Abstract
Anabaena sp. strain PCC 7120 is a filamentous cyanobacterium commonly used as a model organism for studying cyanobacterial cell differentiation and nitrogen fixation. For many decades, this cyanobacterium was considered an obligate photo-lithoautotroph. We now discovered that this strain is also capable of mixotrophic, photo-organoheterotrophic, and chemo-organoheterotrophic growth if high concentrations of fructose (at least 50 mM and up to 200 mM) are supplied. Glucose, a substrate used by some facultatively organoheterotrophic cyanobacteria, is not effective in Anabaena sp. PCC 7120. The gtr gene from Synechocystis sp. PCC 6803 encoding a glucose carrier was introduced into Anabaena sp. PCC 7120. Surprisingly, the new strain containing the gtr gene did not grow on glucose but was very sensitive to glucose, with a 5 mM concentration being lethal, whereas the wild-type strain tolerated 200 mM glucose. The Anabaena sp. PCC 7120 strain containing gtr can grow mixotrophically and photo-organoheterotrophically, but not chemo-organoheterotrophically with fructose. Anabaena sp. PCC 7120 contains five respiratory chains ending in five different respiratory terminal oxidases. One of these enzymes is a mitochondrial-type cytochrome c oxidase. As in almost all cyanobacteria, this enzyme is encoded by three adjacent genes called coxBAC1. When this locus was disrupted, the cells lost the capability for chemo-organoheterotrophic growth.
INTRODUCTION
Cyanobacteria are prokaryotes capable of oxygenic photosynthesis. Therefore, all cyanobacteria can grow photo-litho-auto-trophically. Some cyanobacteria are facultative photo-organoheterotrophs, of these a few can grow chemo-organoheterotrophically (21, 22, 26). The number of substances that can be used for heterotrophic growth by cyanobacteria is limited, only glucose, fructose, sucrose, ribose, a few other sugars, and glycerol are known substrates (22, 31). The filamentous strain Anabaena (also referred to as Nostoc) sp. strain PCC 7120 (hereafter referred to as PCC 7120) has been widely used as a model organism for studying cyanobacterial cell differentiation and nitrogen fixation (for a review, see reference 33). It was characterized as an obligate photo-litho-autotroph (22). Introduction of the frtRABC locus encoding a putative fructose transporter from the facultative chemo-organoheterotrophic cyanobacterium Anabaena variabilis ATCC 29413 into strain PCC 7120 led to a strain capable of chemo-organoheterotrophic growth with fructose as the substrate (27). We wanted to test whether the introduction of a glucose carrier into PCC 7120 would yield a strain that could use glucose for chemo-organoheterotrophic growth. The glucose carrier chosen was the well-characterized Gtr enzyme (also called GlcP) (10, 23, 35) from the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. A gene (Npun_R5323) with 71% identity to the Synechocystis sp. PCC 6803 gtr gene is present in the genomic sequence of the filamentous cyanobacterium Nostoc punctiforme ATCC 29133 (5) that was isolated from a symbiosis with Macrozamia sp. It has been shown that Nostoc strains isolated from Macrozamia symbioses are capable of chemo-organoheterotrophic growth with both glucose and fructose as the substrate (11). While the PCC 7120 strain that carries the gtr gene was found not to be capable of chemo-organoheterotrophic growth (see below), these experiments led to the discovery that PCC 7120 wild type can grow photo-organoheterotrophically and chemo-organoheterotrophically when unusually high concentrations of fructose are supplied.
Chemo-organoheterotrophic growth in cyanobacteria is critically dependent on active respiration. All cyanobacteria respire when subjected to complete darkness (for a review, see reference 24). Cyanobacterial respiratory chains are intimately linked to the photosynthetic electron transport chain, even sharing some components. The only respiratory electron transport components that have no direct function in photosynthesis are the respiratory terminal oxidases (RTOs) that catalyze the reduction of dioxygen to water. The RTOs can therefore be considered the key enzymes of cyanobacterial respiration. Cyanobacterial RTOs belong to three protein families: (i) homologs of the heme-copper oxidases, (ii) homologs of the cytochrome bd-type quinol oxidases, and (iii) homologs of the plastidic alternative oxidases. The homologs of the heme-copper oxidases can be further divided into three different subcategories: (i-a) mitochondrial-type genuine cytochrome c oxidases, (i-b) so-called ARTOs (alternate respiratory terminal oxidases) that have characteristic sequence deviations from mitochondrial-type cytochrome c oxidases (see reference 24), and (i-c) homologs of cytochrome cbb3, cytochrome c oxidases well known from purple bacteria.
The number and types of RTOs present in cyanobacteria differ widely from strain to strain, even among closely related strains. The complete genomic sequence of PCC 7120 (13) has revealed that this strain contains five different RTOs: one mitochondrial-type cytochrome c oxidase (Cox), two ARTOs, one cytochrome bd-type quinol oxidase (Qox), and one plastidic-type alternative oxidase (Ptox). The specific function of these five RTOs has only been partially elucidated. The two ARTOs have been shown to play an important role in the cell differentiation process of normal vegetative cells to heterocysts that are specialized for nitrogen fixation (28, 29). They probably function as dioxygen scavengers so that microaerobic conditions can be maintained in the interior of the heterocyst. This is essential because nitrogenase, the enzyme reducing dinitrogen to ammonia, is one of the most dioxygen-sensitive enzymes known. The Qox in Anabaena sp. strain PCC 7120 was shown to be upregulated under micro-oxic conditions (16). In two other cyanobacteria capable of chemo-organoheterotrophic growth, Synechocystis sp. strain PCC 6803 (17) and Anabaena variabilis ATCC 29413 (25) that contain 3 and 10 RTOs, respectively, it was demonstrated that the inactivation of the single gene locus encoding a mitochondrial-type cytochrome c oxidase was sufficient to generate strains incapable of chemo-organoheterotrophic growth. Therefore, we constructed a mutant of PCC 7120 lacking the coxA1 gene to check whether also in this strain the mitochondrial-type Cox is essential for chemo-organoheterotrophic growth.
MATERIALS AND METHODS
Growth conditions.
