Abstract
Advances in high-throughput, genome-wide profiling technologies have allowed for an unprecedented view of the cancer genome landscape. Specifically, high-density microarrays and sequencing-based strategies have been widely utilized to identify genetic (such as gene dosage, allelic status, and mutations in gene sequence) and epigenetic (such as DNA methylation, histone modification, and micro-RNA) aberrations in cancer. Although the application of these profiling technologies in unidimensional analyses has been instrumental in cancer gene discovery, genes affected by low-frequency events are often overlooked. The integrative approach of analyzing parallel dimensions has enabled the identification of (a) genes that are often disrupted by multiple mechanisms but at low frequencies by any one mechanism and (b) pathways that are often disrupted at multiple components but at low frequencies at individual components. These benefits of using an integrative approach illustrate the concept that the whole is greater than the sum of its parts. As efforts have now turned toward parallel and integrative multidimensional approaches for studying the cancer genome landscape in hopes of obtaining a more insightful understanding of the key genes and pathways driving cancer cells, this review describes key findings disseminating from such high-throughput, integrative analyses, including contributions to our understanding of causative genetic events in cancer cell biology.
Keywords: Integrative analysis, Cancer genome, Sequencing, Microarray
1 Introduction
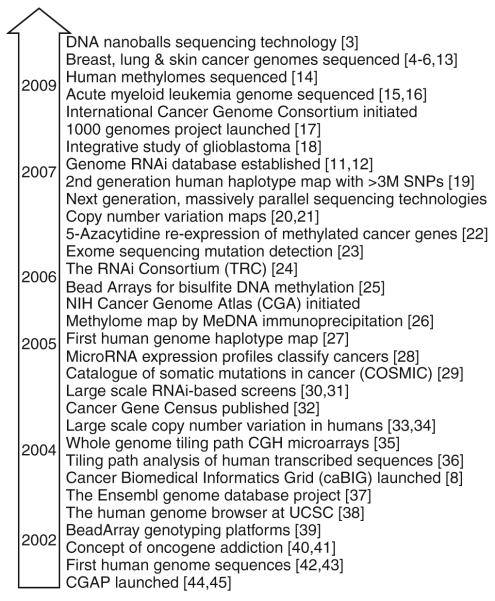
In the past decade, advancements in genome profiling technologies have greatly improved our ability to understand the landscape of cancer genomes. From the emergence of array-based comparative genomic hybridization (CGH) and spectral karyotyping (SKY) to the current state of next generation sequencing, the improvement in resolution at which the genome can be described has been over a million fold [1–6]. Likewise, the recent development of integrative platforms to relate multiple dimensions of DNA features (such as copy number, allelic status, sequence mutations, and DNA methylation) to gene expression patterns has dramatically improved our ability to identify causal genetic events and decipher their downstream consequences in the context of gene networks and biological functions [7, 8] (Table 1). Landmark events in cancer genomics, from the launch of Cancer Genome Anatomy Project at the beginning of the decade to the recent publications of complete cancer genome sequences, are highlighted in Fig. 1 [3–6, 8, 11–45].
Table 1.
List of software for integrative analysis
| Software | Source: commercial (C) or academic (A) |
Genome | Epigenome | Transcriptome | Integrative | Citation | Website (http://www.) |
|---|---|---|---|---|---|---|---|
| Agilent Genomic Workbench 5.0 |
C | X | X | X | X | N/A | chem.agilent.com/en-us/products/instruments/dnamicroarrays/ |
| dnaanalyticssoftware/pages/default.aspx | |||||||
| SIGMA2 | A | X | X | X | X | [7] | flintbox.com/technology.asp?page=3716 |
| Integrative Genomics Viewer |
A | X | X | X | N/A | broadinstitute.org/igv/ | |
| Nexus Copy Number | C | X | X | X | N/A | biodiscovery.com/index/nexus | |
| CGH Fusion | C | X | X | N/A | infoquant.com/index/cghfusion | ||
| ISA-CGH | A | X | X | X | [9] | isacgh.bioinfo.cipf.es | |
| VAMP | A | X | X | X | X | [10] | bioinfo-out.curie.fr/projects/vamp/ |
| Partek Genomics Suite | C | X | X | X | X | N/A | partek.com/partekgs |
Fig. 1.
Advances in cancer genomic landscape post Y2K. The timeframe of events are estimated based on time of publication
Multiple levels of genetic and epigenetic disruption are instrumental to cancer development, whereby specific genes may be altered by a variety of mechanisms. For example, the tumor suppressor CDKN2A can be inactivated through copy number loss, DNA hypermethylation, or sequence mutation. These mechanisms of disruption can occur in a tumor-specific manner or may occur concurrently in the same tumor, i.e., a two-hit scenario. Moreover, in the former situation, if a given gene or pathway’s frequency of alteration is low when examined by one mechanism or dimension, it is likely that the gene/pathway would be overlooked by the analysis. However, when multiple dimensions of disruption are considered in the analyses, alteration of the gene in question may be detected at a high frequency, albeit at low frequencies by any one mechanism. This illustrates the need for and the benefit of integrative analytical approaches. In this article, we discuss the impact of multidimensional genomic analyses on our view of the cancer genome landscape and the contribution of such new knowledge to our understanding of cancer progression and metastasis.
2 Genomic alterations
2.1 Chromosomal aberrations
Chromosomal aberrations and rearrangements, such as translocations and gains/losses of whole or portions of chromosome arms, are detected through direct examination using molecular cytogenetic techniques such as G-banding, SKY, fluorescence in situ hybridization (FISH), and CGH [2, 46–50]. The manifestation of such alterations is generally attributed to mitotic errors, where centrosomal aberrations and telomere dysfunction play key causative roles [51–55].
Aberrations such as gains and losses have been further refined using technologies such as microarray CGH (see below). While primarily associated with different types of leukemia and lymphomas, recent genomic studies have identified translocations in epithelial tumors such as prostate and lung cancer [56–63]. A compilation of cumulative cytogenetic data from three main sources—NCI/NCBI SKY/M-FISH & CGH Database, NCI Mitelman Database of Chromosome Aberrations in Cancer, and NCI Recurrent Aberrations in Cancer—is now integrated into NCBI’s Entrez system as Cancer Chromosomes [64] (Table 2).
Table 2.
List of genomic resources and databases
| Name | Description | Citation | Website (http://www.) |
|---|---|---|---|
| ArrayExpress Gene Expression Atlas | Gene expression analysis of public datasets |
[65] | ebi.ac.uk/gxa |
| BioDrugScreen | Protein/small molecule interaction database |
[66] | biodrugscreen.org |
| Catalogue of Somatic Mutations in Cancer (COSMIC) |
Listing of somatic mutations in cancer | [67] | sanger.ac.uk/cosmic |
| Cancer Gene Expression Database (CGED) | Gene expression analysis of cancer | [68] | cged.hgc.jp |
| Database of Differentially Expressed Proteins in human Cancers (dbDEPC) |
Differentially expressed proteins in cancer | [69] | dbdepc.biosino.org/index |
| Database of Genomics Variants | Reported normal copy number variations | [33] | projects.tcag.ca/variation |
| European Bioinformatics Institute (EBI) | Integrated database of multiple biological resources | [70] | ebi.ac.uk |
| GeneCards | Integrated database of multiple biological resources |
[71] | genecards.org |
| GenomeRNAi | RNAi experiment results | [12] | rnai2.dkfz.de/GenomeRNAi |
| Human DNA Methylome | Whole genome methylation sequences of multiple individuals |
[14] | neomorph.salk.edu/human_methylome |
| Human Histone Modification Database (HHMD) |
Histone modification database | [72] | bioinfo.hrbmu.edu.cn/hhmd |
| microRNA.org | Annotated microRNAs and their targets | [73] | microRNA.org |
| miR2Disease | Deregulated microRNAs in cancer | [74] | miR2Disease.org |
| miRDB | Annotated microRNAs and their targets | [75] | mirdb.org |
| miRGen | Annotated microRNAs and their targets | [76] | diana.cslab.ece.ntua.gr/mirgen |
| National Center for Biotechnology Information (NCBI) |
Integrated database of multiple biological resources |
[77] | ncbi.nlm.nih.gov |
| NCBI Cancer Chromosomes | Curated cytogenetic alterations in cancer | [77] | ncbi.nlm.nih.gov/sites/entrez?db=cancerchromosomes |
| NCBI GEO Profiles | Gene expression analysis of public datasets | [78] | ncbi.nlm.nih.gov/sites/entrez?db=geo |
| Oncomine | Gene expression analysis of public datasets | [79] | oncomine.org |
| PROGENETIX | Copy number aberrations in cancer by CGH | [80] | progenetix.net |
| PRoteomics IDentifications Database (PRIDE) | Mass spectrometry results | [81] | ebi.ac.uk/pride |
| Sanger CGP LOH and Copy Number Analysis | Copy number and LOH profiles of cancer cell lines |
– | sanger.ac.uk/cgi-bin/genetics/CGP/cghviewer/CghHome.cgi |
| siRecords | RNAi experiment results | [82] | siRecords.umn.edu/siRecords |
| System for Integrative Genomic Microarray Analysis (SIGMA) |
Array CGH profiles of cancer cell lines | [83] | sigma.bccrc.ca |
| The Cancer Genome Anatomy Project (CGAP) | Gene expression analysis of cancer | [45] | cgap.nci.nih.gov/ |
| The Cancer Genome Atlas (TCGA) | Multidimensional description of cancer genomes |
[18] | cancergenome.nih.gov/dataportal/data/about/ |
| UCSC Genome Browser | Integrated database of multiple biological resources |
[84] | genome.ucsc.edu/cgi-bin/hgNear |
2.2 Gene dosage, allelic imbalance, and mutational status
Gene dosage
Genomic DNA copy number alterations are a prominent mechanism of gene disruption that contributes to tumor development [85]. Segmental amplification may lead to an increase in gene and protein expression of oncogenes, while deletions may lead to haploinsufficiency or the loss of expression of tumor suppressor genes. Since its development in the mid-1990s, advances in microarray-based CGH technology have dramatically increased genome coverage and target density, improving both the resolution and sensitivity of detection of copy number alterations [86, 87]. The first genome-wide array CGH analysis utilized cDNA microarrays originally designed for gene expression profiling [88]. Since these first experiments, whole genome tiling path arrays with tens of thousands of bacterial artificial chromosome clones, oligonucleotide (25–80-bp nucleotide probes), and single-nucleotide polymorphism (SNP) arrays with over one million DNA elements and the essential bioinformatics tools for visualization and analysis of high-density array CGH data have been developed (Fig. 1) [7, 35, 89–93]. These innovations have enabled increasingly precise mapping of the boundaries and magnitude of genetic alterations throughout the genome in a single experiment, greatly increasing our understanding of the cancer genome landscape in the context of DNA copy number [35, 94–98]. While early attempts have been made utilizing sequence-based approaches [99–102], recent studies have begun to illustrate the improvement in detection resolution through the advances in high-throughput sequencing technologies [6, 13, 15, 16]. The popularity of genome sequencing will depend on further cost reduction in data generation and major advancements in analysis [103].
Copy number variation
The discovery of a vast abundance of germ line segmental DNA copy number variation (CNV) in the normal human population has not only provided a baseline for interpretation of cancer genome data but also highlighted the need for comparison against paired normal tissue [20, 21, 33, 34, 104–111]. Moreover, it has been shown that many of the reported CNVs overlap with loci involved with sensory perception and more importantly, disease susceptibility. While the role of CNV in cancer is not well understood, a recent study showed that these regions are more susceptible to genomic rearrangement and may initiate subsequent alterations during tumorigenesis [112]. Moreover, CNV at 1q21.1 was recently shown to be associated with neuroblastoma and implicated NBPF23, a new member of the neuroblastoma breakpoint family, in tumorigenesis [113]. A database of all known CNVs is available at http://projects.tcag.ca/variation [33]. In addition, as copy number profiles of cancer genomes accumulate, hotspots for amplification and deletion are becoming evident, and signature alterations associated with specific diseases and cancer histologic subtypes are emerging [114–118]. The manifestation of “oncogene addiction” through lineage-specific DNA amplification is a case in point [40, 41, 119–122].
Allelic status
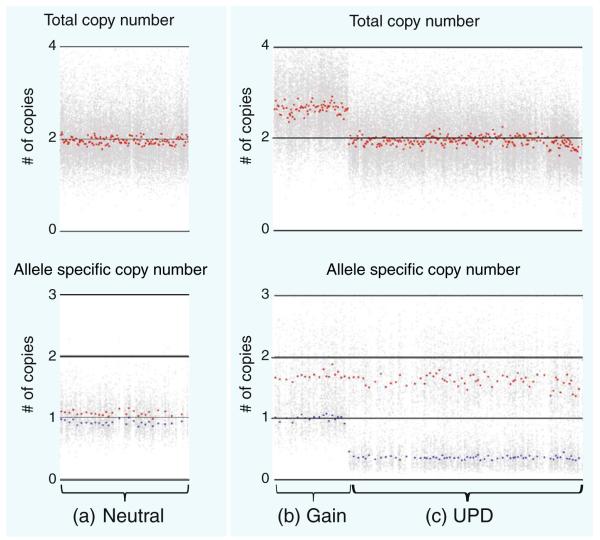
SNP arrays are best known for their application in genome-wide association studies (GWAS), where the correlation of haplotype with phenotype implicates disease susceptibility [123, 124]. SNP array platforms have shown tremendous advances in resolution, with the number of SNPs that can be simultaneously measured increased by 1,000-fold since initial development. Currently, for example, the Affymetrix SNP 6.0 array platform measures 1.8 million elements representing 906,600 SNP elements and >946,000 CNV elements. Likewise, on the Illumina HumanOmni1 platform, over 1,000,000 sites (representing a mixture of SNP and CNV elements) can be simultaneously assessed. In addition to their application in GWAS, SNP arrays can also be used to detect somatic alterations and, when applied in this context, can allow for the simultaneous detection of copy number alteration and allele imbalance in tumor genomes. In the example in Fig. 2, when the SNP array profile of a lung cancer genome is compared against that of its paired noncancerous lung tissue, it is not only possible to distinguish regions of allelic balanced copy neutrality (Fig. 2a) from allelic imbalance (Fig. 2b, c), but also regions of allelic imbalance due to segmental DNA copy number alteration (Fig. 2b) from those without change in total copy number (Fig. 2c).
Fig. 2.
