Abstract
Despite early reports of excellent diagnostic characteristics of [131I]/[123I]-metaiodobenzylguanidine (MIBG) in the evaluation of pheochromocytomas/paragangliomas (PHEOs/PGLs) or medullary thyroid cancer (MTC) as experience with it was accumulated the sensitivity dropped. Nevertheless, this modality is still useful in the diagnostic workup of PHEOs/PGLs since it is widely available and in case of positive scans may indicate patients that are potential candidates for [131I]-MIBG therapy.
Pheochromocytoma/paraganglioma
Chromaffin cells (CC) produce catecholamines; oxidation of fresh tissue samples gives them their characteristic grey-brown stain (“pheos” in Greek). Although CC are mainly located in the adrenal medulla, accessory adrenal tissue comprising both cortical and medullary elements has been reported to be localized in the celiac plexus area in 16% of autopsies (1). Extra-adrenal chromaffin cells give rise to paragangliomas (PGLs; tumors situated along the paravertebral and paraaortic axes) (2). Paragangliomas that are localized in the adrenal medulla are called pheochromocytomas (PHEOs; or more uncommonly termed adrenal medullary PGLs) (3). In the absence of universally established criteria for defining malignancy, a PHEO is considered to be malignant in the presence of metastases (4).
The annual incidence of PHEOs is approximately 1–4/106 population; also 0.5% of subjects with hypertension and 4% of those with an adrenal incidentaloma have PHEO (5). Most, but not all, tumors are adrenal, sporadic and solitary. In 40%–80% of patients with PHEOs tachycardia with diaphoresis and cephalalgia are encountered while hypertension is a common (in over 90% of patients) but non-specific finding (5).
Paragangliomas (PGL) are mostly intraabdominal (adjacent to the adrenals in 85% of patients) (6). Interestingly, head and neck chromaffin-negative tumors, which are related to the parasympathetic nervous system (such as those originating from the carotid bodies or the jugular bulbs) are also termed PGLs (7). Although these rare tumors synthesize and store catecholamines, only 1% are clinically functional and they usually follow an indolent and symptom-free course as painless neck masses (7).
Diagnostic imaging for PHEOs/PGLs follows – for most patients – biochemical proof of disease (at least for PHEOs). The sensitivity of computed tomography (CT) for detecting intraadrenal PHEOs at least 0.5 cm in diameter is 93%–100% and approximately 90% for localizing extraadrenal tumors at least 1 cm in diameter (5). Magnetic resonance imaging (MRI) has slightly better sensitivity although the characteristic high T2-weighted signal on MRI is not seen as often as previously considered (8–9). Although the reported sensitivity for CT/MRI is high, the specificity of CT/MRI may vary from 50%–90%, indicating various degrees of false negative studies (10). In this context, anatomical imaging studies (i.e. CT/MRI) may not be diagnostic (11). Furthermore, previous surgery may hamper imaging, which is important in case of possible recurrence (12). Thus, recurrence or suspicion of extraadrenal or malignant/metastatic disease is the niche for functional (nuclear medicine) methods.
[131I]/[123I]MIBG
The expression of catecholamine plasma membrane and vesicular transporter systems in PHEO cells is usually abundant; this enables imaging with [131I]/[123I] metaiodobenzylguanidine (MIBG), which like norepinephrine, is taken into sympathomedullary tissues (mainly by a norepinephrine transporter system) and into intracytoplasmic vesicles (through a vesicular transporter system). MIBG is thus accumulated within adrenergic tissues (13). [131I]/[123I]MIBG shows no appreciable binding to adrenergic receptors and is minimally metabolized. Plasma membrane and vesicular uptake are Na+-dependent; they can be influenced by medications such as nasal decongestants, antihypertensives (such as labetalol), antidepressants, and antipsychotics, as well as by cocaine; all have to be withheld for 1–3 days (exception being the depot forms of antipsychotics: their withdrawal period should be one month) (12). The half life of [131I] is long (8.2 days); it emits high energy gamma-radiation (364 keV). For imaging, [131I]MIBG is given iv at doses ranging from 0.5–1.0 mCi (18.5–37 MBq) or 0.5 mCi (18.5 MBq)/1.7 m2; this results in an absorbed dose at the adrenals of 1 Gy/mCi (14). The half-life of [123I] is shorter (13 h); it emits lower energy gamma-radiation (159 keV) than 131I. [123I]MIBG is given iv at doses of 3 mCi (in children) to 10 mCi (in adults) (15). The absorbed radiation dose from 10 mCi (370 MBq) [123I]MIBG compares to that of 0.5 mCi (18.5 MBq) [131I]MIBG (16).
