Genes Are Highly Conserved. But Where Does Novelty Arise?
“The central problem for evolutionary biologists interested in development has been how morphology is transformed in evolution. In 1922, Walter Garstang made the very basic observation that because the morphology of animals arises anew in each generation, evolution of new animal forms has to be viewed as a problem in the evolution of development.” R. A. Raff (1)
For most of the past 100 years, the evolutionary origins of the mammalian cortex and the development of the mammalian cortex have been studied as if the two issues were completely unrelated to each other. The paper by Kuan et al. (2) in the current issue of the Proceedings raises a number of important issues for the fields of evolution and cortical development and attempts to bridge this gap. This paper also comes at a time in which molecular genetics has forced us to deal with the “meaning” of major gene families found across all vertebrates and invertebrates.
The Telencephalon of Nonmammals and the Phylogeny of Neocortex
Where Did Neocortex Come From?
Until the latter part of the twentieth century, the prevailing view of the origins of the mammalian neocortex consisted of a series of loosely conceived proposals, with the common theme that the mammalian neocortex arises within the thin pallial mantle region as found in reptiles. This supposed that all the specific neurons of the cortex, including those in receipt of thalamic input, interneurons, and output neurons, were newly evolved in mammals and were constitutively organized as a laminar structure. This hypothesis also implied that the specific sensory nuclei of the thalamus that provide the inputs to the cortex were also uniquely mammalian.
In attempting to understand the origins of the mammalian “neocortex,” the issue may be viewed as two separate experimental problems.
(i) How is the nonmammalian brain organized? This may tell us what is new and what is old. This requires the study of how the cells and circuitry of the nonmammalian forebrain is organized and which common features are shared by mammals and their close nonmammalian relatives (i.e., reptiles/birds).
(ii) What were the evolutionary transformations that account for the differences between mammals and nonmammals? This requires interpretation of the differences and similarities observed in the preceding studies and an analysis of the development of the forebrain in amniotes at molecular, cellular, and multicellular levels of organization.
While the analysis of cells and circuitry can easily stand on its own, the analysis of evolutionary transformations cannot be achieved in the absence of an understanding of what exactly has changed in the course of the evolution of the cortex.
The Evolutionary Origins of the Mammalian Neocortex
Based largely only on the descriptive macroanatomy of a limited number of nonmammalian brains, earlier workers concluded that nonmammals did not possess a neocortex. Consequently, previous hypotheses of the evolution of the cortex assumed that both the component circuitry and its particular configuration evolved as a single event. Thus, studies of the development of the mammalian neocortex proceeded on the assumption that it is a structure unique to mammals, without antecedents in nonmammalian vertebrates.
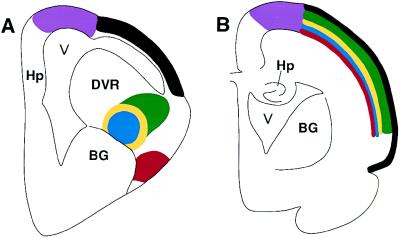
Recent comparative neuroanatomical and physiological studies have now shown that many of the specific cells and circuits that are found in mammalian neocortex also exist in the telencephalon of nonmammalian vertebrates (Fig. 1). This is particularly clear in regards to the auditory and visual systems.
Figure 1.
Schematic showing the organization of a sensory system in the bird/reptilian telencephalon (A) and the location of the homologous neurons in the mammalian cortex (B). The avian telencephalon has only a thin lateral cortex, and a prominent protrusion into the lateral ventricle (V), the dorsal ventricular ridge (DVR). The dorsal ventricular ridge contains several different neuronal populations, directly corresponding to those found in different layers of the mammalian neocortex, including thalamic recipient neurons, interneurons, and descending projections that leave the cortex to contact brainstem and spinal cord neurons. The dorsal ventricular ridge protrudes into the ventricle of birds and reptiles, and hence for many years was erroneously thought to be homologous to the mammalian basal ganglia (BG). In mammals (B), homologous populations of neurons are found within distinct layers of the cortex. The striking similarities in basic neuronal properties and circuits in reptiles, birds, and mammals led to the proposal that the specific neurons evolved prior to the evolutionary appearance of mammalian cortex. Thus, Karten and Shimizu (3) postulated that lamination occurred as an independent event in the course of amniote evolution. Hp, hippocampus.
The Demonstration of “Cortical Equivalent” Circuits in the Nonmammalian Forebrain.