Anabaena (Nostoc) sp. strain PCC 7120 was obtained from the Pasteur Culture Collection in Paris, France. All cultures were grown in 50-ml batches in 100-ml Erlenmeyer flasks on a gyratory shaker at 100 rpm at 32°C. All cultures used medium BG11 (22) as the basis. Phototrophic cultures were illuminated with fluorescent white light (10 μE m−2 s−1). For photo-organoheterotrophic growth experiments, 10 μM DCMU [3-(3,4-dichlorophenyl)-1,1-dimethylurea] was added. Chemo-organoheterotrophic cultures were wrapped completely in aluminum foil and incubated in a dark room. When appropriate, neomycin was added at a concentration of 30 μg/ml, and sucrose was added at 5% (wt/vol). All growth experiments were performed at least three times. The figures show typical results. Both the absorbance at 730 nm and the chlorophyll content of the cultures (according to the method of Mackinney [14]) were determined. The results obtained by both methods were essentially consistent (for examples, see Fig. 3A and B and Fig. 4A and B). For cultures on agar, Difco Bacto agar was purified by the method of Braun and Wood (6).
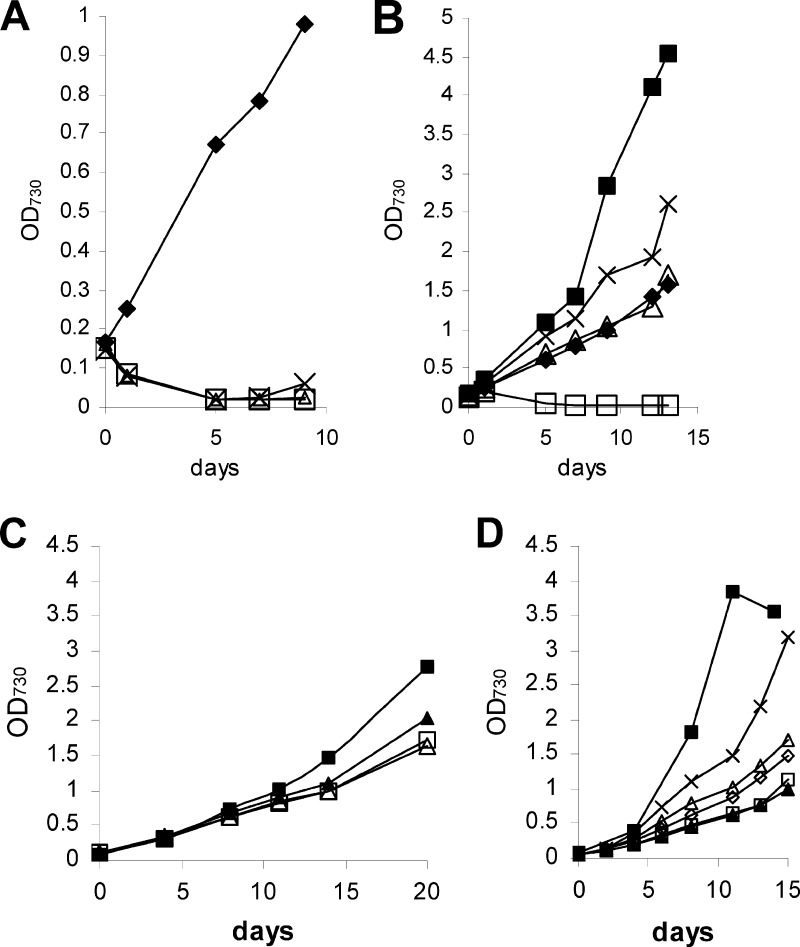
Fig 3.
Cultivation of PCC 7120 strains in darkness. (A and B) PCC 7120 wild type. (A) Measurement of OD730. (B) Measurement of chlorophyll content. Symbols: △, 0 mM fructose; □, 10 mM fructose; ▲, 50 mM fructose; ■, 100 mM fructose; ●, 200 mM fructose. (C) PCC 7120G. Symbols: □, 0 mM fructose; ▲, 10 mM fructose; ×, 50 mM fructose; △, 100 mM fructose; ■, 200 mM fructose; ● (dashed line), 200 mM sorbitol. (D) PCC 7120C. Symbols: □, 0 mM fructose; ■, 50 mM fructose; ▲, 100 mM fructose; ×, 200 mM fructose.
Fig 4.
Chemo-organoheterotrophic growth of PCC 7120 wild type in permanent darkness. The four cultures were inoculated at the same time and opened for measurement of OD730 (A) and chlorophyll content (B) for the first time after 1 week (□), 2 weeks (×), 3 weeks (△), or 4 weeks (▲ [heavy line]).
Cytochrome c oxidase activity and [14C]fructose uptake.
Membranes from PCC 7120 and PCC 7120C (see below) were isolated, and the protein concentrations of the resultant suspensions were determined as described earlier (25). In vitro cytochrome c oxidase activity was measured according to the method of Pils et al. (19). Uniformly labeled 14C-d-fructose was purchased from Hartmann Analytic (Braunschweig, Germany). Fructose uptake into Anabaena cells was measured by filtering an aliquot of the cell suspension (in BG11, optical density at 730 nm [OD730] of 2.0) through Whatman nitrocellulose filters (pore diameter, 0.45 μm) that were immersed in a dioxane-based scintillation liquid and counted in a Perkin-Elmer Tri-Carb 2800 TR liquid scintillation counter.
Respiration measurements.
Cultures intended for dioxygen uptake measurements in the dark with a Clark-type electrode (17) were inoculated at an OD730 of <0.1 and grown to an OD730 of 4 to 5 under photo-litho-autotrophic conditions. Cells were harvested and resuspended in BG11 medium with 10 mM HEPES buffer (pH 8.8) added.
Construction of plasmids and mutants.
Plasmid pBWUV6 (see Fig. S1 in the supplemental material) carrying the gtr gene (23) (also known as glcP [35]) from Synechocystis sp. PCC 6803 was constructed starting from pRL25 (30) that contains the complete plasmid pDU1 from Nostoc sp. PCC 7524 (20), a kanamycin/neomycin resistance cassette, and the bom site necessary for coconjugative mobilization with conjugative plasmid RP4 (9). Plasmid pDU1 has been shown to replicate autonomously in PCC 7120 (32). pRL25 linearized with BamHI and the 2,055-bp BamHI fragment containing the Synechocystis gtr gene from pGT12 (35) were ligated and the resultant plasmid, in which the open reading frame (ORF) of the gtr gene was antiparallel to the ORF of the kanamycin/neomycin resistance gene, was called pBWUV6. pBWUV6 was conjugated into PCC 7120 from Escherichia coli (32) with selection for neomycin resistance. The resulting strain was called PCC 7120G. The presence of the gtr gene in the cells was verified by two PCRs using primers at the gtr gene locus. The primer pairs used were gtr-5_1 (5′-ATGAATCCCTCCTCTTCTCC-3′) and gtr-3 (5′-GTCACCAAAGCAATTATCCCAGC-3′) for a predicted product of 1,124 bp and gtr-5_2 (5′-GGGCGGATTAAAACGATG-3′) and gtr-3 for a predicted product of 881 bp with total DNA from PCC 7120 wild type or total DNA from PCC 7120G as templates (see Fig. S2 in the supplemental material).
