Abstract
BACKGROUND
Life history models suggest that biological preparation for current versus longer term reproduction is favored in environments of adversity. In this context, we present a model of reproductive aging in which environmental adversity is proposed to increase the number of growing follicles at the cost of hastening the depletion of the ovarian reserve over time. We evaluated this model by examining psychological stress in relation to reproductive aging indexed by antral follicle count (AFC), a marker of total ovarian reserve. We hypothesized that stress would be related to (i) higher AFC in younger women, reflecting greater reproductive readiness as well as (ii) greater AFC loss across women, reflecting more accelerated reproductive aging.
METHODS
In a multi-ethnic, community sample of 979 participants [ages 25–45 (mean (standard deviation) = 35.2 (5.5)); 27.5% Caucasian] in the Ovarian Aging study, an investigation of the correlates of reproductive aging, the interaction of age-x-stress was assessed in relation to AFC to determine whether AFC and AFC loss varied across women experiencing differing levels of stress. Stress was assessed by the perceived stress scale and AFC was assessed by summing the total number of antral follicles visible by transvaginal ultrasound.
RESULTS
In linear regression examining AFC as the dependent variable, covariates (race/ethnicity, socio-economic status, menarcheal age, hormone-containing medication for birth control, parity, cigarette smoking, bodymass index, waist-to-hip ratio) and age were entered on step 1, stress on step 2 and the interaction term (age-x-stress) on step 3. On step 3, significant main effects showed that older age was related to lower AFC (b = −0.882, P = 0.000) and greater stress was related to higher AFC (b = 0.545, P = 0.005). Follow-up analyses showed that the main effect of stress on AFC was present in the younger women only. A significant interaction term (b = −0.036, P = 0.031) showed the relationship between age and AFC varied as function of stress. When the sample was divided into tertiles of stress, the average follicle loss was −0.781, −0.842 and −0.994 follicles/year in the low-, mid- and high-stress groups, respectively.
CONCLUSIONS
Psychological stress was related to higher AFC among younger women and greater AFC decline across women, suggesting that greater stress may enhance reproductive readiness in the short term at the cost of accelerating reproductive aging in the long term. Findings are preliminary, however, due to the cross-sectional nature of the current study.
Keywords: life history theory, psychological stress, reproductive aging, ovarian reserve, antral follicle count
Introduction
Life history theory suggests that rates of female reproductive senescence and the timing of major reproductive events are shaped by selection pressures to produce the largest number of viable offspring (Chisholm, 1993; Ellis et al., 2009). In environments with finite resources, investments in reproduction are proposed to result in trade-offs, curtailing the potential for investments in other areas of growth and development. As reviewed in Ellis (2004), several lower order theoretical models have been developed that apply life history principles to understanding the specific phenomenon of pubertal timing. In particular, the psychosocial acceleration hypothesis proposes that environmental cues shape reproductive development by biasing girls toward greater reproductive readiness when the environment signals that the risks associated with the delay of reproduction outweigh the potential costs associated with earlier reproductive development (Belsky et al., 1991; Ellis, 2004). Empirical support for the psychosocial acceleration hypothesis is provided by numerous longitudinal investigations in which girls living in more adverse circumstances were found to experience puberty earlier than their more advantaged peers (Moffitt et al., 1992; Wierson et al., 1993; Campbell and Udry, 1995; Graber et al., 1995; Ellis et al., 1999; Ellis and Garber, 2000; Belsky et al., 2007; Ellis and Essex, 2007; Saxbe and Repetti, 2009). The costs of earlier reproductive maturation among these girls include shorter adulthood height as well as several problematic reproductive (e.g. teenage pregnancy) and psychosocial (e.g. depression/anxiety) outcomes (Fisher et al., 1991; Biro et al., 2001; Deardorff et al., 2005; Mendle et al., 2007; Dunbar et al., 2008).
In the current investigation, principles of life history theory and specifically the psychosocial acceleration hypothesis were applied to examine variability in reproductive aging, a process characterized by ovarian follicle loss leading to the eventual cessation of menses (i.e. menopause). Notably, indicators of greater environmental adversity have been linked to younger age at menopause. Epidemiological studies have found that independently of confounding factors such as cigarette smoking and bodymass index (BMI), aspects of the physical environment (e.g. crowded living conditions), markers of socio-economic status (SES) (e.g. lower educational attainment), family dynamics (e.g. early parental divorce) and psychological well-being (e.g. feeling overwhelmed or out of control) all predicted earlier onset menopause (Stanford et al., 1987; Luoto et al., 1994; Torgerson et al., 1994; Bromberger et al., 1997; Gold et al., 2001; Wise et al., 2002; Lawlor et al., 2003). If, as the psychosocial acceleration hypothesis suggests, environmental adversity biases women toward enhanced biological readiness for current versus longer term reproduction, it is possible that earlier onset menopause reflects a history of accelerated ovarian follicle loss due to stress-related increases in the number of dormant follicles leaving the finite pool of primordial follicles to enter the pool of growing follicles. That is, we forward a model of reproductive aging in which environmental adversity is proposed to promote the allocation of resources toward greater reproductive readiness via increases in the volume of growing follicles which may enhance fertility in the short term but hasten depletion of the ovarian reserve in the long term.
The proposed model makes two primary assumptions: (i) first, that the process of follicle growth (i.e. folliculogenesis) is responsive to environmental cues and (ii) secondly, that having a greater number of growing follicles is adaptive in promoting reproduction. With respect to the first assumption, we suggest that it is biologically plausible that the processes that regulate the growth of ovarian follicles are responsive to environmental cues, increasing or decreasing rates of follicle growth based on environmental demands for reproductive readiness. During folliculogenesis, dormant primordial follicles enter the pool of growing follicles, maturing through several stages of development until (through follicular atresia) only a single dominant follicle remains (McGee and Hsueh, 2000). The movement of dormant primordial follicles into the growing follicle pool, termed initial recruitment (McGee and Hsueh, 2000), may be regarded as a decision point from an evolutionary perspective. A ‘decision’ is made to recruit a particular number of primordial follicles based in part on environmental demands necessitating the prioritization of current versus longer term reproduction. A larger number of recruited follicles may enhance fertility at the cost of increased follicle loss while a smaller number of recruited follicles may limit depletion of the ovarian reserve but risk delaying reproduction. With respect to the second assumption relating follicle number to reproductive capacity, recent evidence has demonstrated that having a greater number of antral follicles, the subset of growing follicles rescued during cyclic recruitment, was related to an increased probability of conception in a non-infertile, community-based sample of women (Rosen et al., 2011).
