Abstract
Interest in the use of poly(ethylene glycol)-b-polycaprolactone diblock copolymers in a targeted, magnetically triggered drug delivery system has led to this study of the phase behavior of the polycaprolactone core. Four different diblock copolymers were prepared by the ring opening polymerization of caprolactone from the alcohol terminus of poly(ethylene glycol) monomethylether, Mn ~ 2,000. The critical micelle concentration depended on the degree of polymerization for the polycaprolactone block and was in the range of 2.9 to 41 mg/L. Differential scanning calorimetry curves for polymer solutions with a concentration above the critical micelle concentration showed a melting endotherm in the range of 40 to 45°C, indicating the polycaprolactone core was semicrystalline. Pyrene was entrapped in the micelle core without interfering with the ability of the polycaprolactone to crystallize. When the polymer solution was heated above the melting point of the micelle core, the pyrene was free to leave the core. Temperature dependent measurements of the critical micelle concentration and temperature dependent dynamic light scattering showed the micelles remain intact at temperatures above the melting point of the polycaprolactone core.
Introduction
There is a vast and growing interest in the medical applications of polymer micelles containing diblock copolymers that consist of hydrophilic and hydrophobic blocks. In aqueous media, the hydrophobic block forms the core and the hydrophilic block provides a shell that disperses the micelles. Diblock and triblock copolymers containing a poly(ethylene glycol) hydrophilic block and a hydrophobic block of either poly(propylene glycol) (PEO-PPO-PEO),1 poly(L-histidine) (His-PEG),2 poly(lactic acid) (PEG-PLA),3–6 poly(D,L-lactide-co-glycolide) (PEG-PLGA),7,8 poly(β-benzyl-L-aspartate) (PEG-PBLA),9 or polycaprolactone (PEG-PCL)10–16 form micelles in aqueous solution. The micelle cores can be loaded with a hydrophobic cancer drug, such as taxol3,5 or doxorubicin.9,12 The drug can be released by a change in pH for pH-responsive poly(L-histidine)-PEG polymer micelles.2 For polymer micelles containing polyester blocks (i.e. PEG-PLA,4 PLA-PEG-PLA,5,6 PEG-PLGA,7 or PEG-PCL10–16), hydrolytic degradation of the ester block erodes the polymer core, affecting release of the drug. For a PEG-PCL diblock where the degree of polymerization for the PEG block was 113, the micelles degraded over a period of days to tens of days, depending on the degree of polymerization of the PCL block.15 For all polymer micelles once they are injected into the bloodstream at concentrations below the CMC, thermodynamics dictates that the micelles will fall apart by dissolution of the polymer chains, albeit at a slow rate. In both cases, there is a possibility for premature release of the cancer drug. This is not desired, especially since these drugs are toxic and premature release would cause undesirable side effects. This motivates our search for a means of triggering release by an external stimulus.
We envision a polymer micelle drug delivery system with cancer drugs trapped in the micelle core. Heating the core above a critical temperature would cause a transformation allowing the drug to be released. The transformation in the core may be a glass transition, or a phase change, such as melting, or phase separation. The prototype thermoresponsive polymer system is based on copolymers of N-isopropylacrylamide (NiPAAm), having a lower critical solution temperature (LCST) in aqueous solution17. This transformation involves going from a homogeneous solution in water at temperatures below the LCST to a two-phase mixture above the LCST. Diblock copolymers of NiPAAm and poly(methylmethacrylate) gave micelles that showed enhanced drug release when heated to 40°C, above the LCST18. Poly(ethylene glycol)-poly(lactic acid) diblock copolymer micelles show a glass transition for the hydrophobic poly(lactic acid) block at 60°C19 and one envisions enhanced drug release from the core when the it was heated above the Tg for the poly(lactic acid) block. We prefer thermoresponsive micelles containing a crystalline micelle core with a moderate melting point. We expect the crystalline core will provide a well-defined, sharp transition allowing release at temperatures above the melting point.
Our interest in poly(ethylene glycol-b-caprolactone) diblock copolymers arises from their potential use in a targeted, magnetically-triggered drug delivery system.20 Such a drug delivery system consist of a polymer micelle loaded with magnetic nanoparticles and anti-cancer drugs trapped in the semicrystalline polycaprolactone core. A targeting moiety, such as an RGD peptide, can be conjugated to the micelles through the terminus of the poly(ethylene glycol) block. These targeted magnetic micelles can be injected into the blood stream and localized to cancer cells through a combination of the enhanced permeation and retention (EPR) effect and the binding of RGD peptides to integrin receptors, which are heavily expressed in many kinds of cancer, including breast and prostate. An external radio frequency AC magnetic field can be used to heat the magnetic nanoparticles21 in the core of the micelles and, in turn, melt the polycaprolactone core, thereby allowing the trapped drug to be released at the cancer site. For such a drug delivery system to be realized, we must first understand the phase behavior of the polycaprolactone core and demonstrate that a small molecule trapped in the core can be released when the core is heated above its melting point. The state of the micelles when heated above the melting point of the polycaprolactone core is of particular interest. If the micelles fall apart, then the encapsulated drug will be released in one burst. If the micelles remain intact, then the drug will diffuse out of the micelle core at a rate dependent on core size and the porosity of the molten polycaprolactone core. Here we report experiments to determine structural changes of the polymer micelles when they are heated above the melting point of the polycaprolactone core.
Methods
All chemicals were reagent grade or better and were purchased from the Sigma-Aldrich Chemical Company. The chemicals were used as received, except for poly(ethylene glycol) monomethyl ether and ε-caprolactone. The ε-caprolactone was freshly distilled from calcium hydride. The poly(ethylene glycol) monomethyl ether, Mn ~ 2,000, was dried by heating to 60°C in the vacuum oven overnight. The dried polymer was stored in a desiccator over indicating Drierite.
MeO-(EG)43-(CL)19-OH
The diblock copolymers were prepared by the tin catalyzed ring-opening polymerization of ε-caprolactone using a procedure adapted from Sosnik and Cohn.22 The ratio of the number of moles of ε-caprolactone to poly(ethylene glycol) monomethylether was either 5 to 1, 10 to 1, 20 to 1, or 40 to 1. The polymerization was initiated from the alcohol terminus of poly(ethylene glycol) monomethylether. The catalyst was dibutyltin dilaurate, instead of stannous 2-ethylhexanoate. The crude polymers were first recrystallized from acetone/hexane. The filtrate was evaporated and 1H NMR showed the residue mostly contained unreacted caprolactone. The polymer was recrystallized a second time from acetone/ether. The synthesis, isolation and purification of one of the diblock copolymers are described in detail.
