Abstract
BACKGROUND
Fetal cells (microchimerism) are acquired by women during pregnancy. Fetal microchimerism persists decades later and includes cells with pluripotent capacity. Persistent microchimerism has the capacity for both beneficial and detrimental maternal health consequences. Both miscarriage and termination of pregnancy can result in fetal microchimerism. We sought to determine whether cellular fetal microchimerism is acquired during management of pregnancy loss and further explored factors that could influence fetal cell transfer, including viability of fetal tissue, surgical versus medical management and gestational age.
METHODS
Pregnant women (n= 150 samples from 75 women) with singleton pregnancies undergoing a TOP (n= 63) or treatment for embryonic or fetal demise (miscarriage, n= 12) were enrolled. Mononuclear cells were isolated from blood samples drawn before, and 30 min after, treatment. Fetal cellular microchimerism concentrations were determined using quantitative PCR for a Y chromosome-specific sequence, expressed as genome equivalents of fetal DNA per 100 000 maternal cell equivalents (gEq/105). Detection rate ratios were determined according to clinical characteristics.
RESULTS
Cellular fetal microchimerism was found more often in post- compared with pretreatment samples, 24 versus 5% (P= 0.004) and at higher concentrations, 0–36 versus 0–0.7 gEq/105 (P< 0.001). Likelihood of microchimerism was higher in surgical than medical management, detection rate ratio 24.7 (P= 0.02). The detection rate ratio for TOP versus miscarriage was 16.7 for known male fetuses (P= 0.02). Microchimerism did not vary with gestational age.
CONCLUSIONS
Significant fetal cell transfer occurs during miscarriage and TOP. Exploratory analyses support relationships between obstetric clinical factors and acquisition of fetal cellular microchimerism; however, our limited sample size precludes definitive analysis of these relationships, and confirmation is needed. In addition, the long-term persistence and potential consequences of fetal microchimerism on maternal health merit further investigation.
Keywords: pregnancy termination, cellular fetal microchimerism, fetal cell transfer, pregnancy, miscarriage
Introduction
Microchimerism refers to the presence of a small amount of genetically foreign material within the circulation or tissues of a person. Natural acquisition of microchimerism primarily occurs through transplacental exchange during pregnancy (Gammill and Nelson, 2010). Both cell-free fetal (cff) DNA and fetal cells get transferred into the maternal system during pregnancy; however, the two differ significantly in terms of timing, concentration and clearance, as well as potential for long-term health impact. Soon after conception, cffDNA is detectable (Thomas et al., 1994) with increasing concentrations over gestation (Lo et al., 2000) and rapid post-partum clearance (Lo et al., 1999a,b). In contrast, cellular trafficking can occur early in pregnancy, but in most uncomplicated pregnancies, the majority of transfer probably occurs late in gestation, primarily as a peripartum event (Adams Waldorf et al., 2010) and the concentration of cells is lower than the concentration of cffDNA (Ariga et al., 2001). Fetal cells, unlike cffDNA, can persist for decades (Bianchi et al., 1996). The long-term consequences of persistent cellular fetal microchimerism are incompletely understood, but available studies support the likelihood of both beneficial and adverse effects (Nelson et al., 1998; Gadi and Nelson, 2007; Gadi, 2010).
Pregnancy loss is a potential source of significant fetal microchimerism, and fetal microchimerism from both miscarriage and termination of pregnancy (TOP) could impact subsequent maternal health. Miscarriage and TOP occur frequently, with 31–73% of conceptions resulting in miscarriage (Boklage, 1990; Wilcox et al., 1988) and nearly one-quarter of all pregnancies in the USA ending in TOP (Jones and Kooistra, 2011; Guttmacher Institute, 2011). Although cellular fetal microchimerism has not been specifically examined immediately after a TOP, fetal microchimerism has been detected in maternal whole blood (Bianchi et al., 2001). Furthermore, a history of pregnancy loss has been shown to correlate with a higher likelihood of detection of fetal microchimerism in maternal tissues years later compared with other reproductive outcomes (Khosrotehrani et al., 2003). It has been speculated that the differential viability of fetal cells in miscarriage compared with TOP influences potential engraftment underscoring the importance of examining each of these types of pregnancy loss (McGrath, 2004). In fact, years after pregnancy loss, cellular fetal microchimerism appears to be more common following a TOP compared with a miscarriage (Yan et al., 2005).
We prospectively investigated the extent of acquisition of fetal cells by a woman during miscarriage and TOP. We specifically focused on peripheral blood mononuclear cells (PBMC) as this compartment contains pluripotent and immune competent fetal cells with the potential for engraftment and influence on subsequent maternal health. We hypothesized that significant fetal cell transfer occurs during pregnancy loss and is increased by several obstetric factors, including fetal viability (miscarriage versus TOP), obstetric management (surgical versus medical approach) and gestational age.
