Abstract
Background
Impaired profiles of neurocognitive function have been consistently demonstrated among pediatric patients with bipolar disorder (BD), and may aid in the identification of endophenotypes across subtypes of the disorder. This study aims to determine phenotypic cognitive profiles of patients with BD Type I and II.
Methods
Subjects (N=79) consisted of BD I (n=27) and BD II (n=19) patients and demographic and intellectually matched healthy controls (HC; n=33) that completed a battery of neurocognitive tasks.
Results
BD I patients performed significantly more poorly compared to HC on all domains of cognitive function including attention, executive function, working memory, visual memory, and verbal learning and memory. BD I patients also performed more poorly compared to BD II patients on all domains of cognitive functioning with the exception of working memory, whereas BD II patients did poorly relative to HC only on verbal learning and memory.
Conclusions
Findings from the current study indicate that BD I patients are characterized by more severe cognitive impairment relative to BD II patients who show an intermediate pattern of performance between BD I patients and HC. Verbal learning and memory may effectively differentiate pediatric BD patients and controls, regardless of the subtype of BD, and may serve as a cognitive endophenotype for the disorder. Additionally, these findings move us closer to developing effective cognitive interventions tailored to specific subtypes of pediatric BD patients.
Keywords: Pediatric bipolar disorder, neurocognitive function, bipolar I disorder, bipolar II disorder, clinical subtypes
Recently, there has been increased interest in identifying possible endophenotypes of pediatric bipolar disorder (BD; Leibenluft & Rich, 2008). In particular, greater attention is being given to the identification of disease associated traits that are more directly linked to the underlying molecular genetics of BD and its associated subtypes than the phenotypic disease state itself (Balanza–Martıez et al., 2008). Indeed, the identification of neurocognitive profiles across differing subtypes of pediatric BD is one way to elucidate the underlying pathophysiological domains that may be loosely linked to specific genes (Deo, Costa, DeLisi, DeSalle, & Haghighi, 2010; Glahn et al., 2004, 2010; Schulze, 2010). Understanding cognitive profiles across separate subtypes of BD can also aid in the development of more tailored cognitive interventions (Cahill et al., 2009; Henin et al., 2009a; Joseph et al., 2008).
Deficits in attention, executive function, working memory, verbal learning and memory, visual memory, response inhibition, and processing speed have been documented in both child and adult BD patients (Arts et al., 2007; Cahill et al., 2009; Joseph et al., 2008; Henin et al., 2009a; Kurtz & Gerraty, 2009). Cognitive deficits are observed in first-degree relatives (Bora, Yucel, & Pantelis, 2009; Doyle et al., 2009), as well as symptomatic and euthymic patients, and appear to be a “trait-like” feature of the disorder (Martinez-Aran et al., 2004; Pavuluri et al., 2006). Deficits in verbal memory and executive functioning are the most prominent and consistent finding in BD patients across the life span (Joseph et al., 2008; Kurtz & Gerraty, 2009; Robinson et al., 2006). These deficits persist even after remission in adults (Martinez-Aran et al., 2004; van Gorp et al, 1998), and become more severe over the course of development in early onset pediatric BD patients (Pavuluri et al., 2009a).
While there are a limited number of adult studies that have attempted to differentiate cognitive profiles among BD I versus II patients, none exist in pediatric BD. The majority of investigations of cognitive dysfunction among pediatric BD youth have focused exclusively on BD I patients (Bearden et al., 2007; McClure et al., 2005; Pavuluri et al., 2006) or have used heterogeneous samples of varying BD subtypes (Dickstein et al., 2004; Doyle et al., 2005; Glahn et al., 2005; McClure et al., 2005; Rucklidge et al., 2006). Differentiating cognitive profiles among pediatric BD subtypes may therefore help us to understand potential differences between BD I and II patients and may also help to clarify past findings on neurocognitive deficits. The majority of findings in BD adults lean towards a more impaired pattern of cognitive dysfunction among BD I patients, most notably on tasks of verbal learning and memory and executive functioning (Hsiao et al., 2009, Simonsen et al., 2008; Torrent et al., 2006). However, results have been inconsistent, with some studies reporting more impaired cognitive dysfunction among BD II patients (Harkavy-Friedman et al., 2006; Summers et al., 2006). Methodological issues such as the inclusion of patients in heterogeneous clinical states and medication effects may have contributed to some of the discrepancies.
