Abstract
Since an epizootic and detection of clinical cases of tularemia (Francisella tularensis) in 1996 at the Oregon National Primate Research Center, only 8 cases were identified in the succeeding 13 y. However, within a period of 7 mo, primarily during Winter 2010, 6 rhesus macaques were confirmed positive for Francisella tularensis type B by the Centers for Disease Control and Prevention by culture and fluorescent antibody testing. All cases had similar gross pathologic findings, which included necrotizing splenitis and lymphadenitis. Recent colony management efforts have focused on minimizing nonhuman primate exposure to commonly observed reservoir species and controlling rodent access to corral-style housing. Strategies continue to evolve with regard to managing a large breeding colony of nonhuman primates in the presence of this threat.
Abbreviation: ONPRC, Oregon National Primate Research Center
Francisella tularensis, the causative agent of tularemia, is a small pleomorphic gram-negative coccobacillus.11 Severe disease and potentially death in humans can result from exposure to as few as 10 cfu of this highly infectious organism.7,10 The disease is also known as rabbit fever and deer-fly fever, reflecting 2 common sources of infection for humans.3 F. tularensis is classified by the United States Department of Health and Human Services as a Category A Select Agent.6 It is considered a potential agent of biologic warfare, and in fact, has been weaponized and stockpiled in the past.10 The 2 biovars that are referenced most frequently in published human and nonhuman primate literature are tularensis (type A) and holarctica (formerly paleartica; type B).4,12,13,23 An additional biovar, novicida (type C), has been described, but its virulence in humans is decreased due to its lack of a capsule.10
Tularemia is endemic to many parts of the northern hemisphere, which includes the region surrounding the Oregon National Primate Research Center (ONPRC), an AAALAC-accredited facility.20 Tularemia has one of the broadest host ranges of all bacteria, encompassing well over 200 mammalian species primarily, in addition to birds, amphibians, fish, and various arthropods such as fleas, ticks, mosquitoes, and flies.10,15,19,20 The ONPRC is located in a mixed forest and field environment which is bordered by wetlands and residential neighborhoods outside of Portland. More than 4500 nonhuman primates are housed here, and most live outdoors in breeding groups. Therefore, exposure to this potentially life-threatening and zoonotic pathogen is inevitable, due to its persistence in the environment and the close proximity of several reservoir species. Presumed reservoir species commonly observed at ONPRC include meadow voles (Microtus pennsylvanicus), brown rats (Rattus norvegicus), deer mice (genus Peromyscus), house mice (Mus musculus), and California ground squirrels (Spermophilus beecheyi). Potential arthropod vectors that are monitored regularly at ONPRC include biting flies and mosquitoes. At this time, testing of prospective rodent carriers for tularemia is ongoing; therefore, in the interest of caution, all of the rodent and arthropod species listed are considered potential carriers of the disease.
Tularemia was first recognized at the ONPRC in 1996 during an epizootic that resulted in 24 deaths among corral-housed rhesus macaques. Serology results from banked sera and sera collected during and after the outbreak demonstrated a seroconversion rate of approximately 25% in 723 animals. During the succeeding 13 y, only 8 sporadic cases were diagnosed. However, within a period of 3 mo during the winter of 2010, 5 rhesus macaques were diagnosed with Francisella tularensis type B by the Centers for Disease Control and Prevention (Atlanta, GA). Four months later, an additional case was confirmed. All 6 macaques were younger than 1 y and were assigned to a breeding colony protocol approved by the ONPRC Animal Care and Use Committee. The current report describes the clinical signs and gross and histologic findings associated with these cases, as well as methods for prevention and control of future cases of disease.
Case Report
Clinical presentation.
Of the 6 macaques, 4 presented to the colony hospital with dehydration and diarrhea; 3 of these animals also presented with clinical signs consistent with respiratory infection, including coughing, nasal discharge, tachypnea, and lethargy. The fourth hospitalized macaque was febrile and exhibited peripheral lymph node enlargement. These 4 animals responded poorly to supportive and therapeutic care for common fecal and respiratory pathogens. Euthanasia was elected, and these macaques were sedated with ketamine, deeply anesthetized with sodium pentobarbital intravenously and exsanguinated via the abdominal aorta. The remaining 2 animals were found dead, having died within a day of birth (Table 1).
Table 1.