SNP array analysis to identify areas of altered copy number and allelic composition in a clinical lung cancer specimen. Shown here are a a region that is copy-neutral with no observed allelic imbalance and regions containing a b segmental gain and c UPD. Examining the allele-specific copy number plot, the gain (in b) is likely a single-copy change, and the UPD event (in c) is signified by the shift in allele levels while maintaining total copy number neutral status
Mutational profiling and whole genome sequencing
In cancer, oncogenes are thought to harbor mutations which lead to increased protein expression or constitutive protein activation while tumor suppressor genes are thought to harbor mutations which are inactivating, either through total loss of protein expression or expression of mutant, nonfunctional protein. In addition, activating and inactivating mutations can also be accompanied by changes in gene dosage or allele status (see below). Traditionally, mutation screening has been focused on specific oncogene and tumor suppressor loci. With the availability of newer and cheaper sequencing technologies [125], recent studies have expanded from single gene analyses to genome-wide screens [6, 13, 15, 16, 126]. For example, in studies using small cell lung cancer and melanoma cell lines, tens of thousands of somatic mutations were identified in each cell line, with a proportion of these mutations being attributed to cigarette smoke (G to T substitutions) and UV exposure (C to T), respectively [4, 5]. It will be interesting to see if other cancers have such mutation signatures. Another observation made in both studies was that the uneven distribution of mutations suggests that DNA sequence integrity is largely maintained by transcription-associated DNA repair. While these and future studies will uncover a vast number of mutations, the contribution of those mutations to tumorigenesis will need to be determined [127, 128].
2.3 Genomic landscape: gains, losses, and uniparental disomy
Individually, the study of genomic dimensions has yielded a global description of cancer genomes in terms of gene dosage, allelic status, and somatic mutation. Collectively, however, the integration of these three dimensions has brought two concepts to the forefront: allele-specific copy number alterations and uniparental disomy (UPD; Fig. 2). Typically, the relationship between somatic mutation and allele-specific copy number alterations has been associated with tumor suppressor genes (e.g., RB1 and TP53) whereby mutation is combined with loss to achieve biallelic inactivation [129, 130]. However, recent studies have shown preferential amplification of alleles encoding mutated oncogenes as well [131–136]. In non-small cell lung cancer, mutant allele specific imbalance (MASI) is frequently present in mutant EGFR and KRAS tumor cells and is associated with increased mutant allele transcription and gene activity [136].
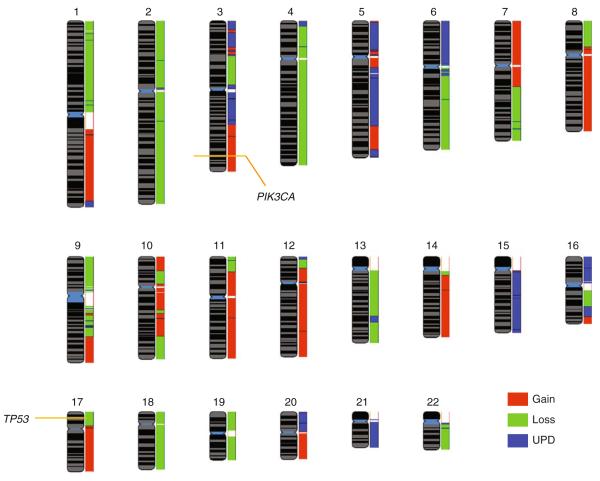
UPD is the presence of two copies of a chromosome segment from one parent and the absence of that DNA from the other parent. Somatic UPD, also known as copy-neutral loss of heterozygosity (LOH), results in loss of heterozygosity (tumor versus normal), without a change in total DNA copy number [137–139]. UPD is observed at tumor suppressor gene loci whereby upon loss of the wild type allele, the mutated allele is duplicated resulting in a diploid state with homozygous mutation of the target gene [140]. Interestingly, UPD events are also detected at mutated oncogenes [136, 141–143]. Until recently, due to limitations in the resolution of genomic array platforms, the prevalence of this event has been widely underestimated and underappreciated. Recent studies have shown that UPD events are frequently observed in tumor genomes, with most of the findings reported from hematological malignancies [144–153]. Our genome-wide analysis of segmental gain, loss, and UPD in the T47D breast cancer cell line genome identified that a significant portion of the genome exhibits UPD, rivaling the proportion of the genome affected by segmental gain and loss and highlighting the potential of UPD as a prominent mechanism of gene disruption in epithelial cancer (Fig. 3). Interestingly, PIK3CA and TP53 mutations in T47D are noted in the Catalogue of Somatic Mutations in Cancer [67]. Integrative analysis at these loci detected copy number increase at PIK3CA and copy number loss at TP53 illustrating the MASI concept described above (Fig. 3).
Fig. 3.
Overlay of chromosomal regions of gain, loss, and UPD (copy number neutral LOH) inherent to the T47D breast cancer cell line. The chromosomal loci for PIK3CA and TP53 (modified by activating and inactivating mutations, respectively, in this cell line) are indicated. The majority of the genome is affected by any one of the three genomic alterations. Raw SNP 6.0 array data were obtained from the Sanger database with mutation status obtained from the COSMIC database [67]. Copy number and allelic status changes were determined using Partek Genomics Suite, and reference genomes used were 72 individuals from the HapMap collection. Data were visualized using the SIGMA2 software [7]
Somatic UPD also exists at genes without mutation. The potential significance of this somatic event is not readily apparent, but it raises the intriguing possibility of allelic conversion of epigenetic status [139, 144, 154].
3 Epigenomic alterations
3.1 The cancer methylome
Abnormal DNA methylation patterns occur in cancer, whereby focal hypermethylation at many CpG islands is evident in a background of global DNA hypomethylation [155–158]. Broad hypomethylation may lead to genomic instability, while hypermethylation of CpG islands silences transcription of specific genes [157, 159–161]. Nonrandom methylation of multiple CpG islands observed in colon cancer led to the discovery of CpG island methylator phenotype, which is causally linked to microsatellite instability via silencing of the mismatch repair gene, MLH1 [162–164].
The determination of DNA methylation status relies on the ability to discriminate between methylated and unmethylated cytosines. This is achieved by exploiting methylation-sensitive/insensitive isoschizomer restriction enzyme pairs [165–171], chemical conversion of unmethylated cytosine to uracil [172–177], and the affinity for methylated DNA of specially developed antibodies and methylated DNA binding proteins [26, 178–184]. Several computational methods have been developed for deriving approximations of actual methylation levels from the relative levels generated by most microarray and locus-specific sequencing assays [168, 183, 185, 186]. However, it is important to note that CpG targets represented on microarrays may or may not be the only elements controlling gene expression. Recently, it was shown that in the human colon cancer methylome sequences up to 2 kb away from CpG islands, termed CpG island shores, exhibited more methylation than CpG islands and had greater influence on gene expression than CpG islands [187]. Furthermore, while excess promoter methylation is typically associated with transcriptional repression, the loss of required methylation within gene bodies, proximal to promoters, can have the same effect [188]. DNA methylation of epigenetic neighborhoods in the megabase size range has also been reported [189]. Validation of methylation-mediated control of gene-specific expression and evaluation of biological significance can be achieved via pharmacologic manipulation of DNA methylation, for example by 5-azacytidine treatment, to relieve methylation silencing and invoke re-expression [22, 190].
The first single-base resolution maps of the human methylome have recently been generated by sequencing of bisulfite converted DNA from human embryonic stem cells and fetal fibroblasts [14, 191]. This landmark study will greatly advance the analysis of DNA methylation by providing whole genome reference maps of methylation in these specific cells. However, it is well known that DNA methylation is tissue-specific and that it changes throughout development; thus, methylome maps for all tissues at various stages of development may be necessary to provide adequate maps of “normal” methylation patterns for use in deciphering aberrant methylation patterns characteristic of tumors [192–197]. In recognition of this, the Human Epigenome Project was launched in 2004 to map the methylomes of all major human tissues [198].
3.2 Integration of cancer genomic and epigenomic events
DNA methylation and genomic instability
Cancer-specific aberrant DNA methylation is associated with reduced genomic stability and subsequent copy number alterations, including preferential loss of certain imprinted alleles (LOI) [199–205]. Mechanistically, this instability may be related to the susceptibility of hypomethylated DNA to undergo inappropriate recombination events [206]. Another mechanism known to negatively impact genomic integrity in lung cancer is the relaxation of transposable element control that is mediated by DNA methylation [207–211].
DNA hypomethylation and DNA amplification
Preliminary evidence of specific demethylation of somatic segmental amplifications (or amplicons) has been put forth in lung cancer, perhaps representing a novel mechanism of aberrant oncogene activation [210, 212]. Further studies using large-scale sequencing of bisulfite-treated DNA will help to clarify this phenomenon [14]. Hypomethylation has also been implicated in the formation of specific copy number alterations in glioblastoma multiforme [213]. One potentially interesting application for DNA methylation profiling of cancer amplicons such as these is in the discrimination between “driver” and “passenger” genes within the amplified sequence. It may be that DNA methylation within the promoters or gene bodies of these genes is responsible for the lack of uniform overexpression of genes residing within amplicons.
DNA hypermethylation and copy number loss
The relationship between DNA hypermethylation and allelic loss is well documented. Tumor suppressor genes are frequently found in regions of common LOH, and these same TSGs are frequently found to be hypermethylated, perhaps best exemplified by the FHIT gene on chromosome 3p [214]. Although it is unclear whether loss or hypermethylation occurs first, both are known to be very early events in tumorigenesis preceding any histologic alterations [215–217]. With the advent of high resolution genome-wide technologies, it has become possible to comprehensively search for genes that are inactivated by both mechanisms simultaneously [218].
Histone modification states
While DNA methylation and gene dosage profiling technologies have become accessible, technologies for global assays of other key epigenetic marks including histone modifications are not widely available. One of the main challenges to conducting the highest quality studies of genome-wide chromatin immunoprecipitation on microarray (ChIP-chip) or on sequencing platform (ChIP-seq) experiments is the requirement of high-quality DNA from pure cells— which essentially means growing cells in culture. It is thus difficult to analyze these dimensions from clinical specimens. However, much has been learned from studies of the relationship between different histone modification states and transcriptional activation or repression in model systems. Such examples utilizing ChIP-chip include: cell or context-specific histone modification patterns related to cell or context-specific gene expression; histone 3 lysine 27 (H3K27) trimethylation patterns associated with prostate, lung, and breast cancers; and H3K9 and H3K79 modification patterns in leukemia [219–225]. Examples utilizing ChIP-seq include: the analysis of the growth inhibition program of the androgen receptor and the chromatic interaction network of the estrogen receptor [226, 227].
4 Relating genetic and epigenetic events to changes in the transcriptome through integrative analysis
Aberrations in individual genetic or epigenetic dimensions are prominent across various cancer types, culminating in changes to the transcriptome. However, for a given gene, most of the events documented previously, such as copy number amplification, homozygous deletion, somatic mutation, or DNA hypermethylation, do not occur in 100% of tumors for a given cancer type. Moreover, it has been observed that the same gene may be activated or inactivated by different mechanisms. Since most of the studies described above analyzed single DNA dimensions, it is likely that many genes would be overlooked due to a low frequency of alteration in a single dimension; the same gene may be detected at a high frequency when multiple dimensions are considered. Thus, analysis of more dimensions may reveal higher frequency gene-specific disruption with corresponding transcriptome aberrations for particular cancer types, as would be expected for genes causative to cancer development.
4.1 Multiple mechanisms of gene disruption
Expression profiling studies have been instrumental in detecting genes dysregulated in cancer [228–230]. However, aberrant expression of some genes may simply reflect incidental genome instability or secondary dysregulation. Global gene expression profiling alone may not distinguish causal events and bystander changes. One of the first studies to relate gene expression changes with gene dosage status on a global scale was a parallel analysis of DNA and mRNA [88, 231]. The same cDNA microarray platform was used to investigate impact of DNA copy number alterations on the expression of over 6,500 genes. This study determined that 62% of genes located within regions of DNA amplification showed elevated expression in breast cancer. Subsequent studies in other cancer types revealed a broad range in the correlation between increased gene dosage and expression levels for protein coding genes (19% to 62%) [114, 228, 231–234]. Studies integrating gene dosage and gene expression have identified cancer subtype-specific pathway activation and signatures associated with clinical outcome [118, 235–238]. In addition, when examining known disease-relevant pathways, it has been shown that even though individual components of a pathway are disrupted at a low frequency, collectively, these alterations can result in frequent disruption of a given pathway [18, 114]. Similarly, alterations in DNA methylation or histone modification status can also affect gene expression and have subsequent pathway level consequences (see above).
4.2 Multiple mechanisms of disrupting noncoding RNA levels
Segmental DNA copy number alterations also affect the expression of noncoding RNAs [239–243]. MicroRNAs (miRNA) have been shown to have a significant role in cancer development with specific miRNAs implicated in a number of different cancer types [28, 244–246]. Specific miRNA expression signatures are associated with critical steps in tumor initiation and development including cell hyperproliferation, angiogenesis, tumor formation, and metastasis [247]. High-throughput analysis of micro-RNAs has been of interest, and microarrays have been developed to assess essentially all annotated microRNAs. To date, >700 miRNAs have been annotated in the genome (http://mirdb.org/miRDB/statistics.html, [75]), with more likely to be discovered. For example, we recently demonstrated that a deletion on chromosome 5q leads to the reduced expression of two miRNAs that are abundant in hematopoietic stem/progenitor cells. This study revealed haploinsufficiency and reduced expression of miR-145 and miR-146a as mediators of a subtype of myelodysplastic syndrome [242]. Although the genomic loss and underexpression implicates a tumor-suppressive role for these specific miRNAs, others undergo activating genomic alterations and elevated expression and hence are thought to be oncogenic [248, 249].
Just as copy number alterations can alter miRNA activity, epigenetic alterations have also been shown to affect miRNA expression [250–252]. Aberrant methylation of miRNAs has been reported in a variety of cancer types, and the disruption of epigenetically mediated miRNA control has been shown to have oncogenic effects due to downstream gene deregulation [253]. For example, abnormal DNA methylation of miRNAs has been associated with tumor metastasis, leading to the appreciation of a group of metastasis-related miRNAs [249].
4.3 Multidimensional integration of genome, epigenome, and transcriptome
Large-scale initiatives
Since multiple genomic/epigenomic mechanisms can influence gene expression and lead to disruption of a given function, an integrative multidimensional analysis is necessary for a more comprehensive understanding of the cancer phenotype (Fig. 4). Specific programs and initiatives such as those by The Cancer Genome Atlas project and the cancer Biomedical Informatics Grid enable parallel and multidimensional analysis of cancer genomes [8, 18] (Table 2). Recently, studies in glioblastoma and osteosarcoma have shown that integrative genomic and epigenomic approaches can indeed reveal the specific genetic pathways involved in different cancers [18, 254].
Fig. 4.
