Abstract
Recent insights into sleep apnoea pathogenesis reveal that a low respiratory arousal threshold (awaken easily) is important for many patients. As most patients experience stable breathing periods mediated by upper-airway dilator muscle activation via accumulation of respiratory stimuli, premature awakening may prevent respiratory stimuli build up as well as the resulting stabilization of sleep and breathing. The aim of the present physiological study was to determine the effects of a non-benzodiazepine sedative, eszopiclone, on the arousal threshold and the AHI (apnoea/hypopnoea index) in obstructive sleep apnoea patients. We hypothesized that eszopiclone would increase the arousal threshold and lower the AHI in patients with a low arousal threshold (0 to −15 cmH2O). Following a baseline overnight polysomnogram with an epiglottic pressure catheter to quantify the arousal threshold, 17 obstructive sleep apnoea patients, without major hypoxaemia [nadir SaO2 (arterial blood oxygen saturation) >70%], returned on two additional nights and received 3 mg of eszopiclone or placebo immediately prior to each study. Compared with placebo, eszopiclone significantly increased the arousal threshold [−14.0 (−19.9 to −10.9) compared with −18.0 (−22.2 to −15.1) cmH2O; P < 0.01], and sleep duration, improved sleep quality and lowered the AHI without respiratory event prolongation or worsening hypoxaemia. Among the eight patients identified as having a low arousal threshold, reductions in the AHI occurred invariably and were most pronounced (25 ± 6 compared with 14 ± 4 events/h of sleep; P < 0.01). In conclusion, eszopiclone increases the arousal threshold and lowers the AHI in obstructive sleep apnoea patients that do not have marked overnight hypoxaemia. The greatest reductions in the AHI occurred in those with a low arousal threshold. The results of this single night physiological study suggest that certain sedatives may be of therapeutic benefit for a definable subgroup of patients. However, additional treatment strategies are probably required to achieve elimination of apnoea.
Keywords: apnoea/hypopnoae index (AHI), lung, sedative medication, sleep-disordered breathing, therapeutic target, upper-airway physiology
INTRODUCTION
OSA (obstructive sleep apnoea) is a serious condition with major cardiovascular and neurocognitive morbidity [1–3]. Existing treatments are often inadequate due to poor adherence or variable efficacy [4–6]. There are currently no pharmacological therapies to treat OSA [7]. Thus identification of novel therapeutic targets for OSA remains an important objective.
The pathophysiological causes of OSA vary considerably between patients [8,9]. As such, the concept of individualized therapeutic approaches is emerging such that treatment of the major underlying abnormality may be beneficial in the appropriately targeted patient subgroups [8,10]. Most OSA patients have anatomical compromise yielding a compensatory increase in pharyngeal dilator muscle activity during wakefulness to maintain airway patency [11]. During sleep, these muscles generally lose activity leading to pharyngeal collapse among susceptible individuals. However, accumulation of physiological stimuli (carbon dioxide and negative pharyngeal pressure) can recruit dilator activity in many OSA patients if sleep can be maintained [12–15]. Indeed, periods of breathing stability, observed in most OSA patients during sleep [16], are associated with increased activity of the genioglossus muscle [13]. This finding suggests that, if sleep can be maintained for adequate duration to facilitate sufficient endogenous respiratory stimuli accumulation, the pharyngeal dilator muscles are both necessary and sufficient to enable breathing stability [13]. Thus the ease with which an individual wakes up to respiratory stimuli (respiratory arousal threshold) can become a critical characteristic [14,15]. Premature arousal may lead to repetitive apnoea due to inadequate accumulation of respiratory stimuli to enable upper-airway muscle recruitment. On the other hand, a very high arousal threshold (difficult to wake up) may be deleterious if profound blood gas disturbance occurs prior to arousal.
The stimulus for arousal during respiratory events is likely negative intrathoracic pressure [17] and is quantified as the nadir esophageal or epiglottic pressure preceding arousal [8]. Recent findings indicate that the arousal threshold varies considerably between patients with a substantial proportion having a low arousal threshold (defined as between 0 and −15 cmH2O) and others with much more negative values [18–20]. Thus strategies to manipulate the arousal threshold may be beneficial for some patients (those with low threshold), but potentially deleterious for others (those with a high threshold).
Several sedative agents increase the arousal threshold, including ethanol [21], flurazepam [22], triazolam [23], pentobarbital [24] and trazodone [25]. To be an ideal therapeutic tool, the agent must increase the arousal threshold without impairing upper-airway dilator muscle responsiveness as may occur with alcohol and some benzodiazepines [26–28]. In unselected patients, sedative medications have variable results, with apnoea severity falling in some patients and increasing in others [29–35]. We believe that the variability in therapeutic responsiveness to sedative agents relates to differences in their upper-airway myorelaxant properties and in the underlying causes of OSA between patients. Therefore the aim of the present study was to examine the effect of the non-benzodiazepine sedative, eszopiclone, on the arousal threshold and sleep apnoea severity. We hypothesized that eszopiclone would increase the arousal threshold and reduce the AHI (apnoea/hypopnoea index) in patients with a low arousal threshold.
