Abstract
Background:
The present study evaluated whether magnetic nanoparticles containing Fe3O4 could enhance the activity of gambogic acid in human colon cancer cells, and explored the potential mechanisms involved.
Methods:
Cytotoxicity was evaluated by MTT assay. The percentage of cells undergoing apoptosis was analyzed by flow cytometry, and cell morphology was observed under both an optical microscope and a fluorescence microscope. Reverse transcriptase polymerase chain reaction and Western blot assay were performed to determine the transcription of genes and expression of proteins, respectively.
Results:
Gambogic acid could inhibit proliferation of LOVO cells in a dose-dependent and time-dependent manner and induce apoptosis, which was dramatically enhanced by magnetic nanoparticles containing Fe3O4. The typical morphological features of apoptosis in LOVO cells were observed after treatment comprising gambogic acid with and without magnetic nanoparticles containing Fe3O4. Transcription of cytochrome c, caspase 9, and caspase 3 genes was higher in the group treated with magnetic nanoparticles containing Fe3O4 and gambogic acid than in the groups that received gambogic acid or magnetic nanoparticles containing Fe3O4, but transcription of phosphatidylinositol 3-kinase, Akt, and Bad genes decreased. Notably, expression of cytochrome c, caspase 9, and caspase 3 proteins in the group treated with gambogic acid and magnetic nanoparticles containing Fe3O4 was higher than in the groups receiving magnetic nanoparticles containing Fe3O4 or gambogic acid, while expression of p-PI3K, p-Akt, p-Bad, pro-caspase 9, and pro-caspase 3 degraded.
Conclusion:
Magnetic nanoparticles containing Fe3O4 can enhance apoptosis induced by gambogic acid which may be closely related to regulation of the PI3K/Akt/Bad pathway in the treatment of human colon cancer.
Keywords: Fe3O4, magnetic nanoparticles, gambogic acid, LOVO cells, apoptosis, PI3K, Akt, Bad, pathway
Introduction
Despite the availability of chemical and molecular targeted drugs, almost 80% of patients with colorectal cancer in China rely on traditional Chinese medicine therapy. A number of studies have been performed recently to assess the therapeutic efficacy and safety of traditional Chinese medicine for treating cancer.1,2 It is noteworthy that gambogic acid, a major ingredient isolated from the Chinese herb Gamboge hanburyi, can not only inhibit cell growth, induce apoptosis, and arrest the cell cycle in various carcinoma cell lines, including pulmonary carcinoma,3 hepatocellular carcinoma,4 breast cancer,5 prostate cancer,6 and gastric cancer,7 but it can also inhibit tumor cell proliferation and inhibit angiogenesis by suppressing the phosphorylation of Akt, Erk, c-Src, Fak, and vascular endothelial growth factor receptor 2.8,9 These findings suggest that gambogic acid may indeed be an effective agent in the treatment of cancer. However, to date, the potential mechanisms that account for the beneficial effects of gambogic acid remain largely unknown.
Recently, magnetic nanoparticles containing Fe3O4, which have excellent biocompatibility10 and improved intracellular penetration,11,12 have become a focus for potential application in human medicine, including the delivery of anticancer drugs to overcome drug resistance and enhance the effectiveness of chemotherapy.12,13 Accumulating research has shown that not only can a combination of magnetic nanoparticles containing Fe3O4 and adriamycin have a significant cytotoxic effect on drug-resistant K562/A02 leukemia cells,14,15 but also that a combination of magnetic nanoparticles containing Fe3O4 and antitumor drugs may interact synergistically to induce apoptosis in cancer cells. Further, it has been shown that the cytotoxicity and apoptosis induced by gambogic acid in cancer cells can be dramatically enhanced if gambogic acid is combined with magnetic nanoparticles containing Fe3O4,16 but the signal transduction pathways accounting for the beneficial effects of gambogic acid remain largely unknown.
It is well known that apoptosis is a major mechanism of cell death. The phosphatidylinositol 3-kinase (PI3K/Akt) pathway acts as a critical regulator of cell survival by stimulating cell proliferation and inhibiting apoptosis, and abnormal signal transduction pathways are important in tumorigenesis and tumor progression.17 Bad, a member of the Bcl-2 family, is one of several substrates for Akt.18 Phosphorylation of Bad by Akt suppresses its auxo-apoptotic functions, accounting in part for the potent survival function of Akt.18 Close attention has been paid to identify new target drugs that block signal transduction pathways in cancer cells.19 Overexpression of PI3K/Akt pathway components, such as PI3K/p85α, Akt1, and Akt2, may contribute to the growth and progression of colorectal cancer.20,21 Therefore, it may be that a strategy of targeted inhibition in PI3K/Akt pathway components could increase sensitivity to chemotherapy and decrease the metastatic capacity of colorectal cancer cells.19,22–24 At present, there are many compounds that target PI3K (or Akt) progressing through clinical trials. However, to the best of our knowledge, there has been no study of the effect of magnetic nanoparticles containing a combination of gambogic acid and Fe3O4 in the PI3K/Akt/Bad pathway in colon cancer cells. Given the role of the PI3K/Akt signaling pathway, our aim in these experiments was to investigate the effect of magnetic nanoparticles containing Fe3O4 and gambogic acid on LOVO cells and the potential role of this signaling pathway in the treatment of human colon cancer.
Materials and methods
Main materials
Gambogic acid (Kanion Pharmaceutical Co, Ltd, Jiangsu, China) was dissolved in dimethyl sulfoxide (Sigma-Aldrich, St Louis, MO), stored at −20°C, and then diluted as needed in RPMI 1640 medium (Gibco/BRL, Carlsbad, CA). Methylthiazol tetrazolium (MTT) and propidium iodide were purchased from Sigma-Aldrich (St Louis, CA). Fe3O4 magnetic nanoparticles (State Key Laboratory of Bioelectronics, Nanjing, China) were well distributed in RPMI 1640 medium containing 10% (v/v) heat-inactivated new-born calf serum (Sijiqing, Hangzhou, China) using an ultrasonic generator (Kudos SK3300HP, 53 kHz, maximum power 180 W, Kudos Ultraphonic Instrument Limited, Shanghai, China) to obtain a colloidal suspension of Fe3O4 magnetic nanoparticles. Gambogic acid was conjugated with the magnetic nanoparticles containing Fe3O4 by mechanical absorption polymerization at 4°C for 48 hours,16 and their size was approximately 50 nm. 4, 6-diamidino-2-phenylindole (DAPI) was obtained from Santa Cruz Biotechnology Inc (Santa Cruz, CA). An Annexin V-fluorescein isothiocyanate kit was obtained from Nanjing Keygen Biotech Co, Ltd (Nanjing, China), and Trizol reagent was from Invitrogen Life Technologies (Carlsbad, CA). Monoclonal antibodies of PI3K, p-PI3K, Akt, p-Akt, Bad, p-Bad, cytochrome c, caspase 9, caspase 3, and β-actin were from Santa Cruz Biotechnology Inc.
