Abstract
Patients with dementia often have neuropsychiatric symptoms. The objective of this study was to evaluate the relationship between neuropsychiatric symptoms and progressive cognitive decline by assessing cognitive impairment, depressive symptoms, and aggressive behavior in a sample of elderly subjects. The study sample consisted of 201 subjects admitted to nursing homes. For the purpose of the present study each subject was evaluated using the Mini-Mental State Examination, the Geriatric Depression Scale, and the Modified Overt Aggression Scale. The results show that aggressive behavior and depressive symptoms are associated with progressive cognitive decline in elderly subjects. Early assessment of these conditions can promote rational therapeutic strategies that may improve the quality of life and delay institutionalization for elderly patients.
Keywords: neuropsychiatric symptoms, dementia, behavioral and psychological syndromes of dementia (BPSD), progressive cognitive decline
Introduction
Neuropsychiatric symptoms are common in dementia and are important predictors of institutionalization. Indeed, patients with dementia often have neuropsychiatric symptoms collectively termed “behavioral and psychological symptoms of dementia” (BPSD).1 Depression is quite common in elderly patients with dementia.2,3 Aggressive behaviors that cause great difficulties in the social functioning of patients are also common, significantly increasing rates of nursing home placement.4,5 Verbal and physical aggression could be strictly connected with depressive symptoms,6–8 but the relationship between depression, aggressive behavior, and dementia is not well defined.
The objective of this study was to evaluate the relationship between neuropsychiatric symptoms and progressive cognitive decline by assessing cognitive impairment, depressive symptoms, and aggressive behavior in a sample of elderly nursing home residents. Increased knowledge of the interaction between aggression, cognitive impairment, and depressive symptoms could lead to the identification of specific strategies in the management of health care for elderly patients.
Methods
Subjects and assessment
The study sample consisted of 201 men and women over the age of 65 years who were residing in one of three different care homes for the elderly. Informed consent to take part in the study was provided by each subject or, in some cases, by a subject’s legal guardian. The authors collected the subjects’ sociodemographic and health status data from medical records and through physical examination, with a particular focus on medical comorbidity. The authors also reported the functional status and psychopathologic symptoms using the Activities of Daily Living Scale and the Neuropsychiatric Inventory. For the purpose of the present study, each subject was evaluated using the Mini-Mental State Examination (MMSE),9 the Geriatric Depression Scale (GDS),10 and the Modified Overt Aggression Scale (MOAS).11
The MMSE was developed as a brief screening tool to provide a quantitative assessment of cognitive impairment and to record cognitive changes over time. The MMSE consists of eleven simple questions or tasks. Typically, these are grouped into seven cognitive domains: (1) orientation to time, (2) orientation to place, (3) registration of three words, (4) attention and calculation, (5) recall of three words, (6) language, and (7) visual construction. Administration by a trained interviewer takes approximately 10 minutes. The test yields a total score of 30 and provides a picture of the subject’s present cognitive performance based on direct observation of completion of test items or tasks. Levels of impairment have been classified as mild (MMSE score 24–29), slight (MMSE score 16–23), moderate (MMSE score 11–15), and severe (MMSE score 0–10).
The GDS is a 30-item self-report assessment designed specifically to identify depression in the elderly. The assessment requires yes or no answers – this is thought to be simpler than scales that use a five-category response set – and is generally recommended as a routine part of a comprehensive geriatric assessment. One point is assigned to each answer and corresponds to a scoring grid. A score of 10 or lower is the usual threshold to separate depressed from nondepressed patients. Levels of depression have been classified as borderline (GDS score 10–14) and present (GDS score > 14).
The MOAS, developed by Kay and colleagues11 is based on the Overt Aggression Scale.12 The MOAS is divided in four domains: (1) verbal aggression, (2) aggression against objects, (3) aggression against self, and (4) aggression against other people. A score from 0 to 4 is assigned to each act: 0 indicates no aggressive behavior and higher scores indicate increasing severity. The score in each category is multiplied by a factor assigned to that category: 1 for verbal aggression, 2 for aggression against objects, 3 for aggression against self, and 4 for aggression against other people. Thus, the total MOAS score ranges from 0 (no aggression) to 40 (maximum grade of aggression). Margari et al.13 validated the psychometric properties of the MOAS in Italy by assessing its inter-rater reliability (P > 0.90) and predictive power.
