Abstract
Background
Chronic hyperglycemia in type 2 diabetes increases the risk of microvascular events. However, there is continuing uncertainty about its effect on macrovascular outcomes and death. We conducted a meta-analysis of prospective studies to estimate the association of glycosylated hemoglobin level with the risk of all-cause mortality and cardiovascular outcomes among patients with type 2 diabetes.
Methodology/Principal Findings
We systematically searched the MEDLINE database through April 2011 by using Medical Subject Heading search terms and a standardized protocol. We included prospective cohort studies that reported data of glycosylated hemoglobin level on the risk of incident cardiovascular events and all-cause mortality. Relative risk estimates (continuous and categorical variables) were derived or abstracted from each cohort study. Twenty six studies were included in this analysis with a mean follow-up rang of 2.2–16 years. The pooled relative risk associated with a 1% increase in glycosylated hemoglobin level among patients with type 2 diabetes was 1.15 (95% CI, 1.11 to 1.20) for all-cause mortality, 1.17 (95% CI, 1.12 to 1.23) for cardiovascular disease, 1.15 (95% CI, 1.10 to 1.20) for coronary heart disease, 1.11 (95% CI, 1.05 to 1.18) for heart failure, 1.11 (95% CI, 1.06 to 1.17) for stroke, and 1.29 (95% CI, 1.18 to 1.40) for peripheral arterial disease, respectively. In addition, a positive dose-response trend existed between glycosylated hemoglobin level and cardiovascular outcomes.
Conclusions/Significance
Chronic hyperglycemia is associated with an increased risk for cardiovascular outcomes and all-cause mortality among patients with type 2 diabetes, likely independently from other conventional risk factors.
Introduction
Type 2 diabetes continues to be one of the most common and important public health crises worldwide. It has been estimated that the global health expenditure on diabetes is at least $376 billion in 2010 and will be $490 billion in 2030 [1]. Type 2 diabetes is a major risk factor for cardiovascular disease (CVD). Patients with type 2 diabetes have a 2–4 times higher risk of CVD mortality than those without diabetes [2], [3]. CVD accounts for approximately 70% of death among patients with type 2 diabetes [4], [5].
The key risk factor associated with diabetes complications is poor glycemic control [6]. Growing evidence has linked chronic hyperglycemia to microvascular complications [7]–[9]. Although improving glycemic control has been demonstrated to reduce microvascular complications in patients with type 2 diabetes [9], [10], the relationship between glycosylated hemoglobin (GHb) level and macrovascular complications and all-cause mortality is still uncertain. In three meta-analyses [11]–[13] of published randomized controlled trials (RCTs), intensive glycemic control showed positive effects on some cardiovascular outcomes, but did not reduce the risk of death from CVD and all causes. However, these studies were constrained by inherent limitations of the clinical trials, which might have been underpowered to show clinical benefits - especially if event rates were lower than expected or the studies had a high lost follow-up rate [14].
A meta-analysis of 10 prospective studies evaluating the association of GHb level with CVD risk among patients with type 2 diabetes has found that every 1% increase in GHb was associated with an 18% increase in the risk of CVD events [15]. However, this meta-analysis did not estimate the association of GHb level with the risk of all-cause mortality. Moreover, among all prospective studies included, only two studies had a baseline sample size greater than 2,000 [16], [17]. In recent years, several high quality and large prospective studies have assessed the association of GHb level with the risks of CVD outcomes and/or all-cause mortality in patients with type 2 diabetes, but the results are inconsistent. With respect to all-cause mortality, a U-shape association [18] and a non-linear positive association [19] have been reported in these cohort studies. For CVD outcomes, most studies reported a positive association [19]–[24], whereas one study found no relation [25]. In addition, some data from previous cohort studies have been updated recently [17], [26], [27].
To clarify whether lowering long-term GHb level can reduce the risks for CVD outcomes and all-cause mortality, we performed a systematic review and meta-analysis with the most updated prospective data to evaluate the association of GHb level with the risks of incident CVD outcomes and all-cause mortality in patients with type 2 diabetes.
Methods
Data Sources and Searches
We searched the MEDLINE database for articles published in English from January 1974 to April 2011 by using Medical Subject Heading (MeSH) terms cardiovascular diseases; coronary heart disease; heart failure; stroke; peripheral arterial disease; all-cause mortality; and diabetes mellitus, type 2, as well as glycemic control, and glycosylated hemoglobin or HbA1c. We also performed a manual searching of references cited by original studies and relevant review articles and queried experts to identify any additional studies. This search provided 3123 articles, which were further screened for inclusion from abstracts or full texts.
Study Selection
We selected the studies based on the following conditions: 1) study design: prospective cohort studies; 2) study population: patients with type 2 diabetes; 3) studies reported at least one of the outcomes of interest: cardiovascular outcomes (CVD, CVD mortality, coronary heart disease (CHD), fatal CHD, heart failure, and stroke), peripheral arterial disease (PAD), and all-cause mortality; and 4) studies reported a measure of GHb (HbA1C, HbA1, and total GHb). We first identified 55 full-text articles and then excluded some if they 1) had no original data (review, editorials, meta-analyses), 2) involved non prospective analysis (e.g., nest case–control studies), 3) had follow-up time <1 year, 4) included patients with type 2 diabetes receiving dialysis, or with heart failure, or with disability, or 5) were duplicate publications. If separate articles from the same study were published, the article with the most updated data was selected for use in this study. In the case of duplicate publications, only one publication was included.
Data Extraction and Quality Assessment
Data were extracted by two independent reviewers (ZY and GH) using standardized data abstraction forms. Disagreements between reviewers were resolved by repeated examination of the original articles and discussion until consensus was achieved. Information on surname of the first author, year of publication, country of origin, mean age, percentage of male of study participants, sample size, number of study participants included in the final analysis, duration of follow-up, outcomes, estimate of the risk of association, variables adjusted in the analyses was extracted. For assessment of study quality, we evaluate 6 major items of each study: 1) is the instrument for measuring GHb validated? 2) does GHb allow quantification as both continuous and categorized variables? 3) are the outcomes determined by the specified criteria (i.e., medical record) or physician’s or patient’s judgments such as registry, death certificate, questionnaire, and patients’ self-report? 4) is the total follow-up duration ≥5 years? 5) are major CVD risk factors for in the statistical analyses, such as age, sex, blood pressure (hypertension), dyslipidemia (or LDL/total cholesterol level), smoking, duration of diabetes, treatment, albuminuria, etc? and 6) are subjects lost-to-follow up excluded from the analysis?
