Abstract
Autoimmune diseases are caused by self-reactive lymphocytes that have escaped deletion. Here we have determined the structure of the trimolecular complex for a T cell receptor (TCR) from a patient with multiple sclerosis that causes autoimmunity in transgenic mice. The structure showed a TCR topology notably different from that of antimicrobial TCRs. Rather than being centered on the peptide–major histocompatibility complex, this TCR contacted only the N-terminal peptide segment and made asymmetrical interactions with the major histocompatibility complex helices. The interaction was dominated by the hypervariable complementarity-determining region 3 loops, indicating that unconventional topologies are possible because of the unique complementarity-determining region 3 sequences created during rearrangement. This topology reduces the interaction surface with peptide and alters the geometry for CD4 association. We propose that unusual TCR-binding properties can permit autoreactive T cells to escape deletion.
Autoimmune diseases are caused by aberrant responses of self-reactive T cells and B cells, which are present in every person despite elaborate mechanisms for eliminating or silencing such cells1. T cell receptors (TCRs) recognize peptide fragments bound to major histocompatibility complex (MHC) glycoproteins, and rearrangement of TCR gene segments during T cell development in the thymus produces a highly diverse repertoire2. This enormous diversity ensures that almost any pathogen-derived peptide can be recognized during an infection, but also creates an autoimmunity hazard. Immature thymocytes undergo selection based on recognition of self peptide–MHC complexes, and the outcome represents a delicate balance: survival of thymocytes depends on weak interactions with self peptide–MHC complexes (positive selection), whereas apoptosis is induced by stronger TCR signals (negative selection)3–5. Almost all ‘tissue-specific’ antigens are presented to immature T cells in the thymus by a specialized subpopulation of medullary epithelial cells6. ‘Promiscuous’ antigen expression by these cells is in part regulated by the transcription factor Aire, and deficiency in this protein results in autoimmunity against multiple organs in mice and humans7,8.
Why do some autoreactive T cells escape negative selection? For some antigens, failure of negative selection has been attributed to an unusually low affinity of the self peptide for the MHC9 or to alternative splice forms in the thymus that do not contain the T cell epitope10. However, these mechanisms do not account for most autoimmune T cell epitopes. They also do not explain the presence of T cells that recognize a peptide consisting of myelin basic protein (MBP) residues 85–99 in patients with multiple sclerosis, because this peptide binds with high affinity to HLA-DR molecules11 and the antigen is expressed in the thymus6,12. Failure of negative selection and ensuing autoimmunity can result from an altered TCR signaling threshold, as has been demonstrated in a spontaneous autoimmune arthritis model (SKG mice)13. These mice have a point mutation in the gene encoding an important TCR signaling molecule, the tyrosine kinase Zap70, and the weakened TCR signal results in positive selection of normally negatively selected autoimmune T cells. In theory, alterations in thymic selection thresholds could also result from changes in the interaction of TCR and its coreceptor with self peptide–MHC, but all reported crystallographic studies of TCR-peptide-MHC complexes have demonstrated a similar position of the TCR on the peptide-MHC complex14–21. This binding mode maximizes the contact surface with the bound peptide and positions the TCR diagonally across the peptide-MHC surface14,15,22,23 such that the most diverse loops of both TCR chains (complementarity-determining region 3 (CDR3) loops) converge on the central peptide residue.
The TCRs studied so far are mainly from infectious disease14,17,18,24 and alloreactivity15,16,20,21 settings characterized by intense T cell competition. The Ob.1A12 TCR studied here emerged in a biological context very different from that of other human receptors studied by X-ray crystallography and represents one of the best-characterized TCRs from a human autoimmune disease. This TCR was isolated from a patient with relapsing-remitting multiple sclerosis11,25 by stimulation of blood mononuclear cells with MBP in conditions in which only a fraction of the wells contained MBP-reactive T cells (the ‘split-well’ technique)26,27, thus avoiding in vitro competition among MBP-reactive T cells. The Ob.1A12 T cell clone recognizes the immunodominant peptide of MBP residues 85–99 bound to the disease-associated HLA-DR2 (DRA, DRB1*1501) molecule11, and experiments using a monoclonal antibody specific for this peptide-MHC combination have demonstrated that this MBP peptide is displayed by antigen-presenting cells in active multiple sclerosis lesions28. The pathogenic potential of the Ob.1A12 TCR has been demonstrated in transgenic mice that express this human TCR and HLA-DR2, which after immunization with the MBP peptide develop central nervous system–specific autoimmunity at sites frequently affected in the human disease, such as the optic nerve, cerebellum, brainstem and spinal cord29. Spontaneous central nervous system inflammation was noted in approximately 4% of these mice, increasing to 100% when the mice were bred onto a recombination activating gene 2–deficient background. We demonstrate here that this TCR engages its self peptide–MHC target with a topology notably different from that of previously studied antimicrobial TCRs. The differences in topology between antimicrobial and an autoimmune TCR may reflect distinct selection pressures: whereas T cells with an optimal fit for a microbial peptide–MHC complex are favored during an infection, autoreactive T cells whose TCRs have an optimal fit for a self peptide–MHC complex are the most likely to be deleted in the thymus.
RESULTS
Initial efforts to crystallize the complex using separately expressed Ob.1A12 TCR and MBP-DR2 did not yield crystals of the complex, and we therefore used the strategy for the creation of stable TCR-peptide-MHC complexes initially described for the influenza hemagglutinin–reactive HA1.7 TCR24. The MBP peptide was attached to the N terminus of the TCRβ chain through a flexible linker so that binding of the MBP peptide to DR2 created a stable complex. We determined the structure to a resolution of 3.5 Å by molecular replacement (Table 1). The structure showed many unusual features with respect to the overall topology and the interaction of TCR with both peptide and MHC (Fig. 1a).
Table 1.
