SUMMARY
Aim.
The aim of the clinical-statistic study was to evaluate the prevalence of the different oral manifestations in a sample of coeliac patients, in comparison with a control group of healthy subjects. Moreover, a second objective was to determine if the clinical oral examination is useful as a diagnostic tool of screening for atypical forms of coeliac disease (CD).
Methods.
The enrolment of 300 coeliac patients, aged between 4 and 13 years (mean age 8.16), was carried out at the Pediatric Dentistry Unit in patients sent from the Pediatric Gastroenterology Unit of the PTV Hospital, University of Rome Tor Vergata. The control group was composed of 300 healthy subjects, age-matched (mean age 8.29), enrolled from the Pediatric Dentistry Unit.
The patients were examined for hard tissues (enamel hypoplasia, dental caries), soft tissues (recurrent aphthous stomatitis RAS, atrophic glossitis, geographic tongue) and delay dental eruption. Enamel defects were classified according to Aine’s criteria, while dental caries was recorded as dmft/DMFT indices.
Statistical analysis was carried out by using SPSS/PC+ Software. Differences between case and control groups were tested using Paired samples T-test, and Chi-Square Test, depending on the variable considered. The minimal level of significance of the differences was fixed at p≤0.05 for all the procedures.
Results.
Statistical differences between groups were observed for the prevalence of enamel defects (p=0.0001), RAS (p=0.005), delay in dental eruption (p=0.0001), but not for the prevalence of atrophic glossitis (p=0.664). Differences in symmetrical distribution and a chronologic coherence of enamel defects were statistically significant between CD and control groups (p=0.0001). Regarding dental caries, the coeliac patients had higher indexes of caries than healthy subjects, both in deciduous teeth (dmft 2.31±1.84 vs 1.42±1.13; p= 0.021) and permanent teeth (DMFT 2.97±1.74 vs 1.74±1.64; p=0.0001).
Conclusions.
The clinical oral examination should be considered a diagnostic tool for the characterization of subjects affected by silent-atypical forms of CD.
Keywords: coeliac disease, dental enamel hypoplasia, recurrent aphtous stomatitis, dental eruption delay
RIASSUNTO
Obiettivi.
Lo scopo dello studio clinico-statistico è stato quello di valutare la prevalenza delle diverse manifestazioni orali in un campione di pazienti celiaci, in confronto con un gruppo di controllo di soggetti sani. Inoltre, un secondo obiettivo è stato quello di determinare se l’esame clinico orale è utile come strumento diagnostico di screening per le forme atipiche di malattia celiaca.
Metodi.
Presso il Reparto di Odontoiatria Pediatrica del Policlinico di Tor Vergata - Università degli Studi di Roma Tor Vergata, sono stati selezionati 300 pazienti celiaci, di età compresa tra i 4 e 13 anni (età media 8.16), inviati dal Reparto di Gastroenterologia Pediatrica e 300 soggetti sani come gruppo controllo, di età compresa tra i 4 e i 13 anni (età media 8.29).
Per ogni paziente sono stati esaminati i tessuti duri (ipoplasia dello smalto, patologia cariosa), i tessuti molli (stomatiti aftose ricorrenti, glossite atrofica, lingua a carta geografica) ed i ritardi di eruzione dentale. I difetti dello smalto venivano classificati dal grado I al grado IV in base alla classificazione di Aine, mentre per la patologia cariosa veniva calcolato l’indice dmft/DMFT.
L’analisi statistica è stata effettuata utilizzando il Software SPSS/PC+. Le differenze tra il campione dei casi ed il gruppo controllo venivano valutate utilizzando il T-Test ed il Chi-Square Test, a seconda della variabile considerata. Il livello minimo di significatività statistica è stato fissato a p≤0.05 per tutte le procedure.
Risultati.
Differenze statisticamente significative sono state osservate tra i due gruppi in rapporto alla prevalenza dei difetti dello smalto (p = 0.0001), delle stomatiti aftose ricorrenti (p = 0.005), dei ritardi dell’eruzione dentale (p = 0.0001), ma non per la prevalenza di glossite atrofica (p = 0.664). È stata osservata alta significatività nella distribuzione simmetrica e coerenza cronologica dei difetti dello smalto dei soggetti celiaci rispetto al gruppo controllo (p=0.0001).
Dall’analisi della frequenza della patologia cariosa, è risultato che i pazienti celiaci presentavano indice di carie più elevato rispetto ai soggetti sani, sia nei denti decidui (dmft 2.31 ± 1.84 vs 1.42 ± 1.13, p=0.021) che nei denti permanenti (DMFT 2.97 ± 1.74 vs 1.74 ± 1.64, p=0.0001).
