Abstract
Captive-raised red drum fish were observed with phenotypic abnormalities, including deformities of the spine, jaw, and cephalic region, that were consistent with vitamin C deficiency during the larval stage. In light of their visible exterior skeletal abnormalities, we suspected that the affected fish would also have abnormal otoliths. Otoliths are dense calcareous structures that function in fish hearing. We hypothesized that abnormal fish would have irregular otoliths that would alter behavior and cortisol levels as compared with those of phenotypically normal fish. The normal and abnormal fish had statistically significant differences in behavior, cortisol levels, and otolith volume and density. MicroCT assessment of abnormal fish revealed operculum abnormalities, malocclusions, and several types of otolith malformations. Therefore, the affected fish had not only an abnormal skeletal appearance but also significantly abnormal behavior and cortisol responses.
Red drum (Sciaenops ocellatus), a perciform teleost species of the family Sciaenidae, are commonly used for endocrine and nutrition research and are a recreationally important species common in the Gulf of Mexico and southeastern United States.7 Since 1983, hatchery-reared red drum have been stocked to help restore depleted populations in Texas. Unlike other commonly cultured species like salmonids, juvenile red drum inhabit warm waters (as high as 30 °C), grow at extremely rapid rates, and are freely euryhaline. In addition, red drum have excellent aquacultural potential because they can be cultured at a range of temperatures and salinities.21
On their arrival to our institution, a new group of red drum was noted to have a specific set of phenotypic abnormalities consistent with vitamin C deficiency during the larval stage. The exact cause of the abnormalities in these fish was unknown. However, because vitamin C (ascorbic acid) is a water-soluble and heat-labile vitamin that can degrade quickly in the diet, vitamin C deficiency was a reasonable explanation for the observed deformities.6 Vitamin C acts as a reducing agent and antioxidant in teleosts and is a dietary requirement for most fish, including red drum, because they are unable to synthesize it.7 In addition, ascorbic acid is a cofactor in hydroxylating amino acids for collagen synthesis, which is required for wound repair, formation of connective tissue and bone matrix.9 Common signs of vitamin C deficiency in teleost fish include deformities of the spine, jaw, and cephalic region as well as anophthalmia and shortened opercula.5 These signs were present in all of our phenotypically abnormal fish. Because of the visible skeletal malformations of the cranium and spine, we hypothesized that the abnormal fish had abnormal otoliths, because bone deposition lesions can be associated with poor collagen formation.7
Fish hear in a similar fashion to other vertebrates.14 Otoliths (or ‘ear stones’) are dense calcareous structures in the 3 chambers associated with the ear in teleost fishes. The saccular otolith, also called sagittal otolith or sagitta, is the largest in most fishes and is considered the primary auditory organ.13 Otoliths are considered to be involved in both auditory and vestibular functions. These calcareous structures detect motion and indirectly sense fluctuations of swim-bladder volume in a pressure field. In addition, otoliths relay information about sound source characteristics, including distance and location.10 The precise pattern of otolith motion likely is affected by characteristics of the otolith, including its mass and center of gravity.14
Red drum have very large otoliths and, as a result, tend to be very responsive to external stimuli.13 Based on the skeletal deformities we observed, we hypothesized that these phenotypically abnormal fish would have abnormal otoliths, which would lead to behavioral differences resulting from impaired sensory function. We also hypothesized that abnormal fish would have increased acute cortisol responses compared with those of normal fish due to a greater startle response when netted, because they are unable to hear the approach of the net.21,23 The elevation of plasma corticosteroids, mainly cortisol, in teleosts in response to various types of stressful stimuli has been well documented and constitutes an important hormonal or primary response to stress.4
Materials and Methods
Animals and housing.