The potential impact of pharmacotherapy on cognitive functioning in BD patients has been gaining increased attention (Donaldson et al., 2003; Holmes et al., 2008; Pavuluri et al., 2010). Recent evidence indicates that higher doses of mood stabilizers (Henin et al., 2009b; Holmes et al., 2008) and antipsychotic medications (Donaldson et al., 2003), can lead to greater cognitive impairment in adult as well as pediatric patients. Because BD I and II patients often vary in the type and number of medications they receive, we sought to study unmedicated acutely ill patients as an important first-step in identifying possible cognitive endophenotypes of the disorder, prior to investigating this in medicated euthymic patients. Therefore, the aim of this study was to investigate neurocognitive functioning in unmedicated BD I and II youth and matched healthy controls (HC). Given the findings from adult studies of BD, we hypothesized that BD I patients would show more severe and widespread cognitive dysfunction, displaying greater deficits on tasks of attention, executive function, working memory, visual memory, and verbal learning and memory compared to BD II patients and HC. Given that there have been no prior studies of this issue in pediatric BD populations, we expected that this study would further inform us on the commonalities and differences in cognitive dysfunction across subtypes of BD, beyond the level of symptom severity.
METHOD
Subjects
Patients with pediatric BD were recruited from the pediatric mood disorders program at the University of Illinois at Chicago (UIC). This study was approved by the Institutional Review Board at UIC. Verbal or written assent was provided by all children in addition to the written informed consent by parents and adolescents over 15 years of age. The BD I (n=27) and BD II (n=19) unmedicated patients and HC (n=33) were between the ages of 8 and 18 years, and were matched on age, sex, parental socio-economic status, and intelligence as assessed by the 2-subtest version of the Wechsler Abbreviated Scale of Intelligence (WASI; Psychological Corporation, 1999). Inclusion criteria for the pediatric BD group were mixed, manic, or hypomanic state according to DSM-IV criteria (American Psychiatric Association, 1994) and medication free for at least one week prior to testing. Among the patient group, 19 participants (41%) had a comorbid diagnosis of attention deficit hyperactivity disorder (ADHD), 6 (13%) had a diagnosis of oppositional defiant disorder (ODD), 4 (.09%) had a diagnosis of generalized anxiety disorder (GAD), and 1 (.02%) had a diagnosis of social phobia. There were five patients who were medication naïve. There were 12 patients who participated in this study who went on to participate in a medication trial receiving lamotrigine (Type I=4; Type II=8) and another 7 (all Type I) that went on to a double blind placebo controlled trial of divalproex and risperidone. HC did not meet DSM IV criteria for any major present or lifetime psychiatric disorder (see Table 1).
Table 1.
Demographic and clinical characteristics of pediatric bipolar disorder type I (BD I) and type II (BD II), and healthy comparison subjects (HC). Means, standard deviations (SD), percentages, and significance values are presented below.
| Healthy Subjects | BDI | BDII | Analysis | |
|---|---|---|---|---|
|
| ||||
| Mean (SD) | Mean (SD) | Mean (SD) | F(p) | |
| Variables | ||||
| Age (years) | 13.03 (3.29) | 12.33 (3.27) | 14.42 (2.50) | 2.53 (.10) |
| Socioeconomic statusa | 1.94 (0.91) | 2.00 (0.96) | 2.00 (0.94) | 0.77 (.45) |
| YMRS | 1.85 (2.00) | 22.89 (8.60) | 12.32 (6.71) | 84.78 (<.0001) |
| CDRS-R | 19.52 (2.33) | 49.89 (15.76) | 52.47 (18.66) | 56.17 (<.0001) |
| WASIIQ | 108.55 (16.44) | 102.67 (18.54) | 100.26 (15.23) | 1.70 (.19) |
| N (%) | N (%) | N (%) | χ2(p) | |
| Sex | 2.10 (.35) | |||
| Male | 19 (58%) | 14 (52%) | 7 (37%) | |
| Female | 14 (42%) | 13 (48%) | 12 (63%) | |
| Race | 2.86 (.24) | |||
| Caucasian | 17 (52%) | 18 (67%) | 13 (68%) | |
| Other | 16 (48%) | 9 (33%) | 6 (32%) | |
| Comorbid Diagnoses | N (%) | N (%) | ||
| bADHD | 15 (56%) | 4 (21%) | ||
| cGAD | 2 (7%) | 2 (11%) | ||
| dODD | 4 (15%) | 2 (11%) | ||
| eSP | 1 (4%) | 0 | ||
Rated with Hollingshead Index of Social Position
Attention Deficit Hyperactivity Disorder
Generalized Anxiety Disorder
Oppositional Defiant Disorder
Social Phobia
Procedure
Each child and at least one of their parents were interviewed using the Washington University St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS; Geller et al., 1998) supplemented by the episode characterization of bipolar disorder from the KSADS- Present and Lifetime version (KSADS-PL; Kaufman et al., 1997), along with a comprehensive clinical interview. Clinical information from all available sources was combined to provide a consensus clinical diagnosis. The semi-structured interviews were completed by three doctoral level clinicians, with established inter-rater reliability, who had no knowledge of cognitive test performance. Live diagnostic interviews of ten cases were coded by the three clinicians to establish inter-rater reliability. By Cohen's Kappa, reliability of diagnoses was 0.96 between the raters. Clinical information from all sources was combined and further discussed to arrive at the final diagnosis in a weekly consensus conference involving the research team. Exclusion criteria for the entire sample were active substance abuse, serious medical problems, IQ <70, or the presence of another DSM-IV axis I diagnosis that required psychiatric intervention of any kind, with the exception of ADHD.