The construction of plasmid pRStUV3 is graphically shown in Fig. S3 in the supplemental material. DNA from PCC 7120 strains was prepared according to the method of Cai and Wolk (7). With PCC 7120 wild-type DNA as the template, we amplified by PCR a region containing the complete coxA1 gene and parts of the coxB1 and coxC1 genes using the primers 7120coxB (5′-CGTCTAGAGCTGAACTTTGCGGCCCCTACCACGGCGC-3′) and 7120coxC (5′-CCTCTAGAGAAGGCCAGATATGCTCCGAACAAACC-3′). This product was cut with XbaI (at the sites underlined in the primer sequences) and ligated to the large fragment (containing the pBR322 origin of replication, the kanamycin/neomycin resistance cassette, and the sacB gene) resulting from a complete digest of pRL278 (4) with XbaI. The plasmid, in which the ORF of the coxA1 gene was antiparallel to that of the sacB gene, was called pBWUV1. The cat gene (encoding chloramphenicol resistance) was amplified by PCR from plasmid pBR328 using the primers Cm5 (5′-CCATCGATCGTTGATCGGCACGTAAGA-3′) and Cm3 (5′-GGAGACCGGTTTTTATCAGGCTCTGGGAGG-3′). The resulting product was cut with PvuI (underlined in primer Cm5) and AgeI (underlined in primer Cm3) and ligated to pBWUV1 cut with AsiSI and SgrAI. The resulting plasmid, called pBWUV2, was cut with EagI and BamHI, treated with mung bean nuclease, and religated to yield pBWUV3. Plasmid pRL6 (32) was then used as the template to amplify by PCR the npt gene with the primers Kmcassette2 (5′-GCTTGCGATCGAACCTTTCATAGAAGGCG-3′) and Kmcassette3 (5′-GCATAGTACTGGGCTATCTGGACAAGG-3′). The resulting product was then cut with PvuI (underlined in primer Kmcassette2) and ScaI (underlined in primer Kmcassette3) and ligated to pBWUV3 cut with the same enzymes. The final plasmid was called pRStUV3. This plasmid was transferred from E. coli into PCC 7120 using conjugative plasmid RP4 and helper plasmid pRL528 as described previously (8). The first selection was on neomycin, and positive selection for double recombination was achieved by selecting for sucrose-resistant cells that had lost the sacB gene (7). Segregation of the mutated chromosomes to homozygosity was verified in a two-step procedure: cells that were resistant to both neomycin and sucrose were grown in liquid culture, total DNA was isolated, and the absence of wild-type chromosomes was checked by PCR (data not shown). From a culture that appeared homozygous in this test, single colonies were grown on an agar plate without selective agents. Material from one of the colonies obtained was incubated in a liquid culture containing no selective agent, i.e., pure BG11 medium. When this culture had grown to stationary growth phase, DNA preparation and PCR were repeated, and the absence of wild-type chromosomes was confirmed (see Fig. S4 in the supplemental material).
RESULTS
Heterotrophic capacities of Anabaena sp. PCC 7120.
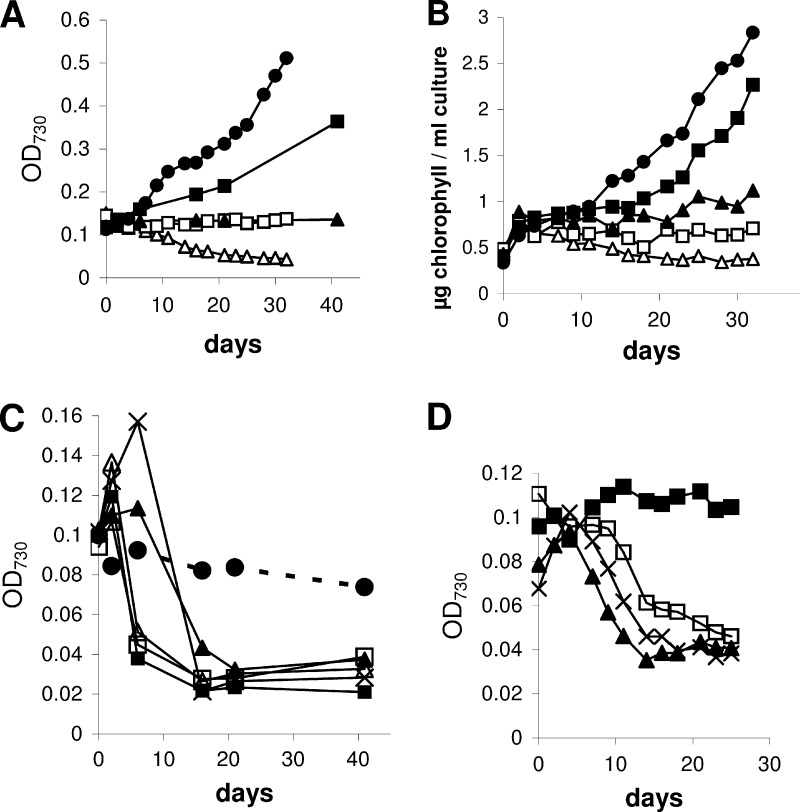
Attempting to generate an Anabaena sp. PCC 7120 mutant potentially capable of heterotrophic growth on glucose, strain PCC 7120G carrying the glucose transporter from Synechocystis sp. PCC 6803 was constructed (see Materials and Methods) and then checked for its heterotrophic capacities using the wild-type PCC 7120 strain as a control. Mixotrophic conditions were attained by adding glucose or fructose to medium BG11 (22) that allows photo-litho-autotrophic growth. Figure 1A shows that PCC 7120G is not able to grow on glucose. Instead, the strain is highly sensitive to this sugar. In contrast, PCC 7120 wild type tolerates up to 200 mM glucose (Fig. 1C) and even shows a slight growth enhancement compared to photo-litho-autotrophic conditions, as has been observed earlier (34). Since the Gtr protein transports not only glucose but also fructose (10), mixotrophy was also tested with fructose. Both PCC 7120G (Fig. 1B) and PCC 7120 (Fig. 1D) show a clear mixotrophic growth (faster than photo-litho-autotrophic growth) on fructose with one significant difference at the highest fructose concentration tested (200 mM). PCC 7120 grew quickly for about 2 weeks, whereas PCC 7120G died immediately. Adding fructose together with glucose to PCC 7120G cultures did not alleviate the toxic effect of glucose (Fig. 1A).