Materials and Methods
Study population
We investigated a prospective cohort of subjects undergoing treatment for a miscarriage or seeking a TOP. Patients were recruited from several locations in our community including a tertiary academic referral center, a county hospital and a private family planning clinic. The study was approved by the Institutional Review Board of the University of Washington; all participants provided informed consent prior to enrollment.
Subjects with known multiple gestation were not eligible for participation. Chart abstraction was performed for clinical variables including patient demographics, medical and obstetric history. We defined miscarriage and TOP according to fetal or embryonic status by ultrasound, rather than by spontaneous passage of tissue. Specifically, miscarriage was defined as treatment that occurred after embryonic or fetal demise (i.e. lack of cardiac activity observed by ultrasound prior to the procedure), and TOP was defined by the presence of fetal cardiac activity at the time of intervention. Obstetric management was categorized as surgical or medical, defined as treatment by dilation and curettage/evacuation or the use of medication to initiate spontaneous passage of pregnancy tissue, respectively. Gestational age was determined by the last menstrual period corroborated by a first or second trimester ultrasound or the earliest ultrasound available. The first trimester was defined as <14 weeks and the second trimester as 14 weeks gestational age or more (maximum 24 weeks in this population). Medical record abstraction was performed to collect information regarding fetal karyotype and anomalies.
Surgical and medical terminations were performed according to standard obstetric practice. Surgical procedures utilized gradual cervical dilation techniques appropriate for gestational age, vacuum aspiration and curettage. Medical approaches included standard mifepristone/misoprostol, misoprostol or oxytocin-based protocols, at the discretion of the provider and as appropriate for gestational age.
Sample collection and isolation of PBMC
Two peripheral blood draws were collected from each subject, the first prior to treatment (before placement of cervical dilators when applicable) and the second ∼30 min post-operatively or after placental delivery. For two subjects who underwent medical management in the first trimester, the second sample was drawn after completion of tissue passage. For all blood draws, venous blood was collected in acid citrate dextrose tubes. PBMC were isolated from maternal whole blood by Ficoll Hypaque (Pharmacia Biotech, Uppsala, Sweden) gradient centrifugation at a density of 1.077 g/ml.
Detection and quantification of male DNA
Genomic DNA was extracted from PBMC using Wizard Genomic DNA Purification Kits (Promega, Madison, WI, USA) according to the instructions from the manufacturer. From each blood draw, a stochastic sample of ∼100 000 female cells was tested for fetal microchimerism. Quantitative PCR (Q-PCR) was performed to measure the prevalence of the Y chromosome-specific sequence, DYS14, and to quantify male fetal microchimerism (Lambert et al., 2002). Six aliquots of DNA from PBMC were tested from each blood draw, with total reaction volumes of 50 μl including 5 μl of extracted DNA. The maximum amount of DNA tested per aliquot was 35 000 genome equivalents (gEq) as higher concentrations of DNA can inhibit the PCR reaction. A calibration curve for the DYS14 assay with known increasing amounts of male DNA was run for each test against which the amount of fetal microchimerism in each specimen was determined. Every sample was also tested for a non-polymorphic gene, betaglobin. A betaglobin calibration curve (obtained from standard human genomic DNA [Promega]) was concurrently evaluated on each plate to quantify the number of gEq of DNA tested in each reaction. For ease of expression, DNA quantities were reported as the DNA gEq number of fetal cells per 100 000 maternal cells by using a conversion factor of 6.6 pg of DNA per cell.
Strict precautions were taken to avoid contamination. All samples were collected and processed by female technicians, nurses and physicians. Processing took place under a biosafety hood. Several negative controls were used in the Q-PCR assays including nulligravid female DNA and water. Analysis was performed by investigators blinded to sample characteristics.
Statistical analysis
Differences in prevalence of fetal microchimerism detection before and after treatment were assessed using McNemar's test, which accounts for the correlation between paired samples from each subject. We also examined differences in the quantitative concentrations of fetal microchimerism detected pre- and post-treatment. By definition, microchimeric cells occur at low concentrations, and detection from a single aliquot of maternal cells is uncommon; therefore, the data distribution is skewed to the right and approximates a Poisson distribution. For this reason, we analyzed the fetal microchimerism concentrations as the outcome in log-linear regression models, estimating a rate of fetal microchimerism detection as the number of gEq of fetal DNA as a proportion of the number of maternal cells tested. Negative-binomial models were fit to account for the higher level of variability in the data than expected in a Poisson model. Interpretation of the resulting estimates is identical to those of a Poisson model.
