Abstract
Ulcerative dermatitis (UD) is a common cause of morbidity and euthanasia in mice with a C57BL/6 (B6) background. The purposes of the current study were to determine whether UD lesions could be reliably produced in B6 mice lacking stearoyl-CoA desaturase 1 (SCD1–/– mice), to ascertain whether the UD lesions in SCD1–/– mice were similar to those found in other B6 mice, and to characterize the cell invasion phenotype of Staphlococcus xylosus cultured from the lesions. S. xylosus isolates from the environment and human skin were used as controls. SCD1–/– (n = 8 per group) and nontransgenic B6 control mice (n = 22 mice pooled from 3 groups that received different concentrations of conjugated linoleic acid) were fed standard rodent chow or a semipurified diet (NIH AIN76A) for 4 wk. Samples from other B6 mice with UD (field cases; n = 7) also were submitted for histology and culture. All of the SCD1–/– mice developed UD lesions by 4 wk on NIH AIN76A. None of SCD1–/– fed standard rodent chow and none of the wildtype B6 mice fed NIH AIN76A developed UD. Supplementation with conjugated linoleic acid did not affect ulcerogenesis. UD lesions in SCD1–/– mice and field cases were grossly and histologically similar. S. xylosus was isolated from SCD1–/– mice with UD (71%) and field cases of UD (43%). These isolates were the most cell-invasive, followed by the environmental isolate, and finally the human skin isolate. Our results provide a basis for further pathologic and clinical study of UD.
Abbreviations: B6, C57BL/6; CLA, conjugated linoleic acid; iNOS, inducible nitric oxide synthase; SCD1, stearoyl-CoA desaturase 1; UD, ulcerative dermatitis
Ulcerative dermatitis (UD) is a chronic and common disease of laboratory mice that often leads to euthanasia of affected animals due to its debilitating nature. The disease often affects mice with the C57BL/6 (B6) background, female mice have an increased propensity for disease, and disease occurrence is increased in iNOS–/– mice.11,13,32 In addition, the incidence of UD increases with age, and some seasonality has been ascribed to the disease, although these reports are conflicting.1,11,13,31,32
UD is diagnosed clinically by observation of excoriations involving the face, ears, or dorsal cervicothoracic skin that are accompanied by pruritus and a negative ectoparasite exam. The histologic description classically includes chronic ulceration with adherent serocellular crust and adjacent epidermal hyperplasia and marked inflammation involving neutrophils, lymphocytes, macrophages, and mast cells.1,11 UD has been postulated to have a multifaceted etiology, with interactions between environmental (dietary vitamin E and humidity11,13,32), immunologic (preferential production of Th1 response by B6 mice1,11), and bacteriologic (overgrowth of Staphylococcus xylosus)37 factors.
The normal skin flora of mice includes staphylococcal species, including S. xylosus.33,37 However, examinations of various dermal staphylococcal species in humans and dogs have revealed differences in their adherence to skin cells once the cells become involved in an inflammatory process.4,18,19 Both adherence and cellular invasion are associated with pathogenesis in gram-positive cocci.4,8,18,19 Documentation of the pathogenicity of S. xylosus is not extensive, but the organism can be used to induce lesions in the tails of SJL/J mice.33 Like UD in B6 mice, diabetic foot ulcers in human beings are also difficult to treat and have a complex etiology. The initial lesion in a diabetic foot ulcer may be vascular, but colonization and infection of the spreading ulcerative lesions by bacteria is part of the pathogenesis.5,36 Likewise, whatever the initiating event in UD, bacterial colonization may be important for the maintenance and spread of the ulcerative lesions.
Stearoyl Co-A desaturase 1 (SCD1) is the enzyme that catalyzes the rate-limiting step in the creation of monounsaturated fatty acids. Mice that lack the SCD1 gene (SCD1–/– mice) have decreased expression of genes coding for enzymes used to synthesize lipids and have increased expression of genes encoding enzymes that oxidize fatty acids. These mice have increased insulin sensitivity, decreased body adiposity, and a resistance to diet-induced obesity.20,25 In addition, SCD1–/– mice have changes in their skin, including a sparse pelage with absence of guard hairs, hypoplasia of meibomian and sebaceous glands, and progressive scarring (nonulcerative) alopecia.21,28,38
Conjugated linoleic acid (CLA) is a naturally occurring lipid that has several potentially therapeutic effects.2,7,26 CLA had antiinflammatory effects in a mouse model of inflammatory bowel disease and in 2 mouse models of autoimmune disease.2,7,26 New Zealand white mice, which are used as a model of systemic lupus erythrematosis, that were fed a standard semipurified rodent diet (NIH AIN76A) plus 1% CLA survived longer than did control mice, and DBA/1J mice with collagen-induced arthritis that were fed the same diet and concentration of CLA had less joint inflammation than did controls.7 Recently, CLA has been shown to enhance wound healing in mice.26
During CLA nutritional studies using SCD1–/– mice, the incidence of UD increased when the mice were changed from a standard chow to a semipurified diet (NIH AIN93).10 This observation initiated an investigation to determine whether SCD1–/– mice fed a similar semipurified diet that was based on NIH AIN76A may serve as a useful model to study UD.7 The nutrients in semi-purified diets are derived from individual single-source components, so that the lipid, protein, or carbohydrate source can be changed by varying a single component. Semipurified diets are used to study conditions like coronary artery disease, where the dietary lipid source plays a role in the pathogenesis of the lesion. In this type of study, less atherogenic fish oils may be substituted for more atherogenic beef tallow as the sole lipid source in the diet.12,16
We hypothesized that at least one of the components of AIN76A was associated with the development of skin ulcers in SCD1–/– mice and that the addition of CLA to the base NIH AIN76A diet would inhibit this ulcerative process. We also hypothesized that the ability of skin bacteria to invade skin cells is part of the pathogenesis underlying ulcerative dermatitis in B6 mice and that diet can affect bacterial invasion. To test our hypotheses, B6 and SCD1–/– mice were fed standard rodent chow and then were switched to the NIH AIN76A semipurified diet, in which corn oil was the fat source. Microbial isolates from the skin of these 2 groups were examined by using a cell invasion assay to determine the ability of the different bacteria to invade NIH 3T3 cells of murine fibroblast origin.
