Abstract
Endometriosis is one of the most frequently encountered gynecologic diseases and a common cause of chronic pelvic pain and infertility. The pathophysiology of this syndrome can best be described as the presence of ectopic endometrium and a pelvic inflammatory process with associated immune dysfunction and alteration in the peritoneal environment. Macrophages play an important role in the progression and propagation of endometriosis. Alternative macrophage activation occurs in rodents and women with endometriosis but had not been examined previously in nonhuman primates. This case–control study aimed to characterize macrophage polarization in the ectopic and eutopic endometrial tissue of nonhuman primates with and without endometriosis. In addition, circulating cytokines in endometriosis cases and normal controls were investigated in an effort to identify serum factors that contribute to or result from macrophage polarization. Endometriosis lesions demonstrated increased infiltration by macrophages polarized toward the M2 phenotype when compared with healthy control endometrium. No serum cytokine trends consistent with alternative macrophage activation were identified. However, serum transforming growth factor α was elevated in macaques with endometriosis compared with healthy controls. Findings indicated that the activation state of macrophages in endometriosis tissue in nonhuman primates is weighted toward the M2 phenotype. This important finding enables rhesus macaques to serve as an animal model to investigate the contribution of macrophage polarization to the pathophysiology of endometriosis.
Abbreviations: HLA, human leukocyte antigen; Iba1, ionized calcium binding adaptor molecule 1; M1, classically activated macrophage; M2, alternatively activated macrophage; sCD40L, soluble cluster of differentiation 40 ligand; TGF, transforming growth factor; VEGF, vascular endothelial growth factor
Endometriosis is a common cause of chronic pelvic pain and infertility and affects more than 5.5 million women in North America alone.41 Although endometriosis is one of the most frequently encountered gynecologic health problems among women of reproductive age, the pathophysiology of this disease remains elusive due to its complexity and multifactorial etiology. The presence of functional endometrial glands and stroma outside the uterine cavity defines endometriosis. Currently, the most widely accepted theory for the origin of ectopic endometrial tissue is a combined effect of retrograde menstruation and associated implantation of endometrial fragments at an ectopic site. Progression of endometriosis lesions is thought to then be supported by peritoneal factors that allow cell adhesion and growth.44 Although endometriosis is not a neoplastic disease, it exhibits aggressive features such as cellular proliferation, invasion, and vascular proliferation.12 Strong evidence indicates that endometriosis involves a pelvic inflammatory process, with immune dysfunction and alteration in the peritoneal environment.13,27 Numerous studies have demonstrated marked increases in macrophage populations and activity in the peritoneum of endometriosis patients.6,54,59 Although macrophages are integral to homeostasis of the peritoneal environment, during endometriosis they mediate inflammation and facilitate the establishment and maintenance of the disease.
Macrophages can be classified into 2 main populations: classically activated macrophages (M1), whose activating stimuli include IFNγ and LPS, and alternatively activated macrophages (M2), whose activating stimuli includes IL4, IL13, IL10, and transforming growth factor (TGF) β.55 These polar phenotypes are not expressed together, but the activation state of tissue macrophages can change over time. This phenotypic switch is possible because macrophages retain plasticity, resulting in macrophage polarization that is transient and reversible.40 A key component in determining the phenotype of the differentially activated macrophage is their response to microenvironmental signals, and this response allows for expression of a spectrum ranging from the M1 to M2 extremes.51 M1- and M2-activated macrophages perform different functions by producing pro- or antiinflammatory factors. M1 macrophages have enhanced endocytic functions and an enhanced ability to kill intracellular pathogens; they also secrete large amounts of proinflammatory cytokines such as IL1α, IL6, IL12, and TNFα.7 In contrast, M2 macrophages are involved in resolution of inflammation and promotion of tissue repair, and they secrete antiinflammatory and immunosuppressive cytokines including IL10 and TGFβ.32 M2 cells also express proangiogenic factors, such as coagulation factor XIII and vascular endothelial growth factor (VEGF) and have been associated with a high degree of vascularization in vivo.1 The pathogenesis of endometriosis is therefore a likely combination of inappropriate or sustained polarization, leading to tissue damage (increased M1 response) and immune dysfunction (increased M2 response) and allowing for persistence of ectopic endometrial tissue.
The use of animal models in endometriosis research is crucial. Work done with rodents involves the study of induced disease.53 Despite this caveat, rodent models have been the basis for important contributions. Global macrophage depletion in a rat model of endometriosis effectively inhibits the initiation and growth of endometriosis implants.15 Attenuation of endometriosis has recently also been demonstrated in a mouse model of endometriosis.4 In that study, systemic depletion of macrophages was associated with failure of endometrial lesion development and defective angiogenesis of established lesions. Further evaluation of specific roles of differentially activated macrophages in that study4 showed that adoptive transfer of alternatively activated macrophages (M2) was associated with enhanced endometriosis progression. Conversely, adoptive transfer of inflammatory macrophages (M1) was associated with abrogated progression. In addition to evaluating murine lesions, the authors of the cited study4 investigated markers for alternative macrophage activation in women with endometriosis and matched controls which revealed increased expression of CD163 and CD206 (2 markers of M2 polarized macrophages) in endometriosis lesions as compared with disease-free peritoneum. Although many studies have been published about the pivotal role of macrophages in the pathophysiology of endometriosis, only a few have dealt with activation of the M1 and M2 macrophage phenotypes.4,57 Furthermore, few studies have examined tissue infiltration of macrophages in eutopic endometrium of human subjects with endometriosis.6,23 An exhaustive literature search failed to identify studies that investigate the role of M1 and M2 macrophage populations in eutopic endometrium.