The procedure was the same for all block copolymers, except the mole ratio of ε-caprolactone to poly(ethylene glycol) monomethylether was varied. The glassware was dried in an oven at 120°C. A 250 mL round bottom flask was equipped with a condenser, magnetic stirring, an oil bath and a nitrogen atmosphere. To the flask was added 17.17 g of poly(ethylene glycol) monomethylether (8.6mmol), 19.59 g ε-caprolactone (172 mmol) and 0.2 mL dibutyltin dilaurate. The mixture was heated to 140°C and allowed to stir at 155 to 160°C for three hours. The reaction mixture was allowed to cool over night. The polymer was dissolved in just enough warm acetone to give a homogeneous solution. Hexane was added until the polymer just began to precipitate. The solution was placed in the freezer overnight. The next day the polymer had precipitated and was isolated by suction filtration. The polymer was rinsed with hexane and allowed to dry. Yield 28.53 g.
The polymer was recrystallized from acetone/ether. Six grams of polymer were dissolved in 9 mL hot acetone. Diethyl ether was added (~4 mL) until the point of imminent precipitation. The mixture was slightly heated to give a homogeneous solution. The solution was allowed to cool to room temperature and then placed in a freezer (−20 °C) to chill overnight. The next morning the polymer had crystallized from the solution and was isolated by suction filtration. The polymer was rinsed with cold ether and allowed in dry in vacuum at room temperature.
Characterization
Micelle solutions were prepared using Type I 18 MG pure water from a Thermo Scientific Barnstead Easypure II Ultrapure water purification system. Polymer micelles were prepared by direct dissolution in H2O for dynamic light scattering (DLS) by heating a solution of ultrapure water with the diblock copolymer up to 60 °C. The solution was allowed to cool back to room temperature and was then diluted to the desired concentration.
To prepare micelles via solvent evaporation for DSC and DLS the appropriate amount of copolymer was dissolved in THF (2 mL, filtered through a 0.2 μm filter) in a volumetric flask so that the final concentration would be 0.05mM. Ultrapure water (20 mL) was then added dropwise using an addition funnel at the rate of 1 drop every 10 seconds while stirring the solution. The solution was then allowed to sit overnight and then kept at reduced pressure for a week or until all of the THF was gone. Finally the solution was heated to 60 °C for 5 min to clear turbidity, allowed to cool, and diluted to the final concentration using ultrapure water.
Polymer micelle samples for TEM imaging were prepared by the solvent evaporation method, described above. The micelle solutions were dropped onto silicon monoxide coated copper TEM grids and allowed to dry overnight. The micelles were stained with phosphomolybdic acid and imaged using a FEI Technai F-20 transmission electron microscope. ImageJ software from the NIH was used to obtain histograms of the micelle size distributions.
Differential scanning microcalorimetry experiments were performed using a MicroCal VP-DSC microcalorimeter at a temperature range of 10 °C to 90 °C at a ramp rate of 60 °C per hour. Ultrapure water was used in the reference cell. From DSC thermograms, values of enthalpy of fusion (ΔHf) for melting the PCL cores were determined in Joules per gram of polymer in solution. The degree of crystallinity of the polycaprolactone core were determined by dividing the heat of melting determined from the endotherms of the micelles by the product of the polycaprolactone weight fraction in the sample and the heat of fusion for pure polycaprolactone (139.5 J/g).23 Dynamic light scattering data was collected with a Malvern Zetasizer Nano ZS at a scattering angle of 173°. All measurements were taken at 25°C and were performed in triplicate with an average of 18 runs per measurement. Data were analyzed using Malvern software. Temperature dependent DLS data was obtained from 20 to 60 °C with a heating rate of 1 °C per minute.
Fluorescence measurements were made using a Varian Cary Eclipse fluorescence spectrometer with a water-thermostatted cell holder for variable temperature measurements. To determine CMCs, pyrene, a hydrophobic probe that resides in the core of the micelle,24 was added at 0.010 mM to an aqueous solution of 2 mg/mL MeO-PEG36-PCL16-OH (0.59 mM). The critical micelle concentration was determined by measuring the fluorescence of pyrene in aqueous solutions containing the diblock copolymers at 25°C. Pyrene (61.0 mg, 0.302 mmol) was diluted to 100 mL with acetone to make a 3.02 mM solution. One milliliter of the acetone solution was placed in a 500 mL volumetric flask and the flask was filled with Type 1 water to give a 6.04 × 10−6 M stock solution of pyrene in water after evaporation of the acetone. The aqueous pyrene solution was used to prepare a series of solutions containing different concentrations of the diblock copolymers. The fluorescence intensity at an emission wavelength of 390 nm was measured for excitation wavelengths of 333 nm (I333) and 338 nm (I338). The ratio of I338/I333 was plotted as a function of polymer concentration. For each polymer solution the temperature was ramped from 10 to 70° C at a rate of 10°C/min. Measurements of I338 and I333were made at one degree intervals. This provided data for determining the temperature dependence of the CMC.
The capability of the micelles to release a drug when heated was demonstrated using pyrene (0.010 mM), a fluorescent probe, which was added to an aqueous solution of MeO-PEG36-PCL16-OH (0.59 mM) and allowed to equilibrate overnight. The fluorescence intensity at 373 nm was measured using 330 nm excitation during a 10°C/min ramp. This gave a plot of the fluorescence intensity at 373 nm as a function of temperature.
Results and Discussion
The degree of polymerization for the diblock copolymers was determined by 1H NMR, Table 1. The degree of polymerization for the poly(ethylene glycol) block was determined by integrating the terminal methyl peak at 3.33 ppm (3 protons) and the methylene peak at 3.64 ppm. As purchased from Aldrich, the PEG oligomer was specified to have Mn ~ 2,000, with a degree of polymerization of ~45. For the diblock copolymer the presence of a triplet at 4.22 ppm (2 protons) arising from the terminal methylene from the poly(ethylene glycol) block as it links to the ester group in the polycaprolactaone block confirmed the formation of the diblock. The degree of polymerization for the polycaprolactone block was determined from the integration of the triplet at 2.30 ppm for the methylene peak at next to the carbonyl. Since the caprolactone polymerization was incomplete (as indicated by the presence of some unreacted caprolactone), the average degree of polymerization for the polycaprolactone blocks were less than the target, i.e. n = 5, 10, 20, 40.