Fig 1.
Mixotrophic growth of PCC 7120G (A and B) and PCC 7120 wild type (C and D). (A) BG11 plus glucose: ◆, 0 mM; □, 5 mM; △, 10 mM; ×, 10 mM glucose plus 50 mM fructose. (B) BG11 plus fructose: ◆, 0 mM; △, 20 mM; ×, 50 mM; ■, 100 mM; □, 200 mM. (C) BG11 plus glucose: □, 0 mM; △, 10 mM; ▲, 50 mM; ■, 200 mM. (D) BG11 plus fructose: □, 0 mM; ▲, 10 mM; ♢, 20 mM; △, 50 mM; ×, 100 mM; ■, 200 mM.
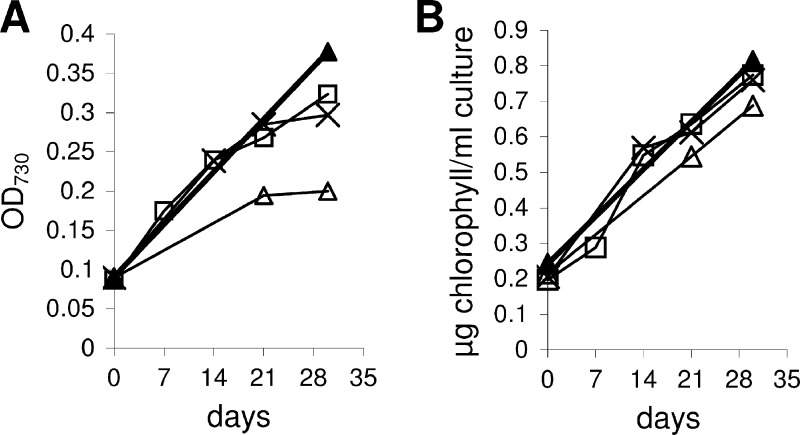
When photosystem II is blocked with DCMU, cyanobacteria lose the capacity for photo-litho-autotrophic growth and become obligate organoheterotrophs (22). Based on the results obtained with mixotrophic growth, both PCC 7120 and PCC 7120G were tested for photo-organoheterotrophic growth with fructose as the substrate. Both strains clearly are capable of photo-organoheterotrophic growth with fructose concentrations of 50 or 100 mM (Fig. 2). A dramatic difference was observed, however, with 200 mM fructose plus DCMU. Whereas the PCC 7120 wild-type strain (Fig. 2B) attained growth rates as high as under photo-litho-autotrophic conditions, the PCC 7120G strain died immediately, just as with DCMU without fructose (Fig. 2A).
Fig 2.
Photo-organoheterotrophic growth of PCC 7120 strains. (A) PCC 7120G: △, 0 mM fructose plus 10 μM DCMU; ■, 50 mM fructose plus 10 μM DCMU; □, 100 mM fructose plus 10 μM DCMU (a growth experiment with 200 mM fructose plus 10 μM DCMU was also performed, but the cells died quickly, exactly as in the experiment with 0 mM fructose plus 10 μM DCMU); ▲, 0 mM fructose plus 0 μM DCMU (photo-litho-autotrophy). (B) PCC 7120 wild type: △, 0 mM fructose plus 10 μM DCMU; ■, 50 mM fructose plus 10 μM DCMU; □, 100 mM fructose plus 10 μM DCMU; ×, 200 mM fructose plus 10 μM DCMU; ▲, 0 mM fructose plus 0 μM DCMU (photo-litho-autotrophy).
The discovery of photo-organoheterotrophic growth in strains PCC 7120 and PCC 7120G prompted us to check for the capacity for chemo-organoheterotrophic dark growth in both strains. The results for PCC 7120G are shown in Fig. 3C. No sustained growth was observed for any fructose concentration. In contrast, PCC 7120 grew chemo-organoheterotrophically with fructose concentrations higher than 50 mM (Fig. 3A and B). As in all growth experiments, we verified whether the cultures were still axenic at all growth stages by microscopic inspection but, in addition, the chlorophyll content was measured, as shown in Fig. 3B. In order to take samples from the chemo-organoheterotrophic cultures for measurement of the OD730 and chlorophyll content, the cultures had to be transferred to a sterile hood. This necessarily exposed the cultures for a few minutes to room light. In Synechocystis sp. PCC 6803, an unusual growth mode has been described, light-activated heterotrophic growth (LAHG) (1). This strain is capable of chemo-organoheterotrophic growth, but only, if 5 to 15 min of light are supplied per 24 h. We therefore conceived an experiment to determine whether the results shown in Fig. 3A and B are due to genuine dark growth or LAHG. Four cultures of PCC 7120 in BG11 plus 100 mM fructose were inoculated at time zero with equal amounts of cells from the same clonal liquid culture, completely wrapped independently in aluminum foil, and incubated in the dark growth room. They were opened for the first time after 1, 2, 3, and 4 weeks, respectively, and the OD730 and chlorophyll content were determined. The results are presented in Fig. 4. Clearly, within the statistical variation observed in all organoheterotrophic growth experiments, the growth rate was independent of the number of times the culture had been exposed to light. Therefore, Anabaena sp. strain PCC 7120 wild type is capable of genuine chemo-organoheterotrophic dark growth.
A striking result obtained with PCC 7120G was that 200 mM fructose was deleterious under all growth modes tested (mixotrophy, photo-organoheterotrophy, and chemo-organoheterotrophy). This led us to check whether PCC 7120G might be sensitive to high osmotic stress. We therefore incubated this strain under chemo-organoheterotrophic conditions with 200 mM sorbitol (Fig. 3C). Not only was this condition not deleterious to the strain, it was actually beneficial, because it prevented the death of the culture that occurred both without fructose and with different concentrations of fructose.