Statistics for data collection and refinement
| Data collection | |
| Space group | F222 |
| Unit cell (Å) | a = 137.3; b = 212.6; c = 278.2 |
| Molecule/asymmetric unit | 1 |
| Resolution limit (Å)a | 20–3.5 (3.62–3.5) |
| Number of reflections | 24,963 |
| Multiplicity | 4.0 |
| Average I/σ(I) | 10.2 (3.8) |
| Completeness (%) | 96.3 (97.6) |
| Rsym (%)b | 8.5 (39.9) |
| Model refinement | |
| Rcryst (%)c | 27.4 |
| Rfree (%) | 31.8 |
| r.m.s deviations from ideality | |
| Bond lengths (Å) | 0.013 |
| Bond angles (°) | 2.0 |
| Dihedrals (°) | 27.0 |
| Impropers (°) | 1.2 |
| Ramachandran plot statistics (%) | |
| Favored | 71.9 |
| Allowed | 25.9 |
| Generously allowed | 2.2 |
| Disallowed | 0 |
| Temperature factors (Å2) | |
| DRα | 46.8 |
| DRβ | 46.7 |
| TCRα | 103.7 |
| TCRβ | 107.4 |
| MBP-peptide | 60.6 |
Values in parentheses refer to the shell of highest resolution.
Rsym= Σ| I − 〈I〉|/ΣI, where I is the measured intensity of a reflection and 〈I〉 is the average intensity of that reflection.
R = Σ| |Fo| − |Fc| |/Σ|Fo|, where Fo and Fc are the observed and calculated structure factor amplitudes, respectively. Rcryst is based on 95% of data; Rfree is used for cross-validation, based on the remaining 5% of data.
Figure 1.
Unconventional binding site for a human autoreactive TCR on its peptide-MHC target. Top and side views of the complex of the Ob.1A12 TCR plus the peptide of myelin basic protein residues 85–99 bound to HLA-DR2 (a) compared with the complex of the HA1.7 TCR plus a peptide of influenza hemagglutinin residues 306–318 bound to HLA-DR1 (ref. 24; b). Yellow, TCR Vα domain; red, TCR Vβ domain; blue, HLA-DR; green, peptide; gray, central P5 residue; pink, P2 residue. Dotted lines are drawn through center of mass of the Vα and Vβ domains. TCR constant domains have been omitted for clarity.
Topology of the complex
The HA1.7 TCR is the only other human MHC class II–restricted TCR for which the structure of the trimolecular complex has been determined to our knowledge24 (Fig. 1b), and its binding topology is very similar to that of the mouse D10 TCR19 that recognizes a conalbumin peptide bound to I-Ak. These TCRs are centered over the peptide-MHC surface, and the CDR3 loops create a ‘pocket’ for the centrally located peptide side chain at position 5 (P5; Fig. 1b). The approximately diagonal-to-orthogonal orientation over the peptide-MHC surface (70° for HA1.7; 80° for D10) permits close contacts with the bound peptide by avoidance of the high points of the MHC helices and the ridge created by the N terminus of the peptide. A very similar topology has been noted for all MHC class I–restricted TCRs, with the crossing angle ranging from 45° to 70° (refs. 14–18,20,21).
The topology of the complex between Ob.1A12 TCR and MBP-DR2 is notably different in many important aspects. Rather than being centered over the peptide-MHC surface, this TCR only contacts the N-terminal peptide segment (Fig. 1a and Supplementary Fig. 1 online). This lateral translation is considerable: 11.4 Å between the centers of mass of Ob.1A12 and HA1.7 (as a reference, the distance between the Cα atoms of the P1 and P9 peptide residues is 24.8 Å). As a result, the Ob.1A12 TCR makes only a lateral interaction with the central P5 peptide residue and is instead positioned over the P2 peptide residue (histidine; Fig. 1a). The interaction with the MHC helices is also not symmetrical because of a shift (Fig. 1a, top, downward shift) and considerable tilt toward the DRβ helix (Fig. 1a, bottom, tilt toward viewer; 26.7° relative to HA1.7). Finally, the line drawn through the center of mass of variable (V) regions Vα and Vβ (Fig. 1) pointed into an opposite direction compared with that of HA1.7 (rotation of 40° relative to HA1.7). This crossing angle (110°) was far outside the range noted in previously determined structures for MHC class I– or MHC class II–restricted TCRs. This binding mode positions Ob.1A12 TCR over the high point of the DRα helix and the ridge created by the N-terminal extension of the peptide, indicating that these topological features do not preclude TCR binding. It has been argued that avoidance of these surface features represents a general property of TCR binding19, but this structure demonstrates that there is more than one topological solution for productive binding of TCR to peptide-MHC. The interaction of the Ob.1A12 TCR with its self peptide–MHC target is thus characterized by an off-center binding mode in which only the N-terminal segment of the peptide is contacted and the interaction with the MHC helices is highly asymmetrical.
The CDR3 dome
The ‘footprint’ of the Ob.1A12 TCR on the MBP-DR2 surface (Fig. 2) demonstrates that a large fraction of the interaction surface is formed by the CDR3 loops of TCRα (28.9%) and TCRβ (37.6%). The CDR3 loops converge on the P2 peptide side chain (histidine), and functional experiments with the Ob.1A12 T cell clone have demonstrated a high degree of specificity for this peptide residue11,30. In contrast, the contact surfaces for the germline-encoded CDR1 and CDR2 loops of TCRα and TCRβ are considerably smaller (Fig. 2). In comparison, the HA1.7 structure shows contributions to the contact surface to be more evenly shared among all CDR loops (discussed below).
Figure 2.
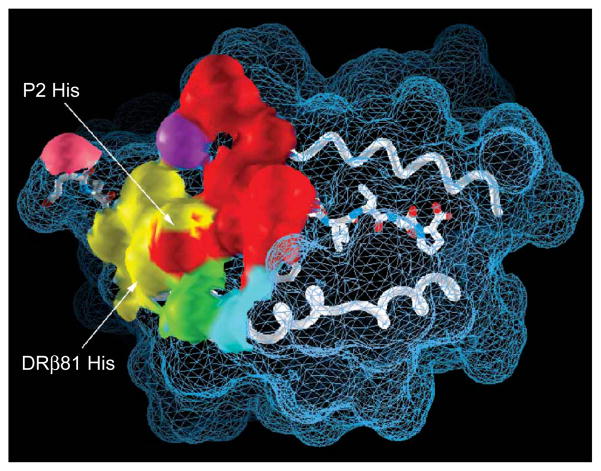
Footprint of the Ob.1A12 TCR on the MBP peptide-DR2 surface. The Ob.1A12 TCR contacts only the N-terminal segment of the MBP peptide. The TCR CDR3α and CDR3β loops (yellow and red, respectively) dominate the interaction and converge over peptide residue P2 His. The CDR3α loop also contacts a histidine from the DRβ chain helix (DRβ81 His). The CDR1 and CDR2 loops of TCRα (green and cyan, respectively) and TCRβ (purple and pink, respectively) cover smaller surface areas.