The examination of variability in reproductive aging has been limited by the availability of methodological tools to adequately measure ovarian reserve. Until recently, approaches largely relied on observations of changes in menstrual cycle and hormonal indicators that are only evident after a woman has already experienced a significant decline in her reproductive capacity (Soules et al., 2001). In contrast, antral follicle count (AFC), defined as the number of follicles (fluid collections between 2 and 10 mm) visible by transvaginal ultrasound (TVUS), reflects the number of dormant primordial follicles remaining in the ovary (Hansen et al., 2007, 2008). As such, AFC provides a marker of total follicular reserve that can be assessed in women of all ages. AFC has been shown to covary with chronological age (Reuss et al., 1996a, b; Scheffer et al., 1999; Rosen et al., 2010) with AFC found to be 60% higher on average among women aged 22 compared with those aged 42 (Reuss et al., 1996a, b). In addition, AFC predicts ovarian response in treatments using assisted reproductive technologies (Frattarelli et al., 2003; Klinkert et al., 2005; Aflatoonian et al., 2009), and, in statistical models, predicts menopausal timing (Broekmans et al., 2004; Giacobbe et al., 2004; Hansen et al., 2008). While AFC is stable across consecutive menstrual cycles and across the follicular and luteal phases of the menstrual cycle for an individual woman (Pache et al., 1990; Scheffer et al., 1999, 2002), it has been shown to be widely variable between women at any given age. For example, in a subsample of 33-year old women, the range in AFC was 7–38 (Rosen et al., 2010).
In the current study, the proposed model was tested by examining women's reports of psychological stress in relation to reproductive aging in a multi-ethnic sample of 979 women ages 25–45 derived from the Ovarian Aging (OVA) study. We predicted that greater perceptions of stress would relate to greater reproductive readiness as indexed by higher AFCs among younger women as well as more accelerated reproductive aging as indexed by higher rates of AFC loss across women. Hypotheses were tested cross-sectionally by examining the interaction of age and self-reported perceptions of stress to determine whether AFC and AFC decline would vary significantly across women experiencing differing levels of stress. All analyses included statistical adjustment for potential confounding factors, including race/ethnicity, SES, menarcheal age, use of hormone-containing medication for birth control, parity, cigarette smoking, BMI and waist-to-hip ratio.
Materials and Methods
Participants
The current sample was derived from the OVA study, a community-based investigation of reproductive aging, which includes women belonging to Kaiser Permanente (KP) of Northern California, a large, integrated health-care delivery system that provides medical care to approximately one-third of the population of Northern California. Comparison of the KP membership with the population of Northern California indicates that the KP membership is generally representative in its socio-demographic and health-related characteristics, particularly if the comparison is limited to those with health insurance (Gordon 2006). Selection criteria for the OVA study were that participants be between ages 25 and 45, have regular menses and have their uterus and both ovaries intact. All participants were required to self-identify as one of five race/ethnicities: Caucasian, African-American, Latina, Chinese or Filipina and speak/read English, Spanish or Cantonese. Participants were excluded if they reported a major medical illness, were taking medications affecting the menstrual cycle within the 3 months prior to study participation or were currently pregnant or breastfeeding. The OVA study protocol included an in-person interview, anthropometric assessment, TVUS and blood draw. Of 1019 women who completed the OVA study protocol, 979 participants (Caucasian: n = 269; African-American: n = 234; Latina: n = 220; Chinese: n = 216 and Filipina: n = 40) were retained for inclusion in the current analysis. Excluded women were those missing TVUS data for the following reasons: presence of ovarian cyst >30 mm on either ovary invalidating AFC measurement (n = 24); presence of other masses (e.g. fibroid) obstructing view of either ovary (n = 10); obesity or anatomical anomalies preventing visualization of either ovary (n = 4) and loss of ultrasound images due to technical error (n = 2). The study protocol was approved by the University of California San Francisco Committee on Human Research as well as the KP of Northern California Institutional Review Board. Informed, written consent was obtained from all study participants.
Measures
Reproductive aging
TVUS assessment of AFC was performed between menstrual cycle days 2 and 4 by one of two reproductive endocrinologists (M.I.C., M.P.R.). The transverse, longitudinal and anteroposterior diameters of each ovary were measured with electronic calipers using a Shimadzu SDU-450XL machine with a variable 4- to 8-mHz vaginal transducer. Follicles (defined as all echo-free structures in the ovaries) with a mean diameter across two dimensions of 2–10 mm were counted. Each measurement was taken twice and the average was taken. The total number of follicles across both ovaries was summed to calculate AFC. Evaluation of a sub-sample of 50 OVA study participants showed that inter-rater reliability between the two reproductive endocrinologists was excellent (r = 0.92) as was test-retest reliability for each reproductive endocrinologist measured over 2 consecutive months (average r = 0.91).
Psychological stress
Psychological stress was evaluated using the 4-item perceived stress scale (PSS), a self-report questionnaire assessing the extent to which individuals appraise their lives as being stressful over the past month (Cohen et al., 1983; Cohen and Williamson, 1988). Two positively worded items (reversed scored) and two negatively worded items are scored on a 5-point scale (0 = never, 1 = almost never, 2 = sometimes, 3 = fairly often, 4 = very often) and summed to produce a total score. The two positively worded items are ‘…how often have you felt confident about your ability to handle your personal problems?’ and ‘… how often have you felt that things were going your way?’ The two negatively worded items are ‘… how often have you felt that you were unable to control the important things in your life?’ and ‘… how often have you felt difficulties were piling up so high that you could not overcome them?’ Higher scores indicate greater perceived stress. The PSS has been widely used and its internal reliability and construct validity are well established (Cohen et al., 1983; Cohen and Williamson, 1988; Cohen and Janicki-Deverts, 2012). In the present sample, internal reliability was adequate according to Cronbach's coefficient alpha (α = 0.75). Additionally, principal components analysis showed the presence of a single factor to account for 57.1% of the total variability in the individual questionnaire items. Factor loadings for the individual items were all high, ranging between 0.72 and 0.77.
Although the PSS asks respondents to reference the past month in reporting their perceptions of stress, an abundant literature suggests that PSS scores may reflect stress experienced more chronically, supporting the validity of its examination in relation to reproductive aging in the present study. Stress, as measured by the PSS, has been related to a variety of health outcomes (e.g. health behaviors, risk factors for cardiovascular disease, infectious diseases and mortality; Cohen et al., 1999; Ng and Jeffery, 2003; Fredman et al., 2010; Yu et al., 2010) as well as biological processes underlying risk for disease (e.g. cellular aging, anti-body responses to vaccination, wound-healing and chronic inflammation; Glaser et al., 1999; Epel et al., 2004; Moynihan et al., 2004; McDade et al., 2006; Li et al., 2007; Parks et al., 2009). In addition, the PSS is commonly used to monitor changes in stress levels in response to stress-reduction programs (Kirby et al., 2006; Lane et al., 2007), with some of these interventions designed to specifically target reductions in stress as a way of improving parameters of health and long-term prognoses for illness (Cruess et al., 1999; Michalsen et al., 2005).
