Abstract
Adolescents often report shorter time in bed and earlier wake-up times on school days compared to weekend days. Extending sleep on weekend nights may reflect a “recovery” process as youngsters try to compensate for an accumulated school-week sleep debt. The authors examined whether the circadian timing system of adolescents shifted after keeping a common late weekend “recovery” sleep schedule; it was hypothesized that a circadian phase delay shift would follow this later and longer weekend sleep. The second aim of this study was to test whether modifying sleep timing or light exposure on weekends while still providing recovery sleep can stabilize the circadian system. Two experiments addressed these aims. Experiment 1 was a 4-wk, within-subjects counterbalanced design comparing two weekend sleep schedule conditions, “TYPICAL” and “NAP.” Compared to weeknights, participants retired 1.5 h later and woke 3 h later on TYPICAL weekends but 1 h later on NAP weekends, which also included a 2-h afternoon nap. Experiment 2 was a 2-wk, between-subjects design with two groups (“TYPICAL” or “LIGHT”) that differed by weekend morning light exposure. TYPICAL and LIGHT groups followed the TYPICAL weekend schedule of Experiment 1, and the LIGHT group received 1 h of light (454–484 nm) upon weekend wake-up. Weekend time in bed was 1.5 h longer/night than week-nights in both experimental protocols. Participants slept at home during the study. Dim light melatonin onset (DLMO) phase was assessed in the laboratory before (Friday) and after (Sunday) each weekend. Participants were ages 15 to 17 yrs. Twelve participants (4 boys) were included in Experiment 1, and 33 (10 boys) were included in Experiment 2. DLMO phase delayed over TYPICAL weekends in Experiment 1 by (mean ± SD) 45 ± 31 min and Experiment 2 by 46 ± 34 min. DLMO phase also delayed over NAP weekends (41 ± 34 min) and did not differ from the TYPICAL condition of Experiment 1. DLMO phase delayed over LIGHT weekends (38 ± 28 min) and did not differ from the TYPICAL group of Experiment 2. In summary, adolescents phase delay after keeping a commonly observed weekend sleep schedule. Waking earlier or exposure to short-wavelength light on weekend mornings, however, did not stabilize circadian timing in this sample of youngsters. These data inform chronotherapy interventions and underscore the need to test circadian phase-shifting responses to light in this age group.
Keywords: Adolescent, Circadian rhythms, DLMO phase, Light, Nap, Weekend sleep pattern
INTRODUCTION
The circadian timing and homeostatic sleep systems manifest altered physiological outputs across human adolescence, defined here as the second decade of life. Psychosocial factors are also changing during this time; for example, older adolescents start to seek independence from parents, and they experience greater academic demands, more social opportunity, and often earlier school start times. As a result, sleep patterns emerge as an interaction between the intrinsic biological drives for sleep and many intrinsic and extrinsic psychosocial factors. This interaction is especially apparent in differences between school and weekend night sleep patterns.
Differences between school and weekend night sleep timing and duration in adolescents are seen in studies from countries such as the United States (National Sleep Foundation, 2006; O’Brien & Mindell, 2005; Wolfson & Carskadon, 1998), Germany (Loessl et al., 2008; Randler, 2008), Belgium (Van den Bulck, 2004), Australia (Henschel & Lack, 1987), Finland (Saarenpaa-Heikkila et al., 1995), Brazil (Beijamini et al., 2008), Italy (Giannotti et al., 2002; Russo et al., 2007), Korea (Yang et al., 2005), Switzerland (Urner et al., 2009), and Iceland (Thorleifsdottir et al., 2002). According to several U.S. survey studies published in the last 15 yrs, older adolescents aged 14–20 yrs report school-night sleep durations between 7 and 7.5 h/night (Hansen et al., 2005; National Sleep Foundation, 2006; O’Brien & Mindell, 2005; Wolfson & Carskadon, 1998; Wolfson et al., 2003). The same studies show that most adolescents report 1–2 h more sleep on weekend nights than on school nights, whereas mean reported bedtimes range from 1.5 to 2 h later on weekend nights compared to school nights (National Sleep Foundation, 2006; O’Brien & Mindell, 2005; Wolfson & Carskadon, 1998), and reported wake-up times show pronounced delays on weekend mornings, ranging between 3 and 4 h later compared to school days in older adolescents (National Sleep Foundation, 2006; Wolfson & Carskadon, 1998).
The older adolescent’s sleep regulatory systems appear “permissive” of late bedtimes on school and weekend nights. Studies indicate that homeostatic sleep “pressure” builds at a faster rate for prepubertal adolescents, whereas the older adolescent maintains waking and alertness longer across the day with greater ease (Jenni et al., 2005; Taylor et al., 2005). More mature adolescents also show a later circadian phase of the salivary melatonin rhythm compared to prepubertal adolescents when sleep (dark) is held fixed (Carskadon et al., 1997, 2004); thus, circadian-dependent alerting (Edgar et al., 1993) is sustained later into the evening in older adolescents compared to their younger peers. Similar findings of a pubertal phase-delay shift of the circadian timing system have also been reported in other mammals (Hagenauer et al., 2009).
The adolescent psychosocial milieu also contributes to the timing of sleep on school-nights and weekend nights. First, parental influence over bedtimes usually lessens for older adolescents (Carskadon, 1990; National Sleep Foundation, 2006; Wolfson & Carskadon, 1998), allowing youngsters to set their own bedtimes. Homework (Manber et al., 1995), extracurricular activities, part-time work (Carskadon, 1989–1990), and socializing (Manber et al., 1995) also contribute to late bedtimes. Other environmental, and usually stimulating, factors, such as watching TV, playing video games, and using the computer may also keep youngsters awake later at night (National Sleep Foundation, 2006; Van den Bulck, 2004). School start time is the most significant environmental factor that affects wake-up times on school days in older adolescents. Most school systems in the United States are organized so that high schools start the earliest, followed by middle schools, and elementary schools start last, and reported wake-up times of adolescents on school days reflect this pattern (National Sleep Foundation, 2006).
Late school-night bedtimes (driven by intrinsic and extrinsic factors), combined with early school-day rise times (driven primarily by the school bell), reduce the amount of time teens spend in bed trying to sleep on school nights. As a result, many students accumulate a substantial sleep debt over the course of a school week. Whether this sleep loss over the school week can be “recovered” on weekends remains unclear; however, sleep extension on weekend nights seems to reflect a “behavioral sleep rebound” by which adolescents try to compensate for an accumulated sleep loss. In addition to longer sleep, delayed bedtimes and wake-up times supported by intrinsic sleep-regulating systems also manifest when extrinsic constraints are reduced, such as on weekend days. In summary, many adolescents devote less time to sleep on school nights versus weekend nights, and the timing of sleep alternates between relatively early and short (school night) and relatively late and long (weekend night).
The shift of light/dark (LD) exposure associated with late weekend “recovery” sleep may exacerbate the delayed circadian phase of older adolescents. Previous studies of young adults (Burgess & Eastman, 2005; Burgess et al., 2003; Martin & Eastman, 2002) and adolescents (Crowley et al., 2006) found that later sleep (dark) timing is correlated with later dim light melatonin onset (DLMO) phase. Moreover, the circadian system responds to acute sleep/wake timing changes that may occur on a weekend. Yang and colleagues (2001), for example, examined a late weekend sleep pattern in young adults with a 2-h delay of both bedtime and wake-up time on Friday and Saturday nights. A circadian phase delay of 31 min was shown by a change in the timing of DLMO phase. Another study showed that a stable bedtime on weekend nights accompanied by ad libitum wake-up times on weekend mornings produced an average DLMO phase delay of 30 min in young adults (Taylor et al., 2008). The adult circadian timing system, therefore, is sensitive to delay shifts of bedtime and wake-up time occurring over just two nights. Whether the adolescent circadian timing system responds similarly to such acute weekend sleep/wake schedule shift remains unclear.