Because detection of fetal microchimerism occurred rarely in the pretreatment samples (5%) and at very low concentrations (<1 gEq of fetal microchimerism per 100 000 maternal cells), we treated the post-treatment fetal microchimerism concentrations as approximate values of the change in concentration from pre- to post-treatment. Therefore, we tested whether fetal microchimerism concentration was increased after treatment using an intercept-only Poisson regression model to assess the statistical significance of the difference between the estimated rate of fetal microchimerism detection and zero. The statistical significance of differences in the rate of fetal microchimerism detection between groups based on clinical characteristics was assessed by including indicator variables for such groups in the negative-binomial model. Groups were defined by miscarriage versus TOP, surgical versus medical management and first versus second trimester. Gestational age was also considered as a continuous predictor variable. Potential confounders of the relationships between fetal microchimerism and clinical groups included maternal age, gestational age and gravidity. A factor was included as a confounder if there was a difference of 10% in the estimated coefficient of interest between the multivariable model including the factor and the model without it. The primary analyses were also specifically considered in subjects with confirmed male fetuses. Paired samples from 75 subjects provides 88% power to detect a difference in microchimerism prevalence of 20% points, based on a McNemar's test with 30% discordant pairs and two-sided significance level of 0.05. All statistical analyses were performed by K.A.G., biostatistician.
Results
A total of 150 samples were studied from 75 subjects who underwent treatment for miscarriage or TOP and completed pre- and post-sampling. TOP was more common than miscarriage, with 63 (84%) subjects in the TOP group and 12 (16%) subjects in the miscarriage group. Surgical management was more common than medical, with 61 (81%) total surgical procedures and 14 (19%) medically managed. Of the 63 subjects undergoing TOP, 31 (49%) were performed for fetal indications and 32 (51%) were performed for elective reasons or maternal indications.
Table I shows the clinical and demographic characteristics of our study population. The median maternal age was 29 years; 33% of the women were primigravid. Fetal sex was confirmed by cytogenetic evaluation or autopsy in 53% (40 of 75) with 17 male and 23 female fetuses (unknown in 35). The median gestation age was 16.6 weeks (range 5.0–24.0 weeks), with a fairly even distribution of data points across gestational ages as shown in Table II. No study participant had a history of a prior blood transfusion. The majority of subjects were cared for at our tertiary care center (49/75, 65%), compared with 26/75 (35%) who were cared for in the community setting. Individual subject characteristics are available as a supplementary resource in Supplementary data, Table SI.
Table I.
Demographics of study population (n = 75).
| Age: median (range) | 29 (16–42) years |
| Gestational age: median (range) | 16.6 (5.0–24.0) weeks |
| Gravidity: median (range) | 2 (1–9) |
| Parity: median (range) | 0 (0–7) |
| Caucasian race: n (%) (61 of 75 known) | 50 (82) |
| Male fetal sex: n (%) (40 of 75 known) | 17 (43) |
| Aneuploid: n (%) (30 of 75 known) | 10 (33) |
| Anomalous: n (%) (40 of 75 known) | 28 (70) |
Table II.
Microchimerism (Mc) prevalence by gestational age.
| Gestational age | n | Mc positive, n (%) |
|---|---|---|
| 5 to <10 weeks | 19 | 5 (26) |
| 10 to <14 weeks | 8 | 2 (25) |
| 14–24 weeks | 48 | 11 (23) |
Data on karyotypic abnormalities were available for 30 (40%) of 75 subjects, with 10 of 30 (33%) showing fetal chromosomal abnormalities. The most common karyotypic abnormalities included three fetuses with Trisomy 21 and two fetuses with Trisomy 13. One fetus had Trisomy 18, and one fetus had monosomy X. The remaining three cytogenetic abnormalities included deletion/duplications or translocations. Of the 40 subjects for whom tissue examination or formal autopsy was available, fetal anomalies were present in 28 of 40 (70%). Fetal anomalies were initially characterized by appearance on antenatal ultrasound and corroborated by post-natal examination or formal autopsy. The most common anomalies included central nervous system defects (9 of 28, 32%), cystic hygromas (5 of 28, 18%) and complex cardiac defects (5 of 28, 18%). Among the 23 subjects for whom both karyotype and fetal examination were performed, concurrent cytogenetic abnormalities and fetal anomalies were found in 5 of 23 (22%). All subjects who underwent karyotype and/or fetal evaluation were cared for in our tertiary care center.