Statistical analysis
Descriptive statistics were used to summarize the variables studied and the characteristics of the subjects. The authors analyzed correspondence between mean GDS scores and score subgroups of the MMSE and between mean MOAS scores and score subgroups of the MMSE and the GDS.
Correlation analysis was used to describe the strength and direction of relationship between variables. The Pearson’s correlation coefficient (r), which can only take on values between −1 and 1, was used; this could indicate a positive (as one variable increases, so too does the other) or a negative correlation (as one variable increases, the other decreases). Furthermore, student’s t-test was used to compare the means of two samples (male and female). With this test the authors evaluated the role of gender on depressive symptoms, aggressive behavior, and cognitive impairment.
A P-value of <0.05 was considered significant for all the tests. The authors used the data processing program Statistical Package for Social Science (v 19.0; SPSS Inc, Chicago, IL) for statistical processing.
Results
The main sociodemographic and clinical features of subjects are summarized in Table 1.
Table 1.
Characteristics of study participants (N = 201)
| Characteristic | Subjects |
|---|---|
| Gender [n (%)] | |
| Female | 165 (82.09) |
| Male | 36 (17.91) |
| Age (years) | |
| Mean | 81.85 |
| Range | 65–103 |
| Marital status (%) | |
| Unmarried | 27.54 |
| Married | 17.87 |
| Widowed | 52.66 |
| Divorced | 1.93 |
| Education level (%) | |
| Illiterate | 28.54 |
| Primary school | 42.06 |
| Secondary school | 23.57 |
| Bachelor’s degree | 5.83 |
| Organic disease (%) | |
| Degenerative pathologies of SNpc | 43.05 |
| Cardiovascular pathologies | 42.51 |
| Articular degenerative pathologies | 20.29 |
| Genitourinary disturbances | 17.39 |
| Endocrine pathologies | 17.39 |
| Recent osteoarticular trauma | 16.43 |
| NPI (n)* | 8.9 ± 12.8 |
| ADL (n)* | 2.6 ± 2.3 |
Note:
Data presented as mean plus or minus standard deviation.
Abbreviations: SNpc, substantia nigra pars compacta; NPI, Neuropsychiatric Inventory; ADL, Activities of Daily Living Scale.
The MMSE showed that only 4.97% of the subjects did not present cognitive impairment (MMSE score 30), while 95.02% of the subjects showed cognitive impairment. In particular, 31.34% achieved a score indicative of mild cognitive impairment (MMSE score 24–29), 27.86% achieved a score indicative of slight dementia (MMSE score 16–23), 13.93% achieved a score indicative of moderate dementia (MMSE score 11–15), and 4.97% achieved a score indicative of severe dementia (MMSE score 0–10). It was not possible to assess the cognitive level in 16.91% of the subjects because of the severity of clinical impairment. Males showed a higher cognitive impairment than females, with a statistically significant difference (F = 4.96; P = 0.01).
For the GDS test, 27.86% of the subjects achieved a GDS score < 10, which corresponds to the absence of depressive symptoms, 11.44% of the subjects achieved a borderline score for depressive symptoms (GDS score 10–14), and 40.79% of the subjects achieved a GDS score > 14, indicating the presence of depressive symptoms; the evaluation was not possible in 19.90% of the subjects because of the severity of clinical impairment. Females showed more depressive symptoms than males (F = 13.21; P = 0.008).
For the MOAS test, 20.89% of subjects showed self- or hetero-directed aggressive behavior (MOAS score > 0). In these subjects, the mean MOAS score was 5.19, corresponding to expressions of verbal aggression and aggression against objects. The mean MOAS score for the entire sample was 1.08. No gender differences were found for aggressive behavior (F = 10.18; P = 0.1).
Cognitive impairment, depressive symptoms, and aggressive behavior in elderly subjects are summarized in Table 2.
Table 2.