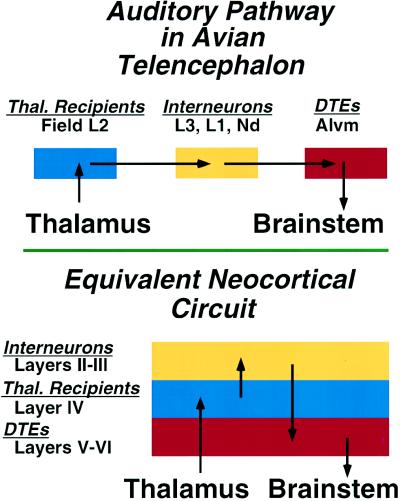
Studies of the organization of the auditory and visual pathways in the forebrain of nonmammals (4–6) have led me to postulate that the basic organization of sensory systems in the forebrain is similar in all amniotes—i.e., the fundamental telencephalic circuitry of auditory and visual pathways is common to all amniotes (Fig. 2). In reptiles/birds, the components are mainly found in discrete nuclei of the dorsal ventricular ridge. In mammals, the components are distributed in lamina, forming the cortex (Fig. 1). These findings have led to the proposal that the mammalian neocortex evolved in at least two separate steps.
Figure 2.
Schematic summary comparing the neuronal circuitry of auditory pathways in the dorsal ventricular ridge of the avian telencephalon and the equivalent neocortical circuit of the mammalian auditory cortex. In the avian forebrain, the populations of neurons corresponding to the individual laminae of cortex are organized as clusters, rather than as laminae. This circuit in the mammal is represented as a simplified three-layered cortex, consisting of a layer of thalamic recipient neurons (blue) forming layer IV, a group of interneurons (yellow) forming the more superficial layers, and a group of descending telencephalic efferents (DTEs) (red), the output neurons of this cortical region, forming layers V and VI. The morphology of individual neurons, their transmitters, and physiological properties at each parallel step of the circuit are virtually identical in bird dorsal ventricular ridge and mammalian cortex (6).
Circuits and Laminae Evolve Independently
In the first step, the constituent populations of neurons of each sensory system evolved as distinct cell types, with modality-specific connections. These populations are common to all amniotes and are found in the cortex of mammals and in the dorsal ventricular ridge of birds and reptiles, though they may have been specified much earlier in the course of vertebrate evolution. The second step, unique to the mammalian lineage, occurred with the lamination of these populations. Thus, lamination may be construed as an independent event in evolution, separate of the elaboration of the component cells and major connections (3). The objective functional benefits of the cortical versus the noncortical arrangement of homologous components is not immediately evident.
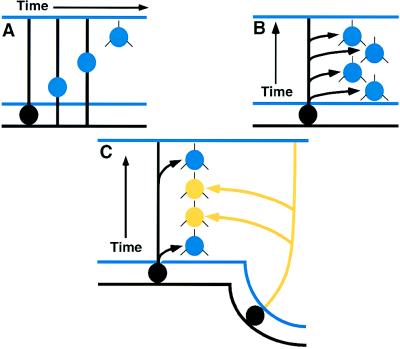
However, those of us concerned with evolutionary developmental neurobiology found that the models of cortical development in mammals could not be easily reconciled with recent information regarding the organization and development of the nonmammalian brain. Why not? In birds and reptiles, these presumably homologous neurons were found in the dorsal ventricular ridge and arose from the local ependyma. In mammals, the cortical neurons were thought to arise exclusively by radial migration from the underlying ependyma (Fig. 3). The growing body of evidence emphasized that the cells and circuits of the mammalian cortex and the nonmammalian forebrain are very similar and probably homologous (3). If the mammalian cortex was developing as reported in the literature, I could not imagine how to transform the nonmammalian embryonic telencephalon to that of mammals. A number of evolutionary neurobiologists suggested that the widespread similarities in forebrain organization between reptiles, birds, and mammals were evidence of convergent evolution, since the developmental data on mammalian cortex was impossible to reconcile with the models of embryological transformation (5) required to evolve a mammalian cortex from nonmammalian forebrains.
Figure 3.