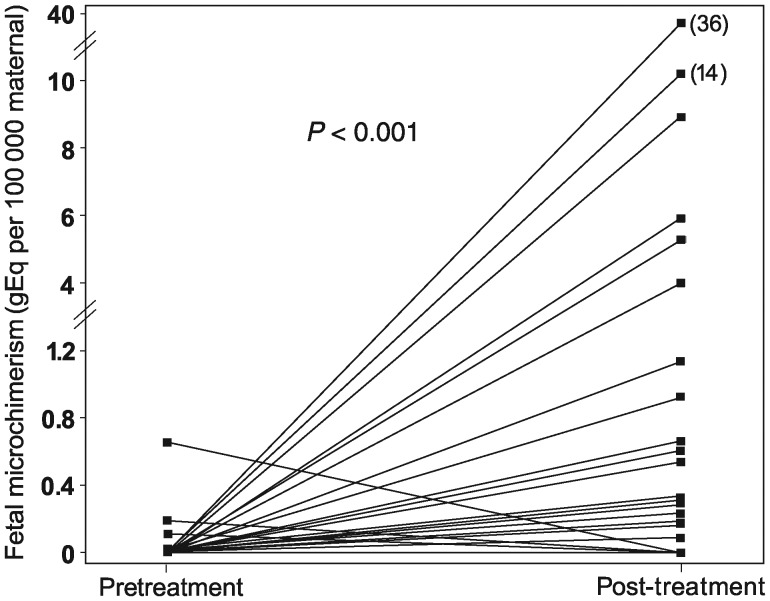
Fetal cellular microchimerism was more likely to be found in post- than pretreatment maternal blood (24 versus 5%, P= 0.004). All 18 subjects with fetal microchimerism post-treatment had no fetal microchimerism prior to treatment. Fetal microchimerism concentrations before and after treatment are shown in Fig. 1. There was a significant increase in the concentration of fetal cellular microchimerism in the post-treatment samples (median 0 gEq per 100 000 maternal cells, range 0–36 gEq) compared with the pretreatment samples (median 0 gEq per 100 000 maternal cells, range 0–0.7 gEq, P< 0.001). Mean concentrations for the two groups, although not evaluated in the statistical model, were 10.6 and 0.1 gEq per 100 000 maternal cells for the post-treatment and pretreatment samples, respectively.
Figure 1.
Microchimerism concentrations before and after treatment for all study participants. Data points (n= 150) show fetal microchimerism concentrations before and after treatment. Lines between points indicate each subject evaluated (n= 75).
Fetal microchimerism was detected at low concentrations prior to treatment in four subjects, all of whom had negative post-treatment values. Of these four subjects, maternal age ranged from 20 to 40 years, all pregnancies were in the second trimester, and fetal sex was confirmed male in two, confirmed female in one and unknown in one. Obstetric histories showed one woman had had three prior term vaginal deliveries and three prior TOPs, one had had three prior term vaginal deliveries and three prior miscarriages, one had had a prior ectopic pregnancy and one was primigravid.
Exploratory analyses of the relationships between clinical characteristics and concentration of post-treatment fetal cellular microchimerism are summarized in Table III. Women whose pregnancies were managed surgically showed significantly higher fetal microchimerism concentrations compared with those managed medically (detection rate ratio 24.7, P= 0.02). The amounts of fetal microchimerism in the TOP and miscarriage groups were not statistically significantly different in the overall population (detection rate ratio 5.9, P= 0.11). There was no statistically significant variability in post-treatment fetal microchimerism concentration according to gestational age, whether dichotomized into first and second trimesters (detection rate ratio 1.7, P= 0.54) or treated as a continuous variable (detection rate ratio 1.0, P= 0.64). Microchimerism prevalence by gestational age is shown in Table II.
Table III.
Adjusted rate ratios of post-treatment fetal microchimerism detection by clinical characteristics (n= 75).
| Sample | Rate ratio | 95% CI | P-value | |
|---|---|---|---|---|
| Surgical versus medical | Total | 24.7a | 1.6–389.8 | 0.02 |
| Confirmed males | 26.9 | 1.6–456.4 | 0.02 | |
| TOP versus miscarriage | Total | 5.9b | 0.7–51.8 | 0.11 |
| Confirmed males | 16.7 | 1.6–173.3 | 0.02 | |
| Second versus first trimester | Total | 1.7c | 0.3–8.5 | 0.54 |
| Confirmed males | d | |||
| Gestational age (continuous) | Total | 1.0c | 0.9–1.2 | 0.64 |
| Confirmed males | 1.4 | 0.8–2.2 | 0.21 |
CI, confidence interval; TOP, termination of pregnancy.
aModel adjusted for maternal age and gestational age.
bModel adjusted for maternal age.
cModel adjusted for maternal age and gravidity.
dEstimate does not exist: only 2 of 17 subjects in first trimester, both without fetal microchimerism.