Statistical analyses
In cross-sectional analyses, hierarchical linear regression was performed to test the interaction of age and stress in predicting AFC. The use of linear regression was justified on the basis of an earlier report showing rates of AFC loss to be linear (Rosen et al., 2010). Covariates as well as age were entered on the first step, stress was entered on the second step and the interaction term (age-x-stress) was entered on the third step of the regression equation. Variables included in the interaction term were centered (Aiken and West, 1991). Age was centered at age 25 and stress was centered at its mean, enabling, in the context of a significant interaction term, the examination of the effect of age on AFC at the mean level of stress and the effect of stress on AFC at age 25. Age 25 was selected in accordance with the hypothesis that stress would be related to higher AFC among younger women. In addition, in the case of a significant interaction term, custom contrasts were planned to aid interpretation by estimating the effects of stress on AFC at pre-specified ages across the full age range (25, 30, 35, 40 and 45).
Covariates included race, individual-level SES, menarcheal age (in years), use of hormone-containing medication for birth control (BC; 0 = no history of use; 1 = positive history of use), parity (0 = no live births; 1 = 1+ live births), cigarette smoking (0 = never smoked; 1 = current/past smoking), BMI (weight in kg/height in m2) and waist-to-hip ratio (WHR). Race/ethnicity was dummy coded (0 versus 1) into four (k–1) variables using Caucasian as the reference group. Individual-level SES was computed by adding standardized education and household income variables. Education was coded 1=<HS/some HS; 2 = HS grad/GED; 3 = some college/AA/vocational school; 4 = college graduate; 5 = graduate school (PhD, MS); 6 = professional degree (MD, JD, DDS, MBA). Household income was coded 1=<$5000; 2 = $5000–$15 999; 3 = $16 000-$24 999; 4 = $25 000-$34 999; 5 = $35 000-$49 999; 6 = $50 000–$74 999; 7 = $75 000–$99 999; 8 = $100 000–$149,999; 9 = $150 000–$199 999; 10 = $200 000+ and divided by the number of individuals in the household who were dependent on the reported income. For three individuals who reported not knowing their household income and two individuals who refused to report their household income, only education contributed to the individual-level SES composite. BMI was normalized using a logarithmic transformation.
Results
Sample characteristics
In Table I, information pertaining to the socio-demographics, reproductive history, health behaviors and self-reported stress levels of women in the full sample (n = 979) as well as women divided into tertiles of low (n = 303), mid (n = 368) and high (n = 308) levels of stress is reported. In the full sample [27.5% Caucasian, 23.9% African-American, 22.5% Latina, 22.0% Chinese, 4.1% Filipina; ages 25–45, mean (standard deviation (SD)) = 35.2 (5.5)], 57.8% of women held a college degree or greater and 53.9% reported an annual household income of $50 000 or greater. The majority (70.1%) of women reported having ever used a hormone-containing medication for BC and 42.8% experienced at least one live birth. Regarding cigarette smoking, 23.4% smoked currently or in the past. On average, women were overweight [BMI: mean (SD) = 27.02 (6.9)], although the sample mean for WHR [mean (SD) = 0.80 (0.1)] was in the low risk range. The sample mean (SD) for self-reported stress was 4.3 (2.8), which is significantly lower (t(2404) = 3.23, P = 0.001) than the average reported in a probability sample of 1427 women [mean (SD) = 4.7 (3.1); Cohen and Williamson, 1988].
Table I.
Sample characteristics among all women and in women across tertiles of the stress distribution reflecting low, mid, and high levels of stress.
| Total (n= 979) | Low stress (n= 303) | Mid stress (n= 368) | High stress (n= 308) | Test statistic | Pa | |
|---|---|---|---|---|---|---|
| Socio-demographics | ||||||
| Age | 35.16 (5.5) | 35.70 (5.4) | 35.04 (5.6) | 34.76 (5.5) | F(2,976) = 2.37 | 0.094 |
| Caucasian (%) | 27.5 | 33.0 | 26.6 | 23.1 | χ2= 7.803 | 0.020 |
| African-American (%) | 23.9 | 24.8 | 19.3 | 28.6 | χ2= 8.110 | 0.017 |
| Latina (%) | 22.5 | 19.5 | 24.2 | 23.4 | χ2= 2.330 | 0.312 |
| Chinese (%) | 22.1 | 19.1 | 25.3 | 21.1 | χ2= 3.872 | 0.144 |
| Filipina (%) | 4.0 | 3.6 | 4.6 | 3.6 | χ2= 0.625 | 0.732 |
| Educational levelb | 3.57 (1.2) | 3.77 (1.1) | 3.61 (1.3) | 3.33 (1.2) | F(2,976) = 10.41 | 0.000 |
| Income/# of dependentsc | 3.29 (2.0) | 3.46 (1.9) | 3.38 (2.1) | 3.01 (2.0) | F(2,971) = 4.34 | 0.013 |
| Reproductive factors | ||||||
| Antral follicle count | 15.08 (9.6) | 14.50 (9.2) | 14.57 (8.9) | 16.27 (10.7) | F(2,976) = 3.43 | 0.033 |
| Menarcheal age | 12.57 (1.6) | 12.56 (1.7) | 12.64 (1.6) | 12.51 (1.5) | F(2,976) = 0.62 | 0.540 |
| BCd (% with hx of BC use) | 70.1 | 71.9 | 69.8 | 68.5 | χ2= 0.878 | 0.645 |
| Parity (% 1+ live births) | 42.8 | 41.3 | 42.1 | 45.1 | χ2= 1.048 | 0.592 |
| General health | ||||||
| Smoking (% current/past) | 23.4 | 26.7 | 20.4 | 23.7% | χ2= 3.766 | 0.152 |
| BMI | 27.02 (6.9) | 26.80 (6.9) | 26.23 (6.3) | 28.19 (7.5) | F(2,976) = 6.98 | 0.001 |
| WHR | 0.80 (0.1) | 0.79 (0.1) | 0.79 (0.1) | 0.81 (0.1) | F(2,976) = 4.12 | 0.017 |
| Psychological stress | ||||||
| PSSe (total score) | 4.25 (2.8) | 1.16 (0.8) | 4.02 (0.8) | 7.56 (1.7) | F(2,976) = 2252.1 | 0.000 |
aAll statistically significant comparisons are detailed in the following. For ANOVAs P-values are reported from post hoc comparisons. For chi-squares, P-values are corrected for alpha inflation using a modified Bonferroni correction. Race: significant differences were between low- versus high-stress groups for Caucasian (versus all other; P = 0.006) and between mid- versus high-stress groups for African-American (versus all other; P = 0.005); education: significant differences were between the low- versus high-stress groups (P = 0.000) and the low- versus mid-stress groups (P = 0.013); income: significant differences were between the low- versus high-stress groups (P = 0.024); AFC: no significant differences between the stress groups were found. BMI: significant differences were between the low- versus high-stress groups (P = 0.049) and the mid- versus high-stress groups (P = 0.002); WHR: significant differences were between the low- versus high-stress groups (P = 0.046) and the mid- versus high-stress groups (P = 0.042).
bEducation was coded 1 =<HS/some HS; 2 = HS grad/GED; 3 = some college/AA/vocational school; 4 = college graduate; 5 = graduate school (PhD, MS); 6 = professional degree (MD, JD, DDS, MBA).
cHousehold income was coded 1 =<$5000; 2 = $5000–$15 999; 3 = $16 000$–$24 999; 4 = $25 000–$34 999; 5 = $35 000–$49 999; 6 = $50 000–$74 999; 7 = $75 000–$99 999; 8 = $100 000–$149 999; 9 = $150 000–$199 999; 10 = $200 000+ and divided by the number of individuals in the household who were dependent on the reported income.
dBC = hormonal methods for BC.
ePSS = perceived stress scale; PSS score ranges were 0–2, 3–5 and 6–14 for low, mid and high levels of stress, respectively.