Cell culture
A human colorectal cancer cell line (LOVO) purchased from Nanjing Keygen Biotech Co, Ltd was cultured in RPMI 1640 medium containing 10% (v/v) heat-inactivated new-born calf serum, penicillin G 100 U/mL, and streptomycin 100 μg/mL at 37°C in a humidified environment containing 5% CO2.
Cell proliferation assay
Cytotoxicity was determined by MTT assay. Exponentially growing LOVO cells were seeded in 96-well plates (Costar, Fisher Scientific, Hampton, NH) in RPMI 1640 medium containing 10% new-born calf serum at a density of 5 × 103 cells/well for 24 hours. Based on our previous studies16 of the characteristics of magnetic nanoparticles containing Fe3O4, the cells were treated with different concentrations of magnetic nanoparticles containing Fe3O4 and/or gambogic acid for 12, 24, and 48 hours. Meanwhile, RPMI 1640 medium was used as the blank control. Then, 20 μL of MTT 0.5 mg/mL were added to each well and cultured for another 4 hours. Thereafter, the formazan was dissolved with 150 μL of dimethyl sulfoxide after blotting the culture medium. Finally, the plates were shaken lightly for 10 minutes and the reduction of MTT was quantified by absorbance at a wave length of 540 nm using a microplate reader (Model-550, Bio-Rad Laboratories, Hercules, CA). The cell inhibition ratio (%) was calculated as (1 – Atreated group/Acontrol group) × 100. Each assay was repeated at least three times.
Apoptosis assay by flow cytometry
Based on the results of the cytotoxicity assay, the minimum inhibition concentration of gambogic acid and its combination with magnetic nanoparticles containing Fe3O4 was used to measure the apoptosis rate in LOVO cells by flow cytometry. Briefly, after treatment with gambogic acid 0.35 μmol/L, 60 μg/mL Fe3O4 magnetic nanoparticles, or nanoparticles containing gambogic acid and Fe3O4 (0.35 μmol/L gambogic acid with 60 μg/mL Fe3O4 magnetic nanoparticles) for 48 hours. LOVO cells were collected, washed twice with ice-cold phosphate-buffered solution, and then suspended in 200 μL of binding buffer and 10 μL of Annexin V-fluorescein isothiocyanate for 15 minutes in the dark. Thereafter, 300 μL of binding buffer and 5 μL of propidium iodide were added to each sample. Finally, the cells were analyzed using BD FACS Diva flow cytometry (BD FACS Canto TM II) with FACSCalibur CellQuest™ software, and the results are expressed as the mean of three individual experiments.
Assessment of cell morphology
For assessment of cell morphology, LOVO cells were incubated for 48 hours as described earlier. The cells were then collected and stained with Wright’s stain to observe the morphological changes in cells undergoing apoptosis by optical microscopy. Meanwhile, cells on other slides were fixed with methanol for 15 minutes, stained with DAPI fluorochrome dye, and then observed under a fluorescence microscope (I × 51; Olympus, Tokyo, Japan) with a peak excitation wave length of 340 nm.
Reverse transcriptase PCR assay
As described for the apoptosis assay, the treated LOVO cells were harvested and their total RNA content was then isolated using Trizol reagent according to the manufacturer’s protocol. The reverse transcription reactions were performed using SuperScript™ II reverse transcriptase, and the newly synthetic cDNA was amplified within target and control sequences (Table 1). Polymerase chain reaction (PCR) products were separated on 1% agarose gel and visualized with ethidium bromide staining using a Molecular Imager Gel Doc XR system (Bio-Rad, CA, USA), and quantified using Molecular Analyst software version 1.5 (Bio-Rad), with β-actin as an internal control for all genes. The relative value was calculated as the ratio of each group and the internal control group. The results are expressed as the mean value of three individual experiments.
Table 1.
Sequences of gene-specific primers
| Gene | Primer | Band size (bp) | Annealing temperature (°C) |
|---|---|---|---|
| β-actin | Forward 5′-GTCACCAACTGGGACGACATG-3′ Reverse 5′-GCCGTCAGGCAGCTCGTAGC-3′ |
510 | 55 |
| PI3K | Forward 5′-CTGTGTGGGACTTATTGAGGTGGTGC-3′ Reverse 5′-GGCATGCTGTCGAATAGCTAGATAAGC-3′ |
457 | 52 |
| Akt | Forward 5′-ATGAGCGACGTGGCTATTGTGAAG-3′ Reverse 5′-GAGGCCGTCAGCCACAGTCTGGATG-3′ |
330 | 52 |
| Bad | Forward 5′-GCTCTTCCTTT GTTCATCTCC-3′ Reverse 5′-CATCTGGCTCGGGGTTACTGC-3′ |
304 | 50 |
| Cytochrome c | Forward 5′-CGCCAATAAGAACAAAGG-3′ Reverse 5′-AATCAGGACTGCCCAACA-3′ |
304 | 50 |
| Caspase 9 | Forward 5′-GCTCTTCCTTTGTTCATCTCC-3′ Reverse 5′-CATCTGGCTCGGGGTTACTGC-3′ |
742 | 52 |
| Caspase 3 | Forward 5′-TTCAGAGGGGATCGTTGTAGAAGTC-3′ Reverse 5′-5-CAAGCTTGTCGGCATACTGTTTCAG-3′ |
264 | 57 |
Western blot assay
Western blot analysis was performed to examine the expression of PI3K, p-PI3K, Akt, p-Akt, Bad, p-Bad, cytochrome c, pro-caspase 9, caspase 9, pro-caspase 3, and caspase 3. In brief, total protein was isolated on ice and subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis using modified radioimmunoprecipitation assay buffer, and then transferred to a polyvinylidene difluoride membrane (65421, Pall Corporation, Port Washington, NY). The membrane was blocked with buffer containing 10% fat-free dry milk and performed with 1:200 dilution of monoclonal antibodies against either antihuman PI3K, p-PI3K, Akt, p-Akt, Bad, p-Bad, cytochrome c, pro-caspase 9, caspase 9, pro-caspase 3, caspase 3, or β-actin antibody, and then subsequently incubated with horseradish peroxidase-conjugated goat antirabbit (1:5000) as a secondary antibody. The band was detected using an enhanced chemiluminescence detection system (Amersham, Buckinghamshire, UK). Each value is presented as the relative density of protein bands normalized to β-actin.