In the subset of 17 subjects with a confirmed male fetus, as in the complete cohort, there was a significant increase in the concentration of fetal cellular microchimerism in post-treatment samples compared with pretreatment samples (P< 0.001). Pregnancies managed surgically showed increased microchimerism transfer compared with those managed medically (detection rate ratio 26.9, P= 0.02). Further, post-treatment fetal microchimerism concentration was higher in subjects with TOP compared with miscarriage (detection rate ratio 16.7, P= 0.02). There was no difference in post-treatment fetal microchimerism concentration according to gestational age. (Results summarized in Table III.) Fetal–maternal cell transfer was also not different among the known aneuploid versus euploid groups in our study (P= 0.87).
Discussion
Data in the current study demonstrate that maternal acquisition of fetal cells occurs commonly during miscarriage and pregnancy termination. Cellular fetal microchimerism durably persists and multiple different cell types have been identified in maternal cells and tissues years later. Other studies point to the potential for both beneficial and adverse consequences of cellular fetal microchimerism for long-term maternal health (Nelson et al., 1998; Gadi and Nelson, 2007; Gadi, 2010). Our findings underscore the potential contribution of pregnancy loss to the biological legacy of pregnancy.
The current study further investigated fetal cell transfer according to obstetric clinical factors. Although our limited sample size precluded definitive analysis of these relationships, exploratory analyses supported relationships between obstetric clinical factors and acquisition of fetal cellular microchimerism. Our observation of higher fetal cellular microchimerism concentrations with TOP than miscarriage indicates a possible role for fetal tissue viability in maternal acquisition of fetal cellular microchimerism. Another clinical variable that could impact fetal cellular transfer is surgical versus medical management. We found higher concentrations of fetal cellular microchimerism in cases managed with surgical intervention compared with those who underwent medical management via labor induction. Surgical management is likely to cause more shearing of the placental–decidual interface when compared with medical management, resulting in transfer of more fetal microchimerism. Gestational age is another variable that could affect fetal cell transfer. Increasing gestational age might be anticipated to confer greater fetal cellular microchimerism due to increasing fetal and placental volume with increasing gestational age. An association of fetal microchimerism with gestational age of TOP and miscarriage has been described in other studies (Bianchi et al., 2001; Wataganara et al., 2004). These studies tested cffDNA or whole blood (which contains substantial cffDNA) and are consistent with the well-established correlation of cffDNA levels with gestational age (Lo et al., 1998). The approach of the current study differs in that testing was conducted for cellular fetal microchimerism for which no significant association was observed according to gestational age.
While the high levels of fetal cffDNA that characterize pregnancy are rapidly cleared (Lo et al., 1999 a,b), the kinetics of fetal cellular microchimerism and persistence are less clear. One study of maternal buffy coat showed that fetal material was not commonly detected at 1 week and 1 month after TOP (Sato et al., 2008). In our study, two of five women for whom additional samples were also available a month after treatment had detectable fetal cellular microchimerism (data not shown). Prior studies have demonstrated that long-term persistence of fetal cellular microchimerism can result from miscarriage and TOP. In a study of women who had never given birth to a son, 22% of women with a previous miscarriage and 57% of women with a previous TOP had microchimerism with male DNA, presumed to have originated from prior pregnancy with a male fetus (Yan et al., 2005). Observations in the current study raise the question whether obstetric factors impact long-term engraftment of cellular fetal microchimerism.
Strengths of the current study include the focus on cellular fetal microchimerism and the prospective collection of samples from both before and after treatment. This longitudinal study design included assessment of fetal cellular microchimerism prior to any intervention, allowing direct quantification of the number of fetal cells acquired by a woman during treatment. A disadvantage is that the sample size did not permit comprehensive analysis of clinical subgroups, and our exploratory findings require confirmation. Other future analyses of interest include further assessment of the relationship of cellular microchimerism with aneuploidy and also evaluation of a possible effect of pretreatment induction of fetal demise. Another limitation is that microchimerism is likely underestimated in our study for several reasons. First, microchimerism, by definition, occurs at very low concentrations, and our results represent a stochastically derived aliquot of maternal cells. Microchimerism prevalence would likely be greater if larger amounts of maternal cells could feasibly be tested. Secondly, only fetal microchimerism from a male fetus would be identified in our study because the quantitative assay employed is specific for a Y chromosome sequence. This approach was chosen because gender-independent methods for identifying fetal microchimerism could not be used in this study population. Specifically, it was not possible to collect samples from partners and other family members for genotyping and identification of other non-shared polymorphisms. Although it would have been possible to limit this analysis to male fetuses on the basis of testing tissue for fetal sex (when fetal sex was unknown clinically), we were not able to perform this testing for logistic reasons. Because there is no reason to expect an imbalance in fetal sex distribution in the population as a whole, nor according to studied predictors, the only expected effect of this limitation is a contribution to underestimation of fetal microchimerism overall. Among the 75 participants studied, four had low concentrations of cellular fetal microchimerism before treatment. Preexisting male cells may originate from a prior pregnancy, including an undiagnosed early miscarriage, from a blood transfusion (not applicable to our population specifically), from a twin or older sibling or from sexual exposure (Yan et al., 2005).