Adolescents who live on such a fluctuating weekday/weekend sleep schedule are in a challenging position in which the competing weekend social needs (staying up later to socialize) and sleep needs (sleeping late to get more sleep) result in a vulnerability to phase delay the circadian timing system. Shifting the clock later may negatively impact sleep duration during the subsequent school days, because sleep onset latency may lengthen at school-night bedtime, yet wake-up time remains early to accommodate the school day. The current study describes two experiments in which weekend sleep (dark) or light is altered in an attempt to simultaneously (i) allow a small amount of social adjustment, (ii) provide “recovery” sleep, and (iii) anchor circadian phase.
Experiment 1 compared circadian timing changes after a “typical” delayed weekend sleep schedule to a weekend sleep schedule that has a more modest sleep delay and provides compensatory sleep with an afternoon nap. This intervention schedule was designed to minimize the circadian phase shift by allowing earlier morning light exposure (targeting the phase-advance portion of the phase response curve [PRC] to light) and to extend sleep with a midday nap that should not affect circadian timing (Buxton et al., 2000). Experiment 2 examined whether short-wavelength light exposure on weekend mornings would anchor circadian phase under “typical” circumstances that permitted a delay of morning wake-up time to allow for recovery sleep. Given the sensitivity of the human circadian timing system to short-wavelength light (Brainard et al., 2001a, 2001b; Thapan et al., 2001), even at low intensity when monochromatic (Warman et al., 2003), we used short-wavelength light exposure on weekend mornings to enrich the ambient light signal during the phase-advance portion of the light PRC. In both experiments, we expected to see a phase-delay shift after the “typical” weekend schedule, but stable phase positions after the experimental weekends; in all schedules, we expected more sleep on weekend nights compared to school nights.
METHODS
Participants
Thirty-three 10th- and 11th-grade students (ages 15–17 yrs, 10 boys) from the Northeast United States (41°49′N, 71°25′W) were enrolled in these experiments while they were attending school (January through May, September, or October). The experiment was explained to each participant and at least one parent who then completed a telephone interview. As further screening, participants completed the Sleep Habits Survey (Wolfson & Carskadon, 1998), and one parent (usually the mother) completed a questionnaire regarding the participant’s sleep habits, medical history, and family medical history.
Most participants (85%) reported themselves as Caucasian. Parents of participants reported that their child was healthy and medication-free. Parents also reported no first-degree family history of psychotic disorders, neurological disorders, bipolar disorder, or narcolepsy. Participants reported their typical sleep duration on school-nights between 6 and 9 h and that they usually sleep at least 1 h longer on weekends versus week-nights. Participants were excluded if they reported traveling beyond two time zones in the month before the first laboratory circadian phase assessments or if their score on the morningness questionnaire of Smith and colleagues (1989) indicated extreme morning (≥44) or evening (≤20) phase preference, defined as 2 standard deviations above and below the mean score of 148 local 10th- and 11th-grade, high-school students (unpublished data). Extreme morning and evening types were excluded because underlying physiological properties of the circadian timing system may differ between these groups (Duffy et al., 1999). Participants and parents were required to have sufficient knowledge of the English language to read and understand the consent forms and complete the study requirements. A final requirement was that commute time from the laboratory to a participant’s home be ≤30 min to allow compliance with the imposed weekend sleep/wake schedule.
The Brown University and Lifespan Institutional Review Boards approved these study protocols, which meet the ethical standards of this journal outlined by Portaluppi and colleagues (2008). A parent gave informed consent, and participants co-signed to indicate their assent to participate.
Circadian Phase
Evening salivary melatonin concentration was measured from approximately 2 mL of saliva collected every 30 min using Salivettes (Starstedt, Nümbrecht, Germany). Participants were seated for at least 5 min before each sample. Each sample was immediately centrifuged to extract saliva, which was stored in a freezer within 4 h. Light levels from standing lamps (GE Soft White 30-70-100 lamp, 30 watts) were maintained at <20 lux during the session (<16.1 lux in the angle of gaze, measured from the Pocket Light meter 840010; Sper Scientific, Scottsdale, AZ, USA).
Saliva samples were radioimmunoassayed (RIA) for melatonin concentration using Alpco melatonin assay kits (Windham, NH, USA). An individual’s samples were analyzed in the same batch. The intra-assay coefficients of variation for evening and nighttime levels of salivary melatonin were 4.1% and 4.8%, respectively. The interassay coefficients of variation for evening and nighttime levels of salivary melatonin were 6.6% and 8.4%, respectively. The functional least detectable dose of the assay, i.e., the minimal melatonin concentration in saliva measured with an intra-assay coefficient of variation <10% was 0.9 pg/mL.
Dim light melatonin onset (DLMO) phase was computed with a threshold of 4 pg/mL (Carskadon et al., 1997). DLMO phase, expressed in 24-h clock time, was determined by linear interpolation across the timepoints before and after the melatonin concentration increased to and remained >4 pg/mL.
Behavioral Sleep
Participants wore an actigraph monitor (Minimotionlogger; Ambulatory Monitoring, Ardsley, NY, USA) to quantify behavioral sleep variables and to verify compliance with their assigned sleep schedules. Activity data were collected in 1-min epochs using a Zero-Crossing Mode (ZCM) and filter setting 18 (the manufacturer’s setting for frequency bandpass of 2–3 Hz). Participants wore the device except when bathing. Participants completed a daily sleep/wake diary and called the laboratory’s time-stamped answering machine at bedtime and rise time throughout the study. Actigraph data were downloaded and sleep diary records reviewed with the participant weekly.
Actigraph data were analyzed using the Action-W2 software (version 2.3.20; Ambulatory Monitoring) to estimate sleep/wake using the validated “Sadeh” algorithm (Sadeh et al., 1994). Each sleep episode was inspected within a specific “scoring interval,” spanning 15 min before participants reported trying to fall asleep to 15 min after they reported waking up on their daily diary. The following variables were derived according to the procedures of Acebo and colleagues (1999): Sleep Start Time (1st min of ≥3 min of consecutive sleep), Sleep End Time (the last minute of ≥5 min of consecutive sleep before the end of the scoring interval), Sleep Interval (elapsed minutes from Sleep Start Time to Sleep End Time), Sleep Minutes (minutes of Sleep Interval scored as sleep). If periods of low activity persisted outside the scoring interval, three members of the research team trained on actigraphy procedures reviewed the record to achieve consensus scoring.
Procedures
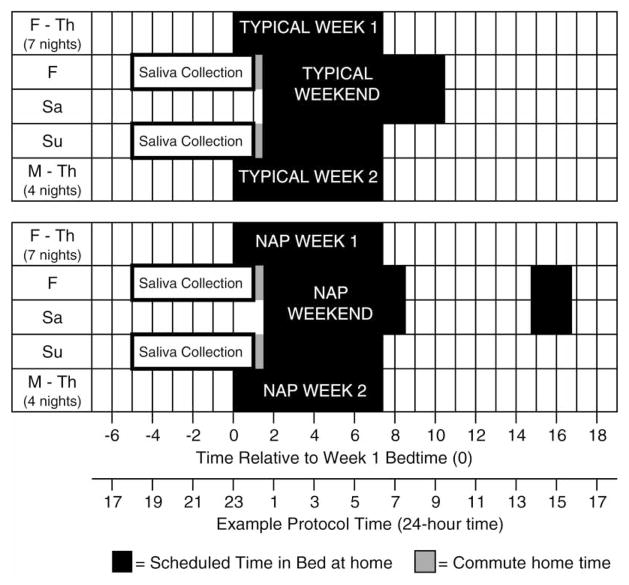
Experiment 1 was a 4-wk within-subjects counterbalanced protocol with two conditions that differed for two weekend sleep schedules (TYPICAL versus NAP). Experiment 2 was a 2-wk between-subjects protocol with two groups having identical sleep schedules and different weekend morning short-wavelength light-exposure (TYPICAL versus LIGHT). Experimental protocols are described separately below and displayed in Figure 1.