The current study uses rhesus macaques, which have been studied extensively in reproductive medicine.58 Because spontaneous development of the disease requires menstrual shedding, endometriosis occurs naturally only in some nonhuman primate species, making development of lesions more comparable to the establishment of disease in humans.14 Compared with rodents, the nonhuman primate model of endometriosis is advantageous due to a close recapitulation of human disease and physiology. Work characterizing M1 and M2 macrophage activation in a species with spontaneous disease development may reflect a closer immunologic characterization to humans. In the current study, macrophage populations were evaluated in archival tissue collected from rhesus macaques with a diagnosis of endometriosis as confirmed by histologic examination. To characterize the phenotype of endometrial tissue macrophages in ectopic endometriosis lesions and eutopic endometrium of both cases and controls, immunohistochemistry was used to quantify cells expressing M1- and M2-specific markers. We hypothesized that endometriosis lesions and eutopic endometrium of rhesus macaques would be associated with a polarized macrophage infiltration consisting of increased numbers of M2 macrophages. This increase in M2 response may cause reduced immune clearance of ectopic endometrial cells, facilitating their implantation and growth. Further we speculated that M2 polarization would be associated with increased serum cytokines including IL10 and VEGF and decreased production of IL6, IL12, and TNFα. The lack of findings that support our hypotheses may suggest that the micro- or peritoneal environment is more important for lesion development or that another component of the systemic milieu is the determining factor in the development of endometriosis.
Materials and Methods
Case and control selection.
All samples used in this study were archival samples previously collected from animals housed at the New England Primate Research Center. The facility is maintained in accordance with the Guide for the Care and use of Laboratory Animals21 and is AAALAC-accredited. Colony animals are maintained under an animal holding and breeding protocol approved by Harvard Medical School's Standing Committee on Animals.
A retrospective analysis of necropsy records from the New England Primate Research Center was performed to identify subjects with (n = 6) and without (n = 4) endometriosis. Cases were included when animals were rhesus macaques (Macaca mulatta) born at NEPRC, were older than 10 y, and had a histologic diagnosis of endometriosis identified at necropsy. In addition, animals selected had no major concurrent diseases and had not received hormonal or other long-term medical therapy. Healthy age-matched controls were selected by a review of their medical record and necropsy report. Subclinical endometriosis was ruled out by histology. Animals were excluded when archival, formalin-fixed, paraffin-embedded tissue blocks were unavailable for immunohistochemistry. Additional rhesus macaques meeting the same inclusion criteria were selected for serum cytokine analysis (endometriosis cases, n = 15; healthy age-matched controls, n = 10). Serum samples were obtained from the serum archive at the New England Primate Research Center. These archival samples are collected for banking during routine preventative health care assessments and stored at −80 °C until the time of analysis. Three time points were selected for each subject and included samples representing ages of 4, 10, and 15 y.
Immunohistochemistry.
Single-label immunohistochemical staining was performed on formalin-fixed, paraffin-embedded tissue sections by using specific antibodies directed against CD163 (clone 10D6, Lab Vision, Fremont, CA), HLA–DP-DQ-DR (clone CR3/43, DakoCytomation, Carpinteria, CA), and Iba1 (polyclonal, Wako Chemicals, Richmond, VA). Prior to immunostaining, tissue sections were deparaffinized in xylene, rehydrated in graded ethanol, and incubated in 3% hydrogen peroxide in PBS to suppress endogenous peroxidase activity. Antigen retrieval consisted of microwave pretreatment in antigen unmasking buffer solution (Vector Laboratories, Burlingame, CA). Sections were immunostained with primary antibody, followed by avidin–biotin blocking (Vector Laboratories, Burlingame, CA) and sequential incubation with biotinylated secondary antibody and horseradish peroxidase-conjugated avidin (ABC Standard or ABC Elite, Vector Laboratories). 3,3′-Diaminobenzidine chromogen (DakoCytomation) was used to visualize antigen–antibody complex formation. Negative control slides were processed identically by using irrelevant, isotype-matched primary antibodies.
Quantification of cells after immunohistochemistry.
Slides immunohistochemically labeled for CD163, human leukocyte antigen (HLA)–DP-DQ-DR, and ionized calcium binding adaptor molecule 1 (Iba1) were examined microscopically (model BX40, Olympus, Irving, TX). Macrophage cell populations within healthy endometrium of control subjects, eutopic endometrium of macaques with endometriosis, and ectopic lesions of macaques with endometriosis were quantified by using automated image analysis. Lesions considered for review consisted of endometriotic glands and periglandular endometriotic stroma including endometriotic cysts and associated surrounding fibrous tissue. Five randomly chosen fields were captured of each slide at 20× magnification by using a microscope-mounted digital camera (Olympus). The images were transferred to the Image J software program (NIH, Bethesda, MD) for automated cell counting. Each image was converted to a grayscale format and subjected to threshold limits and particle size boundaries to quantify the total number of cells stained with 3,3′-diaminobenzidine. The data from each set of 5 images then were compiled to calculate the average cell count per field for each macrophage marker.
Quantification of circulating serum cytokines.