Red drum were obtained as fry and grown to juvenile size (weight, 20 to 80 g) at the Aquacultural Research and Teaching Facility (Texas A and M University, College Station, TX). The standard length and wet mass (mean ± SEM) for all fish (abnormal and normal) were 16.5 ± 0.8 cm and 76.5 ± 1.1 g, respectively. The abnormal and normal groups were maintained in 2 recirculating 1900-L tanks at 25 ± 2 °C and 6 ppt salinity on a 12:12-h light:dark cycle and commercial fish diet. All fish were maintained and treated humanely, and experimental protocols were approved by the Texas A and M University IACUC.
Experimental design.
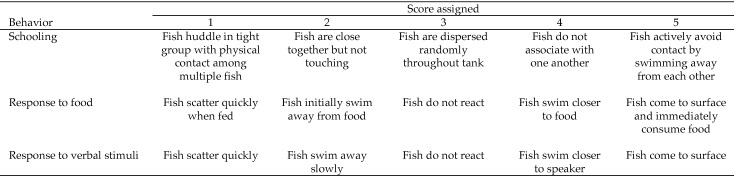
Fish were separated into 2 groups with 75 ± 5 fish per group; the normal group contained only physically normal red drum, whereas the other group consisted of drum with various phenotypic skeletal abnormalities. For 15 d, fish were observed once daily at 0900 for 10 min by a single impartial observer who was unaware of the purpose for monitoring and recording behavior. Fish were scaled as a group on 3 responses: schooling, response to visual stimuli (a standard commercial aquaculture feed; Rangen), and response to acoustic stimuli. Acoustic stimuli consisted of a verbal phrase spoken by the observer after she had been in the room for 5 min—long enough for the fish to acclimate to her presence.21 The speaker was instructed to use the same volume, tone of voice, and phrase during the stimulus. Groups were scored on a scale of 1 to 5 based on their responses (Figure 1).
Figure 1.
Scoring scale for behavioral measurements of normal and abnormal red drum. Fish were scored daily for 15 d and responses were averaged and compared between the 2 fish groups.
For blood collection for cortisol analysis by radioimmunoassay, 8 normal and 7 abnormal red drum were anesthetized by using tricaine methanesulfonate (MS222; Finquel, Argent Chemical Laboratories, Redmond, WA). Blood was collected from the caudal vein and centrifuged to separate the plasma. Plasma was stored at −80 °C until cortisol analysis by using Coat-A-Count Total Cortisol kits (Siemens, Los Angeles, CA); this kit quantifies hormone concentration in diluted samples by using an antibody-coated tube method.
Another 7 abnormal and 3 normal red drum were chosen randomly and euthanized by using tricaine methanesulfonate. They were imaged with microCT (Hawk-160XI, X-Tek Group, Santa Clara, CA). MicroCT scans were reconstructed (version 2.0, VGStudio MAX, Volume Graphics, Heidelberg, Germany) for visualization and qualitative evaluation of otolith morphology. In addition, 8-bit image stacks of the microCT scans were exported to conduct quantitative microCT measurements of the total volume of both sagittal otoliths.
After imaging, all otoliths were removed, air dried, and weighed individually on a gram scale. Otolith volume was measured by water displacement in a 5-mL graduated cylinder. The information was used to calculate density by dividing weight (in grams) by volume (in milliliters). The mean behavioral scores, cortisol levels, and otolith volume, mass, and density of normal and abnormal fish were analyzed by using the Student t test (Excel 2010, Microsoft, Redmond, WA). Differences were considered to be significant when the P value was less than 0.05.
Results
All of the classic signs of vitamin C deficiency, including deformities of the spine, jaw, and cephalic region, anophthalmia, and shortened opercula, were present in fish in the abnormal group. Other than their phenotypic abnormalities, the abnormal fish exhibited similar trends in weight gain and growth as those of normal fish. Over the 15-d observational period, mortality in the normal drum was 32% compared with only 2% in the abnormal fish.