Signalment, location, clinical signs, and pathologic findings of tularemia cases
| Monkey | Sex, age | Location | Clinical Signs | Pathologic findings |
| 1 | Male, 279 d | Corral | Dehydration | Necrotizing splenitis |
| Diarrhea | Necrotizing lymphadenitis and lymphangitis | |||
| Coughing | Pneumonia | |||
| Nasal discharge | Scrotal/preputial edema | |||
| Lethargy | Peritonitis | |||
| 2 | Male, 301 d | Corral | Dehydration | Necrotizing splenitis |
| Diarrhea | Necrotizing lymphadenitis and lymphangitis | |||
| Tachypnea | Pneumonia | |||
| Lethargy | Scrotal/preputial edema | |||
| 3 | Female, 0 d | Corral | Found dead | Necrotizing splenitis |
| 4 | Male, 7 d | Corral | Weight loss | Necrotizing splenitis |
| Dehydration | Necrotizing hepatitis | |||
| Tachypnea | Acute tubular necrosis | |||
| Lethargy | Enterocolitis | |||
| 5 | Female, 0 d | Corral | Found dead | Necrotizing splenitis |
| Necrotizing mesenteric lymphadenitis | ||||
| Necrotizing pneumonia | ||||
| Necrotizing hepatitis | ||||
| 6 | Female, 59 d | Shelter housing | Dehydration | Necrosuppurative splenitis |
| Diarrhea | Necrosuppurative submandibular and mesenteric | |||
| Hyperthermia | lymphadenitis | |||
| Cervical lymph node enlargement | Sepsis | |||
| Typhlitis |
Gross necropsy findings.
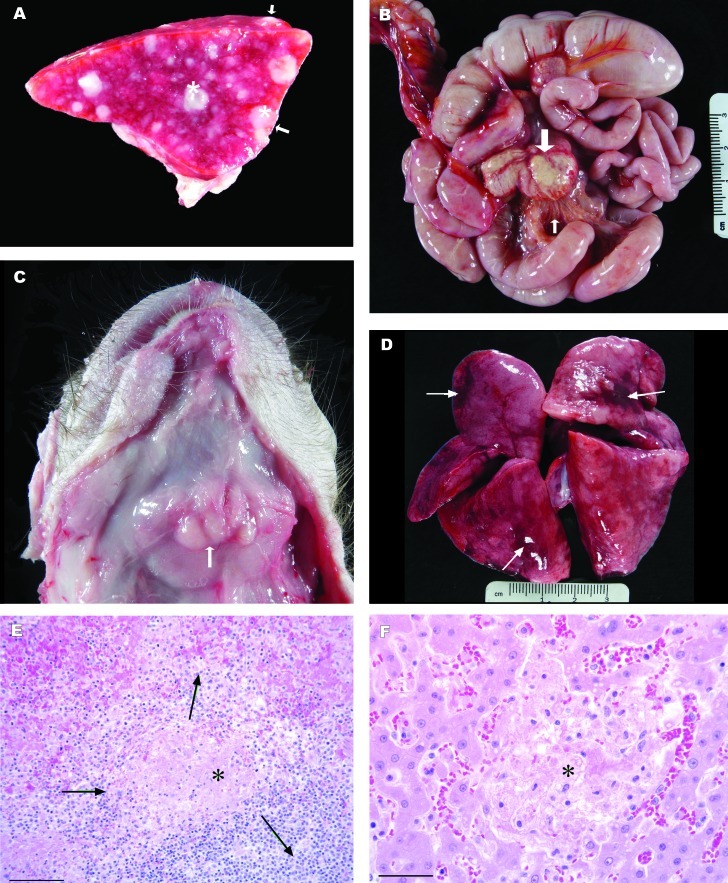
Gross pathologic findings were similar in all cases. All macaques had enlarged spleens with disseminated irregular, dry to moist, white variably sized nodules measuring as large as 0.5 cm (Figure 1 A). Lymph nodes were variably affected in 4 macaques. Lymph nodes affected included submandibular, retropharyngeal, pharyngeal, tracheobronchial, superior mesenteric, and mesenteric lymph nodes (Figure 1 B and C). The nodes were moderately to markedly enlarged and were soft, wet, and gray. On sectioned surface, many affected lymph nodes contained nodules of caseous white material or were completely effaced. In 2 macaques, the lymphatic vessels adjacent to affected mesenteric lymph nodes were prominent with pronounced nodularity (Figure 1 B). In addition, cloudy pink-tinged fluid was present in the peritoneal cavities of both these animals. The lungs in 4 macaques were mottled, pink to dark red, and slightly wet (Figure 1 D). Small numbers of white foci ranging from 0.5 to 2 mm were scattered throughout the hepatic parenchyma in 2 macaques. Additional necropsy findings noted in these 6 cases included scrotal and preputial edema (2 macaques) and enlarged pale, wet kidneys.