Cytokines were evaluated by using the 23-plex Milliplex MAP Nonhuman Primate Cytokine kit (Millipore, Billerica, MA) by using Luminex technology (Millipore). Frozen serum was thawed, mixed by vortexing, and then centrifuged at 8000 × g for 5 min to isolate debris prior to use in the assay. Samples were prepared according to the manufacturer's directions by using a 96-well filter membrane microtiter plate and vacuum filtration unit (Millipore Vacuum Manifold). After the final wash, samples were suspended in 150 µL Luminex Sheath Fluid (Millipore) and analyzed (Luminex 200, Millipore). Acquisition gates were set at 8000 to 15,000; sample volume was 100 µL; and 50 events per bead were acquired. Manufacturer-provided quality-control standards and established expected ranges for each analyte were used for assay validation. Mean fluorescence intensity was analyzed by using Milliplex Analyst software and compared with a standard curve to generate concentration values. Values below the range of the standard curve were set to 0.
Statistical analysis.
Two-tailed Student t tests were used to assess group differences in circulating serum cytokines between cases and controls (Stata software, Stata Press, College Station, TX). P values of less than 0.05 were considered significant. Because of nonnormal distribution and a small sample size, the nonparametric Kruskal–Wallis test was used to determine significant differences in cell counts between healthy uterus of controls, eutopic endometrium of cases, and endometriosis lesions. Pairwise comparisons were performed by using the Wilcoxon rank-sum test. Bonferroni correction was performed to adjust for multiple comparisons. Owing to this correction factor, a P value of less than 0.01 was considered significant. Data are expressed as mean ± 1 SD for normal data and median ± interquartile range for nonnormal data.
Results
The retrospective analysis of necropsy records from January 1997 to April 2010 identified 60 cases of endometriosis among a total of 1042 necropsies performed on female rhesus macaques. This number translates to a prevalence of 5.8%, which is comparable to published human statistics.18 For the part of the study that assessed macrophage populations, 6 adult rhesus macaques with endometriosis were selected and ranged from 12 to 18 y old (median, 15 y) with lesion severity ranging from mild to severe. In addition, 4 healthy female rhesus macaques identified for use as controls ranged in age from 11 to 17 y (median, 15 y). For serum cytokine analysis, 15 macaques with endometriosis were selected and were 12 to 21 y old (median, 16 y); the 10 healthy controls identified ranged in age from 11 to 19 y (median, 15 y).
Comparison of macrophage markers of activation.
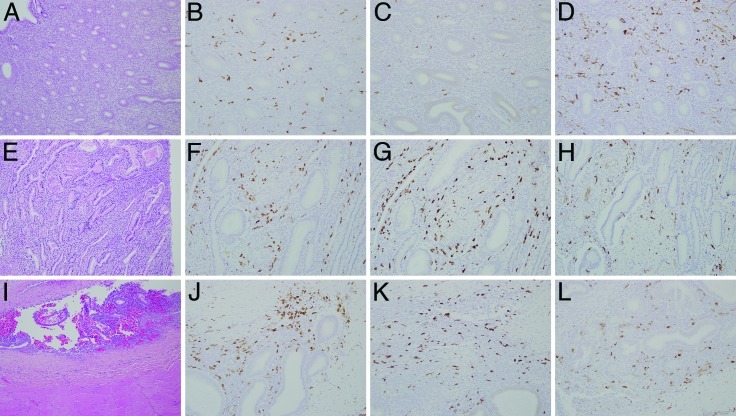
To identify and quantify the phenotype of macrophages in endometriosis, antibodies against antigens commonly expressed on macrophages were used. M2-polarized macrophages were identified by positive immunostaining for the M2-specific cell-surface marker CD163,3,28 whereas HLA–DP-DQ-DR was used as a marker for M1-polarized macrophages.30,42,43 The panmacrophage marker used was Iba1.20,24 A cell with an oval to round nucleus with fine dendritic processes that showed strong membranous and cytoplasmic staining but no nuclear staining was identified as a macrophage. The results of the macrophage quantification are summarized in Table 1. Representative images of histopathology and immunohistochemical staining for macrophage markers are presented in Figure 1.
Table 1.
Quantification of macrophage cell populations (median no. per 20× field ± interquartile range) within the healthy uterus of controls (n= 4), eutopic uterus of macaques with endometriosis (n = 4), and endometriosis lesions of macaques with endometriosis (n = 9)
| Marker | Healthy uterus | Eutopic uterus | Endometriosis lesion | Kruskal–Wallis Pa | |
| Iba1 | 180 ± 47.5b | 287.5 ± 120.5c | 418 ± 119b,c | 0.003 | |
| CD163 | 67.5 ± 65.5d | 288.5 ± 413.5 | 528 ± 191d | 0.02 | |
| HLA–DR-DP-DQ | 93 ± 123.5 | 128.5 ± 152 | 250 ± 168 | 0.21 |
Significance set at P ≤ 0.05.
P = 0.006 (Wilcoxon rank-sum test; significance set at P ≤ 0.01 to account for multiple comparisons) between groups.
P = 0.01 (Wilcoxon rank-sum test) between groups.
P = 0.009 (Wilcoxon rank-sum test) between groups.
Figure 1.
Representative images of histopathology (A, E, and I; hematoxylin and eosin staining; magnification, 10×) and immunohistochemical staining (magnification, 20×) for the macrophage markers Iba1 (B, F, J), CD163 (C, G, and K), and HLA–DP-DQ-DR (D, H, and L). Images include those of healthy control endometrium (A through D), eutopic endometrium from a macaque with endometriosis (E through H), and an ectopic pelvic abdominal wall endometriosis lesion (I through L).