FIGURE 1.
The 4-wk, within-subject protocol for Experiment 1 is depicted relative to school night bedtime (0 h). The TYPICAL condition is illustrated on top and the NAP condition on the bottom. The order of conditions was counterbalanced; five completed the TYPICAL condition first and seven completed the NAP condition first. The protocol schedule timing varied slightly between participants. The second x-axis illustrates an exemplary protocol schedule with bedtime at 23:00 h and wake-up time at 06:30 h. For individuals on this schedule, they went to bed at 00:30 h on Friday and Saturday nights of both experimental weekends. On the “TYPICAL” weekend (top), they got out of bed at 09:30 h. On the “NAP” weekend (bottom), they got out of bed at 07:30 h and took a nap from 13:45 to 15:45 h. Salivary melatonin was collected in 30-min intervals in dim light (<20 lux) from 18:00 to 00:00 h before (Friday) and after (Sunday) each experimental weekend to determine DLMO phase. Participants included in Experiment 2 followed the 2-wk TYPICAL protocol. The LIGHT group was instructed to sit in front of a short-wavelength light device for 1 h on weekend mornings and the TYPICAL group had no light and no instruction about light exposure. See text for more detail.
Experiment 1
Participants
Twelve participants (four boys) ages ranging from 15.1 to 16.7 yrs (mean ± SD: 16.0 ± 0.4 yrs) completed Experiment 1. Of the eight females, one reported she had not begun to menstruate. Based on self-reported menstruation start dates before and after saliva collection to determine DLMO phase, we estimate that five females were in the follicular menstrual phase and two were in the luteal phase during the TYPICAL condition. We estimate that four females were in the follicular menstrual phase and three were in the luteal phase during the NAP condition. Menstrual cycle phase did not change within a given condition, but changed for all menstruating females except two between conditions. It was not possible to control for menstrual phase, and though estimated, contributes to the random variance of the study results.
Protocol
Each participant was given a fixed school-night sleep/wake schedule providing 7.5 h time in bed on seven nights preceding and four nights following a weekend (see Figure 1). The 7.5-h sleep duration was selected to represent the “average” school-night pattern of American adolescents (Carskadon, 1990; Hansen et al., 2005; National Sleep Foundation, 2006; O’Brien & Mindell, 2005; Wolfson & Carskadon, 1998), which restricts sleep to produce a sleep debt. The timing of sleep was based on each participant’s reported school-day wake-up time during the week before the study and differed slightly among participants.
Both “experimental weekends” (TYPICAL and NAP weekends) provided 9 h of “recovery” sleep, the average self-reported sleep duration on weekends for this age group (National Sleep Foundation, 2006; O’Brien & Mindell, 2005; Wolfson & Carskadon, 1998). Participants’ bedtimes were scheduled 1.5 h later on both experimental weekend nights, similar to a common weekend bedtime delay for a teenager (National Sleep Foundation, 2006; O’Brien & Mindell, 2005; Wolfson & Carskadon, 1998). On the TYPICAL weekend condition, participants arose 3 h later than their school-day schedule. On the NAP weekend condition, participants arose only 1 h later and took a 2-h sleep opportunity on Saturday and Sunday afternoons. The midpoint of the nap opportunity occurred 12 h after the midpoint of the school-night sleep period, a schedule that should have little effect on circadian phase (Buxton et al., 2000). Furthermore, this time of day corresponds with increased sleep propensity in teens (Carskadon et al., 1980), providing a strong likelihood for sleep to occur. The order of conditions was randomly counterbalanced for males and females; five participants completed the TYPICAL condition first and seven completed the NAP condition first. Three participants did not complete conditions consecutively: one had 3 wks between conditions and two had 2 wks between conditions.
Before (Friday) and after (Sunday) each experimental weekend, participants spent 6 h in the laboratory for evening saliva collection. Samples were taken at 30-min intervals starting 5 h before and ending 1 h after their scheduled school-night bedtime. This interval was selected to overlap the estimated rising phase of melatonin (Crowley et al., 2006). DLMO phase was computed for Friday and Sunday evenings. DLMO phase shift was defined as the difference between DLMO phase on Sunday evening and Friday evening; using standard nomenclature, positive values indicate a phase advance and negative values indicate a phase delay.
Participants remained awake late on the Sunday nights of the experimental weekends to allow for DLMO phase measurements, and they were instructed to go home and immediately go to bed on these nights. Thus, the 30-min commute time selection criteria meant the latest bedtime was 1.5 h after their school-night bedtime on these Sundays. Participants were instructed to wake at their scheduled school-day rise time on the following Monday mornings, which only allowed for a 6-h sleep opportunity on Sunday night.
Statistical Analysis
Sleep/wake schedule compliance was tested using variables derived from actigraphy. Individually averaged Sleep Start Time, Sleep End Time, and Sleep Minutes were compared across Week 1, Experimental Weekend, and Week 2 using a 2 (within-subjects factor, condition: TYPICAL versus NAP) by 3 (within-subjects factor, time: Week 1 versus Experimental Weekend versus Week 2) repeated-measures analysis of variance (ANOVA). When significant main effects or interactions emerged, paired t tests identified the source of the effect.
Using DLMO phase as the dependent measure, a 2 (within-subjects factor, time: Friday versus Sunday) × 2 (within-subjects factor, condition: TYPICAL versus NAP) repeated-measures ANOVA was used to test the hypothesis that a greater DLMO phase-delay shift after the TYPICAL weekend emerges compared to after the NAP weekend. This hypothesis would be supported by a significant time-by-condition interaction. Effect sizes were computed using Cohen’s d statistic (Cohen, 1988); Cohen’s “small” (d = 0.2), “medium” (d = 0.5), and “large” (d = 0.8) definitions are used to describe effect sizes.
Experiment 2
Participants
Sixteen participants (five boys) ages 15.1–16.7 yrs (16.0 ± 0.4 yrs) were included in the TYPICAL group (Figure 1), 12 of whom were drawn from Experiment 1. Seventeen participants (five boys) ages 15.2–17.0 yrs (15.9 ± 0.5 yrs) were included in the LIGHT group. Of the 11 females in the TYPICAL group, one reported she had not begun to menstruate (same female from Experiment 1), and data for one female are unavailable. Based on self-reported menstruation start dates, we estimate that five females were in the follicular menstrual phase and four in the luteal phase during saliva collection nights. Of the 12 females in the LIGHT group, we estimate six were in the follicular menstrual phase and six in the luteal phase. Menstrual cycle phase did not differ between saliva collection nights for an individual female.
Protocol
The LIGHT group completed a 2-wk protocol that required them to keep the TYPICAL weekend sleep schedule as in Experiment 1 (Figure 1) and to sit in front of a small light-emitting diode (LED)-based, short-wavelength light device (GoLite P2; Apollo Health, Orem, UT, USA) for 1 h upon waking on Saturday and Sunday mornings. The manufacturer specifications indicate that the LEDs of the GoLite device emit light in the 454–484 nm range, and the device is capable of 10 intensity levels ranging from 10% (lowest intensity) and increasing in 10% increments to 100%. Intensity was 30% for the first five participants; however, three of these participants manifested a phase-delay shift. Thus, subsequent participants were instructed to set the intensity level at 100%. When measured at a distance of 18 inches from the eye in darkness, irradiance at 30% measured 156.2 μW/cm2 and at 100% measured 540.8 μW/cm2. These values do not measure irradiance levels of the current experiment, because participants were not in complete darkness; rather, these values illustrate the relative irradiance differences between the 30% and 100% intensity levels.