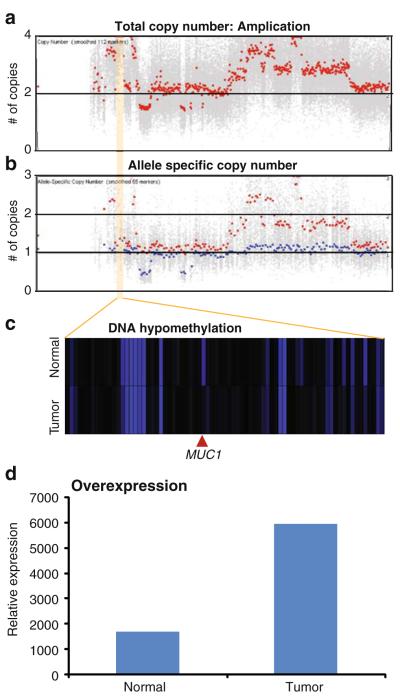
Integration of copy number, allelic status, DNA methylation, and gene expression for a single lung adenocarcinoma sample. a Copy number and b allele status analyses revealed a high level allele-specific DNA amplification (highlighted in yellow, image generated with Partek Genomics Suite); c individual CpG loci within this region were assessed for differential methylation between tumor and nonmalignant tissue. Hypomethylation at the indicated CpG locus, which corresponds to the MUC1 gene, is observed (visualized with Genesis). d Expression analysis revealed fourfold overexpression of the MUC1 transcript when a tumor sample was compared to matched, adjacent nonmalignant tissue. Copy number and allele status profiling was performed using the Affymetrix SNP 6.0 array; DNA methylation profiling using the Illumina Infinium HM27 platform and gene expression using the Affymetrix Human Exon 1.0 ST array
Gene disruption by multiple mechanisms
One of the two key reasons for using an integrative approach is the ability to detect critical genes that are disrupted by multiple mechanisms across a sample set but are disrupted at a low frequency by any one mechanism. These genes would have been overlooked in previous, single dimensional studies. The second key advantage of integrative approaches is the ability to identify genes that are simultaneously disrupted by multiple mechanisms—two hits—in a single sample. Using a dataset comprised of DNA copy number, allelic status, DNA methylation, and gene expression profiles from ten lung adenocarcinomas and matched nonmalignant tissue controls, we illustrate these benefits below.
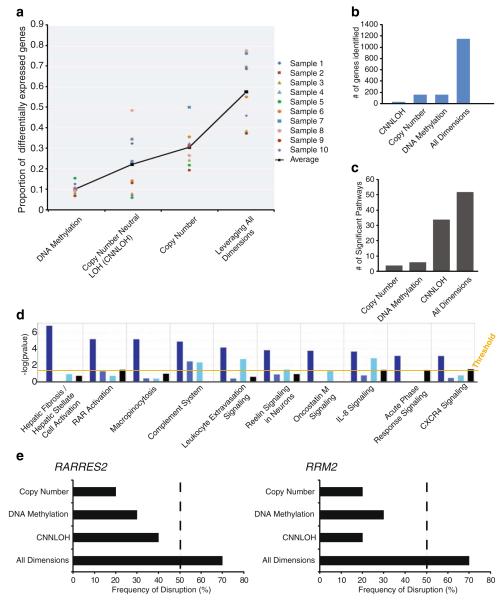
If gene expression changes are a consequence of alterations at the DNA level, then a higher proportion of the observed expression changes can be directly attributed to a defined causal event when multiple types of DNA alterations are examined (Fig. 5a). While some samples have over 70% of the expression associated with DNA level changes (sample 7, sample 8), other samples have only 30% (sample 5, sample 9). Additionally, consequential to associating more gene expression changes with DNA level changes within a sample, more disrupted genes are detected, and in turn, more disrupted pathways are identified across a sample set (Fig. 5b, c). In fact, in our example, nearly five times as many genes (~1,100 compared to ~200) are detected as disrupted in at least 50% of the samples when we account for multiple mechanisms of disruption (versus one mechanism alone; Fig. 5c). This result illustrates that without using an integrative approach, many potentially important genes would be dismissed as they are disrupted by low frequency events when a single DNA dimension is analyzed. This also holds true at the pathway level when the identified genes are grouped based on their biological function (Fig. 5d). For example, the Hepatic Fibrosis/Hepatic Stellate Cell Activation pathway and the RAR Activation pathway, which are identified when all DNA dimensions are considered, would not be detected as significantly altered when using individual DNA dimensions alone.
Fig. 5.
Enhanced analysis of the cancer phenotype using an integrative and multidimensional approach. a On average, a higher proportion of differential gene expression can be associated with genomic alterations when examining multiple DNA dimensions relative to single dimensions. b Using a fixed frequency threshold of 50%, more genes are revealed to be frequently disrupted when multiple mechanisms of genomic alteration (e.g., altered copy number, DNA methylation, or copy number neutral LOH) are considered (~200 genes versus more than 1,000 genes). c Pathway analyses performed using gene lists derived from a multidimensional approach identifies an enhanced number of aberrant pathways relative to those identified from a unidimensional approach. d Functional pathways identified using the integrated gene list are of relatively high significance; the top 10 such pathways are shown. This suggests that the additional identified genes associate with specific pathways rather than with random functions. The four bars represent, from left to right: all dimensions, copy number, DNA methylation, and UPD. Ingenuity Pathway Analysis was used for analyses in c and d. e Example of two genes that are missed when a single DNA dimension is studied but captured when multiple DNA dimensions are examined. Both ribonucleotide reductase M2 (RRM2) [255, 256] and retinoic acid receptor responder (tazarotene-induced) 2 (RARRES2) [257, 258] are known to be deregulated in multiple cancer types
Implications on sample size requirements
In the example above, we illustrate that a significant number of genes and pathways exhibit a low frequency of disruption when examining single dimensions (and thus would be over-looked) but, indeed, exhibit a high frequency of disruption when multiple dimensions are considered (Fig. 5). Notably, these findings imply that integrative multidimensional analysis of individual samples may directly impact the cohort sample size required for gene discovery on the basis of frequency of disruption (Fig. 5e). Reduction in sample size requirements means that one can extend this approach to situations involving rare specimens where accrual of hundreds of samples in a reasonable timeframe is not possible. Moreover, reduced sample sizes are particularly applicable to familial cancers or to isolated populations at increased risk for specific cancers.
Biallelic gene disruption
Two-hit biallelic inactivation of genes and high-level gene amplifications are typically considered to be causal mechanisms that inflict gene expression changes. When examining multiple DNA dimensions, concerted biallelic disruption of a gene in the same sample can be readily identified; copy number loss with hypermethylation resulting in underexpression or copy number gain with hypomethylation and overexpression are examples. Indeed, we do identify genes harboring concerted disruptions using the same lung adenocarcinoma dataset mentioned above. The MUC1 locus exhibits concurrent copy number increase with hypomethylation and overexpression (Fig. 4). MUC1 has previously been shown to be important in lung and breast cancers and is currently a target for therapeutic intervention [259–261]. Collectively, we have demonstrated how an integrative, multidimensional approach can be utilized for cancer gene and pathway discovery.
4.4 Disruption of multiple components in biological pathways
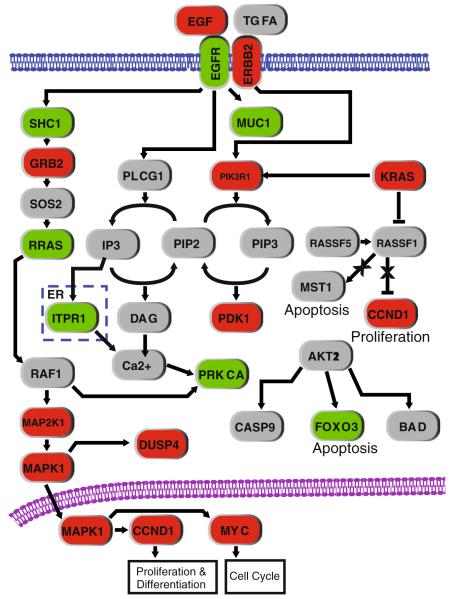
We described above how an integrative, multidimensional approach improves the detection of disrupted genes, especially those affected by multiple low-frequency mechanisms. This concept can be extended to identify biological pathways, where multiple pathway components are disrupted at low frequencies (see above; Fig. 5d). The EGFR signaling pathway is a well-documented dysregulated component of lung cancer. Using the same multidimensional profiling dataset from Fig. 5 above, seven genes were detected with gene dosage alteration at a frequency ≥30%. However, when we considered alterations in gene dosage, allelic status, DNA methylation, and somatic mutation collectively (for KRAS and EGFR only), 18 genes in the pathway were identified to be altered at ≥30% frequency (Fig. 6). The detection of the additional 11 genes illustrates the benefit of employing an integrative approach and extends the sample size reduction argument to the pathway level.
Fig. 6.
Identification of multiple disrupted components in a biological pathway. Integrative analysis identifies more genes affected in the EGFR signaling pathway than a single dimensional analysis alone. In this example, multidimensional profiling data were generated from ten lung adenocarcinomas and their paired noncancerous lung tissue. Analysis of DNA copy number (gene dosage) alterations that affected expression identified seven genes (in green) that are disrupted at ≥30% frequency. However, when alterations in copy number, DNA methylation, sequence mutation, and/or copy-neutral LOH were considered, 17 genes disrupted at ≥30% frequency were identified to be associated with a change in expression, with an additional gene, KRAS, harboring frequent mutation. The 11 additional genes are indicated in red. Genes in gray are not significant in this dataset as they did not meet the frequency criteria
5 Tracking clonal expansion in spatial dimensions
Delineating the clonal relationship between multiple tumors in the same patient is relevant not only to clinical management of disease but also to the understanding of metastasis. Multiple tumors in the same patient may not necessarily share an identical genomic profile. The similarities and differences in genomic landscape between tumors are quantifiable and therefore can be used for delineating relatedness. Whole genome comparison based on array CGH profiles is a new tool for distinguishing metastatic from primary synchronous carcinomas. A multitude of genomic features, for example the boundaries of segmental deletions, are used to delineate the presence and the sequence of events in clonal evolution [262–270].
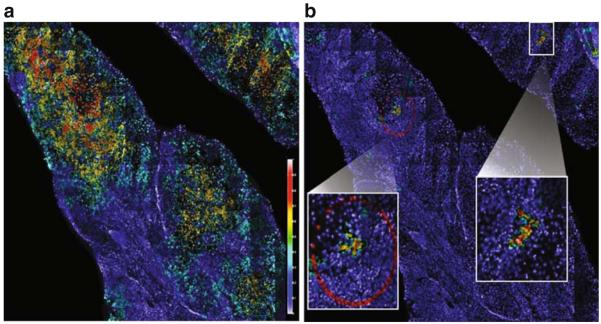
Furthermore, signature genetic alterations can be used to track clonality in a cell population, putting genetic events in the context of tumor tissue architecture. By assessing the appearance of preselected markers in individual nuclei on a tissue section by FISH, the clustering and the expansion of clonally related cells can be delineated by analyzing the marker patterns of neighboring cells (Fig. 7).
Fig. 7.
Automated detection of selected clonal populations of cells within a cancer biopsy tissue section. All nuclei (~150,000 in this example) are detected, and FISH probe signal counts are enumerated for each nucleus. FISH signal pattern for each cell is compared against its neighbor in order to define spatial association (or neighborhood). A mathematical model is then applied to determine clonal cell relationships. a Mapping cancer cells on a tissue section. A gain or loss of any one of three FISH markers indicates a cancer cell. This image shows the density of cancer cells (so defined) in neighborhoods as a color overlay. Red indicates high fraction of cancer cells, yellow indicates medium fraction of cancer cells, and blue indicates low to none (see scale bar). Most of the section is highlighted except for the surrounding normal stromal infiltrates. b Mapping clonal cells. The same image data were analyzed for concurrent gains of each of the three markers. The two clusters of cells, magnified within the white boxes, are cells harboring gain of all three markers
6 Evaluating the biological significance of integrative genomics findings
The utilization of an integrative genomic, epigenomic, and transcriptomic approach will undoubtedly improve our ability to identify gene disruptions and their effects on gene expression. The next challenge is to develop approaches for the determination of functional and phenotypic evidence of the biological relevance of such gene disruptions in a high-throughput manner—for example, functional genomic screens by RNAi, proteomic profiling, and metabolite profiling. Forced expression of genes and RNAi knockdown of gene expression are commonly used methods for assessing growth and invasion phenotypes in cell models. Genome-wide RNAi screens, comprised of large libraries of short hairpin RNA sequences redundantly targeting thousands of genes, have been used to identify genes essential to tumorigenesis, including tumor suppressor genes as well as cooperative genes with oncogenic mutation in several malignancies [24, 30, 31, 271–279]. Animal models are also instrumental to functional validation of genes singly or in combination, but this topic is beyond the scope of this article. Cross referencing genomic findings with proteomic profiles will determine the functional consequences yielding information on expression levels, posttranslational modification, and protein–protein interactions [280–284]. As recent studies have highlighted the importance of the metabolome in cancer, the genomic landscape can also be integrated with metabolome profiles to determine the role of genetic and epigenetic alterations in cellular physiology relevant to cancer development [285–287].
The progress made in the development of technologies and approaches to analyze the genome, epigenome, and transcriptome has allowed for much improved understanding of cancer landscapes. With the increased application of sequence-based approaches to analyze genetic and epigenetic dimensions and the additional complexity with the proteome and metabolome to follow, an unprecedented definition of the cancer cell can be achieved. The next key challenge will be the synthesis of this information to better understand fundamental cancer processes such as progression, metastasis, and drug resistance.
Acknowledgments
This work was supported by funds from the Canadian Institutes for Health Research (MOP 86731, MOP 77903), Canadian Cancer Society (CCS20485), NCI Early Detection Research Network (EDRN; 5U01 CA84971-10), and the Canary Foundation. CEA was supported by the United States National Institutes of Health (HG004663). RC, KLT, WWL, and IMW are supported by scholarships from the Canadian Institutes for Health Research and the Michael Smith Foundation for Heath Research.
Abbreviations
- CGH
Comparative genomic hybridization
Contributor Information
Raj Chari, Genetics Unit - Department of Integrative Oncology, British Columbia Cancer Research Centre, Vancouver, BC, Canada.
Kelsie L. Thu, Genetics Unit - Department of Integrative Oncology, British Columbia Cancer Research Centre, Vancouver, BC, Canada; Interdisciplinary Oncology Program, University of British Columbia, Vancouver, BC, Canada
Ian M. Wilson, Genetics Unit - Department of Integrative Oncology, British Columbia Cancer Research Centre, Vancouver, BC, Canada
William W. Lockwood, Genetics Unit - Department of Integrative Oncology, British Columbia Cancer Research Centre, Vancouver, BC, Canada
Kim M. Lonergan, Genetics Unit - Department of Integrative Oncology, British Columbia Cancer Research Centre, Vancouver, BC, Canada
Bradley P. Coe, Genetics Unit - Department of Integrative Oncology, British Columbia Cancer Research Centre, Vancouver, BC, Canada
Chad A. Malloff, Genetics Unit - Department of Integrative Oncology, British Columbia Cancer Research Centre, Vancouver, BC, Canada
Adi F. Gazdar, Hamon Center for Therapeutic Oncology Research, University of Texas Southwestern Medical Center, Dallas, TX, USA
Stephen Lam, Genetics Unit - Department of Integrative Oncology, British Columbia Cancer Research Centre, Vancouver, BC, Canada; Imaging Unit - Department of Integrative Oncology, British Columbia Cancer Research Centre, Vancouver, BC, Canada.