During data extraction, we abstracted adjusted relative risk (RR) for the association between GHb level either as a continuous or a categorical variable and the major outcomes (see below). Standard errors (SEs) for the estimates were abstracted or derived by using data reported in the original studies. When necessary, the original authors were contacted for additional information (3 authors contacted and 2 responded).
Reviewers recorded the following as the major outcomes of interest: all-cause mortality, incident CVD (non-fatal myocardial infarction, non-fatal stoke, and fatal CVD), CVD mortality, incident CHD (non-fatal myocardial infarction, and fatal CHD), CHD mortality, heart failure (non-fatal and fatal heart failure), incident stroke (non-fatal and fatal stoke), and PAD (lower-extremity peripheral arterial disease, amputation, and claudication).
Data Synthesis and Analysis
Separate meta-analyses of the prospective cohort studies were carried out for the above major outcomes. All RRs estimates included in the pooled analyses were from the most fully adjusted multivariable models.
Most of the studies included in the present analysis reported the RRs of per unit change of GHb level, therefore, we converted studies that used different units in their original analyses based on the method previously published [15]. For example, there were 3 studies [28]–[30] that compared the RR for participants in the 3rd tertile of GHb to participants in the 2 lowest tertiles. In order to make these results comparable to the rest of studies, we assumed that there was a normal distribution for GHb values and used the reported mean and standard deviation (SD) to estimate the 33rd and 83rd percentiles of GHb (corresponding to the midpoints of the 2 lowest and the highest tertiles, respectively). Then, we divided the log RRs by the difference of these 2 values to estimate the effect of a 1% change in HbA1 [31]. Similarly, for one study [32] that compared the reported RR of above and below the median value of GHb, we estimated the effect of a 1% change but calculated the 25th and 75th percentiles instead.
After the RR estimate from each cohort study was converted to reflect a 1% increase in GHb [31], the pooled RRs and 95% confidence intervals (CIs) were calculated using the random-effects model. Statistical heterogeneity was assessed using the DerSimonian and Laird’s Q statistic and I2 statistic. The Q test provides information about the presence or absence of between-study heterogeneity, whereas the I2 statistic quantifies the degree of heterogeneity and is interpretable as the percentage of the total association that may be due to heterogeneity between studies (I2>50% was considered a meaningful level of heterogeneity). We also conducted a sensitivity analysis in which each prospective cohort study was excluded in turn to evaluate the influence of that prospective cohort study on the overall estimate. Publication bias was examined using funnel plots and Begg’s test. A meta-regression analysis was conduced to explore the sources of statistical heterogeneity in the meta-analyses. Subgroup analyses were conducted by stratifying the analysis according to studies that in different areas. All analyses were conducted by using STATA 10.0 (Stata Corporation, College Station, TX).
Results
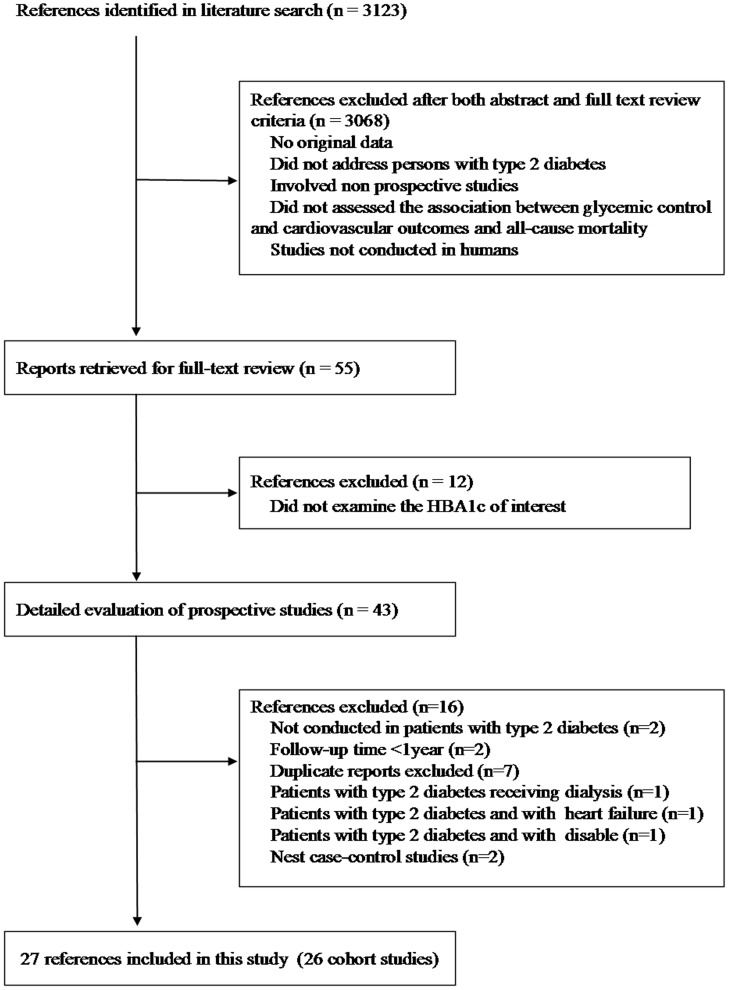
Of 3123 articles that were identified from the literature search, 3068 were excluded after an abstract or full-text review (Figure 1). Of the 55 articles for further review, 43 articles were relevant to GHb, macrovascular outcomes and all-cause mortality. Of these 43 articles, we excluded two that were not conducted in patients with type 2 diabetes [33], [34], two with follow-up time less than 1 year [35], [36], seven that were duplicated reports [5], [26], [37]–[41], one that had type 2 diabetes patients with heart failure at baseline [42], one with hemodialysis [43], and one with disable older women [44]. In addition, we excluded two nest case-control studies because of we were unable to perform the pooled analysis with other studies [45], [46]. Finally, 27 articles [16]–[25], [27]–[30], [32], [47]–[58] that reported 26 independent prospective cohorts were included in the present meta-analysis. Of them, two articles reported the same outcome with the same cohort study but using different analyses: one used continuous variable [56] and the other used categorical variable [22].
Figure 1. Flow diagram of studies assessed and included.