Participants were instructed to place the light device 24 inches from their eyes by using a 24-inch string attached to the device to measure the distance. Participants also viewed their faces with a small pocket mirror to see a bluish hue, which meant that the device was angled toward the face when the light was on. Participants were asked to turn the light on only for the scheduled times, during which they were permitted to watch television, read, or work on a computer, and were allowed to leave the light briefly (<10 min) after the first 30 min of exposure. Participants recorded the time of lights-on, lights-off, and times they left the light if any.
Participants were not randomly assigned to the LIGHT group or the TYPICAL group, because Experiment 2 was a follow-up study to Experiment 1. With the exception of three participants, the TYPICAL group completed the study before the LIGHT group.
Statistical Analysis
Results from the LIGHT group were compared to a TYPICAL group, which included participants from Experiment 1 and additional participants subsequently recruited to complete the 2-wk TYPICAL protocol only. A 2 (between-subjects factor, group: TYPICAL versus LIGHT) × 3 (within-subjects factor, time: Week 1 versus Experimental Weekend versus Week 2) mixed-model ANOVA tested sleep/wake schedule compliance using the same actigraphy variables as Experiment 1. When significant main effects or interactions emerged, independent t-tests identified the source of the effect. A 2 (within-subjects factor, time: Friday versus Sunday) × 2 (between-subjects factor, condition: TYPICAL versus LIGHT) mixed-model ANOVA tested the hypothesis that a larger DLMO phase-delay shift emerges after the TYPICAL weekend compared to after the LIGHT weekend. As in Experiment 1, effect sizes were computed using Cohen’s d statistic (Cohen, 1988).
RESULTS
Experiment 1
Study Schedule Verification
Sleep schedules were verified for each condition using actigraphic measurements, as shown in Table 1. As designed, nocturnal Sleep Start Times were significantly later on the experimental weekends compared to Weeks 1 and 2 (time effect: F(2, 22) = 881.50, p < .001), Sleep Start Time did not differ between conditions (F(1, 11) = 0.01, p = .939), and a condition-by-time interaction was not found (F(2, 22) = 1.37, p = .276). Nocturnal Sleep End Times also reflected the study design, with a significant condition-by-time interaction (F(2, 22) = 136.07, p < .001), such that sleep ended later on the TYPICAL weekend compared to Week 1 (t(11) = 20.98, p < .001) and Week 2 (t(11) = 24.73, p < .001), and sleep ended later on the NAP weekend compared to Week 1 (t(11) = 19.10, p < .001) and Week 2 (t(11) = 30.03, p < .001). Also by design, the TYPICAL weekend Sleep End Times were later compared to the NAP condition (t(11) = 13.35, p < .001). Actigraphically recorded sleep also confirmed that participants had fewer nocturnal Sleep Minutes on the NAP weekend nights compared to the TYPICAL weekend nights (t(11) = 11.23, p < .001), but when nocturnal and daytime nap minutes were combined for the NAP weekend, total Sleep Minutes (488 ± 37 min) did not differ from the TYPICAL weekend (489 ± 27 min) (t(11) = 0.07, p = .943). Total daily sleep minutes showed a significant time effect, such that sleep minutes were greater on experimental weekends compared to Weeks 1 and 2 (time effect: F(2, 22) = 117.06, p < .001). Also as expected, Sunday night Sleep Start Time (t(11) = 0.63, p = .539), Sleep End Time (t(11) = 1.05, p =.315), and Sleep Minutes (t(11) =1.94, p = .078) did not differ between conditions. These analyses confirm that participants were able to maintain the prescribed sleep/wake schedules, and were compliant.
TABLE 1.
Experiment 1 actigraphy.
| TYPICAL (N = 12) | ||||
|---|---|---|---|---|
| Week 1 (7 nights) | Experimental weekend (2 nights) | Sunday (1 night) | Week 2 (4 nights) | |
| Sleep Start Time* | ||||
| Mean | 22:57 h | 00:33 h | 00:34 h | 23:01 h |
| SD | 23 min | 23 min | 21 min | 20 min |
| Minimum | 22:28 h | 00:04 h | 00:07 h | 22:42 h |
| Maximum | 23:41 h | 01:12 h | 01:13 h | 23:49 h |
| Sleep End Time*a | ||||
| Mean | 06:16 h | 09:09 h | 06:25 h | 06:18 h |
| SD | 23 min | 31 min | 22 min | 21 min |
| Minimum | 05:48 h | 07:52 h | 05:55 h | 05:50 h |
| Maximum | 07:05 h | 09:57 h | 07:01 h | 07:07 h |
| Sleep Minutes | ||||
| Mean | 419 | 489 | 338 | 420 |
| SD | 16 | 27 | 26 | 14 |
| Minimum | 391 | 438 | 299 | 395 |
| Maximum | 445 | 522 | 381 | 444 |
| NAP (N = 12)
|
|||||
|---|---|---|---|---|---|
| Week 1 (7 nights) | Experimental Weekend (2 nights/days) | Sunday (1 night) | Week 2 (4 nights) | ||
| Nocturnal | Daytime | ||||
| Sleep Start Time* | |||||
| Mean | 23:00 h | 00:30 h | 13:51 h | 00:37 h | 23:01 h |
| SD | 20 min | 21 min | 19 min | 23 min | 20 min |
| Minimum | 22:38 h | 00:05 h | 13:23 h | 00:08 h | 22:35 h |
| Maximum | 23:50 h | 01:17 h | 14:33 h | 01:32 h | 23:44 h |
| Sleep End Time*a | |||||
| Mean | 06:19 h | 07:19 h | 15:30 h | 06:18 h | 06:15 h |
| SD | 19 min | 23 min | 26 min | 28 min | 22 min |
| Minimum | 05:50 h | 06:46 h | 14:46 h | 05:48 h | 05:53 h |
| Maximum | 06:57 h | 08:05 h | 16:20 h | 07:35 h | 07:11 h |
| Sleep Minutes | |||||
| Mean | 420 | 394 | 94 | 325 | 414 |
| SD | 17 | 16 | 24 | 15 | 15 |
| Minimum | 396 | 367 | 45 | 299 | 378 |
| Maximum | 455 | 415 | 119 | 350 | 437 |
Times given in 24-h clock time. SDs given in minutes and computed across participants.
Significant condition-by-time interaction; significant differences from post hoc tests: TYPICAL weekend > TYPICAL Week 1 = TYPICAL Week 2; NAP weekend > NAP Week 1 = NAP Week 2; TYPICAL weekend > NAP weekend.
Circadian Phase
Because DLMO phase computation was not possible on at least one evening for 2 participants in Experiment 1, we examined DLMO data of 10 participants. As shown in Table 2, mean DLMO phase on Friday was 21:04 h for both conditions. Identical schedules for the first week of each condition allowed us to examine the reliability of DLMO phase as a circadian phase marker in this sample by computing correlation coefficients for DLMO phases measured during the Friday sessions (Figure 1). A significant positive correlation was found between the two assessments (r = .79, p = .004), confirming the reliability of DLMO phase under conditions of a fixed sleep/wake schedule before sampling. Furthermore, the absence of a DLMO phase difference across these assessments provides evidence that circadian phase changes seen after an experimental weekend did not carry over to the subsequent assessment.
TABLE 2.
Experiment 1 DLMO phase.
| TYPICAL (N = 10) | NAP (N = 10) | |
|---|---|---|
| Friday DLMO phase* | ||
| Mean | 21:04 h | 21:04 h |
| SD | 69 min | 65 min |
| Minimum | 18:50 h | 19:22 h |
| Maximum | 22:40 h | 22:40 h |
| Sunday DLMO phase* | ||
| Mean | 21:49 h | 21:45 h |
| SD | 83 min | 79 min |
| Minimum | 19:23 h | 19:25 h |
| Maximum | 23:20 h | 23:51 h |
| Phase shift (min) | ||
| Mean | −45 | −41 |
| SD | 31 | 34 |
| Minimum | 4 | −3 |
| Maximum | −97 | −92 |
Times given in 24-h clock time. SDs given in minutes and computed across participants.