Cathie Garnis, Genetics Unit - Department of Integrative Oncology, British Columbia Cancer Research Centre, Vancouver, BC, Canada.
Calum E. MacAulay, Imaging Unit - Department of Integrative Oncology, British Columbia Cancer Research Centre, Vancouver, BC, Canada
Carlos E. Alvarez, Center for Molecular and Human Genetics, The Research Institute at Nationwide Children’s Hospital, Columbus, OH, USA; Department of Pediatrics, The Ohio State University College of Medicine, Columbus, OH, USA
Wan L. Lam, Interdisciplinary Oncology Program, University of British Columbia, Vancouver, BC, Canada
References
- 1.Pinkel D, Segraves R, Sudar D, Clark S, Poole I, Kowbel D, et al. High resolution analysis of DNA copy number variation using comparative genomic hybridization to microarrays. Nature Genetics. 1998;20(2):207–211. doi: 10.1038/2524. [DOI] [PubMed] [Google Scholar]
- 2.Schrock E, du Manoir S, Veldman T, Schoell B, Wienberg J, Ferguson-Smith MA, et al. Multicolor spectral karyotyping of human chromosomes. Science. 1996;273(5274):494–497. doi: 10.1126/science.273.5274.494. [DOI] [PubMed] [Google Scholar]
- 3.Drmanac R, Sparks AB, Callow MJ, Halpern AL, Burns NL, Kermani BG, et al. Human genome sequencing using unchained base reads on self-assembling DNA nanoarrays. Science. 2009;327(5961):78–81. doi: 10.1126/science.1181498. [DOI] [PubMed] [Google Scholar]
- 4.Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2009 doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pleasance ED, Stephens PJ, O’Meara S, McBride DJ, Meynert A, Jones D, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature. 2009 doi: 10.1038/nature08629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephens PJ, McBride DJ, Lin ML, Varela I, Pleasance ED, Simpson JT, et al. Complex landscapes of somatic rearrangement in human breast cancer genomes. Nature. 2009;462(7276):1005–1010. doi: 10.1038/nature08645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chari R, Coe BP, Wedseltoft C, Benetti M, Wilson IM, Vucic EA, et al. SIGMA2: a system for the integrative genomic multi-dimensional analysis of cancer genomes, epigenomes, and transcriptomes. BMC Bioinformatics. 2008;9:422. doi: 10.1186/1471-2105-9-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Eschenbach AC, Buetow K. Cancer informatics vision: caBIG. Cancer Informatics. 2007;2:22–24. [PMC free article] [PubMed] [Google Scholar]
- 9.Conde L, Montaner D, Burguet-Castell J, Tarraga J, Medina I, Al-Shahrour F, et al. ISACGH: a web-based environment for the analysis of Array CGH and gene expression which includes functional profiling. Nucleic Acids Research. 2007;35(Web Server issue):W81–W85. doi: 10.1093/nar/gkm257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Rosa P, Viara E, Hupe P, Pierron G, Liva S, Neuvial P, et al. VAMP: visualization and analysis of array-CGH, transcriptome and other molecular profiles. Bioinformatics. 2006;22(17):2066–2073. doi: 10.1093/bioinformatics/btl359. [DOI] [PubMed] [Google Scholar]
- 11.Horn T, Arziman Z, Berger J, Boutros M. GenomeRNAi: a database for cell-based RNAi phenotypes. Nucleic Acids Research. 2007;35:D492–D497. doi: 10.1093/nar/gkl906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilsdorf M, Horn T, Arziman Z, Pelz O, Kiner E, Boutros M. GenomeRNAi: a database for cell-based RNAi phenotypes. 2009 update. Nucleic Acids Research. 2010;38:D448–D452. doi: 10.1093/nar/gkp1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah SP, Morin RD, Khattra J, Prentice L, Pugh T, Burleigh A, et al. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature. 2009;461(7265):809–813. doi: 10.1038/nature08489. [DOI] [PubMed] [Google Scholar]
- 14.Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462(7271):315–322. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ley TJ, Mardis ER, Ding L, Fulton B, McLellan MD, Chen K, et al. DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature. 2008;456(7218):66–72. doi: 10.1038/nature07485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. New England Journal of Medicine. 2009;361(11):1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wise J. Consortium hopes to sequence genome of 1000 volunteers. BMJ. 2008;336(7638):237. doi: 10.1136/bmj.39472.676481.DB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Cancer Genome Atlas Research Network Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, Gibbs RA, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449(7164):851–861. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redon R, Ishikawa S, Fitch KR, Feuk L, Perry GH, Andrews TD, et al. Global variation in copy number in the human genome. Nature. 2006;444(7118):444–454. doi: 10.1038/nature05329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong KK, deLeeuw RJ, Dosanjh NS, Kimm LR, Cheng Z, Horsman DE, et al. A comprehensive analysis of common copy-number variations in the human genome. American Journal of Human Genetics. 2007;80(1):91–104. doi: 10.1086/510560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shames DS, Girard L, Gao B, Sato M, Lewis CM, Shivapurkar N, et al. A genome-wide screen for promoter methylation in lung cancer identifies novel methylation markers for multiple malignancies. PLoS Medicine. 2006;3(12):e486. doi: 10.1371/journal.pmed.0030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314(5797):268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 24.Root DE, Hacohen N, Hahn WC, Lander ES, Sabatini DM. Genome-scale loss-of-function screening with a lentiviral RNAi library. Nat Methods. 2006;3(9):715–719. doi: 10.1038/nmeth924. [DOI] [PubMed] [Google Scholar]
- 25.Bibikova M, Lin Z, Zhou L, Chudin E, Garcia EW, Wu B, et al. High-throughput DNA methylation profiling using universal bead arrays. Genome Research. 2006;16(3):383–393. doi: 10.1101/gr.4410706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber M, Davies JJ, Wittig D, Oakeley EJ, Haase M, Lam WL, et al. Chromosome-wide and promoter-specific analyses identify sites of differential DNA methylation in normal and transformed human cells. Nature Genetics. 2005;37(8):853–862. doi: 10.1038/ng1598. [DOI] [PubMed] [Google Scholar]
- 27.International HapMap Consortium A haplotype map of the human genome. Nature. 2005;437(7063):1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 29.Bamford S, Dawson E, Forbes S, Clements J, Pettett R, Dogan A, et al. The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. British Journal of Cancer. 2004;91(2):355–358. doi: 10.1038/sj.bjc.6601894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paddison PJ, Silva JM, Conklin DS, Schlabach M, Li M, Aruleba S, et al. A resource for large-scale RNA-interference-based screens in mammals. Nature. 2004;428(6981):427–431. doi: 10.1038/nature02370. [DOI] [PubMed] [Google Scholar]
- 31.Schlabach MR, Luo J, Solimini NL, Hu G, Xu Q, Li MZ, et al. Cancer proliferation gene discovery through functional genomics. Science. 2008;319(5863):620–624. doi: 10.1126/science.1149200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Futreal PA, Coin L, Marshall M, Down T, Hubbard T, Wooster R, et al. A census of human cancer genes. Nature Reviews Cancer. 2004;4(3):177–183. doi: 10.1038/nrc1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iafrate AJ, Feuk L, Rivera MN, Listewnik ML, Donahoe PK, Qi Y, et al. Detection of large-scale variation in the human genome. Nature Genetics. 2004;36(9):949–951. doi: 10.1038/ng1416. [DOI] [PubMed] [Google Scholar]
- 34.Sebat J, Lakshmi B, Troge J, Alexander J, Young J, Lundin P, et al. Large-scale copy number polymorphism in the human genome. Science. 2004;305(5683):525–528. doi: 10.1126/science.1098918. [DOI] [PubMed] [Google Scholar]
- 35.Ishkanian AS, Malloff CA, Watson SK, DeLeeuw RJ, Chi B, Coe BP, et al. A tiling resolution DNA microarray with complete coverage of the human genome. Nature Genetics. 2004;36(3):299–303. doi: 10.1038/ng1307. [DOI] [PubMed] [Google Scholar]
- 36.Bertone P, Stolc V, Royce TE, Rozowsky JS, Urban AE, Zhu X, et al. Global identification of human transcribed sequences with genome tiling arrays. Science. 2004;306(5705):2242–2246. doi: 10.1126/science.1103388. [DOI] [PubMed] [Google Scholar]
- 37.Hubbard T, Barker D, Birney E, Cameron G, Chen Y, Clark L, et al. The ensembl genome database project. Nucleic Acids Research. 2002;30(1):38–41. doi: 10.1093/nar/30.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, et al. The human genome browser at UCSC. Genome Research. 2002;12(6):996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliphant A, Barker DL, Stuelpnagel JR, Chee MS. BeadArray technology: enabling an accurate, cost-effective approach to high-throughput genotyping. Biotechniques. 2002;(Suppl):56–58. 60–51. [PubMed] [Google Scholar]
- 40.Weinstein IB. Cancer. Addiction to oncogenes—the Achilles heal of cancer. Science. 2002;297(5578):63–64. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 41.Weinstein IB, Joe A. Oncogene addiction. Cancer Research. 2008;68(9):3077–3080. doi: 10.1158/0008-5472.CAN-07-3293. discussion 3080. [DOI] [PubMed] [Google Scholar]
- 42.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409(6822):860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 43.Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, et al. The sequence of the human genome. Science. 2001;291(5507):1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 44.Riggins GJ, Strausberg RL. Genome and genetic resources from the cancer genome anatomy project. Human Molecular Genetics. 2001;10(7):663–667. doi: 10.1093/hmg/10.7.663. [DOI] [PubMed] [Google Scholar]
- 45.Strausberg RL, Buetow KH, Emmert-Buck MR, Klausner RD. The cancer genome anatomy project: Building an annotated gene index. Trends in Genetics. 2000;16(3):103–106. doi: 10.1016/s0168-9525(99)01937-x. [DOI] [PubMed] [Google Scholar]
- 46.Bayani JM, Squire JA. Applications of SKY in cancer cytogenetics. Cancer Investigation. 2002;20(3):373–386. doi: 10.1081/cnv-120001183. [DOI] [PubMed] [Google Scholar]
- 47.Kallioniemi A, Kallioniemi OP, Sudar D, Rutovitz D, Gray JW, Waldman F, et al. Comparative genomic hybridization for molecular cytogenetic analysis of solid tumors. Science. 1992;258(5083):818–821. doi: 10.1126/science.1359641. [DOI] [PubMed] [Google Scholar]
- 48.Garnis C, Buys TP, Lam WL. Genetic alteration and gene expression modulation during cancer progression. Molecular Cancer. 2004;3:9. doi: 10.1186/1476-4598-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gebhart E. Genomic imbalances in human leukemia and lymphoma detected by comparative genomic hybridization (Review) International Journal of Oncology. 2005;27(3):593–606. [PubMed] [Google Scholar]
- 50.Gebhart E, Liehr T. Patterns of genomic imbalances in human solid tumors (Review) International Journal of Oncology. 2000;16(2):383–399. doi: 10.3892/ijo.16.2.383. [DOI] [PubMed] [Google Scholar]
- 51.Cahill DP, Lengauer C, Yu J, Riggins GJ, Willson JK, Markowitz SD, et al. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392(6673):300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- 52.Fukasawa K. Centrosome amplification, chromosome instability and cancer development. Cancer Letters. 2005;230(1):6–19. doi: 10.1016/j.canlet.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 53.Lingle WL, Lukasiewicz K, Salisbury JL. Deregulation of the centrosome cycle and the origin of chromosomal instability in cancer. Advances in Experimental Medicine and Biology. 2005;570:393–421. doi: 10.1007/1-4020-3764-3_14. [DOI] [PubMed] [Google Scholar]
- 54.Chin K, de Solorzano CO, Knowles D, Jones A, Chou W, Rodriguez EG, et al. In situ analyses of genome instability in breast cancer. Nature Genetics. 2004;36(9):984–988. doi: 10.1038/ng1409. [DOI] [PubMed] [Google Scholar]
- 55.O’Hagan RC, Chang S, Maser RS, Mohan R, Artandi SE, Chin L, et al. Telomere dysfunction provokes regional amplification and deletion in cancer genomes. Cancer Cell. 2002;2(2):149–155. doi: 10.1016/s1535-6108(02)00094-6. [DOI] [PubMed] [Google Scholar]
- 56.Green AR. Transcription factors, translocations and haematological malignancies. Blood Reviews. 1992;6(2):118–124. doi: 10.1016/0268-960x(92)90013-g. [DOI] [PubMed] [Google Scholar]
- 57.Rowley JD. Chromosomal translocations: revisited yet again. Blood. 2008;112(6):2183–2189. doi: 10.1182/blood-2008-04-097931. [DOI] [PubMed] [Google Scholar]
- 58.Watson SK, deLeeuw RJ, Horsman DE, Squire JA, Lam WL. Cytogenetically balanced translocations are associated with focal copy number alterations. Human Genetics. 2007;120(6):795–805. doi: 10.1007/s00439-006-0251-9. [DOI] [PubMed] [Google Scholar]
- 59.Brenner JC, Chinnaiyan AM. Translocations in epithelial cancers. Biochimica et Biophysica Acta. 2009;1796(2):201–215. doi: 10.1016/j.bbcan.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mani RS, Tomlins SA, Callahan K, Ghosh A, Nyati MK, Varambally S, et al. Induced chromosomal proximity and gene fusions in prostate cancer. Science. 2009;326(5957):1230. doi: 10.1126/science.1178124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310(5748):644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 62.Dang TP, Gazdar AF, Virmani AK, Sepetavec T, Hande KR, Minna JD, et al. Chromosome 19 translocation, overexpression of Notch3, and human lung cancer. Journal of the National Cancer Institute. 2000;92(16):1355–1357. doi: 10.1093/jnci/92.16.1355. [DOI] [PubMed] [Google Scholar]