(A) Radial migration of a single neuroblast from the underlying pallial ependyma. (B) Hypothesis of radial formation of the neocortex. Progressive waves of migration arise from a small local region of ependyma, forming a radial, or columnar, collection of cortical neurons of affiliated nature. (C) “Pallio-neuromeric” or “multimeric” origins of the neocortex. Tangential migrations, in conjunction with radial migrations, contribute to the formation of the cortex (4, 5). The cortex has been generally suggested to arise exclusively from the pallial ependyma, by events summarized in A and B. The source of the cells contributing to the tangential migration has been uncertain. Karten (4) postulated that such tangentially migrating neurons were derived from early embryonic neuromeres of the telencephalon (C). This notion was inconsistent with a strict radial migration hypothesis. Recent findings in mammals, however, are now compatible with this older hypothesis by Nauta and Karten (5) but still do not prove it to be true.
Homeobox Genes and Prosomeres: Development of Forebrain at Early Stages When Evolutionary Divergence May Be Most Explicitly Manifest
Although there is a large body of literature on the development of specific phenotypic characteristics of the mammalian cortex (including when particular populations of cortical neurons undergo final division, when various transmitters, receptors, and peptides are first evident and when thalamo-cortical connections are established), the events during the very early stages of development of the forebrain have received little attention until recently. The discovery of the expression of Hox genes in neuromeres of the brainstem (7, 8) has increased interest in the prospect of understanding the cellular and molecular events underlying the development of the forebrain. Thus, Rendahl’s (9) description of segmentation in the embryonic diencephalon has attracted new attention, as have the studies by Källén (10–13), Vaage (14), Keyser (15), and Figdor and Stern (16). Studies of the selective expression of various homeobox genes in these regions, as well as in the telencephalon (17–19), have attracted much attention. In conjunction with the work of Walsh and Cepko (20, 21) and others, issues long thought resolved have been subject to reexamination.
The Källén Hypothesis.
Between 1951 and 1960, Bengt Källén, the Swedish embryologist, published a series of intricate papers on the embryogenesis of the vertebrate brain. He emphasized the striking similarity in developmental organization of the brain stem and the thalamus of all vertebrates. Although largely ignored for 35 years, his work on the brainstem has recently been re-discovered in light of the recent work on the development of rhombomeres and the expression of Hox genes. However, his most complex, and most obscure, studies were those on the amniote telencephalon. He directly compared the development of reptiles, birds, and mammals, and suggested that subdivisions of the avian and reptilian dorsal ventricular ridge, the dorsal lateral ventricular ridge (4), and the archistriatum contained “proliferation/migration areas” that are “homologous to the cortex.” The basis for his conclusion was not clearly stated. His later papers on the development of the telencephalon contained only limited documentation, and the basis of his conclusions were not explicitly stated. Despite Källén’s obvious familiarity with the concept of neuromeres, he expressed uncertainty as to whether the “proliferation/migration areas” of the forebrain should be classified as neuromeres, despite their many similarities to those of the rhombencephalon. Källén’s reluctance to classify these zones as prosomeres may be consequent to the difficulties associated with attempting to define a longitudinal axis of segmentation with dorsal to ventral boundaries in the prenotochordal regions, comparable to that of the segmentation observed in the rhombencephalon. Vaage (14), upon reviewing the embryogenesis of chicken brainstem, concluded that he could identify regions of the telencephalon that could justifiably be classified as neuromeres, using Meek’s (22) term of “prosomeres”—i.e., neuromeres of the pros-encephalon. Although he also gave no basis for his conclusions, he included a summary diagram suggesting the presence of six to eight “prosomeres.” A precise definition of prosomeres is lacking (23), and though its use is disputed, I will use “prosomeres” interchangeably with Källén’s “proliferation/migration areas” (14).
A number of recent studies (17, 19, 24, 25) have attempted to broach the problem of telencephalic development by studying the regional expression domains of various transcription factor and homeobox genes in the mammalian embryo. This area of research is still rapidly evolving, and no clear formulations can be advanced that are acceptable to all workers. The mechanism by which many of these genes exert their effects is not at all evident at this time. However, they have been used very effectively to justify postulated regional subdivisions (9, 16) based on the restricted expression of these genes within morphologically defined areas at specific stages of development (17). Though such measures of validity are rather tenuous, the unique nature of many of these genes, and their phylogenetic similarities based as positional “markers” in Drosophila and mammals, is most remarkable. In comparison to the difficulties in accounting for similarities in gene expression in fruit flies and humans, mere differences between birds and mammals must surely appear trivial in extent. But my concern is with this now seemingly minor chasm.
Does Cortex Arise by Reshuffling Prosomeres?