A small percentage of [131I]/[123I] in [131I]/[123I]MIBG is free (approximately 5%, with a further 3% released after administration). Thyroid blocking is advocated for all procedures involving [131I]/[123I]MIBG -- unless the thyroid is the organ of interest – with saturated solution of potassium iodide (100 mg twice a day). In case of allergy to the latter, potassium perchlorate can be given (200–300 mg or 15 drops of perchlorate solution twice a day), starting one day before the radionuclide’s administration and for 1–3 or for 2–7 days after administration of [123I]- or [131I]MIBG, respectively (12, 17). Minimal risk of decreased thyroid function persists regardless of blockade.
[131I]MIBG imaging is performed after 24 hours, 48 hours and, if necessary, at 72 hours; only planar views are obtained. [123I]MIBG scintigraphy is performed after 24 hours and, if necessary, at 48 hours; single-photon emission computed tomography (SPECT) can be performed.
MIBG uptake after 24 h is normally seen in the myocardium, spleen, liver, urinary bladder, lungs, and salivary glands (which are rich in sympathetic innervation) and occasionally in the large intestine and the cerebellum (12). Rarely, thrombocytopenia may occur after [131I]-MIBG has been taken up by platelets (12). [131I]MIBG may show uptake in normal adrenal medulla (in 16% of scans after 48 h). Normal adrenal medulla uptake is more common with [123I]MIBG (in as many as 32–75% of patients after 24 h); caution should be applied in interpreting the scans since the pattern of [123I]MIBG uptake may be asymmetrical between the left and right adrenals (12). Most of the injected MIBG is excreted via the kidneys, with minimal salivary and fecal excretion (12). PHEOs/PGLs are expected to be seen as foci of abnormal and/or increased [131I]/[123I]MIBG uptake.
Diagnostic utility of [131I]/[123I]MIBG in PHEOs/PGLs
Until recently, the gold standard functional imaging method for PHEOs/PGLs was scintigraphy with [131I]-MIBG, with reported sensitivity of 77%–90% and specificity of 95%–100% (18). Scintigraphy with [123I]-MIBG, was reported to have a sensitivity of 83% 100% and specificity of 95%–100% for detecting PHEOs (19). The use of [123I]-MIBG outside the USA has superseded that of [131I]-MIBG, primarily due to the lower radiation burden for patients, as well as for its superior imaging quality (and particularly for SPECT). Availability of [123I]-MIBG compared to [131I]-MIBG in the USA was limited until 2008, when this radiopharmaceutical obtained FDA approval and its use began expanding.
As recently as five years ago [123I]-MIBG appeared to be a peerless functional imaging for PHEOs/PGLs. As a matter of fact, a meta-analysis calculated the sensitivity of [123I]-MIBG to be as high as 98% for adrenal and extraadrenal PHEOs alike (20). However, until recently, there was a paucity of large studies comparing various anatomical and/or functional imaging modalities to [131I]/[123I]-MIBG scintigraphy in the evaluation PHEOs/PGLs.
In a report of 76 patients with PHEOs who were studied with CT, MRI and [123I]-MIBG the sensitivity of the latter was 85% for adrenal PHEOs and 58% for extra-adrenal PHEOs (21).
A large prospective study yielded good results for [123I]-MIBG (planar and SPECT imaging combined): in 140 subjects the overall sensitivity was 84% and the specificity was 73% and more in detail, for PHEOs the sensitivity was 88% and the specificity was 70%, while for PGLs the sensitivity was 75% and the specificity was 100%; the sensitivity for metastatic disease was 83% (22). In a study of 117 patients with PGLs [123I]-MIBG had 56% sensitivity and 84% specificity (23). In the same line of results, in a meta-analysis of 15 well controlled clinical studies of [123I]-MIBG (with clearly defined inclusion criteria) the sensitivity was calculated to be 94% and the specificity to be 92% (the authors acknowledge that this type of analysis has shortcomings though) (24).