Comparisons of women across the low-, mid- and high-stress groups (tertiles) showed there to be proportionately fewer Caucasian women (χ2= 7.803, P = 0.020) and more African-American women (χ2= 8.110, P = 0.017; versus all others) in the high-stress group with the converse evident in the low-stress group. Significant differences between women in the low-, mid- and high-stress groups were also found on educational level (F(2,976) = 10.41, P = 0.000), household income/number of dependents (F(2,971) = 4.34, P = 0.013), AFC (F(2,976) = 3.43, P = 0.033), BMI (F(2,976) = 6.98, P = 0.001) and WHR (F(2,976) = 4.12, P = 0.017). Post hoc tests showed that significant differences in educational level were between the low- and high-stress groups (P = 0.000) as well as the mid- and high-stress groups (P = 0.013). In contrast, significant differences on household income/number of dependents were between the low- and high-stress groups only (P = 0.024). Regarding AFC, post hoc tests showed no comparisons reached statistical significance (all P's > 0.05). Finally, post hoc tests showed that significant differences in BMI were between the low- and high-stress groups (P = 0.049) as well as the mid- and high-stress groups (P = 0.002); this pattern was similar for WHR with significant differences found between the low- and high-stress groups (P = 0.046) as well as the mid- and high-stress groups (P = 0.042).
Linear regression
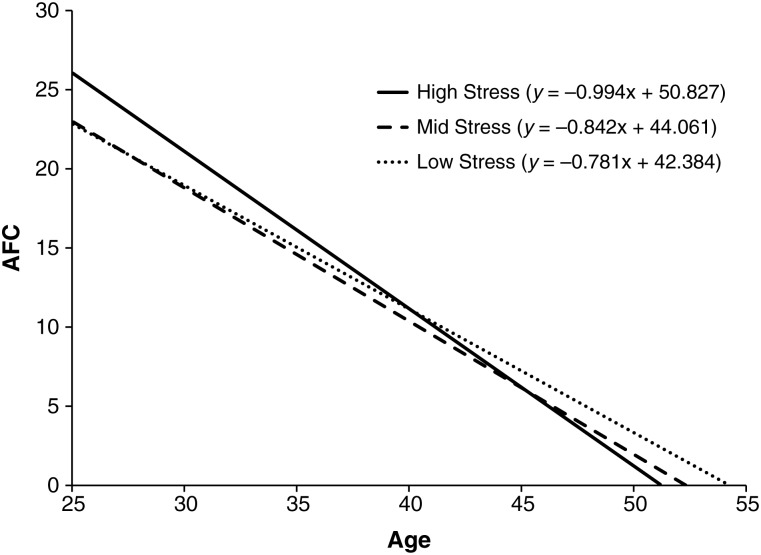
In Table II, results of analyses assessing the interaction of age and stress in relation to AFC are reported using the total score of the PSS as a continuous variable. Covariates as well as age accounted for 26.9% of the variance in AFC with stress and the interaction term (age-x-stress) accounting for an additional 0.3 and 0.4% of the variance in AFC, respectively. As reported from Step 3 (Model 3) of the regression equation, there was an independent association between Chinese (compared with Caucasian) race/ethnicity and lower AFC (b = −1.770, P = 0.045) as well as an independent association between a positive history of hormone-containing contraceptive use and lower AFC (b = −1.820, P = 0.003). In addition, the interaction term (age-x-stress) was related to AFC significantly (b = −0.036, P = 0.031), suggesting the association between age and AFC varied as a function of stress level. To characterize the significant interaction effect, separate regression equations assessing relations between age and AFC were performed among women in the low-, mid- and high-stress tertiles and the corresponding slopes were plotted in Fig. 1. Slopes (unstandardized coefficients) showed rates of ovarian follicle loss to increase progressively across the low- (−0.781 follicles/year), mid- (−0.842 follicles/year) and high-stress (−0.994 follicles/year) groups. Additionally, the x-intercepts, ages at which AFC is estimated to be zero (i.e. menopause) were 54.3, 52.3 and 51.1, for the low-, mid- and high-stress groups, respectively, although these are projections falling outside the age range (25–45) of the current sample.
Table II.
Among 979 women, results of linear regression analyses assessing age-x-stress effects on AFC adjusted for covariates.
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| ba (betab) | ba (betab) | ba (betab) | |
| Variables | |||
| African-Americanc | 0.443 (.020) | 0.390 (0.017) | 0.304 (0.014) |
| Latinac | −0.173 (−0.008) | −0.145 (−0.006) | −0.139 (−0.006) |
| Chinesec | −1.720 (−0.074)♦ | −1.764 (−0.076)* | −1.770 (−0.076)* |
| Filipinac | −2.188 (−0.045) | −2.193 (−0.045) | −2.322 (−0.047) |
| SESd | 0.286 (0.051) | 0.352 (0.063) | 0.359 (0.064) |
| Menarcheal age | 0.035 (0.006) | 0.027 (0.004) | 0.029 (0.005) |
| BCe use (0 = none, 1 = hx of use) | −1.780 (−0.085)** | −1.777 (−0.085)** | −1.820 (−0.087)** |
| Parity (0 = none, 1 = 1+ live births) | 1.042 (0.054) | 1.129 (0.058) | 1.143 (0.059) |
| Smoking (0 = never, 1 = current/past) | 0.959 (0.042) | 0.952 (0.042) | 0.943 (0.042) |
| BMI | 1.033 (0.025) | 0.916 (0.022) | 1.087 (0.026) |
| WHR | −5.796 (−0.045) | −5.938 (−0.046) | −6.140 (−0.048) |
| Age | −0.890 (−0.512)*** | −0.882 (−0.508)*** | −0.882 (−0.507)***,f |
| Stress | 0.180 (0.052)♦ | 0.545 (0.159)**,g | |
| Age-x-stress | −0.036 (−0.122)* | ||
| Change statistics | |||
| R2 | 0.269 | 0.272 | 0.275 |
| R2change | 0.269 | 0.003 | 0.004 |
| Fchange | 29.654 | 3.445 | 4.663 |
| Significant Fchange | 0.000 | 0.064 | 0.031 |
aUnstandardized regression coefficient.
bStandardized regression coefficient.
cCaucasian is the reference group.
dSum of standardized education and household income variables.
eBC = hormonal methods for BC.
fThe effect of age on AFC was estimated at the sample mean of stress.
gThe effect of stress on AFC was estimated at age 25. Additional results are provided in the text regarding custom contrasts that were performed to further evaluate the effect of stress on AFC at five pre-specified ages (i.e. 25, 30, 35, 40 and 45).
