patients than for younger patients. This finding is consistent with the results of a study by Hurria et al. which showed that poor physical functioning was the best predictor of distress in older cancer patients.27 This information has implications for clinical practice and research. Interventions to improve or reduce the impact of cancer and cancer treatment on physical functioning in older patients could improve overall quality of level and reduce distress.
There are limitations to this study. Our study did not collect detailed information about comorbidities and functional status through geriatric assessment. Therefore, it is unclear how much other non-cancer health problems outside the cancer contribute to the higher interference of symptoms with walking in older patients. Our study also had inherent heterogeneity in terms of types of cancer and stages included. A significant limitation of this study was that the data collection did not include whether the intent of RT was curative or palliative, and therefore we cannot accurately assess whether symptoms and/or interference differ for patients in these groups. However, the older and younger patients in this sample did not have differences in days on RT or RT dosing (which would reflect goals of RT), which likely reflects that the younger and older subgroups had similar underlying characteristics in terms of goals of therapy. In addition, we did not have information on treatments (chemotherapy or otherwise) that could affect symptoms of patients prior to RT. Another study limitation was that the patients were primarily white and from a single institution. Despite these limitations, the strengths of the study include the size of the sample and longitudinal assessment of symptoms and interference with daily function and QOL.
The majority of previous symptom research has focused on single symptoms. In reality, patients experience symptoms simultaneously, and many symptoms co-occur.28 Symptom clusters of fatigue, pain, insomnia, and mood disturbance have been shown to negatively impact function and QOL in older patients during cancer therapy over and above the effects of each symptom independently.29 Future work with the database utilized in this study will evaluate whether a difference exists in symptom clusters and their impact on function between older and younger patients.
In summary, this study finds that for the most part, the prevalence and severity of symptoms in younger and older cancer patients are similar. The impact of symptoms on walking is more pronounced in older patients with cancer receiving RT than in younger patients. Severity of distress also increased more during RT for older patients. Assessment of older cancer patients should include an evaluation and assessment of physical performance before therapy is initiated and continued throughout the treatment course.
Acknowledgments
Supported by: Hartford Health Outcomes Research Scholars Award (Supriya G. Mohile, M.D., M.S.), National Cancer Institute grant 1R25CA102618 (Gary Morrow, Ph.D., M.S.), and Clinical and Translational Science 5KL2 RR024136-04 (Supriya G. Mohile, M.D, M.S.)
Footnotes
The data included in this manuscript were presented at the 2008 American Geriatrics Society Presidential Poster Session and won the Presidential Poster Award in the Quality of Life category
The authors had full responsibility for the design of the study, the collection of the data, the analysis and interpretation of the data, the decision to submit the manuscript for publication, and the writing of the manuscript.
Author Contributions: All authors have a significant role in analysis and interpretation of data and preparation of manuscript. Drs. Mohile, Mustian, and Morrow also have roles in study concept and design and preparation of manuscript.
Sponsor's Role: The sponsor had no role in the design, methods, subject recruitment, data collections, analysis and preparation of paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cleeland CS, Sloan JA. Assessing the Symptoms of Cancer Using Patient-Reported Outcomes (ASCPRO): searching for standards. J Pain Symptom Manage. Jun;39(6):1077–1085. doi: 10.1016/j.jpainsymman.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 2.Rao A, Cohen HJ. Symptom management in the elderly cancer patient: fatigue, pain, and depression. J Natl Cancer Inst Monogr. 2004;(32):150–157. doi: 10.1093/jncimonographs/lgh031. [DOI] [PubMed] [Google Scholar]
- 3.Wang XS, Fairclough DL, Liao Z, et al. Longitudinal study of the relationship between chemoradiation therapy for non-small-cell lung cancer and patient symptoms. J Clin Oncol. 2006 Sep 20;24(27):4485–4491. doi: 10.1200/JCO.2006.07.1126. [DOI] [PubMed] [Google Scholar]
- 4.Hickok JT, Morrow GR, Roscoe JA, Mustian K, Okunieff P. Occurrence, severity, and longitudinal course of twelve common symptoms in 1129 consecutive patients during radiotherapy for cancer. J Pain Symptom Manage. 2005 Nov;30(5):433–442. doi: 10.1016/j.jpainsymman.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Hickok JT, Roscoe JA, Morrow GR, Mustian K, Okunieff P, Bole CW. Frequency, severity, clinical course, and correlates of fatigue in 372 patients during 5 weeks of radiotherapy for cancer. Cancer. 2005 Oct 15;104(8):1772–1778. doi: 10.1002/cncr.21364. [DOI] [PubMed] [Google Scholar]