Contrary to the above reports the results of a recent retrospective study of 98 subjects were less supportive of [131I]/[123I]-MIBG: the sensitivity was calculated at 73% and the specificity at 69% (with minimal adrenal uptake considered to be abnormal) or 90% (with minimal adrenal uptake considered to be normal); likelihood ratios (LRs) for positive tests ranged between 2.3–17.0 and LRs for negative tests ranged between 0.29–0.39. The authors acknowledge that this “real world” performance of [131I]/[123I]-MIBG is biased because a number of inappropriately chosen subjects who were examined with [131I]/[123I]-MIBG were included, even with negative biochemical testing (25).
In an older study of patients with metastatic PHEO, we found that [18F]-fluorodopamine (DA; a positron emission tomography – PET – ligand) was superior to [131I]-MIBG (with sensitivities of 100% and 56%, respectively) (26). The subsequent availability of [123I]-MIBG prompted us to compare it with [18F]-DA PET and somatostatin-receptor scintigraphy (SRS; Octreoscan): in patients with non-metastatic (mainly adrenal) PHEOs, [18F]-DA and [123I]-MIBG had equivalent sensitivities for tumor detection, and both were superior to SRS, whereas in patients with metastatic disease, [18F]-DA was superior to [123I]-MIBG and detected more lesions. Furthermore, in a minority of these patients, SRS showed impressively more lesions compared to [123I]-MIBG (27). Further studies with PET ligands (specific and non-specific for chromaffin tumors) have consistently indicated their superiority against [131I/[123I]-MIBG for imaging PHEOs/PGLs: metastatic PGLs are better imaged with PET using [18F]-DA, [18F]-DOPA or [18F]-fluorodeoxyglucose (FDG)(28) and in a report of 25 patients with PHEOs/PGLs (13 with hereditary disease) the sensitivity of [123I]-MIBG was an abysmal 15% versus 100% for [18F]-DOPA PET (29). In a study of 29 patients with succinate dexydrogenase subunit B (SDHB)-associated metastatic PGLs sensitivity of [131I/[123I]-MIBG was 41%–65% while sensitivity for [18F]-DA or [18F]-FDG PET was 71%–94% (30). For head and neck PGLs (n=29 patients) SRS has been shown to be better than [123I]-MIBG (sensitivity 89%–93% vs 42%–44%, respectively) (31). In a well-controlled comparison of four functional imaging modalities of PHEOs/PGLs [18F]-DA was the best modality vs CT/MRI with sensitivity of 76%–82% whereas [123I]-MIBG lagged behind with sensitivity of 57%–78% (32).
“Carrier-free” MIBG
Until recently, in commercially available MIBG, only approximately 0.05% of its molecules were radiolabeled, the rest being unlabeled MIBG (“cold-carrier”). The latter is considered to competitively inhibit radiolabeled MIBG uptake by its target tissues (33). The Ultratrace method, a newer radiopharmaceutical technique, has enabled the production of very pure carrier-free MIBG, with very high specific activity (>1200 mCi/μmol vs approximately 1 mCi/μol for conventional commercially available [123I]-MIBG) (34). Although the primary aim of implementing Ultratrace is to produce [131I]-MIBG for therapy (there is an ongoing clinical trial; ClinicalTrials.gov identifier: NCT00874614) there is also scope for diagnostic functional imaging (34).