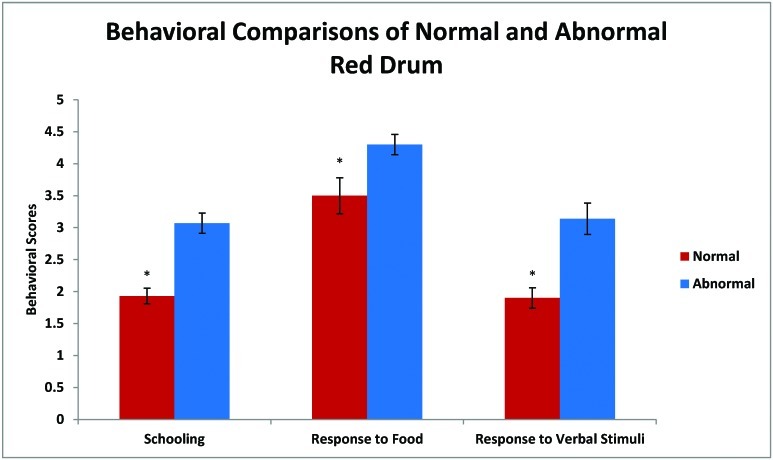
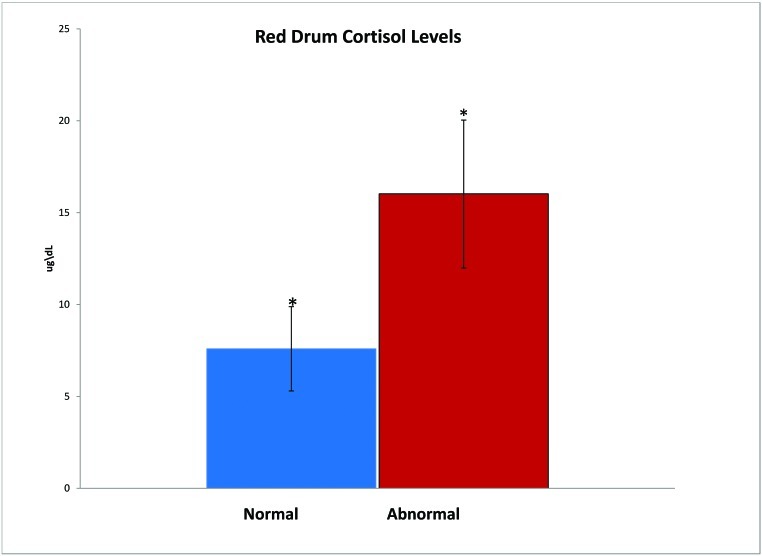
The normal and abnormal fish had statistically significant (P < 0.05) differences in behavior (Figure 2). Normal fish had more schooling behavior (P < 0.001) and swam close together without touching, whereas abnormal fish were dispersed randomly throughout the tank. The abnormal group was more responsive (P < 0.04) to visual stimuli of food than was the normal group. The abnormal fish swam closer to food and more quickly, whereas normal drum were slower to react to the presence of food. The abnormal group showed no response to acoustic stimuli, whereas normal fish swam away from the source of the noise (P < 0.001). Plasma cortisol levels of the abnormal drum group were significantly (P < 0.001) higher than those of the normal group (Figure 3).
Figure 2.
Behavioral differences between abnormal and normal red drum were quantified for 2 wk by an unbiased observer using the previously described scoring system. Compared with normal fish, abnormal fish had significantly (*, P < 0.05) higher scores for each of the 3 criteria.
Figure 3.
Radioimmunoassay of collected fish plasma indicated that abnormal fish had significantly (*, P < 0.05) higher cortisol levels than did normal fish.