♦P < 0.10.
*P < 0.05.
**P < 0.01.
***P < 0.001.
Figure 1.
AFC decline across 979 women (ages 25–45) reporting low, mid, and high levels of stress.
The main effects of age and stress in relation to AFC were also statistically significant. Greater chronological age was related to lower AFC (b = −0.882, P = 0.000) estimated at the sample mean of stress (due to centering the stress variable at the sample mean) and greater self-reported stress was related to higher AFC (b = 0.545, P = 0.005) estimated at age 25 (due to centering the age variable at age 25). In addition, custom contrasts were performed to estimate AFC mean differences across the low-, mid- and high-stress groups at five pre-specified ages (25, 30, 35, 40 and 45). Results showed that among women aged 25, those in the low- and mid-stress groups had a significantly lower AFC than women in the high-stress group (P = 0.013 and P = 0.024, respectively). Similarly, among women aged 30, those in the low- and mid-stress groups had a significantly lower AFC than women in the high-stress group (P = 0.013 and P = 0.012, respectively). Among women aged 35, only those in the mid-stress group had a significantly lower AFC than women in the high-stress group (p = 0.038) and among women aged 40 and 45 there were no differences in AFC means across the stress groups (all P's > 0.05).
Discussion
In the current investigation we applied principles of life history theory and specifically the psychosocial acceleration hypothesis to the examination of variability in reproductive aging. Specifically, we proposed a model in which environmental adversity is hypothesized to promote the allocation of resources toward greater reproductive readiness via increases in the volume of growing follicles (i.e. initial recruitment) at the cost of hastening the depletion of the ovarian reserve over time. In a large, community-based sample of 979 premenopausal women ages 25–45, we evaluated this model by examining the interaction of age and self-reported perceptions of stress in relation to AFC, a subset of growing follicles rescued during cyclic recruitment, that represent proportionately the finite pool of primordial follicles (i.e. ovarian reserve). Results showed that the rate of AFC loss across women was higher among women reporting more psychological stress. Associations were independent of statistical adjustment for potential confounding factors, including race/ethnicity, SES, menarcheal age, use of hormone-containing medication for BC, parity, cigarette smoking, BMI and WHR. When the sample distribution of psychological stress was divided into tertiles, women in the low-stress group showed an average follicle loss of −0.781/year, women in the mid-stress group showed an average follicle loss of −0.842/year and women in the high-stress group showed an average follicle loss of −0.994/year. Calculation of the x-intercepts, ages at which AFC is estimated to be zero (or projected ages of menopausal onset), were 54.3, 52.3 and 51.1 for the low-, mid- and high-stress groups, respectively.
In addition to the significant interaction showing psychological stress was related to a higher rate of AFC loss across women, a significant main effect was found in which greater psychological stress was related to higher AFC. Follow-up tests at pre-specified ages showed that the main effect of stress on AFC was present in the younger (ages 25, 30 and 35) but not in the older (ages 40 and 45) women. That psychological stress was related both to higher AFC among younger women and a higher rate of AFC loss across women suggests that fertility may initially be potentiated among women experiencing greater stress. Increased reproductive readiness (marked by higher AFC) among women experiencing greater stress appears to occur at the cost of having a higher rate of AFC loss. Although speculative, this may ultimately lead to earlier onset menopause and increased risk for diseases of aging that increase in prevalence post-menopausally. This apparent trade-off is consistent with life history theory and specifically the psychosocial acceleration hypothesis which proposes that environmental adversity biases women toward enhanced biological readiness for current versus longer term reproduction even at the cost of longer term consequences.
Although the current findings are consistent with the psychosocial acceleration hypothesis, a model derived from life history theory that has garnered a substantial amount of empirical support in its previous application to the investigation of pubertal timing (Moffitt et al., 1992; Wierson et al., 1993; Campbell and Udry, 1995; Graber et al., 1995; Ellis et al., 1999, Ellis and Garber, 2000; Ellis and Essex, 2007; Belsky et al., 2007; Saxbe and Repetti, 2009), it is notable that alternate life history models would suggest that environmental adversity may actually suppress reproductive functioning based on evidence supporting the hypothesis that reproduction is forestalled when environmental conditions threaten the viability of the offspring (Wasser and Barash, 1983; Ellison 1990). This model is additionally supported by studies of Cynomolgus monkeys in which socially subordinate females (presumed to experience psychological stress) displayed suppressed ovarian function (Kaplan et al., 1996, Kaplan and Manuck, 2004), as well as by studies showing stress-related activation of the hypothalamic–pituitary axis inhibits certain reproductive functions both in animal models and among women (Rivier and Rivest, 1991; Kalantaridou et al., 2004). Although speculative, it is possible that whether fertility, operationalized here as being reflected by the number of antral follicles, is enhanced or suppressed may depend on the severity of the adversity exposure. Because only normative variability in psychological stress was examined in the current investigation, it remains unclear whether the experience of more significant stressors or even traumatic events (that are more likely to threaten offspring viability) might have a different impact on folliculogenesis and reproductive aging. Future work is required to reconcile the current findings with previous evidence suggesting adversity exposures may have a suppressive effect on ovarian function and whether these conflicting outcomes may be explained by the severity of stress experienced.
Two primary weaknesses of the current study were that the analysis was cross-sectional and that the evaluation of psychological stress was limited to the self-report of stress measured over the past month. First, the cross-sectional nature of the current analysis limits the conclusions that can be drawn regarding whether stress relates to rates of AFC loss over time. Rather, the current findings demonstrate an association between stress and AFC loss as examined across women. A longitudinal component of the OVA study is currently underway in which women are returning for a 3-year follow-up visit at which time the study measures, including AFC, are repeated. In the future when these data become available, it will be possible to examine stress in relation to the actual change in the AFC over this period. Secondly, the measurement of psychological stress was limited to the use of the PSS which assesses appraisals of stress over the past month only. Although the measurement of a psychological state or transient appraisal of stress would not be expected to relate to a biological process that unfolds over many years, PSS scores are likely to reflect stress experienced more chronically as is suggested by previous investigations relating PSS to longer term health outcomes and biological processes relevant to disease development (Cohen et al., 1999; Ng and Jeffery, 2003; Epel et al., 2004; McDade et al., 2006; Fredman et al., 2010; Yu et al., 2010) and/or to reflect a trait-like dimension (Federenko et al., 2006; Conard and Matthews, 2008; Bogdan and Pizzagalli, 2009; Ebstrup et al., 2011) which may increase one's proneness to experience stress.