Table 1.
Diblock Copolymers, MeO-EGm-CLn-OH, where m is the degree of polymerization for the poly(ethylene glycol) block and n is the degree of polymerization for the polycaprolactone block. The CMC is reported at a temperature of 25 °C. The hydrodynamic diameter was determined by dynamic light scattering and is reported for measurements at 25 °C. The number average diameter is reported.
| Polymer | m | n | Mn | CMC (mg/L) | Dh (nm) |
|---|---|---|---|---|---|
| MeO-EG44-CL3-OH | 44 | 3 | 2,350 | 41 | 106, 459 |
| MeO-EG43-CL9-OH | 43 | 9 | 2,980 | 3.9 | 59 |
| MeO-EG42-CL19-OH | 42 | 19 | 4,230 | 4.0 | 122 |
| MeO-EG53-CL49-OH | 53 | 49 | 7,970 | 2.9 | 26 |
Critical micelle concentrations (CMC) were determined from measurements of the fluorescence intensity of pyrene as a function of polymer concentration, Fig. 1. The fluorescence intensity was measured at an emission wavelength of 390 nm for excitation wavelengths of 333 nm (I333) and 338 nm (I338). The ratios of I338/I333 were plotted as a function of polymer concentration. At low concentration, below the CMC, the ratio was less than 1, while at concentrations above the CMC the ratio was greater than 2. As expected, the value of the CMC decreased when the length of the polycaprolactone block increased, Table 1. The values obtained at 25 °C were in agreement with published values for other poly(ethylene glycol)-b-polycaprolactone diblock copolymers.14,25 Dynamic light scattering for polymer solutions having concentrations well above the CMC showed that the polymers formed micelles. The values of average hydrodynamic diameter are reported in Table 1.
Figure 1.
Plot of the pyrene fluorescence intensity ratio as a function of concentration for MeO-EG42-CL19-OH.
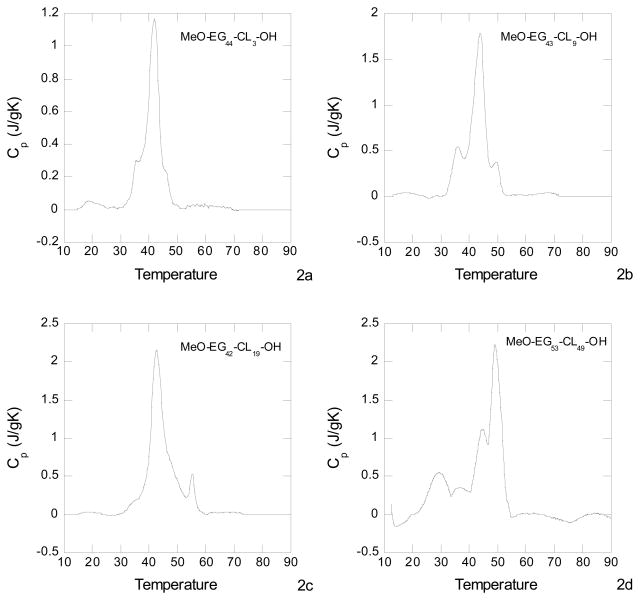
The diblock copolymers were dissolved in water at a concentration of 2 mg/mL, well above the CMC. The DSC curves in Fig. 2 show melting endotherms for all four polymers in the temperature range of 40 to 45 °C. This gives strong evidence that polycaprolactone blocks in the core of the micelles were semicrystalline. Melting endotherms were also reported for liposomes made from poly(ethylene glycol)-b-polycaprolactone, where the average molecular weight of the poly(ethylene glycol) block was 2,000 and Mn for the polycaprolactone block was 12,000.26 For the polymers reported here the degree of crystallization decreased with increasing polycaprolactone content. This decrease in the degree of crystallization and consequent increase is the amount of amorphous phase for the higher polymers, provides the promise of increased capacity for cancer drugs. This assumes that the cancer drugs are trapped in the amorphous regions of the polycaprolactone, an assumption to be examined in future work.
Figure 2.
DSC curves for the diblock copolymers in water at concentrations above the CMC, MeO-EG44-CL3-OH (Fig. 3A), MeO-EG43-CL9-OH (Fig. 3B) and MeO-EG42-CL19-OH (Fig. 3C) and MeO-EG53-CL49-OH (Fig. 3d). The temperature is in degrees centigrade.