- 63.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 64.Knutsen T, Gobu V, Knaus R, Padilla-Nash H, Augustus M, Strausberg RL, et al. The interactive online SKY/M-FISH & CGH database and the Entrez cancer chromosomes search database: Linkage of chromosomal aberrations with the genome sequence. Genes, Chromosomes, and Cancer. 2005;44(1):52–64. doi: 10.1002/gcc.20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kapushesky M, Emam I, Holloway E, Kurnosov P, Zorin A, Malone J, et al. Gene expression atlas at the European bioinformatics institute. Nucleic Acids Research. 2010;38:D690–D698. doi: 10.1093/nar/gkp936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li L, Bum-Erdene K, Baenziger PH, Rosen JJ, Hemmert JR, Nellis JA, et al. BioDrugScreen: a computational drug design resource for ranking molecules docked to the human proteome. Nucleic Acids Research. 2010;38:D765–D773. doi: 10.1093/nar/gkp852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Forbes SA, Tang G, Bindal N, Bamford S, Dawson E, Cole C, et al. COSMIC (the Catalogue of Somatic Mutations in Cancer): A resource to investigate acquired mutations in human cancer. Nucleic Acids Research. 2010;38:D652–D657. doi: 10.1093/nar/gkp995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kato K, Yamashita R, Matoba R, Monden M, Noguchi S, Takagi T, et al. Cancer gene expression database (CGED): A database for gene expression profiling with accompanying clinical information of human cancer tissues. Nucleic Acids Research. 2005;33:D533–D536. doi: 10.1093/nar/gki117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li H, He Y, Ding G, Wang C, Xie L, Li Y. dbDEPC: a database of differentially expressed proteins in human cancers. Nucleic Acids Research. 2010;38:D658–D664. doi: 10.1093/nar/gkp933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brooksbank C, Cameron G, Thornton J. The European Bioinformatics Institute’s data resources. Nucleic Acids Research. 2010;38:D17–25. doi: 10.1093/nar/gkp986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Safran M, Chalifa-Caspi V, Shmueli O, Olender T, Lapidot M, Rosen N, et al. Human gene-centric databases at the Weizmann institute of science: GeneCards, UDB, CroW 21 and HORDE. Nucleic Acids Research. 2003;31(1):142–146. doi: 10.1093/nar/gkg050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y, Lv J, Liu H, Zhu J, Su J, Wu Q, et al. HHMD: the human histone modification database. Nucleic Acids Research. 2010;38:D149–D154. doi: 10.1093/nar/gkp968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Betel D, Wilson M, Gabow A, Marks DS, Sander C. The microRNA.org resource: targets and expression. Nucleic Acids Research. 2008;36:D149–D153. doi: 10.1093/nar/gkm995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang Q, Wang Y, Hao Y, Juan L, Teng M, Zhang X, et al. miR2Disease: A manually curated database for microRNA deregulation in human disease. Nucleic Acids Research. 2009;37:D98–D104. doi: 10.1093/nar/gkn714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang X. miRDB: A microRNA target prediction and functional annotation database with a wiki interface. RNA. 2008;14(6):1012–1017. doi: 10.1261/rna.965408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alexiou P, Vergoulis T, Gleditzsch M, Prekas G, Dalamagas T, Megraw M, et al. miRGen 2.0: A database of microRNA genomic information and regulation. Nucleic Acids Research. 2010;38:D137–D141. doi: 10.1093/nar/gkp888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sayers EW, Barrett T, Benson DA, Bolton E, Bryant SH, Canese K, et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Research. 2010;38:D5–D16. doi: 10.1093/nar/gkp967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barrett T, Troup DB, Wilhite SE, Ledoux P, Rudnev D, Evangelista C, et al. NCBI GEO: Archive for high-throughput functional genomic data. Nucleic Acids Research. 2009;37:D885–D890. doi: 10.1093/nar/gkn764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rhodes DR, Kalyana-Sundaram S, Mahavisno V, Varambally R, Yu J, Briggs BB, et al. Oncomine 3.0: Genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9(2):166–180. doi: 10.1593/neo.07112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baudis M. Genomic imbalances in 5918 malignant epithelial tumors: an explorative meta-analysis of chromosomal CGH data. BMC Cancer. 2007;7:226. doi: 10.1186/1471-2407-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vizcaino JA, Cote R, Reisinger F, Barsnes H, Foster JM, Rameseder J, et al. The proteomics identifications database: 2010 update. Nucleic Acids Research. 2010;38:D736–D742. doi: 10.1093/nar/gkp964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ren Y, Gong W, Zhou H, Wang Y, Xiao F, Li T. siRecords: A database of mammalian RNAi experiments and efficacies. Nucleic Acids Research. 2009;37:D146–D149. doi: 10.1093/nar/gkn817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chari R, Lockwood WW, Coe BP, Chu A, Macey D, Thomson A, et al. SIGMA: A system for integrative genomic microarray analysis of cancer genomes. BMC Genomics. 2006;7:324. doi: 10.1186/1471-2164-7-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rhead B, Karolchik D, Kuhn RM, Hinrichs AS, Zweig AS, Fujita PA, et al. The UCSC genome browser database: Update 2010. Nucleic Acids Research. 2010;38:D613–D619. doi: 10.1093/nar/gkp939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Albertson DG, Collins C, McCormick F, Gray JW. Chromosome aberrations in solid tumors. Nature Genetics. 2003;34(4):369–376. doi: 10.1038/ng1215. [DOI] [PubMed] [Google Scholar]
- 86.Coe BP, Ylstra B, Carvalho B, Meijer GA, Macaulay C, Lam WL. Resolving the resolution of array CGH. Genomics. 2007;89(5):647–653. doi: 10.1016/j.ygeno.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 87.Lockwood WW, Chari R, Chi B, Lam WL. Recent advances in array comparative genomic hybridization technologies and their applications in human genetics. European Journal of Human Genetics. 2006;14(2):139–148. doi: 10.1038/sj.ejhg.5201531. [DOI] [PubMed] [Google Scholar]
- 88.Pollack JR, Perou CM, Alizadeh AA, Eisen MB, Pergamenschikov A, Williams CF, et al. Genomewide analysis of DNA copy-number changes using cDNA microarrays. Nature Genetics. 1999;23(1):41–46. doi: 10.1038/12640. [DOI] [PubMed] [Google Scholar]
- 89.Almagro-Garcia J, Manske M, Carret C, Campino S, Auburn S, Macinnis BL, et al. SnoopCGH: software for visualizing comparative genomic hybridization data. Bioinformatics. 2009;25(20):2732–2733. doi: 10.1093/bioinformatics/btp488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chari R, Lockwood WW, Lam WL. Computational methods for the analysis of array comparative genomic hybridization. Cancer Information. 2007;2:48–58. [PMC free article] [PubMed] [Google Scholar]
- 91.Chi B, DeLeeuw RJ, Coe BP, MacAulay C, Lam WL. SeeGH—a software tool for visualization of whole genome array comparative genomic hybridization data. BMC Bioinformatics. 2004;5:13. doi: 10.1186/1471-2105-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chi B, deLeeuw RJ, Coe BP, Ng RT, MacAulay C, Lam WL. MD-SeeGH: A platform for integrative analysis of multi-dimensional genomic data. BMC Bioinformatics. 2008;9:243. doi: 10.1186/1471-2105-9-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Venkatraman ES, Olshen AB. A faster circular binary segmentation algorithm for the analysis of array CGH data. Bioinformatics. 2007;23(6):657–663. doi: 10.1093/bioinformatics/btl646. [DOI] [PubMed] [Google Scholar]
- 94.Bignell GR, Huang J, Greshock J, Watt S, Butler A, West S, et al. High-resolution analysis of DNA copy number using oligonucleotide microarrays. Genome Research. 2004;14(2):287–295. doi: 10.1101/gr.2012304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iacobucci I, Storlazzi CT, Cilloni D, Lonetti A, Ottaviani E, Soverini S, et al. Identification and molecular characterization of recurrent genomic deletions on 7p12 in the IKZF1 gene in a large cohort of BCR-ABL1-positive acute lymphoblastic leukemia patients: On behalf of Gruppo Italiano Malattie Ematologiche dell’Adulto Acute Leukemia Working Party (GIMEMA AL WP) Blood. 2009;114(10):2159–2167. doi: 10.1182/blood-2008-08-173963. [DOI] [PubMed] [Google Scholar]
- 96.Niini T, Lopez-Guerrero JA, Ninomiya S, Guled M, Hattinger CM, Michelacci F, et al. Frequent deletion of CDKN2A and recurrent coamplification of KIT, PDGFRA, and KDR in fibrosarcoma of bone—an array comparative genomic hybridization study. Genes, Chromosomes, and Cancer. 2009;49(2):132–143. doi: 10.1002/gcc.20727. [DOI] [PubMed] [Google Scholar]
- 97.Selzer RR, Richmond TA, Pofahl NJ, Green RD, Eis PS, Nair P, et al. Analysis of chromosome breakpoints in neuroblastoma at sub-kilobase resolution using fine-tiling oligonucleotide array CGH. Genes, Chromosomes, and Cancer. 2005;44(3):305–319. doi: 10.1002/gcc.20243. [DOI] [PubMed] [Google Scholar]
- 98.Zhao X, Li C, Paez JG, Chin K, Janne PA, Chen TH, et al. An integrated view of copy number and allelic alterations in the cancer genome using single nucleotide polymorphism arrays. Cancer Research. 2004;64(9):3060–3071. doi: 10.1158/0008-5472.can-03-3308. [DOI] [PubMed] [Google Scholar]
- 99.Wang TL, Maierhofer C, Speicher MR, Lengauer C, Vogelstein B, Kinzler KW, et al. Digital karyotyping. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(25):16156–16161. doi: 10.1073/pnas.202610899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tuzun E, Sharp AJ, Bailey JA, Kaul R, Morrison VA, Pertz LM, et al. Fine-scale structural variation of the human genome. Nature Genetics. 2005;37(7):727–732. doi: 10.1038/ng1562. [DOI] [PubMed] [Google Scholar]
- 101.Volik S, Raphael BJ, Huang G, Stratton MR, Bignel G, Murnane J, et al. Decoding the fine-scale structure of a breast cancer genome and transcriptome. Genome Research. 2006;16(3):394–404. doi: 10.1101/gr.4247306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Volik S, Zhao S, Chin K, Brebner JH, Herndon DR, Tao Q, et al. End-sequence profiling: Sequence-based analysis of aberrant genomes. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(13):7696–7701. doi: 10.1073/pnas.1232418100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McPherson JD. Next-generation gap. Nature Methods. 2009;6(11 Suppl):S2–S5. doi: 10.1038/nmeth.f.268. [DOI] [PubMed] [Google Scholar]
- 104.Alkan C, Kidd JM, Marques-Bonet T, Aksay G, Antonacci F, Hormozdiari F, et al. Personalized copy number and segmental duplication maps using next-generation sequencing. Nature Genetics. 2009;41(10):1061–1067. doi: 10.1038/ng.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Conrad DF, Andrews TD, Carter NP, Hurles ME, Pritchard JK. A high-resolution survey of deletion polymorphism in the human genome. Nature Genetics. 2006;38(1):75–81. doi: 10.1038/ng1697. [DOI] [PubMed] [Google Scholar]
- 106.Conrad DF, Pinto D, Redon R, Feuk L, Gokcumen O, Zhang Y, et al. Origins and functional impact of copy number variation in the human genome. Nature. 2009 doi: 10.1038/nature08516. doi:10.1038/nature08516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fiegler H, Redon R, Andrews D, Scott C, Andrews R, Carder C, et al. Accurate and reliable high-throughput detection of copy number variation in the human genome. Genome Research. 2006;16(12):1566–1574. doi: 10.1101/gr.5630906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jakobsson M, Scholz SW, Scheet P, Gibbs JR, VanLiere JM, Fung HC, et al. Genotype, haplotype and copy-number variation in worldwide human populations. Nature. 2008;451(7181):998–1003. doi: 10.1038/nature06742. [DOI] [PubMed] [Google Scholar]
- 109.Kidd JM, Cooper GM, Donahue WF, Hayden HS, Sampas N, Graves T, et al. Mapping and sequencing of structural variation from eight human genomes. Nature. 2008;453(7191):56–64. doi: 10.1038/nature06862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McCarroll SA, Kuruvilla FG, Korn JM, Cawley S, Nemesh J, Wysoker A, et al. Integrated detection and population-genetic analysis of SNPs and copy number variation. Nature Genetics. 2008;40(10):1166–1174. doi: 10.1038/ng.238. [DOI] [PubMed] [Google Scholar]
- 111.Shaikh TH, Gai X, Perin JC, Glessner JT, Xie H, Murphy K, et al. High-resolution mapping and analysis of copy number variations in the human genome: a data resource for clinical and research applications. Genome Research. 2009;19(9):1682–1690. doi: 10.1101/gr.083501.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hastings PJ, Ira G, Lupski JR. A microhomology-mediated break-induced replication model for the origin of human copy number variation. PLoS Genetics. 2009;5(1):e1000327. doi: 10.1371/journal.pgen.1000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Diskin SJ, Hou C, Glessner JT, Attiyeh EF, Laudenslager M, Bosse K, et al. Copy number variation at 1q21.1 associated with neuroblastoma. Nature. 2009;459(7249):987–991. doi: 10.1038/nature08035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lockwood WW, Chari R, Coe BP, Girard L, Macaulay C, Lam S, et al. DNA amplification is a ubiquitous mechanism of oncogene activation in lung and other cancers. Oncogene. 2008;27(33):4615–4624. doi: 10.1038/onc.2008.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Myllykangas S, Himberg J, Bohling T, Nagy B, Hollmen J, Knuutila S. DNA copy number amplification profiling of human neoplasms. Oncogene. 2006;25(55):7324–7332. doi: 10.1038/sj.onc.1209717. [DOI] [PubMed] [Google Scholar]
- 116.Teschendorff AE, Caldas C. The breast cancer somatic ‘muta-ome’: tackling the complexity. Breast Cancer Research. 2009;11(2):301. doi: 10.1186/bcr2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chin SF, Teschendorff AE, Marioni JC, Wang Y, Barbosa-Morais NL, Thorne NP, et al. High-resolution aCGH and expression profiling identifies a novel genomic subtype of ER negative breast cancer. Genome Biology. 2007;8(10):R215. doi: 10.1186/gb-2007-8-10-r215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Coe BP, Lockwood WW, Girard L, Chari R, Macaulay C, Lam S, et al. Differential disruption of cell cycle pathways in small cell and non-small cell lung cancer. British Journal of Cancer. 2006;94(12):1927–1935. doi: 10.1038/sj.bjc.6603167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nature Genetics. 2009;41(11):1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Garraway LA, Widlund HR, Rubin MA, Getz G, Berger AJ, Ramaswamy S, et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature. 2005;436(7047):117–122. doi: 10.1038/nature03664. [DOI] [PubMed] [Google Scholar]