Figure 1.
Gross and histologic images from rhesus macaques with naturally acquired tularemia. (A) Transverse section of the spleen from a 7-d-old rhesus macaque with tularemia. The spleen is markedly enlarged with rounded margins and variably-sized, disseminated white foci that protrude above the splenic capsule (arrows). Dry to moist white necrotic nodules measuring as large as 2 mm elevate the splenic capsule and are scattered throughout the splenic parenchyma (asterisks). (B) Intestines and mesenteric lymph nodes from a 279-d-old rhesus macaque with tularemia. The lymph nodes are markedly enlarged with alteration of the normal architecture by foci of necrosis (large arrow). Lymphatics within the mesentery are prominent and yellow-white (small arrow). (C) Submandibular lymph nodes from 59-d-old female rhesus macaque with tularemia. The skin has been reflected from the throat, exposing the submandibular salivary glands. The associated lymph nodes are increased 3 to 5 times normal size (arrow). (D) Lungs (dorsal surface) from a 7-d-old rhesus macaque with tularemia. There are multifocal areas of hemorrhage, edema, and acute inflammation. Arrows indicate the most severely affected areas. (E) Photomicrograph of spleen from 7-d-old rhesus macaque with tularemia. There is lytic necrosis (asterisk) centered on the splenic white pulp (arrows). Hematoxylin and eosin stain; bar, 100 µm. (F) Photomicrograph of liver from 7-d-old rhesus macaque with tularemia. Centrally, there is sharply demarcated focus of lytic necrosis (asterisk) of hepatocytes with minimal inflammatory response. Hematoxylin and eosin stain; bar, 50 µm.
Histologic findings.
Microscopic changes in the spleen consisted of areas of necrosis with small numbers of macrophages and neutrophils, admixed with fibrin, which primarily were centered on the white pulp and extended into adjacent red pulp (Figure 1 E). Multifocal to diffuse areas of necrosis of lymphoid tissue were present in lymph nodes and gut- and airway-associated lymphoid tissue. Necrosis of lymphatic vessels with thrombosis and intravascular fibrin thrombi was present microscopically in more cases than was appreciated grossly. In the lungs, alveolar walls were diffusely thickened and infiltrated with neutrophils and smaller numbers of histiocytes. Multifocally, there were regions of septal necrosis. Alveoli contained fibrin, proteinaceous fluid, and variable numbers of neutrophils and macrophages. Foci of lytic necrosis with fibrin accumulation were scattered randomly throughout the liver (Figure 1 F). Most of the tissue samples examined demonstrated intravascular fibrin thrombi, consistent with disseminated intravascular coagulopathy.
Bacteriologic testing.
F. tularensis was isolated by bacterial culture in 3 animals from tissues including the spleen, liver, lymph nodes, and lung. Culture identification was based on characteristic colony morphology of organisms grown on cysteine heart agar. All 6 cases were positive for F. tularensis, as determined by fluorescent antibody testing using FITC-labeled rabbit antiFrancisella tularensis whole-cell antibody. The organism was classified as subspecies type B via glycerol fermentation: type A is glycerol-positive, but type B is glycerol-negative. All testing was performed at the Centers for Disease Control and Prevention's Division of Vector-Borne Diseases (Fort Collins, CO).
Discussion
The 6 clinical forms of tularemia commonly distinguished in humans include ulceroglandular, oculoglandular, oropharyngeal, gastrointestinal, pulmonary/pneumonic, and typhoidal. The first form, ulceroglandular, applies to 80% of human cases and is characterized by a papule or ulcer at the site of inoculation.10 Sudden onset of fever is common to all of the forms, and lymphadenopathy in the area of inoculation is another common feature.3 Symptoms usually appear 3 to 5 d after exposure but may take as long as 14 d to appear.5 The course of infection depends on the virulence of the infectious strain, the portal of entry, the extent of systemic involvement, and the immune status of the host.13 Although cases at the ONPRC do not strictly fall into any of the human clinical forms, the cases described here are similar to the typhoidal form, which has features of sepsis.11 However, unlike humans with this form of tularemia, macaques may show peripheral lymphadenopathy. The clinical signs of tularemia in macaques may range from asymptomatic to severe illness with sudden death. Previous testing performed at the ONPRC has revealed that most adult animals asymptomatically seroconvert, whereas clinical signs of this disease in younger animals are nonspecific and can easily resemble a respiratory tract infection or gastroenteritis.13 Given that almost all of the clinical cases observed at the ONPRC have been younger than 1 y, younger outdoor housed animals appear to be more susceptible to this disease. Although 5 of the 6 cases we describe here originated in the same corral, outdoor-housed macaques at this facility are moved periodically from corral to corral and between shelter houses for the purposes of behavior modification and repairs. In the past, cases of disease have occurred in other corrals, suggesting that risk of disease is related to a particular location's rodent activity. Thus far, there has been no evidence to suggest that it is related to familial lines. In addition, outdoor breeding groups are taken apart and redistributed every 6 to 8 y to maintain genetic heterogeneity. Whether this cluster of cases was due to waning immunity of the colony and therefore increased susceptibility to disease or to increased exposure to either the reservoir hosts or to contaminated water is unknown. Proliferation of arthropod vectors is considered unlikely, because much of this outbreak occurred during the winter months.