Cognitive impairment, depressive symptoms, and aggressive behavior
| Test and range | Results | Mean scorea | Significant differences | ||
|---|---|---|---|---|---|
|
|
|
||||
| Female | Male | F-value | P-value | ||
| MMSE (cognitive impairment) | 16.7 ± 10 | 21.4 ± 8.3 | 4.96 | 0.01* | |
| None [n (%)] | 10 (4.97) | ||||
| MCI [n (%)] | 63 (31.34) | ||||
| Slight [n (%)] | 56 (27.86) | ||||
| Moderate [n (%)] | 28 (13.93) | ||||
| Severe [n (%)] | 10 (4.93) | ||||
| ND [n (%)] | 34 (16.91) | ||||
| GDS (depressive symptoms) | 12.1 ± 10 | 7.4 ± 6.7 | 13.21 | 0.008* | |
| Absent [n (%)] | 56 (27.86) | ||||
| Borderline [n (%)] | 23 (11.44) | ||||
| Present [n (%)] | 82 (40.79) | ||||
| ND [n (%)] | 40 (19.90) | ||||
| MOAS (aggressive behavior) | 2.25 ± 5 | 0.8 ± 2.2 | 10.18 | 0.1 | |
| Present [n (%)] | 42 (20.89) | ||||
| Mean aggression score | 5.19 | ||||
| Mean total score | 1.08 | ||||
Notes:
Data presented as mean plus or minus standard deviation;
P ≤ 0.05.
Abbreviations: MMSE, Mini-Mental State Examination; MCI, mild cognitive impairment; ND, not determined; GDS, Geriatric Depression Scale; MOAS, Modified Overt Aggression Scale.
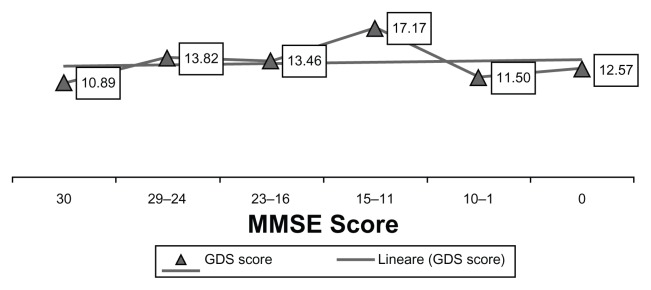
Figure 1 presents the distribution of the mean GDS score across the different ranges of MMSE score. Subjects with MMSE scores at either end of the range reported lower MOAS scores than subjects with MMSE scores in the central range. The borderline GDS score (10–14) was associated with mild cognitive impairment (MMSE score 24–29) and slight dementia (MMSE score 16–23); a higher mean score on the GDS (>14) corresponded to moderate dementia (MMSE score 11–15). The positive correlation between MMSE and GDS scores was not statistically significant (r = 0.087; P = 0.217).
Figure 1.
Mean Geriatric Depression Scale (GDS) score obtained by subjects divided into groups according to Mini-Mental State Examination (MMSE) score.
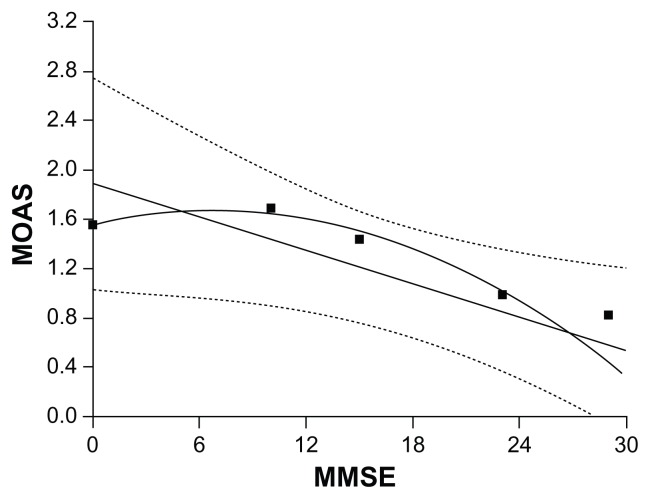
The range of distribution of mean scores between the MMSE and the MOAS showed major impairment of cognitive functioning corresponded to an increase in aggressive behavior (Figure 2). The subgroup without cognitive impairment (MMSE score 30) corresponded to an absence of aggressive behavior (MOAS score 0), the subgroup with mild cognitive impairment (MMSE score 24–29) corresponded to a mean MOAS score of 1.1, the subgroup with slight dementia (MMSE score 16–23) corresponded to a mean MOAS score of 1.2, the subgroup with moderate dementia (MMSE score 11–15) corresponded to a mean MOAS score of 1.5, and the subgroup with severe dementia (MMSE score 0–10) corresponded to a mean MOAS score of 1.7. Analysis of the relationship between MMSE and MOAS scores showed a statistically significant negative correlation (r = −0.440; P = 0.000).
Figure 2.
Mean Modified Overt Aggression Scale (MOAS) score corresponding to score subgroups of the Mini-Mental State Examination (MMSE).