In conclusion, selection bias may have exaggerated the apparent performance of [131I]/[123I]-MIBG in the past; PET with newer ligands, such as with [18F]-DA, is better than [123I]-MIBG, particularly in the evaluation of hereditary or metastatic/extra-adrenal PHEOs (35–36) (Figures 1 and 2), and is gradually used more often. Nevertheless, the availability of [131I]/[123I]-MIBG and the advent of [123I]-MIBG/CT (37) currently prevent this modality from being completely phased out in the diagnostic workup of PHEOs/PGLs (38). Diagnostic imaging with high-specific-activity Ultratrace [123I]-MIBG has yet to be extensively evaluated in PHEOs/PGLs. Furthermore, clinicians should bear in mind that patients with metastatic PHEOs/PGLs who are [131I]/[123I]-MIBG-positive may benefit from [131I]-MIBG therapy (39–40). The latter has not been overtly successful until now, however, [131I]-MIBG therapy may be revitalized since in vitro studies have shown that treatment of PHEO cells with histone deacetylase inhibitors (HDAC; romidepsin and trichostatin A) upregulates the norepinephrine transporter system and increases uptake of [123I]-MIBG (41). In the future, pretreatment with HDAC of patients with metastatic PHEOs who show no or low [123I]-MIBG uptake may permit more effective therapeutic administration of [131I]-MIBG.
Figure 1.
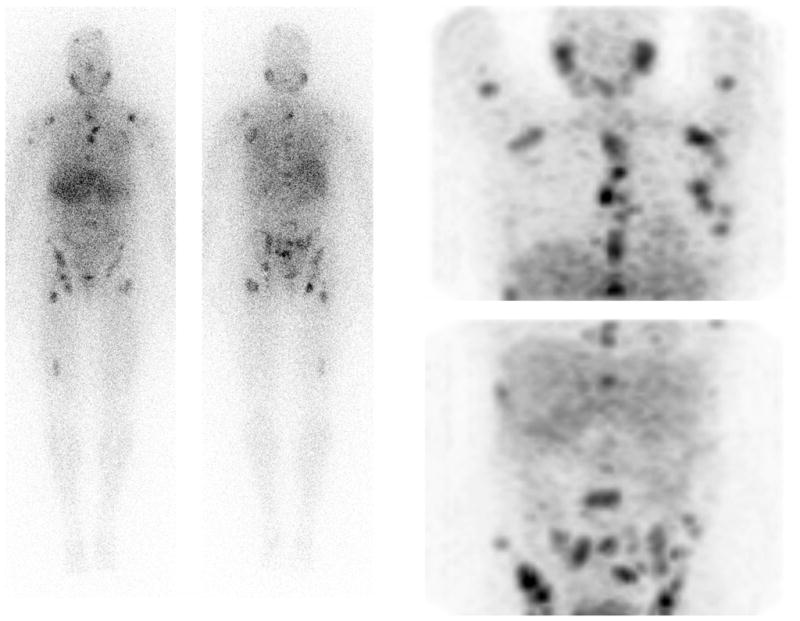
Whole body (left) and anterior reprojected images (right) with [123I]-MIBG of a patient with PHEO; multiple metastatic lesions are seen
Figure 2.
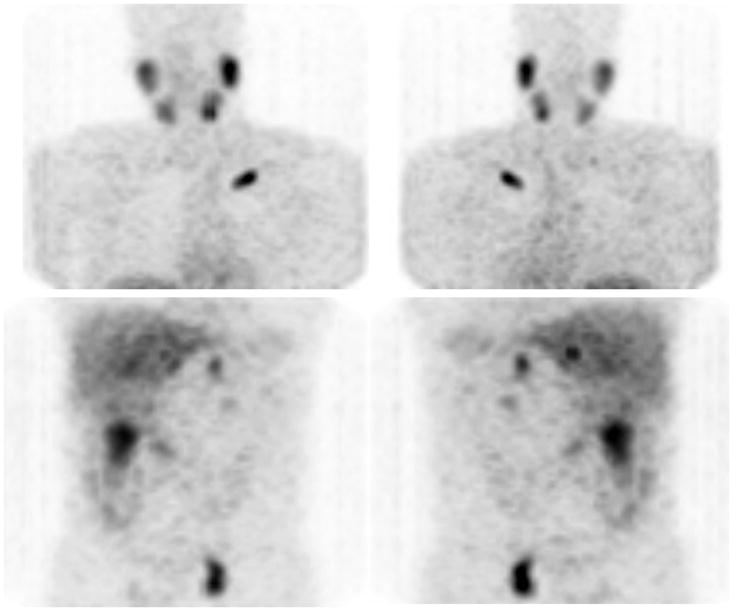
Anterior (left) and posterior (right) reprojected images with [123I]-MIBG of a patient with metastatic PHEO; uptake is seen in the left clavicle, the left pelvis whereas bilateral adrenal uptake is normal. Additional lesions were seen with [18F]-DA, [18F]-DOPA and [18F]-FDG PET in the thoracic spine and pulmonary carina.