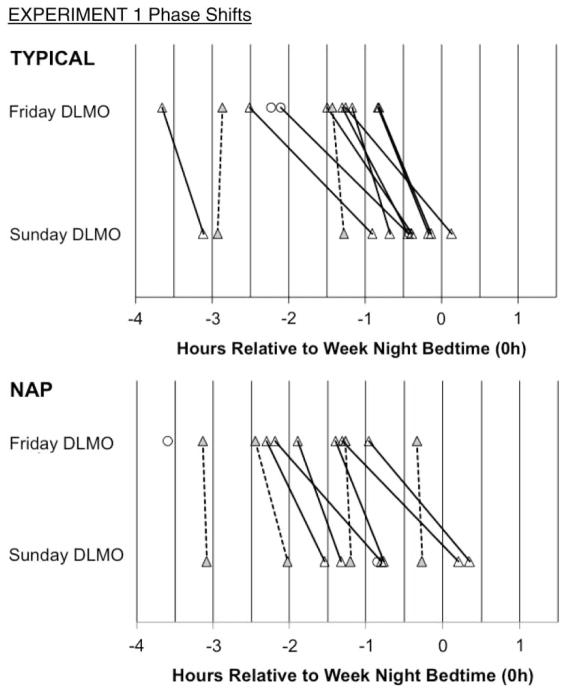
Figure 2 illustrates the Friday and Sunday DLMO phases relative to participants’ scheduled bedtime on weeks preceding (Week 1) and following (Week 2) the experimental weekends, as well as phase shifts over experimental weekends. As shown, DLMO phase shifted to a later time across the weekend for both conditions in nearly every participant, and none manifested a phase advance. Two participants in the TYPICAL condition and four different participants in the NAP condition showed minimal phase changes (weekend phase shift <30 min, the salivary melatonin sampling rate) across the experimental weekend. Mean DLMO phase shifted later by 45 (± 31) min after the TYPICAL weekend and by 41 (± 34) min after the NAP weekend (time effect: F(1, 9) = 34.00, p < .001, effect size = 1.35, “large”), with no significant differences between the experimental weekends (time-by-condition interaction: F(1, 9) = 0.07, p = .769).
FIGURE 2.
Individual DLMO phases measured on Friday and Sunday evenings are plotted relative to scheduled bedtime on school nights (negative = earlier than bedtime; positive = later than bedtime) for Experiment 1. Lines connect Friday and Sunday values to illustrate individual phase shifts; however, we do not assume a linear time trajectory. Solid lines indicate a phase delay shift ≥30 min, and hashed lines indicate a phase delay shift <30 min. Circles illustrate those whose incomplete DLMO on a night excluded them from statistical analyses.
Experiment 2
Study Schedule Verification
As in Experiment 1, the manipulation of weekend sleep timing and duration changes were confirmed with actigraphy (Table 3): nocturnal Sleep Start Times were later for the experimental weekends compared to Weeks 1 and 2 (time effect: F(2, 62) = 1201.82, p < .001), Sleep Start Times did not differ between groups (F(1, 31) = 0.08, p = .777), and a group-by-time interaction was not found (F(2, 62) = 0.93, p = .399). Furthermore, Sleep End Times reflected the study design—later on both experimental weekends compared to Weeks 1 and 2 (time effect: F(2, 62) = 1755.77, p < .001), no difference between groups (group effect: F(1, 31) = 0.14, p = .710), and no group-by-time interaction (F(2, 62) = 0.94, p = .395). Sleep Minutes were greater on weekends compared to the weeks preceding and following (time effect: F(2, 62) = 200.83, p < .001), did not differ between groups (group effect: F(1, 31) = 0.12, p = .727), and showed no group-by-time interaction (F(2, 62) = 0.60, p = .551). Finally, Sunday night Sleep Start Times (t(31) = 0.14, p = .890), Sleep End Times (t(31) = 0.17, p = .864), and Sleep Minutes (t(31) = 0.42, p =.678) did not differ between groups.
TABLE 3.
Experiment 2 actigraphy.
| TYPICAL (N = 16) | ||||
|---|---|---|---|---|
| Week 1 (7 nights) | Experimental weekend (2 nights) | Sunday (1 night) | Week 2 (4 nights) | |
| Sleep Start Time* | ||||
| Mean | 22:59 h | 00:37 h | 00:41 h | 23:03 h |
| SD | 25 min | 29 min | 37 min | 22 min |
| Minimum | 22:28 h | 23:56 h | 23:57 h | 22:42 h |
| Maximum | 23:55 h | 01:50 h | 02:32 h | 23:49 h |
| Sleep End Time* | ||||
| Mean | 06:18 h | 09:11 h | 06:23 h | 06:18 h |
| SD | 25 min | 37 min | 27 min | 26 min |
| Minimum | 05:48 h | 07:52 h | 05:30 h | 05:25 h |
| Maximum | 07:09 h | 10:33 h | 07:04 h | 07:07 h |
| Sleep Minutes | ||||
| Mean | 420 | 489 | 330 | 415 |
| SD | 14 | 25 | 30 | 22 |
| Minimum | 391 | 438 | 268 | 350 |
| Maximum | 445 | 522 | 381 | 444 |
| LIGHT (N = 17) | ||||
|---|---|---|---|---|
| Week 1 (7 nights) | Experimental weekend (2 nights) | Sunday (1 night) | Week 2 (4 nights) | |
| Sleep Start Time* | ||||
| Mean | 23:04 h | 00:36 h | 00:39 h | 23:06 h |
| SD | 25 min | 24 min | 28 min | 31 min |
| Minimum | 22:33 h | 23:58 h | 00:00 h | 22:20 h |
| Maximum | 00:01 h | 01:26 h | 01:40 h | 00:24 h |
| Sleep End Time* | ||||
| Mean | 06:25 h | 09:09 h | 06:25 h | 06:24 h |
| SD | 26 min | 29 min | 23 min | 25 min |
| Minimum | 05:53 h | 08:25 h | 05:50 h | 05:46 h |
| Maximum | 07:14 h | 10:10 h | 07:08 h | 07:18 h |
| Sleep Minutes | ||||
| Mean | 417 | 483 | 334 | 417 |
| SD | 24 | 35 | 15 | 25 |
| Minimum | 345 | 390 | 305 | 360 |
| Maximum | 457 | 526 | 357 | 446 |
Times given in 24-h clock time. SDs given in minutes and computed across participants.