Measures
The neuropsychological test battery consisted of the Trail Making Test, the Digit Span subtest from the Weschler Memory Scale-Third Edition (WMS-III; Wechsler, Wycherley, & Benjamin, 1998), and the California Verbal Learning Test-Child Version (CVLT-C; Delis et al., 1994). The computerized neurocognitive test battery was compiled from the University of Pennsylvania Computerized Neuropsychological Battery (Gur et al., 2001) and Cogtest (Ventura et al., 2008). The Penn battery included the Continuous Performance Test, the Conditional Exclusion Test, and the Visual Object Learning Test. The Cogtest battery included the Set Shifting Test, the Controlled Oral Word Association Test, and the Spatial Span Test.
Statistical Analysis
To provide a standard metric for comparison across neurocognitive domains, test scores were standardized to z scores using the means and standard deviations from the healthy comparison group (Herbener et al., 2005; Pavuluri et al., 2006). Standardized test scores were combined to form domain composites for attention, executive functioning, working memory, visual memory, and verbal learning and memory. Internal consistencies of the scores comprising each neurocognitive domain were calculated using Cronbach's α and were reported in a previous article (Pavuluri et al., 2006).
A 3 (group) × 5 (neurocognitive domain) mixed model multivariate analyses of variance (MANOVA), with Greenhouse-Geisser correction, was used to assess for group differences between the BD and healthy comparison groups on the five neurocognitive composites along with follow-up post-hoc pairwise comparisons to assess for group differences between the BD I and II groups and healthy comparison subjects. Analyses were repeated with the presence of any comorbid DSM-IV axis I diagnosis (including ADHD) included as a covariate. Fisher's p was used to compare rates of ADHD diagnoses between the two BD groups because Fisher's p is less influenced by imbalanced cells or small sample sizes than the Chi-Square statistic. A 3 (group) × 5 (cognitive domain) MANOVA post-hoc analysis was done to examine group differences on each of the neurocognitive domains among healthy comparison subjects and BD youth with and without comorbid ADHD. Finally, associations between performance on the each of the neurocognitive domains and level of symptomatology were analyzed separately for each of the two BD groups using Pearson Correlation Coefficients.
RESULTS
As expected, there were significant differences between the groups on the YMRS (F(2,76)=84.78, p<.0001) and CDRS-R scores (F(2,76)=56.17, p<.0001) (see Table 1 for means and standard deviations). Both BD groups had higher YMRS scores compared to controls (ps<.0001), and BD I patients had significantly higher YMRS scores compared to BD II patients (p<.0001). Similarly, both BD groups had significantly higher CDRS-R scores compared to controls (ps<.0001), however, the BD I and II groups did not differ from each other on CDRS-R severity (p=.79).
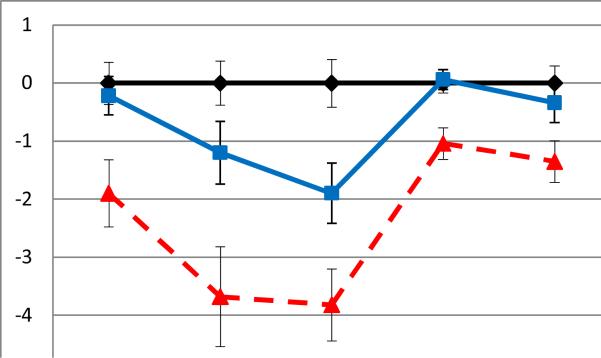
On the neurocognitive composites, there was a significant main effect of group (F(2, 76)=17.28, p<.0001, ηp2 =.31) and cognitive domain (F(3.28, 244.47)=10.46, p<.0001, ηp2 =.12). There was also a significant group × condition interaction (F(6.43, 244.47)=3.88, p<.001, ηp2 =.09), indicating that the profile of abilities across cognitive domains differed across the three groups. Post-hoc Tukey HSD tests indicated that BD I patients performed significantly more poorly compared to controls on all domains of cognitive functioning including attention (p<.01), executive function (p<.0001), verbal learning and memory (p<.0001), visual memory (p<.01), and working memory (p<.01). BD I patients also performed more poorly compared to BD II patients on attention (p<.01), executive function (p<.05), verbal learning and memory (p<.05), and visual memory (p<.01), but there were no differences between the groups on working memory (p=.13). BD II patients performed more poorly than controls on verbal learning and memory (p<.05); no other significant group differences between the BD II group and controls were observed (ps>.05). In terms of the observed pattern of overall performance between the three groups, the BD II patients had an intermediate pattern of performance between BD I patients and controls (see Figure 1). To test this, scores from the five cognitive domains were combined and averaged to form an overall cognitive functioning composite, and tests for a linear trend across the three groups indicated a significant effect (F(1, 78)=5.27, p<.05).