Endometriosis lesions defined by endometriotic glands and stroma were infiltrated extensively by CD163-immunopositive macrophages (Figure 1 K; 528 cells per 20× field), whereas healthy control endometrium shows only sparse CD163 immunopositive macrophage infiltration (Figure 1 C; 67.5 cells per 20× field; P = 0.009). Although not statistically significant, there was an upward trend in CD163-immunopositive macrophages (Figure 1 G; 288.5 cells per 20× field; P = 0.15) in the eutopic endometrium of macaques with endometriosis. Cell counts for Iba1 positivity differed significantly between endometriosis lesions (Figure 1 J; 418 cells per 20× field) and healthy control endometrium (Figure 1 B; 180 cells per 20× field; P = 0.006) and between endometriosis lesions and eutopic endometrium from macaques with endometriosis subjects (Figure 1 F; 287.5 cells per 20× field; P = 0.01).
Comparison of circulating serum cytokine levels.
The results of quantification of circulating serum cytokines are summarized in Table 2. Save for a statistically significant increase in TGFα in macaques with endometriosis (12.69 pg/mL) relative to healthy controls (7.18 pg/mL; P = 0.02) and a marginally significant trend toward increased soluble cluster of differentiation 40 ligand (sCD40L) in macaques with endometriosis (27.20 pg/mL) relative to healthy controls (7.18 pg/mL; P = 0.06), there were few appreciable differences in the levels of circulating serum cytokines (Table 2). These differences were observed at the aged sampling point (15 y) only, with no significant differences in circulating cytokines observed between cases and controls at puberty (4 y) or at peak breeding age (10 y). Several cytokines circulate at very low levels in both affected and unaffected rhesus macaques, in which at least 50% of samples were below the limit of detection for granulocyte colony stimulating factor, granulocyte–macrophage colony stimulating factor, IL4, IL17, IL10, IL1b, TNFα, IL1ra, IL6, and IFNγ. Statistical comparison of these analytes was not performed.
Table 2.
Circulating serum cytokine levels (mean ± 1 SD) at the aged (15 y) time point of controls and macaques with endometriosis
| Analyte (pg/mL) | Macaques with endometriosis | Controls | Pa | Samples below range of standard curve (%) |
| G-CSF | not determined | not determined | not determined | 80 |
| GM-CSF | not determined | not determined | not determined | 100 |
| IL4 | not determined | not determined | not determined | 100 |
| IL17 | not determined | not determined | not determined | 100 |
| TNFα | not determined | not determined | not determined | 100 |
| IL1ra | not determined | not determined | not determined | 92 |
| IL6 | not determined | not determined | not determined | 92 |
| IFNγ | not determined | not determined | not determined | 68 |
| VEGF | 345.70 ± 796.31 | 1656.19 ± 3607.52 | 0.28 | 48 |
| IL10 | 2.05 ± 4.03 | 3.77 ± 7.20 | 0.45 | 32 |
| IL18 | 12.50 ± 19.24 | 15.42 ± 20.35 | 0.72 | 28 |
| sCD40L | 27.20 ± 48.18 | 1.10 ± 3.48 | 0.06 | 20 |
| IL12/23 | 40.81 ± 77.90 | 20.57 ± 13.48 | 0.34 | 8 |
| IL15 | 20.25 ± 6.97 | 19.81 ± 10.71 | 0.90 | 4 |
| IL2 | 14.80 ± 6.99 | 11.03 ± 8.83 | 0.24 | 4 |
| MIP1a | 9.12 ± 0.55 | 10.42 ± 2.12 | 0.09 | 4 |
| IL1b | 2.83 ± 0.06 | 2.90 ± 0.17 | 0.24 | 0 |
| IL5 | 4.76 ± 5.86 | 5.05 ± 3.38 | 0.89 | 0 |
| IL8 | 1173.27 ± 1275.02 | 845.21 ± 1075.61 | 0.51 | 0 |
| MCP1 | 477.92 ± 192.94 | 415.86 ± 386.62 | 0.65 | 0 |
| MIP1b | 6.00 ± 0.23 | 6.69 ± 1.59 | 0.65 | 0 |
| TGFα | 12.69 ± 7.44 | 7.18 ± 3.39 | 0.02 | 0 |
| IL13 | 13.25 ± 0.74 | 13.31 ± 0.94 | 0.88 | 0 |
G-CSF, granulocyte colony stimulating factor; GM-CSF, granulocyte macrophage colony stimulating factor; MCP1, monocyte chemoattractant protein; MIP1a, macrophage inflammatory protein 1a; MIP1b, macrophage inflammatory protein 1b
P value determined by using 2-tailed Student t tests.
Discussion
In the current study, we demonstrate that the presence of endometriosis is associated with significant alterations in the number of tissue macrophages expressing M1 or M2 surface markers. The data demonstrate that the M2 macrophage phenotype occurs more frequently in endometriosis lesions compared with control endometrium in rhesus macaques.
CD163 shows high specificity for the monocyte–macrophage lineage and is highly expressed in immunosuppressive M2- polarized macrophages.22,28 Alternative macrophage activation leads to stimulation of antiinflammatory cytokine production and inhibition of proinflammatory cytokine expression, thus reducing inflammation. Macrophages may act to suppress the immune response to endometriosis and provide an environment permissive to the growth and progression of endometriosis lesions. We surmise that recruited macrophages largely develop an immunosuppressive phenotype (M2), thereby supporting endometrial tissue survival, attachment, and invasion through matrix remodeling, angiogenesis, and lesion maintenance.