Table 1 summarizes the characteristics of the studies included in the present analysis. The sample size ranged from 94 to 48,858 participants, 10 studies (38%) had more than 3,000 patients of type 2 diabetes, and 3 studies [18], [21], [24] (12%) had more than 45,000. The mean follow-up time ranged from 2.2 to 16 years. The studies included were geographically heterogeneous: 5 were conducted in the United States (US), 7 in United Kingdom (UK), 4 in Finland, 2 in The Netherlands, 1 in Sweden, 1 in Denmark, 1 in Italy, 1 in Germany, 2 in New Zealand, and 2 in China. Most studies had primary care or clinic-based patient populations. Both men and women were included in 25 of the 26 studies; the remaining study included only men [51].
Table 1. Design Characteristics of Prospective cohort Studies of Glycosylated Hemoglobin and Cardiovascular Outcomes and All-cause Mortality, 1974–2011. * .
| Study name | Country | Project | No. of subjects | Age(y) | Men(%) | Outcome | Follow-uptime, y | HbA1c variable | Adjusted covariates | |
| Continuous | Category | |||||||||
| Stratton et al.,2006 (47) | United Kingdom | UKPDS 75 | 4320 | 53 | 60 | All-cause mortality,CVD, CHD, Stroke | 10.4 | √ | Age, sex, BP, lipid, smoking, | |
| Adler et al., 2002 (16) | United Kingdom | UKPDS 59 | 3834 | 60 | 60 | PAD | 6 | √ | Age, BP, lipid, smoking, BMI | |
| Adler et al., 1999 (25) | United Kingdom | UKPDS 47 | 5063 | 53 | 59 | CVD, CHD, Stroke | 10–10.3 | √ | Age, sex | |
| Stratton et al., 2000 (17) | United Kingdom | UKPDS 35 | 3642 | 60 | 53 | Heart Failure | 10.4 | √ | Age, sex, BP, lipid, smoking, albuminuria | |
| Mattock et al., 1998 (48) | United Kingdom | London DiabetesClinic | 146 | 59 | 56 | All-cause mortalityCHD mortality | 7 | √ | Age, sex, BP, lipid, albuminuria, duration of DM | |
| Currie et al., 2010 (18) | United Kingdom | Primary care GPRD | 47970 | 64 | 55 | All-cause mortality | 3.9–4.4 | √ | Age, sex, lipid, smoking, DM medication | |
| Donnan et al. 2006 (58) | United Kingdom | Primary care | 4569 | 54.7 | 52.6 | CHD | 9.5 | √ | Age, sex, BP, lipid, smoking, BMI, duration of DM | |
| Moss et al., 1999 (49) | United States | Primary care (WESDR) | 1370 | 64.4 | 43.6 | PAD | 14 | √ | √ | Age, sex, BP |
| Hirai et al, 2008 (50) | United States | Primary care (WESDR) | 1007 | 68.6 | 44.9 | All-cause mortality,CVD, CHD, Stroke | 16 | √ | Age, sex, BP, smoking, BMI, duration of DM | |
| Selvin et al., 2005 (20) | United States | The ARIC study | 1626 | 45–64 | – | CHD | 8–10 | √ | √ | Age, sex, BP, lipid, smoking, BMI |
| Selvin et al., 2005 (23) | United States | The ARIC study | 1635 | 45–64 | – | Stroke | 9 | √ | Age, sex, BP, lipid, smoking, BMI | |
| Iribarren et al.,2001 (21) | United States | The Kaiser PermanenteMedical care | 48 858 | 58 | 52 | Heart Failure | 2.2 | √ | √ | Age, sex, BP, lipid, smoking, BMI, DM medication, duration of DM |
| Agewall et al.,1997 (51) | Sweden | Hypertensionintervention trial | 94 | 67 | 100 | CVD | 6.3 | √ | Age, sex, BP, lipid, smoking, duration of DM | |
| Gall et al., 1995 (52) | Denmark | Hvidore Hospital | 328 | 54 | 61.6 | All-cause mortality CVD | 5.3 | √ | Age, BP | |
| Kuusisto et al.,1994 (32) | Finland | Population registry,East Finland | 229 | 69 | 32.3 | CHD | 3.5 | √ | Sex, BP, lipid, smoking, BMI, duration of DM | |
| Lehto et al., 1996 (28) | Finland | Population registry,East and west Finland | 1044 | 58 | 55 | PAD | 7 | √ | Age, sex | |
| Lehto et al., 1996 (29) | Finland | Population registry,East and west Finland | 1059 | 58 | 55 | Stroke | 7 | √ | Age, sex | |
| Lehto et al., 1997 (30) | Finland | Population registry,East and west Finland | 1059 | 58 | 55 | CHD | 7 | √ | Age, sex, lipid | |
| VAN Hateren et al.,2011 (53) | Netherlands | ZODIAC-20(Primary care) | 374 | 80 | 34.8 | All-cause mortalityCVD mortality | 10 | √ | Age, sex, BP, lipid, smoking, BMI, albuminuria, duration of DM | |
| Landman et al., 2010 (19) | Netherlands | ZODIAC-11(Primary care) | 1145 | 68.7 | 46 | All-cause mortalityCVD mortality | 5.8 | √ | √ | Age, sex, BP, lipid, smoking, BMI, albuminuria, DM medication, duration of DM |
| Roselli della Rovere et al.,2003 (54) | Italy | Diabetic outpatientclinic | 120 | 67 | 44 | CVD | 9 | √ | Age, sex, BP, lipid, smoking | |
| Elley et al., 2008 (24) | New Zealand | Primary care | 48 444 | 60 | 49 | CVD | 2.4 | √ | √ | Age, sex, BP, lipid, smoking, BMI, albuminuria, duration of DM, ethnicity |
| Florkowski et al., 2001(27) | New Zealand | Christ church Hospital Diabetes Center | 447 | 62 | 46.5 | All-cause mortality CHD | 6 | √ | Age, sex, BP, lipid, smoking, BMI, albuminuria, DM medication, duration of DM | |
| Standl et al., 1996 (55) | Germany | Munich General practitioner Project | 290 | 65 | 36 | CVD | 10 | √ | Age, BP, lipid, BMI | |
| Yang et al., 2007 (56) | China | The prince of Wales Hospital | 7209 | 57 | 45.5 | Stroke | 5.37 | √ | Age, sex, BP, lipid, smoking, albuminuria | |
| Yang et al., 2008 (22) | China | The prince of Wales Hospital | 6969 | 57 | 44.8 | Stroke | 5.36 | √ | Age, sex, BP, lipid, BMI, albuminuria | |
| Yang et al., 2008 (57) | China | The prince of Wales Hospital | 7067 | 57 | 45.4 | HF | 5.52 | √ | Age, sex, BP, lipid, smoking, albuminuria, duration of DM | |
CHD, coronary heart disease; CVD, cardiovascular disease; HF, heart failure; PAD, peripheral arterial disease; DM, diabetes mellitus; BMI, body mass index; BP, blood pressure.