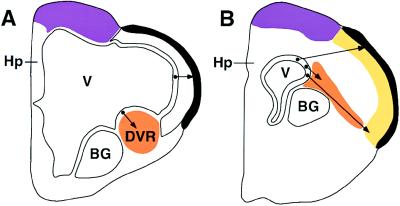
The number of proliferative areas in the lateral wall of the telencephalon representing individual prosomeres is still disputed. Two major early prosomeres form the basal and dorsal ventricular ridges. The basal ganglia and bed nucleus of the stria terminalis arise within the basal ventricular ridge. A number of prosomeres in the dorsal ventricular ridge generate the neuronal populations homologous with individual laminae of cortical neurons of mammals. I suggest that these regions can be traced back to Källén’s separate proliferation areas in early embryogenesis, and that early stages of mammalian embryogenesis will reveal a similar pattern of development, with similar prosomeres. I suggest that these prosomeres can be found in either of two configurations: (i) in birds and reptiles, reflecting the ancestral condition common to all amniotes, they contribute to the dorsal ventricular ridge, with neurons migrating into the various regions as found in the adult bird; and (ii) in mammals, these prosomeres are transposed and become components of the “subventricular zones” described by Stensaas and Gilson (26) as areas of proliferation separate of the ependymal zone and contribute to the formation of the neocortex (Figs. 3 and 4). The population of cells identified by Kuan et al. (2) in the current issue of the Proceedings may represent the derivatives of one of the prosomeres.
Figure 4.
More than one mechanism may be involved in the embryogenesis of the mammalian cortex. Different cortical areas may be derived from quite different proliferation zones. The pattern of migration of neurons suggested in Fig. 3C may help clarify the differing proposals regarding cortical development of the lateral neocortex of mammals. (A) In birds and reptiles, the thin lateral cortex arises by radial migration from the lateral ependyma. Neurons arising from the ependyma of the ventrolateral wall of the ventricle (V) migrate ventrolaterally to form the dorsal ventricular ridge (DVR) (orange). Neurons of the dorsal ventricular ridge form the discrete populations of thalamic recipients, interneurons, and descending telencephalic efferent homologous to those of the mammalian cortex. (B) In the mammalian embryonic forebrain, the subventricular zone of Stensaas and Gilson (26) (orange) may directly contribute to the formation of the neocortex (yellow), by lateral or tangential migration, merging with radially migrating cells of the cortical ependyma, as postulated in Fig. 3C. The dorsomedial cortex (purple) of birds and mammals may form by a different combination of founder cells, and generate the so-called primary visual cortex, and the more medial hippocampal (Hp) formation. BG, basal ganglia.
This also implies that the cortex does not arise by a single unitary developmental mechanism. Different cortical areas may receive greater contributions from one prosomere, and little or none from another. The puzzle may be what makes them share so many organizational properties, if they are generated by different developmental mechanisms?
What is the prospect that there is a master gene that determines which direction these multiple prosomeres will choose—the avian or mammalian form of migration? Although we cannot be too optimistic about being able to uncover such a gene in the next few years, in light of the dramatic rate of current progress, we finally have the prospect of a concrete set of testable experiments that will allow us to evaluate this long-standing riddle of the evolutionary origins of the mammalian neocortex.
Columnar Organization: Emergent Property or Morphological Reality?
One of the most compelling issues regarding cortical development over the past 30 years has centered on the notion of columnar organization of the neocortex. Mountcastle, Hubel, Wiesel, and others have all emphasized the importance of radial organization in cortical function. In attempting to provide a morphological substrate for their observations, many developmental studies were interpreted to support the notion that most of the neurons within a functional radial column were derived from a limited number of precursor neuroblasts. Thus, from a developmental point of view, the laminar location of a cell in the cortex was presumed to be secondary to the vertical migration from a common precursor (27). Most recently, the use of retroviral vector markers in primates, as well as chimeric and transgenic rodents appears to add support to Rakic’s radial migration hypothesis (28–32). The concept of the “clonal” origin of a radial column has enjoyed great popularity over the past two decades. This “radial unit hypothesis” posed particularly thorny problems for my hypothesis that suggested that individual laminae of cortex had distinctive identities with independent evolutionary origins. How could I reconcile the possibility that a common precursor cell acted to generate all the layers, when my model of reptiles/birds proposed that different layers arose from different proliferation areas?