Statistical analysis
All data are expressed as the mean ± standard deviation from triplicate experiments and analyzed using the statistical Package for Social Science (SPSS Release 13.0, IBM, Chicago, IL). The statistical significance of differences was determined by the Student’s t-test in two groups and one-way analysis of variance among multiple groups. A P value < 0.05 was considered to be statistically significant.
Results
Inhibition of cell growth
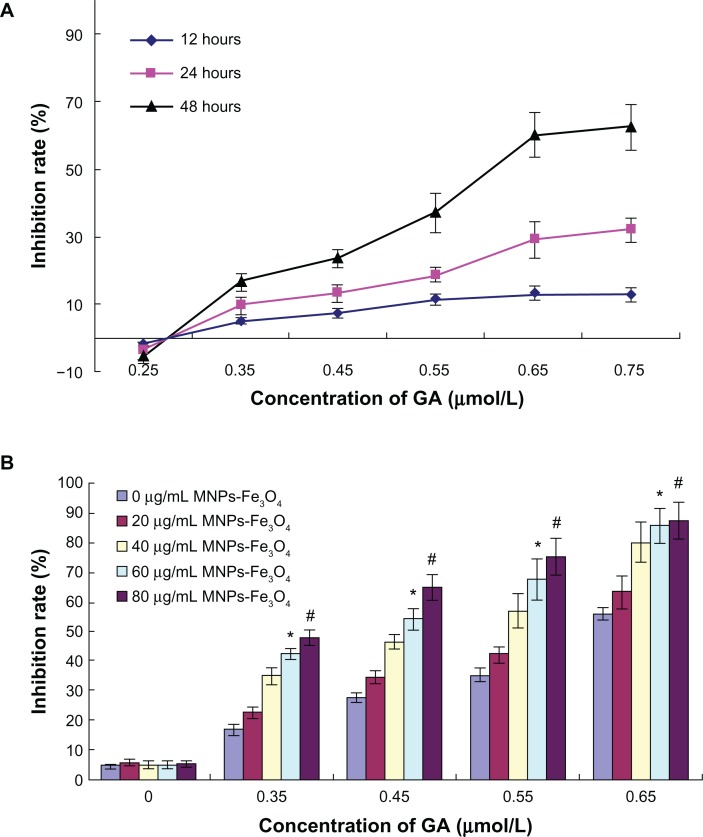
Inhibition of LOVO cell growth was markedly lower when the concentration of gambogic acid was less than 0.35 μmol/L (Figure 1A); however, the inhibition rate was increased in a dose-dependent and time-dependent manner when the dosage of gambogic acid was more than 0.35 μmol/L, suggesting that the minimum inhibition rate of gambogic acid was 0.35 μmol/L. Further, it was observed that addition of magnetic nanoparticles containing Fe3O4 enhanced the inhibition of LOVO cells by gambogic acid, and 50% inhibition achieved by gambogic acid was reduced from 0.60 μmol/L to 0.41 μmol/L by 60 μg/mL magnetic nanoparticles containing a combination of Fe3O4 and gambogic acid (P < 0.05, Figure 1B), suggesting that this combination had a synergistic effect on apoptosis of LOVO cells.
Figure 1.
(A) Inhibition rate of LOVO cells treated with various concentrations of GA for different times. (B) Inhibition rate of LOVO cells treated with the combination of GA and Fe3O4 magnetic nanoparticles for 48 hours.
Notes: *P < 0.05 when compared with controls; #P > 0.05 when compared with the group treated with Fe3O4 magnetic nanoparticles.
Abbreviations: MNPs-Fe3O4, magnetic nanoparticles of Fe3O4; GA, gambogic acid.
Synergistic effect on apoptosis of LOVO cells
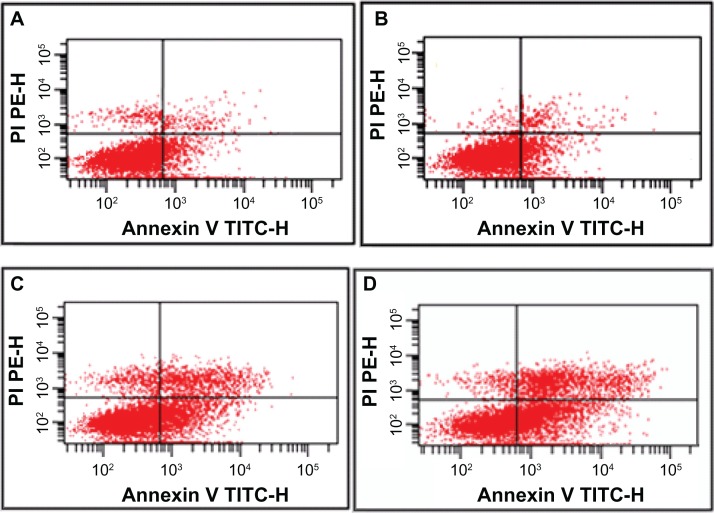
The data show that the proportion of apoptotic cells was higher in the gambogic acid group than that in the control group (20.12% ± 2.32% versus 9.04% ± 2.16%, P < 0.05), but there was no significant difference between the group treated with magnetic nanoparticles containing Fe3O4 and the control group (8.21% ± 1.67% versus 9.04% ± 2.16%, P > 0.05). Most notably, the apoptosis ratio was significantly increased (50.63% ± 4.95%) when 0.35 μmol/L gambogic acid was combined with 60 μg/mL magnetic nanoparticles containing Fe3O4, indicating that magnetic nanoparticles containing Fe3O4 could enhance the apoptosis induced by gambogic acid (Figure 2).
Figure 2.
Apoptosis ratio of LOVO cells treated with gambogic acid, Fe3O4 magnetic nanoparticles, or magnetic nanoparticles containing Fe3O4 and gambogic acid for 48 hours. (A) Controls, (B) 60 μg/mL magnetic nanoparticles containing Fe3O4, (C) 0.35 μmol/L gambogic acid, and (D) magnetic nanoparticles containing Fe3O4 and gambogic acid (0.35 μmol/L gambogic acid and 60 μg/mL magnetic nanoparticles containing Fe3O4).