Materials and Methods
Animals and diets.
Female SCD1–/– mice on a B6 background (age, 4 mo; n = 8) were a generous gift from the laboratory of Dr James Ntambi,20,21,28 and adult C57BL/6 mice of undetermined age with clinical cases of UD (n = 7) were obtained from various facilities on the University of Wisconsin-Madison campus. Control B6 female mice (age, 4 mo ; n = 22) for the initial feeding experiment were obtained from a commercial source (Harlan, Indianapolis, IN). These B6 mice were fed different concentrations of CLA in the semipurified diet described following. Because none of these control mice developed ulcers, the results from these mice were pooled for all of the analyses. Additional unaffected B6 control mice used for culture samples were obtained from campus breeding colonies.
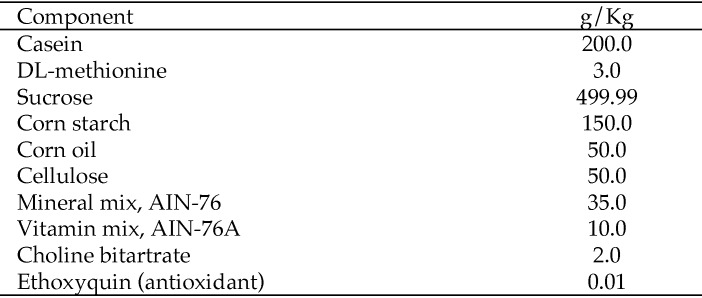
Mice were fed either standard commercial rodent chow (5008, Purina Mills International, St Louis, MO) or a standard semipurified diet (NIH AIN76A) in which corn oil was the lipid source and which was modified with additional calcium carbonate in the mineral mix.7 NIH AIN76A is a standard semipurified diet used in nutritional research. The base diet includes casein as the protein source, corn oil as the fat source, and sucrose and cornstarch as the major carbohydrate sources (Figure 1). Half of the mice (wildtype, n = 11; SCD1–/–, n = 4) received AIN76A to which CLA was added to a concentration of 1%. Because the addition of CLA to the diet of the SCD1–/– mice did not affect the development, histology, or bacterial characterization of skin ulcers, the data from groups receiving or not receiving CLA supplementation were pooled. There was a week of transition from standard chow to AIN76A, after which mice were fed AIN76A for 4 wk. Mice were housed in conventional open-topped shoebox-type cages on hardwood bedding, on a static rack, in social groups with SCD1–/– and wildtype mice housed at a density of 4 or 5 mice per cage. Tap water was provided in bottles ad libitum. Cages were changed once weekly.
Figure 1.
Composition of NIH AIN76A.
Sentinel surveillance indicated that the mice used for these experiments were free of epidemic diarrhea of infant mice virus, Mycoplasma pulmonis, mouse hepatitis virus, murine parvovirus, minute virus of mice, Sendai virus, and Theiler murine encephalomyelitis virus. Sentinels were serologically positive for mouse norovirus. The Animal Care and Use Committees at the University of Wisconsin–Madison approved all animal experiments.
UD lesion occurrence.
Mice on feeding trials were observed at least once daily for evidence of skin ulceration. Once ulcers were observed, mice were observed at least twice daily. Mice with UD were euthanized when the lesions interfered with their locomotion or food consumption or at the direction of the veterinary staff. Mice were euthanized by intraperitoneal injection of commercial euthanasia solution (150 mg/kg; Fatal Plus, Midwest Veterinary Supply, Sun Prairie, WI) or by CO2 asphyxiation.
Pathology.
Pelts from B6 and SCD1–/– mice with UD were removed in a single piece from the carcasses, placed on card stock, and fixed in 10% neutral buffered formalin. Normal SCD1–/– and B6 mice were not included in this experiment. The most important comparison was between the lesions in the B6 and the SCD1–/– mice. The specific abnormalities in the intact skin of SCD1–/– mice have been previously published.6,21,28,30,32 Prior to fixation, small areas of perilesional skin were frozen at −70 °C for later culture. Post fixation, sections of ulcerated skin were excised and placed in cassettes. Cassettes were processed for routine hematoxylin and eosin staining and examined for a defined set of criteria (Table 1). Giemsa-stained slides were used to enumerate mast cells by using a hand counter. A total of 10 high-power (magnification, 400×) fields were enumerated. Masson trichrome stain was used to evaluate the degree of fibrosis, Brown and Hopp stain was used to look for gram-positive cocci and other bacteria; and Alcian blue–periodic acid Schiff staining was used to evaluate slides for the presence of fungi or mucin.
Table 1.