The increase in M2-polarized macrophages in macaque endometriosis lesions as compared with healthy control endometrium is an important finding consistent with a microenvironment that supports implantation of endometrial debris. M2-polarized macrophages have a decreased phagocytic repertoire.25,56 Supporting this concept is the discovery that peritoneal macrophages in women with endometriosis have reduced phagocytic ability.8 This reduced phagocytic capacity of M2 macrophages likely is driven by cues in the tissue microenvironment, including cytokines and growth factors that determine the macrophage phenotype and function.29
In our current study, eutopic endometrium from rhesus macaques with endometriosis showed a trend toward increased expression of M2 macrophages compared with that in endometrium from healthy rhesus. This difference may contribute to the survival of endometrial cells that have refluxed through the fallopian tubes into the peritoneal cavity and thus in the development of endometriosis. Perhaps similar changes in eutopic endometrium predispose to lesion development at the peritoneum or other ectopic sites. Few studies have examined the immunophenotype of the eutopic endometrium of women with and without endometriosis. Evaluation of the panmacrophage marker CD68 has revealed significantly increased macrophage numbers in the eutopic endometrium of women with endometriosis compared with normal controls.6,23 In these cited studies, the menstrual cycle phase was analyzed and was an important factor. A limitation of our current study is that subphases of the menstrual cycle were not differentiated based on the histologic appearance of the endometrium. Identifying correlating features between histology and menstrual subphase is an important area for further investigation. Nonetheless, our findings indicate a connection between various immune changes in eutopic endometrium and the existence of endometriosis. Furthermore, our findings support the idea that distinct immune cell populations are altered in eutopic tissue in endometriosis.
This idea has been confirmed by others using a simulated in vitro pelvic environment, in which expression of RANTES was higher in endometriotic tissue and eutopic endometrium compared with normal endometrium.57 In the cited study, RANTES induced the formation of M2 macrophages, which have an increased capacity to inhibit apoptosis of endometrial stromal cells in the tissue culture system, thereby promoting growth of endometrial debris. Perhaps changing the macrophage balance from the immunosuppressive phenotype to the proinflammatory phenotype is a potential new therapeutic strategy for endometrosis.
Endometriosis has the potential to affect research studies that are unrelated to this disease. For instance, the number of studies using aging colonies of nonhuman primates is increasing, as are associated clinical complications from long-term chronic diseases, such as endometriosis.35 The reported prevalence of endometriosis in animals that have died at national primate centers is as high as 29% when only animals older than 10 y are included.16 Endometriosis is not life-threatening in women; however, in rhesus macaques, untreated disease will result in complications associated with endometriosis lesions and masses, which contribute to the death of the animal.35 As a result, endometriosis causes 3 problems. First, the disease is difficult to diagnose in its early stages. Second, medical or surgical treatment of the disease resulting in hormonal suppression could affect the outcomes of aging studies. Third, a study subject with debilitating disease may be lost due to euthanasia. All of these complications represent potential research confounders. These areas require further research in either women or nonhuman primates.
In light of the findings of the current study, the presence of increased CD163 staining in endometrial biopsies could represent a potential diagnostic marker. Currently, the ‘gold standard’ in the diagnosis of endometriosis involves laparoscopy, biopsy, and histology.48 A noninvasive diagnostic tool that detects early endometriosis before advanced-stage disease occurs has yet to be developed but is critical for research in nonhuman primates. The identification of predictive biomarkers to exclude from aging studies research subjects that have endometriosis will be beneficial. Furthermore, the identification of sensitive and specific markers indicative of endometriosis for use in clinical medicine would be a medical breakthrough that would benefit both nonhuman primate and human populations.
The current study examined cytokines to identify trends consistent with M2 polarization. The investigation involved 23 circulating serum cytokines but failed to demonstrate any trends consistent with the published literature. In addition, trends in serum cytokines compatible with alternative macrophage activation were not identified. IL6, IL8, TNFα, and IFNγ are among the serum biomarkers that have been increased in women with endometriosis34,38,45,49 and would be consistent with M1 polarization. In contrast, higher concentrations of IL4, IL10, IL13, and TGFβ are consistent with M2 polarization, but these cytokines have either shown no difference or inconsistent differences between patients with and without endometriosis.2,5,17,47 None of these M1- or M2-associated biomarkers showed significant differences between controls and macaques with endometriosis in this current study. Perhaps this lack of difference is due to the small number of subjects, thus limiting the statistical power of the comparison. In addition, the cytokine data within the endometriosis group were highly variable; this variability might reflect factors such as severity of disease and stage of the menstrual cycle.
TGFα was the only serum cytokine that was significantly higher at the aged (15 y) time point in subjects with endometriosis. TGFα is a potent mediator of oncogenesis that contributes to tumor-induced angiogenesis; is produced in macrophages, brain cells, and keratinocytes; and functions as an epidermal growth factor.26 TGFα has been investigated as a potential biomarker in diverse cancers, including colorectal cancer9 and oropharyngeal squamous cell carcinoma19 as well as other diseases, such as hepatitis.39 The high level of TGFα in the serum of macaques with endometriosis is consistent with the pathogenesis of the disease. Although we did not examine the localization of TGFα in endometriotic lesions, there are immunohistochemical observations that indicate local production of TGFα occurs in surgically induced endometriosis in rats.52 The endometriotic implants in the cited study52 contained large numbers of macrophages and displayed high immunostaining intensity for TGFα. Macrophages synthesize and release several growth factors like TGFα that serve as mediators of cell proliferation and differentiation.26
The other noteworthy serum cytokine in our current study is sCD40L, in which a marginally significant trend toward increased levels in macaques with endometriosis compared with healthy controls occurred. sCD40L is contained in platelet granules and serves as a marker of platelet activation.10 This cytokine primarily is involved in inflammation, thrombosis, and neoangiogenesis by triggering the release of inflammatory mediators, increasing the activity of matrix metalloproteinases, and activating the coagulation cascade.10 Elevated sCD40L levels have been linked to cardiovascular diseases, especially acute coronary syndrome.50,60 Studies of patients with hypertension have found an association between sCD40L and markers of angiogenesis.46 The proangiogenic effects of sCD40L may contribute to the pathophysiology of endometriosis. sCD40L induces VEGF expression and angiogenesis.37 Although our current study and others11 have not shown elevations in circulating VEGF levels, other studies have shown elevations in VEGF concentrations in the peritoneal fluid of women with endometriosis.31,36 The importance of proangiogenesis in the pathophysiology of endometriosis is well known. However, the role of the polarized M2 macrophage as a known promoter of angiogenesis33 in endometriosis is just being elucidated.