Antemortem definitive diagnosis is difficult in both human and veterinary settings and involves detection of an increasing antibody titer or of the organism in tissue via culture, immunofluorescent staining (direct or indirect), or PCR.14,15 Serologic diagnosis is more common than are other methods, because of the fastidious growth requirements of F. tularensis and the potential for exposure of laboratory personnel.14,22 In addition, growth is slow and may take as long as 3 wk.24 However, in an outbreak situation, serology would be less than ideal, given that serum antibodies typically do not attain diagnostic levels until at least 10 d after onset of illness.7,10 Although additional specialized diagnostic techniques exist, most are performed in research and reference laboratories.7,22 At the ONPRC, definitive diagnosis is performed at the Division of Vector-Borne Diseases by fluorescent antibody testing and culture. Treatment has been attempted in several previously reported veterinary cases (dogs, cats, and several nonhuman primate species) with variable success.1,3,4,8,14,16,17 At the ONPRC, most animals decline so rapidly that euthanasia is often elected.
Traditional methods of disease control such as vaccination are not yet a possibility. Tularemia vaccines have been used previously to protect persons at high risk for exposure, such as military and laboratory personnel.2,7 The website for the Centers for Disease Control and Prevention states that a vaccine is currently under review by the Food and Drug Administration and is not yet available in the United States for humans.5 Vaccines are not available currently for veterinary use.
Regarding the safety of personnel working directly with potentially infected nonhuman primates at the ONPRC, the Office of Environmental Health and Radiation Safety has determined that the personal protective equipment requirements currently in place to prevent the transmission of Macacine herpesvirus 1 (formerly Cercopithecine herpesvirus 1, B virus) effectively prevent transmission of tularemia as well. The community surrounding the primate center is likely to be at a similar risk of exposure as are the macaques, due to the presence of wildlife reservoir hosts and arthropod vectors in this endemic area.
In terms of prevention and control in nonhuman primate colonies, exclusion of potential wildlife reservoirs from outdoor housing areas,18 especially those that are bedded with straw or wood shavings, is paramount, although difficult when a colony is housed in a corral or field cage setting. Recent colony management efforts at ONPRC have focused mainly on controlling the rodent population surrounding the corral-style housing. One such effort to discourage rodents has been habitat modification involving eliminating weeds and bramble, keeping grass mowed, tilling soil to remove ground cover, and destroying existing burrows.18,23 Presently at the ONPRC, the corral walls are undergoing modifications that will allow for tractor access to destroy existing burrows. Correct food storage for the colony is another priority, and storage areas are evaluated regularly for cleanliness and made inaccessible to vermin.13 One report of a tularemia outbreak in a zoo setting was alleviated by removing uneaten food from the enclosure at the end of the day.4 Although this method can be challenging to implement in outdoor corrals, monitoring the amount of food offered and how much the macaques are leaving to avoid overfeeding has been used as a more feasible option. In addition, outdoor-housed nonhuman primates at ONPRC are now offered chow only on concrete pads or in food hoppers, as compared with the past practice of feeding on the ground in the respective housing areas. Consideration also is being given to housing and play structures and their potential for harboring wildlife. Housing structures, such as calf hutches, that are low to the ground and lack a solid floor, are not ideal in that they allow colony animals and wildlife to reside in close proximity, especially during winter months. Bedding, although desirable from the standpoint of promoting normal foraging behaviors, encourages an increase in wildlife presence. In contrast, geodesic domes that are placed on cement pads, similar to those seen on children's playgrounds, have been incorporated successfully, and elevated A-frame housing may be incorporated in the future. In the meantime, in housing areas such as corrals where bedding is used currently, huts are rotated out of dirty bedded areas and rebedded with new straw monthly. This practice has effectively eliminated all rodent tunnels from under the huts.