Histologic characteristics of UD in SCD1–/–(n= 8) and other mice on a C57BL/6 background (n= 7)
| SCD1–/– mice | Other B6 mice | P | |
| Ulcer (present in the section) | 8 | 7 | — |
| Acanthosis | 8 | 6 | 0.467 |
| Hyperkeratosis | 8 | 6 | 0.467 |
| Parakeratosis | 8 | 5 | 0.200 |
| Crusts | 8 | 7 | — |
| Dermal polymorphonuclear cells | 8 | 7 | — |
| Naked hair shafts | 5 | 3 | 0.619 |
| Fibrosis | 8 | 7 | — |
| Giant cells | 7 | 1 | 0.010 |
| Granulomas | 7 | 3 | 0.119 |
| Absence of sebaceous glands | 8 | 0 | <0.001 |
| Pigmentary incontinence | 8 | 1 | 0.001 |
| Bacteria | 1 | 2 | 0.550 |
—, significance not determined
Bacterial culture.
Frozen or fresh skin samples obtained near or including UD lesions or from the skin between the shoulders of normal mice were placed in a tissue macerator with a small amount of brain–heart infusion broth (approximately 1 mL). The tissue was pulverized, and the fluid combined with 50 mL brain–heart infusion broth. Aliquots (0.1 mL each) were streaked onto blood agar plates for isolation and incubated at 37 °C with 5% CO2 overnight. Representative colonies were picked, and organisms were identified by using conventional biochemical methods and API strips (API, Biomerieux, Durham, NC). Isolates of S. xylosus were obtained from ATCC (an environmental isolate [catalog no. 12162] and an isolate from human skin [catalog no., 29966]29), opened, grown for 16 h in brain–heart infusion broth, and streaked for isolation on blood agar plates to confirm the purity of the isolate.
Cell invasion assay.
Isolates to be tested for cell invasiveness were streaked onto blood agar plates and incubated for 16 h. Colonies on the blood agar plates were suspended in Mueller–Hinton broth and the concentration was adjusted to 0.5 McFarland units to produce a suspension of organisms equivalent to 1.0 × 107 bacteria/mL. The bacteria were pelleted by centrifugation, and resuspended in Dulbecco Minimal Essential Medium + 10% Fetal Bovine Serum at a concentration of 1.0 × 1014 bacteria/mL. The bacteria suspended in tissue culture medium were overlaid onto NIH 3T3 cells in duplicate or triplicate wells in 24-well plates. The plates were incubated for 2 h at 37 °C with gentle agitation. The plates were removed from the incubator, the supernatant containing the unattached bacteria was removed and discarded, and the cells were washed 3 times with 1 mL PBS. The NIH 3T3 cells were overlaid with PBS containing 1 mg/mL lysostaphin and incubated for 2 h at 37 °C with gentle agitation to lyse any remaining extracellular bacteria. The lysostaphin solution was removed by aspiration, and the wells were washed 3 times with PBS. The cells were lysed with sterile distilled water, and the cell lysates were collected in 1.5-mL microcentrifuge tubes. Serial dilutions of the cell lysates were plated on blood agar.8,35 Blood agar plates were incubated for 48 h, and colonies were counted by a single person (MR) who was blind to the origin of the isolates.