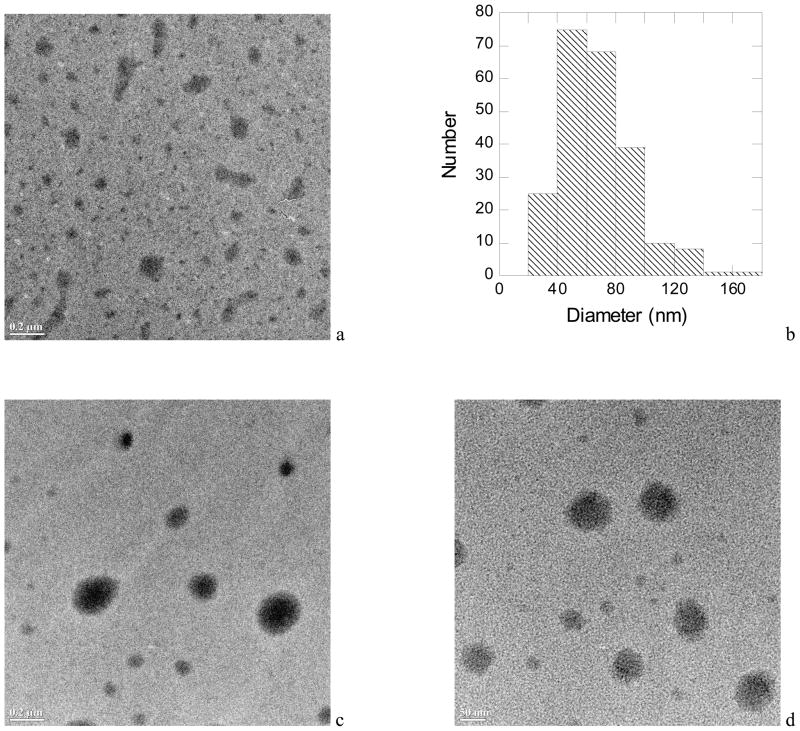
The TEM image in Fig 3a shows the micelles for the diblock MeO-EG42-CL19-OH. The micelles were spherical with a rather large distribution of diameters. There are some nonspherical structures that are attributed to a coalescing of the micelles when the sample was dried. The histogram in Fig 3b represents the size distribution of 227 micelles taken from seven different TEM images. The average diameter was 67 ± 25 nm. Dynamic light scattering gave an average hydrodynamic diameter of 122 nm, which is much larger than the sizes observed by TEM. It is expected that the DLS determination of the hydrodynamic diameter includes the poly(ethylene glycol) corona in its hydrated state around the crystalline polycaprolactone core. The TEM images in Fig. 3c and 3d show that the micelles made from MeO-EG44-CL3-OH and MeO-EG53-CL49-OH were also spherical with a broad distribution of micelle diameters. The literature suggests that when the polycaprolactone core crystallizes, the micelles may assume shapes other than spherical, depending on the relative length of the coronal block and the core block.27 For a series of diblock copolymers containing a poly(ethylene glycol) block with Mn ~ 2,000 (n ~ 44), when the degree of polymerization for the polycaprolactone block was 40, or less, the micelles had a spherical structure.28,29 For diblocks having a higher polycaprolactone to poly(ethylene glycol) ratio, other structures, including worm-like micelles and vesicles were observed.
Figure 3.
In Fig. 3a is a TEM image of polymer micelles made from MeO-EG42-CL19-OH (scale bar 200 nm) and histogram of micelle diameters (Fig. 3b). The number of micelles measured to determine the histogram was 227. In Fig. 3c is a TEM image of polymer micelles made from MeO-EG44-CL3-OH (scale bar 200 nm) and in Fig. 3d is a TEM image of polymer micelles made from MeO-EG53-CL49-OH (scale bar 50 nm).
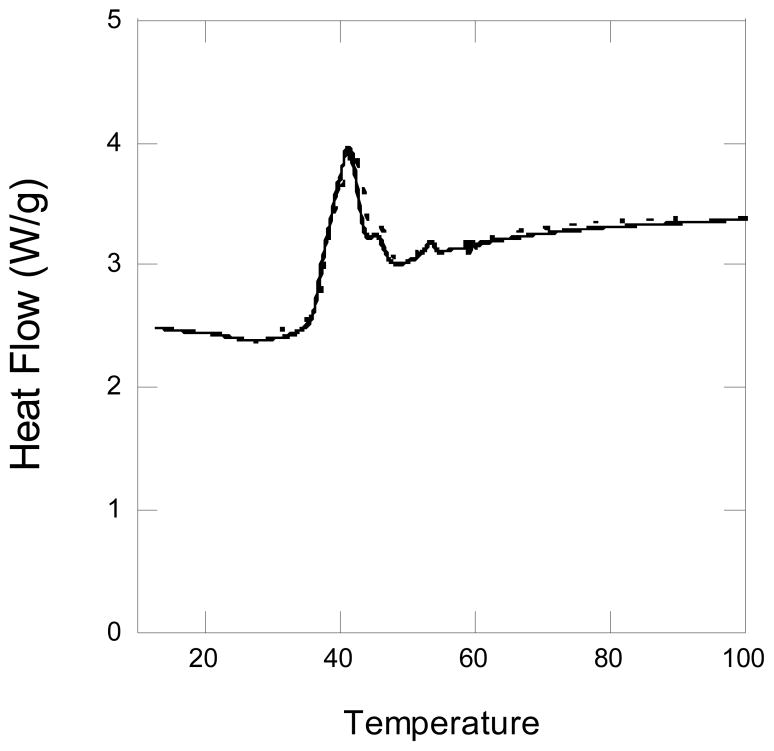
Pyrene-loaded micelles were also investigated by microcalorimetry. The dashed DSC curve in Fig. 4 shows the same sharp melting endotherm seen in the DSC curve for the micelles in the absence of pyrene, solid curve. Indeed, the curves were almost superimposed. Clearly, the pyrene did not interfere with the ability of the polycaprolactone core to crystallize. This leads us to expect that a cancer drug would also not interfere with the ability of the polycaprolactone core to crystallize. However, this may not be the case with high drug loading and experiments with higher loading will be the object of future work.
Figure 4.
DSC curve for a 0.59 mM aqueous solution of MeO-PEG42-PCL19-OH, solid curve. The dashed curve is the DSC for the 0.59 mM MeO-PEG42-PCL19-OH solution containing 10 μM pyrene. The temperature is in degrees centigrade.