MATERIALS AND METHODS
Subjects
Twenty-eight otherwise healthy adults with untreated or high clinical suspicion of suspected OSA gave informed written consent to participate in the study, which was performed in accordance with the Declaration of Helsinki (2000) of the World Medical Association, was approved by the Hospital’s Institutional Review Board and was registered on clinicaltrials.gov (NCT01102270).
Measurements and equipment
PSG (polysomnography)
EEGs, electrooculograms, surface submentalis and tibialis electromyograms, finger pulse oximetry, chest and abdominal motion, position (position monitor and video monitoring) and airflow (thermister plus a nasal pressure) probe were applied to enable sleep staging, score arousals and respiratory event detection. PSG data were acquired using a commercially available system (Nihon Kohden). Analogue signals were simultaneously acquired on a 1401 plus interface and Spike 2 software (Cambridge Electronic Design).
Arousal threshold determination
Both nostrils were decongested (0.05 % oxymetazoline HCl), and the more patent nostril was anaesthetized (4 % lidocaine HCl) for insertion of an epiglottic pressure catheter (model MCP-500; Millar). Briefly, the catheter was advanced 1–2 cm below the base of the tongue under direct visualization and taped securely to the nostril to avoid movement [36]. Using established techniques [8,17,23,37], the arousal threshold was quantified as the average nadir pressure immediately preceding arousal (Figure 1) during 20 replicate respiratory events selected at random (or as many as were available) during each of the sleep stages analysed [stages 1, 2 and REM (rapid eye movement) sleep] in each patient.
Figure 1. A PSG example of the procedure used to quantify the respiratory arousal threshold.
PSG tracings are from a baseline study in a 55-year-old female patient with moderately severe obstructive sleep apnoea (AHI = 23 events/h of sleep). EEG, C3–A2; nasal flow, nasal airflow (arbitrary units); Pepi, pressure at the level of the epiglottis. Results presented show an approx. 45-s segment during stage 2 sleep in which the patient is experiencing an approx. 30-s respiratory event. Note the increasing breathing efforts (more pronounced negative Pepi) during the period of impaired airflow up until the point of arousal from sleep (grey-shaded portion of the EEG). As indicated, the respiratory arousal threshold is quantified as the nadir epiglottic pressure during the effort immediately prior to arousal from sleep (in this example approx. −20 cmH2O).
Protocol
Subjects were instructed to sleep in the supine posture throughout the overnight recordings, which was confirmed via the combination of video monitoring and a position monitor. Initially, an 8-h baseline overnight polysomnogram was performed to confirm the presence and severity of OSA and to define the baseline arousal threshold. Subjects without OSA (AHI ≤5 events/h of sleep), or with severe oxygen desaturation (nadir overnight SaO2 <70 %), were excluded from further participation. All remaining patients returned for two replicate 8-h polysomnograms with an epiglottic pressure catheter to quantify the arousal threshold. During these visits, each patient received placebo or 3 mg of eszopiclone in random order immediately prior to sleep with only a research pharmacist knowing the treatment assignment. Each visit was separated by approx. 1 week. A study flow diagram is shown in Figure 2.
Figure 2. Flow diagram of the enrollment, randomization and analysis procedures.
The present study was a double-blind placebo-controlled cross-over study in which an overnight baseline PSG was initially performed to determine eligibility. If deemed eligible, OSA patients were randomized to the allocation order (placebo first or 3 mg of eszopiclone first) and returned for two additional PSG studies at which time the appropriate intervention was administered prior to sleep (visit 1 and visit 2). Refer to the text for further details.
Data analysis and statistical procedures
All analyses were performed blinded to the study intervention. A registered polysomnographic technician performed sleep staging, scored respiratory events and carefully identified the presence, onset and duration of arousals according to standard criteria [38,39]. For each visit, 20 (or as many as were available) arousals occurring in conjunction with respiratory events were analysed for arousal threshold quantification in each patient. To ensure that the arousal threshold was comprised of a random allocation of arousals across the night, arousal selection was performed by allocating each individual arousal a sequential number and using a random number generator to select the 20 arousals to be analysed. Arousals were excluded from analysis in instances where mucus accumulation yielded artefacts on the epiglottic pressure catheter or where there was <2 cmH2O decrement in the epiglottic pressure catheter in the 30 s prior to arousal as these were deemed to be spontaneous arousals.
For each outcome variable, where the distribution of the delta between eszopiclone and placebo was found to be normally distributed (on the basis of a Shapiro–Wilk test), statistical comparisons were performed using paired Student’s t tests. Non-normally distributed data were compared using a related-samples non-parametric test (Mann–Whitney U test) (SigmaPlot). A power calculation was performed prior to the study using the arousal threshold as the primary outcome. Based on our previous work with trazodone [25], we conservatively estimated that 15 subjects would be required to detect a 4 cmH2O difference in the stage 2 arousal threshold between placebo and eszopiclone (based on a ΔS.D. of 4.5 cmH2O) to achieve 90 % power. Thus 17 patients were randomized to allow for a potential 15 % dropout rate. ANOVA for repeated measures was used to examine sleep state effects (stage 1, 2 and REM sleep) on arousal threshold values during the baseline condition (SPSS). Statistical significance was defined as P < 0.05. All group data are reported as means ± S.E.M. or medians with interquartile range for non-normally distributed variables.