Morphological changes in LOVO cells
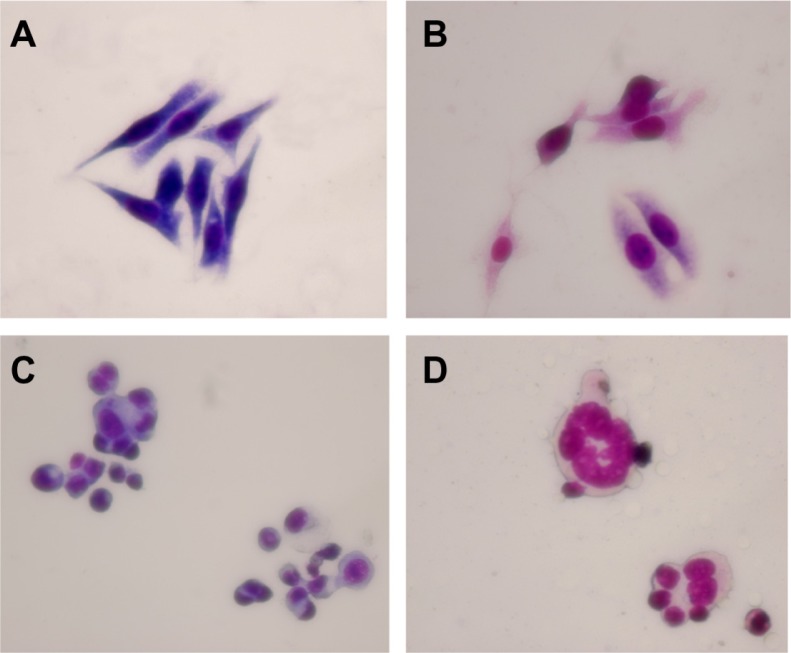
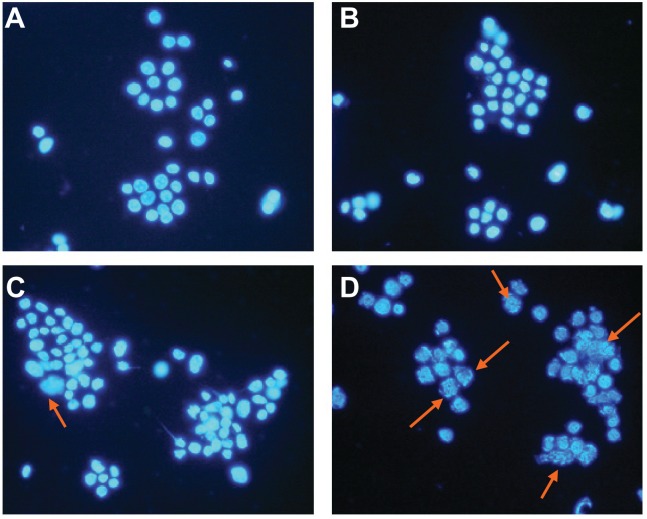
LOVO cells in the control group and in the group treated with magnetic nanoparticles containing Fe3O4 had a normal healthy shape, as demonstrated by their clear cytoskeletons (Figure 3A and B). After treatment consisting of 60 μg/mL magnetic nanoparticles containing Fe3O4 with or without 0.35 μmol/L gambogic acid for 48 hours, typical cytomorphological features of apoptosis in LOVO cells were observed, ie, cell shrinkage, chromatin condensation, margination, and presence of apoptotic bodies (Figure 3C and D). Moreover, under the fluorescence microscope, LOVO cells in the control group and in the Fe3O4 magnetic nanoparticle group stained equally with blue fluorescence, indicating that chromatin was distributed in the nucleolus to a similar extent (Figure 4A and B), but that 0.35 μmol/L gambogic acid induced a proportion of LOVO cells to display chromatin condensation and pyknosis of the nucleolus (Figure 4C). After incubation with magnetic nanoparticles containing Fe3O4 and gambogic acid (0.35 μmol/L gambogic acid with 60 μg/mL Fe3O4 magnetic nanoparticles) for 48 hours, the proportion of cells emitting bright fluorescence increased and showed the typical features of apoptosis, including chromatin condensation, pyknosis of the nucleolus, and nuclear fragmentation (Figure 4D).
Figure 3.
Morphological features of LOVO cells after treatment for 48 hours by optical microscope (×400, Wright staining). (A) Controls, (B) 60 μg/mL magnetic nanoparticles containing Fe3O4, (C) 0.35 μmol/L gambogic acid, and (D) magnetic nanoparticles containing Fe3O4 and gambogic acid (0.35 μmol/L gambogic acid with 60 μg/mL magnetic nanoparticles containing Fe3O4).
Figure 4.
Nucleolus morphological changes of LOVO cells after treatment for 48 hours under fluorescence microscope (×400, DAPI staining). (A) Controls, (B) 60 μg/mL magnetic nanoparticles containing Fe3O4, (C) 0.35 μmol/L gambogic acid, and (D) magnetic nanoparticles containing Fe3O4 and gambogic acid (0.35 μmol/L gambogic acid with 60 μg/mL magnetic nanoparticles containing Fe3O4).
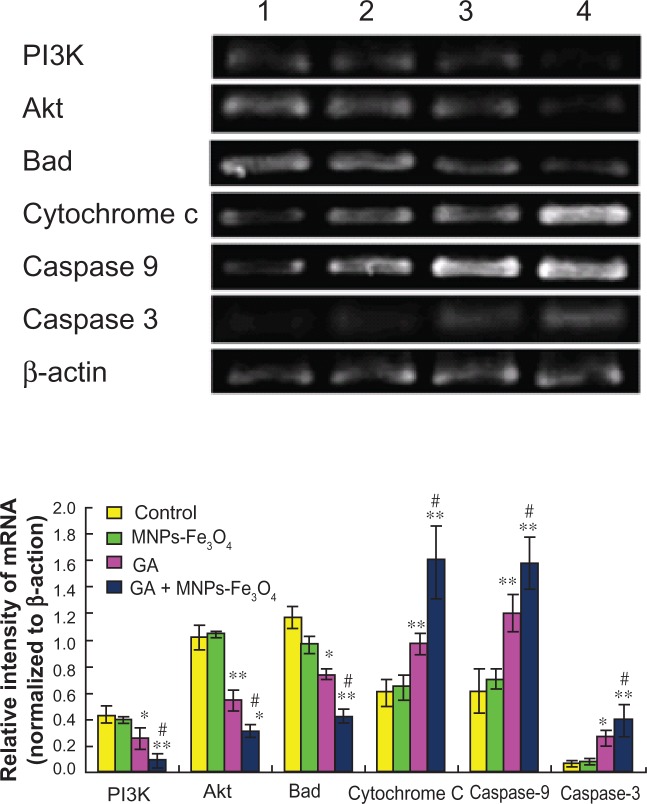
Transcription of apoptosis-related genes by reverse transcriptase PCR
Transcription of apoptosis-related genes was not influenced by 60 μg/mL Fe3O4 magnetic nanoparticles, but nanoparticles containing Fe3O4 and gambogic acid (0.35 μmol/L gambogic acid with 60 μg/mL Fe3O4 magnetic nanoparticles) could dramatically upregulate the transcription of cytochrome c, caspase 9, and caspase 3 genes in LOVO cells when compared with the control group (P < 0.05), surpassing the effects of 0.35 μmol/L gambogic acid alone (Figure 5, P < 0.05), while downregulating transcription of PI3K, Akt, and Bad genes in LOVO cells when compared with the control group (P < 0.05), also surpassing the effects of 0.35 μmol/L gambogic acid alone (P < 0.05, Figure 5).