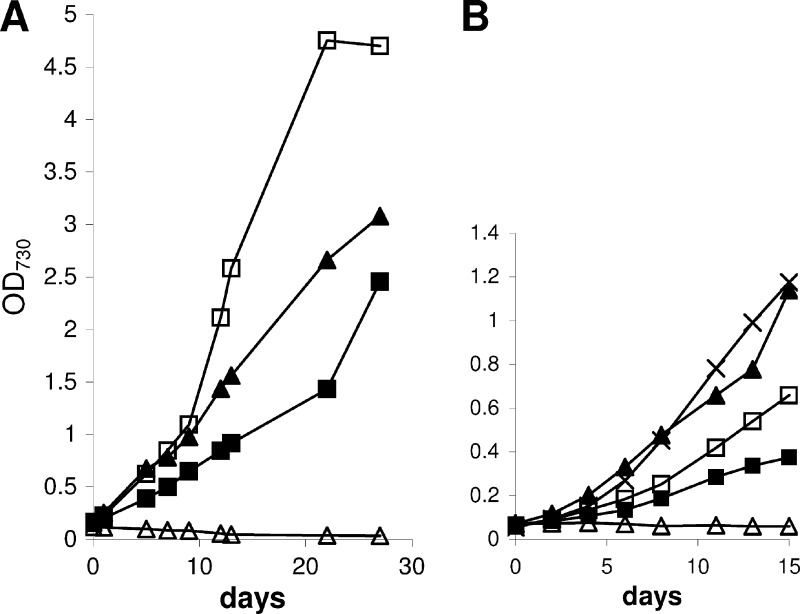
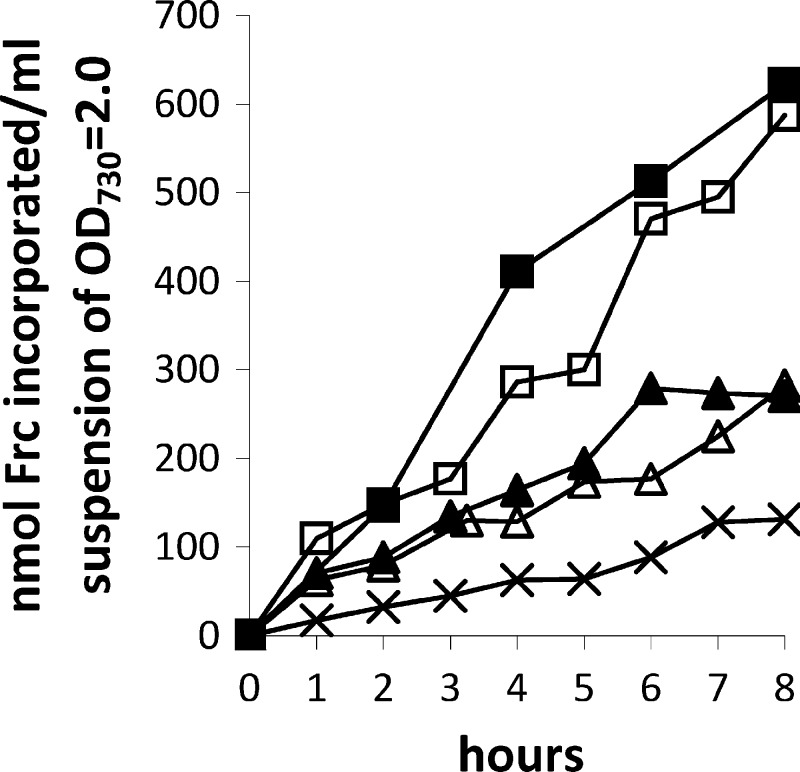
The capacity for heterotrophic growth in strain PCC 7120 implies the existence of a fructose uptake system. In the total genomic sequence of PCC 7120, 16 genes (all0261, all1027, all1823, all1916, all4824, all5282, alr0738, alr0789, alr2532, alr2722, alr3705, alr4277, alr4781, alr5362, alr5367, and alr5368) are currently annotated as components of putative sugar transporters (Cyanobase [http://genome.kazusa.or.jp/cyanobase/Anabaena/genes]). To our knowledge, until now no experimental data on fructose transport in PCC 7120 existed. Therefore, we performed uptake experiments of 14C-labeled d-fructose into PCC 7120 (Fig. 5). In agreement with the growth experiments, the amount taken up by PCC 7120 cells was greater the higher the external fructose concentration was.
Fig 5.
Uptake of [14C]fructose by PCC 7120 is dependent on the external fructose concentration. Symbols: ×, 2 mM; ▲, 10 mM; △, 50 mM; □, 100 mM; ■, 200 mM.
PCC 7120 mutant lacking mitochondrial-type cytochrome c oxidase.
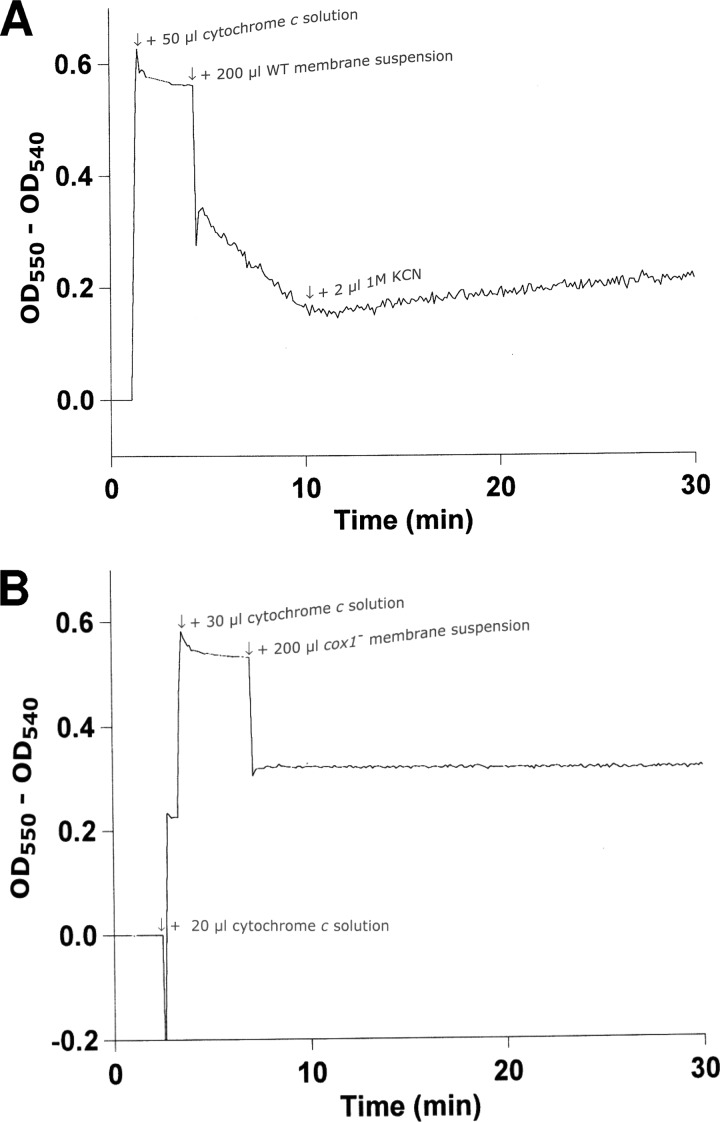
Three genes called coxBAC1 encode subunits II, I, and III, respectively, of the mitochondrial-type cytochrome c oxidase of PCC 7120. Their positions on the PCC 7120 chromosome are alr0950, alr0951, and alr0952 (13). A mutant lacking the central part of the coxA1 gene was constructed by conjugative transfer of plasmid pRStUV3 (see Fig. S3 in the supplemental material) from E. coli strain Top10 to strain PCC 7120. Selection for the first recombination event (integration of pRStUV3 into the chromosome) was performed with neomycin, and selection for the second recombination (loss of the sacB gene on the vector part of the plasmid) was performed with sucrose. The resultant strain was checked for homozygosity by PCR (see Fig. S4 in the supplemental material) and called PCC 7120C. Membranes were isolated from PCC 7120 and PCC 7120C and the in vitro cytochrome c oxidase activity was determined by dual-wavelength spectroscopy (A550 − A540) with prereduced horse heart cytochrome c as the electron donor. The results are presented in Fig. 6. The horse heart cytochrome c oxidation rate in the wild-type membranes (Fig. 6A) was calculated to be 6.23 nmol of cytochrome c per min per mg of protein and inhibited completely by the addition of KCN to a final concentration of 1.3 mM. In contrast, the membranes from PCC 7120C showed no detectable cytochrome c oxidase activity (Fig. 6B).