Most studies modeled the effect of baseline GHb measurements on the risk for incident CVD outcomes; however, 2 studies [47], [50] used updated mean GHb levels and modeled GHb as a time-dependent variable in the multivariable models.
The quality assessments of the included studies were summarized in the Supplementary table 1. The overall quality of included studies was good according to our 6-item evaluation criteria. All the studies adjusted for major CVD risk factors in the statistical analyses, had validated instrument for measuring GHb, had outcomes determined by specified criteria, and excluded participants who were lose during the follow-up (table S1). All studies had follow-up time longer than 1 year and only 4 studies had follow-up less than 5 years [18], [21], [24], [32]. Most studies treated GHb as continuous variables, and 5 studies treated GHb both as continuous and categorized variables in the analyses [19], [20], [21], [24], [49].
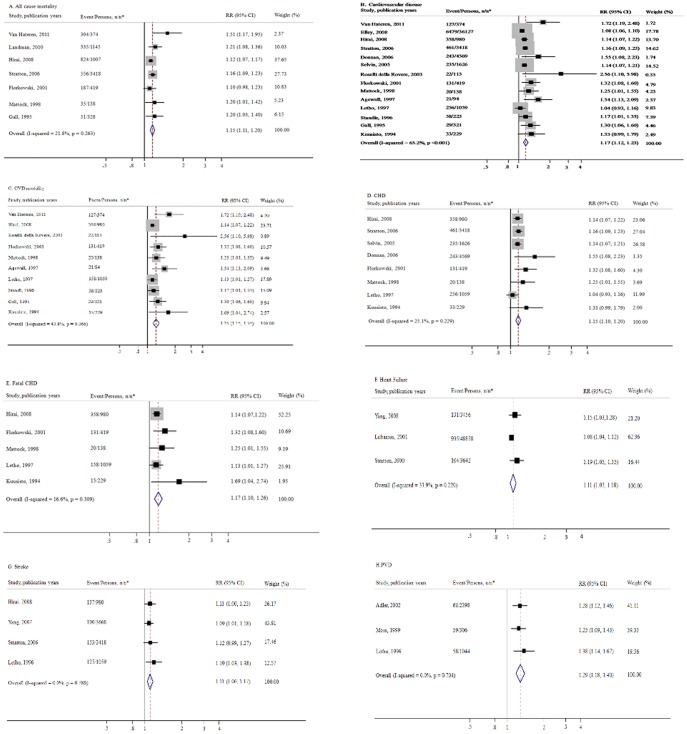
Figure 2 presents the individual and pooled RRs for all-cause mortality and cardiovascular outcomes. The pooled RR associated with a 1% increase in GHb level among patients with type 2 diabetes was 1.15 (95% CI, 1.11 to 1.20) for all-cause mortality in 7 independent studies, 1.17 (95% CI, 1.12 to 1.23) for incident CVD in 14 independent studies, 1.25 (95% CI, 1.15 to 1.35) for CVD mortality in 10 independent studies, 1.15 (95% CI, 1.10 to 1.20) for incident CHD in 8 independent studies, 1.17 (95% CI, 1.10 to 1.26) for fatal CHD in 5 independent studies, 1.11 (95% CI, 1.05 to 1.18) for incident heart failure in 3 independent studies, 1.11 (95% CI, 1.06 to 1.17) for incident stroke in 4 independent studies, and 1.29 (95% CI, 1.18 to 1.40) for incident PAD in 3 independent studies, respectively. The funnel plots and Begg’s test (figure S1) suggested that potential publication bias might be present for the CVD (P = 0.01) and CVD mortality (P = 0.004), but not for all-cause mortality (P = 0.07), CHD (P = 0.11), fatal CHD (P = 0.09), heart failure (P = 0.3), stroke (P = 0.09), and PAD (P = 0.2). In sensitivity analyses, exclusion of any single prospective cohort study from the analysis did not alter the overall findings of a positive association between GHb level and cardiovascular outcomes, and association between GHb level and all-cause mortality.
Figure 2. Forest plot of relative risks (RRs) and 95% confidence intervals (CIs) for the association between glycosylated hemoglobin and the main study outcomes risks in type 2 diabetes.
CVD: cadiovascular diseases; CHD: conoary heart disease; PAD: peripheral arterial disease.
We also analyzed the heterogeneity among the studies of cardiovascular outcomes and all-cause mortality in persons with type 2 diabetes. The I2 statistics (P values for the Q test) in the above analyses were 21.8% (0.26) for all-cause mortality, 65.2% (<0.001) for CVD, 43.8% (0.07) for CVD mortality, 25.1% (0.23) for CHD, 16.6% (0.31) for fatal CHD, 33.9% (0.22) for heart failure, 0.0% (0.79) for stroke, and 0.0% (0.70) for PAD, respectively, which indicated no statistically significant heterogeneity for these pooled results except incident CVD.
To further investigate the potential sources of heterogeneity, we conducted subgroup analyses that compared the relative risk estimates for studies that adjusted for age, sex, blood pressure, and lipids with those that did not: for CVD, the pooled RR was 1.19 (95% CI, 1.11 to 1.27) for 9 studies adjusted for BP and lipids and 1.16 (95% CI, 1.05 to 1.29) for 5 studies that did not adjust for BP and lipids; for CHD, it was 1.17 (95% CI, 1.11 to 1.24; 5 studies adjusted for BP and lipids) vs.1.12 (95% CI, 1.03 to 1.21; 3 studies did not adjust for BP and lipids); for fatal CHD, it was 1.24 (95% CI, 1.06 to 1.46; 3 studies adjusted for BP and lipids) vs. 1.15 (95% CI, 1.08 to 1.21; 3 studies did not adjust for BP and lipids). In addition, we also conducted meta-regression and subgroup analyses to compare the RRs for studies that were conducted in different areas. For 6 outcomes (e.g. all-cause mortality, CVD, CVD mortality, CHD, fatal CHD, stroke) that allowed us to conduct meta-regression, area was not a significant factor that contributed to the heterogeneity (all P values >0.05).