Conclusioni.
L’esame clinico orale deve essere considerato un utile strumento diagnostico di screening per l’identificazione dei soggetti affetti da forme silenti-atipiche di malattia celiaca.
Introduction
Coeliac disease (CD), better known as coeliac sprue or gluten reactive disease, is an autoimmune enteropathy that compromises the small intestinal mucosa by altering proximal villi (1). The damage of gut is caused by an unbalanced immunomediate response that occurs in genetically susceptible individuals, after the ingestion of gluten (2,3).
The prevalence of CD increased notably respect to the past, ranging now from 1:85 to 1:300, according to the population and the area considered (1,4–7). The reason for an increased number of cases detected may be due to improvements in the accuracy of serological markers (measurement of anti-endomysium antibodies EMA, anti-gliadin antibodies AGA and anti-transglutaminase antibodies tTG), used in the early stages of disease screening and diagnosis (8). Then a small-bowel biopsy and an histological examination must be performed to confirm diagnosis (8).
Nonetheless, CD still is diagnosed with a delay, because recently the typical form of CD, characterized by malabsorption and gastrointestinal symptoms, is less frequent respect to atypical forms, often asymptomatic and involving extra-intestinal clinical manifestations (9,10).
Therefore it is important that pediatricians, gastroenterologists and internists have a multidisciplinary approach, because they must pay attention to extra-intestinal manifestations of CD (hematologic, dermatologic, neurologic, gynaecological and oral), in order to make an early diagnosis (11).
Among clinical oral manifestations of CD, enamel hypoplasia (12–20), atrophic glossitis (21), recurrent aphthous stomatitis (RAS) (15) and delay in dental eruption (22) have been described.
The aim of our study was to evaluate the prevalence of the different oral manifestations, related to soft and hard oral tissues, in CD patients, in comparison with a control group of healthy subjects. Moreover, a second objective of the study was to determine if the clinical oral examination is useful as a diagnostic tool of screening for atypical forms of CD.
Materials and methods
The enrolment of 300 coeliac patients, aged between 4 and 13 years (mean age 8.16), was carried out at the Pediatric Dentistry Unit in patients sent from the Pediatric Gastroenterology Unit of the PTV Hospital, University of Rome Tor Vergata.
Inclusion criteria for coeliac patients participating in this study were positivity towards serological tests (Ab-htTG IgA, Ab-htTG IgG, AGA IgA, AGA IgG, EMA IgA, EMA IgG), small-bowel biopsy through esophago-gastro-duodenoscopy (EGDS), and histological evidence of intestinal villous atrophy, crypt hyperplasia and increased intra-epithelial lymphocytes.
Following a gluten free diet (GFD), overall coeliac patients had a disappearance of symptoms and returned to normal ranges of serum values of anti-tTG and/or EMA.
Upon each patient, a complete medical history to gather information about the diagnosis and the beginning of GFD was performed.
Moreover, the study included the selection of a control group, of 300 healthy subjects, age-matched (mean age 8.29), enrolled from the Pediatric Dentistry Unit. Exclusion criteria for control group enrolment were malnutrition status, body growth delay, gastrointestinal diseases and/or familiar coeliac diseases.
Upon all patients a dental check-up was performed by dentists of the Pediatric Dentistry Unit. Data was collected from the patients because medical records were necessary and an intra-extra oral examination was performed. All parents provided a written consent for enrolment in the study.
At each dental visit, the status of hard tissues (enamel hypoplasia, dental caries) and soft tissues (RAS, atrophic glossitis, geographic tongue) was evaluated.
Enamel defects were classified from I to IV degrees, according to Aine’s classification (12). Moreover, particular attention went towards the characteristics, like symmetry and chronological coherence of enamel hypoplasia. Any co-morbidity for enamel defects (pre-term birth, use of some antibiotics and fluoride, dental traumas) were recorded in medical records. The evaluation of a delay in dental change phases was performed through specific dental eruption tables and with a panoramic radiography. Anyone who did not have teeth in dental arch, 8 months after the usual phase of eruption, was classified as a case with delayed eruption.
Regarding the clinical examination of soft tissues, each lesion observed was registered. Aphthous stomatitis was classified as minor, major, herpetic, according to size, shape, localization and time of healing. Frequency of stomatitis, in relation to CD before diagnosis and/or GFD, was also queried to parents.
Finally, an observation of dental caries and the calculation of the index dmft/DMFT were performed.
Statistical analysis. Statistical analysis was carried out by using SPSS/PC+ Software. Differences between case and control groups were tested using Paired samples T-test, and Chi-Square Test, depending on the variable considered. The minimal level of significance of the differences was fixed at p≤0.05 for all the procedures.