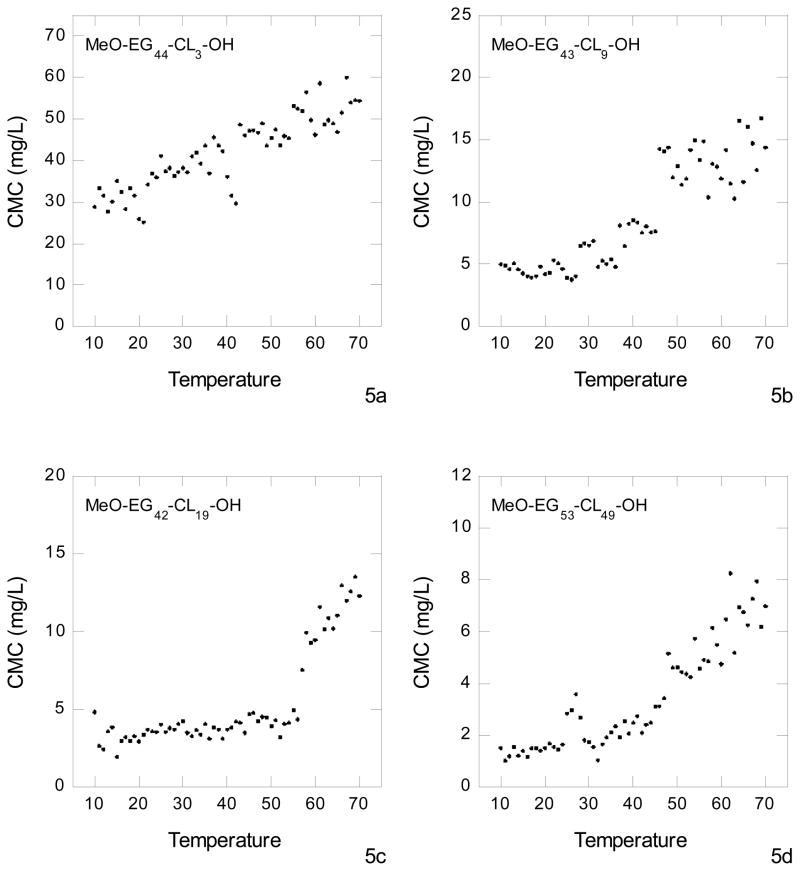
When the micelles are heated above the melting point of the polycaprolactone, the crystals melt, giving micelles with viscous, liquid cores. This would allow the contents (such as a drug) entrapped in the micelle to slowly diffuse out of the core, thereby releasing at a rate dependent on void space within the micelle core and the concentration gradient between the micelle and the surrounding fluid. Alternatively, the micelles may fall apart thereby releasing the contents in one burst. There was a concern that this would be the case for the diblock MeO-EG43-CL3-OH, having a small polycaprolactone block. To gain insight into the events that occur when the cores of the micelles are heated above their melting point, data were collected for the temperature dependence of the value of fluorescence intensity for pyrene in the micelles as well as the temperature dependence of micelle size as determined by dynamic light scattering. Data for the intensity ratio (I338/I333) as a function of temperature were used to determine the temperature dependence of the critical micelle concentration, Figure 5. At any temperature there is an equilibrium between the polymer molecules bound in the micelles and individual polymer molecules dissolved in the continuous aqueous medium. As the temperature increases, entropy favors movement of the polymer molecules from the micelles into the aqueous phase. Accordingly, it is expected that the CMC will increase with increasing temperature. Indeed for all four diblock copolymers the CMC slightly increased in the temperature range below the melting point of the polycaprolactone core. For the case of MeO-EG43-CL3-OH (Fig. 5a), having the lowest polycaprolactone content, this gradual increase in CMC with increasing temperature continued throughout the entire temperature range (10 to 70 °C), even at temperatures above the melting point of the polycaprolactone core. For MeO-EG43-CL9-OH (Fig. 5b), MeO-EG42-CL19-OH (Fig. 5c), and MeO-EG53-CL49-OH (Fig. 5d), there was a significant jump in the value of the CMC as the temperature increased through the range where the polycaprolactone core melted, Fig. 2. At temperatures above the melting point of the core, the CMC continued to increase with increasing temperature. The observation of well-defined CMC’s at temperatures above the melting point of the polycaprolactone core indicates that the micelles remain intact when the core is melted. This was also the case for diblock copolymers containing poly(ethylene glycol) and poly(L-lactic acid)30. There was little variation in the value of critical micelle concentration in the temperature range from 5 to 35°C. The critical micelle concentration increased greatly as the temperature was increased above 35°C, indicating an increase in the mobility of the poly(L-lactic acid) blocks.
Figure 5.
Temperature dependence of the CMC for the diblock copolymers,MeO-EG44-CL3-OH (Fig. 5A), MeO-EG43-CL9-OH (Fig. 5B) and MeO-EG42-CL19-OH (Fig. 5C) and MeO-EG52-CL49-OH (Fig. 5d). The temperature is in degrees centigrade.
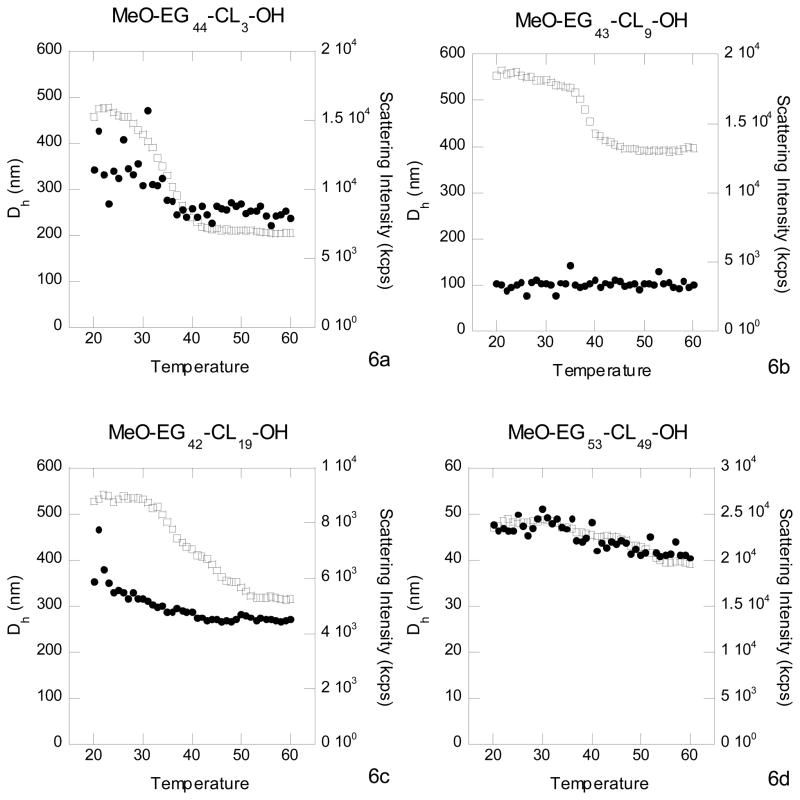
Further insight into the events that occur when the micelles are heated above the melting point of the polycaprolactone core comes from observing the temperature dependence of the dynamic light scattering. In Fig. 6 are double plots of the temperature dependence of the hydrodynamic diameter and the scattering intensity. The micelle diameter decreased slightly with increasing temperature, but did not go to zero when heated above the melting point of the core. The micelles remained intact when heated above the melting point of the polycaprolactone core. The derived count rate is related to the scattering intensity and, as the temperature was increased to above the melting point of the micelle core, the count rate decreased dramatically for MeO-PEG44-PCL3-OH, MeO-PEG43-PCL9-OH and MeO-PEG42-PCL19-OH. We attribute this decrease to the melting of the polycaprolactone core and speculate that the melted micelle had a refractive index closer matched to the continuous aqueous medium. The scattering was due to reflection of photons off the interface between the surface of the micelle and the continuous aqueous phase. According to the Fresnel relation for reflection, the reflectivity depends on the difference in refractive index between the two media. The closer the two refractive indices, the lower the reflectivity, hence the lower the scattering intensity.31 For the case of MeO-EG52-CL49-OH, the polymer having the lowest degree of crystallization, the change in the micelle size and in the scattering intensity were relatively small.