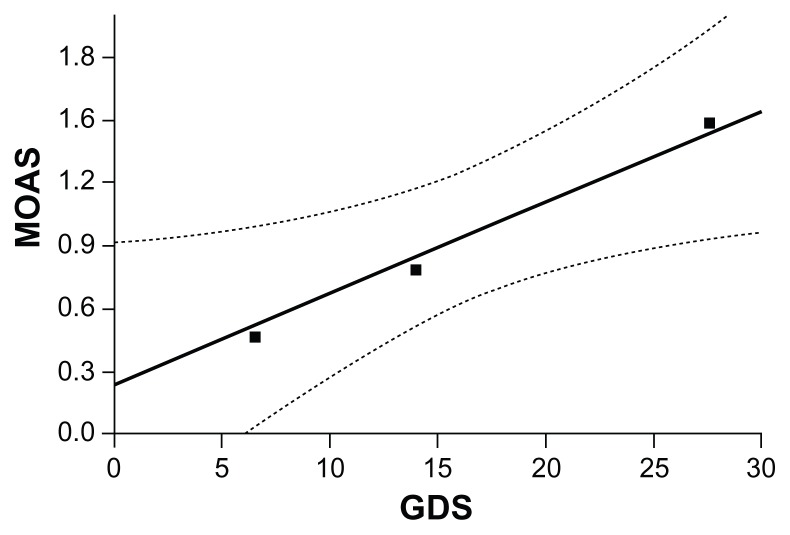
The range of distribution of mean scores between the GDS and the MOAS showed an increase in MOAS score corresponded to an increase in GDS score (Figure 3): the subgroup that showed no depressive symptoms (GDS score 0–10) corresponded to the mean MOAS score of 0.4, the subgroup with a borderline score for depressive symptoms (GDS score 10–14) corresponded to the mean MOAS score of 0.8, and the subgroup with depressive symptoms present corresponded to the mean MOAS score of 1.3. This relationship is statistically significant (r = 0.217; P = 0.002).
Figure 3.
Mean Modified Overt Aggression Scale (MOAS) score corresponding to score subgroups of the Geriatric Depression Scale (GDS).
In addition, the authors analyzed the relationship between age and the results of each rating scale test. The findings showed a statistically significant positive correlation between age and cognitive impairment (r = −0.150; P = 0.033). On the contrary, depressive symptoms and aggressive behavior did not have a significant correlation with age (depressive symptoms, r = 0.092, P = 0.195; aggressive behavior, r = 0.108, P = 0.126).
The relationships between MMSE score, GDS score, MOAS score, and patient age are summarized in Table 3.
Table 3.
Pearson’s correlation coefficient (r) between tests
| MMSE | GDS | MOAS | Age | |
|---|---|---|---|---|
| MMSE | ||||
| r | 1 | 0.087 | −0.440** | −0.150* |
| Sig | 0.217 | 0.000 | 0.033 | |
| GDS | ||||
| r | 1 | 0.217** | 0.092 | |
| Sig | 0.002 | 0.195 | ||
| MOAS | ||||
| r | 1 | 0.108 | ||
| Sig | 0.126 | |||
| Age | ||||
| r | 1 | |||
| Sig | ||||
| Mean | 17.57 | 11.32 | 2 | 82.5 |
| SD | 9.9 | 9.8 | 4.6 | 7.75 |
Notes:
P ≤ 0.05;
P ≤ 0.01.
Abbreviations: MMSE, Mini-Mental State Examination; GDS, Geriatric Depression Scale; MOAS, Modified Overt Aggression Scale; Sig, significant differences value; SD, standard deviation.
Discussion
BPSD may affect up to 90% of patients with dementia during the course of their illness and includes verbal and physical aggression, psychotic symptoms, agitation, anxiety, and depression; these are the major factors of institutionalization and of the associated health care costs.14–17 Depression is one of the most frequent comorbid psychiatric disorders in Alzheimer disease (AD) and other dementias.3,18 In addition, patients with AD seem to have more depressive symptoms than patients with subcortical dementias. The relationship between depression and dementia is still not clear, as dementia and depressive syndromes demonstrate a substantial symptom overlap19– indeed, there are two types of depressive syndrome: (1) depression related to the development of dementia and (2) a reactive depression.
The MMSE detected cognitive impairment in 167 (83%), confirming that cognitive impairment is one of the most common reasons for admission to nursing home care.20–22 The GDS detected symptoms of depression in 105 (52%) of the 201 subjects, revealing a greater frequency than clinical examination.