Results
300 coeliac patients, aged between 4 and 13 years, with mean age of 8.16, were analyzed; 195 were females (65.0%) and 105 were males (35.0%), with a ratio females/males of 2:1 (Table 1). The control group was composed of 300 healthy subjects, age-matched, with a mean age of 8.29±2.95; 199 were females (66.3%) and 101 were males (33.7%), with a ratio females/males of 2:1 (Table 1).
Table 1.
Description of the case-control groups.
| Coeliac subjects (n. 300) | Healthy subjects (n. 300) | ||||
|---|---|---|---|---|---|
|
| |||||
| Frequency | Valid Percent | Frequency | Valid Percent | ||
| Gender | Males | 105 | 35.0 | 101 | 33.7 |
| Females | 195 | 65.0 | 199 | 66.3 | |
|
|
|||||
| Enamel defects | Yes | 99 | 33.0 | 33 | 11.0 |
| No | 201 | 67.0 | 267 | 89.0 | |
|
|
|||||
| RAS | Yes | 25 | 8.3 | 9 | 3.0 |
| No | 275 | 91.7 | 291 | 97.0 | |
|
|
|||||
| Glossitis | Yes | 10 | 3.3 | 12 | 4.0 |
| No | 290 | 96.7 | 288 | 96.0 | |
|
|
|||||
| Delay of eruption | Yes | 60 | 20.0 | 24 | 8.0 |
| No | 240 | 80.0 | 276 | 92.0 | |
|
| |||||
| Age | Mean | Std. Deviation | Mean | Std. Deviation | |
|
| |||||
| dmft DMFT |
8.16 | 2.95 | 8.29 | 2.95 | |
| 2.31 | 1.84 | 1.42 | 1.13 | ||
| 2.97 | 1.74 | 1.74 | 1.64 | ||
The study population was equal distributed for age (p=0.590), sex (p=0.731), and co-morbidities (pre-term birth, use of some antibiotics and fluoride, dental traumas) (p=0.523).
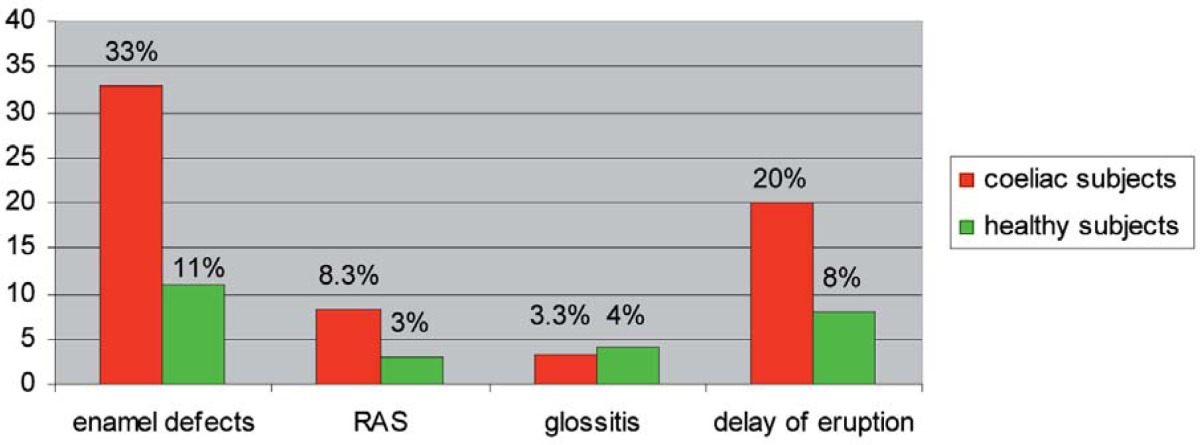
According to the oral visit, 33.0% of coeliac patients were affected by enamel hypoplasia, 8.3% by RAS, 3.3% by atrophic glossitis and 20.0% by delay in dental eruption.
As far as the control group of healthy subjects is concerned it had less oral manifestations: enamel hypoplasia counted for 11.0%, RAS for 3.0%, atrophic glossitis for 4.0% and delay in dental eruption for 8.0% (Table 2).
Table 2.
Distribution of oral manifestations in the case-control groups.
Statistical differences between groups were observed for the prevalence of enamel hypoplasia (p=0.0001), RAS (p=0.005), delay in dental eruption (p=0.0001), but not for the prevalence of atrophic glossitis (p=0.664).