Figure 5.
Transcription of PI3K, Akt, Bad, cytochrome C, caspase 9, and caspase 3 gene in LOVO cells after treatment comprising GA with or without MNPs-Fe3O4 for 48 hours.
Notes: Lane 1, controls; Lane 2, 60 μg/mL MNPs-Fe3O4; Lane 3, 0.35 μmol/L GA; Lane 4, GA-MNPs-Fe3O4 (0.35 μmol/L GA with 60 μg/mL MNPs-Fe3O4). *P < 0.05; **P < 0.01, when compared with control group; #P < 0.05, when compared with the GA group.
Abbreviations: MNPs-Fe3O4, magnetic nanoparticles containing Fe3O4; GA, gambogic acid.
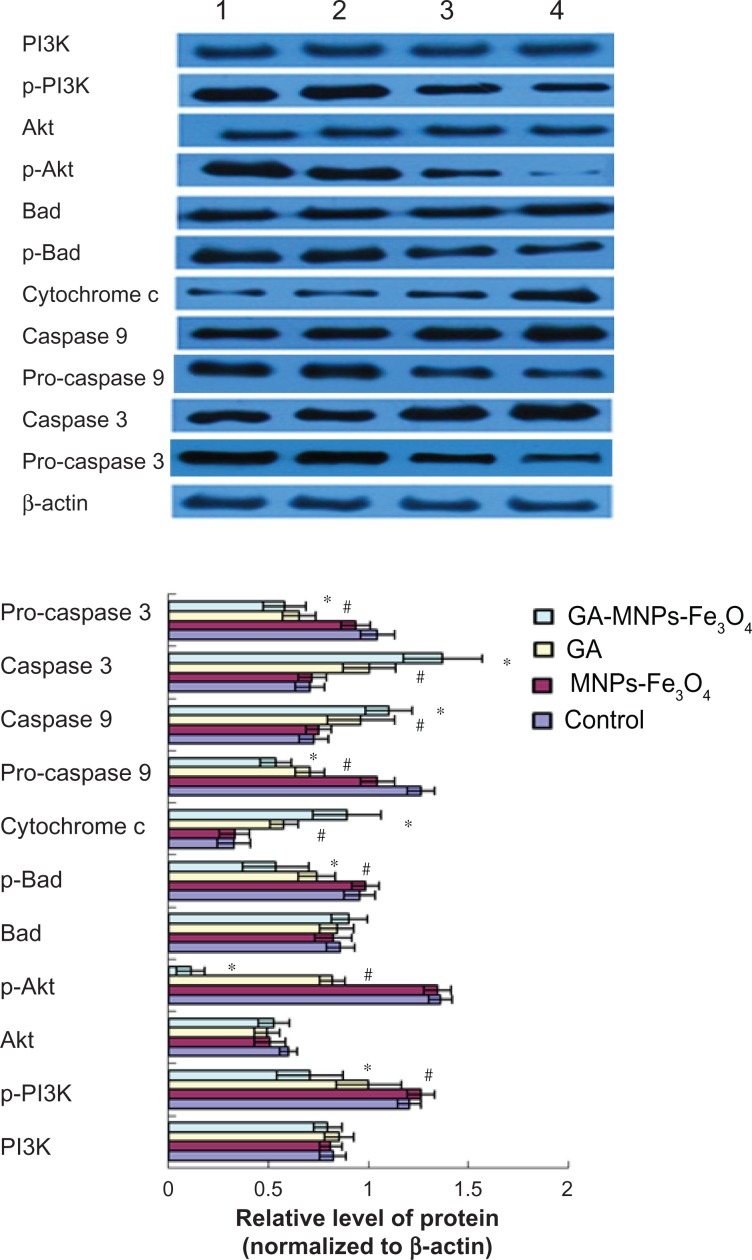
Expression of apoptosis-related proteins by Western blot assay
Based on computer-assisted image analysis, it seems that apoptosis-related proteins in LOVO cells treated with 60μg/mL Fe3O4 magnetic nanoparticles showed no significant changes when compared with the control group (P > 0.05). However, cytochrome c, caspase 3, and caspase 9 protein levels in LOVO cells treated with 0.35 μmol/L gambogic acid increased dramatically when compared with the control group (P < 0.05, Figure 6), and upregulation was enhanced by the magnetic nanoparticles containing Fe3O4 (P < 0.05). Conversely, compared with the control group, p-PI3K, p-Akt, p-Bad, pro-caspase 9, and pro-caspase 3 protein expression in LOVO cells treated with magnetic nanoparticles containing Fe3O4 and gambogic acid was lower than that in cells treated with 0.35 μmol/L gambogic acid alone (P < 0.05, Figure 6), but there were no significant differences in levels of nonactivated proteins, including PI3K, Akt, and Bad in these four groups (P > 0.05, Figure 6).
Figure 6.
Expression of PI3K, p-PI3K, Akt, p-Akt, Bad, p-Bad, cytochrome c, pro-caspase 9, caspase 9, and pro-caspase 3, caspase 3 proteins in LOVO cells after treatment comprising GA with or without MNPs-Fe3O4 for 48 hours.
Notes: Lane 1, control; lane 2, 60 μg/mL MNPs-Fe3O4; lane 3, 0.35 μmol/L GA; lane 4, GA-MNPs-Fe3O4 (0.35 μmol/L GA with 60 μg/mL MNPs-Fe3O4). *P < 0.05, **P < 0.01 compared with the control group; #P < 0.05, when compared with the GA group.
Abbreviations: MNPs-Fe3O4, magnetic nanoparticles containing Fe3O4; GA, gambogic acid.