Table 2 shows the individual RRs for cardiovascular outcomes according to categories of GHb levels. Eight studies reporting ≥3 categories of the GHb level were included. Among them, only 1 study reported both the case number and the total number of each category subgroup, thus we could not carry out the dose-response meta-analysis of the relation between GHb level and the cardiovascular outcomes due to the weight calculation. Except that 1 study showed no association [22], the results from the remaining 7 studies all suggested positive associations between GHb level and the cardiovascular outcomes with 3 studies reported significant P values (P<0.05) for testing the linear trend [23], [24].
Table 2. Hazard ratios for cardiovascular outcomes risks according to glycosylated hemoglobin by different categories (shown by the first author and year of publication).* .
| Cardiovascular outcomes | Categories | Categories(medians) | Hazard ratios(95% CI) | P Valuefor trend | Covariate in Multivariable Model | |||||||||
| Age | Sex | BP | Lipid | Smoking | BMI orWHR | Albuminuria | DMMedications | Durationof DM | ||||||
| Selvin et al., | CHD | <6.2 | 4.95 | 1 | <0.001 | √ | √ | √ | √ | √ | √ | |||
| 2005 (20) | 5.2 to <5.7 | 5.45 | 1.24 (0.77–1.98) | |||||||||||
| 5.7 to <6.5 | 6.10 | 1.57 (0.98–2.52) | ||||||||||||
| 6.5 to <8.2 | 7.35 | 2.04 (1.30–3.19) | ||||||||||||
| ≥8.2 | 9.05 | 2.37 (1.50–3.72) | ||||||||||||
| Landman et al., | CVD mortality | <6.5 | 6.25 | 0.94 (0.47–1.91) | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| 2010 (19) | 6.5–7.0 | 6.75 | 1 | |||||||||||
| 7.0–8.0 | 7.5 | 1.4 (0.84–2.31) | ||||||||||||
| 8.0–9.0 | 8.5 | 1.71 (0.99–2.96) | ||||||||||||
| >9.0 | 9.5 | 3.13 (1.62–6.05) | ||||||||||||
| Elley et al., | CVD | <6.0 | 5.5 | 1 | <0.001 | √ | √ | √ | √ | √ | √ | √ | √ | |
| 2008 (24) | 6.0 to <7.0 | 6.5 | 1.08 (0.97–1.19) | |||||||||||
| 7.0 to <8.0 | 7.5 | 1.13 (1.02–1.25) | ||||||||||||
| 8.0 to <9.0 | 8.5 | 1.26 (1.12–1.41) | ||||||||||||
| 9.0 to <10.0 | 9.5 | 1.31 (1.15–1.50) | ||||||||||||
| ≥10.0 | 10.5 | 1.53 (1.34–1.73) | ||||||||||||
| Iribarren et al., | Heart Failure | <7.0 | 6.5 | 1 | √ | √ | √ | √ | √ | √ | √ | √ | ||
| 2001 (21) | 7.0 to <8.0 | 7.5 | 1.15 (0.93–1.43) | |||||||||||
| 8.0 to <9.0 | 8.5 | 1.10 (0.88–1.38) | ||||||||||||
| 9.0 to <10.0 | 9.5 | 1.39 (1.11–1.74) | ||||||||||||
| ≥10.0 | 10.5 | 1.56 (1.26–1.93) | ||||||||||||
| Yang et al., | Stroke | <6.0 | 5.55 | 0.77 (0.34–1.75) | 0.531 | √ | √ | √ | √ | √ | √ | |||
| 2008 (22) | 6.0–6.9 | 6.45 | 1 | reference | ||||||||||
| 7.0–12.4 | 9.7 | 1.19 (0.71–1.99) | 0.516 | |||||||||||
| ≥12.5 | 15.2 | 1.27 (0.17–9.68) | 0.821 | |||||||||||
| Selvin et al., | Stroke | 5 | 1 | <0.001 | √ | √ | √ | √ | √ | √ | √ | |||
| 2005 (23) | 6 | 1.17 (0.62–2.19) | ||||||||||||
| 9 | 2.33 (1.29–4.21) | |||||||||||||
| Adler et al., | CHD | 6.9 | 1 | √ | √ | √ | √ | √ | ||||||
| 1999 (25) | 9.1 | 1.20 (1.00–1.50) | ||||||||||||
| 11.3 | 1.40 (1.10–1.70) | |||||||||||||
| Adler et al., | Stroke | 6.9 | 1 | √ | √ | √ | √ | √ | ||||||
| 1999 (25) | 9.1 | 1.10 (0.80–1.60) | ||||||||||||
| 11.3 | 1.00 (0.70–1.40) | |||||||||||||
CHD, coronary heart disease; CVD, cardiovascular disease; DM, diabetes mellitus; BMI, body mass index; BP, blood pressure; OR, hazard ratio; CI, confidence interval.
Only two studies have evaluated the association between the categories of GHb level and the all-cause mortality and the results suggested a non-lineal association. One study in United Kingdom including 47,970 participants identified a “U-shape” association [18], while another study in the Netherlands including 1,145 participants detected an “almost positive” association [19]. With the limited numbers of the studies, we could not conduct the dose-response meta-analysis.
Discussion
The meta-analysis of 26 prospective studies provides evidence that chronic exposure to increased glycemic level was associated with increased risks of all-cause mortality and cardiovascular outcomes in type 2 diabetes. We found that every 1% increase in GHb was associated with a 15% increase in hazard of all-cause mortality, 25% in CVD mortality, 17% in CVD, 15% in CHD, 17% in fatal CHD, 11% in heart failure, 11% in stroke, and 29% in PVD event. From the data of 8 prospective studies with 3 or more categories of GHb, we found a positive dose-response association, which provides additional support for our results of GHb level as a continuous variable and the major outcomes.
Our finding that an increased GHb level is associated with an increased risk of cardiovascular outcome is consistent with previous studies. This effect has been shown to be independent of other vascular risk factors. In a meta-analysis [15], Selvin et al. evaluated 10 prospective studies and concluded that every 1% increase in GHb was associated with a 18% increase in hazard of CVD, 13% in CHD, 16% in fatal CHD, and 17% in stroke incidence after controlling for potential confounders. These effect sizes are similar to those estimated in our study for CVD and CHD but higher for stroke. It may be due to the fewer stroke events (396) in the previous study. Our estimates from observational studies are highly consistent with the results from the RCTs. A meta-analysis of 5 RCTs showed, during 5-year treatment, reduction of HbA1c by 0.9% resulted in a 17% significant reduction in non-fatal MI events, 15% in CHD events, but non-significant reduction in stroke events in type 2 diabetic patients [12]. Another two meta-analyses of 4 or 5 relevant RCTs reported similar results, and also found non-significant reduction in HF events in type 2 diabetics [11], [13]. Ray et al. explained that the cases of stroke event in these RCTs were less than that myocardial infarction were reported, which may not have enough power to ascertain whether a significant benefit exists [12]. Due to the lack of case numbers and the total study sample sizes of each category of GHb, we could not conduct a dose-response analysis to identify the relationship between GHb level and the CVD outcomes; however, a positive association between GHb and CVD incident was suggested by carefully summarized findings from 8 studies (Table 2).