Fig 6.
Oxidation of prereduced horse heart cytochrome c by membranes from PCC 7120 wild-type (A) and PCC 7120C (B) strains. The concentration of the cytochrome c solution was 18.7 μM. The cytochrome c solution added to the membranes was 66% pre-reduced. The membrane suspensions contained 2.5 mg of protein per ml (PCC 7120 wild type) and 2.1 mg of protein per ml (PCC 7120C).
An interesting phenotype was observed when respiratory dioxygen uptake in the dark was compared in PCC 7120 wild type and PCC 7120C (Table 1). Within the statistical variance the respiration rates of both strains were identical. This implies that the absence of the mitochondrial-type cytochrome c oxidase does not measurably block electron transport ending in O2 as the terminal acceptor and can be compensated for by one or more of the other RTOs. When 50 mM fructose was added to cells respiring in the dark, both PCC 7120 wild type and PCC 7120C showed no significant change in respiration rate. Also, both strains were essentially completely inhibitable by KCN (Table 1). A small effect was observed in the relative inhibition by HQNO (2-heptyl-4-hydroxy quinoline-N-oxide) of the respiratory dioxygen uptake. This substance is a specific inhibitor of the quinol oxidase of the cytochrome bd type in Synechocystis sp. strain PCC 6803 (18). Assuming that this is also the case in PCC 7120, the higher relative inhibition of respiration in the PCC 7120C mutant compared to that in PCC 7120 wild type means that the contribution of this quinol oxidase to the total respiratory dioxygen uptake is higher in PCC 7120C than in the wild type. However, PCC 7120C shows a very significant growth phenotype, it is unable to grow chemo-organoheterotrophically in the dark (Fig. 3D), independent of the fructose concentration used (up to 200 mM).
Table 1.
Respiratory O2 uptake in strains PCC 7120 and PCC 7120Ca
| Strain | Mean respiratory rateb ± SD (n) | % Inhibition with 0.67 mM KCN | Mean % inhibition with 80 μM HQNO ± SD (n) |
|---|---|---|---|
| PCC 7120 (wild type) | 16.7 ± 5.8 (35) | 98.5 | 26.0 ± 7.1 (5) |
| PCC 7120C (Cox mutant) | 16.6 ± 4.3 (21) | 98.3 | 39.4 ± 8.0 (7) |
n, number of independent experiments. HQNO,2-heptyl-4-hydroxy quinoline-N-oxide.
The respiratory rate is expressed as μmol of O2/h/mg of chlorophyll.
In cyanobacteria, mitochondrial-type cytochrome c oxidase has essentially two functions. (i) In photo-litho-autotrophic conditions, this RTO can remove electrons from the photosynthetic electron transport chain between photosystem II and photosystem I, thus regulating electron flow between the two photosystems. (ii) In chemo-organoheterotrophic conditions mitochondrial type cytochrome c oxidase serves as the terminal electron acceptor of the most important respiratory chain and is therefore essential for this growth mode. Neither function is necessary for photo-organoheterotrophic growth. Therefore, we expected and found that photo-organoheterotrophic growth of PCC 7120C in the presence of 10 μM DCMU and 100 mM fructose was possible (data not shown).
DISCUSSION
The main finding of this study is the discovery that Anabaena sp. strain PCC 7120 is not an obligate photo-litho-autotroph, as was believed for decades, but is capable of mixotrophic (Fig. 1C and D), photo-organoheterotrophic (Fig. 2B), and chemo-organoheterotrophic (Fig. 3A and B) growth. Since this strain is widely used for the study of cyanobacterial cell differentiation and nitrogen fixation, we expect our discovery to be quite useful for new experiments. Heterotrophic growth is dependent on unusually high external concentrations of fructose (>50 mM), which is probably the reason why it has not been observed earlier. Such high concentrations are probably necessary, because the uptake of the sugar into the cells is slow. Indeed, the uptake rate of fructose rises with the concentration (up to 200 mM, the highest concentration used, Fig. 5). Chemo-organoheterotrophic growth is true dark growth (Fig. 4) and not the light-activated heterotrophic growth (LAHG) described in Synechocystis sp. strain PCC 6803 (1).