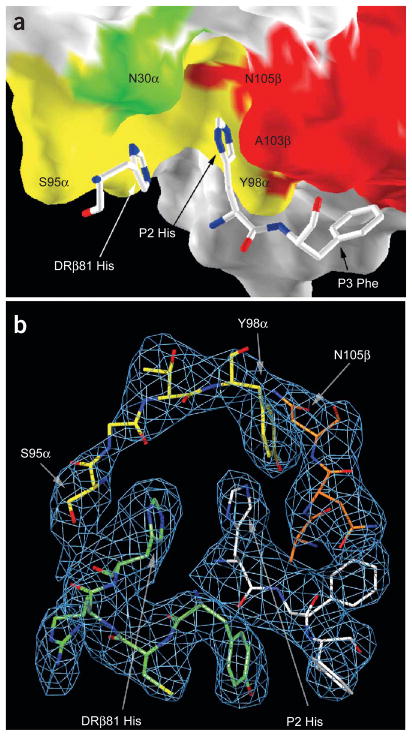
More detailed analysis of the pocket created by the two CDR3 loops showed a highly unusual feature: this dome-shaped cavity is large enough to accommodate both an MHC residue (DRβ81 His) and a peptide side chain (P2 His; Fig. 3). This feature is unprecedented, as the corresponding pocket in other TCRs accommodates a single peptide side chain but no MHC residue. The histidine from the peptide (P2 His) is contacted by both CDR3 loops (Y98α and A103β), whereas the histidine from the MHC is contacted mainly by CDR3α (S95α). The cavity is open to one side and this space is filled by a segment of the DRβ helix (Fig. 3b). Another peptide residue essential for T cell activation, P3 Phe, is nestled between a hydrophobic shelf of the DR molecule and the large surface of the CDR3β loop (Fig. 3a).
Figure 3.
TCR CDR3 loops create a large cavity for histidine residues from peptide and MHC. (a) Dome-shaped cavity created by CDR3α (yellow) and CDR3β (red) loops that accommodates DRβ81 His and P2 His. (b) Omit map showing the electron density for CDR3α (S95α–Y98α; yellow), CDR3β (A103β–N105β; orange), DRβ 78–81 (green) and peptide residues P2 His, P3 Phe and P4 Phe (white).
Relationship between structure and function
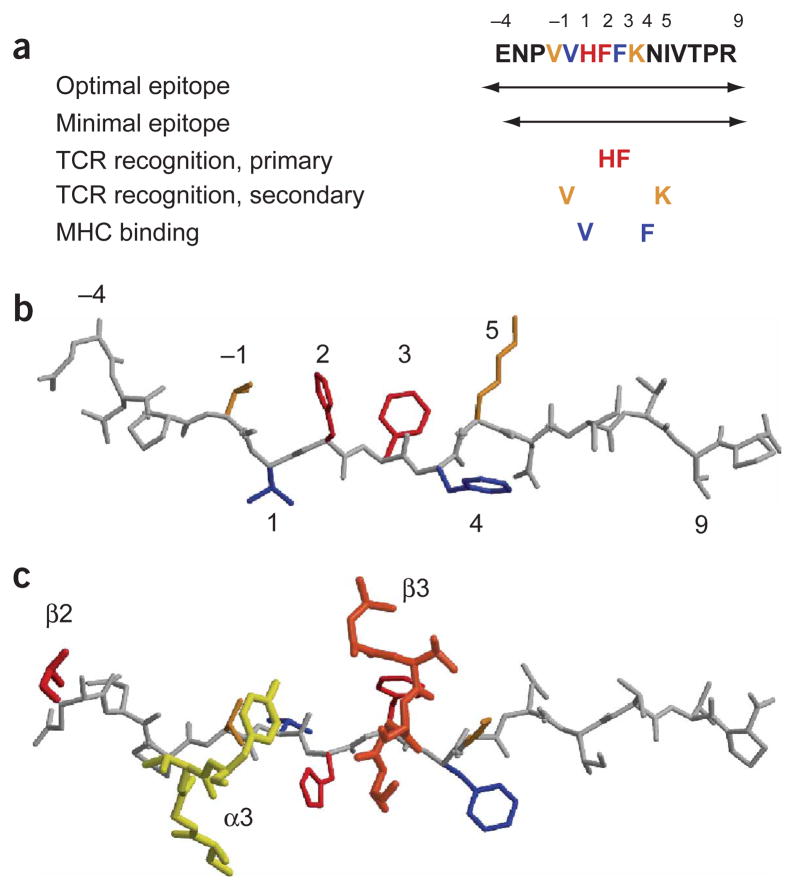
Before determination of this structure, several aspects of the functional data could not be readily explained. First, specificity of TCR recognition was confined to a small segment of the peptide; in particular, residues P2 His and P3 Phe11,30. Substitution of these residues by any other amino acid abolished or greatly reduced the stimulatory capacity of the peptide (Fig. 4a,b). The valine at position −1 (P −1 Val) and P5 Lys make smaller contributions to TCR recognition, and only some substitutions affect T cell activation30. The structure showed that only the CDR3 loops of the Ob.1A12 TCR contact MBP peptide side chains (Figs. 4c and 5), compared with four HA1.7 TCR loops (CDR1 and CDR3 loops from both chains) that make substantial contacts over a nine–amino acid segment of the hemagglutinin peptide (from P −1 to P8; Fig. 5). Moreover, many substitutions in the C-terminal peptide segment that did not affect DR2 binding had little or no effect on T cell stimulation11,30. The absence of any contacts by the Ob.1A12 TCR to this part of the peptide (Fig. 2) accounts for these findings. Furthermore, the N-terminal peptide segment to P −4 was required for efficient T cell activation: without truncation of the P −4 residue the potency was about 10-fold more, and the activity was almost completely lost by truncation of P −3 (ref. 11). This finding was puzzling, because the HA1.7 and D10 TCRs reached only to P −1 (refs. 19,24). The crystal structure of the Ob.1A12 TCR showed that the N-terminal peptide segment ‘arches up’ to the TCR and that the CDR2 loop of TCRβ contacts the peptide backbone at P −4 (Fig. 5). The interaction of the TCR with the peptide backbone also explains why Ala could be substituted for Glu at P −4 without loss of activity. The requirement for the N-terminal extension of the MBP peptide means that T cell stimulation is dependent on the presence of processing products of particular lengths. Thymic epithelial cells and dendritic cells in peripheral lymphoid organs express different proteases (such as cathepsin L versus S in thymic epithelial cells and peripheral dendritic cells, respectively)31, raising the possibility that differential processing of MBP allows T cells that express this TCR to escape negative selection and mediate an inflammatory disease.