The distribution of the mean GDS scores across the MMSE score range might suggest that depressive symptoms have a tendency to increase with the deterioration of cognitive functioning; this correlation is not statistically significant. When the cognitive decline is severe, depressive symptoms tend to decrease. The low GDS scores in those subjects with severe cognitive impairment indicated by the MMSE might be related to the difficulty of recording depressive symptoms in patients with severe dementia who are unable to communicate their feelings and thoughts and who may experience depressive symptoms linked to agitation and aggression. In the severe stages of dementia, the symptoms can only be indirectly observed as overt behavior, such as physically or verbal aggressive behavior. Furthermore, verbal and physical aggression among patients with dementia may be related to a higher frequency of comorbid depressive symptoms.7,8 In addition, cognitive impairment was more common in male subjects. No gender differences were found for aggressive behavior. In agreement with the literature, depression symptoms were more frequent in female subjects.23
In an epidemiologic study of community dwellers and nursing home residents, Lyketsos al24 found that 23.7% of patients with dementia were rated as being agitated or aggressive. Other studies involving older nursing home residents with dementia found a higher rate of verbal aggression than physical aggression17,25,26 and observed that physical aggression is usually preceded by verbal aggression.25
In agreement with these findings, results of the present study show that 20.9% of elderly subjects manifested aggressive behavior with a mean MOAS score of 5.19, corresponding to expressions of verbal aggression and aggression against objects.
According to the literature,15,23,27 analysis of the data of 201 subjects admitted to nursing homes suggests a statistically significant correlation between a greater impairment of cognitive functioning and an increase of aggressive behavior. The increase in MOAS score corresponds to a decrease in MMSE score. This means that subjects with severe dementia tend to exhibit more frequent verbal and physical aggression. Furthermore, analysis of data suggests there is a relationship between depression and aggressive behavior. Indeed, an increase in MOAS score corresponds to an increase in GDS score. This finding suggests that aggressive behavior is correlated with depressive symptoms and the severity of cognitive impairment. In addition, this finding shows that cognitive impairment is correlated with increasing age. In contrast, no significant correlation was found between age and symptoms of depression or aggressive behavior. Therefore, aggressive behavior and depressive symptoms may be part of the clinical picture of senile dementia. For these reasons it is important to understand the neurobiological basis of BPSD. Garcia-Alloza et al28 supposed that cortical cholinergic acetylcholinesterase could be a putative marker of accelerated cognitive decline in AD and may also be implicated in BPSD such as aggressive behavior, supporting the use of cholinesterase inhibitors in alleviating noncognitive behavioral symptoms in patients with AD. Lanctôt et al29 found that the final stages of AD are associated with gamma-aminobutyric acidergic changes, which may contribute to depression and apathy in AD.29 A recent study by Mitchell et al30 suggests that neuropsychiatric behaviors in AD share certain neurochemical features with psychiatric disorders such as major depression, and that distinct or overlapping disease processes leading to similar neurochemical alterations (eg, 5-HT1A receptor deficits) may manifest clinically as behavioral symptoms in both conditions. In a review study, Borroni et al31,32 reported that the involvement of dopamine- or serotonin-related pathways and the associated genetic variability have been demonstrated as being interesting candidates for the neuropsychiatry manifestations of dementia. Moreover, genetic variations of neurotrophins, such as brain-derived neurotrophic factor, have been related to depression susceptibility in AD. These studies on neurobiological mechanisms may explain the relationship observed in the present study between cognitive impairment, aggressive behavior, and depression symptoms.
Limitation and further investigation
The use of self-reports for assessing depression in elderly subjects with cognitive impairments was a limitation of this study. Measurement of depression in dementia is difficult because symptoms such as apathy, indecisiveness, insomnia, and weight loss may be undetected. The GDS test is known to show an adequate diagnostic accuracy in elderly people living in the community but does not show the same accuracy for elderly people in institutions. Therefore, the GDS should be used cautiously as a screening instrument in a population in which dementia is prevalent or in people known to have dementia, because the GDS is not sensitive in detecting depression in demented subjects.33
To resolve these study limitations in any future research, self-reports for the elderly should be specific to elderly with cognitive impairments. These self-reports should be short, easily understood, and appropriate in terms of the size of letters in the items and in terms of the elderly subject’s level of education; they should include relevant age-related items; and they should provide normative data on the elderly population with dementia.