The relationship between GHb level and death has been studied, but the results are inconsistent. A previous meta-analysis of 10 prospective studies [15] did not have available data on all-cause mortality. Another meta-analysis of RCTs showed no association between GHb and CVD and all-cause mortality [11]–[13]. The short follow-up time, the small number of events, and the glucose-lowering drugs used in these clinical trials may actually have adverse cardiovascular effects, which would attenuate macrovascular benefits of improved glycemic control [59]. In the present study, a monotonic positive association between GHb level and the risks of all-cause mortality and CVD mortality was found. Every 1% increase in GHb was associated with a 16% increase in hazard of all-cause mortality, however we cannot assess the dose-response association between GHb and all-cause mortality due to the small number of prospective studies with 3 or more categories of GHb [18], [19]. Currie et al [18] found a U-shape association between the HbA1c level and all-cause mortality: low (6.4%) and high (10.6%) mean HbA1c values were associated with increased all-cause mortality as compared to median mean HbA1c values (7.5%) and this association was independent of the treatment regimen. Landman et al [19] detected an almost positive association between the HbA1c level and the risk of all-cause mortality. The results from RCTs [11]–[13] demonstrated that a low HbA1c therapeutic target level did not result in decreased mortality. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial [60] was halted in February 2008 because of an unexpected excess of all-cause and CVD-related mortality in the intensive treatment group, suggesting that lowered HbA1c concentration might cause an excess risk for all-cause mortality. A clear adverse consequence of tight glycemic control was a 2 to 3 fold increased risk for severe hypoglycemia. These studies samples were composed of mostly elderly subjects with mean age at least 64 years. Nevertheless, these studies have important implications for clinical practice [60].
There are several biologically plausible mechanisms that might account for the finding that chronic hyperglycemia is associated with cardiovascular outcomes and all-cause mortality. Hyperglycemic periods play a major role in the activation of oxidative stress and overproduction of mitochondrial superoxide, which trigger various metabolic pathways of glucose-mediated vascular damage [61], [62]. Glucose can react with various proteins to form advanced glycation end products, which may contribute to long-term complications in diabetes, plaque formation, and atherosclerosis [59]. These effects are gradual and likely to be cumulative, occurring during decades of exposure to chronically elevated blood glucose levels. As explained by Selvin et al [15], this possibility suggests that most previous studies, including clinical trials, may have had insufficient follow-up to detect a moderate increase in risk.
This meta-analysis has notable strengths. We included many large studies with a correspondingly high number of incident cases, which improved the statistical power to detect significant differences. Our study was based on a comprehensive literature search. We believed that the inclusion of large studies, such as the 10-year post-trial monitoring of the UKPDS [47], and larger cohort studies conducted by Currie et al [18] in GPRD, Iribarren et al [21] in Kaiser Permanente Medical care, and Elley et al [24] in New Zealand, could potentially make our analysis more reliable. In addition, our sensitivity analysis showed minimal influence on the combined results for any single study, and the heterogeneity of variance between studies was allowed for by using random effects models. There are several limitations in this review. First, all studies were observational in nature and residual confounding cannot be totally ruled out. Second, the analysis was based on a single measurement of GHb, although GHb is indicative of time-averaged blood glucose concentration over the past 3 months [63]. Third, because of the lacking data, the dose-response relationships between GHb and CVD events and mortality cannot be estimated in the current analysis. Finally, as with any other systematic literature review, a limitation is a potential of publication bias, but the estimates in our study are similar to previous studies.
In conclusion, our results suggest that chronic hyperglycemia is associated with increased risks for cardiovascular outcomes and all-cause mortality among patients with type 2 diabetes and independent from other conventional risk factors. Our finding supports the notion that diabetic patients with higher GHb level should be closely followed due to their higher risks of cardiovascular outcomes and all-cause mortality. In addition, our data suggest that it might be desirable to achieve GHb as close to the normal glycemic range as possible.
Supporting Information
Funnel plots with 95% confidence limits for publication bias. CVD: cadiovascular diseases; CHD: conoary heart disease; PAD: peripheral arterial disease; RR: relative risk; SE: standard error.
(TIF)
Quality assessments* on prospective cohort studies on Glycosylated Hemoglobin Level in relation to Cardiovascular Outcomes and Death in Patients with Type 2 Diabetes.
(DOC)
Acknowledgments
The authors thank Dr. C. Raina Elley and Dr. Xilin Yang for providing additional information.