Statistical analysis.
The frequency of each of the histologic features of ulcerative lesions in SCD1–/– and B6 mice was analyzed by using the Fisher Exact test. Mast cell numbers and colony counts from the cell invasion assay were analyzed by using the Mann–Whitney test. All statistical tests were run by using commercially available software (version 19, SPSS Statistics, SPSS, Armonk, NY).
Results
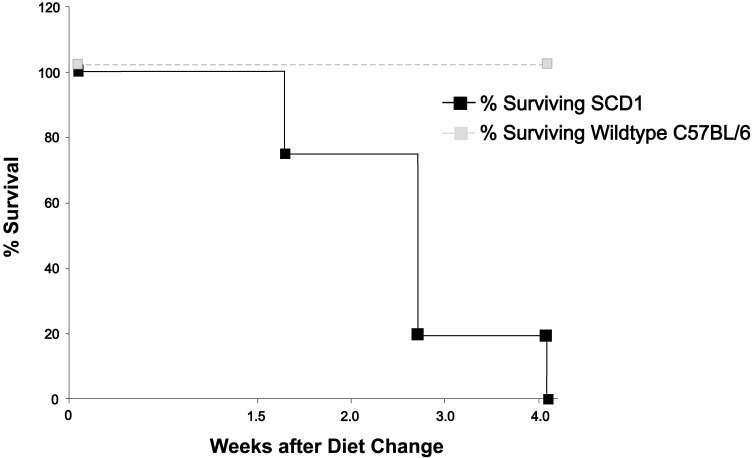
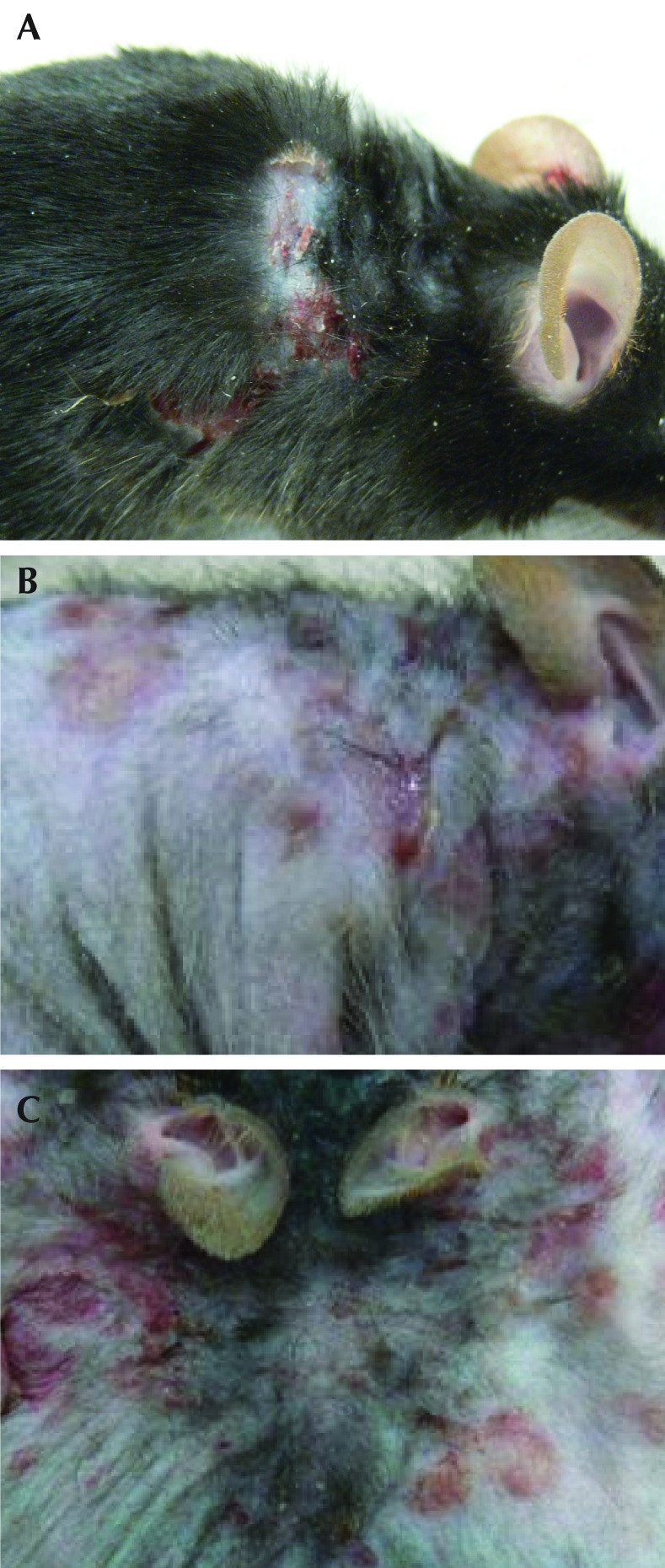
All SCD1–/– mice developed lesions consistent with UD within 4 wk of initiation of NIH AIN76A diet. None of the B6 mice fed this diet developed UD (Figure 2). None of the SCD1–/– or B6 mice maintained on standard chow developed lesions within the 4-wk experimental period. The addition of CLA to the base NIH AIN76A diet did not affect the development of the skin lesions. Gross lesions in field cases of clinical UD and SCD1–/– mice with UD included ulceration over the neck or dorsum, with crusting, scabbing, or oozing (Figure 3). Mice were noted clinically to be pruritic, with self-trauma and occasional lameness. S. xylosus was isolated from 5 of 7 (71%) of the macerated periulcerative skin samples from SCD1–/– mice, 3 of 7 periulcerative skin samples (43%) of field cases of UD in B6 mice, and none of the 6 B6 mice without UD (Table 2). One skin sample from an SCD1–/– mouse was lost during processing. Under the conditions described, UD lesions in SCD1–/– mice grew only pure cultures of S. xylosus. Field cases of UD grew S. xylosus isolates and a single isolate of Streptococcus spp.
Figure 2.
Survival of wildtype (dotted line) and SCD1–/– (solid line) mice (n = 8) after initiation of NIH AIN76A diet. Mortality represents euthanasia of mice due to development of UD.
Figure 3.
Gross appearance of UD during the sporadic, clinical disease in (A) C57BL/6 mice and (B and C) SCD1−/− mice. Ulcerative lesions in SCD1–/– mice are crusted and erythematous and follow the areas of skin motion. Note that the pelage in the SCD1–/– mouse is sparser than in the normal mouse because the genetically modified mice lack guard hairs.
Table 2.
Isolation of S. xylosusfrom clinical cases of UD in SCD1–/–and wildtype C57BL/6 mice (n = 7 each) and from normal C57BL/6 mice (n = 6)
| Mice | No. (%) of mice with S. xylosus | No. of mice with no bacterial growth |
| Clinical UD, C57BL/6 | 3 (43) | 4 |
| UD, SCD1–/– | 5 (71) | 2 |
| Normal skin, C57BL/6a | 0 (0) | 3 |
Includes 3 pelts that were pooled.