RESULTS
Baseline anthropometric and sleep characteristics
Of the 28 subjects recruited, five did not have OSA (AHI = 2 ± 1; overall range 0–5 events/h of sleep) during the baseline polysomnogram and were excluded from further participation and analysis. An additional six subjects had severe OSA (AHI = 82 ± 11; overall range 60 to 136 events/h of sleep), experienced marked oxygen desaturation (nadir SaO2 = 63 ± 3; overall range 54 to 69 %) and had high arousal thresholds (stage 2 arousal threshold = −40 ±6; overall range −25 to −63 cmH2O). These patients were excluded from participating in the cross-over intervention portion of the study (Figure 2). The mean age and the body mass index for the remaining 17 patients (seven females) were 45 ± 4 (overall range 19–62) years and 33 ± 2 (overall range 19–45) kg/m2 respectively (see Table 1 for baseline sleep data). At the time of enrolment, seven subjects had suspected OSA with no prior diagnosis, five were recently diagnosed but not yet treated and five had a prior diagnosis (between 2 and 8 years earlier) but did not tolerate CPAP (continuous positive airway pressure) therapy (two of whom also had uvulopalatopharyngoplasty surgery).
Table 1. Group PSG sleep parameters.
Group PSG data for the 8-h overnight sleep studies during the baseline, placebo and eszopiclone conditions. Data were collected in the supine posture. Values are means ± S.E.M. or medians (interquartile range).
| Parameter | Baseline | Placebo | Eszopiclone |
|---|---|---|---|
| Sleep onset latency (min) | 11 (5–14) | 14 (3–26) | 5 (3–15) |
| Total sleep time (h) | 5.3 ± 0.4 | 5.4 ± 0.4 | 6.8 ± 0.2* |
| Sleep efficiency (% total sleep time) | 66 ± 5 | 68 ± 5 | 84 ± 3* |
| Stage 1 sleep (% total sleep time) | 28 (20–33) | 28 (18–34) | 19 (14–26)* |
| Stage 2 sleep (% total sleep time) | 61 (48–64) | 58 (55–71) | 68 (64–69)* |
| REM sleep (% total sleep time) | 10 ± 2 | 10 ± 2 | 11 ± 1 |
| Total AHI (events/h of sleep) | 31 ± 5 | 31 ± 5 | 24 ± 4* |
| Non-REM AHI (events/h of sleep) | 30 ± 5 | 29 ± 5 | 22 ± 5* |
| REM AHI (events/h of sleep) | 31 ± 5 | 33 ± 6 | 29 ± 5 |
| Central apnoea index (events/h of sleep) | 0 (0–0.4) | 0.2 (0–0.3) | 0 (0–0.1) |
| Arousal index (arousals/h of sleep) | 25 (20–31) | 22 (15–37) | 17 (15–24)* |
| Event duration (s) | 29 ± 1 | 32 ± 1 | 32 ± 2 |
| Overnight SaO2 nadir (%) | 81 ± 1 | 80 ± 1 | 80 ± 1 |
| Average SaO2 during Sleep (%) | 93 ± 0.5 | 93 ± 0.6 | 94 ± 0.4 |
| ODI (oxygen desaturation/h of sleep) | 28 (25–42) | 28 (22–37) | 21 (15–36) |
| Average nadir SaO2 during events (%) | 89 ± 0.4 | 89 ± 0.7 | 90 ± 0.5 |
Significant difference compared with the placebo condition. n = 17 patients. ODI, oxygen desaturation index [number of 3 % oxygen desaturations (lasting > 5 s)/h of sleep].
Effect of eszopiclone on sleep parameters and obstructive sleep apnoea severity
Compared with the placebo visit, 3 mg of eszopiclone prior to the 8-h PSG significantly increased total sleep time by over 1 h (Table 1). Both non-REM (190 ± 20 compared with 270 ± 17 min; P < 0.01) and REM sleep (34 ± 6 compared with 46 ± 5 min; P = 0.08) durations tended to increase. Sleep onset latency was not different between conditions (P = 0.10; Table 1). During the eszopiclone visit, sleep quality improved as reflected by a reduction in the arousal index and less stage 1 with more stage 2 sleep as a percentage of total sleep time (Table 1). There were minimal central apnoeas or SWS (slow-wave sleep) in either condition (<1 % of total sleep time). The proportion of time spent in REM sleep as a percentage of total sleep time was similar between conditions (P = 0.38; Table 1). When brief arousals from sleep (>3 and <15 s) did occur, they were of similar duration during placebo compared with eszopiclone (9.0 ± 0.3 compared with 8.6 ± 0.3 s; P = 0.12) as was the proportion of awakenings (>15 s) compared with brief arousals (38 ± 4 compared with 37 ± 4 %; P = 0.84).
Eszopiclone reduced the total AHI by 23 ± 9 % (Table 1 and Figure 3). The reduction in AHI with eszopiclone occurred in non-REM sleep with no change in the REM AHI (P = 0.43; Table 1). The reduction in apnoea frequency occurred in the absence of respiratory event duration prolongation (P = 0.95) or changes in nadir SaO2 (P = 0.55) or average SaO2 during sleep (P = 0.34) or oxygen desaturation index (P = 0.15) or average nadir SaO2 associated with respiratory events (P = 0.43) (Table 1).