Figure 4.
Relationship between structure and function. (a) Previously generated functional data on the Ob.1A12 T cell clone. Colors match those used in b and c. (b) Structure of the MBP peptide in the trimolecular complex, with MHC binding and TCR recognition sites assigned colors according to the functional data in a. (c) Interaction of CDR3α (α3), CDR3β (β3) and CDR2β (β2) loops with MBP peptide. Only the CDR3 loops bind to peptide side chains; the CDR2β loop makes a contact with the peptide backbone at P −4.
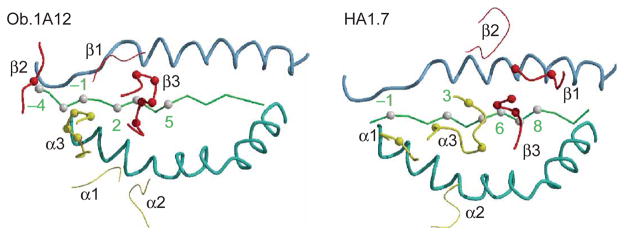
Figure 5.
Differences in peptide recognition by the Ob.1A12 and HA1.7 TCRs. Only the two CDR3 loops of the Ob.1A12 TCR contact peptide side chains; the CDR2β loop contacts the peptide backbone at P −4. In contrast, four TCR loops of HA1.7 (CDR1 and CDR3 loops of both chains) contact peptide side chains. Yellow, TCRα loops (α1, α2 and α3); red, TCRβ loops (β1, β2 and β3). Thick lines, TCR loops that contact peptide; thin lines, TCR loops that do not contact peptide. Peptide positions are numbered as in Figure 4. Blue, MHC helices; green, peptide backbone.
Interaction of the TCR with MHC helices
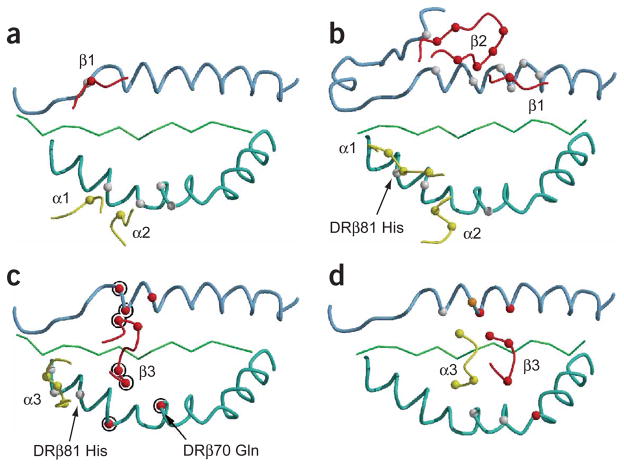
The contribution of the germline-encoded CDR1 and CDR2 loops to MHC binding differs substantially between the Ob.1A12 TCR (Fig. 6a) and HA1.7 TCR (Fig. 6b). In the HA1.7 structure, the germline-encoded CDR1 and CDR2 loops of Vα and Vβ make most of the contacts with the MHC helices (Fig. 6b). Similar findings in other structures led to the proposal that this diagonal orientation is general, even though no universal MHC contact residue was identified in mutagenesis experiments22,32. In contrast to the situation with the HA1.7 TCR, the hypervariable CDR3 loops of the Ob.1A12 TCR make most of the contacts with the MHC helices (seven TCR residues; Fig. 6c), rather than the germline-encoded CDR1 and CDR2 loops (three TCR residues; Fig. 6a). The CDR3β loop of Ob.1A12 TCR spans both MHC helices, and three of these four TCRβ residues are encoded by nucleotides that were randomly inserted between the variable–diversity and diversity–joining junctions during rearrangement of the Ob.1A12 TCRβ chain (Fig. 6c). The hypervariable TCR loops thus seem to be essential in specifying the orientation of this TCR on the MBP-DR2 complex. We noted large differences in the location of particular CDR loops compared with those of HA1.7: the difference in location of the tip of the CDR3α loop for HA1.7 versus Ob.1A12 is 15.6 Å (Fig. 6c,d), and the difference for CDR2β is even larger (18.3 Å). Only the CDR1 and CDR2 loops of TCRα are located in a similar position on the DRβ chain helix in the Ob.1A12 and HA1.7 structures, but only two TCR residues from these loops contact the DRβ helix in the Ob.1A12 structure, compared with five in the HA1.7 complex (Fig. 6a,b). A single MHC residue (DRβ77 Thr) is contacted by the germline-encoded CDR1 and CDR2 loops of both TCRs, and this MHC residue is contacted by different TCR amino acids (N30α for Ob.1A12 TCR; Y31α and S51α for HA1.7 TCR), indicating that the similarity in the overall position of the TCRα CDR1 and CDR2 loops is not due to conserved contacts. A key difference between the two structures is that DRβ81 His is contacted by the germline-encoded CDR1 loop of HA1.7 but by the hypervariable CDR3 loop of the Ob.1A12 TCR. It is possible that a certain general orientation of the TCRα chain on peptide-MHC complexes is required for association of the CD4 coreceptor or other molecules involved in T cell activation.