Figure 6.
The temperature dependence of the dynamic light scattering for MeO-EG44-CL3-OH (Fig 6a), MeO-EG43-CL9-OH (Fig 6b), MeO-EG43-CL19-OH (Fig 6c) and MeO-EG53-CL49-OH (Fig 6d). Plot of the hydrodynamic diameter (closed circles) and derived count rate (open squares) as a function of temperature. The temperature is in degrees centigrade.
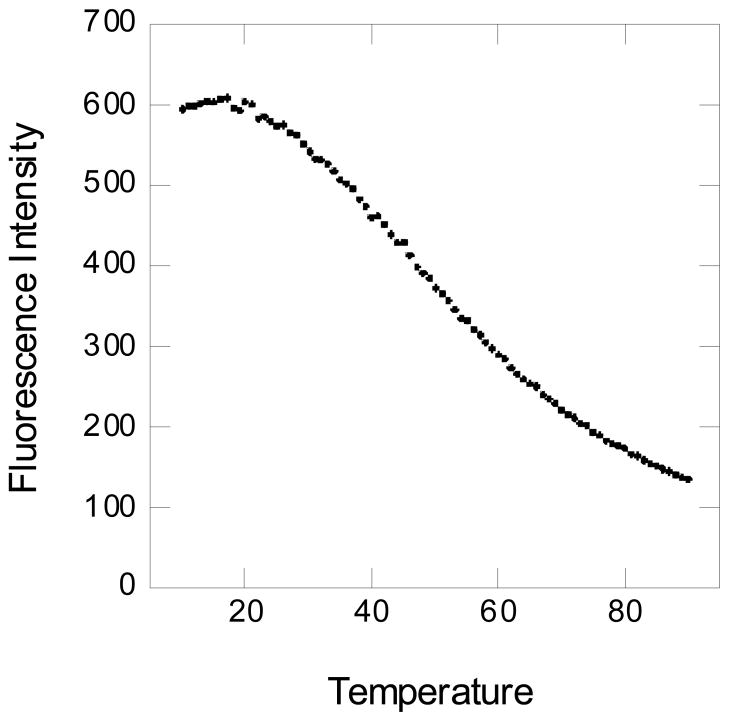
The capability of the micelles to release a drug when heated was demonstrated using pyrene (0.010 mM), a fluorescent probe, which was added to an aqueous solution of MeO-PEG42-PCL19-OH (0.59 mM). The fluorescence spectrum was measured during a 10°C/min ramp, with the fluorescence intensity at 373 nm shown as a function of temperature in Fig. 7. In hydrophobic environments (e.g., inside the micelle), pyrene has a high fluorescence intensity, while in hydrophilic environments the fluorescence intensity decreases.32 In the diblock copolymer the pyrene is largely in the micelle core, but as the temperature is increased above the melting point of the micelle core, the fluorescence intensity decreased, indicating that the pyrene has moved to a hydrophilic environment, the aqueous medium.
Figure 7.
Fluorescence emission intensity at 373 nm as a function of temperature for 10 μM pyrene in the presence of aqueous 0.59 mM MeO-PEG42-PCL19-OH micelles. The temperature is in degrees centigrade.
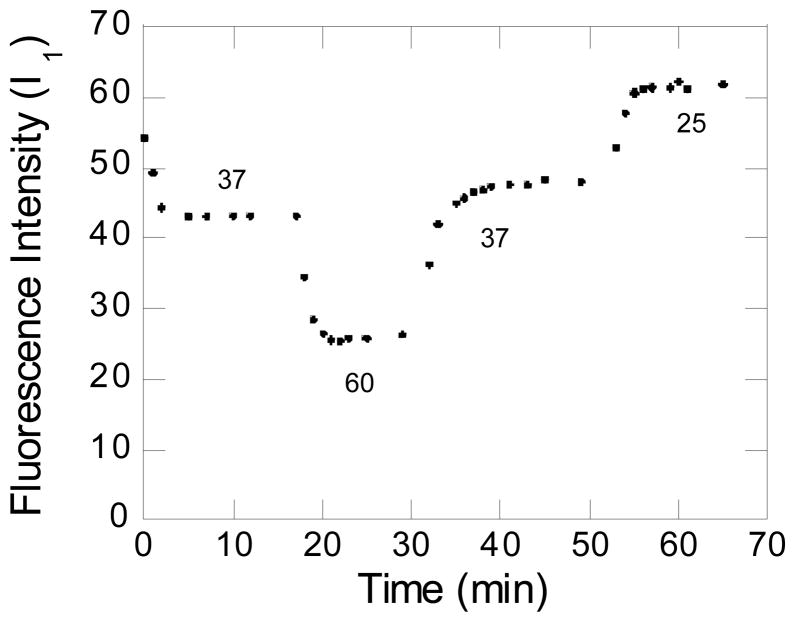
In order to determine the dynamics and reversibility of pyrene release, the fluorescence emission at 373 nm (I1, using an excitation wavelength of 340 nm) was plotted as a function of time (Fig. 8) while the temperature was varied. The polymer concentration was 0.576 mM (well above the CMC) and the pyrene concentration was 0.550 μM. Beginning at a temperature of 25°C, the temperature was raised to 37°C, whereupon I1 decreased rapidly. After 17 minutes, the temperature was further increased to 60°C and I1 further decreased in an exponential fashion. The temperature was then decreased back to 37°C and finally to 25°C, when I1 increased. At any temperature there is a dynamic equilibrium between pyrene bound in the micelle core and pyrene dissolved in the continuous aqueous phase. When the temperature was raised, the value of I1 decreased, indicating that pyrene was leaving the micelle core and entering the aqueous phase. However, when the temperature decreased the value of I1 increased, indicating that pyrene was returning to the micelle core. The time scales for these processes (leaving the micelle core and returning to the micelle core) were on the order of 1 to 3 minutes. Although these time scales included the time required by the instrument to reach the new temperature, these results indicate the potential for fast release. Furthermore, this suggests the possibility of temporal control of the release. Heating above the melting point of the micelle core allows release. Discontinuing heating, thereby allowing the core to recrystallize would stop the release. Further heating and cooling cycles would allow more pulses of release.