Figure 3. AHI scatter plots representing each individual patient’s AHI during the placebo and eszopiclone condition (n = 17).

Mean ± S.E.M. values are presented adjacent to each condition. *Significant difference compared with placebo.
Effect of sleep state and eszopiclone on the respiratory arousal threshold
The arousal threshold was 30 ± 4 % higher (more negative; more difficult to arouse) during stage 2 compared with stage 1 sleep at baseline [−15.6 (−19.9 to −12.5) compared with −9.9 (−14.6 to −7.5) cmH2O; P < 0.01; n = 17]. In the 12 patients in whom REM data were available, the arousal threshold was similar to stage 1 [−11.3 (−14.9 to −9.7) compared with −11.5 (−13.8 to −8.2) cmH2O; P = 0.15] and lower (less negative; easier to arouse) than stage 2 [−11.3 (−14.9 to −9.7) compared with −15.1 (−18.9 to −12.6) cmH2O; P < 0.01].
The arousal threshold was also 18 ± 4 % higher (more negative) during eszopiclone compared with placebo during stage 2 sleep (Figure 4). Paired comparisons for eszopiclone compared with placebo arousal threshold values during stage 1 and REM sleep were available in 14 and eight patients respectively. Unlike stage 2 sleep, there were no statistically significant differences in the arousal threshold between eszopiclone compared with placebo during stage 1 sleep [−13.0 (−18.2 to −9.1) compared with −12.7 (−16.4 to −8.7) cmH2O; P = 0.43] or REM sleep [−14.3 (−17.0 to −9.4) compared with −10.4 (−14.0 to −9.4) cmH2O; P = 0.80]. There were insufficient data to assess SWS arousal thresholds.
Figure 4. Stage 2 sleep arousal threshold scatter plots representing each individual patient’s arousal threshold during the placebo and eszopiclone condition (n = 17).

Median (interquartile range) values are presented adjacent to each condition. *Significant difference compared with placebo.
Effect of eszopiclone in patients with a low respiratory arousal threshold
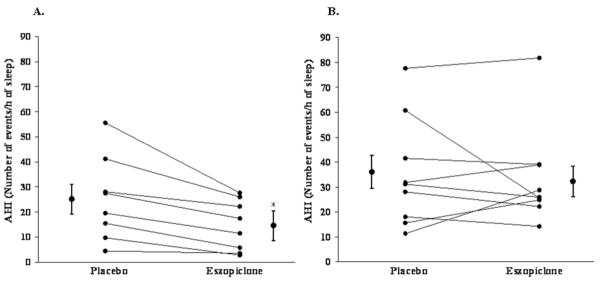
At baseline, eight out of 23 patients had a low arousal threshold (pre-specified stage 2 arousal threshold between 0 and −15 cmH2O). All of the eight out of 17 patients with a low arousal threshold that completed the cross-over portion of the study invariably had a reduction in the AHI during the eszopiclone condition (mean reduction 43 ± 9 %; Figure 5A). Changes in the AHI with eszopiclone were more variable in patients with higher (more negative) arousal thresholds in whom there was no overall change in the AHI (P = 0.52; Figure 5B).
Figure 5. AHI scatter plots during the placebo and eszopiclone condition in (A) the patients with (n = 8) and (B) the patients without (n = 9) a low respiratory arousal threshold (between 0 and −15 cmH2O).
Mean ± S.E.M. values are presented adjacent to each condition. *Significant difference compared with placebo.
DISCUSSION
The results of the present double-blind placebo-controlled cross-over physiological study indicate that, compared with placebo, 3 mg of eszopiclone immediately prior to sleep significantly increases the stage 2 respiratory arousal threshold, sleep duration, improves sleep quality and lowers the AHI without prolonging respiratory events or worsening hypoxaemia. In the patients identified as having a low respiratory arousal threshold, reductions in the AHI occurred invariably and were most pronounced. However, reductions in the AHI to below conventional standards of treatment efficacy were not achieved in the majority of patients.
Effect of eszopiclone on sleep parameters and OSA severity
Although the magnitude of the improvement was quite marked in the present study, the findings of increased sleep duration, in the absence of respiratory event prolongation and worsening of hypoxaemia, are in accordance with the findings of a recently published pilot study by Rosenberg et al. [30]. In that previous study, 3 mg of eszopiclone was administered prior to sleep in unselected mild-moderately severe OSA patients with no overall change in the AHI [30]. We postulate that the larger magnitude change in sleep duration and reduction in the overall AHI in the current study may be explained by differences in patient selection criteria. In particular, patients with marked oxygen desaturation (and therefore likely blunted chemosensitivity and low propensity for arousal) were excluded from participation in the current study. Indeed, the six patients who were excluded from participating in the cross-over portion of the present study all had severe OSA and high arousal thresholds.