Discussion
The ultimate goal of medical cancer research is to be able to manipulate the machinery of cell death. Regulation of apoptosis could lead to new possibilities for cancer therapy. Previous studies25,26 have shown that gambogic acid can induce apoptosis via alteration of different molecules associated with apoptosis, such as Bax/Bcl-2 and nuclear factor-κB. Recently, the synergistic effects of magnetic nanoparticles containing Fe3O4 and gambogic acid in the apoptosis of leukemia cells were demonstrated.16 Accumulating research findings have shown that nanotechnology and nanoparticles are becoming a focus for application in clinical medicine because of their stable biomolecular absorption, high surface energy, and small size comparable with biomolecules.27,28 Our present study shows that gambogic acid can inhibit proliferation of LOVO cells in a dose-dependent and time-dependent manner, and that this is enhanced by magnetic nanoparticles containing Fe3O4. In particular, there was no significant inhibition of LOVO cells when the concentration of magnetic nanoparticles containing Fe3O4 was in the range of 20–80 μg/mL (Figure 1B), indicating that magnetic nanoparticles containing Fe3O4 themselves had no cytotoxicity, which is consistent with current research.29 Our study also provides evidence that the apoptosis ratio in LOVO cells treated with an optimal combination of gambogic acid and Fe3O4 magnetic nanoparticles increased dramatically compared with cells treated with gambogic acid alone (P < 0.05, Figure 2), which confirms that magnetic nanoparticles containing Fe3O4 and gambogic acid have a synergistic effect on LOVO cells. At the same time, under optical microscopy (Figure 3) and fluorescence microscopy (Figure 4), typical features of apoptosis, including chromatin condensation, pyknosis of the nucleolus, and apoptotic bodies, were observed when LOVO cells were treated using gambogic acid with or without magnetic nanoparticles containing Fe3O4 for 48 hours. Of note, the proportion of apoptotic cells in the gambogic acid group was less than that in the group receiving magnetic nanoparticles containing Fe3O4 and gambogic acid. However, no cell morphology typical of apoptosis was seen in either the group receiving magnetic nanoparticles containing Fe3O4 or in the control group, and this result was confirmed by flow cytometry (Figure 2).
In order to explore the potential mechanisms of action of gambogic acid, we investigated the status of the PI3K/Akt signaling pathway, components of which are often overexpressed in colorectal cancer and play important roles in the growth and progression of the disease.20,21 An earlier study demonstrated that 2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one hydrochloride and wortmannin, both known PI3K inhibitors, showed antitumorigenic activity in human colon cancer cells in vivo and could cause induction of apoptosis and cell growth arrest in several colorectal cancer cell lines.30,31 Our present data show that treatment with gambogic acid markedly inhibited the activity of PI3K at the transcriptional and translation levels, and that the mRNA of PI3K and the protein of the activated form of PI3K were substantially reduced (Figures 5 and 6). These variations were more significant when combined with magnetic nanoparticles containing Fe3O4. Nevertheless, differences between the Fe3O4 magnetic nanoparticle and control groups were not found. It is well known that protein translation follows gene transcription, but mRNA and protein were detected simultaneously in our study, and this may explain why no obvious changes were found in total PI3K protein, although the mRNA and activated protein for PI3K were changed. This phenomenon was also found in later indices, including expression of Akt and Bad proteins.
As previously reported, Akt is the best known downstream target of PI3K, which binds PIP3 at the plasma membrane, and this leads to phosphorylation of Akt at Ser-473 in its regulatory domain.32 It has also been reported that inactivation of PI3K inhibits phosphorylation of Akt.33 Consistent with prior research, our study revealed that downregulation of Akt mRNA and the p-Akt (Ser-473) protein was accompanied by inactivation of PI3K in LOVO cells treated with gambogic acid alone. Furthermore, inhibition of Akt was markedly enhanced when combined with magnetic nanoparticles containing Fe3O4 (P < 0.05, Figures 5 and 6). Liu et al34 reported recently that Akt, as a hinge in the signal transduction pathway, can regulate cell proliferation, apoptosis, and the cell cycle via a number of downstream molecules. It is worthwhile noting that it could directly or indirectly regulate apoptosis through the mitochondrial pathway. Bad is the first member of the Bcl-2 family found to be a substrate of Akt, and nonphosphorylated Bad is an activated state. It could be phosphorylated at Ser-136 by activation and phosphorylation of Akt,35 then separated from the heterodimer of Bcl-2/Bcl-xL, bound to 14-3-3 scaffold proteins, and localized in the cytoplasm in an inactive form, thus playing an antiapoptotic effect.36 Kim et al showed that phosphorylated Bad was increased in colorectal cancer cells, which may alter regulation of cell death during colorectal tumorigenesis.37 Therefore, inhibition of Bad phosphorylation could be a promising way of treating or preventing colorectal cancer. To elucidate whether apoptosis induced by gambogic acid was through the mitochondrial pathway associated with the PI3K/Akt/Bad signal transduction pathway, we then explored the status of Bad and its downstream molecules in LOVO cells. Our present study demonstrated that gambogic acid markedly reduced Bad mRNA and inhibited phosphorylation of Bad, especially its protein (Figure 6). Moreover, the literature reports that overexpression of phospho-Akt correlates with phosphorylation of epithelial growth factor receptor, FKHR, and Bad in nasopharyngeal carcinoma.38 Thus, we infer that these variations would be the result of the Akt signal being abrogated by gambogic acid. The crucial step in induction of apoptosis of dephosphorylated Bad requires release of cytochrome c into the cytoplasm, which provokes an apoptotic cascade.39 In our study, it was found that levels of cytochrome c, mRNA, and protein were notably increased along with the decrease of phosphorylated Bad. Most notably, these variations in the group treated with magnetic nanoparticles containing Fe3O4 and gambogic acid were more conspicuous than those in the group treated with gambogic acid alone. It has been shown that cytochrome c catalyzes oligomerization of apoptotic protease activating factor-1, which recruits and promotes activation of pro-caspase 9.39 This, in turn, cleaves and activates the effector caspase 3, leading to apoptosis.39 Our data show that, when accompanied by activation of cytochrome c, the activator caspase 9 and the executor caspase 3 are also activated, along with upregulation of the mRNAs for caspase 9 and caspase 3, and the proteins of cleaved caspase 9 and cleaved caspase 3 are detected. It is noteworthy that levels of mRNA and proteins for caspase 9 and caspase 3 in the group treated with a combination of magnetic nanoparticles containing Fe3O4 and gambogic acid were higher than those in the group treated with gambogic acid alone, which confirms further that gambogic acid and magnetic nanoparticles containing Fe3O4 have a synergistic effect on LOVO cells.