- 121.Weir BA, Woo MS, Getz G, Perner S, Ding L, Beroukhim R, et al. Characterizing the cancer genome in lung adenocarcinoma. Nature. 2007;450(7171):893–898. doi: 10.1038/nature06358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kwei KA, Kim YH, Girard L, Kao J, Pacyna-Gengelbach M, Salari K, et al. Genomic profiling identifies TITF1 as a lineage-specific oncogene amplified in lung cancer. Oncogene. 2008;27(25):3635–3640. doi: 10.1038/sj.onc.1211012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Plomin R, Haworth CM, Davis OS. Common disorders are quantitative traits. Nature Reviews Genetics. 2009;10(12):872–878. doi: 10.1038/nrg2670. [DOI] [PubMed] [Google Scholar]
- 124.Savas S, Liu G. Genetic variations as cancer prognostic markers: Review and update. Human Mutation. 2009;30(10):1369–1377. doi: 10.1002/humu.21078. [DOI] [PubMed] [Google Scholar]
- 125.Ansorge WJ. Next-generation DNA sequencing techniques. New Biotechnology. 2009;25(4):195–203. doi: 10.1016/j.nbt.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 126.Shah SP, Kobel M, Senz J, Morin RD, Clarke BA, Wiegand KC, et al. Mutation of FOXL2 in granulosa-cell tumors of the ovary. New England Journal of Medicine. 2009;360(26):2719–2729. doi: 10.1056/NEJMoa0902542. [DOI] [PubMed] [Google Scholar]
- 127.Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446(7132):153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458(7239):719–724. doi: 10.1038/nature07943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cavenee WK, Hansen MF, Nordenskjold M, Kock E, Maumenee I, Squire JA, et al. Genetic origin of mutations predisposing to retinoblastoma. Science. 1985;228(4698):501–503. doi: 10.1126/science.3983638. [DOI] [PubMed] [Google Scholar]
- 130.Knudson AG., Jr. Mutation and cancer: statistical study of retinoblastoma. Proceedings of the National Academy of Sciences of the United States of America. 1971;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Benz CC, Fedele V, Xu F, Ylstra B, Ginzinger D, Yu M, et al. Altered promoter usage characterizes monoallelic transcription arising with ERBB2 amplification in human breast cancers. Genes, Chromosomes, and Cancer. 2006;45(11):983–994. doi: 10.1002/gcc.20364. [DOI] [PubMed] [Google Scholar]
- 132.LaFramboise T, Weir BA, Zhao X, Beroukhim R, Li C, Harrington D, et al. Allele-specific amplification in cancer revealed by SNP array analysis. PLoS Comput Biol. 2005;1(6):e65. doi: 10.1371/journal.pcbi.0010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Melcher R, Al-Taie O, Kudlich T, Hartmann E, Maisch S, Steinlein C, et al. SNP-Array genotyping and spectral karyotyping reveal uniparental disomy as early mutational event in MSS- and MSI-colorectal cancer cell lines. Cytogenetic and Genome Research. 2007;118(2–4):214–221. doi: 10.1159/000108303. [DOI] [PubMed] [Google Scholar]
- 134.Nomura M, Shigematsu H, Li L, Suzuki M, Takahashi T, Estess P, et al. Polymorphisms, mutations, and amplification of the EGFR gene in non-small cell lung cancers. PLoS Medicine. 2007;4(4):e125. doi: 10.1371/journal.pmed.0040125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sholl LM, Yeap BY, Iafrate AJ, Holmes-Tisch AJ, Chou YP, Wu MT, et al. Lung adenocarcinoma with EGFR amplification has distinct clinicopathologic and molecular features in never-smokers. Cancer Research. 2009;69(21):8341–8348. doi: 10.1158/0008-5472.CAN-09-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Soh J, Okumura N, Lockwood WW, Yamamoto H, Shigematsu H, Zhang W, et al. Oncogene mutations, copy number gains and mutant allele specific imbalance (MASI) frequently occur together in tumor cells. PLoS ONE. 2009;4(10):e7464. doi: 10.1371/journal.pone.0007464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bacolod MD, Schemmann GS, Giardina SF, Paty P, Notterman DA, Barany F. Emerging paradigms in cancer genetics: some important findings from high-density single nucleotide polymorphism array studies. Cancer Research. 2009;69(3):723–727. doi: 10.1158/0008-5472.CAN-08-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Robinson WP. Mechanisms leading to uniparental disomy and their clinical consequences. Bioessays. 2000;22(5):452–459. doi: 10.1002/(SICI)1521-1878(200005)22:5<452::AID-BIES7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 139.Tuna M, Knuutila S, Mills GB. Uniparental disomy in cancer. Trends in Molecular Medicine. 2009;15(3):120–128. doi: 10.1016/j.molmed.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 140.Zhu X, Dunn JM, Goddard AD, Squire JA, Becker A, Phillips RA, et al. Mechanisms of loss of heterozygosity in retinoblastoma. Cytogenetics and Cell Genetics. 1992;59(4):248–252. doi: 10.1159/000133261. [DOI] [PubMed] [Google Scholar]
- 141.Gondek LP, Dunbar AJ, Szpurka H, McDevitt MA, Maciejewski JP. SNP array karyotyping allows for the detection of uniparental disomy and cryptic chromosomal abnormalities in MDS/MPD-U and MPD. PLoS ONE. 2007;2(11):e1225. doi: 10.1371/journal.pone.0001225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tiu RV, Gondek LP, O’Keefe CL, Huh J, Sekeres MA, Elson P, et al. New lesions detected by single nucleotide polymorphism array-based chromosomal analysis have important clinical impact in acute myeloid leukemia. Journal of Clinical Oncology. 2009;27(31):5219–5226. doi: 10.1200/JCO.2009.21.9840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yamamoto G, Nannya Y, Kato M, Sanada M, Levine RL, Kawamata N, et al. Highly sensitive method for genomewide detection of allelic composition in nonpaired, primary tumor specimens by use of affymetrix single-nucleotide-polymorphism genotyping microarrays. American Journal of Human Genetics. 2007;81(1):114–126. doi: 10.1086/518809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Darbary HK, Dutt SS, Sait SJ, Nowak NJ, Heinaman RE, Stoler DL, et al. Uniparentalism in sporadic colorectal cancer is independent of imprint status, and coordinate for chromosomes 14 and 18. Cancer Genetics and Cytogenetics. 2009;189(2):77–86. doi: 10.1016/j.cancergencyto.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Grand FH, Hidalgo-Curtis CE, Ernst T, Zoi K, Zoi C, McGuire C, et al. Frequent CBL mutations associated with 11q acquired uniparental disomy in myeloproliferative neoplasms. Blood. 2009;113(24):6182–6192. doi: 10.1182/blood-2008-12-194548. [DOI] [PubMed] [Google Scholar]
- 146.Gupta M, Raghavan M, Gale RE, Chelala C, Allen C, Molloy G, et al. Novel regions of acquired uniparental disomy discovered in acute myeloid leukemia. Genes, Chromosomes, and Cancer. 2008;47(9):729–739. doi: 10.1002/gcc.20573. [DOI] [PubMed] [Google Scholar]
- 147.Kawamata N, Ogawa S, Gueller S, Ross SH, Huynh T, Chen J, et al. Identified hidden genomic changes in mantle cell lymphoma using high-resolution single nucleotide polymorphism genomic array. Experimental Hematology. 2009;37(8):937–946. doi: 10.1016/j.exphem.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Makishima H, Cazzolli H, Szpurka H, Dunbar A, Tiu R, Huh J, et al. Mutations of e3 ubiquitin ligase cbl family members constitute a novel common pathogenic lesion in myeloid malignancies. Journal of Clinical Oncology. 2009;27(36):6109–6116. doi: 10.1200/JCO.2009.23.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Walter MJ, Payton JE, Ries RE, Shannon WD, Deshmukh H, Zhao Y, et al. Acquired copy number alterations in adult acute myeloid leukemia genomes. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(31):12950–12955. doi: 10.1073/pnas.0903091106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yin D, Ogawa S, Kawamata N, Tunici P, Finocchiaro G, Eoli M, et al. High-resolution genomic copy number profiling of glioblastoma multiforme by single nucleotide polymorphism DNA microarray. Mol Cancer Res. 2009;7(5):665–677. doi: 10.1158/1541-7786.MCR-08-0270. [DOI] [PubMed] [Google Scholar]
- 151.Purdie KJ, Lambert SR, Teh MT, Chaplin T, Molloy G, Raghavan M, et al. Allelic imbalances and microdeletions affecting the PTPRD gene in cutaneous squamous cell carcinomas detected using single nucleotide polymorphism microarray analysis. Genes, Chromosomes, and Cancer. 2007;46(7):661–669. doi: 10.1002/gcc.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Akagi T, Ito T, Kato M, Jin Z, Cheng Y, Kan T, et al. Chromosomal abnormalities and novel disease-related regions in progression from Barrett’s esophagus to esophageal adenocarcinoma. International Journal of Cancer. 2009;125(10):2349–2359. doi: 10.1002/ijc.24620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Andersen CL, Wiuf C, Kruhoffer M, Korsgaard M, Laurberg S, Orntoft TF. Frequent occurrence of uniparental disomy in colorectal cancer. Carcinogenesis. 2007;28(1):38–48. doi: 10.1093/carcin/bgl086. [DOI] [PubMed] [Google Scholar]
- 154.Kerkel K, Spadola A, Yuan E, Kosek J, Jiang L, Hod E, et al. Genomic surveys by methylation-sensitive SNP analysis identify sequence-dependent allele-specific DNA methylation. Nature Genetics. 2008;40(7):904–908. doi: 10.1038/ng.174. [DOI] [PubMed] [Google Scholar]
- 155.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128(4):683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Esteller M. Epigenetics in cancer. New England Journal of Medicine. 2008;358(11):1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 157.Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447(7143):433–440. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- 158.Vucic EA, Brown CJ, Lam WL. Epigenetics of cancer progression. Pharmacogenomics. 2008;9(2):215–234. doi: 10.2217/14622416.9.2.215. [DOI] [PubMed] [Google Scholar]
- 159.Feinberg AP, Gehrke CW, Kuo KC, Ehrlich M. Reduced genomic 5-methylcytosine content in human colonic neoplasia. Cancer Research. 1988;48(5):1159–1161. [PubMed] [Google Scholar]
- 160.Feinberg AP, Tycko B. The history of cancer epigenetics. Nature Reviews Cancer. 2004;4(2):143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 161.Lo PK, Sukumar S. Epigenomics and breast cancer. Pharmacogenomics. 2008;9(12):1879–1902. doi: 10.2217/14622416.9.12.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(15):8681–8686. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Issa JP. CpG island methylator phenotype in cancer. Nature Reviews Cancer. 2004;4(12):988–993. doi: 10.1038/nrc1507. [DOI] [PubMed] [Google Scholar]
- 164.Tanemura A, Terando AM, Sim MS, van Hoesel AQ, de Maat MF, Morton DL, et al. CpG island methylator phenotype predicts progression of malignant melanoma. Clinical Cancer Research. 2009;15(5):1801–1807. doi: 10.1158/1078-0432.CCR-08-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dai Z, Lakshmanan RR, Zhu WG, Smiraglia DJ, Rush LJ, Fruhwald MC, et al. Global methylation profiling of lung cancer identifies novel methylated genes. Neoplasia. 2001;3(4):314–323. doi: 10.1038/sj.neo.7900162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Takai D, Yagi Y, Wakazono K, Ohishi N, Morita Y, Sugimura T, et al. Silencing of HTR1B and reduced expression of EDN1 in human lung cancers, revealed by methylation-sensitive representational difference analysis. Oncogene. 2001;20(51):7505–7513. doi: 10.1038/sj.onc.1204940. [DOI] [PubMed] [Google Scholar]
- 167.Hu M, Yao J, Cai L, Bachman KE, van den Brule F, Velculescu V, et al. Distinct epigenetic changes in the stromal cells of breast cancers. Nature Genetics. 2005;37(8):899–905. doi: 10.1038/ng1596. [DOI] [PubMed] [Google Scholar]
- 168.Irizarry RA, Ladd-Acosta C, Carvalho B, Wu H, Brandenburg SA, Jeddeloh JA, et al. Comprehensive high-throughput arrays for relative methylation (CHARM) Genome Research. 2008;18(5):780–790. doi: 10.1101/gr.7301508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yan PS, Chen CM, Shi H, Rahmatpanah F, Wei SH, Caldwell CW, et al. Dissecting complex epigenetic alterations in breast cancer using CpG island microarrays. Cancer Research. 2001;61(23):8375–8380. [PubMed] [Google Scholar]
- 170.Yamamoto F, Yamamoto M. A DNA microarray-based methylation-sensitive (MS)-AFLP hybridization method for genetic and epigenetic analyses. Mol Genet Genomics. 2004;271(6):678–686. doi: 10.1007/s00438-004-1017-5. [DOI] [PubMed] [Google Scholar]
- 171.Omura N, Li CP, Li A, Hong SM, Walter K, Jimeno A, et al. Genome-wide profiling of methylated promoters in pancreatic adenocarcinoma. Cancer Biol Ther. 2008;7(7):1146–1156. doi: 10.4161/cbt.7.7.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Trinh BN, Long TI, Laird PW. DNA methylation analysis by MethyLight technology. Methods. 2001;25(4):456–462. doi: 10.1006/meth.2001.1268. [DOI] [PubMed] [Google Scholar]
- 173.Fan JB, Gunderson KL, Bibikova M, Yeakley JM, Chen J, Garcia E. Wickham, et al. Illumina universal bead arrays. Methods in Enzymology. 2006;410:57–73. doi: 10.1016/S0076-6879(06)10003-8. [DOI] [PubMed] [Google Scholar]
- 174.Houshdaran S, Cortessis VK, Siegmund K, Yang A, Laird PW, Sokol RZ. Widespread epigenetic abnormalities suggest a broad DNA methylation erasure defect in abnormal human sperm. PLoS ONE. 2007;2(12):e1289. doi: 10.1371/journal.pone.0001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Houseman EA, Christensen BC, Karagas MR, Wrensch MR, Nelson HH, Wiemels JL, et al. Copy number variation has little impact on bead-array-based measures of DNA methylation. Bioinformatics. 2009;25(16):1999–2005. doi: 10.1093/bioinformatics/btp364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Breton CV, Byun HM, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. American Journal of Respiratory and Critical Care Medicine. 2009;180(5):462–467. doi: 10.1164/rccm.200901-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Taylor KH, Pena-Hernandez KE, Davis JW, Arthur GL, Duff DJ, Shi H, et al. Large-scale CpGmethylation analysis identifies novel candidate genes and reveals methylation hotspots in acute lymphoblastic leukemia. Cancer Research. 2007;67(6):2617–2625. doi: 10.1158/0008-5472.CAN-06-3993. [DOI] [PubMed] [Google Scholar]
- 178.Weber M, Hellmann I, Stadler MB, Ramos L, Paabo S, Rebhan M, et al. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nature Genetics. 2007;39(4):457–466. doi: 10.1038/ng1990. [DOI] [PubMed] [Google Scholar]