Conclusion
In conclusion, the results of this study show that aggressive behavior and depressive symptoms are associated with progressive cognitive decline in elderly subjects. Early assessment of these conditions can promote rational therapeutic strategies that may improve the quality of life and delay institutionalization for elderly patients.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Prado-Jean A, Couratier P, Druet-Cabanac M, et al. Specific psychological and behavioral symptoms of depression in patients with dementia. Int J Geriatr Psychiatry. 2010;25(10):1065–1072. doi: 10.1002/gps.2468. [DOI] [PubMed] [Google Scholar]
- 2.Starkstein SE, Jorge R, Mizrahi R, Robinson RG. The construct of minor and major depression in Alzheimer’s disease. Am J Psychiatry. 2005;162(11):2086–2093. doi: 10.1176/appi.ajp.162.11.2086. [DOI] [PubMed] [Google Scholar]
- 3.Starkstein SE, Mizrahi R, Power BD. Depression in Alzheimer’s disease: phenomenology, clinical correlates and treatment. Int Rev Psychiatry. 2008;20(4):382–388. doi: 10.1080/09540260802094480. [DOI] [PubMed] [Google Scholar]
- 4.Kunik ME, Snow AL, Davila JA, et al. Causes of aggressive behavior in patients with dementia. J Clin Psychiatry. 2010;71(9):1145–1152. doi: 10.4088/JCP.08m04703oli. [DOI] [PubMed] [Google Scholar]
- 5.Grochmal-Bach B, Bidzan L, Pachalska M, Bidzan M, Łukaszewska B, Pufal A. Aggressive and impulsive behaviors in frontotemporal dementia and Alzheimer’s disease. Med Sci Monit. 2009;15(5):CR248–254. [PubMed] [Google Scholar]
- 6.Heeren O, Borin L, Raskin A, et al. Association of depression with agitation in elderly nursing home residents. J Geriatr Psychiatry Neurol. 2003;16(1):4–7. doi: 10.1177/0891988703252157. [DOI] [PubMed] [Google Scholar]
- 7.Menon AS, Gruber-Baldini AL, Hebel JR, et al. Relationship between aggressive behaviors and depression among nursing home residents with dementia. Int J Geriatr Psychiatry. 2001;16(2):139–146. doi: 10.1002/1099-1166(200102)16:2<139::aid-gps284>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Volicer L, Frijters DH, Van der Steen JT. Relationship between symptoms of depression and agitation in nursing home residents with dementia. Int J Geriatr Psychiatry. 2012;27(7):749–754. doi: 10.1002/gps.2800. [DOI] [PubMed] [Google Scholar]
- 9.Cockrell JR, Folstein MF. Mini-Mental State Examination (MMSE) Psychopharmacol Bull. 1988;24(4):689–692. [PubMed] [Google Scholar]
- 10.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982–1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 11.Kay SR, Wolkenfeld F, Murrill LM. Profiles of aggression among psychiatric patients: I. Nature and prevalence. J Nerv Ment Dis. 1988;176(9):539–546. doi: 10.1097/00005053-198809000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Yudofsky SC, Silver JM, Jackson W, Endicott J, Williams D. The Overt Aggression Scale for the objective rating of verbal and physical aggression. Am J Psychiatry. 1986;143(1):35–39. doi: 10.1176/ajp.143.1.35. [DOI] [PubMed] [Google Scholar]
- 13.Margari F, Matarazzo R, Casacchia M, Roncone R, Dieci M, Safran S, Fiori G, Simoni L EPICA Study Group. Italian validation of MOAS and NOSIE: a useful package for psychiatric assessment and monitoring of aggressive behaviours. Int J Methods Psychiatr Res. 2005;14(2):109–18. doi: 10.1002/mpr.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrmann N, Lanctôt KL, Sambrook R, et al. The contribution of neuropsychiatric symptoms to the cost of dementia care. Int J Geriatr Psychiatry. 2006;21(10):972–976. doi: 10.1002/gps.1594. [DOI] [PubMed] [Google Scholar]
- 15.Heart Protection Study Collaborative Group. Statin cost-effectiveness in the United States for people at different vascular risk levels. Circ Cardiovasc Qual Outcomes. 2009;2(2):65–72. doi: 10.1161/CIRCOUTCOMES.108.808469. [DOI] [PubMed] [Google Scholar]
- 16.Brodaty H, Ames D, Boundy KL, et al. Pharmacological treatment of cognitive deficits in Alzheimer’s disease. Med J Aust. 2001;175(6):324–329. doi: 10.5694/j.1326-5377.2001.tb143593.x. [DOI] [PubMed] [Google Scholar]