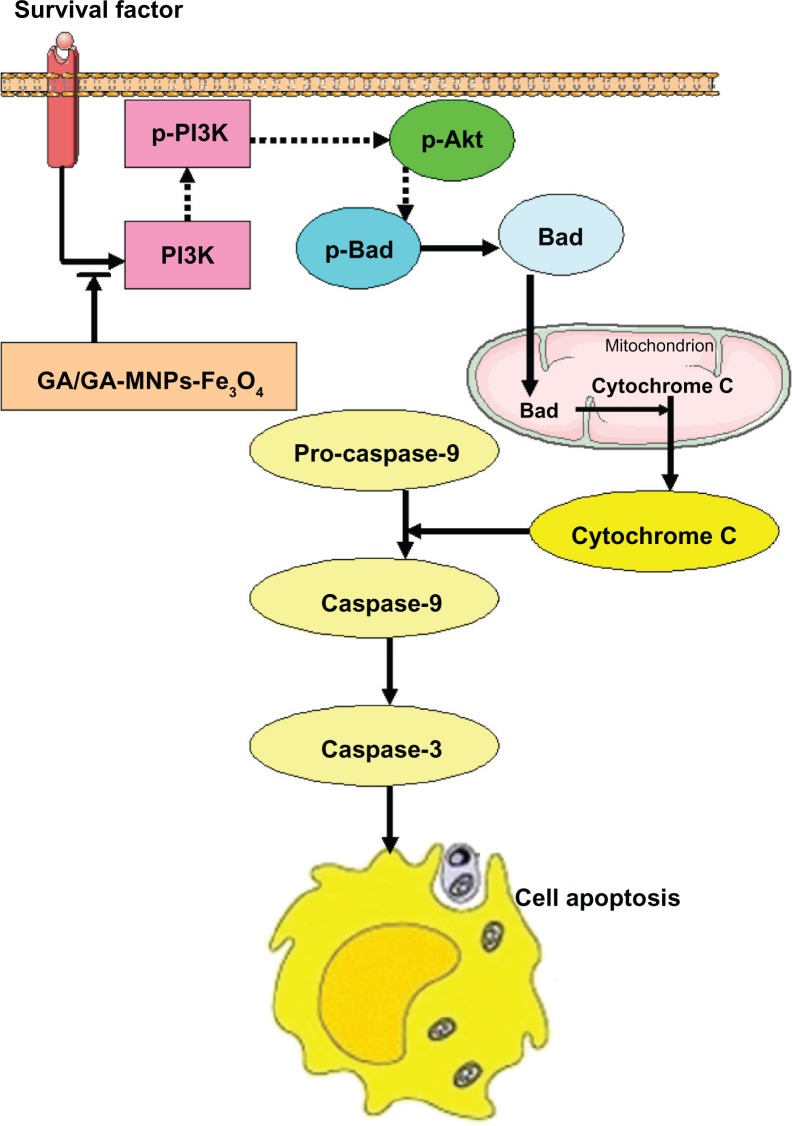
In light of the present data and previous findings, we believe that gambogic acid can stop PI3K transmitting a mitogenic signal to Akt, followed by activation of Bad, leading to release of cytochrome c and activation of caspase 9 and caspase 3. These events may be responsible for the apoptosis shown in Figure 7. Notably, these effects are potentiated by the combination of Fe3O4 magnetic nanoparticles and gambogic acid.
Figure 7.
The mechanism of GA/GA-MNPs-Fe3O4 on apoptosis of LOVO cells.
Notes: →, Promote; ⇢, inhibit; ⇥, block.
Abbreviations: MNPs-Fe3O4, magnetic nanoparticles containing Fe3O4; GA, gambogic acid.
Conclusion
Our study demonstrates for the first time that the combination of Fe3O4 magnetic nanoparticles and gambogic acid has a synergistic effect on the inhibition and apoptosis of LOVO cells, which may be closely associated with regulation of the PI3K/Akt/Bad pathway. This provides theoretical evidence of a clinical benefit for patients with colorectal cancer, and further research is planned in the future.
Acknowledgments
This work was supported by the Priority Academic Development Program of Higher Education Institutions in Jiangsu (012062003010), the Top Talented Personnel in Six Profession in Jiangsu Province project (2010-WS-049), and the Jiangsu Province Hospital of Traditional Chinese Medicine project (2010-Y1008).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Liu A, Chen H, Wei W, et al. Antiproliferative and antimetastatic effects of emodin on human pancreatic cancer. Oncol Rep. 2011;26:81–89. doi: 10.3892/or.2011.1257. [DOI] [PubMed] [Google Scholar]
- 2.Maimon Y, Karaush V, Yaal-Hahoshen N, et al. Effect of Chinese herbal therapy on breast cancer adenocarcinoma cell lines. J Int Med Res. 2010;38:2033–2039. doi: 10.1177/147323001003800617. [DOI] [PubMed] [Google Scholar]
- 3.Li Q, Cheng H, Zhu G, et al. Gambogenic acid inhibits proliferation of A549 cells through apoptosis-inducing and cell cycle arresting. Biol Pharm Bull. 2010;33:415–420. doi: 10.1248/bpb.33.415. [DOI] [PubMed] [Google Scholar]
- 4.Mu R, Lu N, Wang J, et al. An oxidative analogue of gambogic acid-induced apoptosis of human hepatocellular carcinoma cell line HepG2 is involved in its anticancer activity in vitro. Eur J Cancer Prev. 2010;19:61–67. doi: 10.1097/CEJ.0b013e328333fb22. [DOI] [PubMed] [Google Scholar]
- 5.Gu H, Rao S, Zhao J, et al. Gambogic acid reduced Bcl-2 expression via p53 in human breast MCF-7 cancer cells. J Cancer Res Clin Oncol. 2009;135:1777–1782. doi: 10.1007/s00432-009-0624-2. [DOI] [PubMed] [Google Scholar]
- 6.Yi T, Yi Z, Cho SG, et al. Gambogic acid inhibits angiogenesis and prostate tumor growth by suppressing vascular endothelial growth factor receptor 2 signaling. Cancer Res. 2008;68:1843–1850. doi: 10.1158/0008-5472.CAN-07-5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang TT, Wei J, Qian XP, Ding YT, Yu LX, Liu BR. Gambogic acid, a potent inhibitor of survivin, reverses docetaxel resistance in gastric cancer cells. Cancer Lett. 2008;262:214–222. doi: 10.1016/j.canlet.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Lu N, Yang Y, You QD, et al. Gambogic acid inhibits angiogenesis through suppressing vascular endothelial growth factor-induced tyrosine phosphorylation of KDR/Flk-1. Cancer Lett. 2007;258:80–89. doi: 10.1016/j.canlet.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Li R, Chen Y, Zeng LL, et al. Gambogic acid induces G0/G1 arrest and apoptosis involving inhibition of SRC-3 and inactivation of Akt pathway in K562 leukemia cells. Toxicology. 2009;262:98–105. doi: 10.1016/j.tox.2009.04.059. [DOI] [PubMed] [Google Scholar]
- 10.Cheng FY, Su CH, Yang YS, et al. Characterization of aqueous dispersions of Fe3O4 nanoparticles and their biomedical applications. Biomaterials. 2005;26:729–738. doi: 10.1016/j.biomaterials.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 12.Martin W. Natural and synthetic polymers as inhibitors of drug efflux pumps. Pharm Res. 2008;25:500–511. doi: 10.1007/s11095-007-9347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen BA, Lai BB, Cheng J, et al. Daunorubicin-loaded magnetic nanoparticles of Fe3O4 overcome multidrug resistance and induce apoptosis of K562-n/VCR cells in vivo. Int J Nanomedicine. 2009;4:201–208. doi: 10.2147/ijn.s7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Q, Chen BA, Wang XM, et al. Preparation of Fe3O4-magnetic nanoparticles loaded with adriamycin and its reversal of multidrug resistance in vitro. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2007;15:748–751. Chinese. [PubMed] [Google Scholar]
- 15.Wang X, Zhang R, Wu C, et al. The application of Fe3O4 nanoparticle in cancer research: a new strategy to inhibit drug resistance. J Biomed Mater Res A. 2007;80A:852–860. doi: 10.1002/jbm.a.30901. [DOI] [PubMed] [Google Scholar]