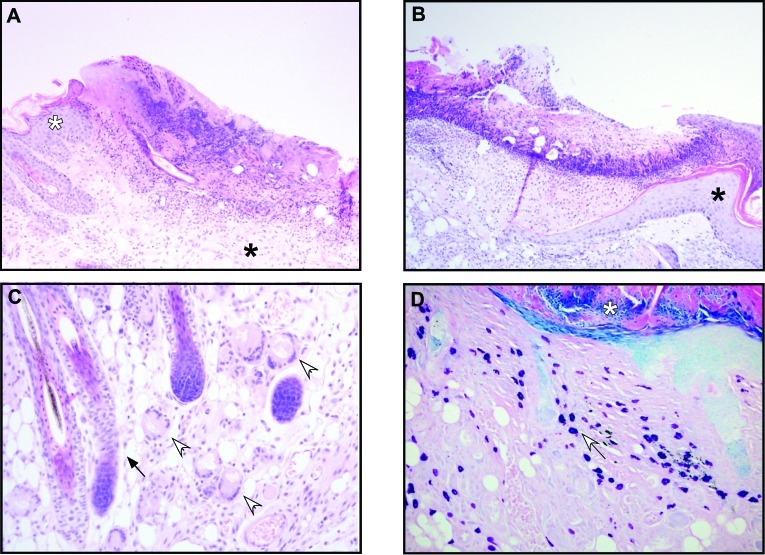
The ulcers were histologically similar between the 2 groups of mice. Histopathologic features common to UD in SCD1–/– and B6 mice included ulceration and reactive changes to the epidermis, including acanthosis, hyperkeratosis, parakeratosis, and pigmentary incontinence. Other changes included serocellular crusts, with and without gram-positive cocci, and reactive fibrosis of the ulcer bed (Table 1, Figure 4). Free hair shafts and hair shafts that penetrated the follicular sheath were seen in the SCD1–/– mice with UD and in field cases of UD (Table 1, Figure 4 B). Consistent with the phenotype of SCD1–/– mice, sebaceous glands were not apparent in the SCD1–/– mice (Table 1, Figure 3 C, black arrow). Inflammatory cells, including neutrophils and mast cells, were common and present in lesions from both groups of mice (Table 1, Figure 4 D), and the median number of mast cells was significantly higher in UD lesions from SCD1–/– mice than B6 mice (SCD1–/–, 305 cells per 10 high-power fields; field cases, 198 cells per 10 high-power fields; P = 0.021, Mann–Whitney test). Giant cells were more common in SCD1–/– mice with UD than in field cases of UD (Table 1, Figure 4 C). The incidence of granulomas did not differ between groups (Table 1). Fungal elements and extracellular mucin were absent.
Figure 4.
(A) UD lesion from an adult female SCD1–/– mouse. S. xylosus was cultured from the ulcer; transition from hyperplastic (white asterisk), acanthotic, and hyperkeratotic epidermis to eroded epidermis to ulcer is evident. The ulcer bed is fibrovascular and cellular; the ulcer itself is covered with proteinaceous and necrotic material. Chronicity is evident in fibrous tissue (black asterisk) underlying ulcer. Hematoxylin and eosin stain; magnification, 100×. (B) Field case of S. xylosus-positive UD in an adult C57BL/6 mouse. The epidermis transitions rapidly from hyperplastic (acanthotic and hyperkeratotic, black asterisk) to eroded to ulcerated. Hematoxylin and eosin stain; magnification, 100×. (C) SCD1–/– adult female mouse. Langhans-type multinucleated giant cells are present in the dermis of mutant skin, adjacent to an ulcer (open arrows). Note the absence of sebaceous glands around hair follicles (black arrow). Hematoxylin and eosin stain; magnification, 200×. (D) SCD1–/– adult female mouse with large numbers of mast cells containing metachromatic granules (white arrow) in the dermis of ulcerated skin with a serocellular crust (white asterisk). Giemsa stain; magnification, 200×.
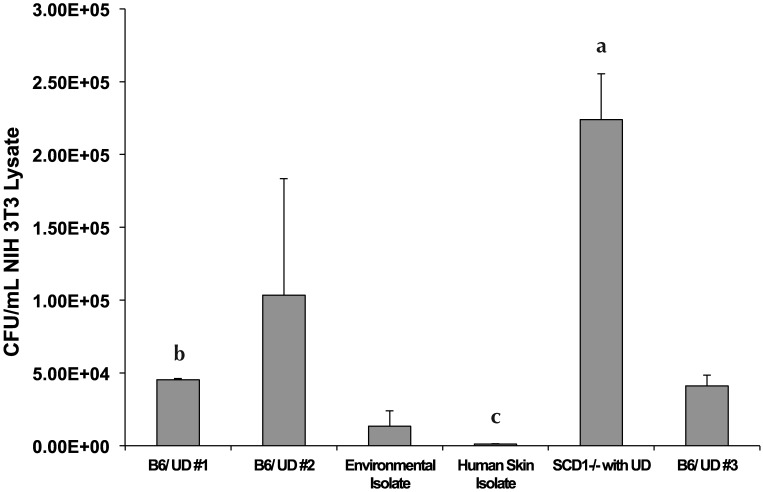
The S. xylosus isolate from a SCD1–/– mouse with UD was the most cell-invasive, followed by isolates from field cases of UD (Figure 5). The environmental isolate was less invasive than were the UD isolates, and the least cell-invasive isolate was the isolate cultured from human skin. The isolate from the SCD1–/– UD lesion had significantly greater cell invasion than did one of the isolates from the field cases of UD in B6 mice (no. 1 in Figure 5; P = 0.050, Mann–Whitney test) and the isolate from human skin (P = 0.050, Mann–Whitney test). In addition, the isolate from the field cases (no. 1) had significantly (P = 0.050, Mann–Whitney test) greater cell invasion than did the isolate from human skin. All other comparisons of cell invasion failed to achieve statistical significance (Figure 5).
Figure 5.
Assessment of cellular invasion potential of different isolates of Staphlococcus xylosus by using NIH 3T3 cells as targets. The isolates characterized included 2 S. xylosus isolates from ATCC (one from an outdoor botanical garden and one from the surface of human skin); 3 isolates from field cases of UD from C57BL/6 mice; and an isolate from UD in a SCD1 –/– mouse. Letters denote significant differences in cell invasiveness as follows: a > b > c. Statistical significance was defined as a P value of 0.05 or less (Mann–Whitney test).
Discussion
Murine UD occurs sporadically in mice with a C57BL/6 background. Genetics, age, and dietary vitamin E content all affect the occurrence of this disease.11,13 The occurrence of UD is unpredictable, with a prevalence in one report of 21% occurring over the course of a 20-mo period.1 All of the SCD1–/– mice in the current study developed UD in 4 wk. This model may provide a predictable method for studying UD and therefore effectively reduce the number of animals for future UD research. UD has a profoundly negative effect on animal welfare, especially in aging studies.1 Loss of animals and data due to clinical euthanasia has been a burden for both researchers and veterinary staff at our institution and elsewhere. The development of a standard model to study this condition has wide-ranging implications for both clinical care and research.