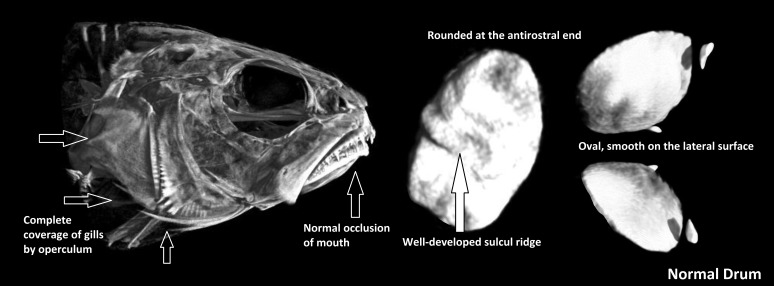
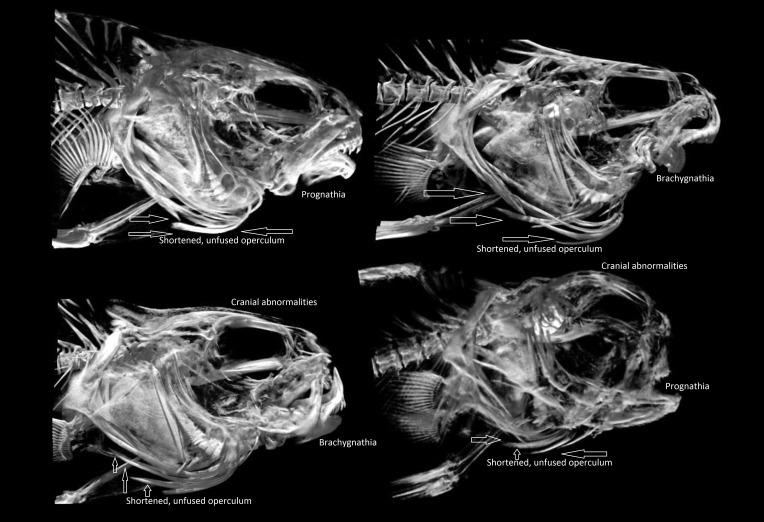
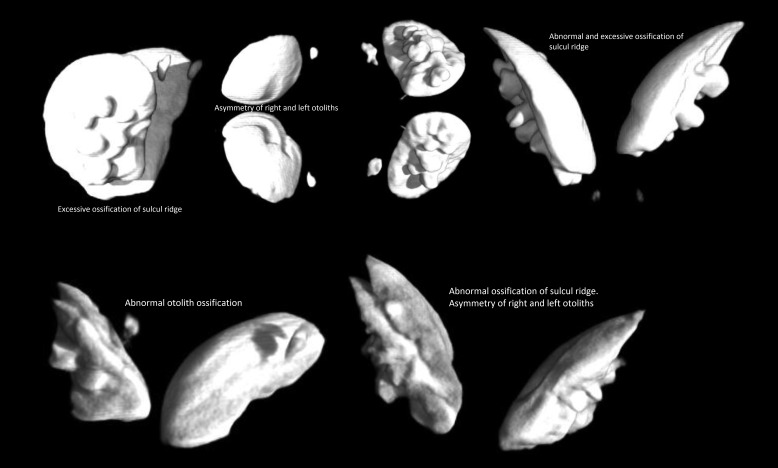
MicroCT of abnormal red drum revealed operculum abnormalities, malocclusions due to brachygnathia or prognathia, different types of otolith malformations, and spinal deformities such as kyphosis when compared with images from the normal group. The mean volume of abnormal sagittal otoliths was 3 times that of normal otoliths: 36.7 mm3 as compared with 12.29 mm3 (P < 0.001). The sagittal otolith was the largest of the otoliths. Normal sagitta are oval, smooth on the lateral surface, rounded at the antirostral end with a well-developed sulcul ridge on the medial surface (Figure 4).7 In normal red drum, normal occlusion of the mouth was present, and the operculum was fused and fully covered the gills (Figure 4). All abnormal drum had shortened, unfused opercula and oral cavity abnormalities; many also exhibited cranial abnormalities (Figure 5). Sagittal otolith abnormalities ranged from abnormal shape, asymmetry, and abnormal ossification, with abnormal ossification being the most common (Figure 6).19
Figure 4.
MicroCT of normal red drum with normal sagittal otoliths. Head and otolith morphology were consistent among all normal fish. Arrows indicate the typical formation of normal operculum.
Figure 5.