Circadian Phase
Fourteen participants in the TYPICAL group and 14 participants in the LIGHT group had useable salivary DLMO phase data on both nights for analysis. Four participants in the LIGHT group set the light device at the 30% intensity level (“30% light group”), and 10 participants set the device at a 100% intensity level (“100% light group”). A comparison of DLMO phase between these groups showed a significant time effect (time effect: F(1, 12) = 16.81, p = .001), indicating that both groups phase delayed over the weekend, yet DLMO phase did not differ between the light intensity groups (group effect: F(1, 12) = 0.02, p = .902), nor was an interaction found (F(1, 12) = 1.09, p = .316). Therefore, data from the 30% and 100% light groups were combined in subsequent
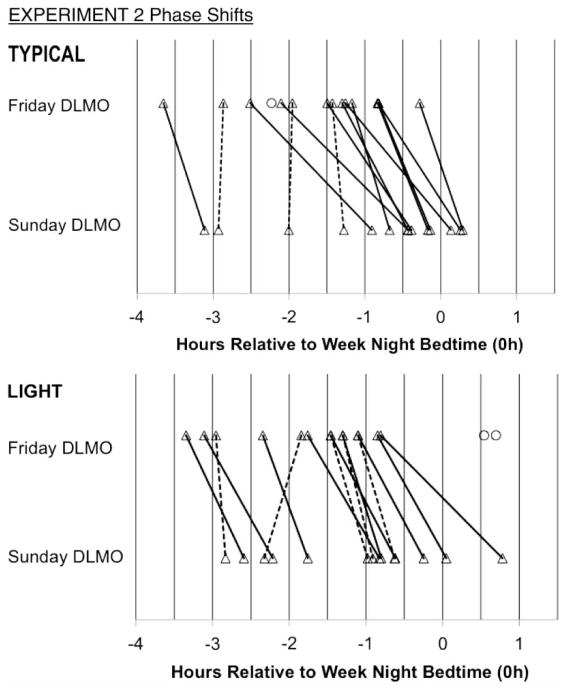
Figure 3 illustrates Friday and Sunday DLMO phases relative to the participants’ scheduled bedtime on Weeks 1 and 2, as well as phase shifts over the weekends. DLMO phase shifted to a later time across weekends in all but three participants in the TYPICAL group and four in the LIGHT group, who showed a minimal phase change (weekend phase shift <30 min). As shown in Table 4, mean DLMO phase shifted later by 46 (± 34) min after the TYPICAL weekend and by 38 (± 28) min after the LIGHT weekend (time effect: F(1, 26) = 51.24, p < .001, effect size = 1.37, “large”). Nevertheless, DLMO phase did not differ between groups (F(1, 26) = 0.18, p = .673), nor did the magnitude of the DLMO phase shift over the experimental weekend (time-by-group interaction: F(1, 26) = 0.48, p = .496) (Table 4).
FIGURE 3.
Individual DLMO phases measured on Friday and Sunday evenings are plotted relative to scheduled bedtime on school nights of Experiment 2. Lines connect Friday and Sunday values to illustrate individual phase shifts; however, we do not assume a linear trajectory. Solid lines indicate a phase delay shift ≥30 min, and hashed lines indicate a phase delay shift <30 min or a phase advance shift (n = 1 in LIGHT group; phase shift = +29 min). Circles illustrate those whose incomplete DLMO on a night excluded them from statistical analyses. DLMO phase shifts from participants in the TYPICAL condition of Experiment 1 are included in the TYPICAL group of Experiment 2.
TABLE 4.
Experiment 2 DLMO phase.
| TYPICAL (N = 14) | LIGHTa (N = 14) | |
|---|---|---|
| Friday DLMO phase* | ||
| Mean | 21:13 h | 21:07 h |
| SD | 67 min | 52 min |
| Minimum | 18:50 h | 19:10 h |
| Maximum | 23:13 h | 21:59 h |
| Sunday DLMO phase* | ||
| Mean | 21:59 h | 21:44 h |
| SD | 78 min | 65 min |
| Minimum | 19:23 h | 19:55 h |
| Maximum | 23:47 h | 23:32 h |
| Phase shift (min) | ||
| Mean | −46 | −38 |
| SD | 34 | 28 |
| Minimum | 4 | 29 |
| Maximum | −100 | −95 |
30% and 100% brightness combined.
Times given in 24-h clock time. SDs given in minutes and computed across participants.
DISCUSSION
The primary aim of these experiments was to examine whether interventions designed to allow older adolescents social time and recovery sleep on weekends could also anchor the timing of the circadian system measured as DLMO phase. In both experiments, the comparison condition was a “typical” weekend sleep schedule, with bedtime 1.5 h later than school days, wake-up time 3 h later, and total sleep 1.5 h longer on Friday and Saturday nights. Most participants exhibited a delay shift of DLMO phase under these conditions, supporting the original expectation that later weekend bedtime and wake-up time commonly observed in older adolescents produce a phase delay. Unexpectedly, however, similar phase delay shifts occurred in the NAP condition of Experiment 1 and the LIGHT group of Experiment 2. In the former case wake-up time on the weekend was set closer to school-day wake-up time, and in the latter case morning short-wavelength light exposure occurred at the more delayed wake-up time. In both cases, the goal was to expose participants to ambient light early in the day, targeting the phase-advance portion of the light-phase response curve (PRC) and provide an entrainment cue to anchor phase.
The delay of DLMO phase after the TYPICAL weekend sleep patterns in both experiments is consistent with previous reports in young adults (Revell et al., 2005; Taylor et al., 2008; Yang et al., 2001). Our findings in adolescents and those in adults indicate that DLMO phase undergoes consistent, moderate delay shifts after late weekend sleep episodes. Thus, many teenagers who keep this late recovery sleep schedule on weekends may feel as if they are jet lagged early in the school week, as they try to advance their rhythms to re-entrain to an early clock time. Furthermore, we designed the TYPICAL protocol to simulate the sleep behavior of the “average” American teenager; however, many teens keep much later and longer weekend sleep schedules than that of the current study (National Sleep Foundation, 2006). For example, of 892 9th- and 10th-grade students surveyed across the United States, 55% reported 9 h or more time in bed on weekends, and 44% reported going to bed 2 h or more later on weekend nights compared to school nights (National Sleep Foundation, 2006). Such schedules may result in greater delay shifts across the weekend than in our experiment and perhaps difficulty readjusting to the subsequent school-week schedule.
The lack of difference in the magnitude of phase shifts between the TYPICAL and NAP conditions and the TYPICAL and LIGHT groups was unexpected and invites further discussion. Because the original hypotheses were derived from general properties of a light-phase response curve, the results will be discussed within this framework also. A PRC to short-wavelength light does not exist in the literature, and a PRC to any type of light does not exist for adolescents. Of the published PRCs to light for adult humans, the three-pulse white light PRC of Revell and Eastman (2005) may be the most appropriate for comparison, because the light stimulus was shorter (2 h) and was not as bright (~3500 lux) as the light stimuli of other published light PRCs (c.f., Czeisler et al., 1989; Khalsa et al., 2003). The Revell and Eastman PRC (2005) shows that advance shifts occur when light begins 8–11 h after the DLMO.
The NAP weekend sleep schedule succeeded in that participants got out of bed earlier than in the TYPICAL condition, and wake-up time occurred 8.8–11.6 h after the DLMO measured on Friday night. This means that ambient light exposure on these mornings was timed within the phase-advance portion of the adult PRC to light for many of these youngsters. Nonetheless, DLMO phase still shifted later in the NAP condition. We did not collect quantitative light exposure data to determine differences in illuminance or duration of ambient light exposure in the morning; thus, participants may not have been exposed to enough morning, phase-stabilizing, light.
We designed the LIGHT weekend intervention to be easy for teenagers to follow; thus, we kept the sleep schedule the same and added 1 h of morning light. When we examine the timing of light administration relative to circadian phase post hoc, however, the light start times ranged between 11.5 and 14.0 h after DLMO phase measured on Friday evening. According to the adult white-light PRC, the timing of the light was too late to target the phase-advance region, and DLMO phase shifted later in the LIGHT group. Therefore, weekend wake-up time and light administration must be earlier for light to phase advance or stabilize the circadian system. Perhaps, a better intervention to stabilize the circadian system, allow recovery sleep, and allow social time is the combination of the NAP and LIGHT schedule by adding a light stimulus to the NAP weekend mornings.
When teens struggle with early school schedules, a common recommendation is to keep a regular sleep schedule on weekends and vacations. One of the National Sleep Foundation’s “tips for teens” (National Sleep Foundation, 2000) is to avoid delaying bedtimes by more than 1 h and to wake within 2 h of their regular sleep schedule on weekends. They also recommend an early afternoon nap to cope with daytime sleepiness. These suggestions are similar to the intervention tested in the NAP condition (stable wake-up time and a nap to obtain more sleep). These recommendations are based on the same rationale we based our study hypotheses—a stable weekend wake-up time and exposure to ambient light in the morning should keep the circadian system from delaying. Results from the current study of healthy adolescents, however, reveal that this intervention does not keep the circadian system entrained. Thus, alternative approaches for weekend sleep recovery may need to be tested.