S. xylosus has been cultured from clinical cases of UD at several institutions.33,37 Our culture results show that S. xylosus was isolated from 43% of field cases examined and from 71% of the SCD1–/– mice with UD. Normal B6 controls had no S. xylosus growth. S. xylosus has been reported to be part of the normal skin flora of conventionally housed mice.33 the mice with field cases of UD cultured for the current study were conventionally housed. Our inability to isolate S. xylosus from conventionally housed B6 mice could be due to the low numbers of organisms present on the on the skin of the mice. S. xylosus isolates from all cases of UD were more cell-invasive than were the control isolates, especially the isolate from human skin, and the isolate from one of the SCD1–/– mice with UD had the greatest number of cell-invasive variants. The SCD1–/– mice used in the current study were not treated once they developed UD lesions and were euthanized as soon the UD lesions interfered with the animals’ welfare. The field cases of UD in B6 mice may or may not have been treated. In addition, the culture conditions for the initial isolation of the organisms may have missed some intracellular or extracellular organisms. Intracellular bacteria and intracellular forms of bacteria, including bacteria that have lost their cell walls (L forms), bacteria present in biofilms, and small-colony variants can be difficult to grow using standard media and culture conditions.5,9,22,27,36 Tailoring the culture conditions to isolate intracellular bacteria or bacterial forms and identifying bacteria by direct pyrosequencing may increase the efficiency of isolation or identification of intracellular bacteria, even in treated mice. Intensive and specialized culture conditions and sequencing experiments were beyond the scope of the current study but may be undertaken in the future. Additional experiments will be needed to determine the effect of treatment on the cell invasiveness of S. xylosus in B6 mice.
The UD lesions in the SCD1−/− and B6 mice were grossly and histologically similar, both to one another and to ulcerative lesions in general, although a few differences were noted. The absence of sebaceous glands in the SCD1–/– mice is characteristic of the phenotype. The significance of the severe sebaceous gland hypoplasia in regard to the histomorphology of UD lesions in SCD1–/– mice is unknown. The frequent presence of Langerhans giant cells in UD lesions of SCD1–/– mice may relate to their abnormal pilosebaceous unit.20 Sebaceous secretions have antibacterial properties, and the lack of normal sebum may be a factor in the pathogenesis of UD in SCD1–/– mice.6,23
The antibacterial functions of sebaceous secretions can be divided into 2 categories: barrier functions that prevent bacteria from entering the keratin layer of the skin and actual antibacterial activity.6,14,23 Squalene, wax esters, cholesterol esters, and monounsaturated fatty acids, including palmitoleate (C16:1) and oleate (C18:1), form a physical barrier that helps protect the skin from bacterial colonization of the lower layers.6,23 Squalene and the monounsaturated fatty acids also have antibacterial properties.6,23 Antimicrobial proteins and peptides present in sebum include histone proteins and peptides such as cathelicidin, psoriasin (that is, S100A7), and β defensins.14 The histone proteins present in sebocytes include H2A, H2B, and H4. Most of the antibacterial activity against Staphylococcus aureus and Propionibacterium acnes was associated with H4.14 Other histone proteins, including H1, H2A, and H2B from fish and frog skin and H2B and H4 in human placenta and colon, have been found to have antibacterial activity.14,23 Flake (flk) mice, which carry a SCD1 mutation on a C57BL/6 background, have impaired clearance of experimental infection with gram-positive bacteria (Streptococcus pyogenes and Staphylococcus aureus) but not gram-negative bacteria (Escherichia coli); this defect can partially be rescued by intradermal administration of palmitoleate.6 We expect that the SCD1–/– mice used in the current study have deficient resistance to gram-positive skin pathogens due to their lack of sebaceous secretions.
The early initiating events in UD in B6 and SCD1–/– mice are unknown but may involve sensitization of skin dendritic cells as part of the initiation and maintenance of the inflammatory process.17,24,34 Skin-derived dendritic cells can be stimulated to produce several inflammatory mediators in response to LPS, granulocyte–acrophage colony-stimulating factor, and 2,4-dinitrofluorobenzene.17,24,34 Treatment of a fetal murine dendritic cell line with LPS or granulocyte–macrophage colony-stimulating factor induces nitrite production in vitro.34 2,4-Dinitrofluorobenzene does not affect inducible nitric oxide synthase (iNOS) pathways but instead activates inflammation-associated kinases, including ERK1/2 and MAPK.17 Similarly, staphylococcal exotoxin α may sensitize dendritic cells and act as a superantigen in atopic dermatitis.3,15 B6 mice that are iNOS–/– have a higher lifetime incidence of UD than do iNOS-competent B6 mice; therefore iNOS must not be the only inflammatory mediator necessary for the development of UD lesions.11 The inflammatory stimulus in SCD1–/– mice may be one (or more) of the components of the AIN76A diet or a metabolite thereof. Conversely, the AIN76A may change the skin integrity or elasticity, leading to increased exposure of deep skin layers to environmental chemicals that would not normally be presented to these cells, including skin bacteria and bacterial components.