Figure 1.
Performance on the on the neurocognitive domains for healthy comparison subjects and bipolar disorder type I (BD I) and type II (BD II) pediatric patients. Average z scores (with standard error bars) are presented.
Youth with BD I displayed significantly more instances of comorbid ADHD compared to those with BD II (X2=5.48, p<.05). A 3 × 5 MANOVA examining differences between healthy controls and BD patients with (BD-ADHD) and without ADHD (BD only) indicated a significant main effect of group (F(2,76)=11.28, p<.0001, ηp2 =.23) and condition (F(3.22, 244.45)=11.47, p<.0001, ηp2 = .13) and a significant group × condition interaction (F(6.43, 244.45)=3.45, p<.005, ηp2 = .08). Post-hoc tests indicated that the BD-ADHD group performed significantly more poorly compared to controls on all five cognitive domains (attention p<.05, executive function p<.01, verbal learning and memory p<.0001, visual memory p<.05, working memory p<.05). The BD only group performed significantly more poorly compared to controls on the executive function (p<.05) and verbal learning and memory (p<.01) domains, but no significant differences were found between the groups on attention (p=.78), visual memory (p=.45), or working memory (p=.28). There were no significant differences between the BD-ADHD and the BD only groups on any of the five neurocognitive domains (ps>.05).
When the analyses were repeated with the presence of any comorbid DSM-IV axis I diagnosis (including ADHD) as a covariate, the findings did not change. Specifically, the main effects of group (F(2,75)=8.57, p<.0001, η2p=.19) and cognitive domain (F(3.21, 240.87)=6.54, p< .0001, ηp2 =.08), and the group × cognitive domain interaction (F(6.42, 240.87)=2.74, p< .05, ηp2=.07) remained significant. Post-hoc follow-up tests for group differences on each of the neurocognitive domains did not change from those reported above. The presence of a comorbid axis I disorder was not a significant covariate (p=.91).
Correlations between performance on the five cognitive domains and depressive and manic symptomatology were computed separately for each BD group. There were no significant associations between YMRS scores or CDRS-R scores and neurocognitive functioning among BD I or BD II pediatric patients (ps>.05).
DISCUSSION
This study is one of the first to examine differences in the neurocognitive profiles of BD I versus BD II pediatric patients. Both BD I and II patients display significant cognitive impairments compared to matched HC, with the most notable deficits in verbal learning and memory which were observed in both the BD groups. Additionally, BD I pediatric patients display more widespread and severe cognitive dysfunction, along with greater ADHD comorbidity compared to those with BD II, which is consistent with the adult BD literature and lends support for theories that BD I is a more severe and debilitating illness, with greater functional impairment and comorbidity (Hsiao et al., 2009; Simonsen et al., 2008; Torrent et al., 2006). Results remained consistent after controlling for additional psychiatric comorbid diagnoses.
Verbal learning and memory deficits were the most prominent finding, with both BD I and II pediatric patients, regardless of comorbid ADHD status, performing significantly more poorly than HC. Problematic performance on verbal-mediated tasks (i.e., learning and remembering auditory information, as well as language comprehension and production), have been one of the most reliable findings across past studies of cognitive dysfunction in pediatric BD patients (Doyle et al., 2005; McClure et al., 2005; Pavuluri et al., 2006, 2009a, Glahn et al., 2005). Findings are also consistent with the adult BD literature on verbal learning and memory deficits (Cavanagh et al., 2002; Glahn et al., 2004; Martinez-Aran et al., 2004) and suggest that these impairments likely represent a reliable cognitive endophenotype for the disorder (Balanza-Martinez et al., 2009). A recent meta-analysis of the literature comparing BD youth with those that have comorbid ADHD and healthy controls, indicate that deficits in verbal memory are the most specific to BD patients (Joseph et al., 2008). Deficits in verbal memory may be associated with abnormalities in the functional connectivity between frontal and mesial temporal systems in pediatric bipolar patients (Botteron et al., 1995; Gogtay et al., 2007). In line with this, structural imaging studies in adolescents with BD indicate reduced volumes in medial temporal lobe structures (Blumberg et al., 2003), and recent data from our laboratory using diffusion tensor imaging indicate abnormalities in frontotempo-occipital and prefrontal-bulbar tracts among pediatric BD patients (Pavuluri et al., 2009b).