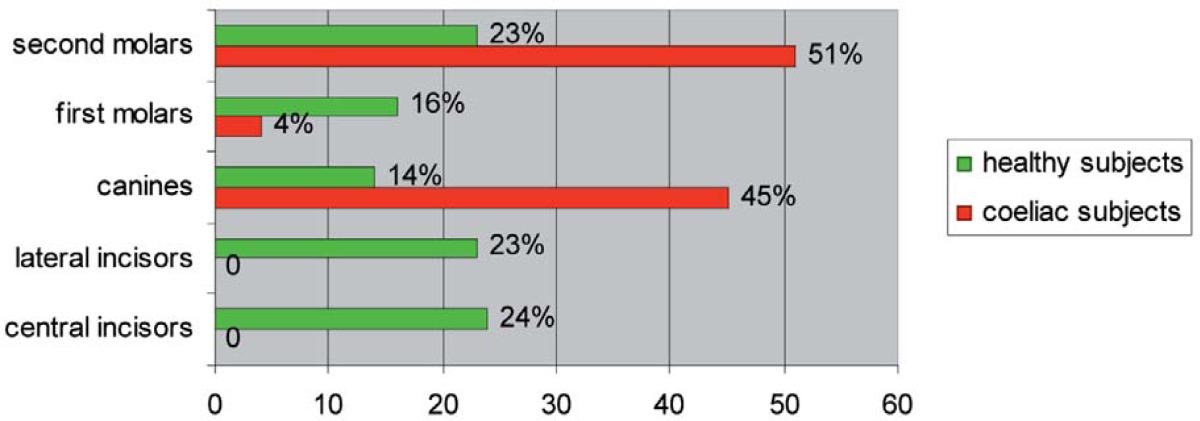
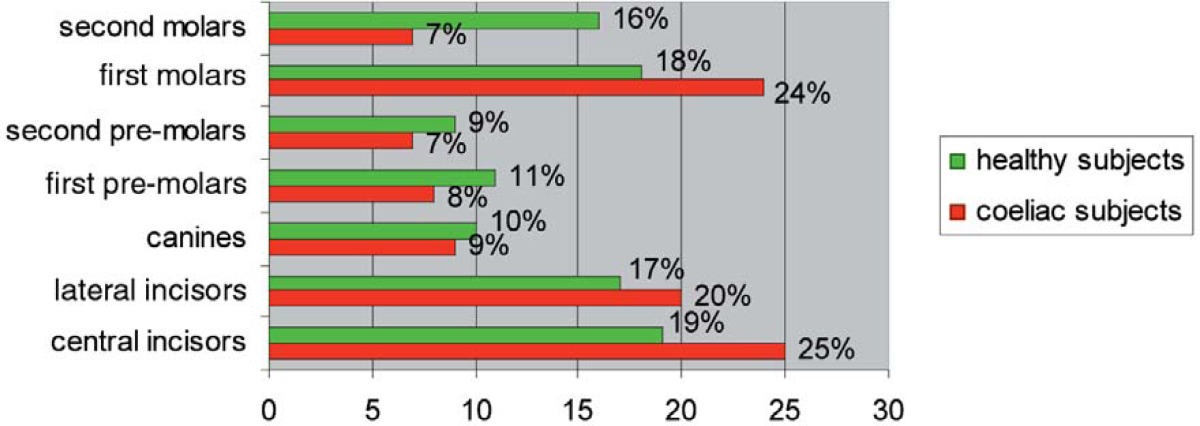
Among coeliac patients, the enamel defects of deciduous teeth occurred in canines (45%) and second molars (51%); whereas the permanent teeth principally involved were central incisors (25%), lateral incisors (20%), first molars (24%), then canines (9%), first premolars (8%), second premolars (7%) and second molars (7%) (Tables 3, 4).
Table 3.
Localization of enamel defects in deciduous teeth in the case-control groups.
Table 4.
Localization of enamel defects in permanent teeth in the case-control groups.