The reason for the development of UD lesions in SCD1–/– mice fed the NIH AIN76 diet is unknown. The use of semipurified diets is standard practice in nutritional studies, and these diets contain all known required nutrients at levels sufficient to meet the needs of a healthy animal. The finding that UD only occurred in mice fed the semipurified diet provides a unique opportunity to discover compounds that either promote or protect against UD. Additional experiments using different fat or protein sources will be useful to determine the influence of these dietary factors on the development of UD in SCD1–/– mice. In particular, the lipid source in AIN76A, corn oil, can be exchanged for fish oil. Corn oil and other vegetable oils rich in omega-6 fatty acids are more proinflammatory than is fish oil, which is high in omega-3 fatty acids.12,16 The S. xylosus cell-invasion phenotype may also play a role in the development of UD. The similarities between the ulcerative skin disease in SCD1–/– mice and other B6 mice warrant further exploration before SCD1−/− mice can be used as a model of UD. Such a model may improve our understanding of UD pathogenesis.
Acknowledgments
We thank the animal care staff of the Department of Animal Sciences and Biochemistry for their care of the mice; Dr Linda Schuler for providing the NIH 3T3 cells; Mariela Quesada for cell culture; and Markus J Wisniewski and Jenaia Delk for technical assistance. This work was supported by RO1DK-62388 (to JMN) and Hatch Funds WIS01073 and the College of Agricultural and Life Sciences, University of Wisconsin–Madison (to MC).
References
- 1.Andrews AG, Dysko RC, Spilman SC, Kunkel RG, Brammer DW, Johnson KJ. 1994. Immune complex vasculitis with secondary ulcerative dermatitis in aged C57BL/6NNia mice. Vet Pathol 31:293–300 [DOI] [PubMed] [Google Scholar]
- 2.Bassaganya-Riera J, Hontecillas R. 2010. Dietary CLA and n-3 PUFA in inflammatory bowel disease. Curr Opin Clin Nutr Metab Care 13:569–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breuer K, Witmann M, Kempe K, Kapp A, Mai U, Dittrich-Breiholz O, Kracht M. 2005. Alpha toxin is produced by skin colonizing Staphylococcus aureus and induces a T helper type 1 response in atopic dermatitis. Clin Exp Allergy 35:1088–1095 [DOI] [PubMed] [Google Scholar]
- 4.Cole GW, Silverberg NL. 1986. The adherence of Staphylococcus aureus to human corneocytes. Arch Dermatol 122:166–169 [PubMed] [Google Scholar]
- 5.Dowd SE, Wolcott RD, Sun Y, McKeehan T, Smith E, Rhoads D. 2008. Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP). PLoS ONE 3:e3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Georgel P, Crozat K, Lauth X, Makrantonaki E, Seltmann H, Sovath S, Hoebe K, Du X, Rutschmann S, Jiang Z, Bigby T, Nizet V, Zouboulis CC, Beutler B. 2005. A Toll-like receptor 2-responsive lipid effector pathway protects mammals against skin infections with gram-positive bacteria. Infect Immun 73:4512–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huebner SM, Campbell JP, Butz DE, Fulmer TG, Gendron-Fitzpatrick A, Cook ME. 2010. Individual isomers of conjugated linoleic acid reduce inflammation associated with established collagen-induced arthritis in DBA/1 mice. J Nutr 140:1454–1461 [DOI] [PubMed] [Google Scholar]
- 8.Hussain M, Haggar A, Peters G, Chhatwal GS, Hermann M, Flock J-I, Sinha B. 2008. More than one tandem repeat domain of the extracellular adherence protein of Staphylococcus aureus is required for aggregation, adherence, and host cell invasion but not for leukocyte activation. Infect Immun 76:5615–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joseleau-Petit D, Liebart J-C, Ayala JA, D'Ari R. 2007. Unstable Escherichia coli L forms revisited: growth requires peptidoglycan synthesis. J Bacteriol 189:6512–6520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang K, Miyazaki M, Ntambi JM, Pariza MW. 2004. Evidence that the antiobesity effect of conjugated linoleic acid is independent of effects on stearoyl-CoA desaturase 1 expression and enzyme activity. Biochem Biophys Res Commun 315:532–537 [DOI] [PubMed] [Google Scholar]
- 11.Kastenmayer RJ, Fain MA, Perdue KA. 2006. A retrospective study of idiopathic ulcerative dermatitis in mice with a C57BL/6 background. J Am Assoc Lab Anim Sci 45:8–12 [PubMed] [Google Scholar]
- 12.Korver DR, Roura E, Klasing KC. 1998. Effect of dietary energy level and oil source on broiler performance and response to an inflammatory challenge. Poult Sci 77:1217–1227 [DOI] [PubMed] [Google Scholar]
- 13.Lawson GW, Sato A, Fairbanks LA, Lawson PT. 2005. Vitamin E as a treatment for ulcerative dermatitis in C57BL/6 mice and strains with a C57BL/6 background. Contemp Top Lab Anim Sci 44:18–21 [PubMed] [Google Scholar]
- 14.Lee DY, Huang CM, Nakatsuji T, Thiboutot D, Kang S-A, Monestier M, Gallo RL. 2009. Histone H4 is a major component of the antimicrobial action of human sebocytes. J Invest Dermatol 129:2489–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin YT, Wang C-T, Chiang B-L. 2007. Role of bacterial pathogens in atopic dermatitis. Clin Rev Allergy Immunol 33:167–177 [DOI] [PubMed] [Google Scholar]
- 16.Massaro M, Scoditti E, Carluccio MA, Campana MC, De Caterina R. 2010. Omega-3 fatty acids, inflammation and angiogenesis: basic mechanisms behind the cardioprotective effects of fish and fish oils. Cell Mol Biol (Noisy-le-grand) 56:59–82 [PubMed] [Google Scholar]