Strengths of the current investigation were its novel focus on reproductive aging during the premenopausal period; use of a well-validated marker of total follicular reserve and recruitment of a sample that is large in size, healthy and community based. To date, while the premenopausal period is well recognized as an important area of study for improved understanding of variability in fertility as well as the emergence of pre-clinical risk factors for disease development post-menopausally (Kaplan and Manuck, 2004; Kaplan and Manuck, 2008), there has been a paucity of research examining the mechanisms underlying the loss of ovarian function during this period. In this respect, the contribution of the current study is unique and timely. The current study also possesses several methodological strengths through its use of AFC, a well-validated marker reflecting reproductive aging on a continuum versus traditional staging methods which provide only categorical determinations of reproductive age (pre-, peri-, post-menopause) on the basis of indicators (e.g. hormones) that can be unreliable or unchanged until the function of the ovary is severely compromised. Lastly, the current sample is large, well-characterized in terms of reproductive history and general health, and includes women screened to be regularly cycling and free from major medical illnesses, maximizing the generalizability of the current results.
To address the limitations of the current study, future investigations should be designed to assess AFC over time, to measure objective as well as subjective aspects of the environment that may contribute to the psychological well-being of women and to characterize features of early environments that might be particularly salient in setting the stage for reproductive development and senescence. Future studies should also attempt to elucidate the biological mechanisms, primarily the hormonal regulation of follicle growth and development, by which such apparent stress effects on the body occur. Lastly, the current analysis was limited in its focus on reproductive aging without specific consideration of how the current findings may relate to other relevant literatures, namely life history models of pubertal timing (Ellis, 2004). Although findings generally suggest that pubertal and menopausal timing are unrelated (Treloar, 1974; Snieder et al., 1998), whether variation in pubertal timing may underlie aspects of ovarian function that are relevant to folliculogenesis and trajectories of reproductive aging is an important question for future research. Moreover, future research should be guided by the over-arching goal of developing better integrated models reflecting the inter-relation of major reproductive processes and events over a woman's life course.
In conclusion, results from the current investigation show that psychological stress was related to higher AFC among younger women and to a higher rate of AFC decline across women. This finding provides preliminary support for the proposed model of reproductive aging which suggests that environmental adversity may enhance the allocation of resources toward greater reproductive readiness via increases in initial recruitment of dormant primordial follicles into the growing pool of follicles, thereby enhancing fertility in the short term at the cost of hastening the depletion of the ovarian reserve in the long term.
Authors’ roles
M.E.B. (primary author) participated in the conceptualization, statistical analysis, manuscript drafting and critical discussion. N.E.A. (co-author) participated in the conceptualization, manuscript drafting and critical discussion. L.A.P. (co-author) participated in the conceptualization, manuscript drafting and critical discussion. B.S. (co-investigator and co-author) participated in the study design, execution, data collection, manuscript drafting and critical discussion. S.E.G. (co-author) participated in the conceptualization, statistical analysis, manuscript drafting and critical discussion: M.P.R. (co-author) participated in the data collection, conceptualization, manuscript drafting and critical discussion. M.I.C. (principal investigator and senior author) participated in the study design, execution, data collection, conceptualization, manuscript drafting and critical discussion.
Funding
The preparation of this manuscript and the research described here were supported by NIH/NICHD and NIH/NIA (R01 HD044876); NIH/NIA (K08 AG03575) and NIH/UCSF-CTSI (UL1 RR024131).
Conflict of interest
None declared.
References
- Aflatoonian A, Oskouian H, Ahmadi S, Oskouian L. Prediction of high ovarian response to controlled ovarian hyperstimulation: anti-Mullerian hormone versus small antral follicle count (2-6 mm) J Assist Reprod Genet. 2009;26:319–325. doi: 10.1007/s10815-009-9319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. 1991. Newbury Park, CA: Sage.
- Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive strategy—an evolutionary theory of socialization. Child Dev. 1991;62:647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg LD, Houts RM, Friedman SL, DeHart G, Cauffman E, Roisman GI, Halpern-Felsher BL, Susman E. Family rearing antecedents of pubertal timing. Child Dev. 2007;78:1302–1321. doi: 10.1111/j.1467-8624.2007.01067.x. [DOI] [PubMed] [Google Scholar]
- Biro FM, McMahon RP, Striegel-Moore R, Crawford PB, Obarzanek E, Morrison JA, Barton BA, Falkner F. Impact of timing of pubertal maturation on growth in black and white female adolescents: the National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr. 2001;138:636–643. doi: 10.1067/mpd.2001.114476. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Pizzagalli DA. The heritability of hedonic capacity and perceived stress: a twin study evaluation of candidate depressive phenotypes. Psychol Med. 2009;39:211–218. doi: 10.1017/S0033291708003619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekmans FJ, Faddy MJ, Scheffer G, te Velde ER. Antral follicle counts are related to age at natural fertility loss and age at menopause. Menopause. 2004;11:607–614. doi: 10.1097/01.gme.0000123643.76105.27. [DOI] [PubMed] [Google Scholar]
- Bromberger JT, Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prospective study of the determinants of age at menopause. Am J Epidemiol. 1997;145:124–133. doi: 10.1093/oxfordjournals.aje.a009083. [DOI] [PubMed] [Google Scholar]
- Campbell BC, Udry JR. Stress and age at menarche of mothers and daughters. J Biosoc Sci. 1995;27:127–134. doi: 10.1017/s0021932000022641. [DOI] [PubMed] [Google Scholar]
- Chisholm JS. Death, hope, and sex—life history theory and the development of reproductive strategies. Curr Anthropol. 1993;34:1–24. [Google Scholar]
- Cohen S, Janicki-Deverts D. Who's stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006 and 2009. J Appl Soc Psychol. 2012;42:1320–1334. [Google Scholar]
- Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapam S, Oskamp S, editors. The Social Psychology of Health: Claremont Symposium on Applied Social Psychology. Newbury Park, CA: Sage; 1988. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Skoner DP. Psychological stress, cytokine production, and severity of upper respiratory illness. Psychosom Med. 1999;61:175–180. doi: 10.1097/00006842-199903000-00009. [DOI] [PubMed] [Google Scholar]
- Conard MA, Matthews RA. Modeling the stress process: personality eclipses dysfunctional cognitions and workload in predicting stress. Pers Individ Differ. 2008;44:171–181. [Google Scholar]
- Cruess DG, Antoni MH, Kumar M, Ironson G, McCabe P, Fernandez JB, Fletcher M, Schneider N. Cognitive-behavioral stress management buffers decreases in dehydroepiandrosterone sulfate (DHEA-S) and increases in the cortisol/DHEA-S ratio and reduces mood disturbance and perceived stress among HIV-seropositive men. Psychoneuroendocrinology. 1999;24:537–549. doi: 10.1016/s0306-4530(99)00010-4. [DOI] [PubMed] [Google Scholar]
- Deardorff J, Gonzales NA, Christopher FS. Early puberty and adolescent pregnancy: the influence of alcohol use. Pediatrics. 2005;116:1451–1456. doi: 10.1542/peds.2005-0542. [DOI] [PubMed] [Google Scholar]
- Dunbar J, Sheeder J, Lezotte D, Dabelea D, Stevens-Simon C. Age at menarche and first pregnancy among psychosocially at-risk adolescents. Am J Public Health. 2008;98:1822–1824. doi: 10.2105/AJPH.2007.120444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebstrup JF, Eplov LF, Pisinger C, Jorgensen T. Association between the five factor personality traits and perceived stress: is the effect mediated by general self-efficacy? Anxiety Stress Coping. 2011;24:407–419. doi: 10.1080/10615806.2010.540012. [DOI] [PubMed] [Google Scholar]
- Ellis BJ. Timing of pubertal maturation in girls: an integrated life history approach. Psychol Bull. 2004;130:920–958. doi: 10.1037/0033-2909.130.6.920. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Essex MJ. Family environments, adrenarche, and sexual maturation: a longitudinal test of a life history model. Child Dev. 2007;78:1799–1817. doi: 10.1111/j.1467-8624.2007.01092.x. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Garber J. Psychosocial antecedents of variation in girls’ pubertal timing: maternal depression, stepfather presence, and marital and family stress. Child Dev. 2000;71:485–501. doi: 10.1111/1467-8624.00159. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, McFadyen-Ketchum S, Dodge KA, Pettit GS, Bates JE. Quality of early family relationships and individual differences in the timing of pubertal maturation in girls: a longitudinal test of an evolutionary model. J Pers Soc Psychol. 1999;77:387–401. doi: 10.1037//0022-3514.77.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Figueredo AJ, Brumbach BH, Schlomer GL. Fundamental dimensions of environmental risk. Hum Nat-Interdiscip Biosoc Perspect. 2009;20:204–268. doi: 10.1007/s12110-009-9063-7. [DOI] [PubMed] [Google Scholar]
- Ellison PT. Human ovarian function and reproductive ecology—new hypotheses. Am Anthropol. 1990;92:933–952. [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federenko IS, Schlotz W, Kirschbaum C, Bartels M, Hellhammer DH, Wust S. The heritability of perceived stress. Psychol Med. 2006;36:375–385. doi: 10.1017/S0033291705006616. [DOI] [PubMed] [Google Scholar]
- Fisher M, Rosenfeld WD, Burk RD. Cervicovaginal human papillomavirus infection in suburban adolescents and young adults. J Pediatr. 1991;119:821–825. doi: 10.1016/s0022-3476(05)80311-7. [DOI] [PubMed] [Google Scholar]
- Frattarelli JL, Levi AJ, Miller BT, Segars JH. A prospective assessment of the predictive value of basal antral follicles in in vitro fertilization cycles. Fertil Steril. 2003;80:350–355. doi: 10.1016/s0015-0282(03)00664-2. [DOI] [PubMed] [Google Scholar]
- Fredman L, Cauley JA, Hochberg M, Ensrud KE, Doros G, Study Osteoporotic F. Mortality associated with caregiving, general stress, and caregiving-related stress in elderly women: results of caregiver-study of osteoporotic fractures. J Am Geriatr Soc. 2010;58:937–943. doi: 10.1111/j.1532-5415.2010.02808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacobbe M, Mendes Pinto-Neto A, Simoes Costa-Paiva LH, Martinez EZ. The usefulness of ovarian volume, antral follicle count and age as predictors of menopausal status. Climacteric. 2004;7:255–260. doi: 10.1080/13697130410001713715. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser PK, Marucha PT, MacCallum RC, Laskowski BF, Malarkey WB. Stress-related changes in proinflammatory cytokine production in wounds. Arch Gen Psychiatry. 1999;56:450–456. doi: 10.1001/archpsyc.56.5.450. [DOI] [PubMed] [Google Scholar]
- Gold EB, Bromberger J, Crawford S, Samuels S, Greendale GA, Harlow SD, Skurnick J. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153:865–874. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- Gordon N. How does the adult Kaiser Permanente Membership in Northern California compare with the larger community? 2006.
- Graber JA, Brooksgunn J, Warren MP. The antecendents of menarcheal age—heredity, family environment, and stressful life events. Child Dev. 1995;66:346–359. doi: 10.1111/j.1467-8624.1995.tb00875.x. [DOI] [PubMed] [Google Scholar]
- Hansen K, Massey J, Craig L. Antral follicle counts obtained by transvaginal ultrasound and histological examination are correlated with ovarian non-growing follicle number. Fertil Steril. 2007;88:S79–S80. [Google Scholar]
- Hansen KR, Knowlton NS, Thyer AC, Charleston JS, Soules MR, Klein NA. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Hum Reprod. 2008;23:699–708. doi: 10.1093/humrep/dem408. [DOI] [PubMed] [Google Scholar]
- Kalantaridou SN, Makrigiannakis A, Zoumakis E, Chrousos GP. Stress and the female reproductive system. J Reprod Immunol. 2004;62:61–68. doi: 10.1016/j.jri.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB. Ovarian dysfunction, stress, and disease: a primate continuum. Ilar J. 2004;45:89–115. doi: 10.1093/ilar.45.2.89. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB. Ovarian dysfunction and the premenopausal origins of coronary heart disease. Menopause. 2008;15:768–776. doi: 10.1097/gme.0b013e31815eb18e. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Adams MR, Clarkson TB, Manuck SB, Shively CA, Williams JK. Psychosocial factors, sex differences, and atherosclerosis: lessons from animal models. Psychosom Med. 1996;58:598–611. doi: 10.1097/00006842-199611000-00008. [DOI] [PubMed] [Google Scholar]
- Kirby ED, Williams VP, Hocking MC, Lane JD, Williams RB. Psychosocial benefits of three formats of a standardized behavioral stress management program. Psychosom Med. 2006;68:816–823. doi: 10.1097/01.psy.0000238452.81926.d3. [DOI] [PubMed] [Google Scholar]
- Klinkert ER, Broekmans FJ, Looman CW, Habbema JD, te Velde ER. The antral follicle count is a better marker than basal follicle-stimulating hormone for the selection of older patients with acceptable pregnancy prospects after in vitro fertilization. Fertil Steril. 2005;83:811–814. doi: 10.1016/j.fertnstert.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Lane JD, Seskevich JE, Pieper CF. Brief meditation training can improve perceived stress and negative mood. Altern Ther Health Med. 2007;13:38–44. [PubMed] [Google Scholar]