Identifying the cognitive profiles across subtypes of pediatric BD is an important step toward identifying candidate genes associated with the disorder. Researchers are beginning to identify cognitive endophenotypes for BD through studies examining neuropsychological functioning among first-degree relatives of BD probands, with verbal learning and memory impairments being identified as the most promising candidate (Balanza-Martinez et al., 2008).
These findings also have important clinical implications. Verbal learning and memory is central for successful academic and psychosocial functioning. The ability to attend to and remember verbal information is essential for more complex cognitive functions such as vocal and subvocal rehearsal of new information, critical thinking, and problem solving. Deficits in these areas would cause significant functional impairment which would likely lead to increased distress and even greater difficulties in the ability to successfully interact with others, as well as study and learn new information (Martinez-Aran et al., 2004). Consistent with this, a recent longitudinal investigation among pediatric BD patients found that these youth show significant lags in the development of executive functions and verbal memory ability over time compared to their same age peers, and that these deficits are associated with greater academic impairment (Pavuluri et al., 2009a). Cognitive dysfunction would also likely interfere with the ability to benefit from psychotherapy techniques, psychoeducation, and social skills training (Simonsen et al., 2008).
Many of the functional impairments reported among BD youth such as difficulty listening to and following directions, remembering verbal instructions given by parents and teachers, and processing and remembering details while engaged in conversations and interactions with others can be explained by deficits in the ability to process verbally-mediated information. Therefore, rehabilitation interventions in pediatric BD patients should take into account cognitive impairments in verbal memory, as well as more general deficits in attention, executive functions, and working memory, and their relationship to real-world functional outcomes. Findings from this study also highlight the need to investigate the potential benefits of cognitive rehabilitation for BD youth, particularly for BD I patients who show the most severe and widespread cognitive dysfunction.
There were no significant differences in neurocognitive functioning between the BD-ADHD and BD only groups. However, the two groups displayed different profiles of cognitive dysfunction when compared to HC, with the BD-ADHD group performing worse on all cognitive domains while the BD only group differed from HC on just the executive function and verbal learning and memory domains. Past work examining neurocogitive functioning among youth with BD and comorbid ADHD has been varied (see Henin et al., 2009 for a review). Consistent with findings from this study, Dickstein et al. (2004) failed to find significant differences in cognitive impairment among pediatric BD patients with and without ADHD. However, earlier findings from our laboratory noted more impaired attention and executive function among comorbid BD youth (Pavuluri et al., 2006). Different sample characteristics among these studies (medicated versus unmedicated patients, BD I and II subtypes) may have accounted for differences in the findings. To clarify this issue, future studies of this should include ADHD symptomatology (in addition to diagnosis) and cognitive dysfunction in medicated and unmedicated subtypes of pediatric BD.
This study focused on an acute group of unmedicated pediatric BD patients, and therefore, findings can not be considered conclusive. However, utilizing an unmedicated sample did allow for careful control of the severity of manic symptoms resulting in a more homogenous sample of patients. It also allowed for the examination of neurocognitive functioning without the potential confounds of medication status. Treatments with mood stabilizers and antipsychotics on cognitive ability have been mixed, with some studies indicating more impaired functioning following treatment in adult and pediatric populations (Donaldson et al. 2003; Henin et al., 2009b; Holmes et al., 2008; Macqueen et al., 2003). Also, we did not find significant associations between manic or depressive symptomatology and neurocognitive performance, suggesting that the observed group differences are not associated with symptom severity and/or mood state. Controlled medication clinical trials using longitudinal follow-up designs are needed to better understand the potential influences of illness chronicity (e.g., illness onset and number of previous episodes), variations in mood state, and medication among different clinical phenotypes of pediatric BD, with specific focus on cognitive enhancement or impediment to cognitive function. Further, these studies should include medication naïve patients to avoid the potential confounds of previous medication status.
In conclusion, results from this investigation suggest that neurocognitive deficits are severe and pervasive among BD I pediatric patients relative to BD II patients and HC, and may represent a more cognitively impaired clinical subtype of the disorder. Verbal learning and memory deficits were common across both BD I and II subtypes, and may effectively differentiate pediatric BD patients and controls, regardless of the subtype of BD. Treatments in BD should target deficits in verbal learning and memory as this may be an important clinical endophenotype for the disorder. Cognitive remediation programs, including the implementation of computer-based cognitive enhancement training, are currently under way in our laboratory, and hold promise for enhancing cognitive skills in BD youth.
Key Points
Both type I and II pediatric bipolar disorder (BD) patients demonstrate cognitive impairment compared to matched healthy controls (HC), however, BD I youth show a more severe and pervasive pattern of dysfunction.
BD II pediatric patients show an intermediate pattern of dysfunction.
Verbal learning and memory deficits were common across both BD I and II subtypes and may effectively differentiate pediatric BD patients and HC.
Treatments in pediatric BD should target cognitive deficits, particularly verbal learning and memory impairments which may be an important endophenotype for the disorder.