- 16.Chen BA, Liang YQ, Wu WW, et al. Synergistic effect of magnetic nanoparticles of Fe3O4 with gambogic acid on apoptosis of K562 leukemia cells. Int J Nanomedicine. 2009;4:251–259. doi: 10.2147/ijn.s7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schatz JH. Targeting the PI3K/AKT/mTOR pathway in non-Hodgkin’s lymphoma: results, biology, and development strategies. Curr Oncol Rep. 2011;13:398–406. doi: 10.1007/s11912-011-0187-7. [DOI] [PubMed] [Google Scholar]
- 18.Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 19.Rychahou PG, Jackson LN, Silva SR, Rajaraman S, Evers BM. Targeted molecular therapy of the PI3K pathway: therapeutic significance of PI3K subunit targeting in colorectal carcinoma. Ann Surg. 2006;243:833–842. doi: 10.1097/01.sla.0000220040.66012.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khaleghpour K, Li Y, Banville D, et al. Involvement of the PI3-kinase signaling pathway in progression of colon adenocarcinoma. Carcinogenesis. 2004;25:241–248. doi: 10.1093/carcin/bgg195. [DOI] [PubMed] [Google Scholar]
- 21.Johnson SM, Gulhati P, Rampy BA, et al. Novel expression patterns of PI3K/Akt/mTOR signaling pathway components in colorectal cancer. J Am Coll Surg. 2010;210:767–768. doi: 10.1016/j.jamcollsurg.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Kuropatwinski K, Hauser J, et al. Colon carcinoma cells harboring PIK3CA mutations display resistance to growth factor deprivation induced apoptosis. Mol Cancer Ther. 2007;6:1143–1150. doi: 10.1158/1535-7163.MCT-06-0555. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Chen L, Ko TC, Fields AP, Thompson EA. Evi1 is a survival factor which conveys resistance to both TGF-β and Taxol-mediated cell death via PI3K/Akt. Oncogene. 2006;25:3565–3575. doi: 10.1038/sj.onc.1209403. [DOI] [PubMed] [Google Scholar]
- 24.Roulin D, Cerantola Y, Dormond-Meuwly A, Demartines N, Dormond O. Targeting mTORC2 inhibits colon cancer cell proliferation in vitro and tumor formation in vivo. Mol Cancer. 2010;9:57. doi: 10.1186/1476-4598-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao W, Zhou SF, Zhang ZP, Xu GP, Li XB, Yan JL. Gambogic acid inhibits the growth of osteosarcoma cells in vitro by inducing apoptosis and cell cycle arrest. Oncol Rep. 2011;25:1289–1295. doi: 10.3892/or.2011.1189. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Yi Y, Chen J, et al. Gambogic acid inhibits Hsp90 and deregulates TNF-α/NF-κB in HeLa cells. Biochem Biophys Res Commun. 2010;403:282–287. doi: 10.1016/j.bbrc.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Pacheco R, Valdivia JG, Ibarra MR. Magnetic nanoparticles for local drug delivery using magnetic implants. Methods Mol Biol. 2009;544:559–569. doi: 10.1007/978-1-59745-483-4_35. [DOI] [PubMed] [Google Scholar]
- 28.Darton NJ, Hallmark B, Han X, Palit S, Slater NK, Mackley MR. The in-flow capture of super-paramagnetic nanoparticles for targeting therapeutics. Nanomedicine. 2008;4:19–29. doi: 10.1016/j.nano.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Wu WW, Chen BA, Cheng J, et al. Biocompatibility of Fe3O4/DNR magnetic nanoparticles in the treatment of hematologic malignancies. Int J Nanomedicine. 2010;5:1079–1084. doi: 10.2147/IJN.S15660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Semba S, Itoh N, Ito M, Harada M, Yamakawa M. The in vitro and in vivo effects of 2-(4-morpholinyl)-8-phenyl-chromone (LY294002), a specific inhibitor of phosphatidylinositol 3′-kinase, in human colon cancer cells. Clin Cancer Res. 2002;8:1957–1963. [PubMed] [Google Scholar]
- 31.Yamaguchi K, Lee SH, Eling TE, Baek SJ. Identification of nonsteroidal anti-inflammatory drug-activated gene (NAG-1) as a novel downstream target of phosphatidylinositol 3-kinase/AKT/GSK-3beta pathway. J Biol Chem. 2004;279:49617–49623. doi: 10.1074/jbc.M408796200. [DOI] [PubMed] [Google Scholar]
- 32.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 33.Gao N, Flynn DC, Zhang Z, et al. G1 cell cycle progression and the expression of G1 cyclins are regulated by PI3K/AKT/mTOR/p70S6K1 signaling in human ovarian cancer cells. Am J Physiol Cell Physiol. 2004;287:C281–C291. doi: 10.1152/ajpcell.00422.2003. [DOI] [PubMed] [Google Scholar]
- 34.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Datta SR, Dudek H, Tao X, et al. Greenberg Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 36.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphory-lation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-XL. Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 37.Kim MR, Jeong EG, Chae B, et al. Pro-apoptotic PUMA and anti-apoptotic phospho-BAD are highly expressed in colorectal carcinomas. Dig Dis Sci. 2007;52:2751–2756. doi: 10.1007/s10620-007-9799-z. [DOI] [PubMed] [Google Scholar]
- 38.Yip WK, Leong VC, Abdullah MA, Yusoff S, Seow HF. Overexpression of phospho-Akt correlates with phosphorylation of EGF receptor, FKHR and BAD in nasopharyngeal carcinoma. Oncol Rep. 2008;19:319–328. [PubMed] [Google Scholar]
- 39.Zimmermann KC, Bonzon C, Green DR. The machinery of programmed cell death. Pharmacol Ther. 2001;92:57–70. doi: 10.1016/s0163-7258(01)00159-0. [DOI] [PubMed] [Google Scholar]