- 6.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000 Oct 1;89(7):1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 7.Hastie TJ, Tibshirani R. Generalized Additive Models. New York: Chapman and Hall; 1990. [Google Scholar]
- 8.West B, Welch KB. Linear mixed models: a practical guide using statistical software. Boca Raton: Chapman & Hall; 2007. [Google Scholar]
- 9.Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr. 2007;(37):16–21. doi: 10.1093/jncimonographs/lgm005. [DOI] [PubMed] [Google Scholar]
- 10.Given CW, Given B, Azzouz F, Kozachik S, Stommel M. Predictors of pain and fatigue in the year following diagnosis among elderly cancer patients. J Pain Symptom Manage. 2001 Jun;21(6):456–466. doi: 10.1016/s0885-3924(01)00284-6. [DOI] [PubMed] [Google Scholar]
- 11.Cooley ME, Short TH, Moriarty HJ. Symptom prevalence, distress, and change over time in adults receiving treatment for lung cancer. Psychooncology. 2003 Oct-Nov;12(7):694–708. doi: 10.1002/pon.694. [DOI] [PubMed] [Google Scholar]
- 12.Gift AG, Jablonski A, Stommel M, Given CW. Symptom clusters in elderly patients with lung cancer. Oncol Nurs Forum. 2004 Mar-Apr;31(2):202–212. doi: 10.1188/04.ONF.202-212. [DOI] [PubMed] [Google Scholar]
- 13.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000 Feb;18(4):743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 14.Kozachik SL, Bandeen-Roche K. Predictors of patterns of pain, fatigue, and insomnia during the first year after a cancer diagnosis in the elderly. Cancer Nurs. 2008 Sep-Oct;31(5):334–344. doi: 10.1097/01.NCC.0000305769.27227.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Given BA, Given CW, Sikorskii A, Hadar N. Symptom clusters and physical function for patients receiving chemotherapy. Semin Oncol Nurs. 2007 May;23(2):121–126. doi: 10.1016/j.soncn.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Soltow D, Given BA, Given CW. Relationship between age and symptoms of pain and fatigue in adults undergoing treatment for cancer. Cancer Nurs. Jul-Aug;33(4):296–303. doi: 10.1097/NCC.0b013e3181ce5a1a. [DOI] [PubMed] [Google Scholar]
- 17.2011 http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 18.Prue G, Rankin J, Allen J, Gracey J, Cramp F. Cancer-related fatigue: A critical appraisal. Eur J Cancer. 2006 May;42(7):846–863. doi: 10.1016/j.ejca.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 19.Ahlberg K, Ekman T, Gaston-Johansson F. Fatigue, psychological distress, coping resources, and functional status during radiotherapy for uterine cancer. Oncol Nurs Forum. 2005 May;32(3):633–640. doi: 10.1188/05.ONF.633-640. [DOI] [PubMed] [Google Scholar]
- 20.van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007 Sep;18(9):1437–1449. doi: 10.1093/annonc/mdm056. [DOI] [PubMed] [Google Scholar]
- 21.Hickok JT, Morrow GR, McDonald S, Bellg AJ. Frequency and correlates of fatigue in lung cancer patients receiving radiation therapy: implications for management. J Pain Symptom Manage. 1996 Jun;11(6):370–377. doi: 10.1016/0885-3924(96)00008-5. [DOI] [PubMed] [Google Scholar]
- 22.Morrow GR. Chemotherapy-related nausea and vomiting: etiology and management. CA Cancer J Clin. 1989 Mar-Apr;39(2):89–104. doi: 10.3322/canjclin.39.2.89. [DOI] [PubMed] [Google Scholar]
- 23.Given B, Given C, Azzouz F, Stommel M. Physical functioning of elderly cancer patients prior to diagnosis and following initial treatment. Nurs Res. 2001 Jul-Aug;50(4):222–232. doi: 10.1097/00006199-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Bylow K, Dale W, Mustian K, et al. Falls and physical performance deficits in older patients with prostate cancer undergoing androgen deprivation therapy. Urology. 2008 Aug;72(2):422–427. doi: 10.1016/j.urology.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohile SG, Bylow K, Dale W, et al. A pilot study of the vulnerable elders survey-13 compared with the comprehensive geriatric assessment for identifying disability in older patients with prostate cancer who receive androgen ablation. Cancer. 2007 Feb 15;109(4):802–810. doi: 10.1002/cncr.22495. [DOI] [PubMed] [Google Scholar]
- 26.Joly F, Alibhai SM, Galica J, et al. Impact of androgen deprivation therapy on physical and cognitive function, as well as quality of life of patients with nonmetastatic prostate cancer. J Urol. 2006 Dec;176(6 Pt 1):2443–2447. doi: 10.1016/j.juro.2006.07.151. [DOI] [PubMed] [Google Scholar]
- 27.Hurria A, Li D, Hansen K, et al. Distress in older patients with cancer. J Clin Oncol. 2009 Sep 10;27(26):4346–4351. doi: 10.1200/JCO.2008.19.9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miaskowski C, Dodd M, Lee K. Symptom clusters: the new frontier in symptom management research. J Natl Cancer Inst Monogr. 2004;(32):17–21. doi: 10.1093/jncimonographs/lgh023. [DOI] [PubMed] [Google Scholar]
- 29.Cheng KK, Lee DT. Effects of pain, fatigue, insomnia, and mood disturbance on functional status and quality of life of elderly patients with cancer. Crit Rev Oncol Hematol. Apr 17; doi: 10.1016/j.critrevonc.2010.03.002. [DOI] [PubMed] [Google Scholar]