Funding Statement
This work was supported by Guanghua Scholarship Fund from Xi’an Jiaotong University (No. GH 0203106), the National Natural Science Foundation of China (No. 50976088), and Louisiana State University’s Improving Clinical Outcomes Network (LSU ICON). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Zhang P, Zhang X, Brown J, Vistisen D, Sicree R, et al. (2010) Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract 87: 293–301. [DOI] [PubMed] [Google Scholar]
- 2. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, et al. (2010) Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375: 2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coutinho M, Gerstein HC, Wang Y, Yusuf S (1999) The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 22: 233–240. [DOI] [PubMed] [Google Scholar]
- 4. Laakso M (1999) Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes 48: 937–942. [DOI] [PubMed] [Google Scholar]
- 5. Moss SE, Klein R, Klein BE (1991) Cause-specific mortality in a population-based study of diabetes. Am J Public Health 81: 1158–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Diabetes Association (2009) Standards of medical care in diabetes–2009. Diabetes Care 32 Suppl 1 S13–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gaster B, Hirsch IB (1998) The effects of improved glycemic control on complications in type 2 diabetes. Arch Intern Med 158: 134–140. [DOI] [PubMed] [Google Scholar]
- 8. Klein R, Klein BE, Moss SE (1996) Relation of glycemic control to diabetic microvascular complications in diabetes mellitus. Ann Intern Med 124: 90–96. [DOI] [PubMed] [Google Scholar]
- 9. UK Prospective Diabetes Study Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352: 837–853. [PubMed] [Google Scholar]
- 10. Shichiri M, Kishikawa H, Ohkubo Y, Wake N (2000) Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care 23 Suppl 2 B21–29. [PubMed] [Google Scholar]
- 11. Turnbull FM, Abraira C, Anderson RJ, Byington RP, Chalmers JP, et al. (2009) Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 52: 2288–2298. [DOI] [PubMed] [Google Scholar]
- 12. Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, et al. (2009) Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet 373: 1765–1772. [DOI] [PubMed] [Google Scholar]
- 13. Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, et al. (2009) Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med 151: 394–403. [DOI] [PubMed] [Google Scholar]
- 14. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA (2008) 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359: 1577–1589. [DOI] [PubMed] [Google Scholar]
- 15. Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, et al. (2004) Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med 141: 421–431. [DOI] [PubMed] [Google Scholar]
- 16. Adler AI, Stevens RJ, Neil A, Stratton IM, Boulton AJ, et al. (2002) UKPDS 59: hyperglycemia and other potentially modifiable risk factors for peripheral vascular disease in type 2 diabetes. Diabetes Care 25: 894–899. [DOI] [PubMed] [Google Scholar]
- 17. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, et al. (2000) Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321: 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Currie CJ, Peters JR, Tynan A, Evans M, Heine RJ, et al. (2010) Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet 375: 481–489. [DOI] [PubMed] [Google Scholar]
- 19. Landman GW, van Hateren KJ, Kleefstra N, Groenier KH, Gans RO, et al. (2010) The relationship between glycaemic control and mortality in patients with type 2 diabetes in general practice (ZODIAC-11). Br J Gen Pract 60: 172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Selvin E, Coresh J, Golden SH, Brancati FL, Folsom AR, et al. (2005) Glycemic control and coronary heart disease risk in persons with and without diabetes: the atherosclerosis risk in communities study. Arch Intern Med 165: 1910–1916. [DOI] [PubMed] [Google Scholar]
- 21. Iribarren C, Karter AJ, Go AS, Ferrara A, Liu JY, et al. (2001) Glycemic control and heart failure among adult patients with diabetes. Circulation 103: 2668–2673. [DOI] [PubMed] [Google Scholar]
- 22. Yang X, So WY, Ma RC, Ko GT, Kong AP, et al. (2008) Thresholds of risk factors for ischemic stroke in type 2 diabetic patients with and without albuminuria: a non-linear approach. Clin Neurol Neurosurg 110: 701–709. [DOI] [PubMed] [Google Scholar]
- 23. Selvin E, Coresh J, Shahar E, Zhang L, Steffes M, et al. (2005) Glycaemia (haemoglobin A1c) and incident ischaemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Lancet Neurol 4: 821–826. [DOI] [PubMed] [Google Scholar]
- 24. Elley CR, Kenealy T, Robinson E, Drury PL (2008) Glycated haemoglobin and cardiovascular outcomes in people with Type 2 diabetes: a large prospective cohort study. Diabet Med 25: 1295–1301. [DOI] [PubMed] [Google Scholar]
- 25. Adler AI, Neil HA, Manley SE, Holman RR, Turner RC (1999) Hyperglycemia and hyperinsulinemia at diagnosis of diabetes and their association with subsequent cardiovascular disease in the United Kingdom prospective diabetes study (UKPDS 47). Am Heart J 138: S353–359. [DOI] [PubMed] [Google Scholar]
- 26. Moss SE, Klein R, Klein BE, Meuer SM (1994) The association of glycemia and cause-specific mortality in a diabetic population. Arch Intern Med 154: 2473–2479. [PubMed] [Google Scholar]
- 27. Florkowski CM, Scott RS, Coope PA, Moir CL (2001) Predictors of mortality from type 2 diabetes mellitus in Canterbury, New Zealand; a ten-year cohort study. Diabetes Res Clin Pract 53: 113–120. [DOI] [PubMed] [Google Scholar]
- 28. Lehto S, Ronnemaa T, Pyorala K, Laakso M (1996) Risk factors predicting lower extremity amputations in patients with NIDDM. Diabetes Care 19: 607–612. [DOI] [PubMed] [Google Scholar]
- 29. Lehto S, Ronnemaa T, Pyorala K, Laakso M (1996) Predictors of stroke in middle-aged patients with non-insulin-dependent diabetes. Stroke 27: 63–68. [DOI] [PubMed] [Google Scholar]
- 30. Lehto S, Ronnemaa T, Haffner SM, Pyorala K, Kallio V, et al. (1997) Dyslipidemia and hyperglycemia predict coronary heart disease events in middle-aged patients with NIDDM. Diabetes 46: 1354–1359. [DOI] [PubMed] [Google Scholar]
- 31. Greenland S (1987) Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 9: 1–30. [DOI] [PubMed] [Google Scholar]
- 32. Kuusisto J, Mykkanen L, Pyorala K, Laakso M (1994) NIDDM and its metabolic control predict coronary heart disease in elderly subjects. Diabetes 43: 960–967. [DOI] [PubMed] [Google Scholar]
- 33. Khaw KT, Wareham N, Bingham S, Luben R, Welch A, et al. (2004) Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 141: 413–420. [DOI] [PubMed] [Google Scholar]
- 34. Khaw KT, Wareham N, Luben R, Bingham S, Oakes S, et al. (2001) Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of european prospective investigation of cancer and nutrition (EPIC-Norfolk). BMJ 322: 15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Valensi P, Benroubi M, Borzi V, Gumprecht J, Kawamori R, et al. (2008) The IMPROVE study–a multinational, observational study in type 2 diabetes: baseline characteristics from eight national cohorts. Int J Clin Pract 62: 1809–1819. [DOI] [PubMed] [Google Scholar]