MicroCT of abnormal red drum illustrating the most common abnormalities seen, including prognathia, brachygnathia, cranial abnormalities, and shortened and unfused operculum (delineated by arrows).
Figure 6.
MicroCT of abnormal drum otoliths illustrating the most common otolith abnormalities seen, including abnormal ossification, excessive ossification of the sulcul ridge, and asymmetry of the right and left otoliths.
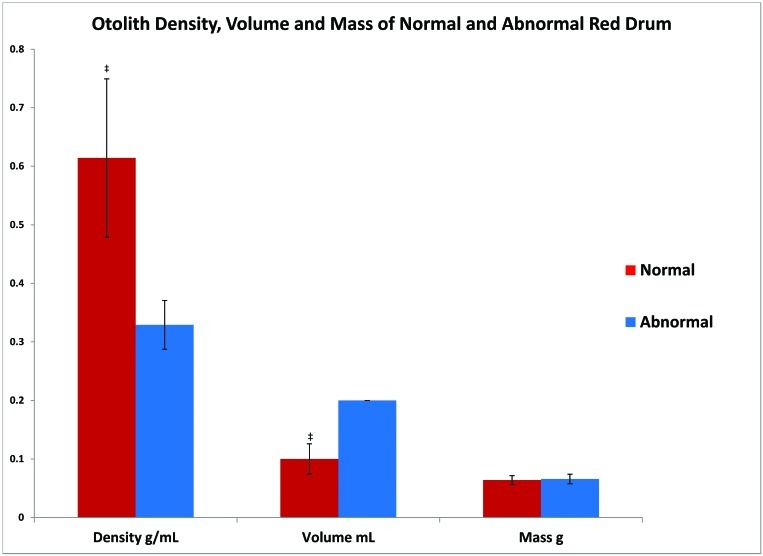
The density of the otoliths from the normal fish was significantly (P < 0.001) higher than of the phenotypically abnormal group; however, the volume of the otoliths was significantly (P < 0.001) higher in the abnormal group (Figure 7). There was no significant difference in the mass of otoliths between the 2 groups (P = 0.922), indicating that the fish were approximately the same age. A direct relationship between otolith mass and linear aging has been well documented, making otolith mass measurements a good indicator of fish age.1,3,11
Figure 7.
Abnormal fish had significantly (‡, P < 0.001) lower mean otolith density and higher mean otolith volume, as compared with the normal group. Mean otolith mass was similar (P = 0.922) between the 2 groups.
Discussion
Comparative studies on fish ear structure may facilitate research regarding how animals and humans interpret complex auditory stimuli.16 By extrapolation from zebrafish studies, the simplest method to detect deficiencies in the auditory structure is to screen for defects in otolith morphology.24 Because the saccular otoliths are the major sound detector in most fish, we elected to image these structures.15 We used microCT to scan 7 of the phenotypically abnormal red drum and confirmed that all 7 fish had abnormal sagittal otoliths, with the most consistent abnormality being abnormal ossification. Abnormal ossification was further confirmed by significant differences in density and volume between the 2 groups of fish. Acoustic functionality (sensitivity, temporal processing, and sound localization) is altered by otolith mass asymmetry.10 We therefore hypothesized that abnormal shape and ossification of otoliths would compromise functionality and resultant behavior. We speculated that abnormal drum would demonstrate atypical behavior because these fish would be less aware of their surroundings than would be normal fish. Behavioral studies of laboratory red drum have taught us to expect consistent schooling behavior and rapid responses to acoustic stimuli.21 Consistent with our hypothesis, abnormal red drum exhibited less schooling behavior and no response to acoustic stimuli, whereas normal fish showed more schooling and swam away from the acoustic source.
The abnormal fish had a greater response to visual stimuli (food) than did normal fish. This finding is consistent with the sensory compensatory mechanism described in humans2,12 and cave fish, in which increased acuteness of hearing compensates for loss of vision.25 The hearing capabilities of fish can be measured behaviorally.15,22 Therefore, the behavior data from the current study support the hypothesis that abnormal red drum perceive their environment differently than normal red drum because the hearing capabilities of the abnormal fish have been compromised.