Medullary thyroid cancer
Medullary thyroid cancer (MTC) stems from thyroid parafollicular C cells. Most cases of MTC are sporadic (75%) with the remaining being hereditary and/or associated with germline mutations of the RET gene and genetic syndromes such as multiple endocrine neoplasia type 2 (MEN2) (42). [131I]/123I]-MIBG shows uptake in MTC via the same molecular mechanisms like in other neuroendocrine tumors such as PHEOs.
The diagnostic utility of [131I]/[123I]-MIBG for MTC has been explored mostly in small case series, following an early report of an [123I]-MIBG-positive MTC case (43). In another study that followed the sensitivity of [131I]-MIBG was 53% for familial/MEN-associated MTC and 87% for sporadic MTC (44). In eight patients the sensitivity of [131I]/123I]-MIBG was approximately 50% (but the combination of [131I]/[123I]-MIBG and SRS had 100% sensitivity) (45). Despite these results, as experience with [131I]/123I]-MIBG was accumulated, its sensitivity for MTC dropped to approximately 30% (46–47). Currently the role of [131I]/[123I]-MIBG in the diagnostic evaluation of MTC is limited in patients with MEN2 (since it may detect lesions either in the thyroid or the adrenals) and patients in whom tentative therapy with [131I]-MIBG is contemplated.
Acknowledgments
Support for this work was provided – in part – by the intramural research program of the Eunice Shriver Kennedy National Institute of Child Health and Development. II and KP would like to thank Ms Millie Whatley, CNMT for her assistance in preparing this manuscript. II would like to thank Dr M. Kesse-Elias for her critical review of the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
Contributor Information
Ioannis ILIAS, Email: iiliasmd@yahoo.com.
Chaitanya DIVGI, Email: chaitanya.divgi@uphs.upenn.edu.
Karel PACAK, Email: karel@mail.nih.gov.
References
- 1.Lack EE. Adrenal cortical nodules and tumor-like lesions, in Tumors of the adrenal gland and extra-adrenal paraganglia. Washington, DC: Armed Forces Institute of Pathology; 1997. [Google Scholar]
- 2.Kimura N, Cappela C, De Krijger RR, et al. Extra-adrenal sympathetic paraganglioma: superior and inferior paraaortic. In: DeLellis RA, Lloyd RV, Heitz PU, et al., editors. Pathology and Genetics of Tumours of Endocrine Organs; WHO Classification of Tumours, Volume 8; IARC WHO Classification of Tumours, No 8. Lyon: IARC Press; 2004. [Google Scholar]
- 3.McNicol AM, Young WF, Jr, Kawashima A, et al. Benign phaeochromocytoma. In: DeLellis RA, Lloyd RV, Heitz PU, et al., editors. Pathology and Genetics of Tumours of Endocrine Organs; WHO Classification of Tumours, Volume 8; IARC WHO Classification of Tumours, No 8. Lyon: IARC Press; 2004. [Google Scholar]
- 4.Thompson LDR, Young WF, Jr, Kawashima A, et al. Malignant adrenal phaeochromocytoma. In: DeLellis RA, Lloyd RV, Heitz PU, et al., editors. Pathology and Genetics of Tumours of Endocrine Organs; WHO Classification of Tumours, Volume 8; IARC WHO Classification of Tumours, No 8. Lyon: IARC Press; 2004. [Google Scholar]
- 5.Ilias I, Pacak K. A clinical overview of pheochromocytomas/paragangliomas and carcinoid tumors. Nucl Med Biol. 2008;35(Suppl 1):S27–34. doi: 10.1016/j.nucmedbio.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lack EE. Tumors of the adrenal gland and extra-adrenal paraganglia. Washington, DC: Armed Forces Institute of Pathology; 1997. Extra-adrenal paragangliomas of the sympathoadrenal neuroendocrine system. [Google Scholar]