A shift from lighter stage 1 to more consolidated stage 2 sleep, as occurred with eszopiclone in the current study, is known to reduce OSA severity [40]. However, the precise underlying mechanisms are uncertain. Apnoea severity has also recently been shown to be reduced markedly during SWS [40], and OSA tends to get worse in REM compared with non-REM sleep [40,41]. However, in the present study, there was minimal SWS in either condition, and the proportion of REM sleep was not different. The non-REM reduction in AHI compared with no change in REM AHI with eszopiclone is consistent with the proposed mechanism yielding a reduction in apnoea severity with a non-benzodiazepine sedative. The upper-airway dilator muscles are known to be responsive during non-REM sleep when provided with sufficient time and respiratory stimulation [12,13] but are much less so during REM sleep [36,42,43].
Effect of sleep state and eszopiclone on the respiratory arousal threshold
We are not aware of any published studies that have systematically quantified the arousal threshold in stage 1 sleep. The novel finding that the arousal threshold increased (more negative) by ~ 30 % from stage 1 to stage 2 sleep is consistent with recently published results demonstrating an ~ 40–50 % reduction in apnoea and arousal frequency respectively from stage 1 to 2 sleep [40]. Together, these observations suggest that the increase in the arousal threshold from stage 1 to 2 sleep may provide greater opportunity for respiratory stimuli to accumulate to enable sufficient upper-airway dilator muscle recruitment and thus, airway patency. Alternatively, stage 2 compared with stage 1 sleep may be inherently more stable from the standpoint of breathing stability for currently unknown reasons.
The arousal threshold was also ~ 20 % higher (more negative) during eszopiclone compared with placebo during stage 2 sleep. Thus, in addition to the associated changes in arousal threshold from a shift in the percentage of stage 1 to stage 2 sleep with eszopiclone, once stage 2 sleep was achieved, there were fewer arousals, and greater levels of negative epiglottic pressure (a known upper-airway dilator muscle stimulus) were tolerated before arousal. Although there were insufficient data to assess the arousal threshold in SWS, a large increase in the arousal threshold associated with a decrease in apnoea severity in this sleep stage has been reported recently [40,44,45]. Thus further reductions in apnoea severity would be predicted in patients with a low arousal threshold if there were a non-myorelaxant sedative medication that could increase SWS to enable further upper-airway dilator muscle recruitment.
Effect of eszopiclone in patients with a low respiratory arousal threshold
The proportion of patients found to have a low arousal threshold in the current study during the baseline visit (eight out of 23) is consistent with the ongoing work within this area in other cohorts in which ~ 30 % of OSA patients were found to have a low arousal threshold [19,20]. The invariable reductions in AHI in OSA patients with a low arousal threshold strongly support the concept that a low arousal threshold is an important contributing factor to OSA pathogenesis for a substantial proportion of patients. In accordance with the proposed role of the arousal threshold in OSA pathophysiology, changes in the AHI with eszopiclone were more variable in patients with higher (more negative) arousal thresholds [14,15,45,46]. Specifically, depending on how close a patient is to their upper-airway muscle recruitment threshold, and their ability to respond to respiratory stimuli during sleep, even patients with moderate to high arousal thresholds may benefit from strategies to manipulate the arousal threshold [15,46]. Alternatively, in patients with poor muscle responsiveness during sleep and a high arousal threshold, a sedative medication may worsen OSA.
These present findings suggest that there is the potential to reduce obstructive sleep apnoea severity with the use of a non-benzodiazepine sedative medication by targeting this therapeutic trait in the appropriately selected patients (i.e. those with a low arousal threshold/recruitable upper-airway dilator muscles). However, although 3 mg of eszopiclone lowered the AHI from moderately severe to mild in the patients with a low arousal threshold (25 ± 6 compared with 14 ± 4 events/h of sleep) in the present study, only two out of eight of these patients had a reduction in AHI from above to below 10 events/h of sleep. Thus, even in patients identified as having a low arousal threshold, other pathophysiological factors are clearly involved. Alternatively, it remains possible that a higher sedative dose may lead to greater reductions in the AHI in these patients, although higher doses may exhibit deleterious effects on upper-airway dilator muscle function.
Ultimately, the concept of individualized therapy tailored at multiple pathophysiological traits may lead to alternative treatment approaches for appropriately characterized patients. For example, combinations of one or more of the following may be effective; a mandibular advancement splint to improve anatomy, oxygen therapy to improve respiratory control instability [47] or, as the present results suggest, a non-benzodiazepine sedative medication to increase the arousal threshold in patients with a low arousal threshold. For this type of approach to be maximally effective, greater knowledge and simplified tools to quantify the key pathophysiological traits in individual patients will be required.
Further considerations and methodological limitations
An important consideration regarding these findings is that, although an increase in the arousal threshold likely facilitates the required stimuli to recruit upper-airway dilator muscle activity, it remains unclear whether the resulting stabilization of breathing yields a major cardiovascular benefit over unstable breathing with repetitive apnoeas/hypopnoeas (with associated catecholamine surges from arousals and hypoxaemia). Although reductions in the frequency of respiratory events and increased sleep duration [48] would be predicted to be beneficial, it is possible that the presence of prolonged large negative intrathoracic pressures during stable, but flow-limited breathing increases left ventricular transmural pressure (and therefore afterload) [49,50]. Thus larger longer-term multicentre trials would be required to examine this possibility before such an approach can be recommended clinically.