In an earlier work, Ungerer et al. (27) introduced the fructose uptake system of Anabaena variabilis ATCC 29413 into strain PCC 7120. This modified strain was capable of chemo-organoheterotrophic growth with 10 mM fructose. Higher concentrations of fructose (50 mM) were deleterious. A somewhat similar result was obtained when we introduced the glucose/fructose carrier Gtr (GlcP) from Synechocystis sp. strain PCC 6803 into strain PCC 7120. Although the resulting strain PCC 7120G was not capable of chemo-organoheterotrophic growth (Fig. 3C), it grew well photo-organoheterotrophically with fructose concentrations of up to 100 mM, but higher concentrations (200 mM) were toxic (Fig. 2A). Incubation of PCC 7120G in 100 mM fructose plus 10 μM DCMU yielded a growth rate that even surpassed that of photo-litho-autotrophic growth. Therefore, fructose is potentially beneficial for this strain. It is therefore surprising that PCC 7120G is unable to grow chemo-organoheterotrophically with any concentration of fructose (Fig. 3C), although PCC 7120G has an ∼3-fold rate of fructose uptake (at 50 mM) compared to PCC 7120 wild type (data not shown). The glucose transporters from Synechocystis strains are sugar/proton cotransporters (3). A possible reason for the inability of PCC 7120G to grow chemo-organoheterotrophically may therefore be that the necessary proton gradient across the cytoplasmic membrane cannot be established under the limited energy regime in darkness. This explanation is corroborated by the repeatedly observed dark growth of PCC 7120G at the beginning of the dark incubation (see Fig. 3C), followed by death later on. In the first few days of darkness the cells might be able to utilize internal carbon reserves (glycogen) accumulated under prior photo-litho-autotrophic growth, until they become dependent on the exogenous substrate. A possible contribution to cell death of toxic metabolites generated from fructose in PCC 7120G can also not be excluded. Thus, in PCC 7120 chemo-organoheterotrophic growth occurs only in the absence of the Gtr carrier (Fig. 3A, B, and C). This was surprising, because in Synechocystis sp. PCC 6803 the Gtr carrier has the opposite effect: only its presence allows chemo-organoheterotrophic growth (on glucose), while in the absence of the Gtr carrier PCC 6803 is an obligate photo-litho-autotroph (10).
Especially striking is the extreme sensitivity of PCC 7120G to glucose. Whereas wild-type PCC 7120 tolerates 200 mM glucose (Fig. 1C), strain PCC 7120G dies in 5 mM glucose (Fig. 1A). Sugar toxicity in cyanobacteria has been reported in several earlier publications, but the exact reason for this is not yet known. As in PCC 7120, cloning of the Synechocystis sp. PCC 6803 gtr (glcP) gene in the obligate photo-litho-autotrophic cyanobacterium Synechococcus sp. PCC 7942 led to a strain highly sensitive to glucose (36). Fructose is toxic not only for Synechocystis sp. PCC 6803 (10) but also for the related strain Synechocystis sp. PCC 6714 (2). Both strains can grow chemo-organoheterotrophically on glucose but strain PCC 6803 only with the LAHG regime (see above), while strain PCC 6714 is capable of true dark growth. In attempts to understand the reason for sugar toxicity in Synechocystis sp. PCC 6803 it was found that the presence of both light and glucose can induce oxidative stress (15) and the histidine kinase hik31 plays an important regulatory role in sensitivity toward glucose (12). The extremely different behavior of fructose and glucose in several cyanobacterial strains (e.g., in Anabaena sp. PCC 7120G and Synechocystis sp. PCC 6803), however, has found no final explanation thus far.
The phenotypes observed in PCC 7120G versus PCC 7120 wild type imply that the gtr locus is expressed in PCC 7120 using its Synechocystis promoter. This is remarkable, because the promoter sequence of this gene has not yet been identified and nothing is known about its exact location. Even the lack of a Shine-Dalgarno sequence in this gene (23) is apparently no obstacle for gtr function in PCC 7120.
Strain PCC 7120C lacking the major subunit I of mitochondrial-type cytochrome c oxidase is unable to grow chemo-organoheterotrophically (Fig. 3D), although all other four RTOs (see Introduction) are still present. This means that the respiratory chains ending in the other four RTOs are not able to support dark growth. Therefore, possibly in all cyanobacteria capable of chemo-organoheterotrophy, the respiratory chain ending in Cox is essential for this process. Interestingly, the three cyanobacteria, in which this has now been verified (besides PCC 7120 in Anabaena variabilis ATCC 29413 and Synechocystis sp. PCC 6803), have a completely different set of respiratory terminal oxidases: Anabaena variabilis ATCC 29413 has one Cox, four ARTOs, four Qoxes, and one Ptox, while Synechocystis sp. PCC 6803 has one Cox, one ARTO, and one Qox. Apparently, only the respiratory chain ending in Cox, which contains three chemiosmotically active components [NAD(P)H dehydrogenase, cytochrome b6f complex, and Cox], is energetically proficient to allow chemo-organoheterotrophy in cyanobacteria.
Supplementary Material
ACKNOWLEDGMENTS
We thank Project Chlorophyll (H. Springer-Lederer) for financial support.
The technical help of Rebecca Neutzner and Manuel Tieber is gratefully acknowledged.
Footnotes
Published ahead of print 22 June 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Anderson SL, McIntosh L. 1991. Light-activated heterotrophic growth of the cyanobacterium Synechocystis sp. strain PCC 6803: a blue-light-requiring process. J. Bacteriol. 173:2761–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Astier C, Joset-Espardellier F, Meyer I. 1979. Conditions for mutagenesis in the cyanobacterium Aphanocapsa 6714: influence of repair phenomena. Arch. Microbiol. 120:93–96 [Google Scholar]
- 3. Beauclerk AAD, Smith AJ. 1978. Transport of d-glucose and 3-O-methyl-d-glucose in the cyanobacteria Aphanocapsa 6714 and Nostoc strain Mac. Eur. J. Biochem. 82:187–197 [DOI] [PubMed] [Google Scholar]
- 4. Black TA, Cai Y, Wolk CP. 1993. Spatial expression and autoregulation of hetR, a gene involved in the control of heterocyst development in Anabaena. Mol. Microbiol. 9:77–84 [DOI] [PubMed] [Google Scholar]
- 5. Blankenship R. 2008. Whole genome sequence of Nostoc punctiforme ATCC 29133. Join Genome Institute, Walnut Creek, CA: http://genome.jgi-psf.org/nospu/nospu.download.ftp.html [Google Scholar]