Table 2.
Means and standard deviations (SD) on the five cognitive domains for the healthy comparison (HC), pediatric bipolar disorder type I (BD I) and type II (BD II) patients.
| Attention | Executive Functioning | Verbal Memory | Visual Memory | Working Memory | |
|---|---|---|---|---|---|
|
| |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| HC | 0.00 (2.08) | 0.00 (2.20) | 0.00 (2.36) | 0.00 (0.98) | 0.00 (1.71) |
| BD I | −1.90 (3.02) | −3.68 (4.47) | −3.82 (3.24) | −1.04 (1.39) | −1.35 (1.87) |
| BD II | 0.23 (1.46) | −1.20 (2.37) | −1.90 (2.26) | 0.06 (0.77) | −0.34 (1.48) |
Acknowledgments
This study is supported by grants MO1-RR-13987 and K23 NIH RR018638 awarded to Dr. Pavuluri.
REFERENCES
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Arts B, Jabben N, Krabbendam L, van Os J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first degree relatives. Psychological Medicine. 2008;38:771–785. doi: 10.1017/S0033291707001675. [DOI] [PubMed] [Google Scholar]
- Balanzá-Martínez V, Rubio C, Selva-Vera G, Martinez-Aran A, Sánchez-Moreno J, Salazar-Fraile J, Vieta E, Tabarés-Seisdedos R. Neurocognitive endophenotypes (endophenocognitypes) from studies of relatives of bipolar disorder subjects: A systematic review. Neuroscience and Biobehavioral Reviews. 2008;32:1426–1438. doi: 10.1016/j.neubiorev.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Glahn DC, Caetano S, Olvera RL, Fonseca M, Najt P, Hunter K, Pliszka SR, Soares JC. Evidence for disruption on prefrontal cortical functions in juvenile bipolar disorder. Bipolar Disorders. 2007;9:145–159. doi: 10.1111/j.1399-5618.2007.00453.x. [DOI] [PubMed] [Google Scholar]
- Blumberg MD, Kaufman J, Andrés M, Whiteman R, Hongyuan Zhang J, Gore JC, Charney DC, Krystal JH, Peterson BS. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Archives of General Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- Bora E, Yücel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. Journal of Affective Disorders. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Botteron KN, Vannier MW, Geller B, Todd RD. Preliminary study of magnetic resonance imaging characteristics in 8-to 16-year-olds with mania. Journal of the American Academy of Child & Adolescent Psychiatry. 1995;34:742–749. doi: 10.1097/00004583-199506000-00014. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Walter G, Malhi GS. Neurocognition in bipolar disorder and juvenile bipolar disorder. Journal of the Canadian Academy of Child and Adolescent Psychiatry. 2009;18:221–230. [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JT, Van Beck M, Blackwood DH. Case-control study of neurocognitive function in euthymic patients with bipolar disorder: An association with mania. British Journal of Psychiatry. 2002;180:320–326. doi: 10.1192/bjp.180.4.320. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober A. California Verbal Learning Test-Children's Version (CVLT-C) Psychological Corporation; San Antonio, TX: 1994. Manual. 1994. [Google Scholar]
- Deo AJ, Costa R, DeLisi LE, DeSalle R, Haghighi F. A novel analytical framework for dissecting the genetic architecture of behavioral symptoms in neuropsychiatric disorders. PLoS One. 2010;5:9714. doi: 10.1371/journal.pone.0009714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DP, Treland JE, Snow J, McClure EB, Mehta MS, Towbin KE, Pine DS, Leibenluft E. Neuropsychological performance in pediatric bipolar disorder. Biological Psychiatry. 2004;55:32–39. doi: 10.1016/s0006-3223(03)00701-7. [DOI] [PubMed] [Google Scholar]
- Donaldson S, Goldstein LH, Landau S, Raymont V, Frangou S. The Maudsley Bipolar Disorder Project: The effect of medication, family history and duration of illness on IQ and memory in bipolar I disorder. Journal of Clinical Psychology. 2003;64:86–93. [PubMed] [Google Scholar]
- Doyle AE, Wilens TE, Kwon A, Seidman LJ, Farone SV, Fried R, Swezey A, Snyder L, Beiderman J. Neuropsychological functioning in youth with bipolar disorder. Biological Psychiatry. 2005;58:540–548. doi: 10.1016/j.biopsych.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Doyle AE, Wozniak J, Wilens TE, Henin A, Seidmen LJ, Petty C, Fried R, Gross LM, Faraone SV, Biederman J. Neurocognitive impairment in unaffected siblings of youth with bipolar disorder. Psychological Medicine. 2009;39:1253–1263. doi: 10.1017/S0033291708004832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller B, Warner K, Williams M, Zimerman B. Prepubertal and young adolescent bipolarity versus ADHD: Assessment and validity using the WASH-U-KSADS, CBCL, and TRF. Journal of Affective Disorders. 1998;51:93–100. doi: 10.1016/s0165-0327(98)00176-1. [DOI] [PubMed] [Google Scholar]