The current study demonstrates the importance of both the local and peripheral environments in supporting the growth and maintenance of endometrial implants. However, the influences of the microenvironment may take precedence over systemic changes. The peritoneal environment must favor the implantation of endometrial cells for endometriosis to become established. Similarly, increased adhesion and angiogenesis must accompany reduced immune surveillance and clearance of endometrial cells for the growth of lesions. The identification of M2 polarization in a rhesus macaque model is an important finding. Because the onset of endometriosis in rhesus macaques closely recapitulates the pathophysiology of the disease in humans, this species likely will become an important animal model for further investigation of the role of macrophage polarization in endometriosis.
Acknowledgment
We thank Keith Mansfield for engaging in helpful scientific discussions on the role of macrophage polarization in the pathophysiology of inflammatory diseases. This work was supported by National Institute of Health funding 5P51OD011103 base grant and 5R25RR024230-03 grant.
References
- 1.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. 2008. The inflammatory microenvironment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol 66:1–9 [DOI] [PubMed] [Google Scholar]
- 2.Antsiferova YS, Sotnikova NY, Posiseeva LV, Shor AL. 2005. Changes in the T-helper cytokine profile and in lymphocyte activation at the systemic and local levels in women with endometriosis. Fertil Steril 84:1705–1711 [DOI] [PubMed] [Google Scholar]
- 3.Aron-Wisnewsky J, Tordjman J, Poitou C, Darakhshan F, Hugol D, Basdevant A, Aissat A, Guerre-Millo M, Clement K. 2009. Human adipose tissue macrophages: M1 and M2 cell-surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab 94:4619–4623 [DOI] [PubMed] [Google Scholar]
- 4.Bacci M, Capobianco A, Monno A, Cottone L, Di Puppo F, Camisa B, Mariani M, Brignole C, Ponzoni M, Ferrari S, Panina-Bordignon P, Manfredi AA, Rovere-Querini P. 2009. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am J Pathol 175:547–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedaiwy MA, Falcone T, Sharma RK, Goldberg JM, Attaran M, Nelson DR, Agarwal A. 2002. Prediction of endometriosis with serum and peritoneal fluid markers: a prospective controlled trial. Hum Reprod 17:426–431 [DOI] [PubMed] [Google Scholar]
- 6.Berbic M, Schulke L, Markham R, Tokushige N, Russell P, Fraser IS. 2009. Macrophage expression in endometrium of women with and without endometriosis. Hum Reprod 24:325–332 [DOI] [PubMed] [Google Scholar]
- 7.Cassol E, Cassetta L, Alfano M, Poli G. 2010. Macrophage polarization and HIV1 infection. J Leukoc Biol 87:599–608 [DOI] [PubMed] [Google Scholar]
- 8.Chuang PC, Wu MH, Shoji Y, Tsai SJ. 2009. Downregulation of CD36 results in reduced phagocytic ability of peritoneal macrophages of women with endometriosis. J Pathol 219:232–241 [DOI] [PubMed] [Google Scholar]
- 9.Daniel CR, Bostick RM, Flanders WD, Long Q, Fedirko V, Sidelnikov E, Seabrook ME. 2009. TGFα expression as a potential biomarker of risk within the normal-appearing colorectal mucosa of patients with and without incident sporadic adenoma. Cancer Epidemiol Biomarkers Prev 18:65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferroni P, Santilli F, Guadagni F, Basili S, Davi G. 2007. Contribution of platelet-derived CD40 ligand to inflammation, thrombosis, and neoangiogenesis. Curr Med Chem 14:2170–2180 [DOI] [PubMed] [Google Scholar]
- 11.Gagne D, Page M, Robitaille G, Hugo P, Gosselin D. 2003. Levels of vascular endothelial growth factor (VEGF) in serum of patients with endometriosis. Hum Reprod 18:1674–1680 [DOI] [PubMed] [Google Scholar]
- 12.Gazvani R, Templeton A. 2002. Peritoneal environment, cytokines, and angiogenesis in the pathophysiology of endometriosis. Reproduction 123:217–226 [DOI] [PubMed] [Google Scholar]
- 13.Giudice LC. 2010. Clinical practice. Endometriosis. N Engl J Med 362:2389–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grummer R. 2006. Animal models in endometriosis research. Hum Reprod Update 12:641–649 [DOI] [PubMed] [Google Scholar]
- 15.Haber E, Danenberg HD, Koroukhov N, Ron-El R, Golomb G, Schachter M. 2009. Peritoneal macrophage depletion by liposomal bisphosphonate attenuates endometriosis in the rat model. Hum Reprod 24:398–407 [DOI] [PubMed] [Google Scholar]
- 16.Hadfield RM, Yudkin PL, Coe CL, Scheffler J, Uno H, Barlow DH, Kemnitz JW, Kennedy SH. 1997. Risk factors for endometriosis in the rhesus monkey (Macaca mulatta): a case–control study. Hum Reprod Update 3:109–115 [DOI] [PubMed] [Google Scholar]
- 17.Hassa H, Tanir HM, Tekin B, Kirilmaz SD, Sahin Mutlu F. 2009. Cytokine and immune cell levels in peritoneal fluid and peripheral blood of women with early- and late-staged endometriosis. Arch Gynecol Obstet 279:891–895 [DOI] [PubMed] [Google Scholar]
- 18.Hummelshoj L, Prentice A, Groothuis P. 2006. Update on endometriosis. Womens Health (Lond Engl) 2:53–56 [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim SO, Lillehaug JR, Johannessen AC, Liavaag PG, Nilsen R, Vasstrand EN. 1999. Expression of biomarkers (p53, transforming growth factor α, epidermal growth factor receptor, c-erbB2/neu and the proliferative cell nuclear antigen) in oropharyngeal squamous cell carcinomas. Oral Oncol 35:302–313 [DOI] [PubMed] [Google Scholar]
- 20.Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. 1996. A novel gene, iba1, in the major histocompatibility complex class III region encoding an EF-hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun 224:855–862 [DOI] [PubMed] [Google Scholar]
- 21.Institute for Laboratory Animal Research. 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies press.