- 6. Braun AC, Wood HN. 1962. On the activity of certain essential biosynthetic systems in cells of Vinca rosea L. Proc. Natl. Acad. Sci. U. S. A. 48:1776–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cai Y, Wolk CP. 1990. Use of a conditionally lethal gene in Anabaena sp. strain PCC 7120 to select for double recombinants and to entrap insertion sequences. J. Bacteriol. 172:3138–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elhai J, Wolk CP. 1988. Conjugal transfer of DNA to cyanobacteria. Methods Enyzmol. 167:747–755 [DOI] [PubMed] [Google Scholar]
- 9. Finnegan J, Sherratt D. 1982. Plasmid ColE1 conjugal mobility: the nature of bom, a region required in cis for transfer. Mol. Gen. Genet. 185:344–351 [DOI] [PubMed] [Google Scholar]
- 10. Flores E, Schmetterer G. 1986. Interaction of fructose with the glucose permease of the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 166:693–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoare DS, Ingram LO, Thurston EL, Walkup R. 1971. Dark heterotrophic growth of an endophytic blue-green alga. Arch. Mikrobiol. 78:310–321 [Google Scholar]
- 12. Kahlon S, et al. 2006. A putative sensor kinase, Hik31, is involved in the response of Synechocystis sp. strain PCC 6803 to the presence of glucose. Microbiology 152:647–655 [DOI] [PubMed] [Google Scholar]
- 13. Kaneko T, et al. 2001. Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 8:205–213 [DOI] [PubMed] [Google Scholar]
- 14. Mackinney G. 1941. Absorption of light by chlorophyll solutions. J. Biol. Chem. 140:315–322 [Google Scholar]
- 15. Narainsamy K, Cassier-Chauvat C, Junot C, Chauvat F. 2011. High-performance analysis of the cyanobacterial metabolism via liquid chromatography coupled to a LTQ-Orbitrap mass spectrometer: evidence that glucose reprograms the whole carbon metabolism and triggers oxidative stress. Metabolomics doi:10.1007/s11306-011-0382-4 [Google Scholar]
- 16. Nicolaisen K, et al. 2010. The interplay between siderophore secretion and coupled iron and copper transport in the heterocyst-forming cyanobacterium Anabaena sp. PCC 7120. Biochim. Biophys. Acta 1789:2131–2140 [DOI] [PubMed] [Google Scholar]
- 17. Pils D, Gregor W, Schmetterer G. 1997. Evidence for in vivo activity of three distinct respiratory terminal oxidases in the cyanobacterium Synechocystis sp. strain PCC6803. FEMS Microbiol. Lett. 152:83–88 [DOI] [PubMed] [Google Scholar]
- 18. Pils D, Schmetterer G. 2001. Characterization of three bioenergetically active respiratory terminal oxidases in the cyanobacterium Synechocystis sp. strain PCC6803. FEMS Microbiol. Lett. 203:217–222 [DOI] [PubMed] [Google Scholar]
- 19. Pils D, Wilken C, Valladares A, Flores E, Schmetterer G. 2004. Respiratory terminal oxidases in the facultative chemoheterotrophic and dinitrogen fixing cyanobacterium Anabaena variabilis strain ATCC 29413: characterization of the cox2 locus. Biochim. Biophys. Acta 1659:32–45 [DOI] [PubMed] [Google Scholar]
- 20. Reaston J, Van den Hondel CAMJJ, Van Arkel GA, Stewart WDP. 1982. A physical map of plasmid pDU1 from the cyanobacterium Nostoc PCC7524. Plasmid 7:101–104 [DOI] [PubMed] [Google Scholar]
- 21. Rippka R. 1972. Photoheterotrophy and chemoheterotrophy among unicellular blue-green algae. Arch. Mikrobiol. 87:93–98 [DOI] [PubMed] [Google Scholar]
- 22. Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. 1979. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111:1–61 [Google Scholar]
- 23. Schmetterer G. 1990. Sequence conservation among the glucose transporter from the cyanobacterium Synechocystis sp. PCC 6803 and mammalian glucose transporters. Plant Mol. Biol. 14:697–706 [DOI] [PubMed] [Google Scholar]
- 24. Schmetterer G, Pils D. 2004. Cyanobacterial respiration, p 261–278 In Zannoni D. (ed), Respiration in Archaea and Bacteria. Springer, Dordrecht, Netherlands [Google Scholar]
- 25. Schmetterer G, et al. 2001. The coxBAC operon encodes a cytochrome c oxidase required for heterotrophic growth in the cyanobacterium Anabaena variabilis strain ATCC 29413. J. Bacteriol. 183:6429–6434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith AJ. 1982. Modes of cyanobacterial carbon metabolism, p 47–85 In Carr NG, Whitton BA. (ed), The biology of cyanobacteria. Blackwell Scientific Publications, Oxford, United Kingdom [Google Scholar]
- 27. Ungerer L, Pratte BS, Thiel T. 2008. Regulation of fructose transport and its effect on fructose toxicity in Anabaena spp. J. Bacteriol. 190:8115–8125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Valladares A, Herrero A, Pils D, Schmetterer G, Flores E. 2003. Cytochrome c oxidase genes required for nitrogenase activity and diazotrophic growth in Anabaena sp. PCC 7120. Mol. Microbiol. 47:1239–1249 [DOI] [PubMed] [Google Scholar]
- 29. Valladares A, Maldener I, Muro-Pastor AM, Flores E, Herrero A. 2007. Heterocyst development and diazotrophic metabolism in terminal respiratory oxidase mutants of the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 189:4425–4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wolk CP, et al. 1988. Isolation and complementation of mutants of Anabaena sp. strain PCC 7120 unable to grow aerobically on dinitrogen. J. Bacteriol. 170:1239–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wolk CP, Shaffer PW. 1976. Heterotrophic micro- and macrocultures of a nitrogen-fixing cyanobacterium. Arch. Microbiol. 110:145–147 [DOI] [PubMed] [Google Scholar]
- 32. Wolk CP, Vonshak A, Kehoe P, Elhai J. 1984. Construction of shuttle vectors capable of conjugative transfer from Escherichia coli to nitrogen-fixing filamentous cyanobacteria. Proc. Natl. Acad. Sci. U. S. A. 81:1561–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu X, Elhai J, Wolk CP. 2008. Transcriptional and developmental responses by Anabaena to deprivation of fixed nitrogen. p 383–422 In Herrero A, Flores E. (ed), The cyanobacteria: molecular biology, genomics, and evolution. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 34. Yu G, Shi D, Cai Z, Cong W, Ouyang F. 2011. Growth and physiological features of cyanobacterium Anabaena sp. strain PCC 7120 in a glucose-mixotrophic culture. Chin. J. Chem. Eng. 19:108–115 [Google Scholar]
- 35. Zhang CC, Durand MC, Jeanjean R, Joset F. 1989. Molecular and genetic analysis of the fructose-glucose transport system in the cyanobacterium Synechocystis PCC6803. Mol. Microbiol. 3:1221–1229 [DOI] [PubMed] [Google Scholar]
- 36. Zhang CC, Jeanjean R, Joset F. 1998. Obligate phototrophy in cyanobacteria: more than a lack of sugar transport. FEMS Microbiol. Lett. 161:285–292 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.