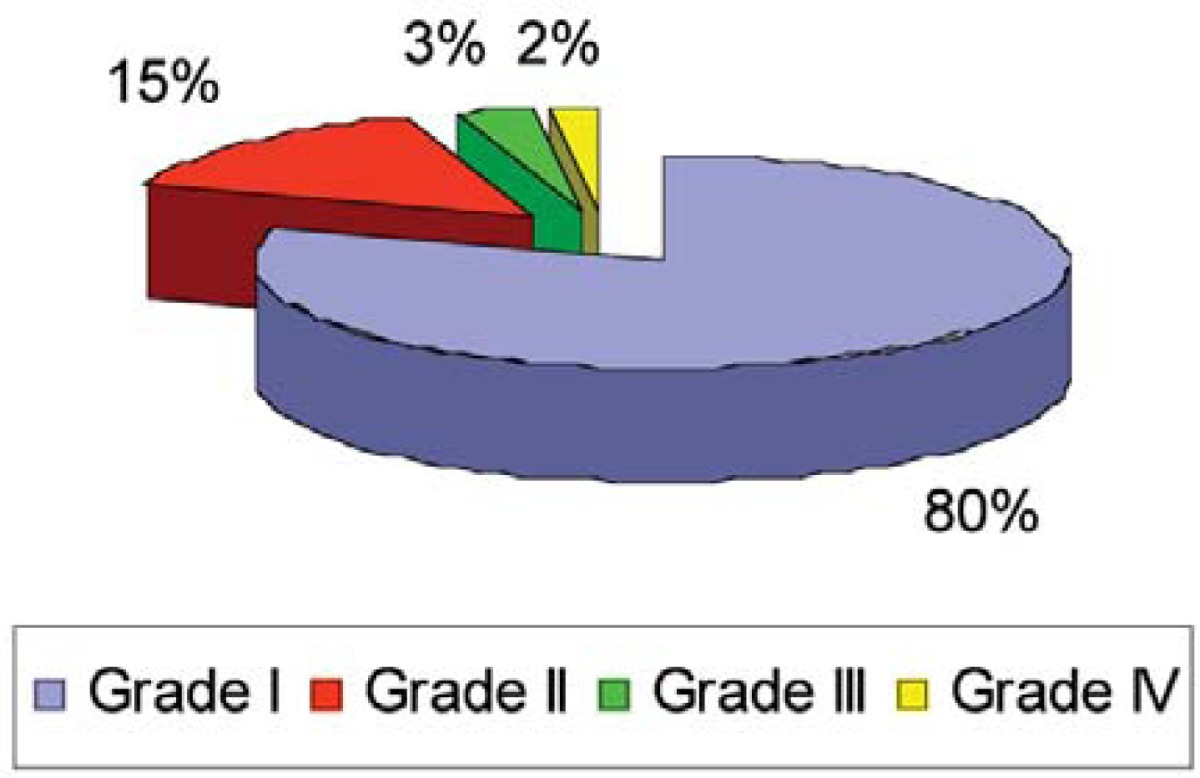
Moreover, the extent of enamel defects of coeliac patients was assessed through Aine’s classification: 80% were classified at the I degree, 15% at the II degree, 3% at the III degree and 2% at the IV degree (Table 5).
Table 5.
Enamel defects of the coeliac subjects, according to Aine’s classification.
60% (n.59) of coeliac subjects affected by enamel defects had a symmetrical distribution and a chronologic coherence of hypoplasia in all hemi-arches, whereas just 15% (n=5) of healthy subjects with enamel defects had such characteristics. The difference between the two groups was statistically significant (p=0.0001).
Regarding dental caries, the coeliac patients had higher indexes of caries than healthy subjects, both in deciduous teeth (dmft 2.31±1.84 vs 1.42±1.13; p=0.021) and permanent teeth (DMFT 2.97±1.74 vs DMFT 1.74±1.64; p=0.0001).
Discussion and conclusion
From the analysis of various studies performed upon enamel defects in CD, it has been demonstrated that enamel hypoplasia is more prevalent in coeliac patients respect to healthy subjects (12–20), whereas others studies agree that there is no association between enamel defect and CD (23, 24). Moreover, there is considerable contradiction on the percentages of frequency reported (Table 6).
Table 6.
Prevalence (%) of enamel defects in the case-control groups in different studies.
| Studies | n. coeliac subjects | n. healthy subjects | Prevalence (%) of enamel defects in coeliac subjects | Prevalence (%) of enamel defects in healthy subjects |
|---|---|---|---|---|
| Aguirre JM et al, 1997 | 137 | 52 | 52.5 | 42.3 |
| Aine L et al, 1990 | 40 | 112 | 83 | 4 |
| Avşar A et al, 2008 | 64 | 64 | 42.2 | 9.4 |
| Bucci P et al, 2006 | 72 | 159 | 20 | 5.6 |
| Campisi G et al, 2007 | 197 | 413 | 23 | 9 |
| Ortega Páez E, 2008 | 30 | 30 | 83.3 | 53.3 |
| Priovolou CH et al, 2004 | 27 | 27 | 83.3 | 50 |
| Procaccini M et al, 2007 | 50 | 50 | 26 | 16 |
| Rasmusson CG et al, 2001 | 40 | 40 | 50 | 38 |
| Wierink CD et al, 2007 | 53 | 28 | 55 | 18 |
Our present case-control study revealed a high prevalence (33%) of enamel hypoplasia, among coeliac patients, significantly greater in comparison with the non-coeliac subjects. Moreover, enamel defects found in coeliac individuals were more “specific” respect to the control group; in fact many of the previous showed a symmetrical distribution, a chronologic coherence and involving all dental hemi-arches (12). In healthy subjects, instead, the enamel defects are mostly “non-specific”, in the absence of previous features. This result is confirmed by other studies (20).