- Glahn DÇ, Bearden CE, Niendam TA, Escamilla MA. The feasibility of neuropsychological endophenotypes in the search for genes associated with bipolar affective disorder. Bipolar Disorders. 2004;6:171–182. doi: 10.1111/j.1399-5618.2004.00113.x. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Caetano S, Fonseca M, Najt P, Hunter K, Pliszka SR, Olvera RL, Soares JC. Declarative memory impairment in pediatric bipolar disorder. Bipolar Disorders. 2005;7:546–554. doi: 10.1111/j.1399-5618.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Winkler AM, Kochunov P, Almasy L, Duggirala R, Carless MA, Curran JC, Olvera RL, Laird AR, Smith SM, Beckmann CF, Fox PT, Blangero J. Genetic control over the resting brain. PNAS Proceedings of the United States of America. 2010;107:1223–1228. doi: 10.1073/pnas.0909969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Ordonez A, Herman DH, Hayashi KM, Greenstein D, Vaituzis C, Lenane M, Clasen L, Sharp W, Giedd JN, Jung D, Nugent TF, Toga AW, Leibenluft E, Thompson PM, Rapoport JL. Dynamic mapping of cortical development before and after the onset of pediatric bipolar illness. Journal of Child Psychology and Psychiatry. 2007;48:852–862. doi: 10.1111/j.1469-7610.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Turner TH, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001;25:766–776. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Harkavy-Friedman JM, Keilp JG, Grunebaum MF, Sher L, Printz D, Burke AK, Mann JJ, Oquendo M. Are BPI and BPII suicide attempters distinct neuropsychologically? Journal of Affective Disorders. 2006;94:255–259. doi: 10.1016/j.jad.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Henin A, Micco JA, Wozniak J, Briesch JM, Narayan AJ, Hirshfeld-Becker DR. Neurocognitive functioning in bipolar disorder. Clinical Psychology: Science and Practice. 2009a;16:231–250. [Google Scholar]
- Henin A, Mick E, Biederman J, Fried R, Hirshfeld-Becker DR, Micco JA, Miller KG, Rycyna CC, Wozniak J. Is psychopharmacological treatment associated with neuropsychological deficits in bipolar youth? Journal of Clinical Psychiatry. 2009b;70:1178–1185. doi: 10.4088/JCP.08m04696. [DOI] [PubMed] [Google Scholar]
- Herbener ES, Hill SK, Marvin RW, Sweeney JA. Effects of antipsychotic treatment on emotion perception deficits in first-episode schizophrenia. American Journal of Psychiatry. 2005;162:1746–1748. doi: 10.1176/appi.ajp.162.9.1746. [DOI] [PubMed] [Google Scholar]
- Holmes M,K, Erickson K, Luckenbaugh DA, Drevets WC, Bain EE, Cannon DM, Snow J, Sahakian BJ, Manji HK, Zarate CA., Jr. A comparison of cognitive functioning in medicated and unmedicated subjects with bipolar depression. Bipolar Disorders. 2008;10:806–815. doi: 10.1111/j.1399-5618.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao Y, Wu Y, Wu JY, Hsu M, Chen H, Lee S, Lee I, Yeh T, Yang Y, Ko H, Lu R. Neuropsychological functions in patients with bipolar I and bipolar II disorder. Bipolar Disorders. 2009;11:547–554. doi: 10.1111/j.1399-5618.2009.00723.x. [DOI] [PubMed] [Google Scholar]
- Joseph MF, Frazier TW, Youngstrom EA, Soares JC. A quantitative and qualitative review of neurocognitive performance in pediatric bipolar disorder. Journal of Child and Adolescent Psychopharmacology. 2008;18:595–605. doi: 10.1089/cap.2008.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kurtz MM, Gerraty RT. A meta-analytic investigation of neurocognitive deficits in bipolar illness: Profile and effects of clinical state. Neuropsychology. 2009;23:551–562. doi: 10.1037/a0016277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E, Rich BA. Pediatric bipolar disorder. Annual Review of Clinical Psychology. 2008;4:163–187. doi: 10.1146/annurev.clinpsy.4.022007.141216. [DOI] [PubMed] [Google Scholar]