Figure 6.
MHC contacts made by the Ob.1A12 and HA1.7 TCRs. (a,b) Differences in the contribution of germline-encoded CDR1 and CDR2 loops from the Ob.1A12 (a) and HA1.7 (b) TCRs to MHC binding. CDR1 loops, α1 and β1; CDR2 loops, α2 and β2. (c,d) Differences in positions of CDR3 loops on the MHC helices for the Ob.1A12 (c) and HA1.7 (d) TCRs. CDR3α and CDR3β loops, α3 and β3, respectively. Ob.1A12 TCRβ residues encoded by N-region residues and corresponding MHC contacts are circled. Red, TCRβ loops; yellow, TCRα loops.
Geometry of CD4 coreceptor binding
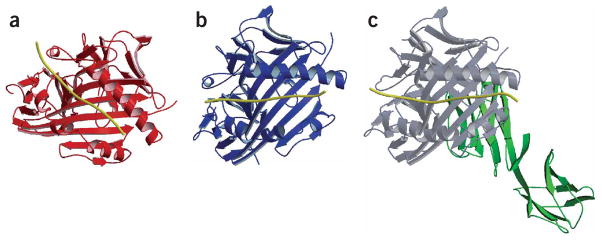
The CD4 coreceptor binds to the membrane-proximal MHC class II domains33 and is essential for T cell development and T cell function by recruiting the tyrosine kinase Lck2. We examined potential consequences of the altered topology to recruitment of the CD4 coreceptor, which is important in early TCR signaling. We superimposed the TCR V domains of the Ob.1A12 TCR (Fig. 7a) and HA1.7 TCR (Fig. 7b) to examine the location of the CD4 binding site on the MHC molecule relative to TCR; we have presented this as the relative position of peptide-MHC in these two complexes when the TCR V domains are superimposed. CD4 binds to the membrane-proximal MHC α2 and β2 domains under the β-sheet of the peptide-binding site33 (Fig. 7c). This alignment showed that the geometry of the interaction with the CD4 coreceptor is altered for the Ob.1A12 TCR. Preliminary functional studies with a blocking antibody to CD4 demonstrated that proliferation of the Ob.1A12 T cell clone was partially inhibited, indicating that the altered geometry does not result in a complete loss of CD4 function (data not shown).
Figure 7.
Consequence of altered topology on the geometry of CD4 coreceptor engagement. The variable domains of the Ob.1A12 (a) and HA1.7 (b) TCRs were superimposed. The relative position of the peptide-MHC component in the two trimolecular complexes is presented. The CD4 coreceptor binds to the membrane-proximal MHC α2 and β2 domains under the β-sheet of the peptide-binding site, as shown for the complex of a mouse MHC class II molecule and CD4 (c; D1 and D2 domains; green, CD4 molecule; gray, MHC molecule33). The MHC-bound peptide is yellow in all structures.
DISCUSSION
This structure demonstrates a TCR-binding topology that is very different from that of previously determined structures for other TCRs. Does this TCR represent an isolated case? Most MBP-reactive T cell lines (60 of 75 lines) from this multiple sclerosis patient recognized the peptide of MBP residues 84–102, and clones established from seven of eight independent lines used the same Vα-Jα, Vβ-Jβ gene segments25. TCRs from six of these clones (represented by clone Ob.2F3) had the same nucleotide sequence, reflecting substantial in vivo expansion. The seventh clone (Ob.1A12) differed from this group at one residue in CDR3α and two residues in CDR3β. Notably, clones Ob.2F3 and Ob.1A12 had an identical ‘fine specificity’ for P −1 Val, P2 His and P3 Phe when analyzed with a large panel of analog peptides30. The clones differed only in the fine specificity for P5 Lys, which is explained by the two amino acid difference in the CDR3β loop that contacts P5 (ref. 30). These clones therefore make very similar contacts to the DR2-bound MBP peptide, indicating that the T cell response to MBP in this multiple sclerosis patient is dominated by T cells with unusual topology.
Given the considerable sequence diversity among TCRs, it is notable that all TCRs previously studied showed a similar position on the peptide-MHC complex. It has been proposed that this topology is the product of coevolution of TCR V genes and MHC genes, and that the germline-encoded CDR1 and CDR2 loops have an inherent bias to bind to the MHC helices34. The structure we have reported here shows that an alternative topology is possible and that T cells that express such a receptor can be functional and induce an inflammatory disease. We propose that the predominant conventional topology is not only the product of coevolution but also the result of selection. Unconventional topologies may be more prevalent among immature thymocytes than is now appreciated, and thymic selection may eliminate substantial numbers of such cells because of affinities outside the appropriate range35 or geometries incompatible with coreceptor binding or formation of higher-order complexes in immunological synapses. Further selection occurs during an infectious challenge where T cells with the highest affinity for relevant peptide-MHC complexes have a competitive advantage over other cells36. T cells selected from an infectious disease setting or animals immunized with an antigen in a microbial adjuvant may thus represent the best available fit for a given peptide-MHC surface, a hypothesis supported by a large body of functional data.