- 22.Kawamura K, Komohara Y, Takaishi K, Katabuchi H, Takeya M. 2009. Detection of M2 macrophages and colony-stimulating factor 1 expression in serous and mucinous ovarian epithelial tumors. Pathol Int 59:300–305 [DOI] [PubMed] [Google Scholar]
- 23.Khan KN, Masuzaki H, Fujishita A, Kitajima M, Sekine I, Ishimaru T. 2004. Differential macrophage infiltration in early and advanced endometriosis and adjacent peritoneum. Fertil Steril 81:652–661 [DOI] [PubMed] [Google Scholar]
- 24.Kohler C. 2007. Allograft inflammatory factor 1/ionized calcium-binding adapter molecule 1 is specifically expressed by most subpopulations of macrophages and spermatids in testis. Cell Tissue Res 330:291–302 [DOI] [PubMed] [Google Scholar]
- 25.Krysko O, Holtappels G, Zhang N, Kubica M, Deswarte K, Derycke L, Claeys S, Hammad H, Brusselle GG, Vandenabeele P, Krysko DV, Bachert C. 2011. Alternatively activated macrophages and impaired phagocytosis of S. aureus in chronic rhinosinusitis. Allergy 66:396–403 [DOI] [PubMed] [Google Scholar]
- 26.Kumar V, Bustin SA, McKay IA. 1995. Transforming growth factor α. Cell Biol Int 19:373–388 [DOI] [PubMed] [Google Scholar]
- 27.Kyama CM, Debrock S, Mwenda JM, D'Hooghe TM. 2003. Potential involvement of the immune system in the development of endometriosis. Reprod Biol Endocrinol 1:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lau SK, Chu PG, Weiss LM. 2004. CD163: a specific marker of macrophages in paraffin-embedded tissue samples. Am J Clin Pathol 122:794–801 [DOI] [PubMed] [Google Scholar]
- 29.Lawrence T, Natoli G. 2011. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol 11:750–761 [DOI] [PubMed] [Google Scholar]
- 30.Ma J, Liu L, Che G, Yu N, Dai F, You Z. 2010. The M1 form of tumor-associated macrophages in non-small-cell lung cancer is positively associated with survival time. BMC Cancer 10:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahnke JL, Dawood MY, Huang JC. 2000. Vascular endothelial growth factor and interleukin 6 in peritoneal fluid of women with endometriosis. Fertil Steril 73:166–170 [DOI] [PubMed] [Google Scholar]
- 32.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. 2004. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25:677–686 [DOI] [PubMed] [Google Scholar]
- 33.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. 2002. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23: 549–555 [DOI] [PubMed] [Google Scholar]
- 34.Martinez S, Garrido N, Coperias JL, Pardo F, Desco J, Garcia-Velasco JA, Simon C, Pellicer A. 2007. Serum interleukin-6 levels are elevated in women with minimal–mild endometriosis. Hum Reprod 22:836–842 [DOI] [PubMed] [Google Scholar]
- 35.Mattison JA, Ottinger MA, Powell D, Longo DL, Ingram DK. 2007. Endometriosis: clinical monitoring and treatment procedures in rhesus monkeys. J Med Primatol 36:391–398 [DOI] [PubMed] [Google Scholar]
- 36.McLaren J, Prentice A, Charnock-Jones DS, Smith SK. 1996. Vascular endothelial growth factor (VEGF) concentrations are elevated in peritoneal fluid of women with endometriosis. Hum Reprod 11:220–223 [DOI] [PubMed] [Google Scholar]
- 37.Melter M, Reinders ME, Sho M, Pal S, Geehan C, Denton MD, Mukhopadhyay D, Briscoe DM. 2000. Ligation of CD40 induces the expression of vascular endothelial growth factor by endothelial cells and monocytes and promotes angiogenesis in vivo. Blood 96:3801–3808 [PubMed] [Google Scholar]
- 38.Mihalyi A, Gevaert O, Kyama CM, Simsa P, Pochet N, De Smet F, De Moor B, Meuleman C, Billen J, Blanckaert N, Vodolazkaia A, Fulop V, D'Hooghe TM. 2010. Noninvasive diagnosis of endometriosis based on a combined analysis of 6 plasma biomarkers. Hum Reprod 25:654–664 [DOI] [PubMed] [Google Scholar]
- 39.Miura N, Kabashima H, Shimizu M, Sato R, Tsukamoto T, Harada T, Takahashi S, Endo R, Nakayama N, Takikawa Y, Mochida S, Suzuki K, Hasegawa J, Shiota G. 2008. Clinical impact of serum transforming growth factor α mRNA as a predictive biomarker for the prognosis of fulminant hepatitis. Hepatol Int 2:213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mosser DM, Edwards JP. 2008. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Institutes of Health. [Internet] 2002. Endometriosis. [Cited 05 December 2011]. Available at: http://www.nichd.nih.gov/publications/pubs/upload/endometriosis.pdf.