Additional strategies of vector control for established wildlife include toxicants, trapping, and other alternative methods. Toxicants generally are not an option, due to the close proximity of the nonhuman primates,23 and fumigants tend to be cost-prohibitive and ineffective due to the complexity and shallowness of vole and mole burrow systems, which allow the fumigant to escape.18 Recently though, smoke cartridges (Giant Destroyer, Atlas Chemical, Cedar Rapids, IA) have been used effectively in some burrows at the ONPRC. Trapping is performed outside of the corrals, by using unbaited snap traps (for example, Trapline Mole Traps),25 which are checked daily. This method has been very successful in controlling the mole population, but the rodent population remains a problem. Further control methods include increasing trapping, burying the fence line, and implementing bait in the traps. Another option, currently in use at the ONPRC, is a system called the Rodenator.21 This equipment uses a precise mixture of propane and oxygen, which is pumped into the burrow. The gases are then ignited, creating an underground shockwave, and the burrow is destroyed. Ideally, this process would be performed at least twice annually or as dictated by the amount of burrowing activity observed. During the past year at the ONPRC, this method of control was used every 3 mo in each corral.
In addition, means of controlling potential arthropod vectors are in place at the ONPRC. All nonanimal areas and areas not currently housing animals are sprayed regularly with biphenthrin by a state-certified exterminator. The exterminator maintains a weekly log of rodent and insect activity in corrals and buildings so that active areas can be targeted more quickly. Standing water is baited to control mosquitoes (BTI Mosquito Dunks, Summit Chemical, Baltimore, MD), and fly bait (QuickBayt, Bayer HealthCare, Animal Health Division, Shawnee Mission, KS) is used to manage the biting fly population. Fleas and ticks have not been seen on any of the outdoor housed macaques or on any of the captured wildlife reservoir species. No additional cases of tularemia have been diagnosed after implementation of these control measures. However, assessing effectiveness is difficult, given the generally sporadic nature of the disease in this colony.
As evidenced by the severity of the gross and histologic lesions in these 6 cases, tularemia can be a devastating disease for susceptible hosts. This case study highlights the need for a multifaceted approach to nonhuman primate colony management when it involves prevention and control of a highly infectious disease in an endemic region.
Acknowledgments
We thank Drs CJ Doane, Jennifer Wilk, Kristine Coleman, Lakshmanan Annamalai, and Michael Axthelm for the invaluable input that they provided in the preparation of this manuscript, and for their assistance in the development of this topic for presentation at the 38th annual Association of Primate Veterinarians Workshop.9
This publication was made possible with support from the Oregon National Primate Research Center core grant 5P51RR000163-51 and the ONPRC Nonhuman Primate Veterinary Clinical Education Program, grant 5R25RR024233-04.
References
- 1.Abril C, Nimmervoll H, Pilo P, Brodard I, Korczak B, Seiler M, Miserez R, Frey J. 2008. Rapid diagnosis and quantification of Francisella tularensis in organs of naturally infected common squirrel monkeys (Saimuri sciureus). Vet Microbiol 127:203–208 [DOI] [PubMed] [Google Scholar]
- 2.Avashia SB, Petersen JM, Lindley CM, Schriefer ME, Gage KL, Cetron M, DeMarcus TA, Kim DK, Buck J, Montenieri JA, Lowell JL, Antolin MF, Kosoy MY, Carter LG, Chu MC, Hendricks KA, Dennis DT, Kool JL. 2004. First reported prairie dog-to-human tularemia transmission, Texas 2002. Emerg Infect Dis 10:483–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckwith CS. 2006. Tularemia as a cause of fever in a squirrel monkey. J Am Vet Med Assoc 229:269–273 [DOI] [PubMed] [Google Scholar]
- 4.Calle PP, Bowerman DL, Pape WJ. 1993. Nonhuman primate tularemia (Francisella tularensis) epizootic in a zoological park. J Zoo Wildl Med 24:459–468 [Google Scholar]
- 5.Centers for Disease Control and Prevention. [Internet] 2003. Key facts about tularemia. [Cited 15 June 2011]. Available at: www.bt.cdc.gov/agent/tularemia/facts.asp.