- 7.Kimura N, Chetty R, Cappela C, et al. Extra-adrenal paraganglioma: carotid body, jugulotympanic, vagal, laryngeal, aortico-pulmonary. In: DeLellis RA, Lloyd RV, Heitz PU, et al., editors. Pathology and Genetics of Tumours of Endocrine Organs; WHO Classification of Tumours, Volume 8; IARC WHO Classification of Tumours, No 8. Lyon: IARC Press; 2004. [Google Scholar]
- 8.Jacques AE, Sahdev A, Sandrasagara M, et al. Adrenal phaeochromocytoma: correlation of MRI appearances with histology and function. Eur Radiol. 2008;18:2885–2892. doi: 10.1007/s00330-008-1073-z. [DOI] [PubMed] [Google Scholar]
- 9.Elsayes KM, Menias CO, Siegel CL, et al. Magnetic resonance characterization of pheochromocytomas in the abdomen and pelvis: imaging findings in 18 surgically proven cases. J Comput Assist Tomogr. 2010;34:548–553. doi: 10.1097/RCT.0b013e3181d529f2. [DOI] [PubMed] [Google Scholar]
- 10.Mittendorf EA, Evans DB, Lee JE, et al. Pheochromocytoma: advances in genetics, diagnosis, localization, and treatment. Hematol Oncol Clin North Am. 2007;21:509–525. doi: 10.1016/j.hoc.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Go AS. Refining Probability: An Introduction to the Use of Diagnostic Tests. In: Friedland DJ, editor. Evidence-Based Medicine. New York, NY: McGraw-Hill; 1998. [Google Scholar]
- 12.Ilias I, Pacak K. Current approaches and recommended algorithm for the diagnostic localization of pheochromocytoma. J Clin Endocrinol Metab. 2004;89:479–491. doi: 10.1210/jc.2003-031091. [DOI] [PubMed] [Google Scholar]
- 13.Shulkin BL, Ilias I, Sisson JC, et al. Current trends in functional imaging of pheochromocytomas and paragangliomas. Ann N Y Acad Sci. 2006;1073:374–382. doi: 10.1196/annals.1353.041. [DOI] [PubMed] [Google Scholar]
- 14.Swanson DP, Carey JE, Brown LE, et al. Human absorbed dose calculations for iodine-131 and iodine-123 labeled meta-iodobenzylguanidine (mIBG): a potential myocardial and adrenal medulla imaging agent. 3rd International Radiopharmaceutical Dosimetry Symposium; Rockville, MD. 1981. [Google Scholar]
- 15.Lynn MD, Shapiro B, Sisson JC, et al. Portrayal of pheochromocytoma and normal human adrenal medulla by m-[123I] iodobenzylguanidine: concise communication. J Nucl Med. 1984;25:436–440. [PubMed] [Google Scholar]
- 16.Shapiro B, Gross MD. Radiochemistry, biochemistry, and kinetics of 131I-metaiodobenzylguanidine (MIBG) and 123I-MIBG: clinical implications of the use of 123I-MIBG. Med Pediatr Oncol. 1987;15:170–177. doi: 10.1002/mpo.2950150406. [DOI] [PubMed] [Google Scholar]
- 17.Bombardieri E, Giammarile F, Aktolun C, et al. 131I/123I-metaiodobenzylguanidine (mIBG) scintigraphy: procedure guidelines for tumor imaging. Eur J Nucl Med Mol Imaging. 2010;37:2436–2446. doi: 10.1007/s00259-010-1545-7. [DOI] [PubMed] [Google Scholar]
- 18.Sisson JC, Shulkin BL. Nuclear medicine imaging of pheochromocytoma and neuroblastoma. Q J Nucl Med. 1999;43:217–223. [PubMed] [Google Scholar]
- 19.Nielsen JT, Nielsen BV, Rehling M. Location of adrenal medullary pheochromocytoma by I-123-metaiodobenzylguanidine SPECT. Clin Nucl Med. 1996;21:695–699. doi: 10.1097/00003072-199609000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Van Der Horst-Schrivers AN, Jager PL, Boezen HM, et al. Iodine-123 metaiodobenzylguanidine scintigraphy in localising phaeochromocytomas--experience and meta-analysis. Anticancer Res. 2006;26:1599–1604. [PubMed] [Google Scholar]