The reason for the variability in the change in arousal threshold with eszopiclone between sleep stages is uncertain but may reflect a specific stage 2 phenomenon. Alternatively, a lack of change in stage 1 and REM sleep may be a reflection of the reduced number of subjects and the decreased proportion of the night in which these sleep stages occurred. Indeed, although the direction of change was consistent with the stage 2 effect, during eszopiclone compared with placebo there were 15 ± 3 compared with 14 ± 3 arousals per patient during stage 1 and only 8 ± 2 compared with 5 ± 1 arousals per patient available for analysis during REM sleep. Larger studies are required to address these questions definitively.
Finally, the total sleep time for the study group at baseline was relatively low. We suspect that this is largely a reflection of the high proportion of patients recruited with a low arousal threshold (and thus increased likelihood to have fragmented/poor sleep efficiency). However, some components of the research setting including standardization of the supine posture and the addition of an epiglottic pressure catheter may have also been potential influential factors. Thus it will be important to conduct future studies during more natural sleep to address these possibilities.
Summary and possible future directions
The results of this single-night physiological study demonstrate that the sedative medication eszopiclone increases the respiratory arousal threshold, sleep duration and lowers the AHI in patients who do not have marked overnight oxygen desaturation at baseline (nadir overnight SaO2 >70 %). The arousal threshold was shown to increase by ~ 30 % from stage 1 to stage 2 sleep, and eszopiclone reduced the proportion of stage 1 sleep and increased stage 2 sleep. Reductions in the AHI occurred in the absence of respiratory event prolongation or increased hypoxaemia. Furthermore, patients identified as having a low arousal threshold (between 0 and −15 cmH2O) experienced an approx. 45 % reduction in the AHI with eszopiclone. These findings also suggest that approx. one-third of untreated obstructive sleep apnoea patients have a low arousal threshold and thus may be amenable to such an approach. However, future novel drugs with stronger stage 2/3-inducing effects in the absence of upper-airway muscle impairment might be required for this group of patients to achieve elimination of apnoea. Thus, although the results of the present physiological study offer promise, large-scale clinical trials assessing hard outcomes will be required before manipulation of arousal threshold can be widely recommended. Ultimately, a combination of strategies that target multiple pathophysiological traits (which can be assessed non-invasively) may be required for this approach to be viable clinically.
Acknowledgments
We thank Karen Stevenson, Lauren Hess, and Erik Smales for their valuable technical support. We are also grateful for the expertise provided by Patricia Kelly of the Brigham and Women’s Hospital Investigational Drug Service in performing the preparation, randomization and blinding code for the study intervention. D.J.E. is a consultant for Apnex Medical. A.W. is a consultant for Philips Respironics. D.P.W. is Chief Medical Officer for Philips Respironics. A.M. has received consulting and/or research income from Philips, Medtronic, Apnicure, Apnex, Itamar Ethicon, Pfizer, Separacor, Merck, Cephalon, SGS and SHC.
FUNDING
This study was supported by the National Institutes of Health (NIH) [grant number P01 HL095491-01 A1] and an unrestricted investigator-initiated research grant from Sepracor Pharmaceuticals. Other support includes the NIH [grant numbers HL73146 R01 HL085188-01A2, R01 HL090897-01A2, K24 HL 093218-01 A1]. D.J.E. is supported by an Overseas Based Biomedical (CJ Martin) Fellowship from the National Health and Medical Research Council of Australia [grant number 510392], and the American Heart Association [grant number 10SDG3510018]. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Abbreviations
- AHI
apnoea/hypopnoea index
- OSA
obstructive sleep apnoea
- PSG
polysomnography
- REM
rapid eye movement
- SaO2
arterial blood oxygen saturation
- SWS
slow-wave sleep
Footnotes
AUTHOR CONTRIBUTION
Danny Eckert was involved in all aspects of the study including study conception and design, acquisition of data, analysis and interpretation of data. Robert Owens, Andrew Wellman, Shilpa Rahangdale, Susie Yim-Yeh and Atul Malhotra provided important assistance with acquisition of data and interpretation. Geoffrey Kehlmann was responsible for arousal threshold data analysis. Atul Malhotra and David White also provided important assistance with study conception and design and interpretation of data. All of the authors contributed to drafting the article and have approved the manuscript.