- 179.Rauch T, Pfeifer GP. Methylated-CpG island recovery assay: a new technique for the rapid detection of methylated-CpG islands in cancer. Laboratory Investigation. 2005;85(9):1172–1180. doi: 10.1038/labinvest.3700311. [DOI] [PubMed] [Google Scholar]
- 180.Jacinto FV, Ballestar E, Ropero S, Esteller M. Discovery of epigenetically silenced genes by methylated DNA immunoprecipitation in colon cancer cells. Cancer Research. 2007;67(24):11481–11486. doi: 10.1158/0008-5472.CAN-07-2687. [DOI] [PubMed] [Google Scholar]
- 181.Ballestar E, Paz MF, Valle L, Wei S, Fraga MF, Espada J, et al. Methyl-CpG binding proteins identify novel sites of epigenetic inactivation in human cancer. EMBO Journal. 2003;22(23):6335–6345. doi: 10.1093/emboj/cdg604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Serre D, Lee BH, Ting AH. MBD-isolated Genome Sequencing provides a high-throughput and comprehensive survey of DNA methylation in the human genome. Nucleic Acids Research. 2009 doi: 10.1093/nar/gkp992. doi:10.1093/nar/gkp992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Down TA, Rakyan VK, Turner DJ, Flicek P, Li H, Kulesha E, et al. A Bayesian deconvolution strategy for immunoprecipitation-based DNA methylome analysis. Nature Biotechnology. 2008;26(7):779–785. doi: 10.1038/nbt1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Thu KL, Pikor LA, Kennett JY, Alvarez CE, Lam WL. Methylation analysis by DNA immunoprecipitation. Journal of Cellular Physiology. 2009;222(3):522–531. doi: 10.1002/jcp.22009. [DOI] [PubMed] [Google Scholar]
- 185.Pelizzola M, Koga Y, Urban AE, Krauthammer M, Weissman S, Halaban R, et al. MEDME: an experimental and analytical methodology for the estimation of DNA methylation levels based on microarray derived MeDIP-enrichment. Genome Research. 2008;18(10):1652–1659. doi: 10.1101/gr.080721.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yamashita S, Hosoya K, Gyobu K, Takeshima H, Ushijima T. Development of a novel output value for quantitative assessment in methylated DNA immunoprecipitation-CpG island microarray analysis. DNA Research. 2009;16(5):275–286. doi: 10.1093/dnares/dsp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Irizarry RA, Ladd-Acosta C, Wen B, Wu Z, Montano C, Onyango P, et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nature Genetics. 2009;41(2):178–186. doi: 10.1038/ng.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lorincz MC, Dickerson DR, Schmitt M, Groudine M. Intragenic DNA methylation alters chromatin structure and elongation efficiency in mammalian cells. Nature Structural & Molecular Biology. 2004;11(11):1068–1075. doi: 10.1038/nsmb840. [DOI] [PubMed] [Google Scholar]
- 189.Frigola J, Song J, Stirzaker C, Hinshelwood RA, Peinado MA, Clark SJ. Epigenetic remodeling in colorectal cancer results in coordinate gene suppression across an entire chromosome band. Nature Genetics. 2006;38(5):540–549. doi: 10.1038/ng1781. [DOI] [PubMed] [Google Scholar]
- 190.Zhong S, Fields CR, Su N, Pan YX, Robertson KD. Pharmacologic inhibition of epigenetic modifications, coupled with gene expression profiling, reveals novel targets of aberrant DNA methylation and histone deacetylation in lung cancer. Oncogene. 2007;26(18):2621–2634. doi: 10.1038/sj.onc.1210041. [DOI] [PubMed] [Google Scholar]
- 191.Lister R, Ecker JR. Finding the fifth base: genomewide sequencing of cytosine methylation. Genome Research. 2009;19(6):959–966. doi: 10.1101/gr.083451.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Byun HM, Siegmund KD, Pan F, Weisenberger DJ, Kanel G, Laird PW, et al. Epigenetic profiling of somatic tissues from human autopsy specimens identifies tissue- and individual-specific DNA methylation patterns. Human Molecular Genetics. 2009;18(24):4808–4817. doi: 10.1093/hmg/ddp445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(30):10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Deng J, Shoemaker R, Xie B, Gore A, LeProust EM, Antosiewicz-Bourget J, et al. Targeted bisulfite sequencing reveals changes in DNA methylation associated with nuclear reprogramming. Nature Biotechnology. 2009;27(4):353–360. doi: 10.1038/nbt.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Costello JF, Fruhwald MC, Smiraglia DJ, Rush LJ, Robertson GP, Gao X, et al. Aberrant CpG-island methylation has non-random and tumour-type-specific patterns. Nature Genetics. 2000;24(2):132–138. doi: 10.1038/72785. [DOI] [PubMed] [Google Scholar]
- 196.Gama-Sosa MA, Midgett RM, Slagel VA, Githens S, Kuo KC, Gehrke CW, et al. Tissue-specific differences in DNA methylation in various mammals. Biochimica et Biophysica Acta. 1983;740(2):212–219. doi: 10.1016/0167-4781(83)90079-9. [DOI] [PubMed] [Google Scholar]
- 197.Richardson B. Impact of aging on DNA methylation. Ageing Research Reviews. 2003;2(3):245–261. doi: 10.1016/s1568-1637(03)00010-2. [DOI] [PubMed] [Google Scholar]
- 198.Eckhardt F, Beck S, Gut IG, Berlin K. Future potential of the human epigenome project. Expert Review of Molecular Diagnostics. 2004;4(5):609–618. doi: 10.1586/14737159.4.5.609. [DOI] [PubMed] [Google Scholar]
- 199.Kohda M, Hoshiya H, Katoh M, Tanaka I, Masuda R, Takemura T, et al. Frequent loss of imprinting of IGF2 and MEST in lung adenocarcinoma. Molecular Carcinogenesis. 2001;31(4):184–191. doi: 10.1002/mc.1053. [DOI] [PubMed] [Google Scholar]
- 200.Kondo M, Suzuki H, Ueda R, Osada H, Takagi K, Takahashi T. Frequent loss of imprinting of the H19 gene is often associated with its overexpression in human lung cancers. Oncogene. 1995;10(6):1193–1198. [PubMed] [Google Scholar]
- 201.Rainier S, Johnson LA, Dobry CJ, Ping AJ, Grundy PE, Feinberg AP. Relaxation of imprinted genes in human cancer. Nature. 1993;362(6422):747–749. doi: 10.1038/362747a0. [DOI] [PubMed] [Google Scholar]
- 202.Pal N, Wadey RB, Buckle B, Yeomans E, Pritchard J, Cowell JK. Preferential loss of maternal alleles in sporadic Wilms’ tumour. Oncogene. 1990;5(11):1665–1668. [PubMed] [Google Scholar]
- 203.Schroeder WT, Chao LY, Dao DD, Strong LC, Pathak S, Riccardi V, et al. Nonrandom loss of maternal chromosome 11 alleles in Wilms tumors. American Journal of Human Genetics. 1987;40(5):413–420. [PMC free article] [PubMed] [Google Scholar]
- 204.Scrable H, Cavenee W, Ghavimi F, Lovell M, Morgan K, Sapienza C. A model for embryonal rhabdomyosarcoma tumorigenesis that involves genome imprinting. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(19):7480–7484. doi: 10.1073/pnas.86.19.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gaudet F, Hodgson JG, Eden A, Jackson-Grusby L, Dausman J, Gray JW, et al. Induction of tumors in mice by genomic hypomethylation. Science. 2003;300(5618):489–492. doi: 10.1126/science.1083558. [DOI] [PubMed] [Google Scholar]
- 206.Rizwana R, Hahn PJ. CpG methylation reduces genomic instability. Journal of Cell Science. 1999;112(Pt 24):4513–4519. doi: 10.1242/jcs.112.24.4513. [DOI] [PubMed] [Google Scholar]
- 207.Daskalos A, Nikolaidis G, Xinarianos G, Savvari P, Cassidy A, Zakopoulou R, et al. Hypomethylation of retrotransposable elements correlates with genomic instability in non-small cell lung cancer. International Journal of Cancer. 2009;124(1):81–87. doi: 10.1002/ijc.23849. [DOI] [PubMed] [Google Scholar]
- 208.Walsh CP, Chaillet JR, Bestor TH. Transcription of IAP endogenous retroviruses is constrained by cytosine methylation. Nature Genetics. 1998;20(2):116–117. doi: 10.1038/2413. [DOI] [PubMed] [Google Scholar]
- 209.Chalitchagorn K, Shuangshoti S, Hourpai N, Kongruttanachok N, Tangkijvanich P, Thong-ngam D, et al. Distinctive pattern of LINE-1 methylation level in normal tissues and the association with carcinogenesis. Oncogene. 2004;23(54):8841–8846. doi: 10.1038/sj.onc.1208137. [DOI] [PubMed] [Google Scholar]
- 210.Rauch TA, Zhong X, Wu X, Wang M, Kernstine KH, Wang Z, et al. High-resolution mapping of DNA hypermethylation and hypomethylation in lung cancer. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(1):252–257. doi: 10.1073/pnas.0710735105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Groudine M, Eisenman R, Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981;292(5821):311–317. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- 212.Wilson IM, Davies JJ, Weber M, Brown CJ, Alvarez CE, MacAulay C, et al. Epigenomics: mapping the methylome. Cell Cycle. 2006;5(2):155–158. doi: 10.4161/cc.5.2.2367. [DOI] [PubMed] [Google Scholar]
- 213.Cadieux B, Ching TT, VandenBerg SR, Costello JF. Genome-wide hypomethylation in human glioblastomas associated with specific copy number alteration, methylenete-trahydrofolate reductase allele status, and increased proliferation. Cancer Research. 2006;66(17):8469–8476. doi: 10.1158/0008-5472.CAN-06-1547. [DOI] [PubMed] [Google Scholar]
- 214.Zabarovsky ER, Lerman MI, Minna JD. Tumor suppressor genes on chromosome 3p involved in the pathogenesis of lung and other cancers. Oncogene. 2002;21(45):6915–6935. doi: 10.1038/sj.onc.1205835. [DOI] [PubMed] [Google Scholar]
- 215.Belinsky SA, Palmisano WA, Gilliland FD, Crooks LA, Divine KK, Winters SA, et al. Aberrant promoter methylation in bronchial epithelium and sputum from current and former smokers. Cancer Research. 2002;62(8):2370–2377. [PubMed] [Google Scholar]
- 216.Palmisano WA, Divine KK, Saccomanno G, Gilliland FD, Baylin SB, Herman JG, et al. Predicting lung cancer by detecting aberrant promoter methylation in sputum. Cancer Research. 2000;60(21):5954–5958. [PubMed] [Google Scholar]
- 217.Belinsky SA. Gene-promoter hypermethylation as a biomarker in lung cancer. Nature Reviews Cancer. 2004;4(9):707–717. doi: 10.1038/nrc1432. [DOI] [PubMed] [Google Scholar]
- 218.Tessema M, Willink R, Do K, Yu YY, Yu W, Machida EO, et al. Promoter methylation of genes in and around the candidate lung cancer susceptibility locus 6q23-25. Cancer Research. 2008;68(6):1707–1714. doi: 10.1158/0008-5472.CAN-07-6325. [DOI] [PubMed] [Google Scholar]
- 219.Heintzman ND, Hon GC, Hawkins RD, Kheradpour P, Stark A, Harp LF, et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature. 2009;459(7243):108–112. doi: 10.1038/nature07829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Komashko VM, Acevedo LG, Squazzo SL, Iyengar SS, Rabinovich A, O’Geen H, et al. Using ChIP-chip technology to reveal common principles of transcriptional repression in normal and cancer cells. Genome Research. 2008;18(4):521–532. doi: 10.1101/gr.074609.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ke XS, Qu Y, Rostad K, Li WC, Lin B, Halvorsen OJ, et al. Genome-wide profiling of histone h3 lysine 4 and lysine 27 trimethylation reveals an epigenetic signature in prostate carcinogenesis. PLoS ONE. 2009;4(3):e4687. doi: 10.1371/journal.pone.0004687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kondo Y, Shen L, Cheng AS, Ahmed S, Boumber Y, Charo C, et al. Gene silencing in cancer by histone H3 lysine 27 trimethylation independent of promoter DNA methylation. Nature Genetics. 2008;40(6):741–750. doi: 10.1038/ng.159. [DOI] [PubMed] [Google Scholar]
- 223.Yu J, Rhodes DR, Tomlins SA, Cao X, Chen G, Mehra R, et al. A polycomb repression signature in metastatic prostate cancer predicts cancer outcome. Cancer Research. 2007;67(22):10657–10663. doi: 10.1158/0008-5472.CAN-07-2498. [DOI] [PubMed] [Google Scholar]
- 224.Wu J, Wang SH, Potter D, Liu JC, Smith LT, Wu YZ, et al. Diverse histone modifications on histone 3 lysine 9 and their relation to DNA methylation in specifying gene silencing. BMC Genomics. 2007;8:131. doi: 10.1186/1471-2164-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Krivtsov AV, Feng Z, Lemieux ME, Faber J, Vempati S, Sinha AU, et al. H3K79 methylation profiles define murine and human MLL-AF4 leukemias. Cancer Cell. 2008;14(5):355–368. doi: 10.1016/j.ccr.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lin B, Wang J, Hong X, Yan X, Hwang D, Cho JH, et al. Integrated expression profiling and ChIP-seq analyses of the growth inhibition response program of the androgen receptor. PLoS ONE. 2009;4(8):e6589. doi: 10.1371/journal.pone.0006589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Fullwood MJ, Liu MH, Pan YF, Liu J, Xu H, Mohamed YB, et al. An oestrogen-receptor-alpha-bound human chromatin interactome. Nature. 2009;462(7269):58–64. doi: 10.1038/nature08497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Coe BP, Chari R, Lockwood WW, Lam WL. Evolving strategies for global gene expression analysis of cancer. Journal of Cellular Physiology. 2008;217(3):590–597. doi: 10.1002/jcp.21554. [DOI] [PubMed] [Google Scholar]
- 229.Liang P, Pardee AB. Analysing differential gene expression in cancer. Nature Reviews Cancer. 2003;3(11):869–876. doi: 10.1038/nrc1214. [DOI] [PubMed] [Google Scholar]
- 230.Nevins JR, Potti A. Mining gene expression profiles: Expression signatures as cancer phenotypes. Nature Reviews Genetics. 2007;8(8):601–609. doi: 10.1038/nrg2137. [DOI] [PubMed] [Google Scholar]
- 231.Pollack JR, Sorlie T, Perou CM, Rees CA, Jeffrey SS, Lonning PE, et al. Microarray analysis reveals a major direct role of DNA copy number alteration in the transcriptional program of human breast tumors. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(20):12963–12968. doi: 10.1073/pnas.162471999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Heidenblad M, Lindgren D, Veltman JA, Jonson T, Mahlamaki EH, Gorunova L, et al. Microarray analyses reveal strong influence of DNA copy number alterations on the transcriptional patterns in pancreatic cancer: implications for the interpretation of genomic amplifications. Oncogene. 2005;24(10):1794–1801. doi: 10.1038/sj.onc.1208383. [DOI] [PubMed] [Google Scholar]
- 233.Hyman E, Kauraniemi P, Hautaniemi S, Wolf M, Mousses S, Rozenblum E, et al. Impact of DNA amplification on gene expression patterns in breast cancer. Cancer Research. 2002;62(21):6240–6245. [PubMed] [Google Scholar]