Figure 8.
Response of a solution containing 0.533 mM MeO-(EG)42-(CL)19-OH and 0.550 μM pyrene to changes in temperature. Plot of the pyrene fluorescence emission intensity (I1) as a function of time while the temperature was raised from 25°C to 37°C, then to 60°C. The temperature was lowered from 60°C to 37°C and finally to 25°C.
Conclusions
Polymeric micelles made from poly(ethylene glycol-b-caprolactone) diblock copolymers have crystalline cores with melting endotherms in the range of 40 to 45°C. Pyrene, a fluorescent probe and surrogate for a cancer drug, can be incorporated into the core without interfering with the ability of the polycaprolactone core to crystallize. The micelles remain intact upon heating to temperatures above the melting point of the polymer core. When heated above the melting point of the core, pyrene was released from the core with a time frame for release on the order of seconds. These observations indicate that a drug delivery vehicle can be built around these diblock copolymers. Incorporating a magnetite nanoparticle into the polycaprolactone core may allow for magnetic triggering of drug release. Application of a radio frequency AC magnetic field will heat the particles by magnetic induction,33 thereby heating the polycaprolactone core above its melting point, allowing the cancer drug to be released.
Table 2.
Melting points and enthalpy of melting for the polycaprolactone cores in the diblock copolymer micelles
| Polymer | Concentration (mg/L) | Tm (°C) | ΔHf (J/g) | Degree of crystallinity |
|---|---|---|---|---|
| MeO-EG44-CL3-OH | 208 | 42 | 7.1 | 45% |
| MeO-EG43-CL9-OH | 410 | 44 | 12.7 | 28% |
| MeO-EG42-CL19-OH | 242 | 43 | 16.3 | 21% |
| MeO-EG53-CL49-OH | 590 | 45 | 14.3 | 14% |
Acknowledgments
This project was supported by the National Cancer Institute through NIH Grant R21CA141388. Yuping Bao of the Department of Chemical & Biological Engineering at the University of Alabama allowed us to use her dynamic light scattering equipment. David E. Graves of the Department of Chemistry at the University of Alabama at Birmingham kindly allowed us to use his microcalorimeter. This work used the instruments in the Central Analytical Facility, which is supported by The University of Alabama.
References
- 1.Almgren M, Bahadur P, Jansson M, Li P, Brown W, Bahadur A. Static and Dynamic Properties of a (PEO-PPO-PEO) Block Copolymer in Aqueous Solution. J Coll Interfac Sci. 1992;151:157–165. [Google Scholar]
- 2.Lee ES, Shin HJ, Na K, Bae YH. Poly(L-histidine)–PEG Block Copolymer Micelles and pH-induced Destabilization. J Control Rel. 2003;90:363–374. doi: 10.1016/s0168-3659(03)00205-0. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Jackson JK, Burt HM. Development of Amphiphilic Diblock Copolymers as Micellar Carriers of Taxol. Int J Pharm. 1996;132:195–206. [Google Scholar]
- 4.Chen W, Luo W, Wang S, Bei J. Synthesis and Properties of Poly(L-lactide)-Poly(Ethylene glycol) Multiblock Copolymers by Coupling Triblock Copolymers. Polym Adv Technol. 2003;14:245–253. [Google Scholar]
- 5.Venkatraman SS, Jie P, Min F, Freddy BYC, Leong-Huat G. Micelle-like Nanoparticles of PLA–PEG–PLA Triblock Copolymer as Chemotherapeutic Carrier. Int J Pharm. 2005;298:219–232. doi: 10.1016/j.ijpharm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal SK, Sanabria-DeLong N, Coburn JM, Tew GN, Bhatia SR. Novel Drug Release Profiles From Micellar Solutions of PLA–PEO–PLA Triblock Copolymers. J Control Rel. 2006;112:64–71. doi: 10.1016/j.jconrel.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Lee CH, Park J, Seo S, Lim EK, Song YS, Suh JS, Yoon HG, Huh YM, Haam S. Antibody Conjugated Magnetic PLGA Nanoparticles for Diagnosis and Treatment of Breast Cancer. J Mater Chem. 2007;17:2695–2699. [Google Scholar]
- 8.Bae Y, Diezi TA, Zhao A, Kwon GS. Mixed Polymeric Micelles for Combination Cancer Chemotherapy Through the Concurrent Delivery of Multiple Chemotherapeutic Agents. J Control Rel. 2007;122:324–330. doi: 10.1016/j.jconrel.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 9.Kataoka K, Matsumoto T, Yokoyama M, Okano T, Sakurai Y, Fukushima S, Okamoto K, Kwon GS. Doxorubicin-loaded Poly(ethylene glycol)–Poly(β-benzyl-L-aspartate) Copolymer Micelles: Their Pharmaceutical Characteristics and Biological Significance. J Control Rel. 2000;64:143–153. doi: 10.1016/s0168-3659(99)00133-9. [DOI] [PubMed] [Google Scholar]
- 10.Allen C, Han J, Yu Y, Maysinger D, Eisenberg A. Polycaprolactone–b-poly(ethylene oxide) Copolymer Micelles as a Delivery Vehicle for Dihydrotestosterone. J Control Rel. 2000;63:275–286. doi: 10.1016/s0168-3659(99)00200-x. [DOI] [PubMed] [Google Scholar]
- 11.Soo PL, Luo L, Maysinger D, Eisenberg A. Incorporation and Release of Hydrophobic Probes in Biocompatible Polycaprolactone-block-poly(ethylene oxide) Micelles: Implications for Drug Delivery. Langmuir. 2002;18:9996–10004. [Google Scholar]