We further hypothesized that defects in the auditory structures and responses to stimuli would result in increased responses to stress in these fish. Cortisol is the predominant corticosteroid released in red drum during acute stress.17 The elevation of plasma corticosteroids, mainly cortisol, in teleosts in response to various types of stressful stimuli has been well documented and constitutes an important hormonal or primary response to stress.22,23 In addition, an appropriate corticosteroid response appears to be essential for resistance to severe trauma via the stimulation of gluconeogenesis and involvement with osmoregulation.4 Handling and anesthesia are expected to result in an acute cortisol stress response that may represent a ‘fright’ reaction to a novel stimulus.23 Both fish groups exhibited increased cortisol responses in response to capture as compared with published normal resting levels for red drum;18 however, the cortisol levels of the abnormal fish were significantly higher than those of the normal group. We propose that the auditory impairment caused by abnormal otoliths was resulted in a heightened startle response when abnormal fish were netted. This fright response may be similar to the increased startle response seen in visually impaired humans.8 In addition, children with hearing loss from an adverse event have increased cortisol levels compared with those of children with normal hearing ability.20 Fish are now well accepted to perceive sound in the same manner as do other vertebrates.14
In summary, the gross morphologic defects in the affected group of fish were only one aspect of the abnormalities present in these animals; they also had significant differences in their behavioral and cortisol responses. Data obtained from studies with these phenotypically abnormal animals may be compromised by their physiologic differences from normal fish. Further studies are needed to define the link between otolith deformities and vitamin C deficiency; however, vitamin C deficiency in red drum might provide a new animal model of hearing impairment.
Acknowledgments
We thank Dr Duncan MacKenzie for allowing us to collect data on the red drum in conjunction with his research and Dr Scott Jaques (Texas Veterinary Medical Diagnostic Laboratory) for his help with the cortisol radioimmunoassay. We appreciate Julie Butler's help collecting the behavior data. We are grateful to Drs Clay Ashley, James Elliott, Vincent Gresham, Ann Kier, Sara Lawhon, and Duncan MacKenzie for reviewing earlier drafts of this manuscript.
References
- 1.Araya M, Cubillos LA, Guzman M, Penailillo J, Sepulveda A. 2001. Evidence of a relationship between age and otolith weight in the Chilean jack mackerel, Trachurus symmetricus murphyi (Nichols). Fish Res 51:17–26 [Google Scholar]
- 2.Arno P, De Volder AG, Vanlierde A, Wanet-Defalque MC, Streel E, Robert A, Sanabria-Bohórquez S, Veraart C. 2001. Occipital activation by pattern recognition in early blind using auditory substitution for vision. Neuroimage 13:632–645 [DOI] [PubMed] [Google Scholar]
- 3.Baker MS, Jr, Wilson CA, VanGent DL. 2001. Testing assumptions of otolith radiometric aging with 2 long-lived fishes from the northern Gulf of Mexico. Can J Fish Aquat Sci 58:1244–1252 [Google Scholar]
- 4.Barton BA, Iwama GK. 1991. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu Rev Fish Dis 1:3–26 [Google Scholar]
- 5.Chavez de Martinez MC. 1990. Vitamin C requirement of the Mexican native cichlid Cichlasoma urophthalmus. Aquaculture 86:409–416 [Google Scholar]
- 6.Dabrowski K, Hinterleitner S, Sturmbauer C, El-Fiky N, Wieser W. 1988. Do carp larvae require vitamin C? Aquaculture 72:295–306 [Google Scholar]