- 234.Wolf M, Mousses S, Hautaniemi S, Karhu R, Huusko P, Allinen M, et al. High-resolution analysis of gene copy number alterations in human prostate cancer using CGH on cDNA microarrays: Impact of copy number on gene expression. Neoplasia. 2004;6(3):240–247. doi: 10.1593/neo.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Adelaide J, Finetti P, Bekhouche I, Repellini L, Geneix J, Sircoulomb F, et al. Integrated profiling of basal and luminal breast cancers. Cancer Research. 2007;67(24):11565–11575. doi: 10.1158/0008-5472.CAN-07-2536. [DOI] [PubMed] [Google Scholar]
- 236.Broet P, Camilleri-Broet S, Zhang S, Alifano M, Bangarusamy D, Battistella M, et al. Prediction of clinical outcome in multiple lung cancer cohorts by integrative genomics: Implications for chemotherapy selection. Cancer Research. 2009;69(3):1055–1062. doi: 10.1158/0008-5472.CAN-08-1116. [DOI] [PubMed] [Google Scholar]
- 237.Chin K, DeVries S, Fridlyand J, Spellman PT, Roydasgupta R, Kuo WL, et al. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell. 2006;10(6):529–541. doi: 10.1016/j.ccr.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 238.Natrajan R, Weigelt B, Mackay A, Geyer FC, Grigoriadis A, Tan DS, et al. An integrative genomic and transcriptomic analysis reveals molecular pathways and networks regulated by copy number aberrations in basal-like, HER2 and luminal cancers. Breast Cancer Research and Treatment. 2009 doi: 10.1007/s10549-009-0501-3. doi: s00280-009-1073-y/s10549-009-0501-3. [DOI] [PubMed] [Google Scholar]
- 239.Deng S, Calin GA, Croce CM, Coukos G, Zhang L. Mechanisms of microRNA deregulation in human cancer. Cell Cycle. 2008;7(17):2643–2646. doi: 10.4161/cc.7.17.6597. [DOI] [PubMed] [Google Scholar]
- 240.Kuo KT, Guan B, Feng Y, Mao TL, Chen X, Jinawath N, et al. Analysis of DNA copy number alterations in ovarian serous tumors identifies new molecular genetic changes in low-grade and high-grade carcinomas. Cancer Research. 2009;69(9):4036–4042. doi: 10.1158/0008-5472.CAN-08-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lionetti M, Agnelli L, Mosca L, Fabris S, Andronache A, Todoerti K, et al. Integrative high-resolution microarray analysis of human myeloma cell lines reveals deregulated miRNA expression associated with allelic imbalances and gene expression profiles. Genes, Chromosomes, and Cancer. 2009;48(6):521–531. doi: 10.1002/gcc.20660. [DOI] [PubMed] [Google Scholar]
- 242.Starczynowski DT, Kuchenbauer F, Argiropoulos B, Sung S, Morin R, Muranyi A, et al. Identification of miR-145 and miR-146a as mediators of the 5q-syndrome phenotype. Natural Medicines. 2009;16:49–58. doi: 10.1038/nm.2054. [DOI] [PubMed] [Google Scholar]
- 243.Zhang L, Volinia S, Bonome T, Calin GA, Greshock J, Yang N, et al. Genomic and epigenetic alterations deregulate microRNA expression in human epithelial ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(19):7004–7009. doi: 10.1073/pnas.0801615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nature Reviews Cancer. 2006;6(11):857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 245.Nicoloso MS, Spizzo R, Shimizu M, Rossi S, Calin GA. MicroRNAs—the micro steering wheel of tumour metastases. Nature Reviews Cancer. 2009;9(4):293–302. doi: 10.1038/nrc2619. [DOI] [PubMed] [Google Scholar]
- 246.Wolf NG, Farver C, Abdul-Karim FW, Schwartz S. Analysis of microsatellite instability and X-inactivation in ovarian borderline tumors lacking numerical abnormalities by comparative genomic hybridization. Cancer Genetics and Cytogenetics. 2003;145(2):133–138. doi: 10.1016/s0165-4608(03)00093-1. [DOI] [PubMed] [Google Scholar]
- 247.Olson P, Lu J, Zhang H, Shai A, Chun MG, Wang Y, et al. MicroRNA dynamics in the stages of tumorigenesis correlate with hallmark capabilities of cancer. Genes and Development. 2009;23(18):2152–2165. doi: 10.1101/gad.1820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Garzon R, Calin GA, Croce CM. MicroRNAs in cancer. Annual Review of Medicine. 2009;60:167–179. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 249.Lujambio A, Esteller M. How epigenetics can explain human metastasis: A new role for microRNAs. Cell Cycle. 2009;8(3):377–382. doi: 10.4161/cc.8.3.7526. [DOI] [PubMed] [Google Scholar]
- 250.Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P, et al. MicroRNA signatures in human ovarian cancer. Cancer Research. 2007;67(18):8699–8707. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- 251.Lujambio A, Esteller M. CpG island hypermethylation of tumor suppressor microRNAs in human cancer. Cell Cycle. 2007;6(12):1455–1459. [PubMed] [Google Scholar]
- 252.Lujambio A, Ropero S, Ballestar E, Fraga MF, Cerrato C, Setien F, et al. Genetic unmasking of an epigenetically silenced microRNA in human cancer cells. Cancer Research. 2007;67(4):1424–1429. doi: 10.1158/0008-5472.CAN-06-4218. [DOI] [PubMed] [Google Scholar]
- 253.Guil S, Esteller M. DNA methylomes, histone codes and miRNAs: Tying it all together. International Journal of Biochemistry and Cell Biology. 2009;41(1):87–95. doi: 10.1016/j.biocel.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 254.Sadikovic B, Yoshimoto M, Chilton-MacNeill S, Thorner P, Squire JA, Zielenska M. Identification of interactive networks of gene expression associated with osteosarcoma oncogenesis by integrated molecular profiling. Human Molecular Genetics. 2009;18(11):1962–1975. doi: 10.1093/hmg/ddp117. [DOI] [PubMed] [Google Scholar]
- 255.Adamovic T, Trosso F, Roshani L, Andersson L, Petersen G, Rajaei S, et al. Oncogene amplification in the proximal part of chromosome 6 in rat endometrial adenocarcinoma as revealed by combined BAC/PAC FISH, chromosome painting, zoo-FISH, and allelotyping. Genes, Chromosomes, and Cancer. 2005;44(2):139–153. doi: 10.1002/gcc.20220. [DOI] [PubMed] [Google Scholar]
- 256.Ferrandina G, Mey V, Nannizzi S, Ricciardi S, Petrillo M, Ferlini C, et al. Expression of nucleoside transporters, deoxycitidine kinase, ribonucleotide reductase regulatory subunits, and gemcitabine catabolic enzymes in primary ovarian cancer. Cancer Chemotherapy and Pharmacology. 2009 doi: 10.1007/s00280-009-1073-y. doi:s00280-009-1073-y/s00280-009-1073-y. [DOI] [PubMed] [Google Scholar]
- 257.Fernandez-Ranvier GG, Weng J, Yeh RF, Khanafshar E, Suh I, Barker C, et al. Identification of biomarkers of adrenocortical carcinoma using genomewide gene expression profiling. Archives of Surgery. 2008;143(9):841–846. doi: 10.1001/archsurg.143.9.841. discussion 846. [DOI] [PubMed] [Google Scholar]
- 258.Segditsas S, Sieber O, Deheragoda M, East P, Rowan A, Jeffery R, et al. Putative direct and indirect Wnt targets identified through consistent gene expression changes in APC-mutant intestinal adenomas from humans and mice. Human Molecular Genetics. 2008;17(24):3864–3875. doi: 10.1093/hmg/ddn286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Joshi MD, Ahmad R, Yin L, Raina D, Rajabi H, Bubley G, et al. MUC1 oncoprotein is a druggable target in human prostate cancer cells. Molecular Cancer Therapeutics. 2009;8(11):3056–3065. doi: 10.1158/1535-7163.MCT-09-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Khodarev NN, Pitroda SP, Beckett MA, MacDermed DM, Huang L, Kufe DW, et al. MUC1-induced transcriptional programs associated with tumorigenesis predict outcome in breast and lung cancer. Cancer Research. 2009;69(7):2833–2837. doi: 10.1158/0008-5472.CAN-08-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Senapati S, Das S, Batra SK. Mucin-interacting proteins: from function to therapeutics. Trends in Biochemical Sciences. 2009 doi: 10.1016/j.tibs.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Buys TP, Aviel-Ronen S, Waddell TK, Lam WL, Tsao MS. Defining genomic alteration boundaries for a combined small cell and non-small cell lung carcinoma. Journal of Thoracic Oncology. 2009;4(2):227–239. doi: 10.1097/JTO.0b013e3181952678. [DOI] [PubMed] [Google Scholar]
- 263.Brommesson S, Jonsson G, Strand C, Grabau D, Malmstrom P, Ringner M, et al. Tiling array-CGH for the assessment of genomic similarities among synchronous unilateral and bilateral invasive breast cancer tumor pairs. BMC Clinical Pathology. 2008;8:6. doi: 10.1186/1472-6890-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Kawanishi H, Takahashi T, Ito M, Matsui Y, Watanabe J, Ito N, et al. Genetic analysis of multifocal superficial urothelial cancers by array-based comparative genomic hybridisation. British Journal of Cancer. 2007;97(2):260–266. doi: 10.1038/sj.bjc.6603850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mhawech-Fauceglia P, Rai H, Nowak N, Cheney RT, Rodabaugh K, Lele S, et al. The use of array-based comparative genomic hybridization (a-CGH) to distinguish metastatic from primary synchronous carcinomas of the ovary and the uterus. Histopathology. 2008;53(4):490–495. doi: 10.1111/j.1365-2559.2008.03107.x. [DOI] [PubMed] [Google Scholar]
- 266.Nakano H, Soda H, Nakamura Y, Uchida K, Takasu M, Nakatomi K, et al. Different epidermal growth factor receptor gene mutations in a patient with 2 synchronous lung cancers. Clinical Lung Cancer. 2007;8(9):562–564. doi: 10.3816/CLC.2007.n.043. [DOI] [PubMed] [Google Scholar]
- 267.Ryoo BY, Na II, Yang SH, Koh JS, Kim CH, Lee JC. Synchronous multiple primary lung cancers with different response to gefitinib. Lung Cancer. 2006;53(2):245–248. doi: 10.1016/j.lungcan.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 268.Speel EJ, van de Wouw AJ, Claessen SM, Haesevoets A, Hopman AH, van der Wurff AA, et al. Molecular evidence for a clonal relationship between multiple lesions in patients with unknown primary adenocarcinoma. International Journal of Cancer. 2008;123(6):1292–1300. doi: 10.1002/ijc.23616. [DOI] [PubMed] [Google Scholar]
- 269.Wa CV, DeVries S, Chen YY, Waldman FM, Hwang ES. Clinical application of array-based comparative genomic hybridization to define the relationship between multiple synchronous tumors. Modern Pathology. 2005;18(4):591–597. doi: 10.1038/modpathol.3800332. [DOI] [PubMed] [Google Scholar]
- 270.Agelopoulos K, Tidow N, Korsching E, Voss R, Hinrichs B, Brandt B, et al. Molecular cytogenetic investigations of synchronous bilateral breast cancer. Journal of Clinical Pathology. 2003;56(9):660–665. doi: 10.1136/jcp.56.9.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Whitehurst AW, Bodemann BO, Cardenas J, Ferguson D, Girard L, Peyton M, et al. Synthetic lethal screen identification of chemosensitizer loci in cancer cells. Nature. 2007;446(7137):815–819. doi: 10.1038/nature05697. [DOI] [PubMed] [Google Scholar]
- 272.Barbie DA, Tamayo P, Boehm JS, Kim SY, Moody SE, Dunn IF, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462(7269):108–112. doi: 10.1038/nature08460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Berns K, Hijmans EM, Mullenders J, Brummelkamp TR, Velds A, Heimerikx M, et al. A large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature. 2004;428(6981):431–437. doi: 10.1038/nature02371. [DOI] [PubMed] [Google Scholar]
- 274.Gobeil S, Zhu X, Doillon CJ, Green MR. A genome-wide shRNA screen identifies GAS1 as a novel melanoma metastasis suppressor gene. Genes and Development. 2008;22(21):2932–2940. doi: 10.1101/gad.1714608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Luo B, Cheung HW, Subramanian A, Sharifnia T, Okamoto M, Yang X, et al. Highly parallel identification of essential genes in cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(51):20380–20385. doi: 10.1073/pnas.0810485105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Luo J, Emanuele MJ, Li D, Creighton CJ, Schlabach MR, Westbrook TF, et al. A genome-wide RNAi screenidentifies multiple synthetic lethal interactions with the Ras oncogene. Cell. 2009;137(5):835–848. doi: 10.1016/j.cell.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Moffat J, Grueneberg DA, Yang X, Kim SY, Kloepfer AM, Hinkle G, et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124(6):1283–1298. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 278.Scholl C, Frohling S, Dunn IF, Schinzel AC, Barbie DA, Kim SY, et al. Synthetic lethal interaction between oncogenic KRAS dependency and STK33 suppression in human cancer cells. Cell. 2009;137(5):821–834. doi: 10.1016/j.cell.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 279.Silva JM, Marran K, Parker JS, Silva J, Golding M, Schlabach MR, et al. Profiling essential genes in human mammary cells by multiplex RNAi screening. Science. 2008;319(5863):617–620. doi: 10.1126/science.1149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Apweiler R, Aslanidis C, Deufel T, Gerstner A, Hansen J, Hochstrasser D, et al. Approaching clinical proteomics: current state and future fields of application in cellular proteomics. Cytometry A. 2009;75(10):816–832. doi: 10.1002/cyto.a.20779. [DOI] [PubMed] [Google Scholar]
- 281.Apweiler R, Aslanidis C, Deufel T, Gerstner A, Hansen J, Hochstrasser D, et al. Approaching clinical proteomics: Current state and future fields of application in fluid proteomics. Clinical Chemistry and Laboratory Medicine. 2009;47(6):724–744. doi: 10.1515/CCLM.2009.167. [DOI] [PubMed] [Google Scholar]
- 282.Peng XQ, Wang F, Geng X, Zhang WM. Current advances in tumor proteomics and candidate biomarkers for hepatic cancer. Expert Review of Proteomics. 2009;6(5):551–561. doi: 10.1586/epr.09.72. [DOI] [PubMed] [Google Scholar]
- 283.Tainsky MA. Genomic and proteomic biomarkers for cancer: A multitude of opportunities. Biochimica et Biophysica Acta. 2009;1796(2):176–193. doi: 10.1016/j.bbcan.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Zamo A, Cecconi D. Proteomic analysis of lymphoid and haematopoietic neoplasms: There’s more than biomarker discovery. J Proteomics. 2009 doi: 10.1016/j.jprot.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 285.Griffin JL, Kauppinen RA. A metabolomics perspective of human brain tumours. FEBS Journal. 2007;274(5):1132–1139. doi: 10.1111/j.1742-4658.2007.05676.x. [DOI] [PubMed] [Google Scholar]
- 286.Spratlin JL, Serkova NJ, Eckhardt SG. Clinical applications of metabolomics in oncology: A review. Clinical Cancer Research. 2009;15(2):431–440. doi: 10.1158/1078-0432.CCR-08-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457(7231):910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]