- 17.Brodaty H, Draper B, Saab D, et al. Psychosis, depression and behavioural disturbances in Sydney nursing home residents: prevalence and predictors. Int J Geriatr Psychiatry. 2001;16(5):504–512. doi: 10.1002/gps.382. [DOI] [PubMed] [Google Scholar]
- 18.Starkstein SE, Jorge R, Mizrahi R, Robinson RG. A prospective longitudinal study of apathy in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2006;77(1):8–11. doi: 10.1136/jnnp.2005.069575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chinello A, Grumelli B, Perrone C, Annoni G. Prevalence of major depressive disorder and dementia in psychogeriatric outpatients. Arch Gerontol Geriatr. 2007;44(Suppl 1):101–104. doi: 10.1016/j.archger.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Snowdon J, Shah A, Halliday G. Severe domestic squalor: a review. Int Psychogeriatr. 2007;19(1):37–51. doi: 10.1017/S1041610206004236. [DOI] [PubMed] [Google Scholar]
- 21.Fullerton CA, McGuire TG, Feng Z, Mor V, Grabowski DC. Trends in mental health admissions to nursing homes, 1999–2005. Psychiatr Serv. 2009;60(7):965–971. doi: 10.1176/appi.ps.60.7.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lezovic M. Analysis of the structure of services provided in the healthcare facilities in long-term care in Slovakia. Bratisl Lek Listy. 2009;110(11):701–704. [PubMed] [Google Scholar]
- 23.Zuidema SU, de Jonghe JF, Verhey FR, Koopmans RT. Predictors of neuropsychiatric symptoms in nursing home patients: influence of gender and dementia severity. Int J Geriatr Psychiatry. 2009;24(10):1079–1086. doi: 10.1002/gps.2225. [DOI] [PubMed] [Google Scholar]
- 24.Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288(12):1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 25.Brodaty H, Luscombe G, Anstey KJ, Cramsie J, Andrews G, Peisah C. Neuropsychological performance and dementia in depressed patients after 25-year follow-up: a controlled study. Psychol Med. 2003;33(7):1263–1275. doi: 10.1017/s0033291703008195. [DOI] [PubMed] [Google Scholar]
- 26.Cohen-Mansfield J, Jensen B. Physicians’ perceptions of their role in treating dementia-related behavior problems in the nursing home: actual practice and the ideal. J Am Med Dir Assoc. 2008;9(8):552–557. doi: 10.1016/j.jamda.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Salzman C, Jeste DV, Meyer RE, et al. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. J Clin Psychiatry. 2008;69(6):889–898. doi: 10.4088/jcp.v69n0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Alloza M, Gil-Bea FJ, Diez-Ariza M, et al. Cholinergic–serotonergic imbalance contributes to cognitive and behavioral symptoms in Alzheimer’s disease. Neuropsychologia. 2005;43(3):442–449. doi: 10.1016/j.neuropsychologia.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Lanctôt KL, Herrmann N, Rothenburg L, Eryavec G. Behavioral correlates of GABAergic disruption in Alzheimer’s disease. Int Psychogeriatr. 2007;19(1):151–158. doi: 10.1017/S1041610206003899. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell JC, Perkinton MS, Yates DM, et al. Expression of the neuronal adaptor protein X11alpha protects against memory dysfunction in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis. 2010;20(1):31–36. doi: 10.3233/JAD-2009-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borroni B, Agosti C, Marcello E, Di Luca M, Padovani A. Blood cell markers in Alzheimer disease: amyloid precursor protein form ratio in platelets. Exp Gerontol. 2010;45(1):53–56. doi: 10.1016/j.exger.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Borroni B, Costanzi C, Padovani A. Genetic susceptibility to behavioural and psychological symptoms in Alzheimer disease. Curr Alzheimer Res. 2010;7(2):158–164. doi: 10.2174/156720510790691173. [DOI] [PubMed] [Google Scholar]
- 33.Montorio I, Wetherell JL, Nuevo R. Beliefs about worry in community-dwelling older adults. Depress Anxiety. 2006;23(8):466–473. doi: 10.1002/da.20199. [DOI] [PubMed] [Google Scholar]