Within the past several years, however, the “radial unit” columnar hypothesis has been increasingly subject to question, and recent developmental studies have seriously challenged one of major the underlying presumptions—i.e., that a limited number of “founder” cells in the ependyma migrate radially to form distinct columns, and thereby establish the overlying cytoarchitecture (33). Initially, Walsh and Cepko (20, 21), using retroviral gene transfer to label clonally related cells, observed that there was tangential migration of developing neurons, with contributions to each cortical area possibly arising from widely separated anlage. The work of O’Rourke et al. (34) further extended this with a direct observation of substantial migrations of neurons in developing cortical slices, thus confirming the earlier work of Stensaas (35–37).
These diverse findings must eventually be reconciled with the earlier suggestion that radial migration alone is an adequate mechanism to explain the formation of the cortex. In the current study, Kuan et al. (2) have shown that individual deep horizontal laminae of the cortex arise from different clonal anlagen than do the more superficial radial columns. Though the origins of individual laminae and their manner of migration are still hotly debated, the recent findings overcome a major obstacle to understanding the evolutionary origins of the mammalian cortex.
The current studies by Kuan et al. (2) provide a hint of the validity of two major aspects of the hypothesis that multiple neuromeres contribute neurons to the developing cortex: (i) they demonstrate that the neurons of the deeper layers of cortex arise as a tangential population from a common anlage, whereas those of the more superficial layers arise from a different anlage and do appear to arise as radial units; and (ii) the comprehensive radial organization may be an emergent property of the cortex, not one intrinsic to the functional populations. Perhaps one of the more important implications of their work, and one that they stress, is the essential role of examining mammalian development in light of evolutionary origins of the cortex.
Conclusions
An understanding of the cellular and molecular bases of the evolutionary transformations associated with the origins of cortex may clarify events occurring in the early stages of embryogenesis of the mammalian cortex. After more than 150 years of speculation regarding the evolutionary origins of the mammalian neocortex, experimental biology may finally be able to approach this issue with direct experimental methods. Work to date has shown that homologous populations of neurons are found in the avian/reptilian dorsal ventricular ridge and the mammalian neocortex. Thus, neuronal specification (e.g., morphology, transmitters, and connections) and lamination are two independent events contributing to the evolutionary origins of the mammalian neocortex. Combined efforts in the area of molecular embryology and traditional experimental embryology may finally permit us to establish the sequence and determinants of morphological changes that have led to the origins of the mammalian neocortex.
Acknowledgments
I would like to express my gratitude to Kevin “Casey” Cox for his patience and determination in trying to convert my priceless prose into comprehensible English. Many thanks to Pasko Rakic for his intellectually provocative ideas and useful discussions on cortical development, as well as to Jerry Chun, Susan McConnell, and William Hodos. This work has benefited by support of the National Institute of Neurological Disorders and Stroke (Grant NS24560-11) and the National Eye Institute (Grant EY06890-12).
References
- 1.Raff R A. The Shape of Life: Genes, Development, and the Evolution of Animal Form. Chicago: Univ. of Chicago Press; 1996. p. 23. [Google Scholar]
- 2.Kuan C, Elliot E A, Flavell R A, Rakic P. Proc Natl Acad Sci USA. 1997;94:3374–3379. doi: 10.1073/pnas.94.7.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karten H J, Shimizu T. J Cognit Neurosci. 1989;4:291–301. doi: 10.1162/jocn.1989.1.4.291. [DOI] [PubMed] [Google Scholar]