- 36. Shah S, Das AK, Kumar A, Unnikrishnan AG, Kalra S, et al. (2009) Baseline characteristics of the Indian cohort from the IMPROVE study: a multinational, observational study of biphasic insulin aspart 30 treatment for type 2 diabetes. Adv Ther 26: 325–335. [DOI] [PubMed] [Google Scholar]
- 37. Kothari V, Stevens RJ, Adler AI, Stratton IM, Manley SE, et al. (2002) UKPDS 60: risk of stroke in type 2 diabetes estimated by the UK Prospective Diabetes Study risk engine. Stroke 33: 1776–1781. [DOI] [PubMed] [Google Scholar]
- 38. Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, et al. (1998) Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 316: 823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stevens RJ, Coleman RL, Adler AI, Stratton IM, Matthews DR, et al. (2004) Risk factors for myocardial infarction case fatality and stroke case fatality in type 2 diabetes: UKPDS 66. Diabetes Care 27: 201–207. [DOI] [PubMed] [Google Scholar]
- 40. Elley CR, Robinson E, Kenealy T, Bramley D, Drury PL (2010) Derivation and validation of a new cardiovascular risk score for people with type 2 diabetes: the new zealand diabetes cohort study. Diabetes Care 33: 1347–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moss SE, Klein R, Klein BE (1996) Long-term incidence of lower-extremity amputations in a diabetic population. Arch Fam Med 5: 391–398. [DOI] [PubMed] [Google Scholar]
- 42. Aguilar D, Bozkurt B, Ramasubbu K, Deswal A (2009) Relationship of hemoglobin A1C and mortality in heart failure patients with diabetes. J Am Coll Cardiol 54: 422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Drechsler C, Krane V, Ritz E, Marz W, Wanner C (2009) Glycemic control and cardiovascular events in diabetic hemodialysis patients. Circulation 120: 2421–2428. [DOI] [PubMed] [Google Scholar]
- 44. Blaum CS, Volpato S, Cappola AR, Chaves P, Xue QL, et al. (2005) Diabetes, hyperglycaemia and mortality in disabled older women: The Women’s Health and Ageing Study I. Diabet Med. 22: 543–550. [DOI] [PubMed] [Google Scholar]
- 45. Colayco DC, Niu F, McCombs JS, Cheetham TC (2011) A1C and cardiovascular outcomes in type 2 diabetes: a nested case-control study. Diabetes Care 34: 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang X, Kong AP, So WY, Ma RC, Ho CS, et al. (2007) Effects of chronic hyperglycaemia on incident stroke in Hong Kong Chinese patients with type 2 diabetes. Diabetes Metab Res Rev 23: 220–226. [DOI] [PubMed] [Google Scholar]
- 47. Stratton IM, Cull CA, Adler AI, Matthews DR, Neil HA, et al. (2006) Additive effects of glycaemia and blood pressure exposure on risk of complications in type 2 diabetes: a prospective observational study (UKPDS 75). Diabetologia 49: 1761–1769. [DOI] [PubMed] [Google Scholar]
- 48. Mattock MB, Barnes DJ, Viberti G, Keen H, Burt D, et al. (1998) Microalbuminuria and coronary heart disease in NIDDM: an incidence study. Diabetes 47: 1786–1792. [DOI] [PubMed] [Google Scholar]
- 49. Moss SE, Klein R, Klein BE (1999) The 14-year incidence of lower-extremity amputations in a diabetic population. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Diabetes Care 22: 951–959. [DOI] [PubMed] [Google Scholar]
- 50. Hirai FE, Moss SE, Klein BE, Klein R (2008) Relationship of glycemic control, exogenous insulin, and C-peptide levels to ischemic heart disease mortality over a 16-year period in people with older-onset diabetes: the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR). Diabetes Care 31: 493–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Agewall S, Wikstrand J, Ljungman S, Fagerberg B (1997) Usefulness of microalbuminuria in predicting cardiovascular mortality in treated hypertensive men with and without diabetes mellitus. Risk Factor Intervention Study Group. Am J Cardiol 80: 164–169. [DOI] [PubMed] [Google Scholar]
- 52. Gall MA, Borch-Johnsen K, Hougaard P, Nielsen FS, Parving HH (1995) Albuminuria and poor glycemic control predict mortality in NIDDM. Diabetes 44: 1303–1309. [DOI] [PubMed] [Google Scholar]
- 53. Van Hateren KJ, Landman GW, Kleefstra N, Drion I, Groenier KH, et al. (2011) Glycaemic control and the risk of mortality in elderly type 2 diabetic patients (ZODIAC-20). Int J Clin Pract 65: 415–419. [DOI] [PubMed] [Google Scholar]
- 54. Roselli della Rovere G, Lapolla A, Sartore G, Rossetti C, Zambon S, et al. (2003) Plasma lipoproteins, apoproteins and cardiovascular disease in type 2 diabetic patients. A nine-year follow-up study. Nutr Metab Cardiovasc Dis 13: 46–51. [DOI] [PubMed] [Google Scholar]
- 55. Standl E, Balletshofer B, Dahl B, Weichenhain B, Stiegler H, et al. (1996) Predictors of 10-year macrovascular and overall mortality in patients with NIDDM: the Munich General Practitioner Project. Diabetologia 39: 1540–1545. [DOI] [PubMed] [Google Scholar]
- 56. Yang X, So WY, Kong AP, Ho CS, Lam CW, et al. (2007) Development and validation of stroke risk equation for Hong Kong Chinese patients with type 2 diabetes: the Hong Kong Diabetes Registry. Diabetes Care 30: 65–70. [DOI] [PubMed] [Google Scholar]
- 57. Yang X, Ma RC, So WY, Kong AP, Ko GT, et al. (2008) Development and validation of a risk score for hospitalization for heart failure in patients with Type 2 diabetes mellitus. Cardiovascular diabetology 7: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Donnan PT, Donnelly L, New JP, Morris AD (2006) Derivation and validation of a prediction score for major coronary heart disease events in a U.K. type 2 diabetic population. Diabetes Care 29: 1231–1236. [DOI] [PubMed] [Google Scholar]
- 59. Sheetz MJ, King GL (2002) Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA 288: 2579–2588. [DOI] [PubMed] [Google Scholar]
- 60. Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, et al. (2008) Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358: 2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Brownlee M, Hirsch IB (2006) Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA 295: 1707–1708. [DOI] [PubMed] [Google Scholar]
- 62. Monnier L, Mas E, Ginet C, Michel F, Villon L, et al. (2006) Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 295: 1681–1687. [DOI] [PubMed] [Google Scholar]
- 63. American Diabetes Association (2000) Standards of medical care for patients with diabetes mellitus. Diabetes Care 23 Suppl 1 S32–42. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Funnel plots with 95% confidence limits for publication bias. CVD: cadiovascular diseases; CHD: conoary heart disease; PAD: peripheral arterial disease; RR: relative risk; SE: standard error.
(TIF)
Quality assessments* on prospective cohort studies on Glycosylated Hemoglobin Level in relation to Cardiovascular Outcomes and Death in Patients with Type 2 Diabetes.
(DOC)