- 6.Centers for Disease Control and Prevention National Select Agent Registry. [Internet] 2011. United States Department of Health and Human Services Select Agents and Toxins. [Cited 3 October 2011]. Available at: http://www.selectagents.gov/Select%20Agents%20and%20Toxins%20List.html.
- 7.Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Friedlander AM, Hauer J, Layton M, Lillibridge SR, McDade JE, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Tonat K. 2001. Tularemia as a biological weapon: medical and public health management. JAMA 285:2763–2773 [DOI] [PubMed] [Google Scholar]
- 8.Eng C, Orr K, Leslie M.2002. Investigation of an outbreak of tularemia among nonhuman primates in a zoological park. Am Assoc Zoo Vet Annual Conference Proceedings 2002:382–383.
- 9.Ferrecchia CE. 2010. Maintaining an outdoor colony of macaques in the presence of endemic tularemia. J Am Assoc Lab Anim Sci 50:119 [Google Scholar]
- 10.Foley JE, Nieto NC. 2010. Tularemia. Vet Microbiol 140:332–338 [DOI] [PubMed] [Google Scholar]
- 11.Geyer SJ, Burkey A, Chandler FW.1997. Tularemia. In: Connor DH, Chandler FW, Schwartz DA, Manz HJ, Lack EE. Pathology of infectious diseases. Stamford (CT): Appleton and Lange.
- 12.Keim PS, Wagner DM. 2009. Humans and evolutionary and ecologic forces shaped the phylogeography of recently emerged diseases. Nat Rev Microbiol 7:813–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matz-Rensing K, Floto A, Schrod A, Becker T, Finke E-J, Seibold E, Splettstoesser W, Kaup F-J. 2007. Epizootic of tularemia in an outdoor-housed group of cynomolgous monkeys (Macaca fascicularis). Vet Pathol 44:327–334 [DOI] [PubMed] [Google Scholar]
- 14.Meinkoth KR, Morton RJ, Meinkoth JH. 2004. Naturally occurring tularemia in a dog. J Am Vet Med Assoc 225:545–547 [DOI] [PubMed] [Google Scholar]
- 15.Morner T, Addison E.2001. Tularemia. In: Williams ES, Barker IK. Infectious diseases of wild mammals. Ames (IA): Iowa State University Press.
- 16.Nayar GP, Crawshaw G, Neufeld J. 1979. Tularemia in a group of nonhuman primates. J Am Vet Med Assoc 175:962–963 [PubMed] [Google Scholar]
- 17.Nelson M, Lever MS, Dean RE, Pearce PC, Stevens DJ, Simpson AJ. 2010. Bioavailability and efficacy of levofloxacin against Francisella tularensis in the common marmoset (Callithrix jacchus). Antimicrob Agents Chemother 54:3922–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Brien JM.1994. Voles. In: Hyngstrom SE, Timm RM, Larson GE. Prevention and control of wildlife damage. Lincoln (NE): University of Nebraska.
- 19.Petersen JM, Mead PM, Schriefer ME. 2009. Francisella tularensis: an arthropod-borne pathogen. Vet Res 40:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen JM, Molins CR. 2010. Subpopulations of Francisella tularensis ssp. tularensis and holarctica: identification and associated epidemiology. Future Microbiol 5:649–661 [DOI] [PubMed] [Google Scholar]
- 21.Rodenator. [Internet] 2011. Rodenator extermination products. [Cited 15 June 2011]. Available at: www.rodenator.com.
- 22.Splettstoesser W, Gugleilmo-Viret V, Seibold E, Thullier P. 2010. Evaluation of an immunochromatographic test for rapid and reliable serodiagnosis of human tularemia and detection of Francisella tularensis-specific antibodies in sera from different mammalian species. J Clin Microbiol 48:1629–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Splettstoesser WD, Matz-Rensing K, Seibold E, Tomaso H, Al Dahouk S, Grunow R, Essbauer S, Buckendahl A, Finke EJ, Neubauer H. 2007. Re-emergence of Francisella tularensis in Germany: fatal tularemia in a colony of semi-free-living marmosets (Callithrix jacchus). Epidemiol Infect 135:1256–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Center for Food Security & Public Health Iowa State University. [Internet] 2005. Animal disease factsheets: tularemia. [Cited 15 June 2011]. Available at: www.ivis.org/advances/Disease_Factsheets/tularemia.pdf.
- 25.Trapline Products. [Internet] 2009. Vole trapping kits. [Cited 15 June 2011]. Available at: www.traplineproducts.com.