Morphological analysis with scansion electron microscopy (SEM) revealed the structural aspect of enamel defects of coeliac patients, both in deciduous and permanent teeth, which were characterized by highly hypomineralization with shorter prisms, distributed irregularly and less interprismatic substance, than observed in non-coeliac subjects (25). The enamel defects, associated with the CD, have been explained by two etiopathogenic mechanisms: malabsorption-hypocalcaemia and autoimmune response.
1. malabsorption-hypocalcaemia. The malabsorption due to the enteropathy determines an alteration of phospho-calcium metabolism and a consequent hypocalcaemia (24, 26). The latter, therefore, could be a cause of enamel defects in coeliac patients, through an influence on dental mineralization during ontogenesis, both in deciduous and permanent teeth (20).
Therefore, according to this theory, an early diagnosis of CD, through the practice of a GFD, could exclude the involvement of other dental elements or at least limit the damage.
2. autoimmune response. According to this etiopathogenic theory, the antigen, i.e the gluten, binding to class II molecules of the major histocompatibility complex, produces an autoimmune response, mediated by lymphocytes, against the enamel organ through the release of antimatrix antibodies (12, 26, 27).
Additionally, a genetic hypothesis was proposed which was confirmed by the association between dental defects and the allele HLA-DR3 (28), related to the locus DQ, specifically the DQW2, i.e. the principal antigenic locus of CD. Mariani et al. (28) demonstrated that the presence of this specific antigen increases the risk for enamel defects.
The frequency of positivity of the DR3 allele among people affected by CD showing enamel defects depends on the geographic area, where the study was performed. In Northern Europe the DR3 allele was characteristic for 93.3% (27), in Italy for 77.2% (28) and in Spain for 53.8% (13) of people screened.
Several lines of evidence support the hypothesis that both etiopathogenic mechanisms could contribute to determine enamel defects.
In the statistical analysis, we observed that enamel defects affected more frequently permanent than deciduous teeth of coeliac subjects.
Moreover, a detailed analysis of enamel defects, in CD patients, clarified that the distribution was in correlation with the chronology of formation of deciduous and permanent dental elements.
In fact, deciduous canines and second molars of coeliac subjects were the only deciduous teeth affected with enamel defects (Table 3), probably because they are the last elements which begin the mineralization process in respect to other deciduous teeth. Meanwhile, permanent incisors and first molars showed a higher percentage of frequency than other permanent teeth (Table 4), probably because they are the first dental elements to begin the process of mineralization and to be affected by enamel defects in cases of CD.
Hence, also the involvement of permanent canines, premolars and second molars in enamel defects could reflect a delayed diagnosis of CD.
An additional result of our study was the finding of an association between dental caries and CD. Coeliac subjects showed a prevalence of dmft-DMFT higher than the control group of healthy subjects (dmft: 2.31±1.84 vs 1.42±1.13; DMFT 2.97±1.74 vs 1.74±1.64) and this difference resulted statistically significant (dmft p=0.021, DMFT p=0.0001).
Previous studies are in contradiction (13,17,19) and only some of them agree with the present result (14). However, the increase of caries in coeliac patients is not a manifestation of CD, but an explanation of this high frequency could be the copresence of risk factors, like the fragility of hypoplasic enamel, alterations in salivary concentrations and reductions in salivary flow.
Moreover, the decrease of salivary flow, occurring in active phase of disease and in concomitance of GFD, determines dryness/soreness of the mouth, soreness/burning sensation in the tongue (29), alterations of oral protective factors and it could increase risk for oral mucosal infections and dental caries.
For these reasons, all coeliac patients should be included in a preventive dental program that provides: professional oral hygiene, motivation-education for home oral hygiene, pits and fissures sealing, fluoride topical application. Beside this, therapeutical actions should be performed in presence of caries or fractures of hypoplasic enamel, through the use of direct or indirect conservative restorations.
Additionally, the present study offered the opportunity to evaluate alterations in the dental eruption. 20% of coeliac subjects showed a delayed dental eruption, in a proportion similar to other previous studies (27%) (16). However, further analyses are needed to confirm this.
No associations of CD with atrophic glossitis were found. Regarding RAS a significant difference between the two groups (p=0.005) was observed, although the low number of cases was certainly a limitation.