Both positive and negative long-term effects of fetal microchimerism on maternal health have been suggested in other studies. Positive effects may include semi-allogeneic cells conferring a protective advantage against some malignancies (Gadi and Nelson, 2007) and possible regeneration of diseased maternal tissues (Johnson et al., 2002). Negative effects may include autoimmune disease (Nelson et al., 1998). However, the potential impact of fetal cellular microchimerism from miscarriage or TOP could differ from that acquired following a birth. This consideration is underscored by the fact that the composition of fetal cells is known to differ in early versus late gestation (Pahal et al., 2000; Shields and Andrews, 1998). Additionally, other studies of disease risk according to reproductive history have observed a difference in associations with parity when compared with gravidity without parity (Guthrie et al., 2010). A final area of consideration relates to the unknown long-term effects of harboring fetal cellular microchimerism that originated from a fetus with aneuploidy or another genetic anomaly. Fetal aneuploidy occurs more commonly in miscarriage and TOP compared with term delivery. In addition, higher concentrations of fetal cffDNA have been found in women carrying an aneuploid fetus compared with a normal fetus (Bianchi et al. 1997; Lo et al., 1999a,b). Although we found no differences in PBMC fetal–maternal transfer among the known aneuploid versus euploid groups in our study, this was not a comparison in our a priori hypothesis. It warrants further investigation with a larger sample size given its possible implications. Acquisition of aneuploid cellular microchimerism might be speculated to convey risk specific to that aneuploidy on its recipient. In this context, it is interesting to consider the relationship between Trisomy 21 (or Down Syndrome) and Alzheimer's dementia. Individuals with Trisomy 21 have an increased risk of Alzheimer's disease (Cutler et al., 1985). Notably, a higher risk of Alzheimer's has also been reported in mothers of Trisomy 21 children (Schupf et al., 1994, 2001). Fetal cells have been demonstrated in maternal tissues in other studies, including maternal brain in murine studies (Khosrotehrani et al., 2004; Tan et al., 2005). These observations raise the question whether aneuploid fetal cells resident in maternal tissues have the potential to impact subsequent maternal health.
Overall, the current study indicates that pregnancy loss is a significant source of fetal cellular microchimerism. Exploratory analyses suggest that obstetric clinical factors impact the prevalence and quantity of fetal cell acquisition. Both miscarriage and TOP are common (Boklage, 1990; Wilcox et al., 1988; Guttmacher Institute, 2011) and the long-term effects of fetal cellular microchimerism acquired from these sources merit further investigation.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
The corresponding author (H.S.G.) had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. S.E.P., J.L.N., K.A.G., V.K.G., D.J.O., S.W.P. and H.S.G. participated in conception and study design. S.E.P., T.M.A. and H.S.G. contributed to laboratory conduct of this research and data collection. S.E.P., J.L.N., K.A.G., V.K.G., T.M.A. and H.S.G. participated in data analysis and interpretation. S.E.P. and H.S.G. wrote the manuscript. S.E.P., J.L.N., K.A.G., V.K.G., T.M.A., D.J.O., S.W.P. and H.S.G. provided significant intellectual contribution to the critical revision of the manuscript and approved the final manuscript as submitted.
Funding
This research was supported by NIH grant HD01264. The funding agency played no role in designing or conducting the study or in the collection, management, analysis and interpretation of the data, nor did they have any input into the preparation, review, or approval of this manuscript.
Conflict of interest
Co-author D.J.O. serves as a consultant at Danco Laboratories, LLC, the U.S. distributor of mifepristone. All other authors confirm that they have no conflicts of interest in relation to this work.
Supplementary Material
Acknowledgements
We gratefully acknowledge the women who agreed to participate in this research, as well as the study coordination efforts of Evangelyn Nkwopara.