- 7.David AW, Isley JJ, Grimes CB. 1994. Differences between the sagitta, lapillus, and astericus in estimating age and growth in juvenile red drum, Sciaenops ocellatus. Fish Bull 92:509–515 [Google Scholar]
- 8.Grillon C, Pellowski M, Merikangas KR, Davis M. 1997. Darkness facilitates the acoustic startle reflex in humans. Biol Psychiatry 42:453–460 [DOI] [PubMed] [Google Scholar]
- 9.Lewis-McCrea LM, Lall SP. 2010. Effects of phosphorus and vitamin C defieciency, vitamin A toxicity, and lipid peroxidation on skeletal abnormalites in Atlantic halibut (Hippoglossus hippoglossus). J Appl Ichthyology 26:334–343 [Google Scholar]
- 10.Lychakov DV, Rebane YT, Lombarte A, Fuiman LA, Takabayashi A. 2006. Fish otolith asymmetry: morphometry and modeling. Hear Res 219:1–11 [DOI] [PubMed] [Google Scholar]
- 11.Megalofonou P. 2006. Comparison of otolith growth and morphology with somatic growth and age in young-of-the-year bluefin tuna. J Fish Biol 68:1867–1878 [Google Scholar]
- 12.Noppeney U. 2007. The effects of visual deprivation on functional and structural organization of the human brain. Neurosci Biobehav Rev 31:1169–1180 [DOI] [PubMed] [Google Scholar]
- 13.Paxton JR. 2000. Fish otoliths: do sizes correlate with taxonomic group, habitat, and/or luminescence? Philos Trans R Soc Lond B Biol Sci 355:1299–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popper AN, Fay RR. 1993. Sound detection and processing by fish: critical review and major research questions. Brain Behav Evol 41:14–38 [DOI] [PubMed] [Google Scholar]
- 15.Popper AN, Lu Z. 2000. Structure–function relationships in fish otolith organs. Fish Res 46:15–25 [Google Scholar]
- 16.Ramcharitar J, Higgs DM, Popper AN. 2001. Sciaenid inner ears: a study in diversity. Brain Behav Evol 58:152–162 [DOI] [PubMed] [Google Scholar]
- 17.Robertson L, Thomas P, Arnold CR. 1988. Plasma cortisol and secondary stress responses of cultured red drum (Sciaenops ocellatus) to several transportation procedures. Aquaculture 68:115–130 [Google Scholar]
- 18.Robertson L, Thomas P, Arnold CR, Trant JM. 1987. Plasma cortisol and secondary stress responses of red drum to handling, transport, rearing density, and a disease outbreak. Prog Fish Cult 49:1–12 [Google Scholar]
- 19.Secor DH, Dean JM, Laban EH. 1991. Position and morphology of otoliths in fishes, p 7-10. In: Secor DH, Dean JM, Laban EH. Manual for otolith removal and preparation for microstructural examination. Columbia (SC): Belle W Baruch Institute for Marine Biology and Coastal Research. [Google Scholar]
- 20.Singhi SC, Bansal A. 2006. Serum cortisol levels in children with acute bacterial and aseptic meningitis. Pediatr Crit Care Med 7:74–78 [DOI] [PubMed] [Google Scholar]
- 21.Smith ME, Fuiman LA. 2004. Behavioral performance of wild-caught and laboratory-reared red drum Sciaenops ocellatus (Linnaeus) larvae. J Exp Mar Biol Ecol 302:17–33 [Google Scholar]
- 22.Smith ME, Kane AS, Popper AN. 2004. Noise-induced stress response and hearing loss in goldfish (Carassius auratus). J Exp Biol 207:427–435 [DOI] [PubMed] [Google Scholar]
- 23.Thomas P, Robertson L. 1991. Plasma cortisol and glucose stress responses of red drum (Sciaenops ocellatus) to handling and shallow water stressors and anesthesia with MS222, quinaldine sulfate, and metomidate. Aquaculture 96:69–86 [Google Scholar]
- 24.Whitfield TT. 2002. Zebrafish as a model for hearing and deafness. J Neurobiol 53:157–171. [Google Scholar]
- 25.Yasuda K. 1973. Comparative studies on the swimming behavior of the blind cave fish and the goldfish. Comp Biochem Physiol A Comp Physiol 45:515–527 [DOI] [PubMed] [Google Scholar]