- 12.Shuai X, Ai H, Nasongkla N, Kim S, Gao J. Micellar Carriers Based on Block Copolymers of Poly(ε-caprolactone) Poly(ethylene glycol) for Doxorubicin Delivery. J Control Rel. 2004;98:415–426. doi: 10.1016/j.jconrel.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Soo PL, Lovric J, Davidson P, Maysinger D, Eisenberg A. Polycaprolactone-block-poly(ethylene oxide) Micelles: A Nanodelivery System for 17β-Estradiol. Molecular Pharm. 2005;2:519–527. doi: 10.1021/mp050049h. [DOI] [PubMed] [Google Scholar]
- 14.Xie W, Zhu W, Shen Z. Synthesis, Isothermal Crystallization and Micellization of mPEG–PCL Diblock Copolymers Catalyzed by Yttrium Complex. Polymer. 2007;48:6791–6798. [Google Scholar]
- 15.Chausson M, Fluchére AS, Landreau E, Aguni Y, Chevalier Y, Hamaide T, Abdul-Malak N, Bonnet I. Block Copolymers of the Type Poly(caprolactone)-b-poly(ethylene oxide) for the Preparation and Stabilization of Nanoemulsions. Int J Pharm. 2008;362:153–162. doi: 10.1016/j.ijpharm.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Cho HK, Lone S, Kim DD, Choi JH, Choi SW, Cho JH, Kim JH, Cheong IW. Synthesis and Characterization of Fluorescein Isothiocyanate (FITC)-labeled PEO–PCL–PEO Triblock Copolymers for Topical Delivery. Polymer. 2009;50:2357–2364. [Google Scholar]
- 17.Ward MA, Georgiou TK. Thermoresponsive Polymers for Biomedical Applications. Polymers. 2011;3:1215–1242. [Google Scholar]
- 18.Akimoto J, Nakayama M, Sakai K, Okano T. Thermally Controlled Intracellular Uptake System of Polymeric Micelles Possessing Poly(N-isopropylacrylamide)-based Outer Coronas. Mol Pharm. 2010;7:926–935. doi: 10.1021/mp100021c. [DOI] [PubMed] [Google Scholar]
- 19.Heald CR, Stolnik S, Kujawinski KS, De Matteis C, Garnett MC, Illum L, David SS, Purkiss SC, Barlow RJ, Gellert PR. Poly(lactic acid)-Poly(ethylene oxide) (PLA-PEG) Nanoparticles: NMR Studies of the Central Solidlike PLA Core and the Liquid PEG Corona. Langmuir. 2002;18:3669–3675. [Google Scholar]
- 20.Brazel CS. Magnetothermally-responsive Nanomaterials: Combining Magnetic Nanostructures and Thermally-Sensitive Polymers for Triggered Drug Release. Pharm Res. 2009;26:644–656. doi: 10.1007/s11095-008-9773-2. [DOI] [PubMed] [Google Scholar]
- 21.Kim DH, Nikles DE, Johnson DT, Brazel CS. Heat Generation of Aqueously Dispersed CoFe2O4 Nanoparticles as Heating Agents for Magnetically Activated Drug Delivery and Hyperthermia. J Magn Magn Matls. 2008;320:2390–2396. [Google Scholar]
- 22.Sosnik A, Cohn D. Poly(ethylene glycol)-poly(epsilon-caprolactone) Block Oligomers as Injectable Materials. Polymer. 2003;44:7033–7042. [Google Scholar]
- 23.Estelles JM, Vidaurre A, Duenas JMM, Corazar IC. Physical Characterization of Polycaprolactone Scaffolds. J Mater Sci: Mater Med. 2008;19:189–195. doi: 10.1007/s10856-006-0101-2. [DOI] [PubMed] [Google Scholar]
- 24.Kalyanasundaram K, Thomas JK. Environmental Effects on Vibronic Band Intensities in Pyrene Monomer Fluorescence and Their Application in Studies of Micellar Systems. J Am Chem Soc. 1977;99:2039–2044. [Google Scholar]
- 25.Lee RL, Hung CB. Synthesis and Characterization of Amphiphilic Block Copolymers From Poly(ethylene glycol)methyl Ether and 4-methyl-ε-caprolactone or 4-phenyl-ε-caprolactone. Polymer. 2007;48:2605–2612. [Google Scholar]
- 26.Ghoroghchian PP, Li G, Levine DH, Davis KP, Bates FS, Hammer DA, Therien MJ. Bioresorbable Vesicles Formed Through Spontaneous Self-Assembly of Amphiphilic Poly(ethylene oxide)-block-polycaprolactone. Macromol. 2006;39:1673–1675. doi: 10.1021/ma0519009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian J, Zhang M, Manners I, Winnik MA. Nanofiber Micelles from the Self-assembly of Block Copolymers. Trends Biotech. 2009;28:84–92. doi: 10.1016/j.tibtech.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Rajagopal K, Mahmud A, Christian DA, Pajerowski JD, Brown AEX, Loverde SM, Discher DE. Curvature-Coupled Hydration of Semicrystalline Polymer Amphiphiles Yields flexible Worm Micelles but Favors Rigid Vesicles: Polycaprolactone-Based Block Copolymers Macromolecules. 2010;43:9736–9746. doi: 10.1021/ma101316w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du ZX, Xu JT, Fan ZQ. Micellar Morphologies of Poly(ε-caprolactone)-b-poly(ethylene oxide) Block Copolymers in Water with a Crystalline Core. Macromolecules. 2007;40:7633–7637. [Google Scholar]
- 30.Jongpaiboonkit L, Zhou Z, Ni X, Wang YZ, Li J. Self-association and Micelle Formation of Biodegradable Poly(ethylene glycol)-poly(L-lactic acid) amphiphilic Di-block copolymers. J Biomater Sci Polym Edn. 2006;17:747–763. doi: 10.1163/156856206777656553. [DOI] [PubMed] [Google Scholar]
- 31.Born M, Wolf E. Principles of Optics. 6. Pergamon Press; Oxford, U.K: 1980. [Google Scholar]
- 32.Mast RC, Haynes KV. The Use of the Fluorescent probes Perylene and Magnesium 8-anilinonaphthalene-1-sulfonate to Determine the Critical Micelle Concentration of Surfactants in Aqueous Solution. J Coll Interfac Sci. 1975;53:35–41. [Google Scholar]
- 33.Cullity BD. Introduction to Magnetic Materials. Addision-Wesley Publishing Company; Reading, Massachusetts, USA: 1972. [Google Scholar]