In contrast, an autoimmune T cell repertoire is shaped by thymic selection9, but a fraction of autoreactive T cells nevertheless evades these elaborate mechanisms. We propose that the altered topology described here has three main consequences. First, it reduces the affinity of the TCR for the peptide-MHC target, in part by reducing the contact surface. The buried surface area for the Ob.1A12 TCR (1,606 Å2) is substantially smaller than that for the HA1.7 TCR (2,054 Å2), and previous studies have shown that the Ob.1A12 TCR binds to DR2-MBP with low affinity37. Comparison of the Ob.1A12 T cell clone to a panel of clones specific for hemagglutinin residues 306–318 demonstrated that the hemagglutinin clones were more sensitive to peptide stimulation (a difference of about 1 log in dose-response curves (data not shown)). Second, it alters the geometry of the complex, which may affect its interaction with the CD4 coreceptor and possibly other molecules during the formation of immunological synapses. The organization of these synapses differs in important ways between thymocytes and mature T cells. In mature T cells, the TCR localizes to the center and the CD4 coreceptor moves only transiently through this zone38–40. In thymocytes in conditions of negative selection, the TCR remains with the CD4 coreceptor in the periphery of the synapse41. These findings raise the possibility that CD4 function is differentially affected in immature and mature T cells by an altered geometry of TCR binding to peptide-MHC. The combination of reduced affinity and altered geometry may have allowed this autoreactive T cell to evade elimination in the thymus and at the same time maintained its ability to induce an inflammatory process. Because rather subtle changes in peptide structure, epitope density or signaling threshold can profoundly affect thymic selection5,13, relatively small changes in the signals resulting from engagement of this unusual TCR may have been sufficient to increase the chance for survival in the thymic micro-environment. Third, a reduced interaction surface with the peptide may increase the risk that such T cells are activated by microbial peptides with sufficient structural similarity to the self-antigen. Five microbial peptides have been identified that activate the Ob.1A12 T cell clone, and only P2 His and P3 Phe are identical to the MBP peptide among all these peptides30,42. It is possible that this TCR can recognize other unrelated peptides in a conventional topology, for example, during positive selection, but this hypothesis has yet to be tested experimentally.
The crystal structure of a mouse autoimmune TCR has been reported43. The TCR was isolated from a T cell clone that induces experimental autoimmune encephalomyelitis and recognizes the acetylated N-terminal peptide of MBP amino acids 1–11 bound to I-Au. This TCR binds to its peptide-MHC target in a conventional diagonal orientation, but the MBP peptide occupies only two thirds of the binding site. Thus, both autoimmune TCRs that have been characterized so far in structural terms show highly aberrant binding properties that result in a reduced TCR contact surface with peptide. The reduced interaction surface with peptide is due to an unconventional topology of TCR on the peptide-MHC complex (human TCR) or an unusual binding register of the peptide that leaves part of the binding site empty (mouse TCR). TCRs with such aberrant binding properties would be ‘out-competed’ by clones with optimal peptide-MHC interactions in an antimicrobial immune response. However, TCRs with aberrant binding properties may not be uncommon in autoimmune settings, because such binding properties may increase the probability that autoreactive T cells escape deletion in the thymus.
Previous structures have demonstrated a conventional TCR topology in which the germline-encoded CDR1 and CDR2 loops make most of the contacts with the MHC helices. The unconventional topology described here for the Ob.1A12 TCR is dependent on the hypervariable CDR3 loops that form unusual interactions with both MHC and peptide. The high degree of structural diversity at the V-(D)-J junctions can thus result in unusual TCR-binding properties, with the potential for pathological consequences.
METHODS
Protein expression and crystallization
The ectodomain of Ob.1A12 TCR was expressed in the baculovirus system with constructs containing the TCRα and TCRβ V and C domains up to the interchain disulfide bond; efforts to express this TCR in Escherichia coli as a two-chain or single-chain molecule were unsuccessful. The MBP peptide sequence (ENPVVHFFKNIVTPR) was covalently linked to the N terminus of the mature TCRβ chain through a flexible linker (GGSGGGGG). Cysteine residues not involved in disulfide bonds were substituted with serine. The protein was purified from the supernatant of infected Sf9 cells with a protein C epitope tag at the C terminus of the TCRβ chain. Fos and Jun leucine zipper dimerization domains at the C terminus used to facilitate heterodimer formation were removed by thrombin cleavage after purification. HLA-DR2 (DRA, DRB1*1501) was purified from baculovirus-infected Sf9 cells as described44. Complexes of TCR-peptide and HLA-DR2 were formed at a pH of 5.8 by incubation of TCR, HLA-DR2 and HLA-DM at a molar ratio of 6:4:1 for 18 h at 22 °C. Unbound TCR was removed by affinity chromatography using the L243 monoclonal antibody to DR44, and complexes were separated from free DR by hydrophobic interaction chromatography. The complex was pure and monodisperse, based on analysis by SDS-PAGE, isoelectric focusing PAGE and dynamic light scattering. The complex was concentrated to 12.6 mg/ml in 25 mM Tris, pH 7.5, 1 mM EDTA, 1 mM PMSF and 1 μM leupeptin. Crystals were grown by the hanging-drop vapor-diffusion method against a reservoir of 1.3 M sodium tartrate, 50 mM ammonium formate and 50 mM HEPES, pH 7.0, at 25 °C. Crystals grew as parallelogram-shaped plates up to a volume of 0.2 × 0.2 × 0.1 mm and were cryoprotected by the addition of glycerol to 15% and LiCl to a concentration of 0.5 M. A total of 441 crystals were screened at the National Synchrotron Light Source at Brookhaven National Laboratories (Upton, New York), and a data set was collected at beamline X29 at a wavelength of 1.1 Å with 1.0-degree oscillation and was evaluated with the HKL2000 program45. The space group was F222 with one complex in the asymmetric unit and a solvent content of 75%. The resolution was 3.5 Å based on an average I/σ(I) of more than 3 (average of 3.8 for the shell of highest resolution). The data extended to 3.2 Å with an average I/σ(I) of 2.0 for the shell of 3.41–3.2 Å (Table 1, data statistics).