- Lawlor DA, Ebrahim S, Smith GD. The association of socio-economic position across the life course and age at menopause: the British Women's Heart and Health Study. BJOG: An International Journal of Obstetrics & Gynaecology. 2003;110:1078–1087. [PubMed] [Google Scholar]
- Li J, Cowden LG, King JD, Briles DA, Schroeder HW, Stevens AB, Perry RT, Chen ZM, Simmons MS, Wiener HW, et al. Effects of chronic stress and interleukin-10 gene polymorphisms on antibody response to tetanus vaccine in family caregivers of patients with Alzheimer's disease. Psychosom Med. 2007;69:551–559. doi: 10.1097/PSY.0b013e3180cc2c61. [DOI] [PubMed] [Google Scholar]
- Luoto R, Kaprio J, Uutela A. Age at natural menopause and sociodemographic status in Finland. Am J Epidemiol. 1994;139:64–76. doi: 10.1093/oxfordjournals.aje.a116936. [DOI] [PubMed] [Google Scholar]
- McDade TW, Hawkley LC, Cacioppo JT. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: the Chicago health, aging, and social relations study. Psychosom Med. 2006;68:376–381. doi: 10.1097/01.psy.0000221371.43607.64. [DOI] [PubMed] [Google Scholar]
- McGee EA, Hsueh AJW. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- Mendle J, Turkheimer E, Emery RE. Detrimental psychological outcomes associated with early pubertal timing in adolescent girls. Dev Rev. 2007;27:151–171. doi: 10.1016/j.dr.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalsen A, Grossman P, Lehmann N, Knoblauch NTM, Paul A, Moebus S, Budde T, Dobos GJ. Psychological and quality-of-life outcomes from a comprehensive stress reduction and lifestyle program in patients with coronary artery disease: results of a randomized trial. Psychother Psychosom. 2005;74:344–352. doi: 10.1159/000087781. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Belsky J, Silva PA. Childhood experience and the onset of menarche—a test of a sociobiological model. Child Dev. 1992;63:47–58. doi: 10.1111/j.1467-8624.1992.tb03594.x. [DOI] [PubMed] [Google Scholar]
- Moynihan JA, Larson MR, Treanor J, Duberstein PR, Power A, Shore B, Ader R. Psychosocial factors and the response to influenza vaccination in older adults. Psychosom Med. 2004;66:950–953. doi: 10.1097/01.psy.0000140001.49208.2d. [DOI] [PubMed] [Google Scholar]
- Ng DM, Jeffery RW. Relationships between perceived stress and health behaviors in a sample of working adults. Health Psychol. 2003;22:638–642. doi: 10.1037/0278-6133.22.6.638. [DOI] [PubMed] [Google Scholar]
- Pache TD, Wladimiroff JW, de Jong FH, Hop WC, Fauser BC. Growth patterns of nondominant ovarian follicles during the normal menstrual cycle. Fertil Steril. 1990;54:638–642. doi: 10.1016/s0015-0282(16)53821-7. [DOI] [PubMed] [Google Scholar]
- Parks CG, Miller DB, McCanlies EC, Cawthon RM, Andrew ME, DeRoo LA, Sandler DP. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiol Biomarkers Prev. 2009;18:551–560. doi: 10.1158/1055-9965.EPI-08-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss ML, Kline J, Santos R, Levin B, Timor-Tritsch I. Age and the ovarian follicle pool assessed with transvaginal ultrasonography. Am J Obstet Gynecol. 1996a;174:624–627. doi: 10.1016/s0002-9378(96)70439-8. [DOI] [PubMed] [Google Scholar]
- Reuss ML, Kolton S, Tharakan T. Transvaginal ultrasonography in gynecologic office practice: assessment in 663 premenopausal women. Am J Obstet Gynecol. 1996b;175:1189–1194. doi: 10.1016/s0002-9378(96)70026-1. [DOI] [PubMed] [Google Scholar]
- Rivier C, Rivest S. Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis -peripheral and central mechanisms. Biol Reprod. 1991;45:523–532. doi: 10.1095/biolreprod45.4.523. [DOI] [PubMed] [Google Scholar]
- Rosen MP, Sternfeld B, Schuh-Huerta SM, Reijo-Pera RA, McCulloch CE, Cedars MI. Antral follicle count: absence of significant midlife decline. Fertil Steril. 2010;94:2182–2185. doi: 10.1016/j.fertnstert.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MP, Johnstone E, Addauan-Andersen C, Cedars MI. A lower antral follicle count is associated with infertility. Fertil Steril. 2011;95:1950–1954. doi: 10.1016/j.fertnstert.2011.01.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxbe DE, Repetti RL. Brief report: fathers’ and mothers’ marital relationship predicts daughters’ pubertal development two years later. J Adolesc. 2009;32:415–423. doi: 10.1016/j.adolescence.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Scheffer GJ, Broekmans FJM, Dorland M, Habbema JDF, Looman CWN, te Velde ER. Antral follicle counts by transvaginal ultrasonography are related to age in women with proven natural fertility. Fertil Steril. 1999;72:845–851. doi: 10.1016/s0015-0282(99)00396-9. [DOI] [PubMed] [Google Scholar]
- Scheffer GJ, Broekmans FJM, Bancsi LF, Habbema JDF, Looman CWN, Te Velde ER. Quantitative transvaginal two- and three-dimensional sonography of the ovaries: reproducibility of antral follicle counts. Ultrasound Obstet Gynecol. 2002;20:270–275. doi: 10.1046/j.1469-0705.2002.00787.x. [DOI] [PubMed] [Google Scholar]
- Snieder H, MacGregor AJ, Spector TD. Genes control the cessation of a woman's reproductive life: a twin study of hysterectomy and age at menopause. J Clin Endocrinol Metab. 1998;83:1875–1880. doi: 10.1210/jcem.83.6.4890. [DOI] [PubMed] [Google Scholar]
- Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N. Stages of Reproductive Aging Workshop (STRAW) J Women Health Gend Base Med. 2001;10:843–848. doi: 10.1089/152460901753285732. [DOI] [PubMed] [Google Scholar]
- Stanford JL, Hartge P, Brinton LA, Hoover RN, Brookmeyer R. Factors influencing the age at natural menopause. J Chronic Dis. 1987;40:995–1002. doi: 10.1016/0021-9681(87)90113-5. [DOI] [PubMed] [Google Scholar]
- Torgerson DJ, Avenell A, Russell IT, Reid DM. Factors associated with onset of menopause in women aged 45-49. Maturitas. 1994;19:83–92. doi: 10.1016/0378-5122(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Treloar AE. Menarche, menopause, and intervening fecundability. Hum Biol. 1974;46:89–107. [PubMed] [Google Scholar]
- Wasser SK, Barash DP. Reproductive suppression among female mammals—implications for biomedicine and sexual selection theory. Q Rev Biol. 1983;58:513–538. doi: 10.1086/413545. [DOI] [PubMed] [Google Scholar]
- Wierson M, Long PJ, Forehand RL. Toward a new understanding of early menarche—the role of environmental stress in pubertal timing. Adolescence. 1993;28:913–924. [PubMed] [Google Scholar]
- Wise LA, Krieger N, Zierler S, Harlow BL. Lifetime socioeconomic position in relation to onset of perimenopause. J Epidemiol Community Health. 2002;56:851–860. doi: 10.1136/jech.56.11.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu RHY, Ho SC, Lam CWK, Woo JLF, Ho SSY. Psychological factors and subclinical atherosclerosis in postmenopausal Chinese women in Hong Kong. Maturitas. 2010;67:186–191. doi: 10.1016/j.maturitas.2010.06.014. [DOI] [PubMed] [Google Scholar]