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 3.Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med. 1999;340:847–851. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 4.Kribbs NB, Pack AI, Kline LR, Smith PL, Schwartz AR, Schubert NM, Redline S, Henry JN, Getsy JE, Dinges DF. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887–895. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 5.Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS) Sleep Med Rev. 2003;7:81–99. doi: 10.1053/smrv.2001.0197. [DOI] [PubMed] [Google Scholar]
- 6.Engleman HM, McDonald JP, Graham D, Lello GE, Kingshott RN, Coleman EL, Mackay TW, Douglas NJ. Randomized crossover trial of two treatments for sleep apnea/hypopnea syndrome: continuous positive airway pressure and mandibular repositioning splint. Am J Respir Crit Care Med. 2002;166:855–859. doi: 10.1164/rccm.2109023. [DOI] [PubMed] [Google Scholar]
- 7.Hedner J, Grote L, Zou D. Pharmacological treatment of sleep apnea: current situation and future strategies. Sleep Med Rev. 2008;12:33–47. doi: 10.1016/j.smrv.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:144–153. doi: 10.1513/pats.200707-114MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172:1363–1370. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- 10.Saboisky JP, Chamberlin NL, Malhotra A. Potential therapeutic targets in obstructive sleep apnoea. Expert Opin Ther Targets. 2009;13:795–809. doi: 10.1517/14728220903005608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan AS, Wellman A, Heinzer RC, Lo YL, Schory K, Dover L, Gautam S, Malhotra A, White DP. Mechanisms used to restore ventilation after partial upper airway collapse during sleep in humans. Thorax. 2007;62:861–867. doi: 10.1136/thx.2006.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordan AS, White DP, Lo YL, Wellman A, Eckert DJ, Yim-Yeh S, Eikermann M, Smith SA, Stevenson KE, Malhotra A. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep. 2009;32:361–368. doi: 10.1093/sleep/32.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–633. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- 15.Younes M, Ostrowski M, Atkar R, Laprairie J, Siemens A, Hanly P. Mechanisms of breathing instability in patients with obstructive sleep apnea. J Appl Physiol. 2007;103:1929–1941. doi: 10.1152/japplphysiol.00561.2007. [DOI] [PubMed] [Google Scholar]
- 16.Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med. 2003;168:645–658. doi: 10.1164/rccm.200302-201OC. [DOI] [PubMed] [Google Scholar]
- 17.Gleeson K, Zwillich CW, White DP. The influence of increasing ventilatory effort on arousal from sleep. Am Rev Respir Dis. 1990;142:295–300. doi: 10.1164/ajrccm/142.2.295. [DOI] [PubMed] [Google Scholar]
- 18.Eckert DJ, Jordan AS, Wellman A, Smith S, Stevenson K, Malhotra A, White DP. The respiratory arousal threshold in obstructive sleep apnea. Am J Respir Crit Care Med. 2008;177:A594. doi: 10.1164/rccm.201312-2115LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kehlmann G, Owens RL, Wellman A, Jordan AS, Rahangdale S, Yim-Yeh S, White DP, Malhotra A, Eckert DJ. Factors influencing the respiratory arousal threshold in obstructive sleep apnea patients. Sleep. 2010;33:A181. [Google Scholar]
- 20.Eckert DJ, Jordan AS, Wellman A, Malhotra A, White DP. Defining physiological phenotypic traits in obstructive sleep apnea: avenues for novel treatment approaches. Respirology. 2009;14:A21. [Google Scholar]
- 21.Berry RB, Bonnet MH, Light RW. Effect of ethanol on the arousal response to airway occlusion during sleep in normal subjects. Am Rev Respir Dis. 1992;145:445–452. doi: 10.1164/ajrccm/145.2_Pt_1.445. [DOI] [PubMed] [Google Scholar]
- 22.Hedemark LL, Kronenberg RS. Flurazepam attenuates the arousal response to CO2 during sleep in normal subjects. Am Rev Respir Dis. 1983;128:980–983. doi: 10.1164/arrd.1983.128.6.980. [DOI] [PubMed] [Google Scholar]
- 23.Berry RB, McCasland CR, Light RW. The effect of triazolam on the arousal response to airway occlusion during sleep in normal subjects. Am Rev Respir Dis. 1992;146:1256–1260. doi: 10.1164/ajrccm/146.5_Pt_1.1256. [DOI] [PubMed] [Google Scholar]
- 24.Eikermann M, Eckert DJ, Chamberlin NL, Jordan AS, Zaremba S, Smith S, Rosow C, Malhotra A. Effects of pentobarbital on upper airway patency during sleep. Eur Respir J. 2010;36:569–576. doi: 10.1183/09031936.00153809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinzer RC, White DP, Jordan AS, Lo YL, Dover L, Stevenson K, Malhotra A. Trazodone increases arousal threshold in obstructive sleep apnoea. Eur Respir J. 2008;31:1308–1312. doi: 10.1183/09031936.00067607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krol RC, Knuth SL, Bartlett D., Jr Selective reduction of genioglossal muscle activity by alcohol in normal human subjects. Am Rev Respir Dis. 1984;129:247–250. [PubMed] [Google Scholar]
- 27.Bonora M, St John WM, Bledsoe TA. Differential elevation by protriptyline and depression by diazepam of upper airway respiratory motor activity. Am Rev Respir Dis. 1985;131:41–45. doi: 10.1164/arrd.1985.131.1.41. [DOI] [PubMed] [Google Scholar]