This finding is probably related to the fact that in some coeliac subjects examined (8.3%) the RAS were not influenced by the GFD. Such evidence has been found in other studies in the literature (15).
The clinical oral examination should be considered a diagnostic tool for the characterization of subjects affected by silent-atypical forms of CD (29,30).
Dental screening is a cheap, easy, no-invasive tool, to identify all the oral manifestations of CD: alterations in the enamel structure, specific enamel defects, delay in dental eruption, atrophic glossitis and RAS.
Therefore, pediatric dentists have a key-role in the early interception of CD, not yet diagnosed, through the help of interdisciplinary collaborators, like pediatricians, gastroenterologists and internists.
Acknowledgments
We thank F.Gloria, MD, Section of Biopathology of Human Populations and Environmental Pathology, Department of Biopathology and Diagnostic Imaging, University of Rome Tor Vergata and F.M.Paone, MD, Pediatric Gastroenterology and Endoscopy Unit, University of Rome Tor Vergata.
References
- 1.Fasano A, Araya M, Bhatnagar S, et al. Celiac disease Working Group, Federation of International Societies of Pediatric Gastroenterology, Hepatology and Nutrition consensus report on celiac disease. J Pediatr Gastroenterol. 2008 Aug;47(2):214–9. doi: 10.1097/MPG.0b013e318181afed. [DOI] [PubMed] [Google Scholar]
- 2.Schuppan D. Current concepts of celiac disease pathogenesis. Gastroenterol. 2000;119:234–42. doi: 10.1053/gast.2000.8521. [DOI] [PubMed] [Google Scholar]
- 3.Schuppan D, Esslinger B, Dieterich W. Innate immunity and coeliac disease. Lancet. 2003;362:3–4. doi: 10.1016/S0140-6736(03)13843-3. [DOI] [PubMed] [Google Scholar]
- 4.Carlsson AK, Axelsson IE, Borulf SK, Bredberg AC, Ivarsson SA. Serological screening for celiac disease in healthy 2.5-year-old children in Sweden. Pediatrics. 2001;107:42–5. doi: 10.1542/peds.107.1.42. [DOI] [PubMed] [Google Scholar]
- 5.Catassi C, Rätsch IM, Fabiani E, et al. High prevalence of undiagnosed coeliac disease in 5280 Italian students screened by antigliadin antibodies. Acta Paediatr. 1995 Jun;84(6):672–6. doi: 10.1111/j.1651-2227.1995.tb13725.x. [DOI] [PubMed] [Google Scholar]
- 6.Korponay-Szabó IR, Kovács JB, Czinner A, Gorácz G, Vámos A, Szabó T. High prevalence of silent celiac disease in preschool children screened with IgA/IgG antiendomysium antibodies. J Pediatr Gastroenterol Nutr. 1999;28(1):26–30. doi: 10.1097/00005176-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Not T, Horvath K, Hill ID, et al. Celiac disease risk in the USA: high prevalence of antiendomysium antibodies in healthy blood donors. Scand J Gastroenterol. 1998;33:494–8. doi: 10.1080/00365529850172052. [DOI] [PubMed] [Google Scholar]
- 8.Korponay-Szabó IR, Sulkanen S, Halttunen T, et al. Tissue transglutaminase is the target in both rodent and primate tissues for celiac disease-specific autoantibodies. J Pediatr Gastroenterol Nutr. 2000 Nov;31(5):520–7. doi: 10.1097/00005176-200011000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Ravikumara M, Tuthill DP, Jenkins HR. The changing clinical presentation of coeliac disease. Arch Dis Child. 2006;91:969–71. doi: 10.1136/adc.2006.094045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torres MI, Lopez Casado MA, Rios A. New aspects in celiac disease. World J Gastroenterol. 2007;13:1156–61. doi: 10.3748/wjg.v13.i8.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterol. 2001;120:636–51. doi: 10.1053/gast.2001.22123. [DOI] [PubMed] [Google Scholar]
- 12.Aine L, Mäki M, Collin P, Keyriläinen O. Dental enamel defects in coeliac disease. J Oral Pathol Med. 1990;19:241–5. doi: 10.1111/j.1600-0714.1990.tb00834.x. [DOI] [PubMed] [Google Scholar]