- 4.Karten H J. Brain Behav Evol. 1991;38:264–272. doi: 10.1159/000114393. [DOI] [PubMed] [Google Scholar]
- 5.Nauta W J H, Karten H J. In: The Neurosciences: Second Study Program. Schmitt F O, editor. New York: The Rockefeller Univ. Press; 1970. pp. 7–26. [Google Scholar]
- 6.Wild J M, Karten H J, Frost B J. J Comp Neurol. 1993;337:32–62. doi: 10.1002/cne.903370103. [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson D G, Krumlauf R. Trends Neurosci. 1990;13:335–339. doi: 10.1016/0166-2236(90)90145-z. [DOI] [PubMed] [Google Scholar]
- 8.Lumsden A, Keynes R. Nature (London) 1989;337:424–428. doi: 10.1038/337424a0. [DOI] [PubMed] [Google Scholar]
- 9.Rendahl H. Acta Zool. 1924;5:241–344. [Google Scholar]
- 10.Källén B. K Fysiogr Saelllsk Lund Handl. 1951;62:5. [Google Scholar]
- 11.Källén B. Acta Soc Med Ups. 1952;57:111–118. [PubMed] [Google Scholar]
- 12.Källén B. J Embryol Exp Morphol. 1953;1:387–392. [Google Scholar]
- 13.Källén B. Ark Zool. 1959;12:137–142. [Google Scholar]
- 14.Vaage S. Ergeb Anat Entwicklungsgesch. 1969;41:3–87. [PubMed] [Google Scholar]
- 15.Keyser A. Acta Anat Suppl. 1972;59:1–178. [PubMed] [Google Scholar]
- 16.Figdor M C, Stern C D. Nature (London) 1993;363:630–634. doi: 10.1038/363630a0. [DOI] [PubMed] [Google Scholar]
- 17.Bulfone A, Kim H J, Puelles L, Porteus M H, Grippo J F, Rubenstein J L. Mech Dev. 1993;40:129–140. doi: 10.1016/0925-4773(93)90071-5. [DOI] [PubMed] [Google Scholar]
- 18.Puelles L, Rubenstein J L. Trends Neurosci. 1993;16:472–479. doi: 10.1016/0166-2236(93)90080-6. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez-Bolado G, Rosenfeld M G, Swanson L W. J Comp Neurol. 1995;355:237–295. doi: 10.1002/cne.903550207. [DOI] [PubMed] [Google Scholar]
- 20.Walsh C, Cepko C L. Experientia. 1990;46:940–947. doi: 10.1007/BF01939387. [DOI] [PubMed] [Google Scholar]
- 21.Walsh C, Cepko C L. Science. 1992;255:434–440. doi: 10.1126/science.1734520. [DOI] [PubMed] [Google Scholar]
- 22.Meek A. Anat Anz. 1907;31:408–415. [Google Scholar]
- 23.Guthrie S. Trends Neurosci. 1995;18:74–79. doi: 10.1016/0166-2236(95)80027-y. [DOI] [PubMed] [Google Scholar]
- 24.Simeone A, Acampora D, Gulisano M, Stornaiuolo A, Boncinelli E. Nature (London) 1992;358:687–690. doi: 10.1038/358687a0. [DOI] [PubMed] [Google Scholar]
- 25.Simeone A, Gulisano M, Acampora D, Stornaiuolo A, Rambaldi M, Boncinelli E. EMBO J. 1992;11:2541–2550. doi: 10.1002/j.1460-2075.1992.tb05319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stensaas L J, Gilson B C. Z Zellforsch Mikrosk Anat. 1972;132:297–322. doi: 10.1007/BF02450711. [DOI] [PubMed] [Google Scholar]
- 27.Rakic P. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 28.Kornack D R, Rakic P. Neuron. 1995;15:311–321. doi: 10.1016/0896-6273(95)90036-5. [DOI] [PubMed] [Google Scholar]
- 29.Natkatsuji M, Kadokawa Y, Suemori H. Dev Growth Differ. 1991;33:571–578. doi: 10.1111/j.1440-169X.1991.00571.x. [DOI] [PubMed] [Google Scholar]
- 30.Tan S-S, Faulkner-Jones B, Breen S J, Walsh M, Bertram J F, Reese B E. Development (Cambridge, UK) 1995;121:1029–1039. doi: 10.1242/dev.121.4.1029. [DOI] [PubMed] [Google Scholar]
- 31.Soriano E, Dumesnil N, Auladell C, Cohen-Tannoudji M, Sotelo C. Proc Natl Acad Sci USA. 1995;92:11676–11680. doi: 10.1073/pnas.92.25.11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rakic P. Proc Natl Acad Sci USA. 1995;92:11323–11327. doi: 10.1073/pnas.92.25.11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bayer S A, Altman J. Neocortical Development. New York: Raven; 1991. [Google Scholar]
- 34.O’Rourke N A, Dailey M E, Smith S J, McConnell S K. Science. 1992;258:299–302. doi: 10.1126/science.1411527. [DOI] [PubMed] [Google Scholar]
- 35.Stensaas L J. J Comp Neurol. 1967;130:149–162. doi: 10.1002/cne.901300204. [DOI] [PubMed] [Google Scholar]
- 36.Stensaas L J. J Comp Neurol. 1967;131:409–422. doi: 10.1002/cne.901310402. [DOI] [PubMed] [Google Scholar]
- 37.Stensaas L J. J Comp Neurol. 1967;131:423–436. doi: 10.1002/cne.901310403. [DOI] [PubMed] [Google Scholar]