- 42.Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P. 2009. Macrophages within NSCLC tumour islets are predominantly of a cytotoxic M1 phenotype associated with extended survival. Eur Respir J 33:118–126 [DOI] [PubMed] [Google Scholar]
- 43.Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P. 2011. The tissue microlocalisation and cellular expression of CD163, VEGF, HLA-DR, iNOS, and MRP 8/14 is correlated to clinical outcome in NSCLC. PLoS ONE 6:e21874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oral E, Olive DL, Arici A. 1996. The peritoneal environment in endometriosis. Hum Reprod Update 2:385–398 [DOI] [PubMed] [Google Scholar]
- 45.Othman E D, Hornung D, Salem HT, Khalifa EA, El-Metwally TH, Al-Hendy A. 2008. Serum cytokines as biomarkers for nonsurgical prediction of endometriosis. Eur J Obstet Gynecol Reprod Biol 137:240–246 [DOI] [PubMed] [Google Scholar]
- 46.Patel JV, Lim HS, Nadar S, Tayebjee M, Hughes EA, Lip GY. 2006. Abnormal soluble CD40 ligand and C-reactive protein concentrations in hypertension: relationship to indices of angiogenesis. J Hypertens 24:117–121 [DOI] [PubMed] [Google Scholar]
- 47.Pizzo A, Salmeri FM, Ardita FV, Sofo V, Tripepi M, Marsico S. 2002. Behaviour of cytokine levels in serum and peritoneal fluid of women with endometriosis. Gynecol Obstet Invest 54:82–87 [DOI] [PubMed] [Google Scholar]
- 48.Scarselli G, Rizzello F, Cammilli F, Ginocchini L, Coccia ME. 2005. Diagnosis and treatment of endometriosis. A review. Minerva Ginecol 57:55–78 [PubMed] [Google Scholar]
- 49.Seeber B, Sammel MD, Fan X, Gerton GL, Shaunik A, Chittams J, Barnhart KT. 2008. Panel of markers can accurately predict endometriosis in a subset of patients. Fertil Steril 89:1073–1081 [DOI] [PubMed] [Google Scholar]
- 50.Setianto BY, Hartopo AB, Achadiono DN, Gharini PP. 2011. Association between levels of circulating soluble CD40 ligand on admission and in-hospital events among acute coronary syndrome patients. Acta Med Indones 43:82–87 [PubMed] [Google Scholar]
- 51.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. 2008. Macrophage polarization in tumour progression. Semin Cancer Biol 18:349–355 [DOI] [PubMed] [Google Scholar]
- 52.Simms JS, Chegini N, Williams RS, Rossi AM, Dunn WA., Jr 1991. Identification of epidermal growth factor, transforming growth factor α, and epidermal growth factor receptor in surgically induced endometriosis in rats. Obstet Gynecol 78:850–857 [PubMed] [Google Scholar]
- 53.Story L, Kennedy S. 2004. Animal studies in endometriosis: a review. ILAR J 45:132–138 [DOI] [PubMed] [Google Scholar]
- 54.Taylor RN, Lebovic DI, Mueller MD.2002. Angiogenic factors in endometriosis. Ann N Y Acad Sci 955: 89–100.
- 55.Varin A, Gordon S. 2009. Alternative activation of macrophages: immune function and cellular biology. Immunobiology 214:630–641 [DOI] [PubMed] [Google Scholar]
- 56.Varin A, Mukhopadhyay S, Herbein G, Gordon S. 2010. Alternative activation of macrophages by IL4 impairs phagocytosis of pathogens but potentiates microbial-induced signalling and cytokine secretion. Blood 115:353–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang XQ, Yu J, Luo XZ, Shi YL, Wang Y, Wang L, Li DJ. 2010. The high level of RANTES in the ectopic milieu recruits macrophages and induces their tolerance in progression of endometriosis. J Mol Endocrinol 45:291–299 [DOI] [PubMed] [Google Scholar]
- 58.Wolfe-Coote S.2005. The laboratory primate. San Diego (CA): Academic Press.
- 59.Wu MY, Ho HN. 2003. The role of cytokines in endometriosis. Am J Reprod Immunol 49:285–296 [DOI] [PubMed] [Google Scholar]
- 60.Yacoub D, Hachem A, Theoret JF, Gillis MA, Mourad W, Merhi Y. 2010. Enhanced levels of soluble CD40 ligand exacerbate platelet aggregation and thrombus formation through a CD40-dependent tumor necrosis factor receptor-associated factor 2/Rac1/p38 mitogen-activated protein kinase signaling pathway. Arterioscler Thromb Vasc Biol 30:2424–2433 [DOI] [PubMed] [Google Scholar]