- 13.Aguirre JM, Rodriguez R, Oribe D, Vitoria JC. Dental enamel defects in celiac patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:646–50. doi: 10.1016/s1079-2104(97)90367-x. [DOI] [PubMed] [Google Scholar]
- 14.Avar A, Kalayci AG. The presence and distribution of dental enamel defects and caries in children with celiac disease. Turk J Pediatr. 2008 Jan-Feb;50(1):45–50. [PubMed] [Google Scholar]
- 15.Bucci P, Carile F, Sangianantoni A, D’Angiò F, Santarelli A, Lo Muzio L. Oral aphthous ulcers and dental enamel defects in children with coeliac disease. Acta Paediatrica. 2006;95:203–7. doi: 10.1080/08035250500355022. [DOI] [PubMed] [Google Scholar]
- 16.Campisi G, Di Liberto C, Iacono G, et al. Oral pathology in untreated coelic disease. Aliment Pharmacol Ther. 2007;26:1529–36. doi: 10.1111/j.1365-2036.2007.03535.x. [DOI] [PubMed] [Google Scholar]
- 17.Farmakis E, Puntis JW, Toumba KJ. Enamel defects in children with coeliac disease. Eur J Paediatr Dent. 2005 Sep;6(3):129–32. [PubMed] [Google Scholar]
- 18.Ortega Páez E, Junco Lafuente P, Baca García P, Maldonado Lozano J, Llodra Calvo JC. Prevalence of dental enamel defects in celiac patients with deciduous dentition: a pilot study. Oral Surg Oral Med Oral Pathol, Oral radio Endod. 2008 Jul;106(1):74–8. doi: 10.1016/j.tripleo.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Priovolou CH, Vanderas AP, Papagiannoulis L. A comparative study on the prevalence of enamel defects and dental caries in children and adolescents with and without coeliac disease. Eur J Paediatr Dent. 2004 Jun;5(2):102–6. [PubMed] [Google Scholar]
- 20.Wierink CD, Van Diermen DE, Aartman IHA, Heymans HAS. Dental enamel defects in children with coeliac disease. Int J Paediatr Dent. 2007;17:163–8. doi: 10.1111/j.1365-263X.2006.00816.x. [DOI] [PubMed] [Google Scholar]
- 21.Pastore L, Lo Muzio L, Serpico R. Atrophic glossitis leading to the diagnosis of celiac disease. N Engl J Med. 2007 Jun 14;356(24):2547. doi: 10.1056/NEJMc070200. [DOI] [PubMed] [Google Scholar]
- 22.Pastore L, Carroccio A, Compilato D, Panzarella V, Serpico R, Lo Muzio L. Oral manifestations of coeliac disease. J Clin Gastroenterol. 2008 Mar;42(3):224–32. doi: 10.1097/MCG.0b013e318074dd98. [DOI] [PubMed] [Google Scholar]
- 23.Procaccini M, Campisi G, Bufo P, et al. Lack of association between celiac disease and dental enamel hypoplasia in a case-control study from an Italian central region. Head & Face Medicine. 2007;3:25. doi: 10.1186/1746-160X-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasmusson CG, Eriksson MA. Celiac disease and mineralisation disturbances of permanent teeth. Int J Paediatr Dent. 2001 May;11(3):179–83. doi: 10.1046/j.1365-263x.2001.00260.x. [DOI] [PubMed] [Google Scholar]
- 25.Bossù M, Bartoli A, Orsini G, Luppino E, Polimeni A. Enamel hypoplasia in coeliac children: a potential clinical marker of early diagnosis. Eur J Paediatr Dent. 2007 Mar;8(1):31–7. [PubMed] [Google Scholar]
- 26.Aine L. Coeliac-type permanent-tooth enamel defects. Ann Med. 1996;28:9–12. doi: 10.3109/07853899608999067. [DOI] [PubMed] [Google Scholar]
- 27.Mäki M, Aine L, Lipsanen V, Koskimies S. Dental enamel defects in first-degree relatives of celiac disease patients. Lancet. 1991;337:763–4. doi: 10.1016/0140-6736(91)91375-5. [DOI] [PubMed] [Google Scholar]
- 28.Mariani P, Mazzilli MC, Margutti G, et al. Coeliac disease, enamel defects and HLA typing. Acta Paediatr. 1994;83:1272–5. doi: 10.1111/j.1651-2227.1994.tb13014.x. [DOI] [PubMed] [Google Scholar]
- 29.Lähteenoja H, Toivanen A, Viander M, et al. Oral mucosal changes in coeliac patients on a gluten-free diet. Eur J Oral Sci. 1998;106:899–906. doi: 10.1046/j.0909-8836.1998.eos106501.x. [DOI] [PubMed] [Google Scholar]
- 30.Pastore L, Campisi G, Compilato D, Lo Muzio L. Orally based diagnosis of celiac disease: current perspectives. J Dent Res. 2008 Dec;87(12):1100–7. doi: 10.1177/154405910808701206. [DOI] [PubMed] [Google Scholar]