- 21.Bhatia KS, Ismail MM, Sahdev A, et al. 123I-metaiodobenzylguanidine (MIBG) scintigraphy for the detection of adrenal and extra-adrenal phaeochromocytomas: CT and MRI correlation. Clin Endocrinol (Oxf) 2008;69:181–188. doi: 10.1111/j.1365-2265.2008.03256.x. [DOI] [PubMed] [Google Scholar]
- 22.Wiseman GA, Pacak K, O’Dorisio MS, et al. Usefulness of 123I-MIBG scintigraphy in the evaluation of patients with known or suspected primary or metastatic pheochromocytoma or paraganglioma: results from a prospective multicenter trial. J Nucl Med. 2009;50:1448–1454. doi: 10.2967/jnumed.108.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milardovic R, Corssmit EP, Stokkel M. Value of 123I-MIBG Scintigraphy in Paraganglioma. Neuroendocrinology. 2010;91:94–100. doi: 10.1159/000242499. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson AF, Deng H, Lombard J, et al. 123I-meta-iodobenzylguanidine scintigraphy for the detection of neuroblastoma and pheochromocytoma: results of a meta-analysis. J Clin Endocrinol Metab. 2010;95:2596–2606. doi: 10.1210/jc.2009-2604. [DOI] [PubMed] [Google Scholar]
- 25.Lev I, Kelekar G, Waxman A, et al. Clinical use and utility of metaiodobenzylguanidine scintigraphy in pheochromocytoma diagnosis. Endocr Pract. 2010;16:398–407. doi: 10.4158/EP09302.OR. [DOI] [PubMed] [Google Scholar]
- 26.Ilias I, Yu J, Carrasquillo JA, et al. Superiority of 6-[18F]-fluorodopamine positron emission tomography versus [131I]-metaiodobenzylguanidine scintigraphy in the localization of metastatic pheochromocytoma. J Clin Endocrinol Metab. 2003;88:4083–4087. doi: 10.1210/jc.2003-030235. [DOI] [PubMed] [Google Scholar]
- 27.Ilias I, Chen CC, Carrasquillo JA, et al. Comparison of 6–18F-fluorodopamine PET with 123I-metaiodobenzylguanidine and 111in-pentetreotide scintigraphy in localization of nonmetastatic and metastatic pheochromocytoma. J Nucl Med. 2008;49:1613–1619. doi: 10.2967/jnumed.108.052373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fliedner SM, Lehnert H, Pacak K. Metastatic paraganglioma. Semin Oncol. 2010;37:627–637. doi: 10.1053/j.seminoncol.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fottner C, Helisch A, Anlauf M, et al. 6–18F-fluoro-L-dihydroxyphenylalanine positron emission tomography is superior to 123I-metaiodobenzyl-guanidine scintigraphy in the detection of extraadrenal and hereditary pheochromocytomas and paragangliomas: correlation with vesicular monoamine transporter expression. J Clin Endocrinol Metab. 2010;95:2800–2810. doi: 10.1210/jc.2009-2352. [DOI] [PubMed] [Google Scholar]
- 30.Timmers HJ, Kozupa A, Chen CC, et al. Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. J Clin Oncol. 2007;25:2262–2269. doi: 10.1200/JCO.2006.09.6297. [DOI] [PubMed] [Google Scholar]
- 31.Koopmans KP, Jager PL, Kema IP, et al. 111In-octreotide is superior to 123I-metaiodobenzylguanidine for scintigraphic detection of head and neck paragangliomas. J Nucl Med. 2008;49:1232–1237. doi: 10.2967/jnumed.107.047738. [DOI] [PubMed] [Google Scholar]
- 32.Timmers HJ, Chen CC, Carrasquillo JA, et al. Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2009;94:4757–4767. doi: 10.1210/jc.2009-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coleman RE, Stubbs JB, Barrett JA, et al. Radiation dosimetry, pharmacokinetics, and safety of ultratrace Iobenguane I-131 in patients with malignant pheochromocytoma/paraganglioma or metastatic carcinoid. Cancer Biother Radiopharm. 2009;24:469–475. doi: 10.1089/cbr.2008.0584. [DOI] [PubMed] [Google Scholar]