References
- Adams Waldorf K, Gammill H, Lucas J, Aydelotte T, Leisenring W, Lambert N, Nelson J. Dynamic changes in fetal microchimerism in maternal peripheral blood mononuclear cells, CD4+ and CD8+ cells in normal pregnancy. Placenta. 2010;31:589–594. doi: 10.1016/j.placenta.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariga H, Ohto H, Busch MP, Imamura S, Watson R, Reed W, Lee T. Kinetics of fetal cellular and cell-free DNA in the maternal circulation during and after pregnancy: implications for noninvasive prenatal diagnosis. Transfusion. 2001;41:1524–1530. doi: 10.1046/j.1537-2995.2001.41121524.x. [DOI] [PubMed] [Google Scholar]
- Bianchi D, Zickwolf G, Weil G, Sylvester S, DeMaria M. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci USA. 1996;93:705–708. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi D, Williams J, Sullivan L, Hanson F, Klinger K, Shuber A. PCR quantitation of fetal cells in maternal blood in normal and aneuploid pregnancies. Am J Hum Genet. 1997;61:822–829. doi: 10.1086/514885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi D, Farina A, Weber W, Delli-Bovi L, DeRiso M, Williams J, Klinger K. Significant fetal–maternal hemorrhage after termination of pregnancy: implications for development of fetal cell microchimerism. Am J Obstet Gynecol. 2001;184:703–706. doi: 10.1067/mob.2001.111072. [DOI] [PubMed] [Google Scholar]
- Boklage C. Survival probability of human conceptions from fertilization to term. Int J Fertil. 1990;35:75–94. [PubMed] [Google Scholar]
- Cutler N, Heston L, Davies P, Haxby J, Schapiro M. Alzheimer's disease and down's syndrome: new insights. Ann Intern Med. 1985;103:566–578. doi: 10.7326/0003-4819-103-4-566. [DOI] [PubMed] [Google Scholar]
- Gadi V. Fetal microchimerism in breast from women with and without breast cancer. Breast Cancer Res Treat. 2010;121:241–244. doi: 10.1007/s10549-009-0548-1. [DOI] [PubMed] [Google Scholar]
- Gadi V, Nelson J. Fetal microchimerism in women with breast cancer. Cancer Res. 2007;67:9035–9038. doi: 10.1158/0008-5472.CAN-06-4209. [DOI] [PubMed] [Google Scholar]
- Gammill H, Nelson J. Naturally acquired microchimerism. Int J Dev Biol. 2010;54:531–543. doi: 10.1387/ijdb.082767hg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie K, Dugowson C, Voigt L, Koepsell T, Nelson J. Does pregnancy provide vaccine-like protection against rheumatoid arthritis? Arthritis Rheum. 2010;62:1842–1848. doi: 10.1002/art.27459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttmacher Institute. Facts on induced abortion in the United States. 2011. http://www.guttmacher.org/pubs/fb_induced_abortion.html#5. (17 May 2011, date last accessed)
- Johnson K, Samura O, Nelson J, McDonnell W, Bianchi D. Significant fetal cell microchimerism in a nontransfused woman with hepatitis C: evidence of long-term survival and expansion. Hepatology. 2002;36:1295–1297. doi: 10.1053/jhep.2002.35622. [DOI] [PubMed] [Google Scholar]
- Jones R, Kooistra K. Abortion incidence and access to services in the United States, 2008. Perspect Sex Reprod Health. 2011;43:41–50. doi: 10.1363/4304111. [DOI] [PubMed] [Google Scholar]
- Khosrotehrani K, Johnson K, Lau J, Dupuy A, Cha D, Bianchi D. The influence of fetal loss on the presence of fetal cell microchimerism: a systematic review. Arthritis Rheum. 2003;48:3237–3241. doi: 10.1002/art.11324. [DOI] [PubMed] [Google Scholar]
- Khosrotehrani K, Johnson K, Cha D, Salomon R, Bianchi D. Transfer of fetal cells with multilineage potential to maternal tissue. J Am Med Assoc. 2004;292:75–80. doi: 10.1001/jama.292.1.75. [DOI] [PubMed] [Google Scholar]
- Lambert N, Lo Y, Erickson T, Tylee T, Guthrie K, Furst D, Nelson J. Male microchimerism in healthy women and women with scleroderma: cells or circulating DNA? A quantitative answer. Blood. 2002;100:2845–2851. doi: 10.1182/blood-2002-01-0295. [DOI] [PubMed] [Google Scholar]