- 17.Matos TJ, Duarte CB, Goncalo M, Lopes MC. 2005. DNFB activates MAPKs and upregulates CD40 in skin-derived dendritic cells. J Dermatol Sci 39:113–123 [DOI] [PubMed] [Google Scholar]
- 18.McEwan NA, Mellor D, Kalna G. 2006. Adherence by Staphylococcus intermedius to canine corneocytes: a preliminary study comparing noninflamed and inflamed atopic canine skin. Vet Dermatol 17:151–154 [DOI] [PubMed] [Google Scholar]
- 19.Mcewan NA. 2000. Adherence by Staphylococcus intermedius to canine keratinocytes in atopic dermatitis. Res Vet Sci 68:279–283 [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki M, Man WC, Ntambi JM. 2001. Targeted disruption of stearoyl-CoA desaturase 1 gene in mice causes atrophy of sebaceous and mebomian glands and depletion of wax esters in the eyelid. J Nutr 131:2260–2268 [DOI] [PubMed] [Google Scholar]
- 21.Miyazaki M, Sampath H, Liu X, Flowers MT, Chu K, Dobrzyn A, Ntambi JM. 2009. Stearoyl-CoA desaturase 1 deficiency attenuates obesity and insulin resistance in leptin-resistant obese mice. Biochem Biophys Res Commun 380:818–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monroe D. 2007. Looking for the chinks in the armor of bacterial biofilm. PLoS Biol 5:e307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagai A, Sato T, Akimoto N, Ito A, Sumida M. 2005. Isolation and identification of histone H3 protein in microvescicles secreted from cultured sebocytes. Endocrinology 146:2593–2601 [DOI] [PubMed] [Google Scholar]
- 24.Neves BM, Cruz MT, Francisco V, Garcia-Rodriguez C, Silvestre R, Corderiro-da-Silva A, Dinis AM, Batista MT, Duarte CB, Lopes MC. 2009. Differential roles of PI3 kinase, MAPKs and NFκβ on the manipulation of dendritic cell Th1–Th2 cytokine–chemokine polarizing profile. Mol Immunol 46:2481–2492 [DOI] [PubMed] [Google Scholar]
- 25.Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JP, Attie AD. 2002. Loss of stearoyl-CoA desaturase 1 function protects mice against adiposity. Proc Natl Acad Sci USA 99:11482–11486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park NY, Valacchi G, Lim Y.2010. Effect of dietary conjugated linoleic acid supplementation on early inflammation responses during cutaneous wound healing. Mediators Inflamm pii: 342328. [DOI] [PMC free article] [PubMed]
- 27.Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, Peters G. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol 4:295–305 [DOI] [PubMed] [Google Scholar]
- 28.Sampath H, Flowers MT, Liu X, Paton CM, Sullivan R, Chu K, Minghui Z, Ntambi JM. 2009. Skin-specific deletion of stearoyl-CoA desaturase 1 alters skin lipid composition and protects mice from high fat diet-induced obesity. J Biol Chem 284:19961–19973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schleifer KH, Kloos WE. 1975. Isolation and characterization of staphylococci from human skin. I. Amended descriptions of Staphyloccus epidermidis and Staphyloccus saprophyticus and descriptions of 3 new species: Staphylococcus cohnii, Staphylococcus haemolyticus, and Staphylococcus xylosus. Int J Syst Bacteriol 25:50–61 [Google Scholar]
- 30.Sundberg JP, Boggess D, Sundberg BA, Eilertsen K, Parimoo S, Filippi M, Stenn K. 2000. Asebia-2J (Scd1ab2J): a new allele and a model for scarring alopecia. Am J Pathol 156:2067–2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sundberg JP, Brown KS, McMahon WM.1994. Chronic ulcerative dermatitis in black mice, p 485–492. In: Handbook of mouse mutations with skin and hair abnormalities. Boca Raton (FL): CRC Press.
- 32.Sundberg JP, Taylor DK, Lorch G, Miller J, Silva KA, Sundberg BA, Roopenian D, Ong D, King LE, Everts H. 2011. Primary follicular dystrophy with scarring dermatitis in C57BL/6 mouse substrains resembles central centrifugal cicatricial alopecia in humans. Vet Pathol 48:513–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thornton VB, Davis JA, St Clair MB, Cole MN. 2003. Inoculation of Staphylococcus xylosus in SJL/J mice to determine pathogenicity. Contemp Top Lab Anim Sci 42:49–52 [PubMed] [Google Scholar]
- 34.Vital AL, Goncalo M, Cruz MT, Figueiredo A, Duarte CB, Lopes MC. 2003. Dexamethasone prevents granulocyte–macrophage colony stimulating factor-induced nuclear factor κβ activation, inducible nitric oxide synthase expression and nitric oxide production in a skin dendritic cell line. Mediators Inflamm 12:71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang B, Li S, Southern PJ, Cleary PP. 2006. Streptococcal modulation of cellular invasion via TGFβ1 signaling. Proc Natl Acad Sci USA 103:2380–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolcott RD, Cox SB, Dowd SE. 2010. Healing and healing rates of chronic wounds in the age of molecular pathogen diagnostics. J Wound Care 19:272–278 [PubMed] [Google Scholar]
- 37.Won YS, Kwon HJ, Ob GT. 2002. Identification of Staphylococcus xylosus isolated from C57BL/6JNos2(tm/Lau) mice with dermatitis. Microbiol Immunol 46:629–632 [DOI] [PubMed] [Google Scholar]
- 38.Zheng Y, Eilersten KJ, Ge L, Zhang L, Sundberg JP, Prouty SM, Stenn KS, Parimoo S. 1999. Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nat Genet 23:268–270 [DOI] [PubMed] [Google Scholar]