- Martínez-Arán A, Vieta E, Reinares M, Colom F, Torrent C, Sánchez-Moreno, Reinares M, Benabarre A, Goikolea JM. Cognitive impairment in euthymic bipolar patients: Implications for clinical and functional outcome. Bipolar Disorders. 2004;6:224–232. doi: 10.1111/j.1399-5618.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- McClure EB, Treland JE, Snow J, Dickstein DP, Towbin KE, Charney DS, Pine DS, Leibenluft E. Memory and learning in pediatric bipolar disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44:461–469. doi: 10.1097/01.chi.0000156660.30953.91. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Schenkel LS, Aryal S, Harral EM, Hill SK, Herbener ES, Sweeney JA. Neurocognitive function in unmedicated manic and medicated euthymic pediatric bipolar patients. American Journal of Psychiatry. 2006;163:286–293. doi: 10.1176/appi.ajp.163.2.286. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Mohammed T, Carbray JA, Sweeney JA. Enhanced working and verbal memory after lamotrigine treatment in pediatric bipolar disorder. Bipolar Disorders. 2010;12:213–220. doi: 10.1111/j.1399-5618.2010.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, West A, Hill SK, Jindal K, Sweeney JA. Neurocognitive function in pediatric bipolar disorder: 3-year follow-up shows cognitive development lagging behind healthy youths. Journal of the American Academy of Child Adolescent Psychiatry. 2009a;48:299–307. doi: 10.1097/CHI.0b013e318196b907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Yang S, Kamineni K, Passarotti AM, Srinivasan G, Harral EM, Sweeney JA, Zhou XJ. Diffusion tensor imaging study of white matter fiber tracts in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. Biological Psychiatry. 2009b;65:586–93. doi: 10.1016/j.biopsych.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski EO, Freeman LN, Mokros HB. Children's depressive rating scale-revised. Psychopharmacology Bulletin. 1985;21:979–989. [Google Scholar]
- Psychological Corporation . Wechsler Abbreviated Scale of Intelligence (WASI) Harcourt Brace & Company; San Antonio, TX: 1999. [Google Scholar]
- Robinson LJ, Thompson JM, Gallagher P, Goswami U, Young AH, Ferrier IN, Moore BP. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. Journal of Affective Disorders. 2006;93:105–115. doi: 10.1016/j.jad.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Rucklidge J. Impact of ADHD on the neurocognitive functioning or adolescents with bipolar disorder. Biological Psychiatry. 2006;60:921–928. doi: 10.1016/j.biopsych.2006.03.067. [DOI] [PubMed] [Google Scholar]
- Savitz J, van der Merwe L, Solms M, Ramesar R. Lateralization of hand skill in bipolar affective disorder. Genes, Brain, & Behavior. 2007;6:698–705. doi: 10.1111/j.1601-183X.2006.00299.x. [DOI] [PubMed] [Google Scholar]
- Schulze TG. Genetic research into bipolar disorder: The need for a research framework integrates sophisticated molecular biology and clinically informed phenotype characterization. Psychiatric Clinics of North America. 2010;33:67–82. doi: 10.1016/j.psc.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen C, Vaskinn A, Birkenaes AB, Engh JA, Hansen CF, Jósdóttir H, Ringen PA, Opjordsmoen S, Friis S, Andreassen OA. Neurocognitive profiles in bipolar I and bipolar II disorders: Differences in pattern and magnitude of dysfunction. Bipolar Disorders. 2008;10:245–255. doi: 10.1111/j.1399-5618.2007.00492.x. [DOI] [PubMed] [Google Scholar]
- Summers M, Papadopoulou K, Bruno S, Cipolotti L, Ron MA. Bipolar I and bipolar II disorder: Cognition and emotion processing. Psychological Medicine. 2006;36:1799–1809. doi: 10.1017/S0033291706008804. [DOI] [PubMed] [Google Scholar]
- Torrent C, Martínex-Arán A, Daban C, Sánchez-Moreno J, Comes Mercè, Goikolea J, Salamero M, Vieta E. Cognitive impairment in bipolar II disorder. British Journal of Psychiatry. 2006;189:254–259. doi: 10.1192/bjp.bp.105.017269. [DOI] [PubMed] [Google Scholar]
- van Gorp WG, Altshuler L, Theberge DC, Wilkins J, Dixon W. Cognitive impairment in euthymic bipolar patients with and without prior alcohol dependence: A preliminary study. Archives of General Psychiatry. 1998;55:41–46. doi: 10.1001/archpsyc.55.1.41. [DOI] [PubMed] [Google Scholar]
- Ventura J, Cienfuegos A, Boxer O, Bilder R. Clinical global impression of cognition in schizophrenia (CGI-CogS): Reliability and validity of a co-primary measure of cognition. Schizophrenia Research. 2008;106:59–69. doi: 10.1016/j.schres.2007.07.025. [DOI] [PubMed] [Google Scholar]
- Wechsler D, Wycherley RJ, Benjamin L. Wechsler Memory Scale: WMS-III. The Psychological Corporation; San Antonio, TX: 1998. Manual. [Google Scholar]
- Young RC, Biggs JT, Ziegeler VE, Mayer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]