Structure solution and refinement
The structure was solved by molecular replacement using the Phaser program46. The HLA-DR2 component44 (Protein Data Bank accession number, 1BX2) could be readily located (Roverall = 49.7%). The TCRβ chain was subsequently located with the 3.0- Å model of TCRβ from the Vβ2.1–streptococcal pyrogenic exotoxin C2 complex (Protein Data Bank accession number, 1KTK)47. By varying the elbow angle between the V and C domains of the TCRβ chain, we created a set of search models of which one gave a satisfactory solution (C domain rotated −9° relative to the V domain around E119). After positioning of the TCRβ chain, the TCRα model of HA1.7 (Protein Data Bank accession number, 1FYT)24 could be manually positioned opposite the β-chain counterpart and rigid body could be refined in the Phaser program. The resulting complex was refined with the crystallography and nuclear magnetic resonance system (CNS) program48 and the CDR loops were built into the electron density with the O program49. Special care was taken to prevent model bias by building crucial parts of the structure including the peptide, CDR loops and MHC helices with the help of omit maps. Subsequent ‘runs’ of positional and temperature factor refinement led to a drop in the Rfree to 31.8% between 20 Å and 3.5 Å. As in the structure for the complex of A6 TCR and HLA-A2–Tax14, Cα had little continuous density and was therefore removed from the model. The linker connecting the C terminus of the peptide with the N terminus of TCRβ as well as the last residue of the MBP peptide were not visible in the electron density map. The distance between the termini was 20 Å, and the octapeptide linker was of sufficient length (about 30 Å) to not constrain the interaction of TCR with the peptide-MHC complex. Also, the same linker had been used in the HA1.7 structure24 for which the topology of TCR-peptide-MHC interaction was very distinct. There was no evidence that crystal contacts influenced the overall topology of the complex. All observed hydrogen bonds and salt bridges between symmetry-related molecules were between MHC chains (DRα-DRα and DRβ-DRβ). A sugar group at Asn118 of DRα was included in the model. Figures 1 and 4–7 were prepared with MolScript (www.avatar.se/molscript/) and Figures 2 and 3, with GRASP (http://trantor.bioc.columbia.edu/grasp/).
Protein Data Bank accession numbers
Coordinates and structure factor files, 1YMM.
Supplementary Material
Acknowledgments
This paper is dedicated to the memory of D.C. Wiley. We thank V. Stojanoff, M. Allaire (beamline X6A), A. Soares, D. Schneider (beamline X12B), A. Saxena and H. Robinson (beamline X29) at Brookhaven National Laboratories for support; M. Eck for access to X-ray facilities at the Dana-Farber Cancer Institute; K. Arnett, M. Call and S. Turley for reading the manuscript; T. Springer and T. Xiao for help and advice in evaluating Fluidigm crystallization chips; D. Zaller for the S2 cell line expressing HLA-DM; and L. Stern and K.C. Garcia for discussions. Supported by the National Institutes of Health (AI045757 and AI064177 to K.W.W.).
Footnotes
Supplementary information is available on the Nature Immunology website.
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
References
- 1.Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 2.Davis MM, Chien Y-H. T-cell antigen receptors. In: Paul WE, editor. Fundamental Immunology. Lippincott Williams & Wilkins; Philadelphia: 2003. pp. 227–258. [Google Scholar]
- 3.Kisielow P, Teh HS, von Bluthmann H, Boehmer H. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature. 1988;335:730–733. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- 4.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 5.Alam SM, et al. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996;381:616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 6.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 7.The Finnish-German APECED Consortium. Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 8.Anderson MS, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 9.Harrington CJ, et al. Differential tolerance is induced in T cells recognizing distinct epitopes of myelin basic protein. Immunity. 1998;8:571–580. doi: 10.1016/s1074-7613(00)80562-2. [DOI] [PubMed] [Google Scholar]
- 10.Anderson AC, et al. High frequency of autoreactive myelin proteolipid protein-specific T cells in the periphery of naive mice: mechanisms of selection of the self-reactive repertoire. J Exp Med. 2000;191:761–770. doi: 10.1084/jem.191.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wucherpfennig KW, et al. Structural requirements for binding of an immunodominant myelin basic protein peptide to DR2 isotypes and for its recognition by human T cell clones. J Exp Med. 1994;179:279–290. doi: 10.1084/jem.179.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pribyl TM, Campagnoni C, Kampf K, Handley VW, Campagnoni AT. The major myelin protein genes are expressed in the human thymus. J Neurosci Res. 1996;45:812–819. doi: 10.1002/(SICI)1097-4547(19960915)45:6<812::AID-JNR18>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi N, et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003;426:454–460. doi: 10.1038/nature02119. [DOI] [PubMed] [Google Scholar]
- 14.Garboczi DN, et al. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature. 1996;384:134–141. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- 15.Garcia KC, et al. An αβ T cell receptor structure at 2.5 Å and its orientation in the TCR-MHC complex. Science. 1996;274:209–219. doi: 10.1126/science.274.5285.209. [DOI] [PubMed] [Google Scholar]
- 16.Garcia KC, et al. Structural basis of plasticity in T cell receptor recognition of a self peptide-MHC antigen. Science. 1998;279:1166–1172. doi: 10.1126/science.279.5354.1166. [DOI] [PubMed] [Google Scholar]
- 17.Ding YH, et al. Two human T cell receptors bind in a similar diagonal mode to the HLA-A2/Tax peptide complex using different TCR amino acids. Immunity. 1998;8:403–411. doi: 10.1016/s1074-7613(00)80546-4. [DOI] [PubMed] [Google Scholar]
- 18.Stewart-Jones GB, McMichael AJ, Bell JI, Stuart DI, Jones EY. A structural basis for immunodominant human T cell receptor recognition. Nat Immunol. 2003;4:657–663. doi: 10.1038/ni942. [DOI] [PubMed] [Google Scholar]