- Lo Y, Tein M, Lau T, Haines C, Leung T, Poon P, Wainscoat J, Johnson P, Chang A, Hjelm N. Quantitative analysis of fetal DNA in maternal plasma and serum: implications for noninvasive prenatal diagnosis. Am J Hum Genet. 1998;62:768–775. doi: 10.1086/301800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Y, Lau T, Zhang J, Leung T, Chang A, Hjelm N, Elmes R, Bianchi D. Increased fetal DNA concentrations in the plasma of pregnant women carrying fetuses with Trisomy 21. Clin Chem. 1999a;45:1747–1751. [PubMed] [Google Scholar]
- Lo Y, Zhang J, Leung T, Lau T, Chang A, Hjelm N. Rapid clearance of fetal DNA from maternal plasma. Am J Hum Genet. 1999b;64:218–224. doi: 10.1086/302205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Y, Lau T, Chan L, Leung T, Chang A. Quantitative analysis of the bidirectional fetomaternal transfer of nucleated cells and plasma DNA. Clin Chem. 2000;46:1301–1309. [PubMed] [Google Scholar]
- McGrath H. Elective pregnancy termination and microchimerism: comment on the article by Khosrotehrani et al. Arthritis Rheum. 2004;50:3058–3059. doi: 10.1002/art.20650. [DOI] [PubMed] [Google Scholar]
- Nelson J, Furst D, Maloney S, Gooley T, Evans P, Smith A, Bean M, Ober C, Bianchi D. Microchimerism and HLA-compatible relationships of pregnancy in scleroderma. Lancet. 1998;351:559–562. doi: 10.1016/S0140-6736(97)08357-8. [DOI] [PubMed] [Google Scholar]
- Pahal G, Jauniaux E, Kinnon C, Thrasher A, Rodeck C. Normal development of human fetal hematopoiesis between eight and seventeen weeks' gestation. Am J Obstet Gynecol. 2000;183:1029–1034. doi: 10.1067/mob.2000.106976. [DOI] [PubMed] [Google Scholar]
- Sato T, Fujimori K, Sato A, Ohto H. Microchimerism after induced or spontaneous abortion. Obstet Gynecol. 2008;112:593–597. doi: 10.1097/AOG.0b013e31818345da. [DOI] [PubMed] [Google Scholar]
- Schupf N, Kapell D, Lee J, Ottman R, Mayeux R. Increased risk of Alzheimer's disease in mothers of adults with Down's syndrome. Lancet. 1994;344:353–356. doi: 10.1016/s0140-6736(94)91398-6. [DOI] [PubMed] [Google Scholar]
- Schupf N, Kapell D, Nightingale B, Lee J, Mohlenhoff J, Bewley S, Ottman R, Mayeux R. Specificity of the fivefold increase in AD in mothers of adults with Down syndrome. Neurology. 2001;57:979–984. doi: 10.1212/wnl.57.6.979. [DOI] [PubMed] [Google Scholar]
- Shields L, Andrews R. Gestational age changes in circulating CD34+ hematopoietic stem/progenitor cells in fetal cord blood. Am J Obstet Gynecol. 1998;178:931–937. doi: 10.1016/s0002-9378(98)70526-5. [DOI] [PubMed] [Google Scholar]
- Tan X, Liao H, Sun L, Okabe M, Xiao Z, Dawe G. Fetal microchimerism in the maternal mouse brain: a novel population of fetal progenitor or stem cells able to cross the blood-brain barrier? Stem Cells. 2005;23:1443–1452. doi: 10.1634/stemcells.2004-0169. [DOI] [PubMed] [Google Scholar]
- Thomas M, Williamson R, Craft I, Yazdani N, Rodeck C. Y chromosome sequence DNA amplified from peripheral blood of women in early pregnancy. Lancet. 1994;343:413–414. doi: 10.1016/s0140-6736(94)91248-3. [DOI] [PubMed] [Google Scholar]
- Wataganara T, Chen A, LeShane E, Sullivan L, Borgatta L, Bianchi D, Johnson K. Cell-free fetal DNA levels in maternal plasma after elective first-trimester termination of pregnancy. Fertil Steril. 2004;81:638–644. doi: 10.1016/j.fertnstert.2003.07.028. [DOI] [PubMed] [Google Scholar]
- Wilcox A, Weinberg C, O'Connor J, Baird D, Schlatterer J, Canfield R, Armstrong E, Nisula B. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- Yan Z, Lambert N, Guthrie K, Porter A, Loubiere L, Madeleine M, Stevens A, Hermes H, Nelson L. Male microchimerism in women without sons: Quantitative assessment and correlation with pregnancy history. Am J Med. 2005;118:899–906. doi: 10.1016/j.amjmed.2005.03.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.