- 34.Barrett JA, Joyal JL, Hillier SM, et al. Comparison of high-specific-activity ultratrace 123/131I-MIBG and carrier-added 123/131I-MIBG on efficacy, pharmacokinetics, and tissue distribution. Cancer Biother Radiopharm. 2010;25:299–308. doi: 10.1089/cbr.2009.0695. [DOI] [PubMed] [Google Scholar]
- 35.Havekes B, King K, Lai EW, et al. New imaging approaches to phaeochromocytomas and paragangliomas. Clin Endocrinol (Oxf) 2010;72:137–145. doi: 10.1111/j.1365-2265.2009.03648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ilias I, Pacak K. Diagnosis, localization and treatment of pheochromocytoma in MEN 2 syndrome. Endocr Regul. 2009;43:89–93. [PubMed] [Google Scholar]
- 37.Meyer-Rochow GY, Schembri GP, Benn DE, et al. The utility of metaiodobenzylguanidine single photon emission computed tomography/computed tomography (MIBG SPECT/CT) for the diagnosis of pheochromocytoma. Ann Surg Oncol. 2010;17:392–400. doi: 10.1245/s10434-009-0850-5. [DOI] [PubMed] [Google Scholar]
- 38.Hicks RJ. Use of molecular targeted agents for the diagnosis, staging and therapy of neuroendocrine malignancy. Cancer Imaging. 2010;10:S83–S91. doi: 10.1102/1470-7330.2010.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giammarile F, Chiti A, Lassmann M, et al. EANM procedure guidelines for 131I-meta-iodobenzylguanidine (131I-mIBG) therapy. Eur J Nucl Med Mol Imaging. 2008;35:1039–1047. doi: 10.1007/s00259-008-0715-3. [DOI] [PubMed] [Google Scholar]
- 40.Scholz T, Eisenhofer G, Pacak K, et al. Clinical review: Current treatment of malignant pheochromocytoma. J Clin Endocrinol Metab. 2007;92:1217–1225. doi: 10.1210/jc.2006-1544. [DOI] [PubMed] [Google Scholar]
- 41.Martiniova L, Perera SM, Brouwers FM, et al. Increased uptake of [123I]meta-iodobenzylguanidine, [18F]fluorodopamine, and [3H]norepinephrine in mouse pheochromocytoma cells and tumors after treatment with the histone deacetylase inhibitors. Endocr Relat Cancer. 2011;18:143–157. doi: 10.1677/ERC-10-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nyström E, Berg GEB, Jansson SKG, et al. Thyroid Disease in Adults. Berlin: Springer-Verlag; 2011. Thyroid cancer. [Google Scholar]
- 43.Endo K, Shiomi K, Kasagi K, et al. Imaging of medullary thyroid cancer with 131I-MIBG. Lancet. 1984;2:233. doi: 10.1016/s0140-6736(84)90525-7. [DOI] [PubMed] [Google Scholar]
- 44.Baulieu JL, Guilloteau D, Delisle MJ, et al. Radioiodinated meta-iodobenzylguanidine uptake in medullary thyroid cancer. A French cooperative study. Cancer. 1987;60:2189–2194. doi: 10.1002/1097-0142(19871101)60:9<2189::aid-cncr2820600913>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 45.Gao Z, Biersack HJ, Ezziddin S, et al. The role of combined imaging in metastatic medullary thyroid carcinoma: 111In-DTPA-octreotide and 131I/123I-MIBG as predictors for radionuclide therapy. J Cancer Res Clin Oncol. 2004;130:649–656. doi: 10.1007/s00432-004-0588-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szakáll SJ, Ésik O, Bajzik G, et al. 18F-FDG PET Detection of Lymph Node Metastases in Medullary Thyroid Carcinoma. J Nucl Med. 2002;43:66–71. [PubMed] [Google Scholar]
- 47.Rufini V, Castaldi P, Treglia G, et al. Nuclear medicine procedures in the diagnosis and therapy of medullary thyroid carcinoma. Biomed Pharmacother. 2008;62:139–146. doi: 10.1016/j.biopha.2007.07.011. [DOI] [PubMed] [Google Scholar]