- 28.Leiter JC, Knuth SL, Krol RC, Bartlett D., Jr The effect of diazepam on genioglossal muscle activity in normal human subjects. Am Rev Respir Dis. 1985;132:216–219. doi: 10.1164/arrd.1985.132.2.216. [DOI] [PubMed] [Google Scholar]
- 29.Hoijer U, Hedner J, Ejnell H, Grunstein R, Odelberg E, Elam M. Nitrazepam in patients with sleep apnoea: a double-blind placebo-controlled study. Eur Respir J. 1994;7:2011–2015. [PubMed] [Google Scholar]
- 30.Rosenberg R, Roach JM, Scharf M, Amato DA. A pilot study evaluating acute use of eszopiclone in patients with mild to moderate obstructive sleep apnea syndrome. Sleep Med. 2007;8:464–470. doi: 10.1016/j.sleep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Berry RB, Kouchi K, Bower J, Prosise G, Light RW. Triazolam in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:450–454. doi: 10.1164/ajrccm.151.2.7842205. [DOI] [PubMed] [Google Scholar]
- 32.Lofaso F, Goldenberg F, Thebault C, Janus C, Harf A. Effect of zopiclone on sleep, night-time ventilation, and daytime vigilance in upper airway resistance syndrome. Eur Respir J. 1997;10:2573–2577. doi: 10.1183/09031936.97.10112572. [DOI] [PubMed] [Google Scholar]
- 33.Series F. Can improving sleep influence sleep-disordered breathing? Drugs. 2009;69:77–91. doi: 10.2165/11532000-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.Lu B, Budhiraja R, Parthasarathy S. Sedating medications and undiagnosed obstructive sleep apnea: physician determinants and patient consequences. J Clin Sleep Med. 2005;1:367–371. [PubMed] [Google Scholar]
- 35.Quadri S, Drake C, Hudgel DW. Improvement of idiopathic central sleep apnea with zolpidem. J Clin Sleep Med. 2009;5:122–129. [PMC free article] [PubMed] [Google Scholar]
- 36.Eckert DJ, Malhotra A, Lo YL, White DP, Jordan AS. The influence of obstructive sleep apnea and gender on genioglossus activity during rapid eye movement sleep. Chest. 2009;135:957–964. doi: 10.1378/chest.08-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sforza E, Krieger J, Petiau C. Arousal threshold to respiratory stimuli in OSA patients: evidence for a sleep-dependent temporal rhythm. Sleep. 1999;22:69–75. [PubMed] [Google Scholar]
- 38.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Brain Information Service/Brain Research Institute, U.C.L.A; Los Angeles: 1968. [Google Scholar]
- 39.American Sleep Disorders Association. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–184. [PubMed] [Google Scholar]
- 40.Ratnavadivel R, Chau N, Stadler D, Yeo A, McEvoy RD, Catcheside PG. Marked reduction in obstructive sleep apnea severity in slow wave sleep. J Clin Sleep Med. 2009;5:519–524. [PMC free article] [PubMed] [Google Scholar]
- 41.Findley LJ, Wilhoit SC, Suratt PM. Apnea duration and hypoxemia during REM sleep in patients with obstructive sleep apnea. Chest. 1985;87:432–436. doi: 10.1378/chest.87.4.432. [DOI] [PubMed] [Google Scholar]
- 42.Eckert DJ, McEvoy RD, George KE, Thomson KJ, Catcheside PG. Genioglossus reflex inhibition to upper-airway negative-pressure stimuli during wakefulness and sleep in healthy males. J Physiol. 2007;581:1193–1205. doi: 10.1113/jphysiol.2007.132332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shea SA, Edwards JK, White DP. Effect of wake–sleep transitions and rapid eye movement sleep on pharyngeal muscle response to negative pressure in humans. J Physiol. 1999;520:897–908. doi: 10.1111/j.1469-7793.1999.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ratnavadivel R, Stadler D, Windler S, Bradley J, Paul D, McEvoy RD, Catcheside PG. Upper airway function and arousability to ventilatory challenge in slow wave versus stage 2 sleep in obstructive sleep apnoea. Thorax. 2010;65:107–112. doi: 10.1136/thx.2008.112953. [DOI] [PubMed] [Google Scholar]
- 45.Saboisky J, Eckert D, Malhotra A. Stable breathing through deeper sleeping. Thorax. 2010;65:95–96. doi: 10.1136/thx.2009.127860. [DOI] [PubMed] [Google Scholar]
- 46.Younes M, Park E, Horner RL. Pentobarbital sedation increases genioglossus respiratory activity in sleeping rats. Sleep. 2007;30:478–488. doi: 10.1093/sleep/30.4.478. [DOI] [PubMed] [Google Scholar]
- 47.Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respir Physiol Neurobiol. 2008;162:144–151. doi: 10.1016/j.resp.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chandola T, Ferrie JE, Perski A, Akbaraly T, Marmot MG. The effect of short sleep duration on coronary heart disease risk is greatest among those with sleep disturbance: a prospective study from the Whitehall II cohort. Sleep. 2010;33:739–744. doi: 10.1093/sleep/33.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fessler HE. Heart–lung interactions: applications in the critically ill. Eur Respir J. 1997;10:226–237. doi: 10.1183/09031936.97.10010226. [DOI] [PubMed] [Google Scholar]
- 50.Hausknecht MJ, Brin KP, Weisfeldt ML, Permutt S, Yin FC. Effects of left ventricular loading by negative intrathoracic pressure in dogs. Circ Res. 1988;62:620–631. doi: 10.1161/01.res.62.3.620. [DOI] [PubMed] [Google Scholar]