- 19.Reinherz EL, et al. The crystal structure of a T cell receptor in complex with peptide and MHC class II. Science. 1999;286:1913–1921. doi: 10.1126/science.286.5446.1913. [DOI] [PubMed] [Google Scholar]
- 20.Reiser JB, et al. A T cell receptor CDR3β loop undergoes conformational changes of unprecedented magnitude upon binding to a peptide/MHC class I complex. Immunity. 2002;16:345–354. doi: 10.1016/s1074-7613(02)00288-1. [DOI] [PubMed] [Google Scholar]
- 21.Reiser JB, et al. Crystal structure of a T cell receptor bound to an allogeneic MHC molecule. Nat Immunol. 2000;1:291–297. doi: 10.1038/79728. [DOI] [PubMed] [Google Scholar]
- 22.Sun R, et al. Evidence that the antigen receptors of cytotoxic T lymphocytes interact with a common recognition pattern on the H-2Kb molecule. Immunity. 1995;3:573–582. doi: 10.1016/1074-7613(95)90128-0. [DOI] [PubMed] [Google Scholar]
- 23.Rudolph MG, Luz JG, Wilson IA. Structural and thermodynamic correlates of T cell signaling. Annu Rev Biophys Biomol Struct. 2002;31:121–149. doi: 10.1146/annurev.biophys.31.082901.134423. [DOI] [PubMed] [Google Scholar]
- 24.Hennecke J, Carfi A, Wiley DC. Structure of a covalently stabilized complex of a human αβ T- cell receptor, influenza HA peptide and MHC class II molecule, HLA-DR1. EMBO J. 2000;19:5611–5624. doi: 10.1093/emboj/19.21.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wucherpfennig KW, et al. Clonal expansion and persistence of human T cells specific for an immunodominant myelin basic protein peptide. J Immunol. 1994;152:5581–5592. [PubMed] [Google Scholar]
- 26.Ota K, et al. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990;346:183–187. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- 27.Pette M, et al. Myelin autoreactivity in multiple sclerosis: recognition of myelin basic protein in the context of HLA-DR2 products by T lymphocytes of multiple-sclerosis patients and healthy donors. Proc Natl Acad Sci USA. 1990;87:7968–7972. doi: 10.1073/pnas.87.20.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krogsgaard M, et al. Visualization of myelin basic protein (MBP) T cell epitopes in multiple sclerosis lesions using a monoclonal antibody specific for the human histocompatibility leukocyte antigen (HLA)-DR2-MBP 85–99 complex. J Exp Med. 2000;191:1395–1412. doi: 10.1084/jem.191.8.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madsen LS, et al. A humanized model for multiple sclerosis using HLA-DR2 and a human T-cell receptor. Nat Genet. 1999;23:343–347. doi: 10.1038/15525. [DOI] [PubMed] [Google Scholar]
- 30.Hausmann S, Martin M, Gauthier L, Wucherpfennig KW. Structural features of autoreactive TCR that determine the degree of degeneracy in peptide recognition. J Immunol. 1999;162:338–344. [PubMed] [Google Scholar]
- 31.Nakagawa T, et al. Cathepsin L: critical role in Ii degradation and CD4 T cell selection in the thymus. Science. 1998;280:450–453. doi: 10.1126/science.280.5362.450. [DOI] [PubMed] [Google Scholar]
- 32.Baker BM, Turner RV, Gagnon SJ, Wiley DC, Biddison WE. Identification of a crucial energetic footprint on the α1 helix of human histocompatibility leukocyte antigen (HLA)-A2 that provides functional interactions for recognition by tax peptide/HLA-A2-specific T cell receptors. J Exp Med. 2001;193:551–562. doi: 10.1084/jem.193.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang JH, et al. Crystal structure of the human CD4 N-terminal two-domain fragment complexed to a class II MHC molecule. Proc Natl Acad Sci USA. 2001;98:10799–10804. doi: 10.1073/pnas.191124098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sim BC, Zerva L, Greene MI, Gascoigne NR. Control of MHC restriction by TCR Vα CDR1 and CDR2. Science. 1996;273:963–966. doi: 10.1126/science.273.5277.963. [DOI] [PubMed] [Google Scholar]
- 35.Savage PA, Davis MM. A kinetic window constricts the T cell receptor repertoire in the thymus. Immunity. 2001;14:243–252. doi: 10.1016/s1074-7613(01)00106-6. [DOI] [PubMed] [Google Scholar]
- 36.Busch DH, Pamer EG. T cell affinity maturation by selective expansion during infection. J Exp Med. 1999;189:701–710. doi: 10.1084/jem.189.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Appel H, Gauthier L, Pyrdol J, Wucherpfennig KW. Kinetics of T-cell receptor binding by bivalent HLA-DR peptide complexes that activate antigen-specific human T-cells. J Biol Chem. 2000;275:312–321. doi: 10.1074/jbc.275.1.312. [DOI] [PubMed] [Google Scholar]
- 38.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 39.Grakoui A, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 40.Ehrlich LI, Ebert PJ, Krummel MF, Weiss A, Davis MM. Dynamics of p56lck translocation to the T cell immunological synapse following agonist and antagonist stimulation. Immunity. 2002;17:809–822. doi: 10.1016/s1074-7613(02)00481-8. [DOI] [PubMed] [Google Scholar]
- 41.Richie LI, et al. Imaging synapse formation during thymocyte selection: inability of CD3ζ to form a stable central accumulation during negative selection. Immunity. 2002;16:595–606. doi: 10.1016/s1074-7613(02)00299-6. [DOI] [PubMed] [Google Scholar]
- 42.Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell-mediated auto-immunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maynard J, et al. Structure of an autoimmune T cell receptor complexed with class II peptide-MHC; insights into MHC bias and antigen specificity. Immunity. 2005;22:81–92. doi: 10.1016/j.immuni.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 44.Smith KJ, Pyrdol J, Gauthier L, Wiley DC, Wucherpfennig KW. Crystal structure of HLA-DR2 (DRA*0101, DRB1*1501) complexed with a peptide from human myelin basic protein. J Exp Med. 1998;188:1511–1520. doi: 10.1084/jem.188.8.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Meth Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 46.Storoni LC, McCoy AJ, Read RJ. Likelihood-enhanced fast rotation functions. Acta Crystallogr D Biol Crystallogr. 2004;60:432–438. doi: 10.1107/S0907444903028956. [DOI] [PubMed] [Google Scholar]
- 47.Sundberg EJ, et al. Structures of two streptococcal superantigens bound to TCR beta chains reveal diversity in the architecture of T cell signaling complexes. Structure (Camb) 2002;10:687–699. doi: 10.1016/s0969-2126(02)00759-1. [DOI] [PubMed] [Google Scholar]
- 48.Brunger AT, et al. Crystallography & NMR system: A new software suite for macro-molecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 49.Jones TA, Zou JY, Cowan SW, Kjeldgaard Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.