Delayed weekend sleep timing is likely to impact daytime functioning, such as cognition, alertness, and mood during the subsequent week. The resulting delayed circadian phase after late weekend sleep drives alertness later into the evening, and it may make falling asleep at an early clock time more difficult on school nights, especially at the beginning of the week. Late sleep onset and early wake times for school reduces the amount of time for sleep, which may, in turn, contribute to decrements in academic performance (Fallone et al., 2005; National Sleep Foundation, 2006; Wolfson & Carskadon, 1998, 2003), alertness (Carskadon et al., 1981; Fallone et al., 2001; O’Brien & Mindell, 2005; Wolfson & Carskadon, 1998), and mood (National Sleep Foundation, 2006; Wolfson & Carskadon, 1998). Indeed, a recent study showed that depression and suicidal ideation are associated with short sleep duration and later parent-set bedtimes in a large group of American adolescents (Gangwisch et al., 2010). Restricted school-night sleep may also increase the likelihood of “recovery” sleep on weekends; thus, the cycle of late and long weekend sleep followed by early and short weekday sleep persists. Survey data indicate that this variability between weekday and weekend sleep patterns is associated with poor academic performance, depressed mood, and risk-taking behavior (O’Brien & Mindell, 2005; Wolfson & Carskadon, 1998). Experimental manipulations of weekend sleep have also shown behavioral changes during subsequent weekdays in adults. For example, Yang and Spielman (2001) reported that after a 2-h weekend bedtime and wake-up time delay, young adults rated themselves as more sleepy, irritable, and angry compared to a group who kept their weekday sleep schedule over the weekend. Furthermore, their weekend delay group also performed worse on a word recall task on Monday morning compared to the habitual sleep group (Yang & Spielman, 2001). More recently, Taylor and colleagues (2008) reported that young adults who delayed wake-up time by an average of 3 h rated themselves sleepier compared to a weekend when they kept their habitual weekday sleep times. This difference in daytime sleepiness persisted for 3 days after the weekend sleep change. We could not make a valid test of the behavioral effects of the moderate phase delay of DLMO in this study, because Sunday night sleep was significantly shorter after the weekend manipulation due to salivary melatonin collection late into the evening. Based on these previous studies, however, anchoring phase over a weekend may help alleviate decrements of cognition, alertness, and mood during the subsequent school week.
If phase anchoring is an important outcome for subsequent school-week behaviors, these experiments indicate that keeping bedtime stable during the weekend may be a critical feature of weekend sleep schedules. Our participants went to bed 1.5 h later on the intervention weekends compared to school nights to allow for social time, and the circadian system showed a phase-delay shift after all of these weekends, even if only a 1-h wake-up-time delay was permitted. Similarly, a previous study of adults (Burgess & Eastman, 2004) who kept either early (22:00 h) or late (01:00 h) bedtimes along with consistent wake-up times (07:00 h) for 7 to 19 days showed that the DLMO phase shifted later by an average of 38 min after late compared to early bedtimes. This study and the current experiments indicate that to anchor DLMO phase, weekend recovery sleep schedules not only need to enforce a stable wake-up time but also need to enforce a stable bedtime.
In summary, data from this study show that older adolescents phase-delay shift after keeping a commonly observed late and long weekend recovery sleep schedule. Neither a relatively early wake-up time nor morning short-wavelength light exposure at a late weekend wake-up time were sufficient to keep circadian phase stable across a weekend. The number of participants in both experiments was relatively small, however, so that these findings need to be replicated with a larger sample size, and by another laboratory. Although the modifications to weekend recovery sleep did not keep the circadian timing system stable as we predicted, these negative results raise important research questions: (i) Are late bedtimes and potentially late exposure to light stronger circadian phase-shifting cues compared to morning ambient light exposure for adolescents? (ii) Is morning ambient light exposure effective to phase shift adolescents? (iii) What combination of factors may contribute to the inability to keep circadian phase stable across weekend recovery sleep interventions? Our findings underscore the need for future studies to understand and describe responses to light in this adolescent age group.
Acknowledgments
This work was supported by a predoctoral Ruth L. Kirschstein National Research Service Award (NRSA) from the National Institutes of Mental Health (NIMH) (1 F31 MH078662-01) awarded to S. Crowley and a grant from Apollo Health, Inc., awarded to S. Crowley. We thank Christine Acebo, PhD, Ronald Seifer, PhD, Russell Church, PhD, and David Berson, PhD, for their insightful comments on this project, Isaac Gross and Jacqueline Muñoz for assisting with data collection, and Denise Maceroni for analyzing saliva samples for melatonin. We would also like to acknowledge the participants and their families for their dedication and cooperation while participating in this research project.
Footnotes
This work was completed at the E.P. Bradley Hospital Sleep and Chronobiology Research Laboratory, Providence, Rhode Island, USA.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Acebo C, Sadeh A, Seifer R, Tzischinsky O, Wolfson AR, Hafer A, Carskadon MA. Estimating sleep patterns with activity monitoring in children and adolescents: how many nights are necessary for reliable measures? Sleep. 1999;22:95–103. doi: 10.1093/sleep/22.1.95. [DOI] [PubMed] [Google Scholar]
- Beijamini F, Silva AG, Peixoto CA, Louzada FM. Influence of gender on psychomotor vigilance task performance by adolescents. Braz J Med Biol Res. 2008;41:734–738. doi: 10.1590/s0100-879x2008000800016. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001a;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Rollag MD, Greeson J, Byrne B, Glickman G, Gerner E, Sanford B. Human melatonin regulation is not mediated by the three cone photopic visual system. J Clin Endocrinol Metab. 2001b;86:433–436. doi: 10.1210/jcem.86.1.7277. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Eastman CI. Early versus late bedtimes phase shift the human dim light melatonin rhythm despite a fixed morning lights on time. Neurosci Lett. 2004;356:115–118. doi: 10.1016/j.neulet.2003.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Eastman CI. The dim light melatonin onset following fixed and free sleep schedules. J Sleep Res. 2005;14:229–237. doi: 10.1111/j.1365-2869.2005.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Savic N, Sletten T, Roach G, Gilbert SS, Dawson D. The relationship between the dim light melatonin onset and sleep on a regular schedule in young healthy adults. Behav Sleep Med. 2003;1:102–114. doi: 10.1207/S15402010BSM0102_3. [DOI] [PubMed] [Google Scholar]
- Buxton OM, L’Hermite-Baleriaux M, Turek FW, Van Cauter E. Daytime naps in darkness phase shift the human circadian rhythms of melatonin and thyrotropin secretion. Am J Physiol Regul Integr Comp Physiol. 2000;278:R373–R382. doi: 10.1152/ajpregu.2000.278.2.R373. [DOI] [PubMed] [Google Scholar]
- Carskadon MA. Adolescent sleepiness: increased risk in a high-risk population. Alcohol Drugs Driving. 1989–1990;5/6:317–328. [Google Scholar]
- Carskadon MA. Patterns of sleep and sleepiness in adolescents. Pediatrician. 1990;17:5–12. [PubMed] [Google Scholar]
- Carskadon MA, Harvey K, Duke P, Anders TF, Litt IF, Dement WC. Pubertal changes in daytime sleepiness. Sleep. 1980;2:453–460. doi: 10.1093/sleep/2.4.453. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Harvey K, Dement WC. Acute restriction of nocturnal sleep in children. Percept Mot Skills. 1981;53:103–112. [Google Scholar]
- Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. An approach to studying circadian rhythms of adolescent humans. J Biol Rhythms. 1997;12:278–289. doi: 10.1177/074873049701200309. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: implications for behavior. Ann N Y Acad Sci. 2004;1021:276–291. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. p. 567. [Google Scholar]
- Crowley SJ, Acebo C, Fallone G, Carskadon MA. Estimating dim light melatonin onset (DLMO) phase in adolescents using summer or school-year sleep/wake schedules. Sleep. 2006;29:1632–1641. doi: 10.1093/sleep/29.12.1632. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Kronauer RE, Allan JS, Duffy JF, Jewett ME, Brown EN, Ronda JM. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–1333. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ, Hall EF, Czeisler CA. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J Invest Med. 1999;47:141–150. [PMC free article] [PubMed] [Google Scholar]
- Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. J Neurosci. 1993;13:1065–1079. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallone G, Acebo C, Arnedt JT, Seifer R, Carskadon MA. Effects of acute sleep restriction on behavior, sustained attention, and response inhibition in children. Percept Mot Skills. 2001;93:213–229. doi: 10.2466/pms.2001.93.1.213. [DOI] [PubMed] [Google Scholar]
- Fallone G, Acebo C, Seifer R, Carskadon MA. Experimental restriction of sleep opportunity in children: effects on teacher ratings. Sleep. 2005;28:1279–1285. doi: 10.1093/sleep/28.12.1561. [DOI] [PubMed] [Google Scholar]
- Gangwisch JE, Babiss LA, Malaspina D, Turner JB, Zammit GK, Posner K. Earlier parental set bedtimes as a protective factor against depression and suicidal ideation. Sleep. 2010;33:97–106. doi: 10.1093/sleep/33.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. J Sleep Res. 2002;11:191–199. doi: 10.1046/j.1365-2869.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- Hagenauer MH, Perryman JI, Lee TM, Carskadon MA. Adolescent changes in the homeostatic and circadian regulation of sleep. Dev Neurosci. 2009;31:276–284. doi: 10.1159/000216538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Janssen I, Schiff A, Zee PC, Dubocovich ML. The impact of school daily schedule on adolescent sleep. Pediatrics. 2005;115:1555–1561. doi: 10.1542/peds.2004-1649. [DOI] [PubMed] [Google Scholar]
- Henschel A, Lack L. Do many adolescents sleep poorly or just too late? [Abstract] Sleep Res. 1987;16:354. [Google Scholar]
- Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005;28:1446–1454. doi: 10.1093/sleep/28.11.1446. [DOI] [PubMed] [Google Scholar]
- Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549.3:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loessl B, Valerius G, Kopasz M, Hornyak M, Riemann D, Voderholzer U. Are adolescents chronically sleep-deprived? An investigation of sleep habits of adolescents in the Southwest of Germany. Child Care Health Dev. 2008;34:549–556. doi: 10.1111/j.1365-2214.2008.00845.x. [DOI] [PubMed] [Google Scholar]
- Manber R, Pardee RE, Bootzin RR, Kuo T, Rider AM, Rider SP, Bergstrom L. Changing sleep patterns in adolescents [Abstract] Sleep Res. 1995;24:106. [Google Scholar]
- Martin SK, Eastman CI. Sleep logs of young adults with self-selected sleep times predict the dim light melatonin onset. Chronobiol Int. 2002;19:695–707. doi: 10.1081/cbi-120006080. [DOI] [PubMed] [Google Scholar]
- National Sleep Foundation. Adolescent sleep needs and patterns: research report and resource guide. 2000 [Cited 27 July 2010]. Available at: www.sleepfoundation.org.
- National Sleep Foundation. 2006 sleep in America poll. A National Sleep Foundation poll. 2006 Available at: www.sleepfoundation.org.
- O’Brien EM, Mindell JA. Sleep and risk-taking behavior in adolescents. Behav Sleep Med. 2005;3:113–133. doi: 10.1207/s15402010bsm0303_1. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Touitou Y, Smolensky MH. Ethical and methodological standards for laboratory and medical biological rhythm research. Chronobiol Int. 2008;25:999–1016. doi: 10.1080/07420520802544530. [DOI] [PubMed] [Google Scholar]
- Randler C. Differences in sleep and circadian preference between Eastern and Western German adolescents. Chronobiol Int. 2008;25:565–575. doi: 10.1080/07420520802257794. [DOI] [PubMed] [Google Scholar]
- Revell VL, Eastman CI. How to trick mother nature into letting you fly around or stay up all night. J Biol Rhythms. 2005;20:353–365. doi: 10.1177/0748730405277233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell VL, Kim H, Tseng CY, Crowley SJ, Eastman CI. Circadian phase determined from melatonin profiles is reproducible after 1 wk in subjects who sleep later on weekends. J Pineal Res. 2005;39:195–200. doi: 10.1111/j.1600-079X.2005.00236.x. [DOI] [PubMed] [Google Scholar]
- Russo PM, Bruni O, Lucidi F, Ferri R, Violani C. Sleep habits and circadian preference in Italian children and adolescents. J Sleep Res. 2007;16:163–169. doi: 10.1111/j.1365-2869.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- Saarenpaa-Heikkila OA, Rintahaka PJ, Laippala PJ, Koivikko MJ. Sleep habits and disorders in Finnish schoolchildren. J Sleep Res. 1995;4:173–182. doi: 10.1111/j.1365-2869.1995.tb00166.x. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17:201–207. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 1989;74:728–738. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Jenni OG, Acebo C, Carskadon MA. Sleep tendency during extended wakefulness: insights into adolescent sleep regulation and behavior. J Sleep Res. 2005;14:239–244. doi: 10.1111/j.1365-2869.2005.00467.x. [DOI] [PubMed] [Google Scholar]
- Taylor A, Wright HR, Lack LC. Sleeping-in on the weekend delays circadian phase and increases sleepiness the following week. Sleep Biol Rhythms. 2008;6:172–179. [Google Scholar]
- Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorleifsdottir B, Bjornsson JK, Benediktsdottir B, Gislason T, Kristbjarnarson H. Sleep and sleep habits from childhood to young adulthood over a 10-year period. J Psychosom Res. 2002;53:529–537. doi: 10.1016/s0022-3999(02)00444-0. [DOI] [PubMed] [Google Scholar]
- Urner M, Tornic J, Bloch KE. Sleep patterns in high school and university students: a longitudinal study. Chronobiol Int. 2009;26:1222–1234. doi: 10.3109/07420520903244600. [DOI] [PubMed] [Google Scholar]
- Van den Bulck J. Television viewing, computer game playing, and Internet use and self-reported time to bed and time out of bed in secondary-school children. Sleep. 2004;27:101–104. doi: 10.1093/sleep/27.1.101. [DOI] [PubMed] [Google Scholar]
- Warman VL, Dijk DJ, Warman GR, Arendt J, Skene DJ. Phase advancing human circadian rhythms with short wavelength light. Neurosci Lett. 2003;342:37–40. doi: 10.1016/s0304-3940(03)00223-4. [DOI] [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69:875–887. [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA. Understanding adolescents’ sleep patterns and school performance: a critical appraisal. Sleep Med Rev. 2003;7:491–506. doi: 10.1016/s1087-0792(03)90003-7. [DOI] [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA, Acebo C, Seifer R, Fallone G, Labyak SE, Martin JL. Evidence for the validity of a sleep habits survey for adolescents. Sleep. 2003;26:213–216. doi: 10.1093/sleep/26.2.213. [DOI] [PubMed] [Google Scholar]
- Yang CK, Kim JK, Patel SR, Lee JH. Age-related changes in sleep/wake patterns among Korean teenagers. Pediatrics. 2005;115:250–256. doi: 10.1542/peds.2004-0815G. [DOI] [PubMed] [Google Scholar]
- Yang C-M, Spielman AJ. The effect of a delayed weekend sleep pattern on sleep and morning functioning. Psychol Health. 2001;16:715–725. [Google Scholar]
- Yang C-M, Spielman AJ, D’Ambrosio P, Serizawa S, Nunes J, Birnbaum J. A single dose of melatonin prevents the phase delay associated with a delayed weekend sleep pattern. Sleep. 2001;24:272–281. doi: 10.1093/sleep/24.3.272. [DOI] [PubMed] [Google Scholar]